Abstract
Background
While corticosteroids and β-2 agonists are effective in managing asthma symptoms, a curative therapy for asthma is lacking. Traditional Chinese medicine (TCM), used in Asia for centuries, is beginning to play a role in Western health care as a complementary and alternative medicine (CAM) modality. There is increasing scientific evidence supporting the use of TCM for asthma treatment.
Objective
This review article discusses promising TCM interventions for asthma and explores their possible mechanisms of action.
Methods
We first reviewed five clinical studies of “anti-asthma” TCM herbal remedies published between 2005–2007. We then summarized possible mechanisms underlying their effects based on data in the original articles, published abstracts, and available data bases. Possible mechanisms include anti-inflammation, inhibition of airway smooth muscle contraction, and immunomodulation. Research on TCM herbal therapy for food allergy is rare, and we therefore focused on the effect and mechanism of action of Food Allergy Herbal Formula-2 (FAHF-2) on a murine model of peanut allergy and preliminary clinical study results.
Conclusion
Evidence from clinical studies supports beneficial effects of TCM herbal therapy on asthma. A number of mechanisms may be responsible for efficacy of these agents. Strong preclinical study data suggest potential efficacy of FAHF-2 for food allergy.
Keywords: Complementary and Alternative Medicine, traditional Chinese herbal medicine, botanical drug, asthma, food allergy, Th1/Th1 balance
Introduction
Asthma is a chronic respiratory disease that affects 300 million adults and children worldwide including, 15.7 million adults and 6.5 million children in the USA (1). The prevalence has increased by 50% in the past few decades, particularly in westernized countries. While corticosteroids and β-2 agonists are effective in managing asthma symptoms, there is no curative therapy. There are also concerns regarding the side effects from chronic use of current drugs, particularly by children. The chronic nature of asthma and lack of preventive and curative therapy is leading asthma patients in western societies to seek complementary and alternative medicine (CAM) treatment (2;3). Although there are wide variations in reported use of CAM, a reasonable estimate is that up to 30% of adults and 60% of children in the US are currently using some form of CAM to treat their asthma (4). Thus there is a need for defining and developing reliable CAM therapy for patients.
Traditional Chinese medicine (TCM) has a long history of human use and is one of the major components of CAM utilized in the US. It has a unique (independent) system of theory, diagnosis and treatment tools. The National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health (NIH) defined TCM as Whole Medical Systems (5) In the US, TCM is mainly provided by licensed practitioners and is playing an increasing role in the health care system. Chinese herbal medicines are one of the major components in TCM practice, and is prescribed in hospitals in China as either monotherapy or complementary therapy to conventional western therapy. However, TCM remains to be established as part of mainstream medicine in the US. Unlike in China and other Asian countries, TCM herbal products are viewed as dietary supplements in the US and the cost is not covered by most insurance policies. This may contribute to the gap between scientific evidence-based medicine and human use-based practice [West meets East].(6;7) The NCCAM/NIH now supports clinical and basic research on CAM. In recent years the US FDA has provided guidance for investigating botanical drug products, including complex formulas containing several focusing on efficacy, safety and consistency (8). NIH support and FDA guidelines will foster the development of botanical drugs in the US. Thus certain beneficial TCM products currently used as dietary supplements for asthma and allergy may be investigated as new botanical drugs.
In contrast to the long human use history and popularity of TCM herbal medicines for asthma, evidence-based research into its efficacy and mechanisms of efficacy is still in its infancy. Although a number of anti-asthma herbal formulas are recorded in TCM text books and utilized in TCM practice, (9;10) only a few have been studied. Several literature reviews of herbal medicines for asthma have reported that evidence for clinical efficacy is weak, although some herbal preparations induced improvement in asthma symptoms and FEV1. Although these early clinical investigations of herbal medicines are far from satisfactory, these reviews concluded that additional well-designed, i.e. double-blind, placebo-controlled trials of herbal medicines are warranted because of the promising findings and increased popularity of herbal use for asthma. It should be noted that previous reviews included findings from all herbal medication publications, including Chinese, Japanese (Kampo), Indian, Latin American, Hawaiian, and Western herbal medicines. Since herbal medicines used in TCM practice differ, including Japanese and Korean herbal medicines that are derivatives of TCM, separate sections specifically summarizing clinical studies relevant to TCM appear to be more positive. Huntley and Ernst (2000) (11) for example, reviewed herbal interventions for asthma, in which 17 controlled studies were included. Six of these studies used traditional Chinese herbal medicines, and four reported significantly increased FEV1 levels (p<0.05-0.01) when compared to controls. However, these studies were not double-blind.
Since 2005, several new English language publications have reported results of double-blind, placebo-controlled clinical studies investigating efficacy and safety of TCM herbal products for asthma. Some publications also provided evidence regarding possible mechanisms underlying the reported clinical efficacy.(12) One of these TCM herbal products is undergoing clinical trials in the US under a US FDA botanical investigational new drug (IND) title. In this review, we will focus on the clinical effects of TCM herbal therapy for asthma and the possible underlying mechanisms reported in English language publications since 2005.
The prevalence of food allergy is increasing in western societies including the United States. It is now estimated that 6% of children under the age of 3 and 4% of adults are allergic to food. (13) At the present time there is no definitive treatment for food allergy. Although research in TCM herbal therapy for food allergy is still very limited, food allergy herbal formula-2 (FAHF-2) has demonstrated excellent efficacy in a murine model of peanut allergy (14–16)and a clinical trial is ongoing in the US (IND 77,468). In this review, we will also review the preclinical efficacy, safety and mechanisms of FAHF-2, and updated information about a FAHF-2 clinical trial..
This review is not a general systematic review and will not address whether earlier herbal medicine studies beginning in the early1980th demonstrated efficacy. This review discusses promising TCM interventions for asthma and allergy, and explores their possible mechanisms of action In addition, according to FDA guidelines for botanical drug development, and the use of advanced chromatographical technique and available TCM phytochemistry data bases (17), we also provide information regarding herbal product quality control, and chemical characteristics of botanicals aimed at botanical drug development for asthma and allergy.
Part I. Efficacy and Mechanisms of actions of traditional Chinese medicine for treating asthma
A. Clinical efficacy of Chinese herbal medicine for asthma
1. Chinese herbal medicine for asthma as monotherapy
Wen et al 2005(12) reported the first double-blind, randomized, placebo-controlled trial investigating the efficacy and tolerability of an anti-asthma herbal medicine intervention (ASHMI, which contains 3 herbs, table 1) compared to oral prednisone therapy on 91 patients 18–60 years of age with moderate-to-severe asthma. Subjects in the ASHMI group (45 patients) received oral ASHMI capsules (4 capsules, tid, 0.3 g/capsule) and placebo tablets similar in appearance to prednisone. Subjects in the prednisone group (46 patients) received oral prednisone tablets (20mg qd in the morning) and “ASHMI placebo capsules” for 4 weeks. Treatment was administered daily over 4 weeks. This study found that following treatment, lung function (FEV1 and peak expiratory flow values) was significantly improved in both ASHMI (64.9± 6 3.6 to 84.2± 6 5.0; P < 0.001) and prednisone (65.2± 6 3.7 to 88.4 ± 6 8.0; P < 0 .001) groups. The improvement was slightly but significantly greater in the prednisone group (P < 0.05). There was a significant and similar degree of reduction in clinical symptom scores in both treated groups (median [range], ASHMI, 5.0 [4–8] to 2.0 [0–4]; P < 0.001; prednisone, (5.0 [4–7] to 2.0 [0–4]; P < 0.001), use of β2-bronchodilators (median [range], ASHMI, 4.7 [3.5–5.7] to 0.9 [0.14–2.3]; P < 0.001; prednisone, 4.7 [3.5–5.6] to 0.6 [0.3–1.0]; P < 0.001). ASHMI had no significant effect on body weight (increases in body weight post-therapy was 2.8 kg in the prednisone group vs 0.8 kg in ASHMI). No significant side effects were observed in either group. All hematological, electrocardiographic and liver and kidney function test results were normal in both groups. Thus ASHMI appeared to be effective and well tolerated, and may offer benefits comparable to standard prednisone therapy for some patients without the undesirable side-effects associated with steroid use.
