Abstract
Inflammation has been implicated in the pathogenesis of ischemic stroke and the recruitment of inflammatory cells appears to exacerbate ischemic brain injury. Since leukocyte-endothelial cell adhesion is a rate-determining step in the recruitment of leukocytes into post-ischemic brain tissue, much attention has been devoted to defining the contribution of different adhesion molecules, expressed either on leukocytes or endothelial cells, to the leukocyte recruitment process. Similarly, a large effort has been made to determine whether interference with leukocyte-endothelial cell adhesion protects the brain against ischemic tissue injury. These efforts have revealed an important contribution of β2-integrins (CD11/CD18), ICAM-1, and P-selectin in the recruitment of leukocytes as well as platelets in the post-ischemic cerebral microvasculature. Immunoblockade or genetic deletion of these adhesion molecules has been shown to reduce infarct volume, edema, behavioral deficits, and/or mortality in different animal models of ischemic stroke. Anti-adhesion agents also appear to widen the therapeutic window for thrombolytic therapy in these experimental models. Emerging evidence on the role of signaling pathways (eg, CD40/CD40L, Notch-1) and immune cells in the regulation of ischemia-reperfusion induced leukocyte recruitment in the cerebral microvasculature offer novel targets for controlling inflammation in stroke. The few clinical trials assessing anti-adhesion therapy in ischemic stroke have all failed to show efficacy. It remains to be determined whether inflammation in general and leukocyte adhesion in particular represent useful targets for therapeutic intervention in stroke patients.
Keywords: Adhesion, leukocytes, platelets, stroke, cerebral ischemia, inflammation
Introduction
Stroke is defined as “rapidly developing clinical signs of focal or global disturbance of cerebral function with symptoms lasting 24 hours or longer, or leading to death with no apparent cause other than of vascular originrdquo; (1) Although this definition includes the hemorrhagic forms of stroke, 80 % of stroke cases occur due to the occlusion of arteries carrying blood to the brain and subsequent ischemia. Ischemic stroke is the third leading cause of death in the United States with an estimated cost of 71.8 billion dollars (2). The mortality rate after an ischemic incident is very high 30% and survivors almost always face disabilities that require costly long term care (3).
Despite the high mortality and morbidity associated with ischemic stroke, current established therapies are limited. To date, the only effective treatment approved for acute ischemic stroke in the U.S. and Canada is thrombolysis achieved by recombinant tissue plasminogen activators (rt-PA). However, this regime needs to be applied within 3 hour of symptom onset, decreasing the availability of treatment to the majority of patients in need (4). In addition to thrombolysis, anti-platelet therapies such as aspirin and glycoprotein IIb-IIIa inhibitors (clopidogrel) or anticoagulants (heparin) have been used in the prevention/treatment of acute ischemic stroke. Aspirin treatment is associated with significantly fewer recurrent ischemic strokes and no significant increase in hemorrhagic strokes at 14 days. A small but a significant improvement at 6 months has also been observed with aspirin in large-scale clinical studies. Heparin treatment, however, does appear to offer any clinical advantage at 6 months (5), and initial efforts to assess glycoprotein IIb/IIIa directed treatment strategies have not shown promising results (6).
After an ischemic insult, the neuronal injury around the ischemic core, called the penumbra, continues to develop over several hours. Neuronal tissue within the penumbra is electrically inactive but viable, and considered to represent salvageable tissue that can be targeted with neuroprotective interventions. The slow evolution of ischemic damage within the penumbra provides a window of opportunity for neuroprotective therapies. Attenuating and/or delaying this time-dependent brain injury may improve neurological outcome and facilitate brain recovery from injury (7).
Experimental interventions that have been used to confer protection to the penumbra include free radical scavengers and synthesis inhibitors, excitotoxicity inhibitors, suppressors of neuronal metabolism (e.g. hypothermia), anti-inflammatory agents, and membrane stabilizers (8). While there is substantial experimental evidence demonstrating the beneficial effects of these interventions in animal models, human trials have either failed or proven inadequate (9, 10).
Anti-Inflammation as a Therapeutic Target for Ischemic Stroke
Ischemic stroke frequently results from thromboemboli blocking the blood supply to neuronal tissue. Immediately after cessation of blood flow, due to the high oxygen and nutrient needs of brain tissue, ATP depletion occurs in the neurons. Consequently, the ionic gradients across the cellular membranes cannot be sustained resulting in calcium and water influx and neurotransmitter release. This sequence of events leads to cytotoxic edema, excitotoxicity and activation of intracellular enzymes. The overall impact of blood flow cessation is cellular damage and initiation of an inflammatory response. While the other triggering events for cellular damage occurs rapidly after the stroke, inflammation occurs over hours to days and provides an excellent window of opportunity for new treatment strategies (11).
Many studies demonstrate that cerebral ischemia is associated with the infiltration of inflammatory cells to the ischemic region (12–20). Infiltration of the ischemic brain region by leukocytes is associated with inflammatory activation of cerebral endothelial cells, microglia/macrophages and astrocytes (3). Activation of these resident cell populations along with immune cells stimulates the production and release of pro-inflammatory cytokines such as TNF-α and IL-1 from the ischemic tissue (22). In this inflammatory environment, cerebral endothelial cells increase their expression of cell surface adhesion molecules that mediate recruitment of leukocytes and platelets to the ischemic region (22–26). A role for leukocytes in the pathogenesis of post-ischemic brain injury is supported by three major lines of evidence: 1) leukocytes (neutrophils and/or lymphocytes) accumulate in the brain prior to or during the onset of tissue injury, 2) depletion of neutrophils from the circulation reduces infarct volume and improves neurological outcome, and 3) immunoneutralization or genetic deletion of cell adhesion molecules that mediate leukocyte recruitment reduces tissue injury and brain dysfunction in animal models of focal and global cerebral ischemia (27–38).
Brain ischemia triggers profound changes in cerebral microvascular endothelium. In microvessels, the endothelial cell activation is accompanied by an upregulation of adhesion molecules, which promotes the rolling, and firm adhesion of both leukocytes and platelets. The leukocyte recruitment may lead to further injury by releasing reactive oxygen species, proteases and inflammatory mediators. The adhesion process may also trigger signaling cascades in cerebral endothelial cells, leukocytes or both which may contribute to the injury (39). The accumulation of platelets, either directly attached to endothelial cells or bound to adherent leukocytes promote a prothrombotic state that further exaggerates the perfusion deficit and state of “no-reflow” after cerebral ischemia (32).
Although there are a large number of reports that implicate leukocytes in the tissue injury associated with ischemic stroke, there are also several reports that do not support this contention (40, 41, 42, 43). For example, it has been reported that brain necrosis precedes the appearance of neutrophils and that further increases in the number of neutrophils in the infarcted region is not associated with a larger infarct size (40). Furthermore, some studies have revealed an inability of neutrophil blocking antibodies to protect against neuronal tissue injury following ischemia reperfusion (I/R) (42, 43). These observations lead to the conclusion that neutrophils may be by-standers rather that the effectors of the tissue injury induced by cerebral ischemia (15). This view is also supported by studies that fail to demonstrate protection against ischemic brain injury in adhesion molecule deficient mice (44, 45). These inconsistent findings may be explained by the differences in experimental models/techniques or by the duration and magnitude of the ischemic insult and reperfusion periods.
Leukocyte Adhesion in Postischemic Cerebral Microvessels
The recruitment of leukocytes and platelets in the cerebral microvasculature is widely regarded as a rate-limiting step in the inflammatory response associated with cerebral ischemia. Under normal conditions (non-inflamed) cerebral endothelium is a poor substrate for the rolling and firm adhesion of circulating cells. Intravital microscopy studies have revealed that the number of rolling leukocytes in normal (non-inflamed) cerebral venules is very low compared to skeletal muscle and mesenteric (46). However, in response to an ischemic (or other inflammatory) insult cerebral endothelium are capable to expressing high levels of adhesion molecules and recruit large numbers of leukocytes.
