Abstract
Glycyrrhizae uralensis (Gan-Cao) commonly called “licorice” is one of the most commonly used herbs in Traditional Chinese Medicine (TCM). In the United States licorice products are most often consumed as flavoring and sweetening agents in food products. The licorice triterpenoid glycyrrhizin has several biological activities, including anti-inflammatory activity. Other potential anti-inflammatory constituents in Glycyrrhizae uralensis have not been fully investigated. Airway eosinophilic inflammation is a major feature of allergic asthma. Eotaxin-1 is involved in the recruitment of eosinophils to sites of antigen induced inflammation in asthmatic airways. Because human lung fibroblasts are the major source of eotaxin, inhibition of eosinophil recruitment by suppression of fibroblast eotaxin production is a potentially valuable approach for the pharmacological intervention in asthma. A systematic bioassay guided purification of G. uralensis yielded 5 flavonoids-liquiritin (1), liquiritigenin (2), isoliquiritigenin (3), 7, 4′-dihydroxyflavone (4) and isoononin (5). The structure of the compounds was established by 1H, 13C NMR and LC-MS. Potential ability of these isolated pure compounds, and glycyrrhizin, to inhibit secretion of eotaxin-1 by human fetal lung fibroblasts (HFL-1) was tested. Liquiritigenin, isoliquiritigenin, and 7, 4′-dihydroxyflavone were more effective than liquiritin, isoononin and glycyrrhizin in suppressing eotaxin secretion. A dose response study showed the IC50 concentration of liquiritigenin (2), isoliquiritigenin (3), 7, 4-dihydroxyflavone (4) were 4.2, 0.92 and 0.21 µg/mL, respectively. We therefore, for the first time, show that Glycyrrhiza flavonoids inhibit eotaxin-1 secretion, suggesting that these compounds may have potential for the development of new therapies for asthma, allergy and other inflammatory diseases.
Keywords: Glycyrrhiza uralensis; Gan-Cao; flavonoids; liquiritigenin, isoliquiritigenin and 7, 4′-dihydroxyflavone; eotaxin-1 inhibition
INTRODUCTION
Eosinophils are important in the pathogenesis of allergic asthma, allergic rhinitis, and other inflammatory diseases such as inflammatory bowel disease and gastrointestinal allergic hypersensitivity (1–4). Interleukin (IL)-5 is the central mediator of eosinophilic proliferation, and differentiation (5). Eotaxin, recruits eosinophils to sites of allergic inflammation. (6). Human lung fibroblast (HFL-1) cells are commonly used to investigate eotaxin secretion and mechanisms of action of eotaxin inhibitory agents (7, 8).
Asthma is currently treated with several classes of drugs, including β2-adrenergic receptor agonists, glucocorticoids, theophylline, cromones and anticholinergic agents (9). Glucocorticoids the most effective antiinflammatory drugs, however systemic side effects and poor compliance show the need for alternate approaches to treat asthma. Use of traditional herbal medicines (TM) is rapidly increasing despite the lack of conclusive scientific evidence regarding safety and efficacy of TMs. Traditional Chinese Medicines (TCMs) are of increasing interest to Western researchers, and a growing number of studies are exploring their efficacy, safety and mechanisms of action. Glycyrrhiza uralensis, Gan-Cao is considered to be a prominent herb in TCM, and is the major constituent in several TCM formulae used to treat asthma, coughs and peptic ulcers (10). An aqueous extract of Glycyrrhiza uralensis rhizomes is one of the constituents in Anti-asthma Herbal Medicine Intervention (ASHMI), which has been shown to have therapeutic effects in an animal model of asthma, and in a controlled clinical trial of moderate to severe asthmatics (11, 12). The animal study found that ASHMI inhibited eosinophil accumulation in lungs of allergic mice following antigen intratracheal challenge (13). As part of an ongoing study of pharmacologically active components in ASHMI we discovered that G. uralensis contains eotaxin-1 inhibitory flavonoids, and report here the isolation, characterization, quantification and the eotaxin-1 inhibitory biological activity of these compounds.
MATERIALS AND METHODS
General Experimental Procedures
1H (600/500 MHz) and 13C (150/125 MHz) on an INOVA VARIAN VRX 500/600 instruments using standard pulse sequences. The chemical shifts were measured in DMSO-d6 and expressed in δ The silica gel used for CC was Merck Silica gel 60 (35–70 µm particle size). Analytical Si gel plates (20 × 20, 500 µm) were purchased from Fisher Scientific. Organic solvents for CC and LC were purchased from Fisher Scientific (Fair Lawn, NJ). Glycyrrhizin ammonium salt was purchased from Sigma-Aldrich (St Louis, MO) and identity was confirmed by LC-MS and NMR spectra. HFL-1 cells were purchased from American Type Collection Culture (ATCC) and maintained in our laboratory.
Plant Material
The extracts of Gan-Cao (Glycyrhiza uralensis) (root & rhizome) were purchased from Sino-lion Pharmaceutical Company (Weifang, China). The extracts were prepared as described below: shade dried roots and rhizome of G. uralensis were soaked in water (1:10, w/v) for 40 min and boiled for 45 min, and the decoction collected. The process was repeated and the combined decoction was filtered and spray-dried and yielded of extract was 22.9%. The powdered GC extract was shipped to Mount Sinai School of Medicine and stored at room temperature until further use.
Extraction and Isolation
500 g of G. uralensis dried hot water extract was mixed with MeOH (1L) at room temperature with occasional stirring, and the soluble portion was filtered. The process was repeated several times and combined extracts were concentrated under reduced pressure to yield 125.0 g of MeOH extract. This extract (23.0 g) was chromatographed over silica gel column and eluted with CHCl3, CHCl3 and MeOH step gradients. A total of 50 fractions of 20 mL each were collected. Based on their TLC profiles, fractions were mixed to yield to get five major fractions Fr. A (2.2 g), Fr. B (1.9 g), Fr. C (4.8 g), Fr. D (5.2 g), and Fr. E (8.0 g). Fr. D contained one major compound and it was purified by repeated solvent wash followed by recrystallization to yield pure compound liquritin (1) (1.2 g). Fraction C (4.0 mg) was chromatographed over silica gel and eluted with CHCl3 and CHCl3:MeOH (9:1) to yield compound isoononin (5) (150 mg). Similarly fraction B was purified by repeatedly CC to yield compounds 7, 4'-dihydroxyflavone (4) (150 mg) and three fractions I–III. Fr. II on purification on CC to yield Liquiritigenin (2) (25.0 mg) and Isoliquiritigenin (3) (37.0 mg).
Characterization of Compounds
Compounds 1–5 were identified as liquiritin (1) (14), liquiritigenin (2) (14, 15), isoliquiritigenin (3) (16, 17), 7, 4'-dihydroxyflavone (4) (18), and isoononin (5) (18) by 1H, 13C NMR and LC-MS (accurate mass) and by comparison of the spectral data with published results.
