Abstract
Micro-Abstract Osteoporosis and atherosclerosis frequently occur in the same individuals and may share similar pathogenic mechanisms. This study examined the relation between severity of aortic calcification in middle-age years and subsequent risk of hip fracture in women and men in the population-based Framingham Study.
Introduction
We assessed vascular calcification in women and men in middle-age and risk of hip fracture at advanced age.
Methods
Participants included 2499 Framingham cohort members (mean age, 61 years; range, 47 to 80 years). Semi-quantitative methods were used to determine severity of abdominal aortic calcification on baseline radiographs. Information on potential confounding factors was obtained from study examinations conducted at, or prior to, baseline radiography. Hip fractures were ascertained by active surveillance and confirmed by medical records.
Results
35-year cumulative incidence of hip fracture was 16% in women and 5% in men with prevalent aortic calcification at baseline (score 1+), and 14% in women and 4% in men without aortic calcification (score 0). Hazard ratios (HRs) and 95% confidence intervals (CIs) for hip fracture did not increase from the lowest to the highest category of aortic calcification. HRs were 1.0, 1.2 (95% CI=0.9-1.8), 1.2 (0.7-1.9), 1.1 (0.7-1.7), and 1.4 (0.8-2.3) in women (p for trend=0.44) and 1.0, 1.8 (0.8-3.8), 1.8 (0.7-4.6), 1.5 (0.6-3.9), and 1.2 (0.2-5.7) in men (p for trend=0.29), for aortic calcification scores 0 (reference), 1-4, 4-5, 6-10, 11+, respectively. However, aortic calcification score was strongly associated with increased risk of death (p for trend < 0.0001 in women and men). HRs (95% CIs) for mortality from the lowest to highest aortic calcification score were 1.0, 1.6 (1.4-1.9), 1.7 (1.4-2.1), 1.8 (1.5-2.2), and 2.1 (1.7-2.6), and for men were 1.0, 1.4 (1.1-1.6), 1.4 (1.2-1.8), 1.6 (1.3-2.0), 1.9 (1.5-2.5).
Conclusions
Vascular calcification in middle-aged adults does not increase long-term hip fracture risk.
Keywords: hip fracture, men, osteoporosis, population studies, vascular calcification, women
Introduction
Osteoporosis and atherosclerosis are both highly prevalent conditions causing major morbidity, mortality, and medical costs in the western world. Moreover, the magnitude of the impact of these chronic conditions will only increase as both the number and relative proportion of elderly persons in the population rise. Evidence for a shared pathogenesis between osteoporosis and atherosclerosis is emerging from both basic and clinical research. Although the pathophysiological mechanisms are not yet established, numerous factors are implicated including regulators of bone turnover, inflammatory cytokines, homocysteine, oxidized lipids, osteoprotegerin and the RANK ligand system, sex steroids, vitamin D, vitamin K, and others.(1-10) Knockout mice with deletions of matrix gla protein, Smad6, Klotho, osteopontin, osteoprotegerin or RANKL, and other genes develop both osteoporosis and vascular calcification, however, these findings in animals are not directly applicable to humans.(11)
In population-based cohort studies, postmenopausal women with the greatest bone loss have the greatest progression of aortic calcification, and those with lower bone mass have greater incidence of coronary heart disease compared to those with greater bone mass.(12-14) Women with low bone mineral density, increased bone loss, and vertebral fracture are also observed to have increased mortality due to cardiovascular disease.(15-17) An inverse relation between bone mineral density and vascular calcification at different sites, including the coronary, carotid, and peripheral arteries, is reported by some, but not all cross-sectional studies.(18-21)
While it is well established that vascular calcification increases risk of cardiovascular disease and mortality independently of the effects of traditional risk factors,(22-24) the clinical implication of the potential link between osteoporosis and vascular calcification is not yet known. Few studies have assessed the relation between vascular calcification and hip fracture, the most serious clinical outcome of osteoporosis. More importantly, the ability of vascular calcification assessments in persons during middle-age years to predict long term risk of hip fracture is not known. This is a particularly important clinical issue to address, as individuals in middle-age years may be most amenable to prevention and treatment efforts to reduce subsequent risk of osteoporosis and asssociated fractures later in life.
