Abstract
Costimulation regulates multiple cellular processes of T cells inducing proliferation, expansion and survival. The molecular targets of costimulation might then be useful to augment T cell activities. Two defined targets of costimulatory signals in primary T cells are the anti-apoptotic bcl-2 family molecule Bcl-xL, and Survivin, an Inhibitor of Apoptosis family member that might regulate both cell division and survival. However, the relative importance of, and relationship between, these molecules in primary T cells is not clear. To understand whether they have overlapping or co-operative functions, we used retrovirus-mediated transduction to introduce Bcl-xL and Survivin separately, or together linked by a 2A picornavirus self-cleaving peptide, into antigen (Ag)-responding CD8+ T cells. We found that CD8+ effector T cells expressing both Bcl-xL and Survivin strongly expanded at an early stage and had a long-term survival advantage over cells transduced with either molecule alone. In vivo, with response to tumor-expressed Ag following adoptive T cell transfer, Ag-reactive CD8+ T cells expressing both Bcl-xL and Survivin displayed greatly enhanced tumor protective activity compared to CD8+ T cells expressing either molecule introduced separately. These results indicate that Bcl-xL and Survivin can critically contribute in a co-operative, non-redundant, manner to augment the accumulation and persistence of CD8+ T cells following encounter with Ag. The data provide new insights into why costimulatory signals might need to be sustained over time and suggest a potential novel approach to augment cellular immunotherapy for cancer.
Keywords: Mouse, CD8 T cells, Survivin, Bcl-xL, Antigens/peptides, Costimulation, Cell activation
Introduction
Costimulatory signals are necessary for T cell proliferation, differentiation, survival, and the establishment of memory T cell populations. The activation of T cells without such costimulation may lead to T cell anergy, T cell deletion, or the development of immune tolerance. Therefore, costimulation is essential for mediating efficient T cell responses (1, 2). Most costimulatory molecules belong to either the immunoglobulin superfamily or the tumor necrosis factor receptor (TNFR) family. The immunoglobulin superfamily, also known as the B7 family, is comprised of eight members, each of which has a defined costimulatory or inhibitory activity. The B7 family includes CD80 (B7-1), CD86 (B7-2), B7-H1/PDL1, B7-DC/PDL2, B7RP-1, B7H3, B7H4/B7S1/B7x, and B7S3 (3, 4). Several ligands or receptors of B7 costimulatory molecules have been identified such as CD28 and CTLA-4 (receptors) for B7-1/B7-2 (ligands), inducible costimulator (ICOS) for B7-RP-1, and PD1 for B7-H1/B7-DC (5–10). Several TNF family members expressed by antigen-presenting cells (APC) can serve a costimulatory function in T cell activation by binding to specific TNFR family members expressed on T cells. For example, the 4-1BB and OX40 ligands are expressed on APC, and their receptors (4-1BB, OX40) on T cells have been shown to regulate anti-tumor activities of T cells (11–14). CD27, HVEM, LT-α and CD30 are additional TNFR family members expressed by T cells that might be critical targets for controlling Ag-specific responses (2, 15–17).
Previously we have shown that OX40 can activate the PI3K/PKB pathway and that sustained PKB (Akt) signaling driven by OX40 leads to upregulation of several Bcl-2 family members, including Bcl-xL, Bcl-2, and Bfl-1, that control T cell longevity (18, 19). In addition, OX40 or CD28-mediated PKB activation also promotes Survivin expression that controls T cell proliferation and expansion (20) in conjunction with a kinase termed Aurora B (21). Furthermore, we determined that nuclear factor-kappa B1 (NF-κB1) is a main target of costimulation, which controls expression of Survivin and Aurora B and bcl-2 anti-apoptotic family members (20–22). However, the relative importance and relationship between these molecules in primary T cells is not clear.
Recent strategies have used the foot- and-mouth disease virus (FMDV) 2A or 2A-like elements to create multicistronic vectors capable of generating multiple proteins from the same transcript (23, 24). Some FMDV viruses encode multiple proteins that are cleaved into individual protein products at 2A or 2A-like sequences. The 2A-like sequence contains a canonical Asp-Val/Ile-Glu-X-Asn-Pro-Gly(2A)–Pro(2B) motif, which results in a cleavage between the 2A glycine and the 2B proline (25, 26). This cleavage mechanism is thought to occur as the result of a ribosomal skipping mechanism whereby ribosome activity is modified by the 2A-like sequences, preventing peptide bond formation between the 2A Gly and the 2B Pro. This causes release of the upstream protein while allowing continued translation of the downstream gene (27, 28).
Published data have shown in various ways that a single 2A peptide-linked retroviral vector can be used to generate reliable and versatile vectors for gene therapy and biomedical research. Using the T-cell receptor (TCR): CD3 complex as a test system, a 2A peptide-linked retroviral vector was used to generate all four CD3 proteins (CD3epsilon, gamma, delta, zeta), and restored T cell development and function in CD3-deficient mice (29). In addition, the 2A-like sequences were also utilized to construct a tricistronic vector bearing the human iduronidase (IDUA) gene along with the firefly luciferase and DsRed2 reporter genes. In this study, efficient cleavage was observed and all three proteins were functional in vitro and in vivo, allowing for supratherapeutic IDUA enzyme levels and the coexpression of luciferase and DsRed2 expression (23). More importantly, in order to improve TCR activity, retrovirus-mediated transfer of the modified TCR (TCR-alpha-2A-beta) using a 2A sequence resulted in efficient surface expression and HLA-A2/LMP2 pentamer binding, which suppressed the cell surface expression of a large proportion of endogenous TCR combinations present in primary human T cells (30). More notably, “retrogenic” (Rg) mice were generated by the rapid expression of TCRs in mice using 2A peptide-linked multicistronic retroviral vectors to transduce stem cells, and the Rg mice were comparable to transgenic mice expressing those commonly used TCRs (OT-I, OT-II and AND, and HY) (31, 32). Collectively, these data suggest that a single 2A peptide-linked retroviral vector can be used to express multiple genes.
To understand whether the targets of costimulation have overlapping or synergistic functions, we therefore used retrovirus-mediated transduction to introduce Bcl-xL and Survivin linked by a 2A peptide, into Ag-responding CD8+ T cells. We found that Bcl-xL and Survivin both critically contribute in a synergistic, non-redundant, manner to augment the accumulation and persistence of CD8+ T cells following encounter with Ag. Most significantly, the co-introduction of these molecules into CD8 T cells resulted in their ability to fully protect against tumor growth. These data indicate that co-operation among molecular targets of costimulation is crucial for T cell activation and function mediated by costimulatory signals. Furthermore, genetic modification with targets of costimulation using vectors containing the 2A sequence is able to generate highly active Ag-specific T lymphocytes that could be used for augmented cellular immunotherapy.