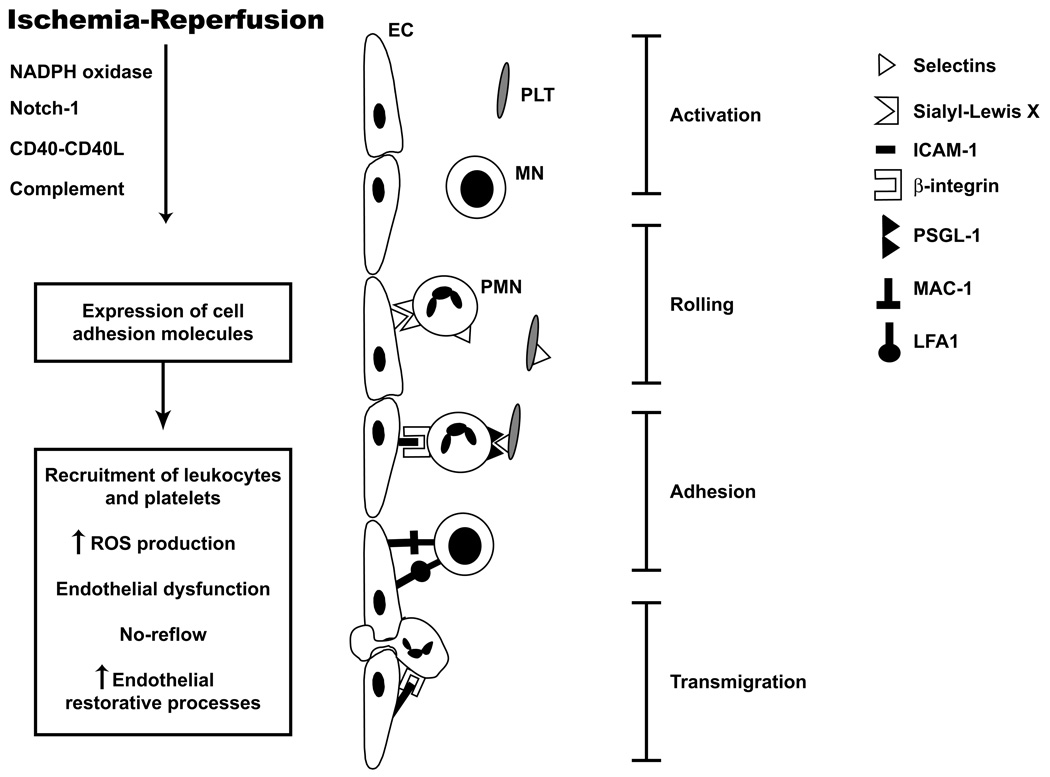
The recruitment of leukocytes and platelets in cerebral venules exposed to ischemia and reperfusion is a highly coordinated and well-regulated process that involves different adhesion molecules expressed on vascular endothelium and circulating cells (Figure 1). This recruitment process involves at least two different stages, i.e., an initial low affinity binding that is manifested as rolling and a later high affinity interaction that results in firm adhesion. The rolling interaction appears to be largely mediated by P-selectin expressed on endothelial cells that engage with sialyl Lex moieties on circulating cells (47, 48), while firm adhesion is mediated by an interaction between β2-integrins on leukocytes with ICAM-1 (49) on cerebral microvascular endothelial cells. Both CD11a/CD18 (LFA-1) or CD11b/CD18 (Mac-1) seem to play a role in the firm adhesion of leukocytes at 24 h of reperfusion of ischemia since CD11a (LFA-1) or CD11b (Mac-1) knock out mice exhibit less accumulation of adherent leukocytes in cerebral venules at 24 hrs (but not at 4 hrs) of reperfusion following occlusion of the middle cerebral artery (50).
Figure 1.
Adhesion of leukocytes and platelets into cerebral microvessels following cerebral ischemia reperfusion (I/R). I/R triggers activation of leukocytes and platelets and expression of adhesion molecules on cerebral endothelium (EC). Initially polymorphonuclear cells (PMN) roll and attach to endothelial cells. Platelets (PLT) adhere directly to endothelial cells or to PMN adhering to cerebral endothelium. At late phases of reperfusion mononuclear cell (MN) adhesion predominates. Adhesion of circulating cells further exacerbates the cerebral injury.
Leukocyte adhesion has been demonstrated in different experimental models of cerebral ischemia and hypoxia (51, 52, 53, 54). In these experiments leukocyte adhesion has been observed as early as 30 min after reperfusion and as late as 48 hours (54, 55). These kinetics are consistent with a report describing P-selectin upregulation as early as 15 min following an ischemic insult, with increased E-selectin expression occurring within 2 h of ischemia (56).
The intensity of the leukocyte adhesion response appears to depend on the nature of the ischemic/hypoxic insult. For example, asphyxia elicits less leukocyte adhesion (57) compared to focal and global ischemia (54, 47) in brain microvessels. In the early stages of reperfusion after ischemia, neutrophils represent the dominant leukocyte population that accumulates at the cerebral microvasculature. This view is supported by studies showing that rendering mice neutropenic anti-neutrophil serum attenuates the recruitment of leukocytes in cerebral venules at 4 hours after I/R but has no effect on leukocyte adhesion at 24 hrs after reperfusion (54). It appears likely that the leukocyte population that accumulates at 24 hrs of reperfusion is mainly mononuclear cells rather than neutrophils (54, 58).
Platelet Adhesion in Postischemic Cerebral Microvessels
Increased leukocyte adhesion after cerebral I/R is also accompanied by the recruitment of rolling and adherent platelets. Immunoblockade of the platelet adhesion molecule glycoprotein/IIb/IIIa has no effect on the platelet adhesion elicited by cerebral I/R. However, adhesion molecule blocking antibodies that inhibit leukocyte adhesion, such as P-selectin, CD18, and ICAM-1, in postischemic cerebral venules also blunt the recruitment of adherent platelets (47, 59). Mice deficient in either endothelial cell or platelet P-selectin exhibit a blunted platelet recruitment response to I/R (54, 47). Collectively, the data generated using different blocking antibodies and mutant mice are consistent with a model of platelet recruitment that involves the binding of platelet-associated P-selectin to its ligand PSGL-1 on leukocytes, which in turn utilize endothelial cell P-selectin and ICAM-1 to attach to the venular walls. This dependency of platelet adhesion on leukocyte adhesion is consistent with the observation that platelet accumulation lags behind the leukocyte accumulation (60) in postischemic cerebral venules and that a large proportion of platelets bind to already adherent leukocytes (61). While the consequences of platelet accumulation to the overall injury response remain poorly understood, there is evidence suggesting the binding of platelets to adherent leukocytes may lead to further activation of both cell types, which may exacerbate the injury response (54, 62). The results of a recent clinical study of genetic variants of PSGL-1 and P-selectin genes have revealed an association between some variants and the risk for ischemic stroke, however it remains unclear if and how this genetic association relates to platelet-leukocyte-endothelium interactions (63).
T-Lymphocytes, Blood Cell Adhesion and Stroke
There is a growing body of evidence in the literature that supports a role for T-lymphocytes in the tissue injury following an ischemic stroke. (58, 64, 65) In a mouse model of cerebral ischemia-reperfusion, T-lymphocytes appear to be major modulators of the adhesion of leukocytes and platelets in cerebral venules, and infarct size. Mice deficient in either CD4+ and/or CD8+ T-lymphocytes exhibit a reduced infarct size, lower number of adherent leukocytes and platelets in the cerebral venules, and improved neurological outcome at 24 hours after reperfusion (58). B-lymphocyte deficient mice did not exhibit protection against the inflammation, tissue injury, and neurological deficit induced by ischemic stroke. While these observations implicate T (but not B-)-lymphocytes in the pathogenesis of experimental stroke, there are no in vivo studies to date that directly demonstrate the adhesion of T-lymphocytes to cerebral endothelium after ischemia. However, in vitro experiments have revealed that the adhesion of lymphocytes, derived from stroke patients, to cerebral endothelium is significantly higher than that noted for lymphocytes isolated from healthy subjects (94). The pathophysiological relevance of this observation is unclear since it is not known whether T-lymphocytes must directly interact with cerebral endothelial cells to exert their deleterious actions during ischemic stroke.