Liquiritin (1)
1H NMR (DMSO-d6, 500 MHz) δ10.60 (1H, s, OH-7), 7.63 (1H, d, J = 9.0 Hz, H-5), 7.42 (2H, d, J = 9.0, H-2′, 6′), 7.05 (2H, d, J = 9.0 Hz, H-3′, 5′), 6.50 (2H, dd, J = 8.5, 2.0 Hz, H-6), 6.34 (1H, d, J = 2.5 Hz, H-8), 5.50 (1H, dd, J = 12.5, 3.0 Hz, H-2), 4.86 (1H, d, J = 7.5, H-1″), 3.04–3.36 (m, sugar protons), 3.67 (1H, dd, J = 12.0, 2.0 Hz, H6a), 3.44 (1H, dd, J = 6.0, 12.0 Hz, H-6b), 3.16 (1H, dd, J = 13.0, 16.5 Hz, H-2trans), 2.66 (1H, dd, J = 2.5, 16.5 Hz, H-3cis). 13C NMR (DMSO-d6, 125 MHz) δ189.9 (C-4), 164.7 (C-7), 163.0 (C-9), 157.4 (C-4′), 132.3 (C-10), 128.4 (C-5), 128.0 (C-2′, 6′), 116.2 (C-3′, 5′), 113.5 (C-10), 110.6 (C-6), 102.6 (C-8), 100.3 (C-1″), 78.6 (C-2), 77.0 (C-5″), 76.6 (C-3″), 73.2 (C-2″), 69.7 (C-4″), 60.7 (C-6″), 43.2 (C-3).
Liquiritigenin (2)
1H NMR (DMSO-d6, 600 MHz) δ10.54 (1H, s, OH-7), 9.54 (1H, s, OH-4′), 7.63 (1H, d, J = 8.4 Hz, 3.16 (1H, dd, J = 13.0, 16.5 Hz, H-2trans), 2.66 (1H, dd, J = 2.5, 16.5 Hz, H-3cis), H-5), 7.31 (2H, d, J = 8.4, H-2′, 6′), 6.77 (2H, d, J = 8.4 Hz, H-3′, 5′), 6.45 (2H, dd, J = 8.4, 2.4 Hz, H-6), 6.31 (1H, d, J = 2.4 Hz, H-8), 5.43 (1H, dd, J = 13.2, 3.0 Hz, H-2), 3.09 (1H, dd, J = 13.2, 16.8 Hz, H-2trans), 2.61 (1H, dd, J = 3.0, 16.8 Hz, H-3cis). 13C NMR (DMSO-d6, 125 MHz) δ190.5 (C-4), 164.6 (C-7), 163.1 (C-9), 157.6 (C-4′), 129.3 (C-10), 128.4 (C-5), 128.2 (C-2′, 6′), 115.1 (C-3′, 5′), 113.5 (C-10), 110.4 (C-6), 102.5 (C-8), 78.9 (C-2), 43.1 (C-3).
Isoliquiritigenin (3)
1H NMR (DMSO-d6, 600 MHz) δ13.59 (1H, s, OH-2′), 10.62 (1H, s, OH-4′), 10.14 (1H, s, OH-4), 8.15 (1H, d, J = 8.4 Hz, H-6′), 7.74 (4H, m, H-α, H-β, H-2, 6), 6.83 (2H, d, J = 8.4 Hz, H-3, 5), 6.39 (1H, dd, J = 8.4, 2.4 Hz, H-5′), 6.27 (1H, d, J = 2.4 Hz, H-3′). 13C NMR (DMSO-d6, 150 MHz) δ 191.5 (C=O), 165.8 (C-4′), 164.9 (C-2′), 160.2 (C-4), 144.2 (C-b), 132.8 (C-6′), 131.2 (C-2, 6), 125.7 (C-1), 117.4 (C-a), 115.8 (C-3, 5), 113.0 (C-1′), 108.1 (C-5′), 102.6 (C-3′).
7, 4'-dihydroxyflavone (4)
1H NMR (DMSO-d6, 500 MHz) δ 10.44 (2H, br s, OH-7, 4′), 7.91 (2H, d, J = 9.0 Hz, H-2′, 6′), 7.86 (1H, d, J = 9.0 Hz, H-5), 6.96 (1H, d, J =2.5 Hz, H-8), 6.92 (2H, d, J = 9.0 Hz, H-3′, 5′), 6.90 (1H, dd, J = 9.0, 2.5 Hz, H-6), 6.60 (1H, s, H-3). 13C NMR (DMSO-d6, 125 MHz) 176.2 (C-4), 162.5 (C-2), 162.4 (C-47, 160.6 (C-3′), 157.3 (C-9), 128.1 (C-2′, 6′), 126.4 (C-5), 121.7 (C-1′), 116.0 (C-10), 115.8 (C-3′, 5′), 114.7 (C-6), 104.4 (C-3), 102.4 (C-8).
Isoononin (5)
1H NMR (DMSO-d6, 500 MHz) δ 8.43 (1H, s, H-2), 8.05 (1H, d, J = 9.0 Hz, H-5), 7.53 (2H, d, J = 8.5 Hz, H-2′, 6′), 7.23 (1H, d, J = 2.5 Hz, H-8), 7.14 (1H, dd, J = 9.0, 2.0 Hz, H-6), 6.99 (2H, d, J = 9.0 Hz, H-3′, 5′), 5.10 (1H, d, J = 7.5 Hz, H-1″), 3.77 (3H, s, 7-OMe), 3.67 (1H, dd, J = 12.0, 2.0 Hz, H-6″a), 3.44 (1H, dd, J = 6.0, 12.0 Hz, H-6″b), 3.12–3.74 (m, sugar protons). 13C NMR (DMSO-d6, 125 MHz) δ 174.6 (C-4), 161.4 (C-4), 159.0 (C-4), 157.0 (C-4), 153.6 (C-4), 130.0 (C-4), 126.9 (C-4), 124.0 (C-4), 123.3 (C-4), 118.4 (C-4), 115.6 (C-4), 113.6 (C-4), 103.4 (C-4), 100.0 (C-1″), 77.2 (C-5″), 76.4 (C-3″), 73.1 (C-2″), 69.6 (C-4″), 60.6 (C-6″), 55.1 (7-OMe).
HPLC Quantification
The biologically active compounds present in Glycyrrhiza extract were quantified by HPLC. The analyses were carried out with a Waters Alliance HT 2695 (Waters Corp., Milford, MA) HPLC instrument equipped with autosampler and PDA detector with Empower software (Waters Corp., Milford, MA). A Zorbax-C18 (4.6 × 150 mm i.d.) column was used with mobile phase consisting of A (H2O:H3PO4, 99.9:0.1, v/v) and B (CH3CN). The linear gradient from 2% B to 46% B over 75 min and back to initial conditions (2% B) in 80 min with flow rate 1.0 mL/min was used for elution of the compounds. Since G. uralensis is a major component of the multi herbal formula ASHMI, an HPLC method was developed to monitor the presence of G. uralensis in ASHMI. Each run was followed 10 min equilibration and automatic needle wash with wash solvent (80% acetonitrile in water). Samples were initially dissolved in MeOH and diluted with H2O. The sample injection volume was 10 µL. Each concentration was assayed by three times.
Standard Solutions
The standard test solutions were prepared at 1.0 mg/mL concentration as stock solutions. Then the required concentrations, 300, 100, 30, 10, 3, and 1 µg/mL were serially diluted with mobile phase. Each concentration was assayed in triplicate. The peak area was measured for chromatograms resulted from triplicates and averaged. Concentration versus peak area was plotted and regression analyses (R2) were performed to assess the linearity. The amounts present in the extracts were estimated by the equation obtained from regression.