Table 1.
Summary of Clinical Studies for Asthma published 2005 to 2007
| TCM Formula | ASHMI (1) | mMMDT (2) | Ding Chuan Tang (3) | STA-1(4) |
|---|---|---|---|---|
| Publication Date | (2005) | (2006) | (2006) | (2006) |
| Number of Herbs | 3 | 5 | 9 | 10 |
| Type of study | RCT | RCT | RCT | RCT |
| Sample size | n = 45ASHMI n = 46 Prednisone |
n = 40 mMMDT 80mg n = 40 mMMDT 40mg n = 20 Placebo |
n = 28 DCT n = 24 Placebo |
n = 50 STA-1 n = 50 STA-2 n = 20 Placebo |
| Ages (years) | 18–65 | 5–18 | 8–15 | 8–15 |
| Indication | Moderate-to-severe persistent asthma | Mild-to-moderate persistent asthma | Mild-to-moderate persistent asthma | Mild-to-moderate persistent asthma |
| Length of Study | 4 weeks | 4 months | 3 months | 6 months |
| Herbal Components (5;6) |
|
|
|
Combined formula of mMMDT wihout the herb #5 with Lui Wei Di Huang Wan (6 hrbs)
|
| Improved FEV1 | yes | yes | yes | yes |
| Improved Symptom score | yes | no | yes | yes |
| Safety and tolerability | yes | yes | yes | yes |
All the herbals are Chinese original. All formulas contain Radix Glycyrrhizae. RCT; Randomized, placebo-controlled, double-blind, clinical trial; This table is a modification of the table in Li, J Allergy. Clin Immunol(7) with permission.
2. Chinese herbal remedies used as complementary therapy
Three recent controlled studies have been published in English language journals. In those studies, Chinese herbal remedies were used as complementary therapy.
Modified Mai Men Dong Tang (mMMDT)
Hsu et al(18) tested modified Mai Men Dong Tang (mMMDT, 5 herbs) for treatment of persistent, mild-to-moderate asthma in children. (Table I). This four-month trial included 100 asthmatics aged 5 to 18. The two active groups received 40 mg (40 patients), or 80 mg mMMDT (40 patients) for 2 months. The control group received placebo capsules (20 patients). Asthma medications were provided gratis and adjusted in a stepwise fashion equally in all three groups as follows: step 1, use of bronchodilator as needed; step 2, regular use of bronchodilator (theophylline or albuterol); step 3, regular use of two or three drugs (theophylline, albuterol and cromolyn); step 4, addition of beclomethasone delivered with a metered-dose inhaler or alternate day methylprednisolone; and, step 5, addition of oral corticosteroids (> 0.5 mg/kg/day, with tapering). Acute exacerbation of asthma was treated as directed by the child’s physician using tapered doses of oral methylprednisolone. The investigators reported that relative to baseline, significantly greater increases in FEV1 were demonstrated in both mMMDT-treated groups in comparison with the placebo group (P < 0.05 for both doses of mMMDT), but no dose response effect was found between the two mMMDT treated groups. However, symptom scores were similarly improved in both mMMDT treatment groups. No drug-related adverse effects were reported. Blood tests, and liver and kidney function test results were within normal ranges during the study.
Ding Chuan Tang (DCT)
Chan et al(19) reported that in a randomized, double-blind clinical trial, Ding Chuan Tang (DCT), a nine herb formula, reduced airway hyper-reactivity (AHR) in stabilized asthmatic children. (Table I). This study enrolled children between 8 and 15 years of age diagnosed with mild-to-moderate persistent asthma. Patients were randomly allocated to receive 6.0 g DCT or placebo daily for 12 weeks. Fifty-two asthmatic children completed the study. Both groups received standard asthma management in a stepwise fashion (5 steps) as outlined above in the Hsu study(11). Twenty-eight patients were assigned to the treatment group and 24 to the placebo group. At the end of the treatment period, AHR determined by log PC(20) was significantly improved in the DCT group (0.51 +/− 1.05 mg/ml vs. 0.26 +/− 0.84 mg/ml, p = 0.034). Clinical and medication scores showed improvement in the DCT group (p = 0.004). The authors concluded that more stable airways were achieved by this add-on complementary therapy.
STA-1
Chang et al(20) reported results of a clinical evaluation of STA-1 and STA-2 herbal formulas [Table 1]. STA-1 and STA2-2 are combinations of mMMDT (10 herbs) and Lui-Wei-Di-Huang Wan (LWDHW, 6 herbs). But the authors did not specify the specific composition. STA-1 and STA-2 reportedly differ only in the preparation procedures of LWDHW. The six herbs in LWDHW were milled to a powder for STA-1, and extracted in boiling water for STA-2. Overall, 120 patients, 5 to 20 years of age with mild-to-moderate asthma were included in this study. Forty four patients were treated with STA-1 at a dose of 80 g/kg/day and forty were treated with STA-2 at a dose of 80 g/kg/day and 16 patients received a placebo. Treatment was administered twice daily for 6 months. All patients were provided with asthma medications adjusted in a stepwise fashion as described above in the Hsu et al. mMDT study.(11) Completion rates were 88%, 80% and 80% for STA1, STA2 and placebo respectively. The results showed a statistically significant reduction of symptom scores, systemic steroid dose, and total IgE and specific IgE levels in the STA-1 group. Furthermore, STA-1 improved pulmonary lung function FEV(1) as compared with the placebo group. STA-2 treated patients showed no significant improvement in any parameter. The authors speculated that some as yet unknown heat-sensitive compounds in LWDHW possess anti-inflammatory activity.
Sophora flavescens Ait
In addition to the multi-herb formulas, Hoang et al. 2007 (21) reported the effect of an extract of Sophora flavescens Ait (also a traditional Hawaiian anti-asthma medicine) and a component of ASHMI, on the management of asthma. An open and selective 3-year follow-up of 14 chronic refractory asthmatic patients ages 22 to 70 years was used. Participants received an aqueous S flavescens Ait extract with a dose equal to 4g of dried root three times daily for 3 months, twice daily for 6 months, and once daily for 27 months. Although this study was not controlled, since it involved unstable refractory asthmatic subjects, the reported clinical outcome appears to be remarkable. Table II summarizes the improvement of symptoms, reduction of β2 agonist use, dose of inhaled corticosteroid, and improvement of PEF from 4 weeks to the 3rd years of treatment. The improvement was greater with longer treatment. Since this study employed a prolonged observation, safety data was an important factor. No side effects were detected by clinical examination, hematology or clinical chemistry.