Materials and Methods
Mice
OT-I TCR-transgenic mice, expressing a TCR composed of variable (Vβ5 and Vα2) chains responsive to an ovalbumin (OVA) peptide 257–264 (SIINFEKL), were bred on a C57BL/6J background. C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME). All experiments were in compliance with the regulations of the Pennsylvania State University College of Medicine Animal Care committee in accordance with guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care.
Peptides, Chemicals, and Antibodies
OVA 257–264 was synthesized by Abgent, Inc. (San Diego, CA). Anti-CD3 (2C11), anti-CD28 (37.51), mouse IL-2 and IFN-γ were from BD PharMingen (San Diego, CA). Anti-human/mouse Survivin (D-8, sc-17779) and Actin (C2, sc-8432) for Western blot were from Santa Cruz Biotech (Santa Cruz, CA). Bcl-xL (#2762), peroxidase-conjugated anti-rabbit (#7054) or anti-mouse Ig (#7056) for Western blot, were from Cell Signaling Technology (Beverly, MA). All FITC-, PE-, Cyt-, and Apc- conjugated antibodies, Annexin V: PE Apoptosis Detection Kit (559763) and Cytofix/Cytoperm™ (555028) were from BD PharMingen. PKH26 Red Fluorescent Cell Linker Kit (PKH26-GL) and Mitomycin C (M0503) were from Sigma (St. Louis, MO).
T Cells and APC
Naive CD8+ T cells were purified from spleen and lymph nodes by nylon wool depletion, followed by antibody and complement treatment (18). The cells were >90% CD8+ and >95% of these cells expressed the appropriate TCR and a naive phenotype. APC were obtained from spleens of syngeneic non-transgenic mice by depleting T cells. APC were treated with Mitomycin C (100 μg/ml) for 30 min at 37° C.
T Cell Cultures
Cultures were in 48-well plates containing 1 ml RPMI 1640 (Invitrogen) with 10% fetal calf serum (Omega Scientific, CA). Naive CD8+ cells were plated at 5 × 105/ml with 2 × 106/ml APCs and various concentrations of Ag. For determining secondary responses, on day 5 of primary stimulation, 5 × 105 T cells were isolated and recultured with 2 × 106 APCs per ml. For Western blot, live CD8+ T cells were isolated from culture with CD8α (Ly-2) MicroBeads by Miltenyi Biotec (#130-049-401).
Retroviral Transduction
cDNA for human Bcl-xL and Survivin was subcloned into the murine bicistronic retroviral expression vector pMig (33, 34). Retroviral transduction was performed as described before (20). 5 × 105 T cells were stimulated with Ag/APCs. After 2 days, the supernatant was replaced with 1 ml viral supernatant containing 5 μg/ml Polybrene (Sigma), and the cells were spun for 1 hr at 32°C and incubated at 32°C for 8 hr. This was repeated the following day. Viral supernatant was removed and replaced with fresh medium, and T cells were re-cultured. Expression of GFP was determined by flow cytometry gating on Vβ5+ T cells. GFP-expressing T cells were purified by cell sorting using a FACS Vantage SE I high-speed cell sorter (BD Immunocytometry Systems, San Jose, CA).
Adoptive Cell Transfer
T cells were cultured with Ag/APC and transduced on day 2/3 with retroviral vectors (20). Cells were recultured for 2 more days. GFP+ CD8+ T cells were sorted, and 3 × 106 cells were injected i.v. into naive C67BL/6J mice. The following day, mice were challenged i.p with 4 × 106 EG.7 OVA tumor cells in PBS, or PBS without EG.7 cells as a control. Numbers of T cells were calculated based on total cell numbers in the spleen, draining lymph nodes (LN; inguinal, mesenteric, and paraaortic), and the peritoneal cavity, together with percentages of GFP+Vβ+ cells visualized using flow cytometry. At days 3 and 20 after adoptive cell transfer, single-cell suspensions from lymph nodes, spleen, and peritoneal cavity were stimulated with OVA peptide with brefeldin A (Golgiplug; BD Biosciences) for 7 hours at 37°C at 5% CO2. INF-γ and granzyme B were analyzed by intracellular staining, after gating on live CD8+ GFP+ T cells.
Cytokine secretion, cell recovery, proliferation and cell division
Cytokines were measured by ELISA (18). T cell survival in vitro was determined by trypan blue exclusion. Proliferation was measured in triplicate cultures by incorporation of 3H-thymidine (1 μCi/well; ICN Pharmaceuticals) during the last 12 hr of culture. Cell division was assessed by prelabeling T cells with PKH26.
Immunoblotting
Live CD8+ cells were recovered by Ficoll treatment and positive selection with anti-CD8 microbeads (Miltenyi Biotec Inc). Cells were lysed in ice-cold RIPA Lysis Buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, and 1 μg/ml leupeptin) for 30 min. Insoluble material was removed and lysates used for Western blotting. Protein content was determined by Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). Equal amounts (30μg) were loaded onto 4–12% NuPage Bis-Tris precasting gels (SDS-PAGE), transferred onto PVDF membrane (Invitrogen), and immunoblotted. All blots were developed with the ECL immunodetection system (Amersham Pharmacia Biotech, Piscataway, NJ).
Results
Expression of Multiple Genes Using 2A Gene Sequence in Primary CD8+ T Cells
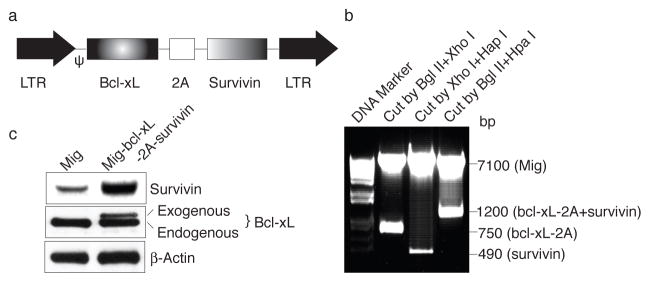
The 2A peptide regions from Picornavirus FMDV (abbreviated herein as F2A), ERAV (E2A) and TaV (T2A) were used to generate several multicistronic cassettes which linked the CD3 or TCR chains to make a single fragment encoding several proteins (29). To generate reliable and versatile constructs to transduce primary CD8+ T cells that permit the expression of multiple genes, we used T2A peptides to generate multicistronic retroviral vectors with efficient translation of two cistrons (e.g., Bcl-xL, Survivin) (Fig. 1A). The human Bcl-xL gene was amplified from a Mig-bcl-xL vector (18) with primers containing F2A gene sequence (Forward: 5′-GAG ATC TAT GTC TCA GAG CAA CCG GGA GCT GGT GGT TGA CTT TCT CTC CTA CAA GCT TTC CCA GAA AGG ATA CAG CTG GAG TCA GTT-3′, Reverse: 5′-GCT CGA GAG GGC CGG GAT TCT CCT CCA CGT CAC CGC ATG TTA GAA GAC TTC CTC TGC CCT CTT TCC GAC TGA AGA GTG A-3′. Italics show the added restriction enzyme sites, and underline shows F2A sequence). Survivin gene was amplified from a Mig-survivin vector (20). Thus, two genes of Bcl-xL and Survivin were linked with the 2A sequence and were subcloned back into the Mig vector. The new construct Mig-bcl-xL-2A-survivin was confirmed by DNA sequencing as well as by restriction digestion, showing fragments of Survivin (~500 bp) and Bcl-xL (~700 bp) (Fig. 1B). Furthermore, naive CD8+ T cells were infected with the retroviral construct, which led to increased expression of both Bcl-xL and Survivin (Fig. 1C).