Methods
Participants
This prospective study of hip fracture is part of the original cohort of the Framingham Heart Study, a population-based cohort of a two-thirds sample of residents in Framingham, Massachusetts, established in 1949 to identify risk factors for cardiovascular disease. Methods of recruitment and data collection have been described elsewhere.(25,26) Briefly, participants completed standardized examinations, laboratory tests, and structured questionnaires, administered by trained physician examiners every two years, at study visits. Cohort members have been followed closely for all major health events, and follow-up is complete for 99%. In 1967-70, 67% of cohort members underwent lumbar spine radiography. No selection criteria were used, rather, the radiographic examinations were conducted until funding was no longer available. Radiographs were not readable for 11 participants. The current study includes 2499 cohort members (mean age, 61 years; range, 47 to 80 years), with no previous hip fracture, who were followed until the first of the following events: hip fracture, last contact, death, or December 31, 2003.
Hip fracture
Hip fracture was ascertained by interview at study examinations as well as by comprehensive review of fracture logs, hosptalization and death records. Hip fracture cases were confirmed by review of medical records as well as radiographic and operative reports. Hip fracture was defined as a first-time fracture of the proximal femur occuring between the time of baseline lumbar spine radiography (1967-69) and the end of follow-up for the current study, December 31, 2003. Rates of hip fracture reported in members of the Framingham Study correspond closely with national rates.(26,27)
Vascular calcification
Readers, blinded to subject characteristics, used a validated, semi-quanititative method to grade severity of vascular calcification in the aorta (intra-rater agreement=0.98, interrater agreement=0.93).(28) Calcific deposits in the abdominal aorta adjacent to each lumbar vetebra (L1-L4) were assessed separately for the posterior and anterior wall of the aorta. The midpoints of the intervertebral spaces above and below the vertebrae were used as boundaries. Each segment was graded systematically, using an illustrated atlas, according to a 4-point severity scale as follows: (0) no calcific deposits, (1) small, scattered calcific deposits filling less than one third of the longitudinal wall of the aorta, (2) one third or more, but less than two thirds of the longitudinal wall of the aorta calcified, (3) two thirds or more of the longitudinal wall of the aorta calcified. The (0-3) grades for each of the 8 segments (anterior and posterior segments for each of four lumbar vertebrae) were then summed for a total score, ranging from a minimum of 0, indicating no aortic calcification, and increasing in severity to a maximum of 24.
Potential confounders
Risk factors for hip fracture and/or vascular calcification were considered as potential confounders including age, weight, height, systolic blood pressure, smoking, total cholesterol, diabetes, coronary heart disease, and estrogen use (in women).(24) Assessments made at the time of baseline radiography were used for the analysis. Height, to the nearest 0.25 inch and weight, to the nearest 0.5 pound, were measured using a stadiometer and balance beam scale, respectively. Body mass index was calculated as weight in kilograms divided by the square of height in meters. Individuals were classified as current, former, or never smokers. Total serum cholesterol was measured on blood drawn from nonfasting participants using nonenzymatic methods.(29) Systolic blood pressure was measured twice at each visit by a physician using a standard sphygmomanometer (and averaged). Diabetes was defined as a casual glucose level of more than 150 mg/dL on two or more study visits or treatment with insulin or oral hypogycemic agents.(30) Coronary heart disease was defined as recognized or unrecognized myocardial infarction (identified by electrocardiogram or enzymes), angina pectoris, or coronary insufficiency. Ascertainment of coronary heart disease was conducted through active surveillance and diagnoses were confirmed by a three-member panel of physicains who conducted comprehesive, standardized reviews of medical records available from study examinations, physician visits, and hospitalizations. Women were categorized according to whether or not they used postmenopausal estrogen treatment at the baseline assessement.
Statistical analysis
Cox proportional hazard regression models were used to calculate hazard ratios and 95% confidence intervals for the relation between aortic calcification and risk of hip fracture. Since age-adjusted and multivariable-adjusted hazard ratios were similar, we present only multivariable results. Multivariable models were adjusted for age, body mass index, smoking, systolic blood pressure, total cholesterol, diabetes, coronary heart disease, and estrogen use (in women). Prior to their inclusion in multivariable models, independent variables were evaluated for concordance with the proportional hazard assumption. We examined aortic calcification as a categorical variable (0, 1-3, 4-5, 6-10, 11+) as well as a continuous, natural-log-transformed variable due to the skewed distribution. Particpants with score 0, indicating the complete absence of aortic calcification, were used as the reference group.
