Abstract
Dietary survey data show that intake of sugar-sweetened beverages is negatively associated with intake of milk, but these findings have yet to be confirmed by laboratory feeding studies. The objectives of the present study were to analyze children’s intake across 2 laboratory-based ad libitum lunches to 1) investigate the relationships between intake of sweetened beverages, milk, and calcium and 2) explore relationships between beverage consumption and child age and weight status. Data were extracted from a cohort of 126, 3–7 year (y.)-old twins from diverse ethnic backgrounds who participated in a cross-sectional study (conducted from November 1999 – September 2002) designed to determine the genetic and environmental contributions to eating and body weight. At 2 visits, children ate ad libitum from lunches that offered a variety of sugar-sweetened and calcium-rich beverages. Total beverage and nutrient intakes were computed from the test meals. Weight, height, and waist circumference were assessed on the final visit. Regression analyses tested the associations among intake of sweetened beverages, calcium, and milk (primary aim) and whether these variables were associated with child age and weight status (secondary aim). Sweetened beverage intake was negatively correlated with both milk (p < 0.01) and calcium (p < 0.01) intakes, and these relationships remained after controlling for age, gender, and ethnicity (p < 0.01). Child age was negatively associated with milk intake (r=−0.22, p < 0.01) but positively associated with intake of sweetened beverages (r=0.27, p < 0.01). Results support the notion that sugar-sweetened beverages displace milk in a single meal, and this phenomenon may vary with child age. Due to the cross-sectional nature of this study, future investigations are needed to determine the long-term implications of this consumption pattern. The possibility that limiting sweetened beverages may help optimize dietary calcium during childhood is a topic that merits further research.
Keywords: Beverage intake, sugar-sweetened beverages, milk intake, childhood obesity
Introduction
Cross-sectional data find that calcium intake in United States (US) children is lower than recommended (1,2), and tends to decrease with age, while intake of sweetened beverages such as soft drinks and juices tends to increase, particularly among adolescents (3,4). Decreases in calcium intake among children can in part be attributed to declines in milk consumption over the past several decades (5). Some investigators have hypothesized that sweetened beverages displace intake of milk in children’s diets (6,7). If this displacement begins in early childhood, children might suffer from inadequate intakes of calcium through adolescence, a dietary pattern which can potentate the development of osteoporosis (8,9) or possibly obesity (10,11) in adulthood. To date, there have been no reports using direct measures of food intake from controlled laboratory settings to support findings from cross-sectional surveys. The present laboratory feeding study is warranted to more rigorously test the reported inverse associations between intake of milk and sweetened beverages.
The primary aim of this study was to test the hypothesis that increased sweetened beverage intake is associated with decreased milk and calcium intake in laboratory-based meal data previously collected from a twin cohort. The secondary objective was to test the hypothesis that sweetened beverage intake at meals is positively associated with child age, while the inverse is true for milk intake. Because several recent studies have found that intake of high-calcium dairy products may be inversely correlated to body weight (12–15), the associations between beverage consumption and weight status were also assessed.
Methods
Children’s data were obtained from a cross-sectional laboratory study designed to test genetic and environmental influences on child eating and body composition. The original study was conducted during the period of November 1999 to September 2002. Participants (n=126, 54 boys, 72 girls) in this convenience sample were same-sex identical and fraternal twins, 3–7 y. old with a mean (± standard deviation) – (SD) age of 4.5 (± 1.5) y. Recruitment was done by advertising in local parent-targeted circulations and was ongoing over the course of the 3 year study. Twins were excluded if they had any medical problems or were taking any medications, had any food allergies, or were lactose intolerant. All parents signed consent for their children’s participation and research authorization forms as defined by the Health Insurance Portability and Accountability Act (HIPAA). The Institutional Review Board of St. Luke’s Roosevelt Hospital approved this study.
For the full study protocol, children came to the child feeding laboratory for 4 lunch-time visits (16). Data for the present study came from the last two visits where children consumed multi-item ad libitum lunches. Each twin ate alone with his or her mother and the lunch was videotaped in order to capture parental feeding interactions associated with the meal (16,17), but these variables were not analyzed in the present study.