Figure 1.
Expression of Bcl-xL and Survivin using a 2A gene sequence in primary CD8+ T cells. (a) Schematic representation of the retrovirus construct expressing Bcl-xL and Survivin. Ψ, packaging signal. 2A, picornavirus self-cleaving 2A sequence. (b) Genes of Bcl-xL and Survivin were linked with T2A sequence and were subcloned into Mig vector (Mig-bcl-xL-2A-survivin), which was confirmed by digestion with restriction enzymes, showing fragments of Survivin (~500 bp), Bcl-xL-2A (~750 bp), and bcl-xL-2A-survivin (~1,200 bp). (c) Naive CD8+ T cells from OT-I TCR transgenic mice were stimulated with peptide/APCs. On day 2/3, T cells were transduced with retroviral vectors expressing GFP (Mig), GFP with Bcl-xL (Mig-bcl-xL), GFP with Survivin (Mig-survivin), or GFP with Bcl-xL and Survivin (Mig-bcl-xL-2A-survivin). On day 5 of primary culture, GFP+ CD8+ T cells were sorted, and protein expression of Bcl-xL, Survivin, and β-actin was determined by western blotting. Data are representative of three independent experiments.
Bcl-xL and Survivin Promote Passive Proliferation and Survival of CD8+ T Cells in vitro
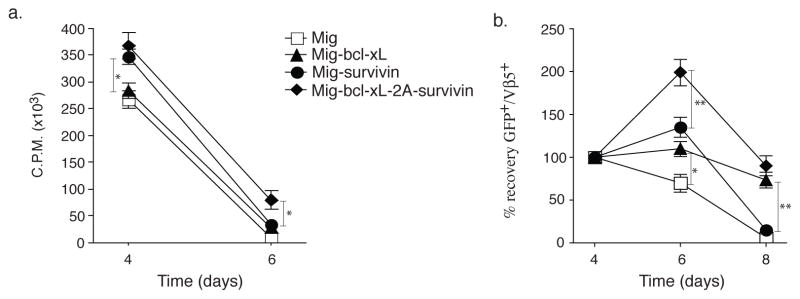
To determine whether enforced co-expression of Bcl-xL and Survivin could contribute to the proliferation and survival of primary CD8+ T cells (20), we transduced Ag-stimulated T cells with the GFP-IRES retroviral vector containing Bcl-xL and Survivin (Mig-bcl-xL-2A-survivin). After transduction on days 2–3, T cells were passively recultured in the absence of further Ag stimulation and their proliferation assessed by thymidine incorporation after 1 (day 4) and 3 days (day 6). Bcl-xL and Survivin forced co-expression in CD8+ T cells resulted in greater passive proliferation at late times at day 6, as measured by thymidine incorporation, compared to forced expression of either molecule alone (Fig. 2a). In line with this, enumerating the recovery of live T cells through monitoring GFP expression showed that expression of Bcl-xL and Survivin allowed CD8+ T cells to expand from day 4 through to day 6 over that engendered by transducing either molecule in isolation. Longer-term culture over 8 days showed that, Bcl-xL and Survivin enhanced the ability of CD8+ T cells to survive comparable to CD8+ T cells transduced with Bcl-xL or Survivin alone (Fig. 2b). Survivin expression in Bcl-xL and Survivin co-transfection was not more than that in Survivin single transfection, indicating that exogenous Bcl-xL did not affect Survivin expression (Fig. 2b). Moreover, the activation marker of CD25 expression was significantly upregulated on day 4 in CD8+ GFP+ T cells, suggesting that recovered T cells were activated CD8+ T cells after T cell priming and gene transfection (Fig. 2c). In addition, the percentage of apoptosis in CD8+ GFP+ T cells was similar compared to transfection of single Bcl-xL and Bcl-xL with Survivin (Fig. 2d). Thus, in this assay, co-expression of both molecules showed features of either Survivin or Bcl-xL when expressed in the single retroviral vectors, but with a strong additive effect when measuring short-term T cell expansion.
Figure 2.
Retroviral transduction of Bcl-xL and Survivin promotes passive proliferation and survival of CD8+ T cells in vitro. Naive CD8+ T cells from OT-I TCR transgenic mice were stimulated with peptide/APCs, and transduced on days 2/3 with retroviral vectors expressing GFP, GFP with Bcl-xL, GFP with Survivin, or GFP with Bcl-xL and Survivin, and then recultured without any further stimulation. (a) Primary passive proliferation on day 4 and day 6 were measured in unseparated cultures by pulsing with tritiated thymidine for 20 hr. Data are representative with mean of three independent experiments (* P<0.05, Student’s unpaired t-test). (b) GFP+Vβ5+ T cell recovery normalized to take into account differences in initial transduction efficiency between cultures. Numbers of GFP+ cells present on day 4 were assigned a value of 100%, and numbers surviving on day 6 and day 8 were used to calculate the percentage recovery relative to day 4. Data represent the mean ± S.D. percentage change from three separate experiments (* P<0.05, ** P<0.01, Student’s unpaired t-test). GFP+ CD8+ T cells on day 6 were sorted, and protein expression of Survivin and β-actin was also determined by western blotting, Data are representative of three independent experiments. (c) CD25 expression on day 4 was analyzed by flow cytometry, after gating on live CD8+ GFP+ T cells. Data are representative of three independent experiments. (d) Apoptosis of GFP+ CD8+ T cells on day 6 based on staining of Annexin V and 7-AAD and analyzed by flow cytometry. Data are representative of three independent experiments.