Table 2.
Summary of Sophora Flavescens Ait on refractory asthmatics
| Visit | 4 weeks | 3 months | 1 year | 3 years |
|---|---|---|---|---|
| Day time asthma improvement | 78% | 87 | 94 | 97 |
| Night time | 72 | 85 | 95 | 98 |
| Reduction of β2 agonist use | 45 | 92 | 95 | 97 |
| Reduction of ICS | 45 | 84 | 92 | 100 (no patient took ICS) |
| Mean PEF rate improvement | 12% | 15 | 18 | 21 |
| Side effect | none | none | NR | NR |
ICS, inhaled corticosteroid; NR, not reported.
In summary, these clinical studies show promising results. All reported improvement in lung functions, an objective measurement of clinical improvement. Furthermore, all addressed safety, and demonstrated that the tested TCM herbal medicines were safe and well tolerated. Although demonstrating significant improvement in asthmatic symptoms compared to the earlier clinical trials of TCM,(11) these studies have limitations. ASHMI, mMMDT and DCT studies were of short duration and while most studies showed significant improvement in symptoms scores, mMMDT did not, which may be due to the small sample size in the placebo arm. Dose dependent effects, except for mMMDT, were not addressed in these studies. Studies of mMMDT, DCT and AST1/AST2 also used conventional therapy based on a step-wise algorithm of asthma management, but not fixed regimens. The DCT and AST1/AST2 studies reported a reduction in conventional medication use when compared to placebo controls. Thus it is unlikely that the positive effect of TCM was due to variations in conventional medications. However, no reductions in conventional medication use was reported in the mMMDT study, and improvements in lung function due to variations in conventional medications use can not be excluded. The study utilizing Sophora flavescens Ait for refractory asthma was a long term study; but since this study did not include a placebo control arm, a placebo effect cannot be ruled-out. Clinical studies of TCM are limited and there are no previous studies investigating possible persistent effects after discontinuation of treatment. Given the positive preliminary results and safety profiles, and the increased use of TCM for asthma treatment, additional studies of TCM formulations, with the goal of botanical drug development should be strongly encouraged. One TCM formulation, ASHMI, has received US FDA IND approval (IND 71 526) for phase I and II clinical trials for treating asthma A phase I study has been completed, which included 20 patients ages 18–40 years with mild to moderate, persistent allergic asthma. This was a double-blind, placebo-controlled, dose escalation study. Based on clinical and laboratory test results, ASHMI was considered safe and well tolerated (Kelly-Piper et al manuscript submitted, 2008). A phase II study, involving 60 patients over a 6 month duration is underway.
B. Mechanism of action of anti-asthma TCM herbal remedies
Asthma is a chronic inflammatory condition of the airways that causes airway hyperresponsiveness. Th1 and Th2 responses are felt to be mutually antagonistic, such that they normally exist in equilibrium and cross-regulate each other. An optimumTh1-Th2 balance has been suggested as necessary to maintain healthy immune homeostasis. Loss of such balance has been hypothesized to underlie allergic asthma through a shift in immune responses from a Th1 (IFN-γ) pattern toward a Th2 (IL-4, IL-5, and IL-13) profile, which promote IgE production, eosinophilic inflammation, activation and survival, and enhanced airway smooth muscle contractility (22) A recent study showed that low IFN-γ production in the first year of life was a predictor of wheeze during childhood(23). In another study, patients with severe asthma exhibited significantly reduced IFN-γ production in response to allergen as compared to control and resolved asthma subjects. (22) It has also been shown that allergen immunotherapy, when effective, results in an increase in antigen-specific Th1-cells and suppression of Th2 cytokine production. (24) Therefore, a shift in balance of cytokines from a dominant Th2 response to a strong Th1 response may help to resolve allergy and asthma. Although corticosteroids (CS) improve asthma symptoms they do not alter the progression of asthma or cure the disease.(25) Guilbert et al (26) reported that prophylactic administration of a corticosteroid to high risk children for one year did not decrease the rate of asthma development as compared with controls. CS withdrawal is often accompanied by increased inflammation in bronchial biopsies and symptomatic disease relapse.(27) This has been suggested to be due to CS induced overall suppression of both Th1 and Th2 responses. Among the 5 anti-asthma herbal remedy studies reviewed above, some interesting pharmacological actions have been generated from ASHMI studies. These findings are summarized in the following 4 sections.
1. Immunomodulation- but not overall immune suppression- data generated from clinical study
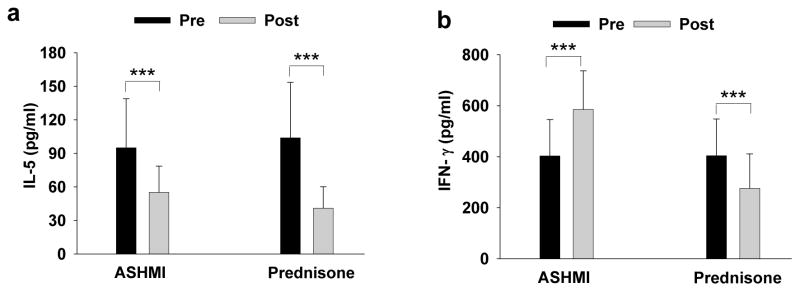
To understand the mechanisms underlying ASHMI’s clinical effects, we evaluated immunological responses secondary to treatment. Both ASHMI and prednisone decreased peripheral blood eosinophils, serum IgE, and Th2 cytokines (IL-5 and IL13) levels (Fig 1a). Inhibition was greater in the prednisone group. However, unlike prednisone which suppressed IFN-γ secretion, ASHMI actually increased IFN-γ secretion(12) Fig 1b.
Fig 1. Effect of 4 weeks of treatment with ASHMI or Prednisone on serum cytokine levels.
All blood samples were drawn between before treatment and 48 hours after treatment. Serum IL-5 (a) and IFN-γ (b) were determined by ELISA. This table is a modification of the table in Li, J Allergy. Clin Immunol(1) with permission.
Since ASHMI was used as monotherapy in this study, we investigated whether ASHMI would also enhance IFN-γ production in patients on inhaled steroid. We recently completed a study to determine the safety, tolerability and immunological benefits of complementary ASHMI in children 5–14 years of age with persistent asthma with or without allergic rhinitis. Subjects were randomly assigned to receive standard inhaled corticosteroid treatment (Budesonide-Pulmicort Turbohaler) plus ASHMI as complementary therapy (complementary ASHMI group, n=28) or inhaled cortical steroid treatment plus placebo (standard group, n=28,). 51 asthma patients completed the trial including 26 patients in the complementary ASHMI group and 25 from the standard group. We found that patients in the complementary ASHMI group showed significantly greater reduction of serum total IgE (p<0.05) and higher serum levels of IFN-γ after 3 months of treatment as compared to the standard therapy alone group (Wen et al. manuscript in preparation).