Liquid Chromatography-Mass Spectrometry
The accurate mass for the isolated pure compounds was estimated by Time of flight (TOF)-mass spectrometer; LCT-Premier XE (Waters Corp., Milford, MA). For LC-MS, we developed a short elution method to monitor the compounds present in G. uralensis and used this method for accurate mass measurement of pure compounds. Mobile phase used was A: H2O (0.1% HCO2H); B: CH3CN (0.1% HCO2H) with linear gradient from 20% to 50% B over 25 min at a flow rate of 1.0 mL/min equipped with peak flow splitter. The LC and the mass spectrometer were controlled by MassLynx 4.1 application manager software. Leucine enkaphaline was used as lock spray reagent for the accurate mass measurements and monitored its non-attenuated peak at m/z 557.2802 where as sodium formate (NaF) is used as calibration standard. The positive electrospray ionization (ESI+) was used to analyze the molecules with the following parameters. The capillary voltage: 3.2~3.5 kV; Source temperature: 100° C; Desolvation temperature: 50 ° C; Desolvation gas flow: 550 L/h; Cone gas flow: 50 L/h; Mass range: 100–1000 amu.
HFL-1 Cell culture and Eotaxin-1 Inhibition Assay
Eotaxin-1 inhibitory assays were conducted by using human fetal lung fibroblast (HFL-1) cells. HFL-1 cells were grown in F-12K medium (ATCC, Rockville, MD) containing 10% fetal bovine serum (Gibco BRL, Grand Island, NY), 1% Penicillin-Streptomycin (BD Bio). The linearly growing cells were detached using trypsin-EDTA and 8 × 104 cells per well were transferred to a 24-well culture plates. After 48 h the growth medium was replaced with medium contained test compounds at the different concentrations. Compounds were initially dissolved in DMSO and added to the medium. The final DMSO concentration in the assay well was 0.1%. Serial dilutions (1:1) were made to establish the dose response curves. Each concentration was tested in triplicate. Supernatants were harvested after 96 h culture. Eotaxin-1 levels were determined in cell supernatant by ELISA (R & D Systems). The IC50 values were calculated by non-linear regression analysis by GraphPad Prism 4.01 (San Diego, CA).
RESULTS AND DISCUSSION
Identification of Compounds
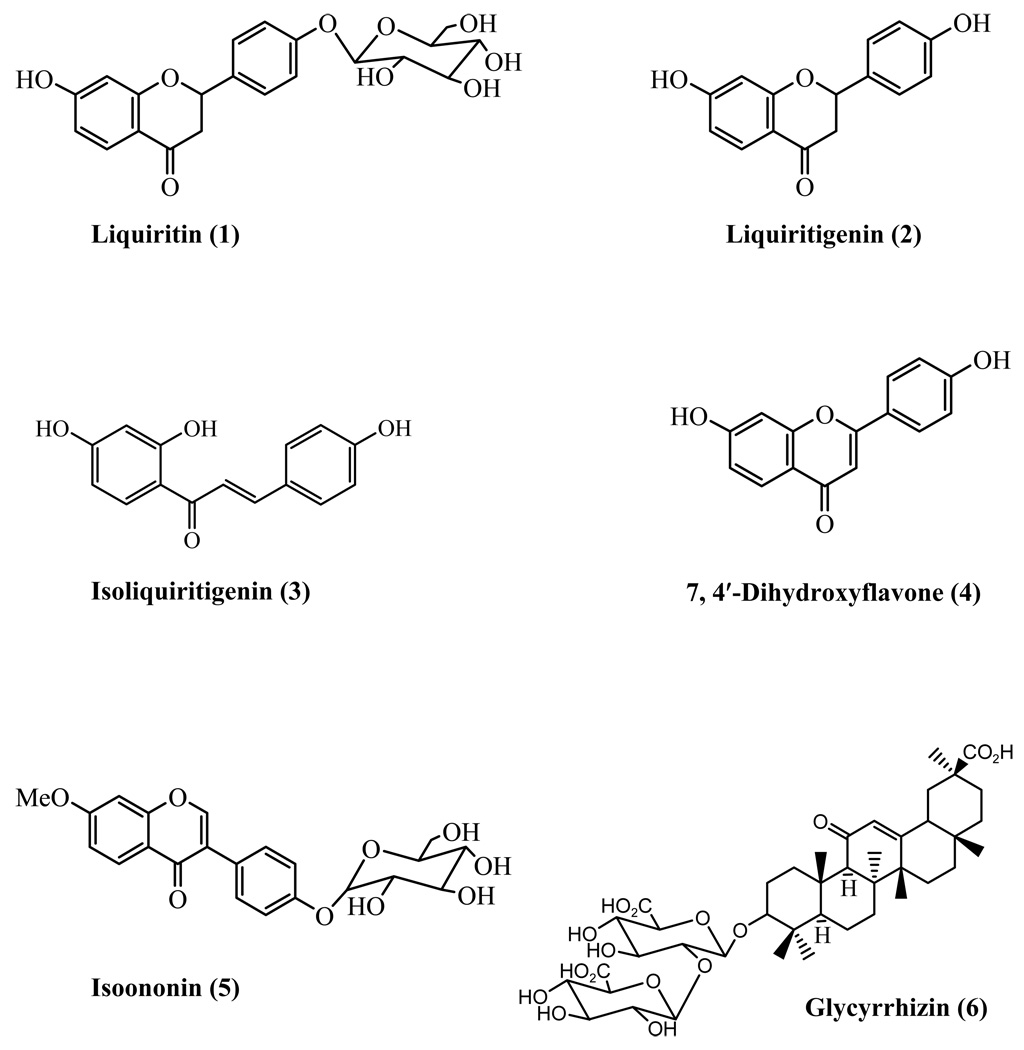
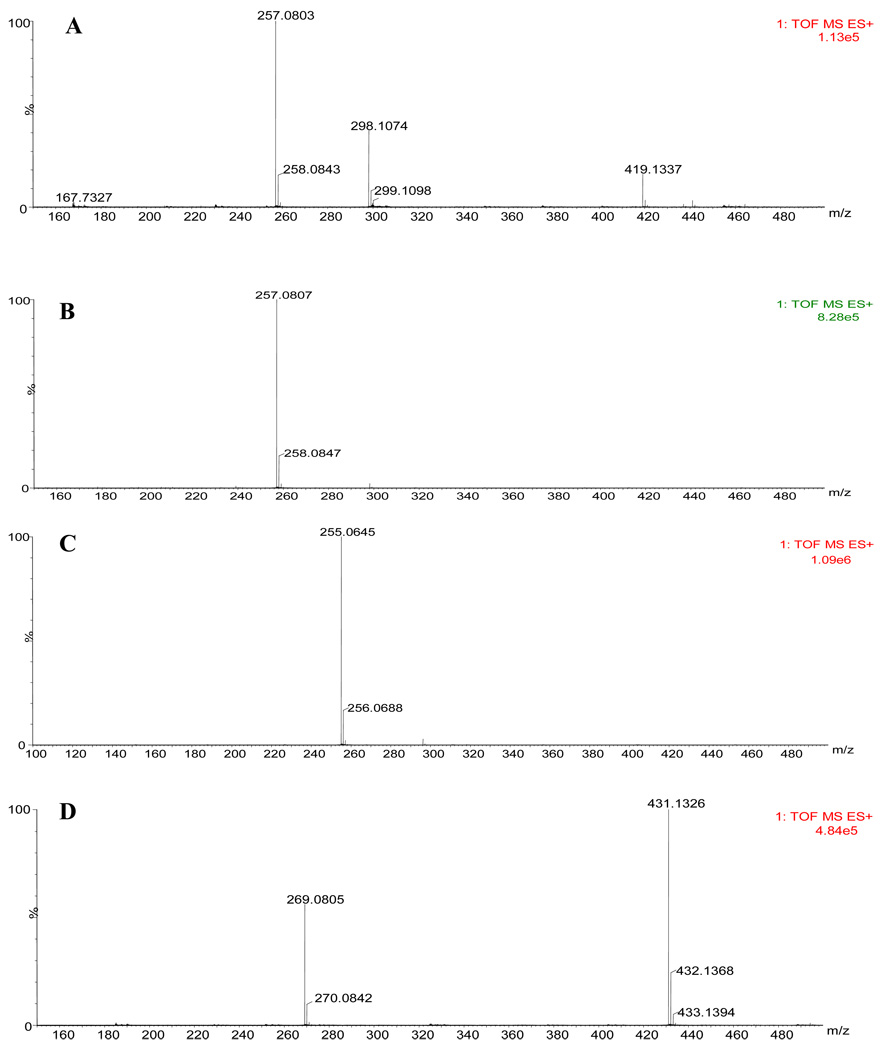
The MeOH soluble portion of a water extract of G. uralensis was chromatographed over a silica gel column to yield structurally related flavonoids. The structures of the compounds were elucidated by 1H, 13C NMR and MS spectra (accurate mass). Compound 1 isolated as major compound and it displayed [M+H]+ ion at m/z 419.1337 (calcd for C21H23O9 419.1342). Further MS spectrum showed a base peak at m/z 257 with loss of 162 amu indicated the presence of hexosyl moiety in the compound. The 1H NMR spectrum showed signals for para-substituted phenyl ring δ 7.42 (2H) and 7.05 (2H); 1, 2, 4-trisubstituted aromatic ring at δ 7.63, 6.50 and 6.34; aliphatic protons coupling to each other at δ 5.50, 3.16 and 2.66. An anomeric carbon signal (100.3 ppm) together with additional five carbons further substantiated the hexosyl moiety. The data indicated that the compound is a flavanone substituted at 7 and 4′ positions. A D2O exchangeable signal at δ 10.6 revealed the presence of hydroxyl group. Thus