Bcl-xL and Survivin Augment Recall Responses of CD8+ T Cells in vitro
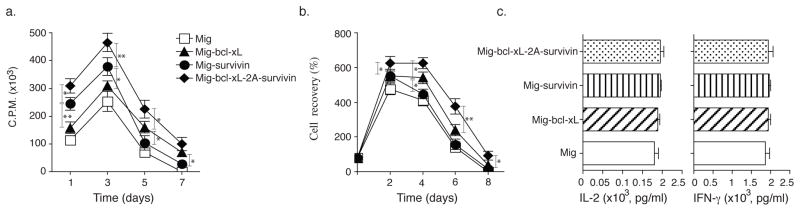
To investigate whether co-expression of Bcl-xL and Survivin promote greater recall responses of CD8+ T cells, effector CD8+ T cells from OT-I TCR transgenic mice expressing Bcl-xL and Survivin from primary naive cultures were sorted based on GFP expression, and equal numbers restimulated with OVA. CD8+ T cells transduced with both Bcl-xL and Survivin displayed enhanced recall proliferation (Fig. 3a), and greater numbers were recovered over time (Fig. 3b) compared to introduction of either molecule in isolation. Also, Survivin expression in Bcl-xL and Survivin co-transfection was not more than that in Survivin single transfection as determined by western blotting (Fig. 3b). In contrast, effector function was not affected in that production of IL-2 or IFN-γ was unaltered regardless of the forced expression of these molecules (Fig. 3c). However, the percentage of apoptosis in CD8+ GFP+ T cells transduced with both Bcl-xL and Survivin was decreased compared to introduction of either molecule in isolation. The percentage of early apoptotic cells (Annexin V+ 7-AAD−) reduced less (3.85% with two molecules versus 5.46% in vector control and 3.93% or 3.98% with Bcl-xL or Survivin), but the percentage of late apoptotic cells (Annexin V+ 7-AAD+) markedly lessened (8.32% with two molecules versus 29.4% in vector control and 13.9% or 15.8% with Bcl-xL or Survivin) (Fig. 3d).
Figure 3.
Retroviral transduction of Bcl-xL and Survivin augments the proliferation and survival of CD8+ T cells in secondary responses in vitro. Naive CD8+ T cells from OT-I TCR transgenic mice were stimulated with peptide/APCs. On day 2/3, T cells were transduced with retroviral vectors expressing GFP, GFP with Bcl-xL, GFP with Survivin, or GFP with Bcl-xL and Survivin. On day 5 of primary culture, GFP+ CD8+ T cells were sorted, and restimulated with APCs/peptide. (a) Recall proliferation on day 1 to day 7 measured by pulsing with tritiated thymidine for the last 20 hr. Data are mean cpm ± S.D. from triplicate cultures and are representative of three experiments (* P<0.05, ** P<0.01, Student’s unpaired t-test). (b) Recall survival, based on recovery of GFP+Vβ5+ T cells over time. Cell numbers present on day 0 were assigned a value of 100%, and cell numbers surviving on day 2 to day 8 were used to calculate the percentage recovery. Data represent the mean ± S.D. percentage change from three separate experiments (* P<0.05, Student’s unpaired t-test). GFP+ CD8+ T cells on day 3 were sorted, and protein expression of Survivin and β-actin was also determined by western blotting, Data are representative of three independent experiments. (c) Recall IL-2 and IFN-γ production were measured by ELISA at 40 h. Data are representative of three independent experiments. (d) Apoptosis of GFP+ CD8+ T cells on day 4 based on staining of Annexin V and 7-AAD and analyzed by flow cytometry. Data are representative of three independent experiments.
Bcl-xL and Survivin Sustain the Persistence of CD8+ T Cells in vivo
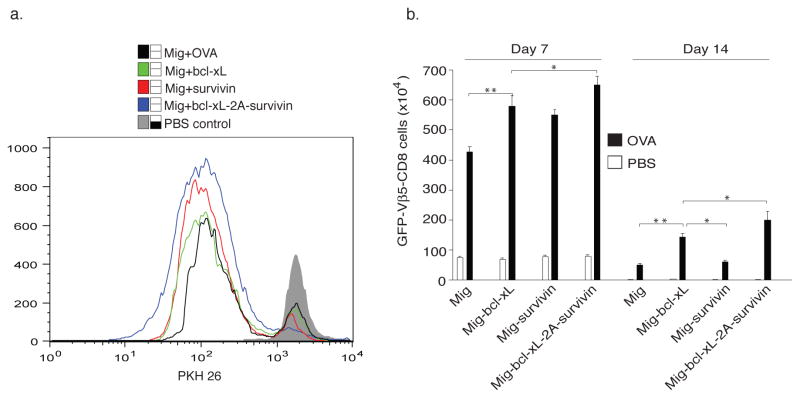
To determine whether Bcl-xL and Survivin were capable of increasing the expansion or persistence of CD8+ T cells in response to antigen presented in vivo, GFP-sorted OT-I T cells, obtained from the in vitro cultures in Fig. 3, were labeled with the dye PKH26, which dilutes as T cells divide, and adoptively transferred into syngeneic recipients. These mice were subsequently challenged with OVA protein. OT-1 T cells transduced with either the vector control or the single genes expanded less over 3 days (lower percentages of diluted PKH26) in lymph nodes and spleen than those CD8 T cells expressing both Bcl-xL and Survivin genes Fig. 4a), supporting the in vitro results (Figs. 1–3). The effect of Bcl-xL and Survivin was additive, and long lasting, with enhanced numbers of Ag-specific T cells not only present 7 days after Ag challenge through the peak of response, but also after 14 days when the secondary in vivo response was over and contraction of T cell populations had occurred in all recipients (Fig. 4b). Overall, these data strongly support the conclusion that a joint action of Bcl-xL and Survivin sustains CD8+ T cell proliferation and long-term survival.
Figure 4.
Retroviral transduction of Bcl-xL and Survivin sustains CD8+ T cell proliferation and long-term survival. Naive CD8+ T cells from OT-I TCR transgenic mice were stimulated with peptide/APCs. On day 2/3, T cells were transduced with retroviral vectors expressing GFP, GFP with Bcl-xL, GFP with Survivin, or GFP with Bcl-xL and Survivin. On day 5 of primary culture, GFP+ CD8+ T cells were sorted, labeled with the dye PKH26, and adoptively transferred into naive recipient mice that were subsequently challenged i.p. with whole OVA protein (100 μg) in PBS (filled bars) or with PBS alone (open bars). (a) Cell division of GFP+ CD8+ T cells on day 3 based on dilution of PKH26. The mean fluorescence intensity of PKH 26 expression, the percentage of diluted PKH 26 in each group and the histogram overlay were shown. Data are representative of three independent experiments. (b) On days 7 and 14, GFP+Vβ5+CD8+ T cells were enumerated from pooled lymph nodes and spleen. Data are mean number of GFP+Vβ5+CD8+ ± S.D. from six individual mice and representative of three independent experiments (* P<0.05, ** P<0.01, Student’s unpaired t-test).