A clinical study by Chang et al(20) also found that total and specific IgE levels were significantly reduced by herbal therapy (STA-1) as compared to placebo treatment. However, effects on T cell responses were not investigated. The clinical studies of mMMDT and DCT did not find a significant reduction of IgE as compared to placebo(18;19)
2. ASHMI enhances adrenal functions-data generated from clinical study
Corticosteroid-induced suppression of the hypothalamic-pituitary-adrenal axis, marked by depressed cortisol levels, has been implicated as an adverse side effect of systemic steroid use.(28;29) In the Wen et al. study, a beneficial effect of ASHMI treatment on adrenal function was found. Pretreatment cortisol levels were slightly below normal (6–23 mg/dL) in both groups (Fig 1c). After treatment, subjects in the prednisone treatment group showed a significant reduction in serum cortisol levels following treatment (5.1±3.0 to 3.7± 2.3 μg/dL, p<0.001. In contrast, patients in the ASHMI treatment group showed increased levels of serum cortisol (5.4±2.8 to 7.7±2.3μg/dL, p<0.001, Figure 2), which is within the normal range. The difference between groups was statistically significant (P < 0.001). Thus, ASHMI restored adrenal homeostasis. This result might be attributed to glycyrrhizin (a component of Gan-Cao), which affects the conversion of cortisol to cortisone, by inhibition of the 11-β-hydroxysteroid dehydrogenase enzyme activity. (30) Gan-Cao has been used for adrenal insufficiency.(31) However, it is also possible that the increase in cortisol levels may be due to the lack of suppression of adrenal functions. Further research is required to understand the precise mechanisms.
Fig 2. Effect of 4 weeks treatment with ASHMI or Prednisone on serum cortisol levels.
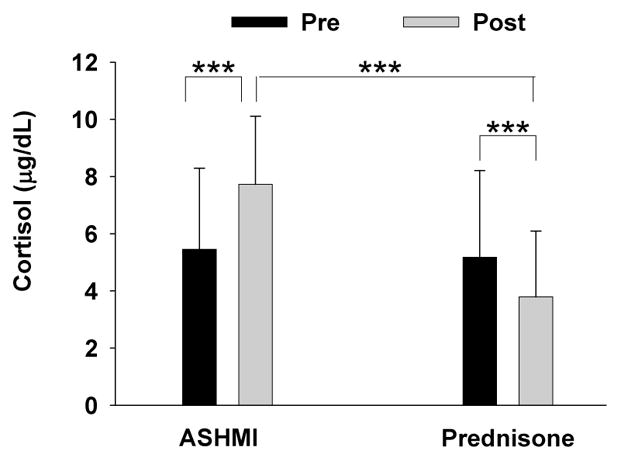
All blood samples were drawn between 7:30–8:30 A.M. before treatment and 48 hours after treatment. Serum cortisol levels were determined by RIA. Data are Means±SD. *** indicates p<0.001 (Normal level of morning serum cortisol levels are 8–23 μg/dL). This table is a modification of the table in Li, J Allergy. Clin Immunol(1) with permission.
3. Broad mechanisms of action of ASHMI in a murine model of asthma
Animal models of asthma are a valuable tool to explore the mechanisms of action of ASHMI and other herbal remedies for treating asthma. There are at least two phases of asthmatic reactions following allergen exposure. The first, an IgE- mediated type 1 hypersensitivity response induces an early-phase airway reaction (EAR), usually within 30 min following relevant antigen (Ag) exposure, and is triggered by mast cell-derived bronchoconstrictors such as histamine and leukotrienes (LT) (32;33). The second phase, a late-phase hypersensitivity reaction (LAR) occurs 6–12 hours after allergen inhalation, and is associated with infiltration of inflammatory cells, primarily eosinophils(33;34). Studies using a murine model of asthma found that ASHMI exhibits a broad spectrum of therapeutic effects on the major pathogenic mechanisms of asthma. It blocked EAR, which was associated with a reduction of histamine and leukotriene release (Zhang et al unpublished data), airway hyperreactivity (AHR), pulmonary inflammation and airway remodeling, which was accompanied by down-regulation of Th2 responses(35). Another murine study found that SAT-1 suppressed Der p 5-induced allergic reactions as evidenced by significantly reduced Der p 5-specific IgE, pulmonary inflammation and AHR.(36). Our initial study of herbal medicine showed that the formula MSSM-002, the precursor of ASHMI (containing more herbs), inhibited the Th2 specific transcription factor GATA-3. (37) ASHMI had the same effect on memory Th2 cells. In addition to anti-inflammatory properties, ASHMI also directly modulates airway smooth muscle contraction ex vivo, as shown by employing myographic techniques using murine tracheal rings.(38) ASHMI both decreased airway smooth muscle contractility in response to acetylcholine and increased airway smooth muscle relaxation in response to prostaglandin E2. These effects were not associated with β2-adrenergic receptors, but were associated with increased prostaglandin E2 production(38). Although the detailed mechanisms underlying ASHMI’s potent effects on numerous asthma-associated mechanisms are unknown, our recent pharmacological study demonstrated that constituents in ASHMI act synergistically in suppression of eotaxin production by human fetal lung fibroblasts.(39)
In summary, modulation of Th1 and Th2 responses, but not overall immune suppression, and lack of inhibition of adrenal function suggest that ASHMI exhibits different therapeutic mechanisms from corticosteroids. However, more studies are required to understand the exact mechanisms by which ASHMI and perhaps other TCM herbal products modulate Th-Th2 responses at the molecular level.
Part II. Development of herbal interventions for food allergy
1. Investigation of herbal interventions from TCM for peanut allergy in animal model and human in vitro study
Peanut allergy accounts for approximately 80% of fatal and near-fatal anaphylactic reactions to foods.(40;41) The prevalence of childhood peanut allergy doubled between 1997 and 2002.(42), and affects ~1% of the American population (43). While tremendous strides have been made in food allergy awareness, there is no satisfactory therapy to prevent or reverse the disease. Peanut allergy imparts a significant psychological burden on the allergic individuals and their families(44). An effective treatment would offer a life-altering option for those affected.