Children were given 30 minutes to consume meals that consisted of a variety of age-appropriate foods listed in Table 1. Foods were selected based on their familiarity to most children in this age group. Although they were not selected for the purposes of studying beverage and calcium intake, per se, the variety of foods was such to allow some exploration of beverage choice.
Table 1.
Foods Served in Buffet Lunch, Serving Sizes, and Nutrient Information
| Food | Approximate Serving Size (g) | Calories; Calcium (mg) |
|---|---|---|
| Chicken Nuggets | 372 g (5 chicken nuggets) | 951 kcal; 43 mg |
| Bologna on Wheat | 150 g (1 small sandwich) | 292 kcal; 58 mg |
| Bologna on White | 150 g (1small sandwich) | 247 kcal; 59 mg |
| Turkey on Wheat | 100 g (1 small sandwich) | 225 kcal; 56 mg |
| Turkey on White | 100 g (1 small sandwich) | 178 kcal; 57 mg |
| Canned Peas | 40 g | 28 kcal; 8 mg |
| Canned Corn | 40 g | 32 kcal; 2 mg |
| Baby Carrots | 35 g | 13 kcal; 8 mg |
| Cheese and Crackers | 195 g | 312 kcal; 94 mg |
| Strawberry Yogurt | 120 g | 118 kcal; 176 mg |
| Raisins | 18 g | 54 kcal; 9 mg |
| Applesauce | 120 g | 91 kcal; 5 mg |
| Potato Chips | 47 g (1 small bag) | 257 kcal; 13 mg |
| Regular Fat Chocolate Milk (3.75 % fat) | 260 g | 217 kcal; 292 mg |
| Whole White Milk (3.75 % fat) | 400 g | 257 kcal; 476 mg |
| Apple Juice | 255 g (1 small juice box) | 120 kcal; 18 mg |
| Orange Juice | 220 g (1 small can) | 92 kcal; 18 mg |
| Fruit Punch | 260 g (1 small juice box) | 122 kcal; 21 mg |
| Caffeine Free Cola | 275 g (1 can) | 151 kcal; 0 mg |
| Banana | 90 g (1 half banana) | 83 kcal; 5 mg |
| Graham Crackers | 25 g | 106 kcal; 20 mg |
| Oreos | 36 g (3 cookies) | 100 kcal; 0 mg |
| Chocolate Cupcakes | 90 g (2 packaged cupcakes) | 275 kcal; 32 mg |
| Hershey Kisses | 50 g | 257 kcal; 96 mg |
| Fruit Cocktail | 145 g | 67 kcal; 12 mg |
| Jello | 110 g | 65 kcal; 2 mg |
| Chocolate Pudding | 120 g | 157 kcal; 119 mg |
| Fruit Roll-up | 15 g | 56 kcal; 0 mg |
|
| ||
| Granola Bar | 30 g | 126 kcal; 28 mg |
Beverage options are shaded.
Anthropometric measures (height, weight, and waist circumference) were performed by a trained technician with experience taking such measures in children. Waist circumference was measured to the nearest 1 centimeter (cm), midway between the lowest point of the ribcage and the iliac crest. Weight was measured to the nearest 0.1 kg by a digital scale (Weight-Tronix, Scale Electronics Development, New York, NY). Height (without shoes) was measured to the nearest 0.5 cm using a stadiometer (Holtain, Crosswell, Wales, UK). Height and weight were converted to body mass index (BMI = kg/m2), and BMI z-scores, using the Centers for Disease Control growth charts conversion program for Statistical Analysis Software (version 9.1, 2002, SAS Institute Inc, Cary, NC).
Statistics
Energy consumption across meals was determined by first calculating the difference between the pre-and post-weights of all foods. Total energy and percent of calories from fat, carbohydrate, and protein were calculated from food label information.
Micronutrient intakes at the meals, in particular calcium and vitamin D, were calculated using FoodWorks (Version 8.0, 2000, The Nutrition Company, LongValley, NJ). FoodWorks contains over 30,000 foods and determines adequacy of each nutrient based on current dietary standards. To attain these values for each child, grams consumed of each food were entered into the program database and micronutrient outputs were extracted for further analyses.