Adoptive Cell Transfer of Bcl-xL and Survivin Transduced CD8+ T Cells Prevents Tumor Growth
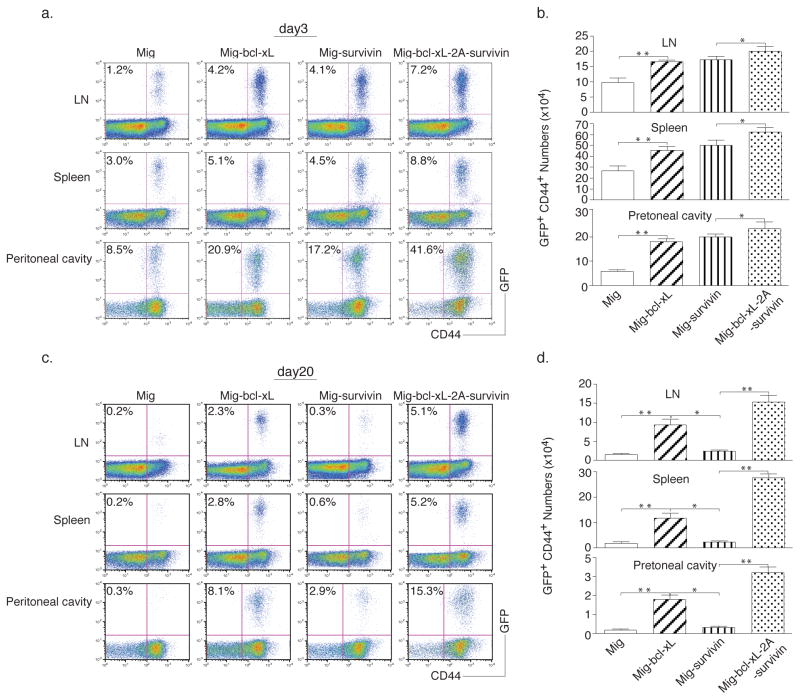
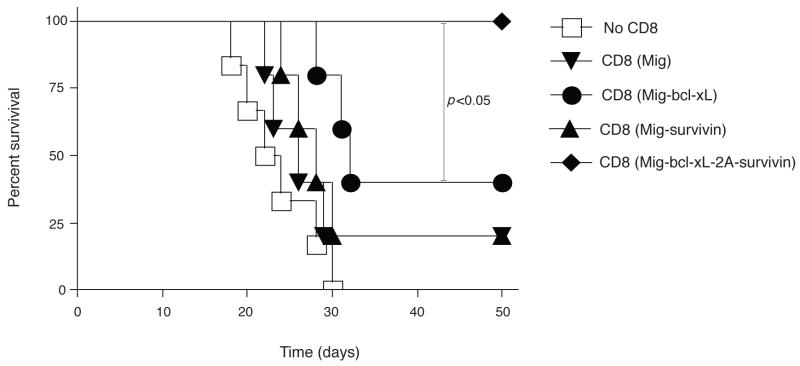
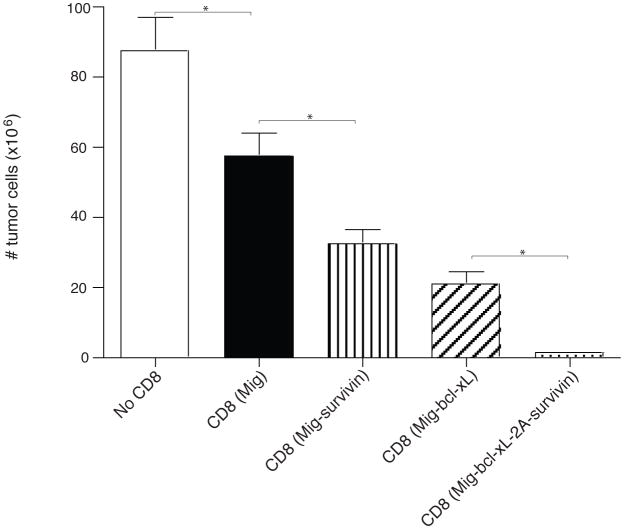
Lastly, to demonstrate that the gene transduction of Bcl-xL and Survivin sustains the CD8+ T cell response in a physiologically and clinically relevant setting, GFP-sorted OT-I T cells were adoptively transferred into syngeneic recipients, and the mice were subsequently challenged with EG.7 tumor cells (EL4-OVA) expressing the OVA antigen recognizable by the T cells. On days 3 and 20 after tumor inoculation, higher numbers of these tumor-reactive CD8+ T cells were observed in the draining lymph nodes (LN), spleen, and peritoneal cavity, as analyzed by flow cytometry gating on CD44+GFP+ cells (Figs. 5a and 5d) and calculating the number of CD8+GFP+ cells (Fig. 5b and 5e). To determine the CTL function of these tumor-reactive CD8+ T cells, CD44+GFP+ cells from LN, spleen and peritoneal cavity were restimulated with the OVA peptide ex vivo and exhibited similar profiles of IFN-γ and Granzyme B by intracellular staining (Fig. 5c and 5f). Most importantly, the mice receiving Survivin and Bcl-xL transduced CD8+ T cells survived up to 50 days after the challenge of EG.7 tumor cells (Fig. 6a) but not the EL4 tumor control cells (without OVA) (Fig. 6b), and remained tumor free (Fig. 7), whereas T cells expressing Survivin or Bcl-xL alone showed more modest effects in protecting against tumor growth. These findings show that the expression of molecular targets of costimulation by retrovirus-mediated transduction can co-operate in CD8+ T cells and promote the persistence of these cells to tumor antigens.
Figure 5.
Retroviral transduction of Bcl-xL and Survivin promotes initial CD8+ T cell expansion in vivo in response to tumor antigen. Naive CD8+ T cells from OT-I TCR transgenic mice were stimulated with APCs/peptide. On day 2/3, T cells were transduced with retroviral vectors expressing GFP, GFP with Bcl-xL, GFP with Survivin, or GFP with Bcl-xL and Survivin. On day 5 of primary culture, GFP+ CD8 T cells were sorted and adoptively transferred into naive recipient mice that were subsequently challenged i.p. with EG.7 tumor cells expressing OVA. At different time points, percentage of GFP+CD44+ T cells was analyzed by flow cytometry, after gating on live CD8+ T cells in the lymph nodes, spleen, and peritoneal cavity (a, d). Only tumor cell challenged mice shown. Results are representative of three experiments. Actual numbers of GFP+CD44+ T cells in pooled lymph nodes (LN: inguinal, mesenteric, and paraaortic; top), spleen (middle), and peritoneal cavity (bottom) (b, e). Data are mean number of GFP+CD44+ T cells ± S.D. from six individual mice (* P<0.05, ** P<0.01, Student’s unpaired t-test). At different time points, single-cell suspensions from pooled lymph nodes, spleen, and peritoneal cavity were stimulated with OVA peptide for 7 hours, INF-γ and granzyme B were analyzed by intracellular staining, after gating on live CD8+ GFP+ T cells (c, f). Data are representative of three independent experiments. (a, b, c) Day 3. (d, e, f) Day 20.