Modulation of Blood Cell Adhesion
Several agents and interventions have been shown to alter the adhesion of platelets and leukocytes in cerebral vessels following I/R. These include physical factors, pharmacological agents, interventions targeting the blockade of adhesion molecules and some of the risk factors for cardiovascular diseases. High venular shear rates (18), platelet activating factor receptor antagonists (51), hypothermia (52), hydroxyethyl starch (57), and estrogen (66) decreases the leukocyte accumulation, while low venular shear rates (61) and ovariectomy increases the leukocyte adhesion into cerebral vessels following I/R.
Some of the risk factors for cardiovascular disease also appear to modulate the adhesion of platelets and leukocytes in the cerebral vessels after I/R. For example, hypercholesterolemia increases the number of adherent leukocytes and platelets in cerebral venules after I/R (59). Evidence from postischemic retinal microvessels suggests that hypertension may also exacerbate the adhesion of leukocytes and platelets following I/R in the CNS (67). A recent study has addressed the contribution of angiotensin II (Ang II) type 1 receptor signaling to platelet-leukocyte-endothelial cell interactions in the cerebral microvasculature. ANG II type 1 signaling results in increased adhesion of leukocytes and platelets by a P-selectin and PSGL-1 mediated mechanism and may represent a mechanism that links hypertension to tissue injury in ischemic stroke (68).
Therapeutic targeting of leukocyte-endothelial cell adhesion
In several experimental studies, adhesion molecules were targeted in order to define the mechanisms of cell recruitment and the related tissue damage after cerebral I/R (Table 1). These interventions include immunoblockade of adhesion molecules with specific antibodies, inhibition with ligands or antagonists, and genetic deletion. The principal targets for these interventions include the selectins, ICAM-1 and the β2-integrins, LFA-1 and Mac-1.
Table 1.
Selected experimental studies targeting adhesion molecules in ischemic stroke.
| Treatment | Model | Animal | Outcome | Reference |
|---|---|---|---|---|
| Nonspecific selectin antagonist | 4 h of focal ischemia and 24 h of reperfusion | Rat | Improved neurological functions, decreased cerebral edema | 70 |
| Anti-L-selectin antibody | 2 h focal ischemia 6 h of reperfusion | Rabbit | No reduction of infarct or neutrophil infiltration | 71 |
| P-selectin knockout | 45 min ischemia 22 h reperfusion | Mice | Smaller infarct, reduced neutrophil influx, improved cerebral reflow | 36 |
| Anti-P-selectin antibodies | 24 hr focal permanent ischemia | Rat | Reduced infarct size, brain water content and leukocyte infiltration | 35 |
| Anti-E-Selectin antibodies | 45 min focal ischemia 24 h reperfusion | Mice | Reductions in neurological deficit, mortality, and infarct volumes | 74 |
| An analogue of Sialyl-LewisX (CY-1503) | 2 h focal ischemia, 46 h reperfusion | Rat | Reduced infarct volume and neutrophil infiltration | 30 |
| Anti-P- and E-selectin | 1 h focal ischemia, 72 h reperfusion | Nonhuman primate | Reduced infarct volume and neutrophil infiltration | 75 |
| Blocking selectins with synthetic oligopeptide | Permanent focal cerebral ischemia 24 h | Spontaneously hypertensive rats | Not effective | 34 |
| Mucosal tolerance to E-selectin | 6 or 48 h permanent cerebral ischemia model | Spontaneously hypertensive stroke-prone rats | Reduction in infarct volume | 78 |
| ICAM-1 knockout animals | 45 min focal cerebral ischemia followed by 22 h of reperfusion | Mice | Reduction in infarct volume and neurological deficit, increase in survival | 38 |
| Anti- ICAM-1 antibodies | Transient focal ischemia | Rat | Reduced infarct volume and neutrophil infiltration | 88 |
| Anti-Mac-1 antibodies | 2 ischemia, 46 hours of reperfusion | Rat | Reduced infarct volume and neutrophil infiltration | 91 |
| Mice deficient in either LFA-1 or Mac-1 | 1 h focal ischemia 24 h of reperfusion | Mice | Reduced infarct volume, neurological deficit, leukocyte & platelet adhesion | 50 |
| tPA with anti-CD11b/CD18 antibodies | Embolic model | Rat | Better outcome with combination of agents | 100 |
Selectin blockade
Following cerebral ischemia, P- and E-selectins are highly expressed at brain. P-selectin can be detected as early as 15 min after reperfusion while E-selectin expression is observed beginning at 2 h after ischemia. The expression of selectins contributes to the early recruitment of circulating cells to the infarct region (56). The link between P-selectin expression and injury following ischemic stroke seems to involve a complement-dependent pathway wherein P-selectin upregulation results from complement activation and can be modulated with a targeted approach to complement receptor 2 without an effect on systemic complement activity (69).
The polysaccharide fucoidin (FCN), a homopolymer of sulfated L-fucose that competitively inhibits P- and L-selectin, has been shown to attenuate the leukocyte accumulation during reperfusion of the rat brain following focal ischemia. Fucoidin also reduced infarct size and improved neurological outcome, suggesting a role for selectin-dependent leukocyte-endothelial cell interactions following cerebral ischemia (70). However, L-selectin blockade with a humanized anti-L-selectin antibody did not decrease the tissue damage or number or infiltrating leukocytes to the ischemic region in a rabbit model of transient cerebral ischemia (71). In another study employing a similar cerebral I/R model, anti-L-Selectin antibodies were found to be effective only when used in combination with tissue plasminogen activators (tPA), which addresses the potential involvement of L-selectin in tissue injury following thrombolytic reperfusion of ischemic brain (72).
Therapies targeting P-selectin have also been shown to be effective in different experimental settings. In a model of cerebral I/R, P-selectin knockout mice exhibited a reduction in infarct volume, better functional outcome and a better return of cerebral blood flow after ischemia (36). Similarly blocking antibodies for P-selectin reduced infarct size with a reduced hemorrhagic transition in cerebral I/R (73). In a permanent ischemia model, P-selectin immunoblockade attenuated both infarct size and brain edema, which were associated with a reduction of leukocyte infiltration (35). In these studies, the anti-P-selectin antibodies were administered 30 min before the ischemic insult, which lessens the therapeutic value of the observed protection.
E-selectin immunoblockade does not need to be done during the pre-ischemic period, thereby enhancing it’s utility as a potential therapeutic strategy. Blocking E-selectin with specific antibodies as long as 90 minutes after onset of ischemia has been shown to reduce infarct size (74). In a rat model of transient ischemia, E-selectin blockade with an analogue of sialyl-LewisX (CY-1503) reduced both infarct size and neutrophil infiltration. The possibility that CY-1503 affords protection by blocking L-selectin on leukocytes or P-selectin on endothelial cells or platelets can not be discounted (30).
A blinded placebo-controlled trial in a nonhuman primate model of reperfused stroke has been performed to test the combination of blocking of both E- and P-selectin using a humanized monoclonal antibody (HuEP5C7). Administration of the blocking antibody during the occlusion period reduced infarct volume and improved neurological outcome. No immune response (complement activation) was noted with the antibody. Despite the promising outcome of this study, there is no evidence supporting the effectiveness HuEP5C7 if it is administered after onset of the stroke (75). Finally, blocking the lectin domain of selectins with a synthetic oligopeptide has been shown to reduce brain injury in a transient, but not in a permanent, model of cerebral ischemia, suggesting that reperfusion is necessary for effective anti-selectin therapy (34).