There is no TCM herbal product for food allergy. Our group developed a food allergy herbal formula I (containing extracts of 11 herbs (45) and then a refined formula FAHF-2, containing 9 herb extracts (16). Using a well established murine model that clinically and immunologically mimics peanut allergy, (46)we found that FAHF-2 completely blocked peanut induced anaphylaxis when administered intragastrically (i.g,) during the development of peanut hypersensitivity (early treatment)(16). We then reported that FAHF-2 also completely blocked peanut anaphylaxis when administered after peanut hypersensitivity was fully established (late treatment protocol) (15) This protection was associated with suppression of histamine release, Th2 responses (IgE and Th2 cytokines) and up-regulation of Th1 responses (IgG2a and IFN-γ). (15) FAHF-2 also showed a high safety profile in an acute toxicity study in which mice fed with 24 times the effective FAHF-2 dose did not show any detectable abnormality(16). Given the potent protection and immunomodulatory effects to food allergens, and given that mice were completely protected for 4–5 weeks post-therapy (15;16), we investigated the long term effects of FAHF-2 and the mechanisms underlying its prolonged protection. We found FAHF-2 completely prevented anaphylactic reactions following multiple peanut re-challenges every 1–2 months for at least 6 months (~25% of the mouse life span(47)). This was also accompanied by continued suppression of histamine release and IgE production post-therapy(47). We then used in vivo depletion techniques to explore the mechanisms of persistent protection. IFN-γ neutralization and CD8+ T cell depletion abolished the FAHF-2’s suppressive effect on IgE and Th2 cytokine production, and significantly attenuated FAHF-2’s protective effect on peanut anaphylaxis, (48;49) demonstrating an important role of IFN-γ and CD8+ T cells in mediating long term protection. A similar immunomodulatory effect has also been found on human cells. In this study, peripheral blood mononuclear cells from children with peanut allergy were cultured in the presence or absence of peanut protein with or without FAHF-2. We found that FAHF-2 significantly suppressed IL-5 production and increased IFN-γ production (50). We recently extended our studies to explore additional effects of FAHF-2 on other mechanisms involved in peanut anaphylaxis and found that FAHF-2 inhibited FcεRI expression on mast cells and basophils in vivo, and inhibited mast cell degranulation in vitro (51). It appears that multiple mechanisms are involved in FAHF-2’s potent and persistent protection. In ongoing studies we found that FAHF-2 is also effective in a mouse model of multiple food allergies in which animals were sensitized to fish and egg, in addition to peanut (manuscript in preparation and abstract accepted for AAAAI annual meeting, 2009).
2. Clinical investigation of FAHF-2 for food allergy
Given the excellent efficacy and safety profile in animal studies, FAHF-2 appears to be an ideal candidate to treat human food allergy. After obtaining an IND approval from the US FDA, and local IRB approval, a FAHF-2 clinical trial was initiated in 2008. This was the first clinical trial of a botanical drug for multiple persistent food allergies, including peanut, and/or tree nut, fish and shellfish allergies, and the first botanical drug trial that included children. A double-blind, dose escalation phase I study was conducted on 12 subjects (8 received FAHF-2 and 4 received placebo) with peanut and other food allergies. The results showed that FAHF-2 is safe and well tolerated. An extended 6 month phase I open label study is currently underway. After completion of this study, we will conduct a double-blind, placebo-controlled phase II study.
Part III. Botanical drug quality control and research into chemical and biological mechanisms of botanicals
1. Botanical drug quality control and IND
Although TCM has been used for thousand of years, the use of TCM herbal products as investigational botanical new drugs began only recently in the US. Unlike synthetic drugs that begin with preclinical laboratory studies, botanical drug development from TCM has the advantage of long term experience in humans and generally an established safe profile.(9;10;52) However, standardization of herbal formulas is challenging because the complex mixtures of herbs contain many constituents that have not been clearly defined. An essential requirement for clinical investigation of a botanical drug is an IND approval by the US FDA [Title 21 Code of Federal Regulation 312.23 (a) [(21 CFR 312.23(a)]. The most unique section in this IND is the Chemical, Manufacturing, and Control (CMC) Data [21 CFR 312.23(a) (7)] requirement, which differs from that required for synthetic drugs. Given the unique characteristics of herbal mixtures, the FDA frequently relies on a combination of tests when the active chemical are not well defined. High pressure liquid chromatography (HPLC) fingerprints, assays of characteristic markers, and biological assays are accepted methods to ensure quality, potency and consistency of botanical drugs. In accordance with the FDA Guidelines, provision of sufficient quality and safety data at three levels – raw herbs, extracts (substance in FDA terminology) and final product – led to the approval of ASHMI and FAHF-2. The 3 dimensional HPLC fingerprint of FAHF-2 and chemical markers identified by liquid chromatography coupled with mass spectrometry (LC-MS) are shown on the cover page of this issue. HPLC fingerprinting was also used as a means of quality control in the studies of mMMDT, DCT and AST-1 & 2 described above.
2. Research into chemical and biological activities of botanicals
Although selected chemical markers and HPLC fingerprints are accepted by the FDA for botanical quality control purposes in preliminary clinical trials, the FDA encourages identification of active ingredients in herbal products, if feasible, to improve the quality control and to understand the pharmacokinetics of herbal products. Isolation and identification of active constituents is essential to obtain better understanding of the mechanisms of action. This goal depends on biological testing-guided isolation and chemical identification of active ingredients. Although herbal products contain many constituents, only a few compounds are responsible for the physiological effects. The development of modern chromatographic techniques, such as HPLC, together with mass spectrometry and nuclear magnetic resonance (NMR), makes isolation and purification of chemical constituents from complex mixtures feasible (53–55). Recently a phytochemical database of Chinese herbal constituents and bioactive plant components was established (17). This database shows that the two classes of phytochemicals most often represented in TCM are the triterpenes (15%) and sesquiterpenes (13%). The remaining classes of phytochemicals include alkaloids (10%), simple phenolics (10%), flavonoids (10%), and monoterpenes (10%). The diterpenes (5%), coumarins (5%), aliphatics (5%), and steroids (5%) are found less often in TCM; and tannins, isoflavonoids, polycylic aromatics, lignans, and carbohydrates are least common (less than 5% each).(17;51;56) Of these phytochemical classes, the non-steroidal alkaloids, polyphenols, and terpenes are constituents of TCMs most frequently used to treat allergy and asthma1.(56) As an example, Table 3 shows the major known chemical constituents of individual herbs in ASHMI. (17;57) However, most of these compounds identified in TCM are not yet commercially available, and their potential pharmacological actions have not been well established. In recent years an increasing number of pharmacological studies of known compounds have been published. As examples, prenylated flavonoids from Sophora flavescens inhibited the release of β-hexosaminidase from cultured RBL-2H3 cells, suggesting an anti-allergy property. (58). Glycyrrhiza uralensis (Gan-Cao) commonly called “licorice” is one of the most commonly used herbs in TCM. The licorice triterpenoid glycyrrhizin and related compounds down-regulate production of the inflammatory chemokines IL-8 and eotaxin 1, and stat 6 expression by a human lung fibroblast cell line(59;60). However, their effects on asthma have not been reported, and other potential anti-inflammatory constituents in G. uralensis have not been fully investigated. Eosinophilic airway inflammation is a major feature of allergic asthma and Eotaxin-1 (eotaxin) is involved in recruitment of eosinophils to sites of antigen-induced inflammation in asthmatic airways(61). Since human lung fibroblasts are a major source of eotaxin (62), inhibition of eosinophil recruitment by suppression of fibroblast eotaxin production is a potentially valuable approach to pharmacological intervention in asthma. Our group has conducted a systematic bioassay-guided purification of G. uralensis which yielded 5 flavonoids: liquiritin, liquiritigenin, isoliquiritigenin, 7, 4′-dihydroxyflavone and isoononin. The structures of the compounds were established by 1H, 13C (NMR) and LC-MS. The potential ability of these isolated compounds, and of glycyrrhizin, to inhibit eotaxin-1 secretion by human fetal lung fibroblasts (HFL-1) was tested. Glycyrrhiza flavonoids inhibited eotaxin-1 secretion, and liquiritigenin, isoliquiritigenin, and 7, 4′-dihydroxyflavone were more effective than liquiritin, isoononin and glycyrrhizin (commercially available) in suppressing eotaxin secretion. (63)
Table 3.