Descriptive statistics (means and standard deviations) were generated for quantitative data, including beverage parameters (ie, calories from sweetened beverages and milk), as well as total energy, macro- and micronutrients (calcium and vitamin D). Pearson’s correlation coefficients were calculated to test the hypotheses that sweetened beverage intake was negatively associated with intake of milk, calcium, and vitamin D. The above analyses were also performed after adjusting for key descriptive variables (ie. age, gender, and ethnicity). Secondary objectives were assessed by determining the Pearson’s correlation coefficients between beverage intake, child age, and weight status.
To account for family dependencies in the data structure, significant correlations were retested using the degrees of freedom (df) from twin pairs instead of individual children in the analyses. Any relationships that were no longer significant after accounting for the family dependency were noted in the results.
Power calculations were not performed prior to these secondary data analyses. For all statistical tests, a p-value of less than or equal to 0.05 was the cut-off for significance, and all hypotheses were two-tailed. Descriptive statistics were reported as means ± SD. All analyses were done using SPSS software program (version 14.0, 2005, SPSS Inc, Chicago, IL).
Statement of Ethics
All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Results & Discussion
Children in this study were on average lean, with a mean (SD) BMI z-score of 0.2 (± 1.4), and waist circumference of 52.2 (± 6.0) cm. Fifty-three percent were non-Hispanic white, 16.2% were African-American, 14.0% were Hispanic-American, 2.9% were Asian-American, and 13.2% identified themselves as “other.” Across meals, children consumed 23.9 (±31.1) kcals from milk (both white and chocolate) and 37.6 (±38.0) kcals from sweetened beverages (all juices, juice drinks, and cola).
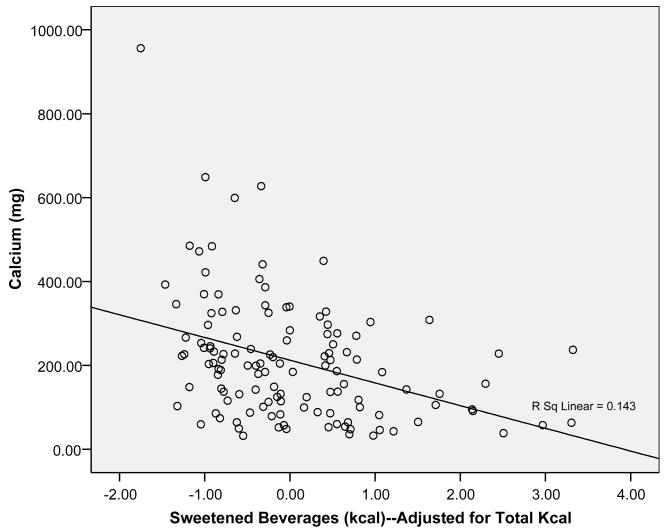
The primary aim of the study was to determine if sweetened beverage and milk/calcium intakes are inversely correlated with one another in a laboratory meal setting. Findings from these test meals show that intake of sweetened beverages (cola, juice, and juice drink) was negatively associated with intake of not only milk, but also calcium and vitamin D, two essential micronutrients. Specifically, sweetened beverage intake was negatively associated with intakes of both milks together (chocolate and white) (r = −0.32, p < 0.01), and with intake of just white milk (r = −0.20, p = 0.02). Also, sweetened beverage intake was negatively associated with total calcium consumed at the test meals (r = −0.24; p < 0.01). This relationship was stronger after adjusting sweetened beverage intake for total calories consumed at the meals (r = −0.38; p < 0.001) [Figure 1].
Figure 1.
Intake of sweetened beverages (kcal), adjusted for total kcal consumed at the test meals, was negatively associated with total meal calcium (mg) (r = −0.38; p ≤ 0.001).
In addition to having a negative relationship to calcium intake, sweetened beverages were also negatively associated with amount of vitamin D consumed (r = −0.28; p < 0.05). All of the above relationships remained after adjusting for child age, gender, and ethnicity.