To determine if the relation between aortic calcification and hip fracture risk may be obscured by the long, 35-year duration of follow-up time, we repeated analyses truncating follow-up time to 15 and 25 years. Since results were unchanged, we present results based upon the full duration of study follow-up time (1967-70 through 2003).
To determine if a potential association between aortic calcification and hip fracture risk might be more apparent in individuals who were free of prevalent coronary heart disease, we repeated our analyses exluding participants with baseline coronary heart disease. Since results were unchanged, we present findings based upon inclusion of all participants (and adjust for coronary heart disease in multivariable models).
We also calculated hazard ratios for mortality associated with aortic calcification in order to evaluate whether or not survival bias may obscure a potential relation between aortic calcification and hip fracture. Further, we performed a competing risk analysis that considered both mortality and hip fracture as possible outcomes, allowing either to occur first.(31) Analyses were conducted using PC-SAS 9.1 (SAS Institute Inc., Cary, North Carolina) and performed separately for women and men.
Results
The study included 1453 women and 1046 men whose baseline age ranged from 47 to 80 years. Mean age of participants was 61 years, and mean duration of follow-up was 21 years and reached a maximum of 36 years. Individuals with aortic calcification were older, had higher cholesterol and blood pressure levels, and had greater prevalence of diabetes, coronary heart disease, and smoking than those without aortic calcification (Table 1). Body mass index was similar among participants with and without prevalent aortic calcification. Women with aortic calcification used postmenopausal estrogen therapy less frequently (12%) than those without aortic calcification (18%).
Table 1.
Baseline characteristics of 1453 women and 1046 men in the Framingham Study according to absence (score 0) or presence (score 1+) of aortic calcification, 1967-70
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Aortic calcification | Aortic calcification | |||||
| Absent | Present | Total | Absent | Present | Total | |
| Number | 602 (41%) | 851 (59%) | 1453 | 340 (33%) | 706 (67%) | 1046 |
| Age (yr) | 57 | 64 | 61 | 56 | 62 | 60 |
| Weight (kg) | 65 | 64 | 64 | 80 | 77 | 78 |
| Height (cm) | 159 | 158 | 158 | 172 | 171 | 171 |
| Body mass index (kg/m2) | 26 | 26 | 26 | 27 | 26 | 27 |
| Cholesterol (mg/dl) | 223 | 244 | 236 | 213 | 218 | 216 |
| Systolic blood pressure (mmHg) | 132 | 146 | 140 | 133 | 142 | 139 |
| Type 2 diabetes mellitus (%) | 1 | 7 | 5 | 4 | 7 | 6 |
| Current smoker (%) | 28 | 35 | 32 | 30 | 39 | 36 |
| Current estrogen use(%) | 18 | 12 | 14 | - | - | |
| Coronary heart disease (%) | 3 | 12 | 8 | 9 | 18 | 15 |
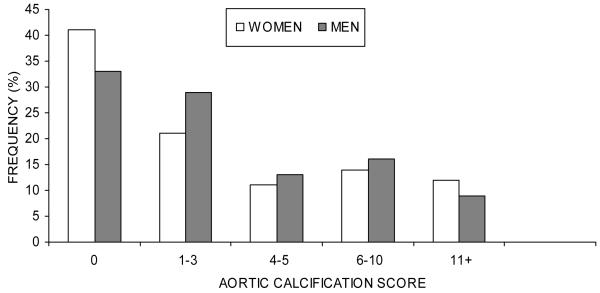
Aortic calcification was present (score 1+) in 59% of women and 67% of men; however, more than three-fifths of participants (62%) scored either 0 or 1-3, the lowest categories of aortic calcification. While men more frequently than women had aortic calcification scores of 1-3 (29% men, 21% women), 4-5 (13% men, 11% women), and 6-10 (16% men, 14% women), a greater frequency of women (12%) than men (9%) scored 11+, the highest category of acortic calcification (Figure 1).
FIG. 1.