Summary of herbal components of ASHMI and known chemical constituents
| Chinese name Synonyms | Ling-Zhi Reishi |
Ku-Shen Light yellow sophora root |
Gan-Cao Licorice root |
| Pharmaceutical name | Ganoderma | Radix Sophorae Flavescentis |
Radix Glycyrrhiza |
| Plant Species Information | Ganoderma lucidum Karst. | Sophora flavescens Ait. | Glycyrrhiza uralensis Fischer. |
| Part used | Fruiting body | Root | Root and rhizome |
| Family | Polyporaceae | Leguminosae | Leguminosae |
| Traditional Uses (ref) | General weakness, cough, asthma, insomnia, indigestion | dysentery, jaundice, pruritis edema, dysuria, eczema | sore throat, cough |
| Modern Uses | Nightmares, neurasthenia, coronary heart disease, arrhythmia, asthma, leukocytopenia | chronic hepatitis B, leukocytopenia | bronchitis, gastroduodenal ulcers |
| Chemical constituents |
|
|
|
Since most previous phytochemical studies of herbal medicine focused on isolation and identification of new compounds and since limited amounts of material were isolated, few isolated compounds are commercially available and few studies on pharmacological actions and mechanisms of action relevant to asthma are limited. Isolation of individual compounds from each individual herb in complex formulas is a major undertaking. Several groups have generated targeted fractions based on the phytochemistry of TCMs. (64;65). One previous study of a murine model of allergic asthma showed that triterpenpenoid-rich extracts of Ganoderma tsugae exhibited anti-inflammatory effects, decreased airway responses and attenuated Th2 responses without causing overall immune suppression.(66) Our approach is to generate targeted fractions of ASHMI and its constituent herbs, and by using various in vitro models representing pathological asthma mechanisms, determine active fractions for purification into single compounds. We have found that certain triterpene rich ASHMI fractions are potent suppressors of TNF-α production by macrophages (Raw 264.7 cell line). Other fractions, flavonoid-rich, inhibit IgE production by B lymphocytes (U266 myeloma cell line), and an alkaloid-rich fraction inhibits acetylcholine induced airway smooth muscle contractility (Brown et al. unpublished data 2008). These procedures may prove to be an efficient approach to identification of anti-inflammatory and other pharmacologically active compounds in the complex ASHMI formula, and other TCM formulas.
Conclusion
Several recent controlled clinical studies have found that some herbal formulas and one individual herb improved lung function and reduced symptoms when used as monotherapy, or as a complement to conventional standard therapy for the treatment of asthma. These findings strongly suggest that TCM herbal remedies are of some value for asthma management. ASHMI produced a beneficial immnunomodulatory effect in asthma patients. Other mechanisms in addition to anti-inflammatory activity require further investigation. Given the lack of any alternative food allergy therapies, and the excellent preclinical safety and efficacy data, continued research into FAHF-2 for food allergy is needed. Both ASHMI and FAHF-2 are entering clinical studies in the US, and may prove to be the first generation of anti-asthma and food allergy botanical drugs. Additional TCM formulas and individual medicinal herbs as well as purified compounds should be investigated using state of the art laboratory and clinical methodologies.
Acknowledgments
The author thanks Kamal Srivaratava, Ming-Chun Wu, TengFei Zhang, ChunFeng Qu, Zhong Mei Zho, Joseph Godthab, Rong Wang, Sylva Wallenstein, Jimmy Ko, Joyce Yu, Meyer Kattan, Sally Noon, Brian Schofield, Julie Wang, and Hugh Sampson for their significant contributions to this work, and Sharon Hamlin for her assistance with manuscript preparation.
Funding: Supported by NIH/NCCAM center grant # 1P01 AT002644725-01 and NIH/NCCAM R01 AT001-14, Food Allergy Initiative, the Rothstein family and The Cornfield Family Foundation.
Abbreviation (alphabetic order)
- AHR
airway hyperresponsiveness
- ASHMI
Anti-asthma herbal medicine intervention
- CAM
complementary and alternative medicine
- CS
corticosteroids
- DCT
Ding Chuan Tang, classical formula
- DP
Dermatophagoides pteronyssinus
- FAHF-1
Food allergy herbal formula-1
- FAHF-2
Food allergy herbal formula-2
- G,mg
gram, milligram
- LWDHW
Lui-Wei-Di-Huang Wan, classical formula
- mMMDT
Modified Mai Men Dong Tang, modified classical formula
- MSSM-002
Herbal formula
- NCCAM
National Center for Complementary and Alternative Medicine
- PN
peanut
- PNA
peanut allergy
- RCT
Randomized controlled trial
- TCM
traditional Chinese medicine
- Wk wks
weeks
Footnotes
U.S. Provisional Patent Applications regarding FAHF-2 (reference number 60554775) and ASHMI (PCT/US05/08600) have been filed.
References
- 1.CDC. [Accessed March 14, 2007 2007.];National Center for Health Statistics: Asthma. http://wwwcdcgov/nchs/fastats/asthmahtm.
- 2.Hassed C. An integrative approach to asthma. Aust Fam Physician. 2005;34(7):573–6. [PubMed] [Google Scholar]
- 3.Bielory L, Russin J, Zuckerman GB. Clinical efficacy, mechanisms of action, and adverse effects of complementary and alternative medicine therapies for asthma. Allergy Asthma Proc. 2004;25(5):283–91. [PubMed] [Google Scholar]
- 4.Slader CA, Reddel HK, Jenkins CR, Armour CL, Bosnic-Anticevich SZ. Complementary and alternative medicine use in asthma: who is using what? Respirology. 2006;11(4):373–87. doi: 10.1111/j.1440-1843.2006.00861.x. [DOI] [PubMed] [Google Scholar]
- 5.nccam. ( http://nccam.nih.gov/health/backgrounds/wholemed.htm. 1-28-2008. Ref Type: Internet Communication.