While these findings are cross-sectional and do not demonstrate a causal relationship, they lend support to the notion that sweetened beverages displace intake of milk in children’s diets. The possibility that children who over-consume sugar-sweetened beverages may do so at the detriment of essential nutrients such as calcium and vitamin D warrants further investigation. In other reports, increased soft drink consumption, in particular, has been associated with lower intakes of calcium and other micronutrients in children and adolescents (7, 18–20). This is of great nutritional concern because calcium and vitamin D work together as key nutrients in achieving optimal bone health (8–9). Further, Zemel and colleagues have observed that adding calcium to the diet can enhance weight loss (10) and improve metabolic profiles (21) in adults. In addition to its potential to displace calcium, consumption of sweetened beverages alone has been proposed as an independent risk factor for obesity because some studies have found that children do not compensate for energy consumed in these highly palatable sources of liquid calories (22). Taken together, one can hypothesize that sweetened beverage consumption might be a contributing risk factor to poor overall diet quality and, potentially, obesity.
Previous reports have demonstrated that intake of sweetened beverages increases as children grow older, while intake of milk tends to decrease (3,23,24). Accordingly, a secondary aim of this project was to test for these associations in the laboratory. Indeed, child age was positively associated with sweetened beverage intake (r = 0.27; p < 0.01) and this association remained after adjusting for total energy intake (r = 0.19; p < 0.05). Further, after adjusting for total energy intake, age was negatively correlated with calories from both milks (white and chocolate) (r = −0.22; p < 0.01). While these relationships were expected, it was surprising that such low intakes of milk were seen in children that were, on average, not yet 5 y. old and who consumed the meal with their mothers present. Average caloric consumption from milk at the meal was around 24 kcal (~15 milliliters) and intake of calcium was around 200 mg. If this is representative of children’s usual consumption, the recommended adequate intake of 800 mg/day for this age group (25) would not be achieved. While other reports have noted similar results, most studies have identified this trend in adolescents (3) or children older than 5 y. of age (23). Results from the present study suggest that younger children may be at risk for this dietary trend as well, but additional studies are warranted to determine how well this test meal predicts children’s usual intakes.
Previous cross-sectional (12–15) and experimental (10,11,21) studies have suggested that calcium can be protective against obesity, despite a lack of relationship in this study. Neither calcium nor milk intake was inversely associated with BMI z-score (p-values ranging from 0.30–0.80), or with waist circumference (p-values ranging from 0.13–0.53). However, children were young and positive effects of calcium on body weight may not be evident yet. Also, sweetened beverage intake (adjusted for milk intake) was not associated with BMI z-score (p = 0.61) but showed a modest positive association with child waist circumference (r = 0.18, p = 0.05). These intriguing findings should be interpreted with caution. Intake of a test meal may not reflect children’s usual intake, and thus foods and nutrients consumed at these meals may not be good predictors of long-term consumption or health risk.
Limitations
There are several factors that might have influenced children’s food choices at the test meal that were not taken into account in this study, but should be noted. First, maternal beverage consumption patterns may influence children’s beverage choices, but these were not factored into the present study design. Fisher et al. found that maternal milk consumption is positively associated with daughters’ milk intakes (26). It is possible that children’s beverage choices in the present study were in part mediated by mothers’ habitual beverage consumption, thus future studies might wish to control for this variable. Furthermore, Birch and colleagues have hypothesized that parental feeding styles that are overly restrictive can encourage overeating of “restricted” foods in free access situations, particularly in young girls (27,28). It may be that children preferred to drink sweetened beverages at the laboratory because they were not regularly offered these beverages at home. The fact that calcium intakes in these meals were low is concerning, given that mothers were present, and this suggests the need to conduct future research on assessing level of parental concern and awareness about this important nutrient.