the structure of the compound elucidated as 7-hydroxyflavanone 4′-O-β-D-glucopyranoside (Liquiritin (1)) reported from G. uralensis earlier (14) (Figure 1). The molecular formula of compound 2 was C15H13O4 [(M+H)+ 257.0807; calcd for C15H12O4: 257.081]. The absence of signals for glucosyl moiety in 1H and 13C NMR spectra together with 162 amu less in its molecular ion indicated the compound 2 could be an aglycone of liquiritin (1). Further comparison of 1H and 13C NMR data (see experimental section) with liquiritigenin confirmed the structure of 2 (14, 15). Compounds 3 exhibited similar spectral characteristics that of 2 except for the absence of aliphatic proton signals and appearance of two olefinic protons at δ 7.74 indicated that the compound is isoliquiritigenin, a chalcone (16, 17). Similarly, absence of aliphatic protons and appearance of one olefinic proton at δ 6.71 in 4 indicated that it is a dehydro derivative of liquiritigenin (2) (Figure 1). It was further substantiated by HRMS showed the molecular ion at m/z 255.0645 accounted the molecular formula C15H11O4. The structure of compound 4 was confirmed as 7, 4′-dihydroxyflavone by comparison with published results (18). Compound 5 showed the [M+H]+ ion at m/z 431.2245 revealed the molecular formula C22H22O9. The 1H NMR spectra showed signals for 7, 4′-disubstituted isoflavone [8.43 (1H, s), 8.05 (2H, d), and 7.53 (2H, d), 7.23 (1H, d), 7.14 (1H, dd), 6.99 (1H, d)]; methoxyl group [3.76 (3H,s)]; and hexosyl moiety [5.10 (1H, d), 3.73-3.15 (m)]. The 13C NMR, spectra also confirmed the presence of isoflavone moiety. The comparison of the data with 7-methoxyisoflavones allowed us to place the methoxyl group at 7-position and this led us to place the sugar moiety at 4′-position. The data indicated that the compound is isononin (5), and its identity was further confirmed by comparison with published data (19).
Figure 1.
Structure of the compounds 1–6
Inhibitory Effect of Licorice Flavonoids on Eotaxin Production
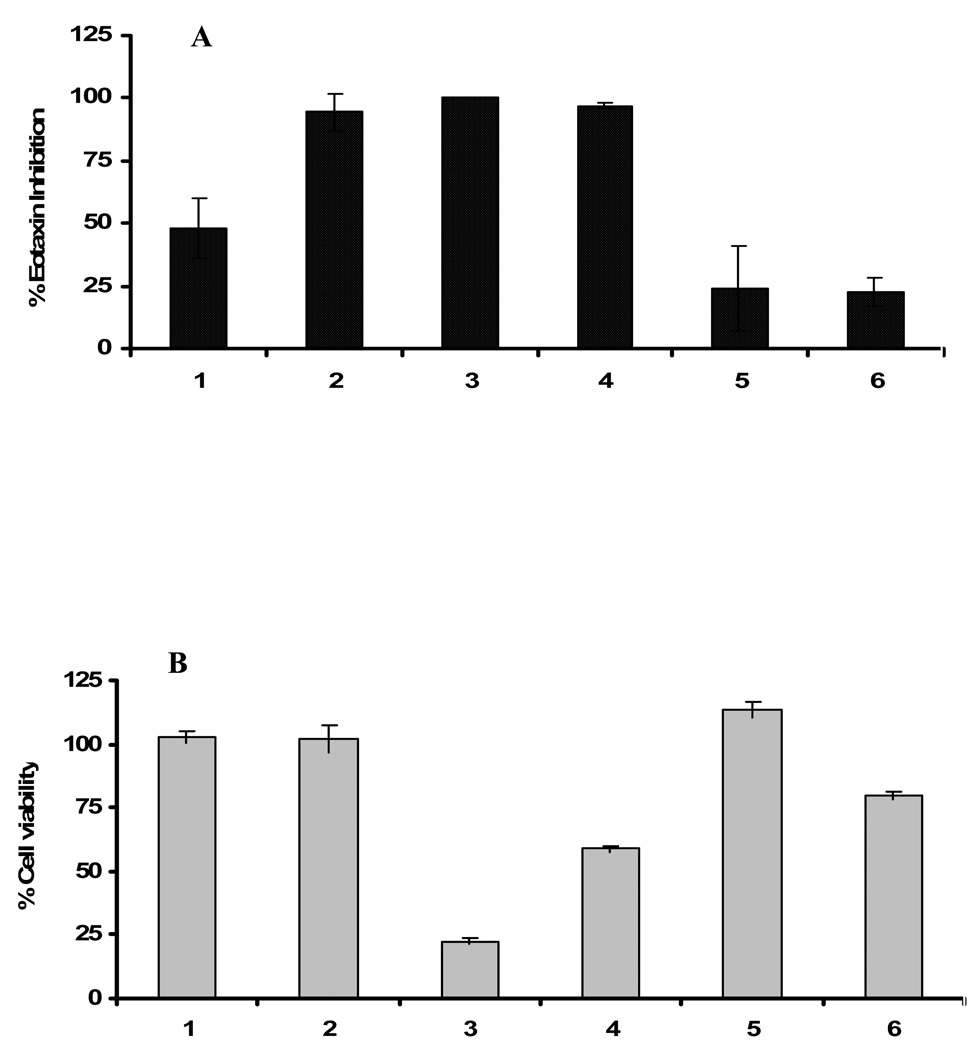
To determine the potential anti-inflammatory effects of the licorice flavonoids identified above, and because eotaxin is the key chemokine involved in eosinophil migration into the lung in asthma, and in other diseases, we tested the effects of these licorice flavonoidal compounds on human lung fibroblasts (HFL-1) eotaxin production. A previous study showed that the concentration of glycyrrhizin and its derivatives that produced approximately 50% inhibition of eotaxin and other inflammatory cytokine was between 25–100 µg /ml (7). To compare the efficacy with glycyrrhizin, we initially tested_25µg/ml concentrations of the 5 isolated compounds and glycyrrhizin (Figures 2A). Liquiritin (1) inhibited eotaxin-1 by approximately 50% and Isoononin (5) inhibited eotaxin-1 by approximately 20%, which is similar to glycyrrhizin (Figure 2A) in our assay. Liquiritigenin (2), isoliquiritigenin (3) and 7, 4′-dihydroxy flavone (4) completely abolished eotaxin production, demonstrating a effective antieotaxin activity. However, isoliquiritigenin (3) and 7, 4′-dihydroxy flavone (4) exhibited significant cytotoxicity at this 25µg/ml (Figure 2B), which may be due to the concentration used was well above the effective dose.