Distribution of aortic calcification in 1453 women and 1046 men in The Framingham Study, 1967-70
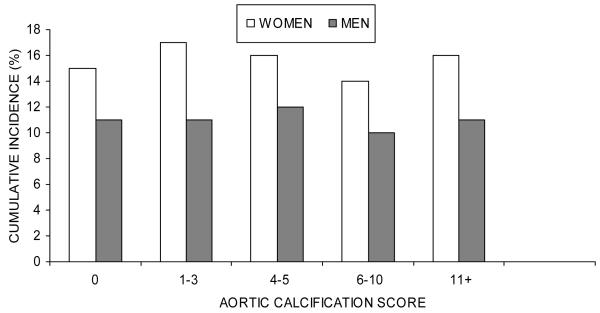
During the study, 223 women (15%) and 48 men (5%) sustained hip fractures. Cumulative incidence of hip fracture, however, did not increase with severity of aortic calcification (Figure 2). Incidence of hip fracture at 35 years of follow-up in women was 15% for aortic calcification score 0, 17% for score 1-3, 16% for score 4-5, 14% for score 6-10, and 16% for score 11+. For men, incidence was 4%, 6%, 5%, and 2%. Cumulative incidence of hip fracture also did not differ between individuals with aortic calcification present (women 16%, men 5%) or absent (women 15%, men 4%).
FIG. 2.
Cumulative incidence of hip fracture, 1967-70 through 2003, according to baseline aortic calcification score in 1453 women and 1046 men, The Framingham Study
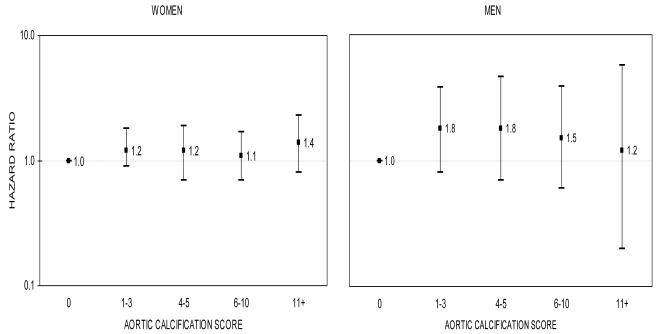
In age- and multivariable-adjusted analyses, aortic calcification was not associated with risk of hip fracture. Figure 3 reveals no clear trend in the relation between severity of aortic calcification and increased risk for fracture in women (p=0.44) or men (p=0.29), and hazard ratios did not statistically differ from 1.00. For example, the hazard ratios associated with the highest aortic calcifcation score (11+) were 1.4 (95% CI: 0.8-2.3) for women and 1.2 (95% CI: 0.2-5.7) for men. Similarly, no association was observed when aortic calcification was treated as a dichotomous variable or as a continuous variable. Hazard ratios for aortic calcification score 1+ versus score 0 were 1.2 (95%CI, 0.9-1.7) for women and 1.7 (95% CI, 0.8-3.4) for men. Hazard ratios for hip fracture per unit increase in log-transformed aortic calcification score were 1.1 (95% CI, 0.9-1.3) for women and 1.3 (95% CI, 0.9-1.8) for men. Results (not shown) were similar when alternative categorization schemes were considered including sex-specific quartiles as well as groupings such as (A) 0, 1-3, 4+, (B) 0, 1-5, 6+, and (C) 1-5, 6+.
FIG. 3.
Multivariable-adjusted hazard ratios* for hip fracture in women (left) and men (right) according to aortic calcification score, The Framingham Study, 1967-70 through 2003
The y-axis is on a log scale. The reference group is aortic calcification score 0. The bars denote 95 percent confidence intervals.
bars denote 95 percent confidence intervals.
* Models included baseline age, body mass index, systolic blood pressure, smoking, total cholesterol, diabetes, coronary heart disease, and estrogen use (in women).
Findings were unchanged when individuals with coronary heart disease at baseline were excluded (results not shown). When duration of follow-up time was restricted to 15 and 25 years, no association between vascular calcification and risk of hip fracture was found, and the magnitudes of the hazard ratios were generally reduced (and confidence intervals increased) due to the reduction in the number of hip fracture cases with shorter duration of follow-up time.