In addition to the parental variables mentioned above, there were other limitations. First, a laboratory meal might not reflect children’s usual intakes, as discussed above. Although the lab setting provided was comfortable for families, the laboratory cannot substitute for children’s natural eating environments. Furthermore, day-to-day and meal-to-meal fluctuations in calcium intake are common in children. Because this study presents data from only two meals, results may not reflect overall patterns of calcium consumption. Second, while children were offered a range of foods, intakes were limited by the choices available. The number of calcium-rich foods offered to children was minimal, and the possibility that greater calcium intake would have been seen with a greater variety of calcium-containing foods cannot be eliminated. Finally, power analyses were not performed on the reported relationships, so it is not possible to rule out the presence of a Type II error in the data. Despite the limitations, this is one of the only laboratory feeding studies to corroborate a wealth of cross-sectional data. Further, there is a lack of observational food intake data on children this age, so the contribution to the literature is thought to be meaningful.
Conclusion
These findings support previous cross-sectional data, and suggest that intake of sweetened beverages and intake of micronutrients such as calcium may be inversely correlated with each other during meals. Because this study was not designed to test causality, future investigations are warranted to determine the implications for consumption of sweetened beverages in the overall diet. Reducing consumption of sweetened beverages in children’s diets might be one strategy for increasing milk and calcium intake, a notion that warrants future testing.
Acknowledgments
This research was supported by NIH grant K08MH01530 (MSF) and a Pilot and Feasibility Grant awarded from the New York Obesity Research Center. Also, the work was made possible by the Obesity Research Center Grant (NIH grant 5P30DK026687-27). There are no conflicts of interest on behalf of any of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Park YK, Meier ER, Bianchi P, Song WO. Trends in children’s consumption of beverages: 1987 to 1998. Fam Econ Nutr Rev. 2002;14:69–79. [Google Scholar]
- 2.Storey ML, Forshee RA, Anderson PA. Associations of adequate intake of calcium with diet, beverage consumption, and demographic characteristics among children and adolescents. J Am Coll Nutr. 2004;23:18–33. doi: 10.1080/07315724.2004.10719339. [DOI] [PubMed] [Google Scholar]
- 3.Bowman SA. Beverage choices of young females: changes and impact on nutrient intakes. J Am Diet Assoc. 2002;102:1234–1239. doi: 10.1016/s0002-8223(02)90273-7. [DOI] [PubMed] [Google Scholar]
- 4.Smiciklas-Wright H, Mitchell D, Mickle S, Cook A, Goldman J. Foods commonly eaten in the United States: quantities consumed per eating occasion and in a day, 1994–96. Beltsville, MD: US Department of Agriculture, Agricultural Research Service; 2002. p. 252. [Google Scholar]
- 5.Rampersaud GC, Bailey LB, Kauwell GP. National survey beverage consumption data for children and adolescents indicate the need to encourage a shift toward more nutritive beverages. J Am Diet Assoc. 2003;103:97–100. doi: 10.1053/jada.2003.50006. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med. 2004;27:205–210. doi: 10.1016/j.amepre.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Harnack L, Stang J, Story M. Soft drink consumption among US children and adolescents: nutritional consequences. J Am Diet Assoc. 1999;99:436–441. doi: 10.1016/S0002-8223(99)00106-6. [DOI] [PubMed] [Google Scholar]
- 8.Wosje KS, Specker BL. Role of calcium in bone health during childhood. Nutr Rev. 2000;58:253–268. doi: 10.1111/j.1753-4887.2000.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 9.Matkovic V. Nutrition, genetics and skeletal development. J Am Coll Nutr. 1996;15:556–569. doi: 10.1080/07315724.1996.10718630. [DOI] [PubMed] [Google Scholar]
- 10.Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC. Regulation of adiposity by dietary calcium. FASEB J. 2000;14:1132–1138. [PubMed] [Google Scholar]