Figure 2.
Effect of the compounds, 1–6, on A) Eotaxin inhibition B) Cell viability tested at 25 µg/mL concentration. Compounds were incubated with Human lung fibroblast (HFL-1) cells for 96 h and amount of eotaxin secreted was estimated by ELISA. Cell viability was determined by MTT assay. Data represents the mean ± SEM.
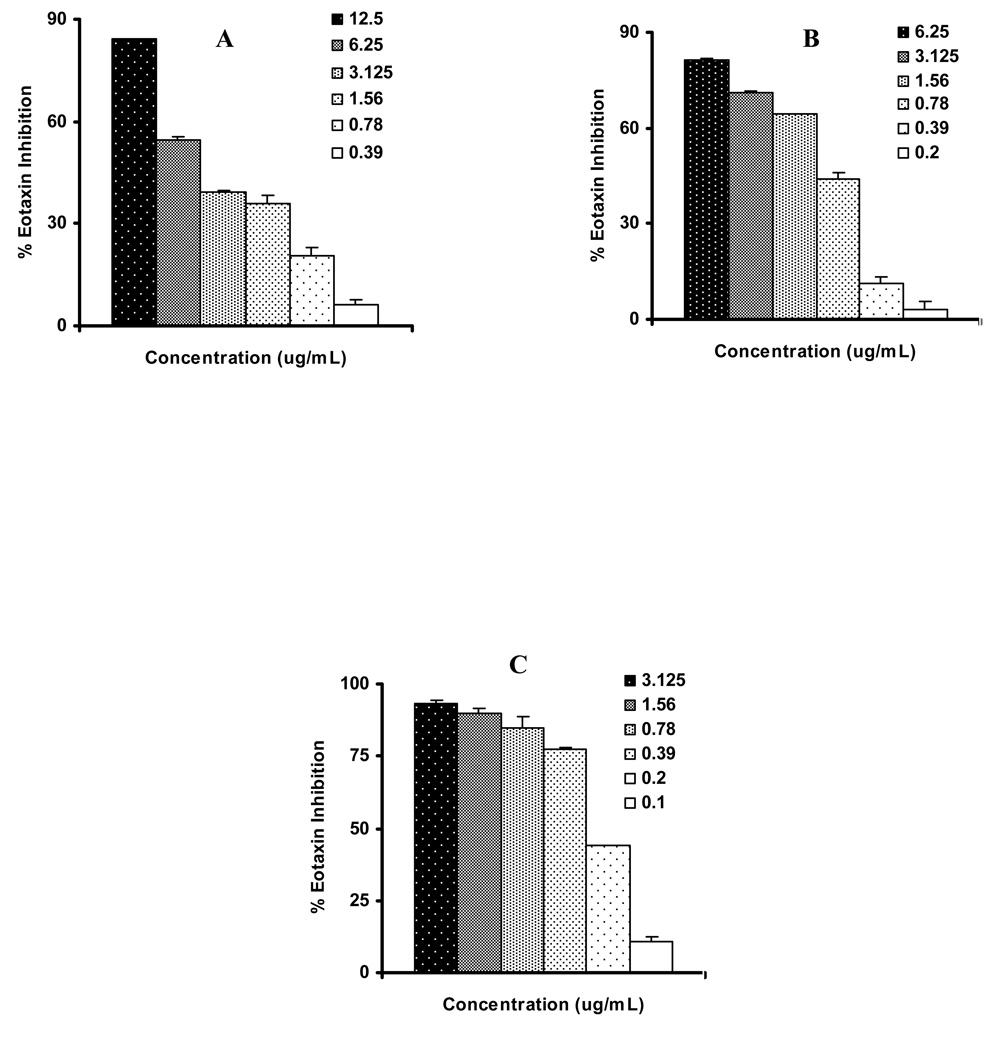
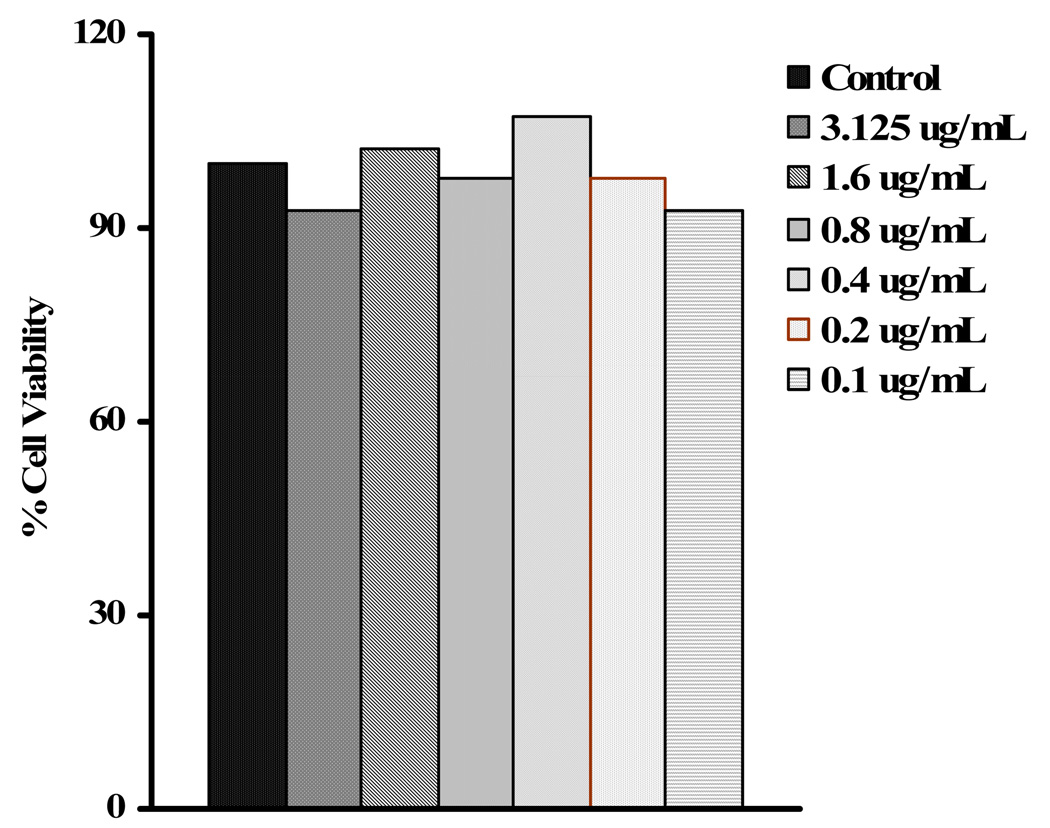
We therfore focused on Liquiritigenin (2), isoliquiritigenin (3) and 7, 4′-dihydroxyflavone (4) to establish dose response curve at non toxic dose (Figure 3A–C) and found IC50 values at 4.2, 0.92, and 0.21 µg/mL concentration, respectively (Table 1). The cells were >95% viable (MTT and Trypan blue exclusion assays) at 12.5, 6.25 and 3.125 µg/mL the maximum tested concentration for dose response studies. The representative results for 7, 4′-dihydroxyflavone (4) shown in Figure 4. These results demonstrated that 7, 4′-dihydroxyflavone (4) is the most effective flavonoid isolated from licorice. We then tested whether 7, 4′-dihydroxyflavone (4) causes apoptosis. At the (5 µg/mL), well higher than the IC50, did not induce apoptosis (data not shown). These results suggest that liquiritigenin (2), isoliquiritigenin (3) and 7, 4′-dihydroxyflavone (4) in G. uralensis exhibits potent anti-inflammatory effect.