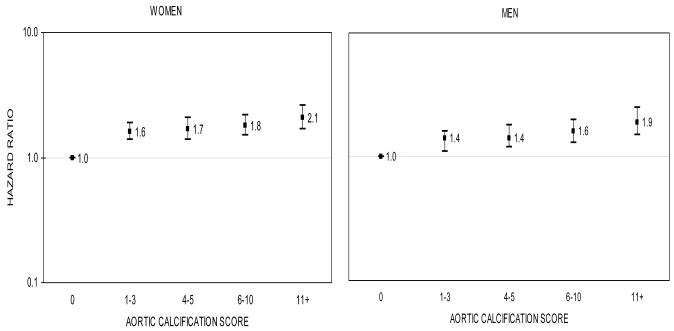
Mortality was 91% (777/851) in women and 94% (663/706) in men with aortic calcification compared to 60% (361/602) in women and 74% (252/340) in men without aortic calcification. In contrast to the null findings for risk of hip fracture, increasing severity of aortic calcification was highly associated with mortality for women and men (Figure 4). Multivariable-adjusted hazard ratios for mortality increased linearly with severity of aortic calcification score (trend, p<.0001). For example, hazard ratios for mortality were 1.6, 1.7, 1, 8 and 2.1 in women, respectively, for aortic calcification score 1-3, 4-5, 6-10, and 11+. A similar pattern was seen in men, however, the magnitudes of the hazard ratios associated with each category of aortic calcification were slightly smaller than found for women. Mortality was double in individuals with the greatest severity of aortic calcification: hazard ratios were 2.1 (95% CI:1.7-2.6) in women and 1.9 (95% CI:1.5-2.5) in men with score 11+ relative to those with score 0. In additional analyses with death modeled as a competing outcome to hip fracture, hazard ratios did not differ from those provided by Cox regression, and no association between vascular calcification and fracture risk was observed.
FIG. 4.
Multivariable-adjusted hazard ratios* for death in women (left) and men (right) according to aortic calcification score, The Framingham Study, 1967-70 through 2003
The y-axis is on a log scale. The reference group is aortic calcification score 0. The bars denote 95 percent confidence intervals.
bars denote 95 percent confidence intervals.
* Models included baseline age, body mass index, systolic blood pressure, smoking, total cholesterol, diabetes, coronary heart disease, and estrogen use (in women).
Discussion
In a large, population-based cohort followed for more than 30 years, we found no evidence that increasing severity of vascular calcification in women and men in middle-age years increased subsequent risk of hip fracture later in life. Results were unchanged when shorter durations of follow-up were considered. We also found no association between vascular calcification and risk of hip fracture in persons without pre-existing coronary heart disease. While we did observe a strong relation between increasing severity of vascular calcification in women and men in middle-age years and risk of death, differential survival in middle-aged individuals with and without aortic calcification does not explain the lack of an association with risk of hip fracture observed in our study.
Our findings do not agree with those of Schulz et al(32) who reported a 2.9 increase in odds of previous hip fracture (95% CI 1.8-4.8) among women with compared to those without aortic calcification. Several methodological differences may account for the discordant results. The cross-sectional design and inclusion of a patient population referred for computed tomography (CT) examination may have introduced a selection bias since subjects having a greater prevalence of both osteoporosis and atherosclerosis may have been overrepresented relative to the co-occurrence of these conditions in the general population. The high prevalence of subjects classified with vascular calcification (76%) or osteoporosis (70%) supports this suggestion.(32,33) In addition, the cross-sectional nature of the study prohibits determination of the temporal sequence with respect to vascular calcification and risk of hip fracture. In contrast, our investigation allowed for us to prospectively evaluate the clinical significance of the severity of vascular calcification in middle-aged individuals with respect to long term risk of hip fracture. Alternatively, CT determination of vascular calcification in the abdominal aorta may be a more accurate measurement of vascular calcification than the radiographic assessment used in our study. Such misclassification, if non-differential, may have biased our results toward the null. However, while the previous study measured aortic calcification from CT scans in degrees of calcified wall from 0-360, in the analysis, women were dichotomized into subjects with and without calcification (cut point not given). Thus, it is difficult to compare the vascular calcification measurements between studies.