Figure 6.
Adoptive transfer of Bcl-xL and Survivin transduced CD8+ T cells into tumor-bearing mice augments mouse survival. Naïve CD8+ T cells from OT-I TCR transgenic mice were stimulated with APCs/peptide. On day 2/3, T cells were transduced with retroviral vectors expressing GFP, GFP with Bcl-xL, GFP with Survivin, or GFP with Bcl-xL and Survivin. On day 5 of primary culture, GFP+ CD8+ T cells were sorted and adoptively transferred into naive recipient mice that were subsequently challenged i.p. with EG.7 (a) or EL4 (b) tumor cells. Percent survival was studied for 50 days. Kaplan Meier survival analysis indicated significantly increased survival in mice (n=6) receiving Bcl-xL and Survivin transduced CD8+ T cells compared to control groups (log rank test, P<0.05).
Figure 7.
Adoptive cell transfer of Bcl-xL and Survivin transduced CD8+ T cells prevents tumor growth. Naïve CD8+ T cells from OT-I TCR transgenic mice were stimulated with APCs/peptide. On day 2/3, T cells were transduced with retroviral vectors expressing GFP, GFP with Bcl-xL, GFP with Survivin, or GFP with Bcl-xL and Survivin. On day 5 of primary culture, GFP+ CD8+ T cells were sorted and adoptively transferred into naive recipient mice that were subsequently challenged i.p. with EG.7 tumor cells. On day 20, the peritoneal cavity was flushed with 10 ml of ice-cold PBS twice to extract tumor cells and recruited lymphocytes. The total number of cells obtained from each site and the numberof tumor cells from the peritoneal cavity were counted by trypanblue exclusion. Data are mean number of tumor cells ± S.D. from six individual mice (* P<0.05, One-way ANOVA test).
Discussion
Advances in our understanding of costimulatory signals have provided a vast array of novel approaches to prevent autoimmune, infectious, and inflammatory diseases, and cancer (35–38). It has become clear that simple recognition of antigen is not sufficient for generating long-lived productive T cell responses, and that costimulatory signals play an important role in determining immunity and disease. However, the extent of integration of costimulatory receptor signaling with TCR signaling is still not clear. Although data already show costimulatory signals are necessary for T cells to display optimal activation, cytokine production, survival, and memory generation, how these signals function still needs to be unraveled. Elucidating the molecular targets of costimulation will provide new insight into understanding the importance of individual molecules in Ag-reactive T cells, and may help to define novel targets for augmenting T-cell immunity against diseases. In this paper, we now show that Bcl-xL and Survivin, two targets of costimulatory signals, can contribute in a co-operative and non-redundant manner to augment the accumulation and persistence of CD8+ T cells following encounter with Ag.
We previously reported that OX40 signals regulate T cell number and viability through the NF–κB1 pathway that controls expression and activity of intracellular targets for proliferation and survival (22). Also, PKB activation from OX40 or CD28 is required for upregulation and maintenance of cell division and survival factors such as Bcl-2, Bcl-xL, Bfl-1, and Survivin or Aurora B (18–20). Thus, the intracellular PI3K/PKB/NF-κB signal transduction pathway is critical for costimulation-mediated T cell activation and function. In the current study, using Picornavirus ‘self-cleaving’ 2A sequence, we linked Bcl-xL and Survivin in a retroviral vector that permitted their equal expression in primary T cells. Our results clearly showed that by using this 2A sequence, Bcl-xL and Survivin could be concomitantly expressed in primary CD8+ T cells after retrovirus-mediated transduction. Furthermore, Bcl-xL and Survivin co-operated to sustain T cell division and survival over time, and hence we conclude they coordinately regulate the extent of clonal expansion of primary effector and memory effector T cells.
The 2A-like sequences are small (63 bp in the case of P2A and 54 bp in T2A), making the multiple cistronic construct ideal for use in size-restricted viral and non-viral vectors, which can promote equal expression of several genes (total size < 4,000 bp) (24, 31). Use of the small 2A-like sequences may then allow for the development of multicistronic vectors that allow for coupled expression of several genes as we have documented here, as well as for the development of tools that can be used for the generation of highly active immune cells even when vector expression is size-restricted. Previously, the 2A-like sequences have been successfully utilized to generate all four CD3 proteins that restored T cell development and function in CD3-deficient mice, as well as to generate Ag-specific TCR Rg mice or T cells (29–31). Our data provide new insights into the potential uses of the 2A-like sequences to generate reliable and versatile vectors that can modulate T cell activation and function.
Generation of long-lasting antigen-specific T cell immunity to human tumors is one of the major challenges faced by cancer immunology research (39–41). Recent advances in the use of in vitro activated tumor-specific T cells that can be re-infused into humans raise the possibility that this strategy may be successfully utilized for the treatment of cancer (42–44). After primed T cells of the effector and/or memory sub-types are generated, they are suitable for adoptive cell transfer. However, results from both mouse studies and clinical trials indicate that intrinsic properties related to the differentiation state of the adoptively transferred T cell populations are crucial to the success of these approaches (45, 46). For adoptive cell transfer, the in vitro generation of less-differentiated, central memory-like tumor-specific T cells for in vivo re-infusion is the optimal approach because these cells have a high proliferative potential and are less prone to apoptosis, and have a greater ability to respond to homeostatic cytokines, such as IL-7 (47–50). Over the last several years, the efficacy of adoptive immunotherapy by transferring tumor-specific CD8+ T cells at various stages of differentiation into tumor-bearing mice has been evaluated. These studies concluded that administration of naive or early effector T cells, in combination with active immunization and IL-2, resulted in the eradication of large, established tumors (51). Also, tumor Ag-reactive CD8+ T cell populations with the phenotypic and functional attributes of central memory T cells were reported to be superior to effector memory T cells for adoptive immunotherapy (52). In addition, memory cell precursors present in the effector CD8+ T cell population have been investigated, and research identified that IL-7 receptor-chain (IL-7R) hi effector CD8+ T cells were able to differentiate into memory cells (53). Thus, intrinsic cellular properties that permit their survival and proliferation are critical for memory T cell development, and hence the generation of optimal anti-tumor immunity.