Induction of mucosal immune tolerance to E-selectin represents another promising strategy for prevention of ischemic and hemorrhagic stroke. Takeda et al. have demonstrated that the induction of mucosal tolerance to E-selectin, by repeated nasal instillation of this antigen, prevents the development of ischemic and hemorrhagic strokes in spontaneously hypertensive stroke-prone rats (76, 77). In a follow-up study, the protective effects of mucosal tolerance to E-selectin were also demonstrated in a permanent model of cerebral ischemia. In the latter study, the neuroprotective effects of mucosal tolerance to E-selectin was linked to immunomodulation of CD8+ T-cell responses addressing a possible link between T-cell modulation and neuroprotection. A better understanding of this link may provide promising selective therapeutic options in stroke (78).
ICAM-1 blockade
The results of several studies have revealed significant ICAM-1 expression in the postischemic brain and a role for this adhesion molecule in the recruitment of inflammatory cells (78–82). In vitro experiments confirm an increase in ICAM-1 in cerebral endothelial cells following ischemia or ischemia-like conditions (25, 83, 84). Human studies provide further support for an increased ICAM-1 expression in the ipsilateral cortex and increased circulating levels of soluble ICAM-1 following ischemic stroke (85, 86).
ICAM-1 expression is an essential step in mediating the firm adhesion of leukocytes in cerebral microvessels after ischemic stroke and there are several studies that address the contribution of ICAM-1 to cerebral injury after stroke (38, 87, 88, 89, 90). ICAM-1 knockout mice exhibit a reduction in leukocyte adhesion, smaller infarcts, decreased leukocyte adhesion (87), improved cerebral blood flow and lower mortality after cerebral ischemia and reperfusion (38). Similarly, ICAM-1 immunoblockade reduces ischemic brain injury and neutrophil accumulation in both rat (88, 90) and rabbit (89) models of cerebral ischemia. The combined use of anti-ICAM antibodies with tPA has been shown to improve neurological outcome under conditions wherein neither of these interventions are independently effective, indicating that ICAM-1 blockade may improve outcome of tPA administration with an extended therapeutic window for thrombolysis (91).
LFA-1/MAC-1 blockade
LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18) are β2-integrins that mediate the firm adhesion of leukocytes on vascular endothelium. LFA-1 is expressed on all leukocytes where Mac-1 expression is found on neutrophils, monocytes and natural killer cells. Both adhesion molecules are constitutively expressed and may exhibit increased expression upon stimulation. ICAM-1 and -2 on endothelial cells are known targets for LFA-1 and Mac-1 (92). The expression of CD11a and CD18 is upregulated in stroke patients and patients with transient ischemic attacks for up to 72 hours after the ischemic incident, revealing an association between cerebral ischemia and the cell surface density of these adhesion molecules (93).
Mac-1 and CD18 knockout mice subjected to stroke followed by reperfusion exhibit a reduced infarct volume and lower mortality, compared to their wild type counterparts (37, 45). In models of transient cerebral ischemia, immunoblockade of either CD11b, CD18, or Mac-1 (28, 33, 95, 96) also affords protection against tissue injury. Similarly, CD18 immunoneutralization in rats reduces the edema, leukocyte infiltration and infarct size resulting from transient ischemia (28). In an in vitro study, the enhanced adhesion of lymphocytes to cerebral endothelial cells appears to be dependent to LFA-1 and ICAM-1 expression following ischemic stroke. Furthermore, lymphocytes isolated from patients with stroke exhibit increased adhesion to cerebral endothelium when compared to lymphocytes derived from healthy donors (94). The contributions of LFA-1 and Mac-1 to I/R-induced brain injury and cerebral microvascular dysfunction at 4 and 24 hrs after reperfusion have been addressed using mice deficient in either leukocyte adhesion molecule. Both mutants exhibited smaller infarcts, improved neurological outcome, and less adhesion of leukocytes and platelets in cerebral microvessels. Interestingly, the lack of LFA-1 or Mac-1 had no effect on leukocyte adhesion at 4 hours of reperfusion but reduced the number of adherent leukocytes at 24 h, suggesting a more important role for these adhesion molecules at later times after reperfusion (50).
Anti-adhesion molecule strategies in ischemic stroke have proven more effective following transient, but not permanent, ischemia (45, 97, 98). This observation suggests that anti-adhesion interventions may offer more therapeutic benefit in patients receiving rt-PA to achieve reperfusion following ischemic stroke. Support for this possibility is provided by the observation that a combination of anti-β2-integrin (CD11/CD18) and tPA extends the therapeutic window of tPA, with a better outcome than the additive effects of these agents in experimental animals (99, 100).
Leukocyte adhesion and signaling pathways
Ischemia elicits changes in different signaling pathways in endothelial cells that allow for the recruitment of leukocytes and platelets in cerebral venules. Increased production of reactive oxygen species (ROS) following I/R is considered to be one of the major mechanisms that links I/R and increased adhesion of blood cells in cerebral microvessels. Enhanced superoxide production is associated with cerebral I/R and this response is exacerbated by hypercholesterolemia (59) and diabetes mellitus (101). NADPH oxidase appears to be a major source of ROS production in the postischemic cerebral microvasculature (59, 68). ROS are known to trigger the expression of endothelial cell adhesion molecules that mediate leukocyte adnd platelet adhesion in postischemic cerebral venules. Superoxide may promote adhesion by inactivating nitric oxide, which is an endogenous inhibitor of leukocyte adherence (102). Nitric oxide donors such as nitroprusside inhibit leukocyte adhesion (103). The opposing actions of superoxide and nitric oxide suggest that the balance between these reactive species in the cerebral microcirculation may be a major determinant of the blood cell adhesion induced by I/R.
CD40-CD40L signaling also appears to play a major role in modulating the recruitment of blood cells in brain microvessels following stroke (53). CD40 and CD40L knockout mice exhibit a smaller infarct volume and lower numbers of rolling and adherent leukocytes and platelets in postischemic venules. While the diminished blood cell recruitment that is observed in CD40-CD40L knockout mice may be a secondary consequence of the smaller infarct volume, a direct effect on blood cell-interactions is also a likely explanation for the protection afforded by these mutations.
Notch-1 is a cell surface receptor that controls a broad number of cellular processes such as cell differentiation, proliferation and apoptosis. The Notch-1 signaling pathway also mediates angiogenesis, T-cell activation and proliferation, synaptic plasticity and cell-fate decisions in nervous system (104, 105). A recent study addressed the contribution of Notch-1 signaling to the inflammatory and tissue injury responses to cerebral I/R (106). Mice treated with inhibitors of the Notch activating enzyme, gamma-secretase, as well as Notch antisense transgenic mice exhibit an attenuated brain injury response to I/R. This protective effect was accompanied by a blunted leukocyte/platelet recruitment response and a reduced number of ICAM-1 and CD11b positive cells in ischemic brain.
Clinical Trials Employing Anti-Adhesion Strategies
ICAM-1 blockade (Enlimomab)
The promising data obtained with anti-adhesion molecule strategies in different animal models of ischemic stroke lead to clinical trials that tested the efficacy of anti-ICAM-1 antibodies in human stroke. The murine monoclonal anti-ICAM-1, Enlimomab was administered over 5 days during an observation period of 30 +/− 10 days and initially found to be safe for use in humans with ischemic stroke (107). However, in a prospective, randomized, blinded, phase III trial, administration of the anti-ICAM antibody within 6 hours after the onset of symptoms increased mortality, infarct volume and side effects (fever, infection) in patients with a worse functional outcome (108). One explanation for the failure of anti-ICAM antibody is the possibility that murine antibody would trigger an immunological response after administration. This hypothesis was further supported with animal studies showing that anti-ICAM-1 antibodies can increase selectin expression and activates complement, which in turn activates neutrophils (109, 110). In a subsequent in vivo study in rats, anti-ICAM-1 antibodies administered prior to cerebral ischemia were found to sensitize the animals, resulting in larger infarcts, activation of complement system, and an upregulation of selectins (111). This experience with Enlimomab underscores the need to consider potentially harmful immune reactions to antibody administration and raises the possibility of improved success if humanized antibodies are employed.