Our findings also do not agree with those of Bagger et al(34) who, in a prospective cohort study of 2662 women in Denmark, found a 2.3 increased risk of hip fracture (95% CI, 1.1-4.8) in women with aortic calcification scores greater than or equal to three, relative to those scoring less than three. Aortic calcification was based upon radiographic assessment using the same methods as our study, however, the previous study included only women who survived from baseline to follow-up (mean follow-up time was seven years; study duration was not provided), and the investigators used logistic regression to estimate relative risk. In contrast, our study used Cox proportional hazards regression to account for varying lengths of follow-up time from baseline to the time of censorship due to hip fracture, death, or end of study.
While evidence for a link between bone and vascular calcification is increasing, the clinical importance remains largely unknown. This prospective cohort study evaluated the effect of radiographic vascular calcification in middle-age on subsequent risk of hip fracture. Participants were selected from a community-based sample that includes large numbers of both women and men making our results highly generalizable to the larger population. In addition, information on important confounders, including risk factors for both osteoporosis and atherosclerosis, is highly accurate and complete due to the highly standardized, rigorous data collection methods used in the Framingham Heart Study. Ascertainment of incident hip fracture was conducted by active surveillance with thorough case confirmation methods and, morever, without knowledege of participant characteristics, including vascular calcification status.
A limitation of our study was the reliance on plain radiographs to assess aortic calcification which may not be as accurate as current technologies, including CT. However, the semi-quantitative method used in our study is both highly reliable,(28,34-36) and the severity score is strongly associated with vascular morbidity and mortality,(24,37) as well as with overall mortality, as shown in our study. Finally, the long, thirty-five year duration of follow-up may have obscured a potential association between aortic calcification and risk of hip fracture. While we found no association when we restricted our analysis to ten and twenty years of follow-up, the reduction in the number of cases in these analyses reduced study power to detect a potential association. However, we found no association between vascular calcification and risk of hip fracture whether we used Cox regression or competing risk analysis. Thus, survival bias does not appear to explain the null findings observed in our study.
In conclusion, we found no evidence that vascular calcification in middle-age increases subsequent risk of hip fracture in women or men. The common radiographic finding of aortic calcification in middle-aged and older adults cannot be recommended in the clinical setting for identifying persons at increased risk of hip fracture.
Acknowledgements
Funding sources for this study included grants K01AR053118-01, R01 AR/AG 41398, and NIH/NHLBI Contract N01-HC-25195.
Footnotes
Publisher's Disclaimer: All authored papers and editorial news and comments, opinions, findings, conclusions or recommendations in JBMR WebFirst papers are those of the author(s) and do not necessarily reflect the views of the JBMR and its publisher, nor does their publication imply any endorsement. No responsibility is assumed, and responsibility is hereby disclaimed, by the American Society for Bone and Mineral Research and the Journal of Bone and Mineral Research for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of methods, products, instructions or ideas presented in these articles. Independent verification of diagnosis and drug dosages should be made. Discussions, views and recommendations as to medical procedures, choice of drugs and drug dosages are the responsibility of the authors. JBMR WebFirst papers have been peer-reviewed, however the articles have not gone through the copyediting process. Papers will not appear in JBMR style and format until the final print and online version is available. The WebFirst publication date is the official date of publication for each paper. There will be minor changes made to the WebFirst paper in the copyediting process, however no scientific content will be changed. The final paper published in the print Journal and on JBMR Online will not change in scientific content, only in presentation and to adhere to JBMR style.
Conflict of Interest: The authors state that they have no conflicts of interest.