Figure 3.
Dose dependent inhibition of Eotaxin-1 by A) Liquiritigenin (2) B) Isoliquiritigenin (3) C) 7, 4′-Dihyroxyflavone (4). Percent inhibition was calculated by comparison of the eotaxin-1 levels of treated groups with controls. Data represents the mean ± SEM. The compounds did not show cytotoxicity even at the maximum tested concentration in dose response study.
Table 1.
IC50 values for compounds 2–4
| Compound | IC50 (µg/mL) |
|---|---|
| Liquiritigenin (2) | 4.2 ± 0.07 |
| Isoliquiritigenin (3) | 0.92 ± 0.05 |
| 7, 4′-Dihyroxyflavone (4) | 0.21 ± 0.06 |
Figure 4.
Dose dependent assay of the cell viability of 7, 4′-Dihyroxyflavone (4) as determined by trypan blue exclusion assay. The compound showed >95% cell viability with out changing morphology of cells.
Quantification
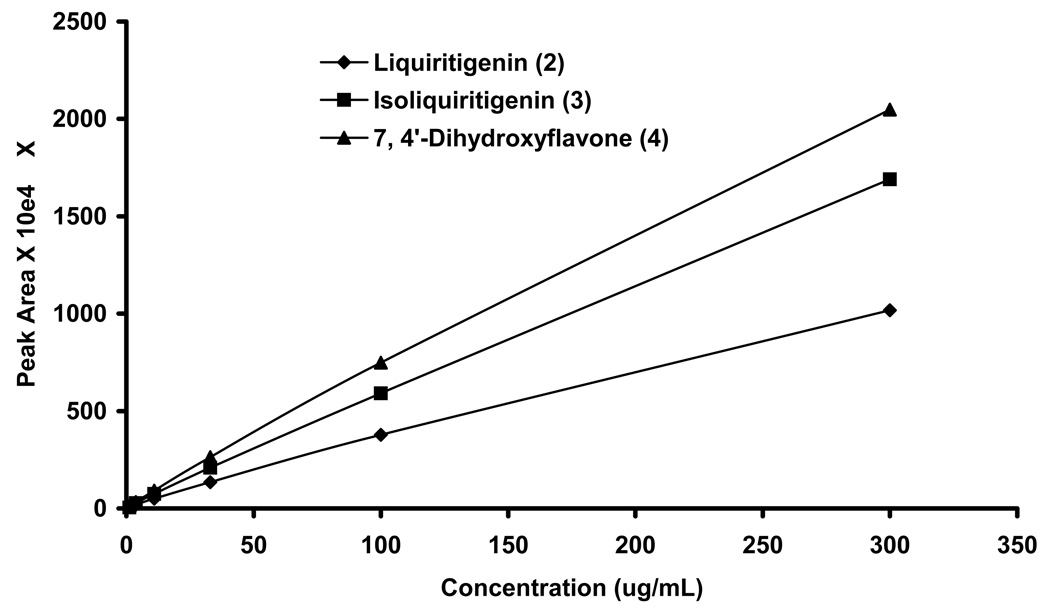
The bioactive flavonoids (1, 2, 3, 4) present in the G. uralensis were quantified by HPLC. The calibration curves were generated for pure compounds by analyzing six concentrations ranging from 300 µg/mL to 1 µg/mL (Figure 5). The areas of the peaks were calculated and plotted over C vs AUC. The isomers, liquiritigenin (2) and isoliquirtigenin (3) were present approximately equal quantity about 30 µg/100 mg of extract. The most active compound, 7, 4′-dihydroxyflavone (4), is minor compound (8 µg/100 mg) where as liquiritin is most abundant (184 µg/100 mg) in our formulation (Table 2). Even though active compounds are in present in lower quantities, considering the IC50 values, they may play major role in overall observed activity of G. uralensis.
Figure 5.
Standard curves for compounds 3–5. The highest concentration of 300 mg/mL was prepared and serial dilution (1:2) was made until 1 µg/mL concentration and 10 µL was analyzed by HPLC. Each concentration was injected in triplicate and area was averaged for all three injections.
Table 2.
Amount of bioactive flavonoids (2, 3 & 4) present in the G. uralensis extracts
| Compound | Concentration µg/ 100 mg of extract |
Linearity (R2) |
|---|---|---|
| Liquiritin (1) | 184 | 1.0 |
| Liquiritigenin (2) | 28 | 0.9987 |
| Isoliquiritigenin (3) | 30 | 0.9997 |
| 7, 4'-dihydroxyflavone (4) | 8 | 0.9990 |
LC-MS analysis of G. uralensis extract and its purified compounds
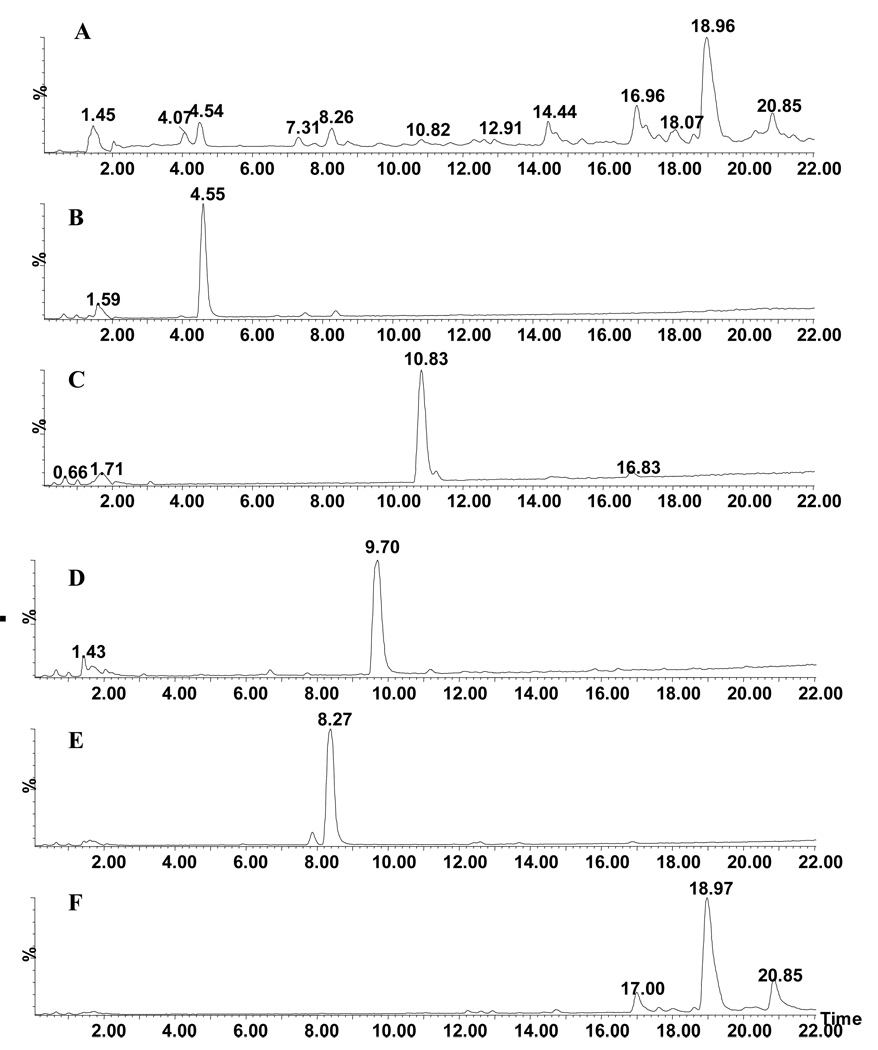
Figure 6A–F represents the total ion chromatogram (TIC) of the crude formulation of G. uralensis and isolated pure compounds. MS spectra showed the presence of flavonoids and triterpenoids in G. uralensis. The isolated pure compounds 1–5, and glycyrrhizin ammonium salt were also analyzed along with G. uralensis extract (Figures 7A–E). The molecular mass for the compounds in extract was displayed <5.0 ppm deviation with calculated mass. The comparison the retention time together MS spectrum revealed the peak at 4.54 min determined as liquiritin (1) with a molecular mass 419.1337. The peak at 4.07 min also showed same molecular formula of 1 indicated that it would isomer of liquiritin. As this is a minor compound, we could not isolate in pure form. The UV spectrum together with accurate mass measurement the structure of the compound was identified as isoliquiritin. Peaks at 10.83 and 14.44 min were identified as liquiritigenin and isoliquiritigenin. A minor peak at 9.70 min was identified as 7, 4′-dihydroxy flavone. Our attempts to isolate the major peak 18.96 min were unsuccessful. This may be due to instability of the molecule under our isolation condition. However, it was identified as glycyrrhizin by high resolution mass spectrum followed comparing the retention time with commercially available standard.
Figure 6.
Total ion chromatogram (TIC) of water extracts of A) G. uralensis B) Liquiritin C) Liquiritigenin D) 7, 4′-Dihydroxy flavone E) Isoononin F) Glycyrrhizin.
Figure 7.
Mass spectrum of A) Liquiritin B) Liquiritigenin C) Isoliquiritigenin D) 7, 4′-Dihydroxy flavone E) Isononin. The accurate mass (high resolution) of the compounds was measured by TOF instrument.