Mac-1 blockade (Leukarrest, UK-279, 276)
Leukarrest (Hu23F2G) is a humanized, anti-Mac-1 (CD11b/18) antibody that had been shown to be effective in limiting infarct size, and neuronal damage following transient focal cerebral ischemia in rabbits (115). In a phase III clinical study, Leukarrest was tested in stroke patients however the trial was halted due to a failure to meet the predefined success criteria (112, 116).
UK-279, 276, a small recombinant glycoprotein that selectively binds to CD11b integrin of Mac-1 (CD11b/CD18), has been shown to be effective in reducing infarct size in reperfused animal models of stroke (100, 113). In a phase 2 clinical study (Acute Stroke Therapy by Inhibition of Neutrophils; ASTIN) approximately 900 patients with ischemic stroke were treated with UK-279, 276 within 6 h of stroke onset (114). In 204 patients UK-279, 276 was co administered with t-PA. No side effects were reported with UK-279, 276. The trial was terminated due to no improvement in outcome of stroke patients with UK-279, 276. However post-hoc analysis showed that there was a slight improvement in patients who received a combination therapy (rt-PA + UK-279, 276), indicating a possible beneficial effect of UK-279,279 in reperfusion injury related to t-PA administration.
CONCLUSIONS
The data generated from several experiments using cell surface adhesion molecules as targets of stroke therapy is promising yet inadequate. An ideal therapeutic agent that targets adhesion molecules for stroke treatment should not elicit an immune response, narrow the therapeutic window, and/or interfere with endogenous restorative processes. Unfortunately, existing reagents do not appear to fulfill all of these requirements.
The diversity of adhesion molecules used by different leukocyte populations to adhere to vascular endothelium warrants more attention as does the relative contributions of these different cell populations to ischemic brain injury. Monocytes and lymphocytes, for example, employ different adhesion glycoproteins than neutrophils to bind to vascular endothelium. While much effort has been devoted to defining the role of neutrophils to brain injury in ischemic stroke, emerging evidence suggests that mononuclear leukocytes play an equal or more important role in this injury process.
Adhesion molecule-directed therapies for stroke are based on the assumption that the recruitment of inflammatory/immune cells in postischemic brain is detrimental (117). However, some of these recruited cells may contribute to CNS regeneration following an ischemic stroke (41). For example, the results of a recent animal study indicate that activation of T-lymphocytes is neuroprotective in the setting of cerebral ischemia (118). Circulating lymphocytes, monocytes, and/or other circulating cells might induce a restorative phenotype following an ischemic insult via mechanisms that involve cell-to-endothelium contact. In a preliminary report (119), we have recently described the ability of selectin-specific antibodies to block the recruitment of exogenous bone marrow stromal cells (stem cells) into postischemic cerebral venules. Since stem cells may have therapeutic benefit in ischemic stroke, interfering with their recruitment or the trafficking of other restorative cells to the injured brain region may impair or delay the resolution of ischemia-induced tissue injury.
A promising, but inadequately studied, aspect of leukocyte adhesion-directed therapies is the combined use of anti-adhesion molecules with thrombolytic agents, such as tPA. The available evidence suggests the combination of rt-PA with a small molecule, non-immunogenic anti-adhesion agent may significantly extend the duration of the therapeutic window of rt-PA and confer some protection against brain injury. With improved and more readily available imaging methods for screening stroke patients and the development of novel thrombolytic agents, a larger proportion of stroke patients are likely to receive thrombolytic therapy in the future. A likely consequence of the increased frequency of aggressive intervention in stroke patients is an increased incidence of reperfusion-related brain pathophysiology that may be more responsive to anti-adhesion therapy. This possibility justifies a continued effort to more clearly define the role of adhesion molecules in the pathophysiology of cerebral ischemia-reperfusion and to develop novel reagents that effectively interfere with the adhesion of leukocytes to vascular endothelium.
References
- 1.WHO MONICA Project Principal Investigators. The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. J Clin Epidemiol. 1988;41:105–114. doi: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed]
- 2.Murphy J. Pharmacological treatment of acute ischemic stroke. Crit Care Nurs Q. 2003;26:276–282. doi: 10.1097/00002727-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated review. Trends in Neuroscience. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 4.Khaja AM, Grotta JC. Established treatments for acute ischaemic stroke. Lancet. 2007;27:319–330. doi: 10.1016/S0140-6736(07)60154-8. [DOI] [PubMed] [Google Scholar]
- 5.International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet. 1997;349:1569–1581. [PubMed] [Google Scholar]
- 6.Ciccone A, Abraha I, Santilli I. Glycoprotein IIb-IIIa inhibitors for acute ischaemic stroke. Cochrane Database Syst Rev. 2006;18 doi: 10.1002/14651858.CD005208.pub2. CD005208. [DOI] [PubMed] [Google Scholar]
- 7.Legos JJ, Barone FC. Update on pharmacological strategies for stroke: prevention, acute intervention and regeneration. Curr Opin Investig Drugs. 2003;4:847–858. [PubMed] [Google Scholar]
- 8.Sacco R, Chong J, Prabhakaran S, et al. Experimental treatments for acute ischaemic stroke. Lancet. 2007;369:331–341. doi: 10.1016/S0140-6736(07)60155-X. [DOI] [PubMed] [Google Scholar]
- 9.McDonald ES, Windebank AJ. Mechanisms of neurotoxic injury and cell death. Neurol Clin. 2000;18:525–540. doi: 10.1016/s0733-8619(05)70209-7. [DOI] [PubMed] [Google Scholar]
- 10.Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 11.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Garcia JH, Kamijyo Y. Cerebral infarction. Evolution of histopathological changes after occlusion of a middle cerebral artery in primates. J Neuropathol Exp Neurol. 1974;33:409–421. doi: 10.1097/00005072-197407000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Hallenbeck JM, Dutka AJ, Tanishima T, et al. Polymorphonuclear leukocyte accumulation in brain region with low blood flow during the early postischemic period. Stroke. 1986;17:246–253. doi: 10.1161/01.str.17.2.246. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Chopp M, Bodzin G. Neutropenia reduces the volume of cerebral infarct after transient middle cerebral artery occlusion in the rat. Neurosci Res Commun. 1992;11:93–99. [Google Scholar]
- 15.Dereski MO, Chopp M, Knight RA, et al. Focal cerebral ischemia in the rat: temporal profile of neutrophil responses. Neurosci Res Comm. 1992;11:179–186. [Google Scholar]