- 6.Engler RJ. Alternative and complementary medicine: a source of improved therapies for asthma? A challenge for redefining the specialty? J Allergy Clin Immunol. 2000;106(4):627–9. doi: 10.1067/mai.2000.110504. [DOI] [PubMed] [Google Scholar]
- 7.Lazar MA. East meets West: an herbal tea finds a receptor. J Clin Invest. 2004;113(1):23–5. doi: 10.1172/JCI200420661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration (FDA) Guidance for Industry Botanical Drug Products. Revised. 2004. Center for Drug Evaluation and Research. [Google Scholar]
- 9.Bensky D, Gamble A. Chinese Herbal Medicine: Materia Medica. Revised. Seattle: Eastland Press; 1993. [Google Scholar]
- 10.Bensky D, Barolet R. Chinese Herbal Medicine: Formulas & Strategies. Seattle: Eastland Press; 1990. [Google Scholar]
- 11.Huntley A, Ernst E. Herbal medicines for asthma: a systematic review. Thorax. 2000;55(11):925–9. doi: 10.1136/thorax.55.11.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen MC, Wei CH, Hu ZQ, Srivastava K, Ko J, Xi ST, et al. Efficacy and tolerability of anti-asthma herbal medicine intervention in adult patients with moderate-severe allergic asthma. J Allergy Clin Immunol. 2005;116(3):517–24. doi: 10.1016/j.jaci.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 13.Sicherer SH, Sampson HA. 9. Food allergy. J Allergy Clin Immunol. 2006;117(2 Suppl MiniPrimer):S470–S475. doi: 10.1016/j.jaci.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 14.Kattan JD, Srivastava KD, Zou ZM, Goldfarb J, Sampson HA, Li XM. Pharmacological and immunological effects of individual herbs in the Food Allergy Herbal Formula-2 (FAHF-2) on peanut allergy. Phytother Res. 2008;22(5):651–9. doi: 10.1002/ptr.2357. [DOI] [PubMed] [Google Scholar]
- 15.Qu C, Srivastava K, Ko J, Zhang TF, Sampson HA, Li XM. Induction of tolerance after establishment of peanut allergy by the food allergy herbal formula-2 is associated with up-regulation of interferon-gamma. Clin Exp Allergy. 2007;37(6):846–55. doi: 10.1111/j.1365-2222.2007.02718.x. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava KD, Kattan JD, Zou ZM, Li JH, Zhang L, Wallenstein S, et al. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol. 2005;115(1):171–8. doi: 10.1016/j.jaci.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Ehrman TM, Barlow DJ, Hylands PJ. Phytochemical databases of Chinese herbal constituents and bioactive plant compounds with known target specificities. J Chem Inf Model. 2007;47(2):254–63. doi: 10.1021/ci600288m. [DOI] [PubMed] [Google Scholar]
- 18.Hsu CH, Lu CM, Chang TT. Efficacy and safety of modified Mai-Men-Dong-Tang for treatment of allergic asthma. Pediatr Allergy Immunol. 2005;16(1):76–81. doi: 10.1111/j.1399-3038.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 19.Chan CK, Kuo ML, Shen JJ, See LC, Chang HH, Huang JL. Ding Chuan Tang, a Chinese herb decoction, could improve airway hyper-responsiveness in stabilized asthmatic children: a randomized, double-blind clinical trial. Pediatr Allergy Immunol. 2006;17(5):316–22. doi: 10.1111/j.1399-3038.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 20.Chang TT, Huang CC, Hsu CH. Clinical evaluation of the Chinese herbal medicine formula STA-1 in the treatment of allergic asthma. Phytother Res. 2006;20(5):342–7. doi: 10.1002/ptr.1843. [DOI] [PubMed] [Google Scholar]
- 21.Hoang BX, Shaw DG, Levine S, Hoang C, Pham P. New approach in asthma treatment using excitatory modulator. Phytother Res. 2007 doi: 10.1002/ptr.2107. [DOI] [PubMed] [Google Scholar]
- 22.Busse WW, Rosenwasser LJ. Mechanisms of asthma. J Allergy Clin Immunol. 2003;111(3 Suppl):S799–S804. doi: 10.1067/mai.2003.158. [DOI] [PubMed] [Google Scholar]
- 23.Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol. 2007;120(4):835–41. doi: 10.1016/j.jaci.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 24.Ray A, Cohn L. Altering the Th1/Th2 balance as a therapeutic strategy in asthmatic diseases. Curr Opin Investig Drugs. 2000;1(4):442–8. [PubMed] [Google Scholar]
- 25.Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med. 2000;343(15):1054–63. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 26.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354(19):1985–97. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 27.Epstein MM. Targeting memory Th2 cells for the treatment of allergic asthma. Pharmacol Ther. 2006;109(1–2):107–36. doi: 10.1016/j.pharmthera.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Sorkness CA, LaForce C, Storms W, Lincourt WR, Edwards L, Rogenes PR. Effects of the inhaled corticosteroids fluticasone propionate, triamcinolone acetonide, and flunisolide and oral prednisone on the hypothalamic-pituitary-adrenal axis in adult patients with asthma. Clin Ther. 1999;21(2):353–67. doi: 10.1016/S0149-2918(00)88292-2. [DOI] [PubMed] [Google Scholar]
- 29.Zora JA, Zimmerman D, Carey TL, O’Connell EJ, Yunginger JW. Hypothalamic-pituitary-adrenal axis suppression after short-term, high-dose glucocorticoid therapy in children with asthma. J Allergy Clin Immunol. 1986;77(1 Pt 1):9–13. doi: 10.1016/0091-6749(86)90315-5. [DOI] [PubMed] [Google Scholar]
- 30.WHO monographs on selected medicinal plants Volume 1: Radix Glycyrrhizae. 1999:183–94. [Google Scholar]
- 31.Peirce A. The American Pharmaceutical Association Practical Guide to Natural Medicines. New York: William Morrow and Company, Inc; 1999. [Google Scholar]
- 32.Barrios RJ, Kheradmand F, Batts L, Corry DB. Asthma: pathology and pathophysiology. Arch Pathol Lab Med. 2006;130(4):447–51. doi: 10.5858/2006-130-447-APAP. [DOI] [PubMed] [Google Scholar]
- 33.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161(5):1720–45. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 34.Barrios RJ, Kheradmand F, Batts L, Corry DB. Asthma: pathology and pathophysiology. Arch Pathol Lab Med. 2006;130(4):447–51. doi: 10.5858/2006-130-447-APAP. [DOI] [PubMed] [Google Scholar]
- 35.Busse PJ, Wen MC, Huang CK, Srivastava K, Zhang TF, Schofield B, et al. Therapeutic effects of the Chinese herbal formula, MSSM-03d, on persistent airway hyperreactivity and airway remodeling. J Allergy Clin Immunol (Abstract) 2004;113:S220. [Google Scholar]
- 36.Chang TT, Huang CC, Hsu CH. Inhibition of mite-induced immunoglobulin E synthesis, airway inflammation, and hyperreactivity by herbal medicine STA-1. Immunopharmacol Immunotoxicol. 2006;28(4):683–95. doi: 10.1080/08923970601067409. [DOI] [PubMed] [Google Scholar]
- 37.Li XM, Huang CK, Zhang TF, Teper AA, Srivastava K, Schofield BH, et al. The chinese herbal medicine formula MSSM-002 suppresses allergic airway hyperreactivity and modulates TH1/TH2 responses in a murine model of allergic asthma. J Allergy Clin Immunol. 2000;106(4):660–8. doi: 10.1067/mai.2000.110102. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava K, Zou ZM, Sampson HA, Dansky H, Li XM. Direct Modulation of Airway Reactivity by the Chinese Anti-Asthma Herbal Formula ASHMI. J Allergy Clin Immunol (Abstract) 2005;115(2):S7. [Google Scholar]