Previously, we have identified Bcl-xL and Survivin as two important targets of costimulation that regulate T cell survival and proliferation. In this study, we generated highly active antigen-specific CTLs that were genetically modified with introduction of Bcl-xL and Survivin genes in vitro, which then persisted after re-infusion. Our results showed that adoptive cell transfer of the generated CTLs provided an efficient treatment for prevention of growth of the lymphoma used within our murine tumor model. This is significant since it suggests the retroviral transfection technique can be used to develop novel strategies for the generation of highly active tumor-specific CTLs in vitro, which will induce long-lasting anti-tumor immunity in vivo.
Acknowledgments
This work was supported by grants from the Pennsylvania Department of Health and the St. Baldrick’s Foundation to J.S, and NIH grants CA91837 and AI49453 to M.C.
We thank Dr. Dario Vignali (Department of Immunology, St. Jude Children’s Research Hospital) for help on designing the construct of Mig-bcl-xL-2A-survivin.
The abbreviations used are
- Ag
antigen
- TCR
T cell receptor
- APC
antigen presenting cells
- CTL
cytotoxic T lymphocytes
- TNFR
tumor necrosis factor receptor
- NF–κB1
nuclear factor of kappa light polypeptide gene enhancer in B-cells 1
- PI3K
phosphoinositide Kinase-3
- PKB
protein kinase B/Akt
- FMDV
foot-and-mouth disease virus
- OVA
ovalbumin
- GFP
green fluorescent protein
- IRES
internal ribosome entry site
- Rg
retrogenic
References
- 1.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 2.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Liu XK, Nguyen T, Bishop C, Graf D, Dong C. Characterization of B7S3 as a novel negative regulator of T cells. J Immunol. 2007;178:3661–3667. doi: 10.4049/jimmunol.178.6.3661. [DOI] [PubMed] [Google Scholar]
- 4.Zang X, Allison JP. To be or not to be B7. J Clin Invest. 2006;116:2590–2593. doi: 10.1172/JCI30103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khoury SJ, Sayegh MH. The roles of the new negative T cell costimulatory pathways in regulating autoimmunity. Immunity. 2004;20:529–538. doi: 10.1016/s1074-7613(04)00116-5. [DOI] [PubMed] [Google Scholar]
- 6.Rietz C, Chen L. New B7 family members with positive and negative costimulatory function. Am J Transplant. 2004;4:8–14. doi: 10.1046/j.1600-6143.2003.00303.x. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 8.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Tuder RM, Grosso JF, Harris TJ, Getnet D, Whartenby KA, Brockstedt DG, Dubensky TW, Jr, Chen L, Pardoll DM, Drake CG. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–192. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, Ma J, Tezuka K, Yagita H, Okumura K. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005;175:2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- 11.Glenney GW, Wiens GD. Early diversification of the TNF superfamily in teleosts: genomic characterization and expression analysis. J Immunol. 2007;178:7955–7973. doi: 10.4049/jimmunol.178.12.7955. [DOI] [PubMed] [Google Scholar]
- 12.Bossen C, Ingold K, Tardivel A, Bodmer JL, Gaide O, Hertig S, Ambrose C, Tschopp J, Schneider P. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem. 2006;281:13964–13971. doi: 10.1074/jbc.M601553200. [DOI] [PubMed] [Google Scholar]
- 13.Serghides L, Bukczynski J, Wen T, Wang C, Routy JP, Boulassel MR, Sekaly RP, Ostrowski M, Bernard NF, Watts TH. Evaluation of OX40 ligand as a costimulator of human antiviral memory CD8 T cell responses: comparison with B7.1 and 4-1BBL. J Immunol. 2005;175:6368–6377. doi: 10.4049/jimmunol.175.10.6368. [DOI] [PubMed] [Google Scholar]
- 14.Dawicki W, Bertram EM, Sharpe AH, Watts TH. 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol. 2004;173:5944–5951. doi: 10.4049/jimmunol.173.10.5944. [DOI] [PubMed] [Google Scholar]
- 15.French RR, V, Taraban Y, Crowther GR, Rowley TF, Gray JC, Johnson PW, Tutt AL, Al-Shamkhani A, Glennie MJ. Eradication of lymphoma by CD8 T cells following anti-CD40 monoclonal antibody therapy is critically dependent on CD27 costimulation. Blood. 2007;109:4810–4815. doi: 10.1182/blood-2006-11-057216. [DOI] [PubMed] [Google Scholar]
- 16.Gavrieli M, Sedy J, Nelson CA, Murphy KM. BTLA and HVEM cross talk regulates inhibition and costimulation. Adv Immunol. 2006;92:157–185. doi: 10.1016/S0065-2776(06)92004-5. [DOI] [PubMed] [Google Scholar]
- 17.Silva-Santos B, Pennington DJ, Hayday AC. Lymphotoxin-mediated regulation of gammadelta cell differentiation by alphabeta T cell progenitors. Science. 2005;307:925–928. doi: 10.1126/science.1103978. [DOI] [PubMed] [Google Scholar]
- 18.Song J, Salek-Ardakani S, Rogers PR, Cheng M, Van Parijs L, Croft M. The costimulation-regulated duration of PKB activation controls T cell longevity. Nat Immunol. 2004;5:150–158. doi: 10.1038/ni1030. [DOI] [PubMed] [Google Scholar]
- 19.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–455. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 20.Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 2005;22:621–631. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Song J, Salek-Ardakani S, So T, Croft M. The kinases aurora B and mTOR regulate the G1-S cell cycle progression of T lymphocytes. Nat Immunol. 2007;8:64–73. doi: 10.1038/ni1413. [DOI] [PubMed] [Google Scholar]
- 22.Song J, So T, Croft M. Activation of NF-kappaB1 by OX40 contributes to antigen-driven T cell expansion and survival. J Immunol. 2008;180:7240–7248. doi: 10.4049/jimmunol.180.11.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osborn MJ, Panoskaltsis-Mortari A, McElmurry RT, Bell SK, Vignali DA, Ryan MD, Wilber AC, McIvor RS, Tolar J, Blazar BR. A picornaviral 2A-like sequence-based tricistronic vector allowing for high-level therapeutic gene expression coupled to a dual-reporter system. Mol Ther. 2005;12:569–574. doi: 10.1016/j.ymthe.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Bosselut R. Retroviral TCR gene transduction: 2A for two. Nat Methods. 2006;3:162–164. doi: 10.1038/nmeth0306-162. [DOI] [PubMed] [Google Scholar]