- 11.Lin Y, Roseann ML, McCabe L, McCabe G, Weaver C, Teegarden D. Dairy calcium is related to changes in body composition during a two-year exercise intervention in young women. J Am Coll Nutr. 2000;19:754–760. doi: 10.1080/07315724.2000.10718075. [DOI] [PubMed] [Google Scholar]
- 12.Barba G, Troiano E, Russo P, Venezia A, Siana A. Inverse association between body mass and frequency of milk consumption in children. Br J Nutr. 2005;93:15–19. doi: 10.1079/bjn20041300. [DOI] [PubMed] [Google Scholar]
- 13.Skinner JD, Bounds W, Carruth BR, Zeigler P. Longitudinaly calcium intake is negatively related to children’s body fat indexes. J Am Diet Assoc. 2003;103:1626–1631. doi: 10.1016/j.jada.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Novotny R, Daida YG, Acharya S, Grove JS, Vogt TM. Dairy intake is associated with lower body fat and soda intake with greater weight in adolescent girls. J Nutr. 2004;134:1905–1909. doi: 10.1093/jn/134.8.1905. [DOI] [PubMed] [Google Scholar]
- 15.Moore LL. Low intakes of dairy products in early childhood may increase body fat acquisition . Obes Res. 2003;11(suppl 9):130-OR. [Google Scholar]
- 16.Faith MS, Keller KL, Matz P, et al. Project Grow-2-Gether: a study of the genetic and environmental influences on child eating and obesity. Twin Research. 2002;5:472–475. doi: 10.1375/136905202320906309. [DOI] [PubMed] [Google Scholar]
- 17.Keller KL, Pietrobelli A, Johnson SL, Faith MS. Maternal restriction of children’s eating and encouragements to eat as the ‘non-shared environment’: a pilot study using the child feeding questionnaire. Int J Obes. 2006;30:1670–1675. doi: 10.1038/sj.ijo.0803318. [DOI] [PubMed] [Google Scholar]
- 18.Striegel-Moore RH, Thomson D, Affenito SG, et al. Correlates of beverage intake in adolescent girls: the National Heart Lung and Blood Institute Growth and Health Study. J Pediatr. 2006;148:183–187. doi: 10.1016/j.jpeds.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Artalego F, Garcia EL, Gorgojo L, et al. Consumption of bakery products, sweetened soft drinks, and yogurt among chilren aged 6–7 years: association with nutrient intake and overall diet quality. Br J Nutr. 2003;89:419–429. doi: 10.1079/BJN2002787. [DOI] [PubMed] [Google Scholar]
- 20.Fray CD, Johnson RK, Wang MQ. Children and adolescents’ choices of foods and beverages high in added sugars are associated with intakes of key nutrients and food groups. J Adolesc Health. 2004;34:56–63. doi: 10.1016/s1054-139x(03)00248-9. [DOI] [PubMed] [Google Scholar]
- 21.Zemel MB, Thompson W, Milstead JA, Morris K, Campbell P. Calcium and dairy acceleration of weight and fat loss during energy restriction in obese adults. Obes Res. 2004;12:582–590. doi: 10.1038/oby.2004.67. [DOI] [PubMed] [Google Scholar]
- 22.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes. 2000;24:794–800. doi: 10.1038/sj.ijo.0801229. [DOI] [PubMed] [Google Scholar]
- 23.Fisher J, Mitchell D, Smiciklas-Wright H, Birch L. Maternal milk consumption predicts the tradeoff between milk and soft drinks in young girls’ diets. J Nutr. 2001;131:246–250. doi: 10.1093/jn/131.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briefel R, Johnson C. Secular trends in dietary intake in the United States. Ann Rev Nutr. 2004;24:401–431. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Medicine, National Academy of Sciences. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Washington, DC: National Academy Press; 1997. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. [PubMed] [Google Scholar]
- 26.Fisher JO, Mitchell DC, Smiciklas-Wright H, Mannino ML, Birch LL. Meeting calcium recommendations during middle childhood reflects mother-daughter beverage choices and predicts bone mineral status. Am J Clin Nutr. 2004;79:698–706. doi: 10.1093/ajcn/79.4.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birch LL, Firsher JO, Davison KK. Learning to overeat: maternal use of restrictive feeding practices promotes girls’ eating in the absence of hunger. Am J Clin Nutr. 2003;78:215–220. doi: 10.1093/ajcn/78.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faith MS, Scanlon KS, Birch LL, Francis LA, Sherry B. Parent-child feeding strategies and their relationships to child eating and weight status. Obes Res. 2004;12:1711–1722. doi: 10.1038/oby.2004.212. [DOI] [PubMed] [Google Scholar]