- 16.Clark RK, Lee EV, Fish CJ, et al. Development of tissue damage, inflammation and resolution following stroke: an immunohistochemical and quantitative planimetric study. Brain Res Bull. 1993;31:565–572. doi: 10.1016/0361-9230(93)90124-t. [DOI] [PubMed] [Google Scholar]
- 17.Zhang RL, Chopp M, Chen H, et al. Temporal profile of ischemic tissue damage, neutrophil response, and vascular plugging following permanent and transient (2H) middle cerebral artery occlusion in the rat. J Neurol Sci. 1994;125:3–10. doi: 10.1016/0022-510x(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 18.Ritter LS, Orozco JA, Coull BM, et al. Leukocyte accumulation and hemodynamic changes in the cerebral microcirculation during early reperfusion after stroke. Stroke. 2000;31:1153–1161. doi: 10.1161/01.str.31.5.1153. [DOI] [PubMed] [Google Scholar]
- 19.Barone FC, Hillegass LM, Price WJ, et al. Polymorpho-nuclear leukocyte infiltration into cerebral focal ischemic tissue: myeloperoxidase activity assay and histologic verification. J Neurosci Res. 1991;29:336–345. doi: 10.1002/jnr.490290309. [DOI] [PubMed] [Google Scholar]
- 20.Campanella M, Sciorati C, Tarozzo G, et al. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33:586–592. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- 21.Liu T, Clark RK, McDonnell PC, et al. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25:1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho-Tavares J, Hickey MJ, Hutchison J, et al. A role for platelets and endothelial selectins in tumor necrosis factor-alpha-induced leukocyte recruitment in the brain microvasculature. Circ Res. 2000;87:1141–1148. doi: 10.1161/01.res.87.12.1141. [DOI] [PubMed] [Google Scholar]
- 23.Eppihimer MJ, Wolitzky B, Anderson DC, et al. Heterogeneity of expression of E-and P-selectins in vivo. Circ Res. 1996;79:560–569. doi: 10.1161/01.res.79.3.560. [DOI] [PubMed] [Google Scholar]
- 24.Henninger DD, Panes J, Eppihimer M, et al. Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J Immunol. 1997;158:1825–1832. [PubMed] [Google Scholar]
- 25.Stanimirovic DB, Wong J, Shapiro A, et al. Increase in surface expression of ICAM-1, VCAM-1 and E-selectin in human cerebromicrovascular endothelial cells subjected to ischemia-like insults. Acta Neurochir Suppl. 1997;70:12–16. doi: 10.1007/978-3-7091-6837-0_4. [DOI] [PubMed] [Google Scholar]
- 26.Stanimirovic D, Shapiro A, Wong J, et al. The induction of ICAM-1 in human cerebromicrovascular endothelial cells (HCEC) by ischemia-like conditions promotes enhanced neutrophil/HCEC adhesion. J Neuroimmunol. 1997;76:193–205. doi: 10.1016/s0165-5728(97)00057-x. [DOI] [PubMed] [Google Scholar]
- 27.Heinel LA, Rubin S, Rosenwasser RH, et al. Leukocyte involvement in cerebral infarct generation after ischemia and reperfusion. Brain Res Bull. 1994;34:137–141. doi: 10.1016/0361-9230(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 28.Matsuo Y, Onodera H, Shiga Y, et al. Correlation between myeloperoxidase-quantified neutrophil accumulation and ischemia brain injury in the rat. Stroke. 1994;25:1469–1475. doi: 10.1161/01.str.25.7.1469. [DOI] [PubMed] [Google Scholar]
- 29.Jiang N, Moyle M, Soule HR, et al. Neutrophil inhibitory factor is neuroprotective after focal ischemia in rats. Ann Neurol. 1995;38:935–942. doi: 10.1002/ana.410380615. [DOI] [PubMed] [Google Scholar]
- 30.Zhang RL, Chopp M, Zhang ZG, et al. E-selectin in focal cerebral ischemia and reperfusion in the rat. J Cereb Blood Flow Metab. 1996;16:1126–1136. doi: 10.1097/00004647-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Zhang RL, Chopp M, Li Y, et al. Anti-ICAM-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in the rat. Neurology. 1994;44:1747–1751. doi: 10.1212/wnl.44.9.1747. [DOI] [PubMed] [Google Scholar]
- 32.Mori E, del Zoppo GJ, Chambers JD, et al. Inhibition of polymorphonuclear leukocyte adherence suppresses no-reflow after focal cerebral ischemia in baboons. Stroke. 1992;23:712–718. doi: 10.1161/01.str.23.5.712. [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Chopp M, Zhang RL, et al. Anti-CD11b monoclonal antibody reduces ischemic cell damage after transient focal cerebral ischemia in rat. Ann Neurol. 1994;35:458–463. doi: 10.1002/ana.410350414. [DOI] [PubMed] [Google Scholar]
- 34.Morikawa E, Zhang SM, Seko Y, et al. Treatment of focal cerebral ischemia with synthetic oligopeptide corresponding to lectin domain of selectin. Stroke. 1996;27:951–955. doi: 10.1161/01.str.27.5.951. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki H, Hayashi T, Tojo SJ, et al. Anti-P-selectin antibody attenuates rat brain ischemic injury. Neurosci Lett. 1999;265:163–166. doi: 10.1016/s0304-3940(99)00229-3. [DOI] [PubMed] [Google Scholar]
- 36.Connolly ES, Jr, Winfree CJ, Prestigiacomo CJ, et al. Exacerbation of cerebral injury in mice that express the P-selectin gene: identification of P-selectin blockade as a new target for the treatment of stroke. Circ Res. 1997;81:304–310. doi: 10.1161/01.res.81.3.304. [DOI] [PubMed] [Google Scholar]
- 37.Soriano SG, Coxon A, Wang YF, et al. Mice deficient in Mac-1 (CD11b/CD18) are less susceptible to cerebral ischemia/reperfusion injury. Stroke. 1999;30:134–139. doi: 10.1161/01.str.30.1.134. [DOI] [PubMed] [Google Scholar]
- 38.Connolly ES, Jr, Winfree CJ, Springer TA, et al. Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest. 1996;97:209–216. doi: 10.1172/JCI118392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishikawa M, Zhang JH, Nanda A, et al. Inflammatory responses to ischemia and reperfusion in the cerebral microcirculation. Front Biosci. 2004;9:1339–1347. doi: 10.2741/1330. [DOI] [PubMed] [Google Scholar]
- 40.Hayward NJ, Elliott PJ, Sawyer SD, et al. Lack of evidence for neutrophil participation during infarct formation following focal cerebral ischemia in the rat. Exp Neurol. 1996;139:188–202. doi: 10.1006/exnr.1996.0093. [DOI] [PubMed] [Google Scholar]
- 41.Kochanek PM, Hallenbeck JM. Polymorphonuclear leukocytes and monocytes/macrophages in the pathogenesis of cerebral ischemia and stroke. Stroke. 1992;23:1367–1379. doi: 10.1161/01.str.23.9.1367. [DOI] [PubMed] [Google Scholar]
- 42.Takeshima R, Kirsch JR, Koehler RC, et al. Monoclonal leukocyte antibody does not decrease the injury of transient focal cerebral ischemia in cats. Stroke. 1992;23:247–252. doi: 10.1161/01.str.23.2.247. [DOI] [PubMed] [Google Scholar]
- 43.Harris AK, Ergul A, Kozak A, et al. Effect of neutrophil depletion on gelatinase expression, edema formation and hemorrhagic transformation after focal ischemic stroke. BMC Neurosci. 2005;6:49. doi: 10.1186/1471-2202-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soriano SG, Wang YF, Wagner DD, et al. P- and E-selectin-deficient mice are susceptible to cerebral ischemia-reperfusion injury. Brain Res. 1999;835:360–364. doi: 10.1016/s0006-8993(99)01637-6. [DOI] [PubMed] [Google Scholar]