- 25.Donnelly ML, Hughes LE, Luke G, Mendoza H, ten Dam E, Gani D, Ryan MD. The ‘cleavage’ activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring ‘2A-like’ sequences. J Gen Virol. 2001;82:1027–1041. doi: 10.1099/0022-1317-82-5-1027. [DOI] [PubMed] [Google Scholar]
- 26.Luke GA, de Felipe P, Lukashev A, Kallioinen SE, Bruno EA, Ryan MD. Occurrence, function and evolutionary origins of ‘2A-like’ sequences in virus genomes. J Gen Virol. 2008;89:1036–1042. doi: 10.1099/vir.0.83428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donnelly ML, Luke G, Mehrotra A, Li X, Hughes LE, Gani D, Ryan MD. Analysis of the aphthovirus 2A/2B polyprotein ‘cleavage’ mechanism indicates not a proteolytic reaction, but a novel translational effect: a putative ribosomal ‘skip’. J Gen Virol. 2001;82:1013–1025. doi: 10.1099/0022-1317-82-5-1013. [DOI] [PubMed] [Google Scholar]
- 28.de Felipe P, Hughes LE, Ryan MD, Brown JD. Co-translational, intraribosomal cleavage of polypeptides by the foot-and-mouth disease virus 2A peptide. J Biol Chem. 2003;278:11441–11448. doi: 10.1074/jbc.M211644200. [DOI] [PubMed] [Google Scholar]
- 29.Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DA. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 30.Hart DP, Xue SA, Thomas S, Cesco-Gaspere M, Tranter A, Willcox B, Lee SP, Steven N, Morris EC, Stauss HJ. Retroviral transfer of a dominant TCR prevents surface expression of a large proportion of the endogenous TCR repertoire in human T cells. Gene Ther. 2008;15:625–631. doi: 10.1038/sj.gt.3303078. [DOI] [PubMed] [Google Scholar]
- 31.Holst J, Szymczak-Workman AL, Vignali KM, Burton AR, Workman CJ, Vignali DA. Generation of T-cell receptor retrogenic mice. Nat Protoc. 2006;1:406–417. doi: 10.1038/nprot.2006.61. [DOI] [PubMed] [Google Scholar]
- 32.Holst J, Vignali KM, Burton AR, Vignali DA. Rapid analysis of T-cell selection in vivo using T cell-receptor retrogenic mice. Nat Methods. 2006;3:191–197. doi: 10.1038/nmeth858. [DOI] [PubMed] [Google Scholar]
- 33.Denk A, Goebeler M, Schmid S, Berberich I, Ritz O, Lindemann D, Ludwig S, Wirth T. Activation of NF-kappa B via the Ikappa B kinase complex is both essential and sufficient for proinflammatory gene expression in primary endothelial cells. The Journal of biological chemistry. 2001;276:28451–28458. doi: 10.1074/jbc.M102698200. [DOI] [PubMed] [Google Scholar]
- 34.Herrmann O, Baumann B, de Lorenzi R, Muhammad S, Zhang W, Kleesiek J, Malfertheiner M, Kohrmann M, Potrovita I, Maegele I, Beyer C, Burke JR, Hasan MT, Bujard H, Wirth T, Pasparakis M, Schwaninger M. IKK mediates ischemia-induced neuronal death. Nat Med. 2005;11:1322–1329. doi: 10.1038/nm1323. [DOI] [PubMed] [Google Scholar]
- 35.Ochi S, Shinohara M, Sato K, Gober HJ, Koga T, Kodama T, Takai T, Miyasaka N, Takayanagi H. Pathological role of osteoclast costimulation in arthritis-induced bone loss. Proc Natl Acad Sci U S A. 2007;104:11394–11399. doi: 10.1073/pnas.0701971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thebeau LG, Vagvala SP, Wong YM, Morrison LA. B7 costimulation molecules expressed from the herpes simplex virus 2 genome rescue immune induction in B7-deficient mice. J Virol. 2007;81:12200–12209. doi: 10.1128/JVI.01224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seshasayee D, Lee WP, Zhou M, Shu J, Suto E, Zhang J, Diehl L, Austin CD, Meng YG, Tan M, Bullens SL, Seeber S, Fuentes ME, Labrijn AF, Graus YM, Miller LA, Schelegle ES, Hyde DM, Wu LC, Hymowitz SG, Martin F. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117:3868–3878. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narni-Mancinelli E, Campisi L, Bassand D, Cazareth J, Gounon P, Glaichenhaus N, Lauvau G. Memory CD8+ T cells mediate antibacterial immunity via CCL3 activation of TNF/ROI+ phagocytes. J Exp Med. 2007;204:2075–2087. doi: 10.1084/jem.20070204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura Y, Watchmaker P, Urban J, Sheridan B, Giermasz A, Nishimura F, Sasaki K, Cumberland R, Muthuswamy R, Mailliard RB, Larregina AT, Falo LD, Gooding W, Storkus WJ, Okada H, Hendricks RL, Kalinski P. Helper function of memory CD8+ T cells: heterologous CD8+ T cells support the induction of therapeutic cancer immunity. Cancer Res. 2007;67:10012–10018. doi: 10.1158/0008-5472.CAN-07-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benson MJ, Dillon SR, Castigli E, Geha RS, Xu S, Lam KP, Noelle RJ. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol. 2008;180:3655–3659. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
- 42.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jorritsma A, Gomez-Eerland R, Dokter M, van de Kasteele W, Zoet YM, Doxiadis, Rufer N, Romero P, Morgan RA, Schumacher TN, Haanen JB. Selecting highly affine and well-expressed TCRs for gene therapy of melanoma. Blood. 2007;110:3564–3572. doi: 10.1182/blood-2007-02-075010. [DOI] [PubMed] [Google Scholar]
- 44.Augustine CK, Yoshimoto Y, Gupta M, Zipfel PA, Selim MA, Febbo P, Pendergast AM, Peters WP, Tyler DS. Targeting N-cadherin enhances antitumor activity of cytotoxic therapies in melanoma treatment. Cancer Res. 2008;68:3777–3784. doi: 10.1158/0008-5472.CAN-07-5949. [DOI] [PubMed] [Google Scholar]
- 45.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 48.Seki Y, Yang J, Okamoto M, Tanaka S, Goitsuka R, Farrar MA, Kubo M. IL-7/STAT5 cytokine signaling pathway is essential but insufficient for maintenance of naive CD4 T cell survival in peripheral lymphoid organs. J Immunol. 2007;178:262–270. doi: 10.4049/jimmunol.178.1.262. [DOI] [PubMed] [Google Scholar]
- 49.Stemberger C, Huster KM, Koffler M, Anderl F, Schiemann M, Wagner H, Busch DH. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Siewert C, Lauer U, Cording S, Bopp T, Schmitt E, Hamann A, Huehn J. Experience-driven development: effector/memory-like alphaE+Foxp3+ regulatory T cells originate from both naive T cells and naturally occurring naive-like regulatory T cells. J Immunol. 2008;180:146–155. doi: 10.4049/jimmunol.180.1.146. [DOI] [PubMed] [Google Scholar]
- 51.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]