G. uralensis, commonly called Gan-Cao meaning “sweet grass” in traditional Chinese medicine practice is used to treat several inflammatory disorders (20). The root of Glycyrrhiza uralensis has been used in TCM since the 3rd century B.C. In many parts of the world, licorice products are most often used as flavoring and sweetening agents in food products (21–23). Previous phytochemical investigations of this plant identified several flavonoids, triterpenoids and polysaccharides (24). The triterpenoids glycyrrhizin and its aglycone glycyrhetinic acid are the major compounds exhibiting anti inflammatory activities including decreased IL-4 and IgE levels in murine asthma models (25). 18α-Glycyrrhizin has shown stronger inhibition than its 18β counterpart at an IC50 of 25 µg/mL (7). However, in the present study glycyrrhizin exhibited only a slight effect at 25 µg/mL. Recent studies also showed that glycyrrhizin and its derivative, hetero-30-OH-Glycyrrhizin (3β-[(2-O- β-D-glucopyranuronosyl- β-D-glucopyranuronosyl)oxy]-olean-11,13(18)-dien-30-ol inhibited activation of STAT-6 and NF-kB, the major transcription factors involved in up regulation of eotaxin synthesis (8). The isoflavonoid, isoononin was less effective in this assay. Since eotaxin-1 is one of the major chemokines involved in recruitment of eosinophils, inhibition of eotaxin-1 secretion would be expected to have a beneficial effect on eosinophil associated inflammatory disorders such as asthma, eosinophilic esophagitis and inflammatory bowel disease, the incidence of which are increasing.
The antiallergic effects of G. uralensis have been reported to be mainly attributed to glycyrrhizin however, other constituents have not systematically evaluated. In the present study liquiritin (1), liquiritigenin (2), isoliquiritigenin (3) and 7, 4′-dihydroxy flavone (4), structurally related flavonoidal compounds, showed potent inhibition of eotaxin-1 secretion. The eotaxin inhibitory activity was in the order of 7, 4′-dihydroxy flavone (4)>isoliquiritigenin (3)>liquirtitigenin (2)>liquiritin (1). Although substantial quantities of liquirtin (1) are present in G. uralensis, it did not exhibit promising biological activities in earlier studies. Its aglycone liquiritigenin, potently inhibited IgE induced degranulation of RBL-2H3 cells in vitro, and blocked anaphylactic reactions in mice (26). Liquiritigenin also showed protective effects against acetaminophen induced acute liver injuries (27). Isoliquiritigenin (3) is an isomer of 2 well known for antitumor promoting activity which induces apoptosis in human prostate cancer cells (28). Isoliquirtigenin (3) also decreased the production of inflammatory prostaglandin, PGE2, and NO through suppression of COX-2 and inducible nitric oxide synthase (iNOS) protein expression (29). Compound 4, the dehydro derivative of 2 was most active in this study. This compound has not been the subject of many bioassay studies. In fact, except for baicalein (IC50: 1.8 µg/mL), none of the flavonoidal compounds have been tested for eotaxin-1 inhibitory activity (30). Biosynthetically, compound 3 is the precursor of 2, which in turn is precursor for 4. Liquiritin, a major compound isolated is less active than its aglycone, 2. Similarly, the dehydrogenation and isomerization of 2, form compounds 4 and 3, respectively. Formation of double bonds at 2, 3-positions in compound 4 enhanced eotaxin inhibitory activity. Even though the compounds examined are too few to draw conclusions about the relationships between structure and activity, the results show that the double bond at 2, 3-position plays a major role in inhibition of eotaxin-1. Our in vitro study showed that Glycyrrhiza flavonoids potently inhibit eotaxin-1 sectretion. Since G. uralensis is widely consumed in herbal medications and food products, exploring the biological active components in this plant will benefit human health. Our study together with earlier studies shows the potential of G. uralensis compounds as anti-inflammatory agent for asthmatics. However, further studies are needed to determine the efficacy and safety of purified components of this herb in vivo. Our study is the first report of inhibition of eotaxin-1 by the reported compounds.
ACKNOWLEDGEMENT
This study was supported by NIH/NCCAM center grant # 1P01 AT002644725-01 “Center for Chinese Herbal Therapy (CHT) for Asthma”.