References
- 1.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. Jama. 2004;292(4):490–5. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 2.Schoppet M, Preissner KT, Hofbauer LC. RANK ligand and osteoprotegerin: paracrine regulators of bone metabolism and vascular function. Arterioscler Thromb Vasc Biol. 2002;22(4):549–53. doi: 10.1161/01.atv.0000012303.37971.da. [DOI] [PubMed] [Google Scholar]
- 3.Jie KG, Bots ML, Vermeer C, Witteman JC, Grobbee DE. Vitamin K status and bone mass in women with and without aortic atherosclerosis: a population-based study. Calcif Tissue Int. 1996;59(5):352–6. doi: 10.1007/s002239900139. [DOI] [PubMed] [Google Scholar]
- 4.Kammerer CM, Dualan AA, Samollow PB, Perisse AR, Bauer RL, MacCluer JW, O’Leary DH, Mitchell BD. Bone mineral density, carotid artery intimal medial thickness, and the vitamin D receptor BsmI polymorphism in Mexican American women. Calcif Tissue Int. 2004;75(4):292–8. doi: 10.1007/s00223-004-0215-9. [DOI] [PubMed] [Google Scholar]
- 5.Parhami F. Possible role of oxidized lipids in osteoporosis: could hyperlipidemia be a risk factor? Prostaglandins Leukot Essent Fatty Acids. 2003;68(6):373–8. doi: 10.1016/s0952-3278(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 6.Luegmayr E, Glantschnig H, Wesolowski GA, Gentile MA, Fisher JE, Rodan GA, Reszka AA. Osteoclast formation, survival and morphology are highly dependent on exogenous cholesterol/lipoproteins. Cell Death Differ. 2004;11(Suppl 1):S108–18. doi: 10.1038/sj.cdd.4401399. [DOI] [PubMed] [Google Scholar]
- 7.Towler DA, Shao J-S, Cheng S-L, Pingsterhaus JM, Loewy AP. Osteogenic regulation of vascular calcification. Ann N Y Acad Sci. 2006;1068 doi: 10.1196/annals.1346.036. [DOI] [PubMed] [Google Scholar]
- 8.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24(7):1161–70. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 9.Price PA, June HH, Buckley JR, Williamson MK. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol. 2001;21(10):1610–6. doi: 10.1161/hq1001.097102. [DOI] [PubMed] [Google Scholar]
- 10.Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA, Jr., Falb D, Huszar D. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet. 2000;24(2):171–4. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- 11.Hofbauer LC, Brueck CC, Shanahan CM, Schoppet M, Dobnig H. Vascular calcification and osteoporosis--from clinical observation towards molecular understanding. Osteoporos Int. 2007;18(3):251–9. doi: 10.1007/s00198-006-0282-z. [DOI] [PubMed] [Google Scholar]
- 12.Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC. Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal study. Arterioscler Thromb Vasc Biol. 2000;20(8):1926–31. doi: 10.1161/01.atv.20.8.1926. [DOI] [PubMed] [Google Scholar]
- 13.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O’Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68(5):271–6. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- 14.Samelson EJ, Kiel DP, Broe KE, Zhang YQ, Cupples LA, Hannan MT, Wilson PWF, Levy D, Williams SA, Vaccarino V. Metacarpal cortical area and risk of coronary heart disease - The Framingham Study. Am J Epidemiol. 2004;159(6):589–595. doi: 10.1093/aje/kwh080. [DOI] [PubMed] [Google Scholar]
- 15.Browner WS, Pressman AR, Nevitt MC, Cauley JA, Cummings SR. Association between low bone density and stroke in elderly women. The study of osteoporotic fractures. Stroke. 1993;24(7):940–6. doi: 10.1161/01.str.24.7.940. [DOI] [PubMed] [Google Scholar]
- 16.von der Recke P, Hansen MA, Hassager C. The association between low bone mass at the menopause and cardiovascular mortality. Am J Med. 1999;106(3):273–8. doi: 10.1016/s0002-9343(99)00028-5. [DOI] [PubMed] [Google Scholar]
- 17.Ensrud KE, Thompson DE, Cauley JA, Nevitt MC, Kado DM, Hochberg MC, Santora AC, 2nd, Black DM, Fracture Intervention Trial Research Group Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. J Am Geriatr Soc. 2000;48(3):241–9. doi: 10.1111/j.1532-5415.2000.tb02641.x. [DOI] [PubMed] [Google Scholar]
- 18.van der Klift M, Pols HA, Hak AE, Witteman JC, Hofman A, de Laet CE. Bone mineral density and the risk of peripheral arterial disease: the Rotterdam Study. Calcif Tissue Int. 2002;70(6):443–9. doi: 10.1007/s00223-001-2076-9. [DOI] [PubMed] [Google Scholar]