- 45.Prestigiacomo CJ, Kim SC, Connolly ES, Jr, et al. CD18-mediated neutrophil recruitment contributes to the pathogenesis of reperfused but not nonreperfused stroke. Stroke. 1999;30:1110–1117. doi: 10.1161/01.str.30.5.1110. [DOI] [PubMed] [Google Scholar]
- 46.Barkalow FJ, Goodman MJ, Gerritsen ME, et al. Brain endothelium lack one of two pathways of P-selectin-mediated neutrophil adhesion. Blood. 1996;88:4585–4593. [PubMed] [Google Scholar]
- 47.Ishikawa M, Cooper D, Russell J, et al. Molecular determinants of the prothrombogenic and inflammatory phenotype assumed by the postischemic cerebral microcirculation. Stroke. 2003;34:1777–1782. doi: 10.1161/01.STR.0000074921.17767.F2. [DOI] [PubMed] [Google Scholar]
- 48.Varki A, Cummings R, Esko J, Freeze H, Hart G, Mart J. Essentials of Glycobiology. New York:: Cold Spring Harbor Laboratory Press; 1999. [PubMed] [Google Scholar]
- 49.Liu L, Kubes P. Molecular mechanisms of leukocyte recruitment: organ-specific mechanisms of action. Thromb Haemost. 2003;89:213–220. [PubMed] [Google Scholar]
- 50.Arumugam TV, Salter JW, Chidlow JH, et al. Contributions of LFA-1 and Mac-1 to brain injury and microvascular dysfunction. Am J Physiol Heart Circ Physiol. 2004;287:H2555–H2560. doi: 10.1152/ajpheart.00588.2004. [DOI] [PubMed] [Google Scholar]
- 51.Park TS, Gonzales ER, Gidday JM. Platelet-activating factor mediates ischemiainduced leukocyte-endothelial adherence in newborn pig brain. J Cereb Blood Flow Metab. 1999;19:417–424. doi: 10.1097/00004647-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Ishikawa M, Sekizuka E, Sato S, et al. Effects of moderate hypothermia on leukocyte-endothelium interaction in the rat pial microvasculature after transient middle cerebral artery occlusion. Stroke. 1999;30:1679–1686. doi: 10.1161/01.str.30.8.1679. [DOI] [PubMed] [Google Scholar]
- 53.Ishikawa M, Vowinkel T, Stokes KY, et al. CD40/CD40 ligand signaling in mouse cerebral microvasculature after focal ischemia/reperfusion. Circulation. 2005;111:1690–1696. doi: 10.1161/01.CIR.0000160349.42665.0C. [DOI] [PubMed] [Google Scholar]
- 54.Ishikawa M, Cooper D, Arumugam TV, et al. Platelet-leukocyte-endothelial cell interactions after middle cerebral artery occlusion and reperfusion. Cereb Blood Flow Metab. 2004;24:907–915. doi: 10.1097/01.WCB.0000132690.96836.7F. [DOI] [PubMed] [Google Scholar]
- 55.Granger DN, Stokes KY. Differential regulation of leukocyte-endothelial cell interactions. In: Aird WC, editor. Endothelial cells in health and disease. Boca Raton:: Taylor & Francis; 2005. pp. 229–243. [Google Scholar]
- 56.Zhang R, Chopp M, Zhang Z, et al. The expression of P-and E-selectins in three models of middle cerebral artery occlusion. Brain Research. 1998;785:207–214. doi: 10.1016/s0006-8993(97)01343-7. [DOI] [PubMed] [Google Scholar]
- 57.Kaplan SS, Park TS, Gonzales ER, et al. Hydroxyethyl starch reduces leukocyte adherence and vascular injury in the newborn pig cerebral circulation after asphyxia. Stroke. 2000;31:2218–2223. doi: 10.1161/01.str.31.9.2218. [DOI] [PubMed] [Google Scholar]
- 58.Yilmaz G, Arumugam TV, Stokes KY, et al. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 59.Ishikawa M, Stokes KY, Zhang JH, et al. Cerebral microvascular responses to hypercholesterolemia: roles of NADPH oxidase and Pselectin. Circ Res. 2004;94:239–244. doi: 10.1161/01.RES.0000111524.05779.60. [DOI] [PubMed] [Google Scholar]
- 60.Tailor A, Cooper D, Granger DN. Platelet-vessel wall interactions in the microcirculation. Microcirculation. 2005;12:275–285. doi: 10.1080/10739680590925691. [DOI] [PubMed] [Google Scholar]
- 61.Russell J, Cooper D, Tailor A, et al. Low venular shear rates promote leukocyte-dependent recruitment of adherent platelets. Am J Physiol Gastrointest Liver Physiol. 2003;284:G123–G129. doi: 10.1152/ajpgi.00303.2002. [DOI] [PubMed] [Google Scholar]
- 62.Zeller JA, Lenz A, Eschenfelder CC, et al. Platelet-leukocyte interaction and platelet activation in acute stroke with and without preceding infection. Arterioscler Thromb Vasc Biol. 2005;25:1519–1523. doi: 10.1161/01.ATV.0000167524.69092.16. [DOI] [PubMed] [Google Scholar]
- 63.Volcik KA, Ballantyne CM, Coresh J, et al. The Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. 2007 Apr 7; doi: 10.1016/j.atherosclerosis.2007.03.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arumugam TV, Granger DN, Mattson MP. Stroke and T-cells. Neuromolecular Med. 2005;7:229–242. doi: 10.1385/NMM:7:3:229. [DOI] [PubMed] [Google Scholar]
- 65.Hurn PD, Subramanian S, Parker SM, et al. T-and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007 Mar 28; doi: 10.1038/sj.jcbfm.9600482. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santizo RA, Anderson S, Ye S, et al. Effects of estrogen on leukocyte adhesion after transient forebrain ischemia. Stroke. 2000;31:2231–2235. doi: 10.1161/01.str.31.9.2231. [DOI] [PubMed] [Google Scholar]
- 67.Hirose F, Kiryu J, Miyamoto K, et al. In vivo evaluation of retinal injury after transient ischemia in hypertensive rats. Hypertension. 2004;43:1098–1102. doi: 10.1161/01.HYP.0000123069.02156.8a. [DOI] [PubMed] [Google Scholar]
- 68.Ishikawa M, Sekizuka E, Yamaguchi N, et al. Angiotensin II type 1 receptor signaling contributes to platelet-leukocyte-endothelial cell interactions in the cerebral microvasculature. Am J Physiol Heart Circ Physiol. 2007 Jan 12; doi: 10.1152/ajpheart.00601.2006. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 69.Zhang R, Chopp M, Zhang Z, Jiang N, Powers C. The expression of P-and E-selectins in three models of middle cerebral artery occlusion. Brain Res. 1998;785:207–214. doi: 10.1016/s0006-8993(97)01343-7. [DOI] [PubMed] [Google Scholar]
- 70.Ruehl ML, Orozco JA, Stoker MB, et al. Protective effects of inhibiting both blood and vascular selectins after stroke and reperfusion. Neurol Res. 2002;24:226–232. doi: 10.1179/016164102101199738. [DOI] [PubMed] [Google Scholar]
- 71.Yenari MA, Sun GH, Kunis DM, et al. L-selectin inhibition does not reduce injury in a rabbit model of transient focal cerebral ischemia. Neurol Res. 2001;23:72–78. doi: 10.1179/016164101101198154. [DOI] [PubMed] [Google Scholar]
- 72.Bednar MM, Gross CE, Russell SR, et al. Humanized anti-L-selectin monoclonal antibody DREG200 therapy in acute thromboembolic stroke. Neurol Res. 1998;20(5):403–408. doi: 10.1080/01616412.1998.11740538. [DOI] [PubMed] [Google Scholar]
- 73.Goussev AV, Zhang Z, Anderson DC, et al. P-selectin antibody reduces hemorrhage and infarct volume resulting from MCA occlusion in the rat. J Neurosci. 1998;161:16–22. doi: 10.1016/s0022-510x(98)00262-7. [DOI] [PubMed] [Google Scholar]
- 74.Huang J, Choudhri TF, Winfree CJ, et al. Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke. Stroke. 2000;31:3047–3053. [PubMed] [Google Scholar]
- 75.Mocco J, Choudhri T, Huang J, et al. HuEP5C7 as a humanized monoclonal anti-E/P-selectin neurovascular protective strategy in a blinded placebo-controlled trial of nonhuman primate stroke. Circ Res. 2002;91:907–914. doi: 10.1161/01.res.0000042063.15901.20. [DOI] [PubMed] [Google Scholar]