Reference List
- 1.Maddox L, Schwartz DA. The pathophysiology of asthma. Annu. Rev. Med. 2001;53:477–498. doi: 10.1146/annurev.med.53.082901.103921. [DOI] [PubMed] [Google Scholar]
- 2.Mould AW, Matthaei KI, Young IG, Foster PS. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. 1997:1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rankin SM, Conroy DM, Williams TJ. Eotaxin and eosinophil recruitment: implications for human disease. 2000:20–27. doi: 10.1016/s1357-4310(99)01635-4. [DOI] [PubMed] [Google Scholar]
- 4.Wardlaw AJ. Eosinophil trafficking in asthma. 2001:214–218. doi: 10.7861/clinmedicine.1-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clutterbuck EJ, Hirst EM, Sanderson CJ. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood. 1989;73:1504–1512. [PubMed] [Google Scholar]
- 6.Mould AW, Ramsay AJ, Matthaei KI, Young IG, Rothenberg ME, Foster PS. The effect of IL-5 and eotaxin expression in the lung on eosinophil trafficking and degranulation and the induction of bronchial hyperreactivity. J. Immunol. 2000;164:2142–2150. doi: 10.4049/jimmunol.164.4.2142. [DOI] [PubMed] [Google Scholar]
- 7.Matsui S, Matsumoto H, Sonoda Y, Ando K, Aizu-Yokota E, Sato A, Kasahara T. Glycyrrhizin and related compounds down-regulate production of inflammatory chemokines IL-8 and eotaxin 1 in a human lung fibroblast cell line. Int. Immunopharmacol. 2004;4:1633–1644. doi: 10.1016/j.intimp.2004.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsui S, Sonada Y, Sekiya T, Aizu-Yokota E, Kasahara T. Glycyrrhizin derivative inhibits eotaxin 1 production via STAT6 in human lung fibroblasts. Int. Immunopharmacol. 2006;6:369–375. doi: 10.1016/j.intimp.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 9.Barnes BJ. Current therapies for asthma. Promise and limitations. Chest. 1997;111:17S–26S. doi: 10.1378/chest.111.2_supplement.17s. [DOI] [PubMed] [Google Scholar]
- 10.Zheng XY. Pharmacopoeia of the People's Republic of China. English ed. ed. Beijing, China: Chemical Industry Press; 2000. [Google Scholar]
- 11.Li XM. Traditional Chinese herbal remedies for asthma and food allergy (Current Perspectives) J. Allergy Clin. Immunol. 2007;120(1):25–31. doi: 10.1016/j.jaci.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen MC, Wei CH, Hu ZQ, Srivastava K, Ko J, Xi ST, Mu DZ, Du JB, Li GH, Wallenstein S, Sampson H, Kattan M, Li XM. Efficacy and tolerability of anti-asthma herbal medicine intervention in adult patients with moderate-severe allergic asthma. J. Allergy Clin. Immunol. 2005;116(3):517–524. doi: 10.1016/j.jaci.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 13.Li XM. Traditional Chinese herbal remedies for asthma and food allergy (Current Perspectives) J. Allergy Clin. Immunol. 2007;120(1):25–31. doi: 10.1016/j.jaci.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu B, Li H, Wang X, Lee FSC, Cui S. Isolation and Identification of Flavonoids in Licorice and a Study of Their Inhibitory Effects on Tyrosinase. J. Agric. Food Chem. 2005;53(7408):7414. doi: 10.1021/jf051258h. [DOI] [PubMed] [Google Scholar]
- 15.Yahara S, Nishioka I. Flavonoid glucosides from licorice. Phytochemistry. 1984;23:2108–2109. [Google Scholar]
- 16.Vaya J, Blinsky PA, Aviram M. Anti-oxidant constituents from licorice roots: Isolation, structure elucidation, and anti-oxidative capacity toward LDL oxidation. Free Radidical Biol. Med. 1997;23:302–313. doi: 10.1016/s0891-5849(97)00089-0. [DOI] [PubMed] [Google Scholar]
- 17.Ma CJ, Li GS, Zhang DL, Liu K, Fan X. One step isolation and purification of liquiritigenin and isoliquiritigenin from Glycyrrhiza uralensis Risch. using high-speed counter-current chromatography. J. Chromatogr. A. 2005;1078:188–192. doi: 10.1016/j.chroma.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 18.Park Y, Moon BH, Lee E, Lee Y, Yoon YAJH, Lim Y. Spectral assignments and reference data: 1H and 13C-NMR data of hydroxyflavone deriv. Mag. Res. Chem. 2007;45:674–679. doi: 10.1002/mrc.2010. [DOI] [PubMed] [Google Scholar]
- 19.Haznagy A, Toth G, Tamas J. Constituents of the aqueous extracts from Ononis spinosa L. Arch Pharm. 1978;311:318–323. doi: 10.1002/ardp.19783110408. [DOI] [PubMed] [Google Scholar]
- 20.Shibata S. A drug over the millennia: Pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi. 2000;120:849–862. doi: 10.1248/yakushi1947.120.10_849. [DOI] [PubMed] [Google Scholar]
- 21.Kitagawa I. Licorice root. A natural sweetener and an important ingredient in Chinese medicines. Pure Appl. Chem. 2002;74:1189–1198. [Google Scholar]
- 22.Kinghorn AD, Compadre CM. Less common high potency sweeteners. In: O'Brien Nabors L, editor. Alternative Sweeteners. Third Edition-Revised and Expanded ed. New York: Marcel Dekker; 2001. pp. 209–231. [Google Scholar]
- 23.US Department of agriculture: GRAS status of licorice (Glycyrrhiza), ammoniated glycyrrhizin, and monoammonium glycyrrhizinate. Fed Reg. 2008;50:19852. 52104321045. [Google Scholar]
- 24.Wang ZY, Nixon DW. Licorice and cancer. Nutr. Cancer. 2001;39:1–11. doi: 10.1207/S15327914nc391_1. [DOI] [PubMed] [Google Scholar]
- 25.Ram A, Mabalirajan U, Das M, Bhattacharya I, Dinda AK, Gangal SV, Ghosh B. Glycyrrhizin alleviates experimental allergic asthma in mice. Int. Immunopharmacol. 2006;6:1468–1477. doi: 10.1016/j.intimp.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Shin YW, Bae EA, Lee B, Lee SH, Kim JA, Kim YS, Kim DH. In vitro and in vivo antiallergic effects of Glycyrrhiza glabra and its components. Planta Med. 2007;73:257–261. doi: 10.1055/s-2007-967126. [DOI] [PubMed] [Google Scholar]
- 27.Kim YW, Ki SH, Lee JR, Lee SJ, Kim CW, Kim SC, Kim SG. Liquiritigenin, an aglycone of liquiritin in Glycyrrhizae radix, prevents acute liver injuries in rats induced by acetaminophen with or without buthionine sulfoximine. Chem. Biol. Interact. 2006;161(2):125–138. doi: 10.1016/j.cbi.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Jung JI, Chung E, Seon MR, Shin HK, Kim EJ, Lim SS, Chung YW, Park KK, Park JHY. Isoliquiritigenin (ISL) inhibits ErbB3 signaling in prostate cancer cells. BioFactors. 2006;28:159–168. doi: 10.1002/biof.5520280302. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi T, Takasuka N, Iigo M, Baba M, Nishino H, Tsuda H, Okuyama T. Isoliquiritigenin, a flavonoid from licorice, reduces prostaglandin E2 and nitric oxide, causes apoptosis, and suppresses aberrant crypt foci development. Cancer Sci. 2004;95:448–453. doi: 10.1111/j.1349-7006.2004.tb03230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakajima T, Imanishi M, Yamamoto K, Cyong J-CI, Hirai K. Inhibitory effect of baicalein, a flavonoid in Scutellaria root, on eotaxin production by human dermal fibroblasts. Planta Med. 2001;67:132–135. doi: 10.1055/s-2001-11532. [DOI] [PubMed] [Google Scholar]