- 19.Barengolts EI, Berman M, Kukreja SC, Kouznetsova T, Lin C, Chomka EV. Osteoporosis and coronary atherosclerosis in asymptomatic postmenopausal women. Calcif Tissue Int. 1998;62:209–213. doi: 10.1007/s002239900419. [DOI] [PubMed] [Google Scholar]
- 20.Banks LM, Lees B, MacSweeney JE, Stevenson JC. Effect of degenerative spinal and aortic calcification on bone density measurements in post-menopausal women: links between osteoporosis and cardiovascular disease? Eur J Clin Invest. 1994;24:813–817. doi: 10.1111/j.1365-2362.1994.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 21.Uyama O, Yoshimoto Y, Yamamoto Y, Kawai A. Bone changes and carotid atherosclerosis in postmenopausal women. Stroke. 1997;28:1730–1732. doi: 10.1161/01.str.28.9.1730. [DOI] [PubMed] [Google Scholar]
- 22.Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. Jama. 2000;283(21):2810–5. doi: 10.1001/jama.283.21.2810. [DOI] [PubMed] [Google Scholar]
- 23.Witteman JC, Kannel WB, Wolf PA, Grobbee DE, Hofman A, D’Agostino RB, Cobb JC. Aortic calcified plaques and cardiovascular disease (the Framingham Study) Am J Cardiol. 1990;66(15):1060–4. doi: 10.1016/0002-9149(90)90505-u. [DOI] [PubMed] [Google Scholar]
- 24.Wilson PW, Kauppila LI, O’Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103(11):1529–34. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 25.Dawber TR, Meadors GF, Moore FE. Epidemiological approaches to heart disease: the Framingham Study. Am J Pub Health. 1951;41:279–286. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiel DP, Felson DT, Anderson JJ, Wilson PW, Moskowitz MA. Hip fracture and the use of estrogens in postmenopausal women. The Framingham Study. N Engl J Med. 1987;317(19):1169–74. doi: 10.1056/NEJM198711053171901. [DOI] [PubMed] [Google Scholar]
- 27.Samelson EJ, Zhang Y, Kiel DP, Hannan MT, Felson DT. Effect of birth cohort on risk of hip fracture: age-specific incidence rates in the Framingham Study. Am J Public Health. 2002;92(5):858–62. doi: 10.2105/ajph.92.5.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kauppila LI, Polak J, Cupples LA, Hannan MT, Kiel DP, Wilson PWF. New indices to classify location, severity, and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132:245–250. doi: 10.1016/s0021-9150(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 29.Abell LL, Levy BB, Brodie BB, Kendall FE. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952;195:357–366. [PubMed] [Google Scholar]
- 30.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28(12):1039–57. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 31.Southern DA, Faris PD, Brant R, Galbraith PD, Norris CM, Knudtson ML, Ghali WA. Kaplan-Meier methods yielded misleading results in competing risk scenarios. J Clin Epidemiol. 2006;59(10):1110–4. doi: 10.1016/j.jclinepi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89(9):4246–53. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 33.Rubin MR, Silverberg SJ. Vascular calcification and osteoporosis--the nature of the nexus. J Clin Endocrinol Metab. 2004;89(9):4243–5. doi: 10.1210/jc.2004-1324. [DOI] [PubMed] [Google Scholar]
- 34.Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. J Intern Med. 2006;259(6):598–605. doi: 10.1111/j.1365-2796.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- 35.Tanko LB, Qin GR, Alexandersen P, Bagger YZ, Christiansen C. Effective doses of ibandronate do not influence the 3-year progression of aortic calcification in elderly osteoporotic women. Osteoporosis Int. 2005;16(2):184–190. doi: 10.1007/s00198-004-1662-x. [DOI] [PubMed] [Google Scholar]
- 36.Jamal SA, Leiter RE, Bauer DC. Hyperhomocysteinaemia and aortic calcification are associated with fractures in patients on haemodialysis. Qjm. 2005;98(8):575–579. doi: 10.1093/qjmed/hci092. [DOI] [PubMed] [Google Scholar]
- 37.Walsh CR, Cupples LA, Levy D, Kiel DP, Hannan M, Wilson PW, O’Donnell CJ. Abdominal aortic calcific deposits are associated with increased risk for congestive heart failure: the Framingham Heart Study. Am Heart J. 2002;144(4):733–9. doi: 10.1067/mhj.2002.124404. [DOI] [PubMed] [Google Scholar]