- 39.Bolleddula J, Goldfarb J, Wang R, Sampson H, Li XM. Synergistic Modulation Of Eotaxin And Il-4 Secretion By Constituents Of An Anti-asthma Herbal Formula (ASHMI) In Vitro. J Allergy Clin Immunol (Abstract) 2007;119(1 Supplement 1):S172. [Google Scholar]
- 40.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107(1):191–3. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 41.Sampson HA, Mendelson L, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992;327:380–4. doi: 10.1056/NEJM199208063270603. [DOI] [PubMed] [Google Scholar]
- 42.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112(6):1203–7. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 43.Sicherer SH, Sampson HA. Peanut allergy: emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007;120(3):491–503. doi: 10.1016/j.jaci.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 44.Primeau MN, Kagan R, Joseph L, Lim H, Dufresne C, Duffy C, et al. The psychological burden of peanut allergy as perceived by adults with peanut allergy and the parents of peanut-allergic children. Clin Exp Allergy. 2000;30(8):1135–43. doi: 10.1046/j.1365-2222.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- 45.Li XM, Zhang TF, Huang CK, Srivastava K, Teper AA, Zhang L, et al. Food Allergy Herbal Formula-1 (FAHF-1) blocks peanut-induced anaphylaxis in a murine model. J Allergy Clin Immunol. 2001;108(4):639–46. doi: 10.1067/mai.2001.118787. [DOI] [PubMed] [Google Scholar]
- 46.Li XM, Serebrisky D, Lee SY, Huang CK, Bardina LSBH, Burk AW, et al. A Murine Model of IgE Mediated Peanut anaphylaxis. J Immunol. 1999 [Google Scholar]
- 47.Srivastava KD, Zhang TF, Qu C, Sampson HA, Li XM. Silencing Peanut Allergy: A Chinese Herbal Formula, FAHF-2, Completely Blocks Peanut-induced Anaphylaxis for up to 6 Months Following Therapy in a Murine Model Of Peanut Allergy. J Allergy Clin Immunol (Abstract) 2006;117(2):S328. [Google Scholar]
- 48.Srivastava KD. SHALX. Contribution Of CD8 T Cells To The Anti-Allergic Effects of the TCM Formula FAHF-2. J Allergy Clin Immunol. 2008;121(2 S1):S246. (Abstract) [Google Scholar]
- 49.Srivastava KD, Sampson HA, Li X. Anti-Allergic Effects of the Traditional Chinese Medicine Herbal Formula FAHF2 are Partially Dependent on Interferon-[gamma] Activity. J Allergy Clin Immunol (Abstract) 2007;119(1 Supplement 1):S197. [Google Scholar]
- 50.Ko J, Busse PJ, Shek L, Noone SA, Sampson HA, Li XM. Effect of Chinese Herbal Formulas on T Cell Responses in Patients with Peanut Allergy or Asthma. J Allergy Clin Immunol (Abstract) 2005;115(2):S34. [Google Scholar]
- 51.Qu C, Srivastava KD, Zou ZM, Sampson HA, Li XM. The Herbal formula FAHF-2 Desensitizes Basophil/Mast Cells in vivo and in vitro. J Allergy Clin Immunol. 2006;115(2):S204. Abstract. [Google Scholar]
- 52.The State Pharmacopoeia Commission of The People’s Republic of China. Pharmacopoeia of the People’s Republic of China. 6. People’s Medical Publishing House; 2005. [Google Scholar]
- 53.Huang X, Kong L, Li X, Chen X, Guo M, Zou H. Strategy for analysis and screening of bioactive compounds in traditional Chinese medicines. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812(1–2):71–84. doi: 10.1016/j.jchromb.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 54.Efferth T, Kahl S, Paulus K, Adams M, Rauh R, Boechzelt H, et al. Phytochemistry and pharmacogenomics of natural products derived from traditional Chinese medicine and Chinese materia medica with activity against tumor cells. Mol Cancer Ther. 2008;7(1):152–61. doi: 10.1158/1535-7163.MCT-07-0073. [DOI] [PubMed] [Google Scholar]
- 55.Zhang HY, Hu CX, Liu CP, Li HF, Wang JS, Yuan KL, et al. Screening and analysis of bioactive compounds in traditional Chinese medicines using cell extract and gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2007;43(1):151–7. doi: 10.1016/j.jpba.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 56.Ehrman TM, Barlow DJ, Hylands PJ. Phytochemical informatics of traditional Chinese medicine and therapeutic relevance. J Chem Inf Model. 2007;47(6):2316–34. doi: 10.1021/ci700155t. [DOI] [PubMed] [Google Scholar]
- 57.Huang KC. The Pharmacology of Chinese Herbs. 2. Boca Raton, Florida: CRC Press LLC; 1998. [Google Scholar]
- 58.Quan W, Lee HJ, Kim CY, Noh CW, Um BH, Oak MH, et al. Anti-allergic prenylated flavonoids from the roots of Sophora flavescens. Planta Med. 2008;74(2):168–70. doi: 10.1055/s-2008-1034285. [DOI] [PubMed] [Google Scholar]
- 59.Matsui S, Sonoda Y, Sekiya T, izu-Yokota E, Kasahara T. Glycyrrhizin derivative inhibits eotaxin 1 production via STAT6 in human lung fibroblasts. Int Immunopharmacol. 2006;6(3):369–75. doi: 10.1016/j.intimp.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 60.Matsui S, Matsumoto H, Sonoda Y, Ando K, izu-Yokota E, Sato T, et al. Glycyrrhizin and related compounds down-regulate production of inflammatory chemokines IL-8 and eotaxin 1 in a human lung fibroblast cell line. Int Immunopharmacol. 2004;4(13):1633–44. doi: 10.1016/j.intimp.2004.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conroy DM, Williams TJ. Eotaxin and the attraction of eosinophils to the asthmatic lung. Respir Res. 2001;2(3):150–6. doi: 10.1186/rr52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sato E, Haniuda M, Numanami H, Ushiyama T, Tsukadaira A, Takashi S, et al. Histamine and serotonin stimulate eotaxin production by a human lung fibroblast cell line. Int Arch Allergy Immunol. 2002;128 (Suppl 1):12–7. doi: 10.1159/000059413. [DOI] [PubMed] [Google Scholar]
- 63.Jayaprakasam B. Licorice FlavonoidsInhibit Eotaxin-1 Secretion by Human Fetal Lung Fibroblasts in vitro. Journal of Agricultural and Food Chemistry. 2008 doi: 10.1021/jf802601j. dSWRHDGJLX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen ML, Lin BF. Effects of triterpenoid-rich extracts of Ganoderma tsugae on airway hyperreactivity and Th2 responses in vivo. Int Arch Allergy Immunol. 2007;143(1):21–30. doi: 10.1159/000098222. [DOI] [PubMed] [Google Scholar]
- 65.Chu X, Xu Z, Wu D, Zhao A, Zhou M, Qiu M, et al. In vitro and in vivo evaluation of the anti-asthmatic activities of fractions from Pheretima. J Ethnopharmacol. 2007;111(3):490–5. doi: 10.1016/j.jep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 66.Chen ML, Lin BF. Effects of triterpenoid-rich extracts of Ganoderma tsugae on airway hyperreactivity and Th2 responses in vivo. Int Arch Allergy Immunol. 2007;143(1):21–30. doi: 10.1159/000098222. [DOI] [PubMed] [Google Scholar]