- 76.Takeda H, Spatz M, Ruetzler C, et al. Induction of mucosal tolerance to E-selectin prevents ischemic and hemorrhagic stroke in spontaneously hypertensive genetically stroke-prone rats. Stroke. 2002;33:2156–2163. doi: 10.1161/01.str.0000029821.82531.8b. [DOI] [PubMed] [Google Scholar]
- 77.Feuerstein N, Goldman S, Feuerstein G. Immune tolerance and stroke: a turning point. Stroke. 2002;33:2163–2164. [PubMed] [Google Scholar]
- 78.Yong Chen, Christl Ruetzler, Sruthi Pandipati, et al. Mucosal tolerance to E-selectin provides cell-mediated protection against ischemic brain injury. PNAS. 2003;100:15107–15112. doi: 10.1073/pnas.2436538100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okada Y, Copeland BR, Mori E, et al. P-selectin and intercellular adhesion molecule-1 expression after focal brain ischemia and reperfusion. Stroke. 1994;25:202–211. doi: 10.1161/01.str.25.1.202. [DOI] [PubMed] [Google Scholar]
- 80.Jander S, Kraemer M, Schroeter M, et al. Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J Cereb Blood Flow Metab. 1995;15:42–51. doi: 10.1038/jcbfm.1995.5. [DOI] [PubMed] [Google Scholar]
- 81.Zhang RL, Chopp M, Zaloga C, et al. The temporal profiles of ICAM-1 protein and mRNA expression after transient MCA occlusion in the rat. Brain Res. 1995;682:182–188. doi: 10.1016/0006-8993(95)00346-r. [DOI] [PubMed] [Google Scholar]
- 82.Wang X, Yue TL, Barone FC, et al. Demonstration of increased endothelial-leukocyte adhesion molecule-1 mRNA expression in rat ischemic cortex. Stroke. 1995;26:1665–1668. doi: 10.1161/01.str.26.9.1665. [DOI] [PubMed] [Google Scholar]
- 83.Hess DC, Zhao W, Carroll J, et al. Increased expression of ICAM-1 during reoxygenation in brain endothelial cells. Stroke. 1994;25:1463–1467. doi: 10.1161/01.str.25.7.1463. [DOI] [PubMed] [Google Scholar]
- 84.Howard EF, Chen Q, Cheng C, et al. NF-kappa B is activated and ICAM-1 gene expression is upregulated during reoxygenation of human brain endothelial cells. Neurosci Lett. 1998;248:199–203. doi: 10.1016/s0304-3940(98)00239-0. [DOI] [PubMed] [Google Scholar]
- 85.Lindsberg PJ, Carpen O, Paetau A, et al. Endothelial ICAM-1 expression associated with inflammatory cell response in human ischemic stroke. Circulation. 1996;94:939–945. doi: 10.1161/01.cir.94.5.939. [DOI] [PubMed] [Google Scholar]
- 86.Bitsch A, Klene W, Murtada L, et al. A longitudinal prospective study of soluble adhesion molecules in acute stroke. Stroke. 1998;29:2129–2135. doi: 10.1161/01.str.29.10.2129. [DOI] [PubMed] [Google Scholar]
- 87.Kitagawa K, Matsumoto M, Mabuchi T, et al. Deficiency of intercellular adhesion molecule 1 attenuates microcirculatory disturbance and infarction size in focal cerebral ischemia. J Cereb Blood Flow Metab. 1998;18:1336–1345. doi: 10.1097/00004647-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 88.Zhang RL, Chopp M, Li Y, Zaloga C, et al. Anti-ICAM-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in the rat. Neurology. 1994;44:1747–1751. doi: 10.1212/wnl.44.9.1747. [DOI] [PubMed] [Google Scholar]
- 89.Bowes MP, Zivin JA, Rothlein R. Monoclonal antibody to the ICAM-1 adhesion site reduces neurological damage in a rabbit cerebral embolism stroke model. Exp Neurol. 1993;119:215–219. doi: 10.1006/exnr.1993.1023. [DOI] [PubMed] [Google Scholar]
- 90.Chopp M, Li Y, Jiang N, et al. Antibodies against adhesion molecules reduce apoptosis after transient middle cerebral artery occlusion in rat brain. J Cereb Blood Flow Metab. 1996;16:578–584. doi: 10.1097/00004647-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 91.Bowes MP, Rothlein R, Fagan SC, et al. Monoclonal antibodies preventing leukocyte activation reduce experimental neurologic injury and enhance efficacy of thrombolytic therapy. Neurology. 1995;45:815–819. doi: 10.1212/wnl.45.4.815. [DOI] [PubMed] [Google Scholar]
- 92.Frijns CJM, Kappelle LJ. Inflammatory Cell Adhesion Molecules in Ischemic Cerebrovascular Disease. Stroke. 2002;33:2115–2122. doi: 10.1161/01.str.0000021902.33129.69. [DOI] [PubMed] [Google Scholar]
- 93.Kim JS, Chopp M, Chen H, et al. Adhesive glycoproteins CD11a and CD18 are upregulated in the leukocytes from patients with ischemic stroke and transient ischemic attacks. J Neurol Sci. 1995;128:45–50. doi: 10.1016/0022-510x(94)00203-z. [DOI] [PubMed] [Google Scholar]
- 94.Zhao H, Dong X, Wang X, et al. Studies on single-cell adhesion probability between lymphocytes and endothelial cells with micropipette technique. Microvasc Res. 2002;63:218–226. doi: 10.1006/mvre.2001.2390. [DOI] [PubMed] [Google Scholar]
- 95.Chopp M, Zhang RL, Chen H, et al. Postischemic administration of an anti-Mac-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in rats. Stroke. 1994;25:869–875. doi: 10.1161/01.str.25.4.869. [DOI] [PubMed] [Google Scholar]
- 96.Bednar MM, Wright SD, Raymond-Russell SJ, et al. IB4, a monoclonal antibody against the CD18 leukocyte adhesion protein, reduces intracranial pressure following thromboembolic stroke in the rabbit. Neurol Res. 1996;18:171–175. doi: 10.1080/01616412.1996.11740398. [DOI] [PubMed] [Google Scholar]
- 97.Garcia JH, Liu KF, Bree MP. Effects of CD11b/18 monoclonal antibody on rats with permanent middle cerebral artery occlusion. Am J Pathol. 1996;148:241–248. [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang RL, Chopp M, Jiang N, et al. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke. 1995;26:1438–1442. doi: 10.1161/01.str.26.8.1438. [DOI] [PubMed] [Google Scholar]
- 99.Zhang RL, Zhang ZG, Chopp M. Increased therapeutic efficacy with rt-PA and anti-CD18 antibody treatment of stroke in the rat. Neurology. 1999;52:273–279. doi: 10.1212/wnl.52.2.273. [DOI] [PubMed] [Google Scholar]
- 100.Zhang L, Zhang ZG, Zhang RL, et al. Effects of a selective CD11b/CD18 antagonist and recombinant human tissue plasminogen activator treatment alone and in combination in a rat embolic model of stroke. Stroke. 2003;34:1790–1795. doi: 10.1161/01.STR.0000077016.55891.2E. [DOI] [PubMed] [Google Scholar]
- 101.Rizk NN, Rafols JA, Dunbar JC. Cerebral ischemia-induced apoptosis and necrosis in normal and diabetic rats: effects of insulin and C-peptide. Brain Res. 2006;1096:204–212. doi: 10.1016/j.brainres.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 102.Kubes P, Suzuki M, Granger DN. Nitric oxide: An endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gidday JM, Park TS, Shah AR, et al. Modulation of basal and postischemic leukocyte-endothelial adherence by nitric oxide. Stroke. 1998;29:1423–1429. doi: 10.1161/01.str.29.7.1423. [DOI] [PubMed] [Google Scholar]
- 104.Baron M. An overview of the Notch signaling pathway. Semin Cell Dev Biol. 2003;14:113–119. doi: 10.1016/s1084-9521(02)00179-9. [DOI] [PubMed] [Google Scholar]
- 105.Bray SJ. Notch signaling: A simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 106.Arumugam TV, Chan SL, Jo DG, et al. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nat Med. 2006;12:621–623. doi: 10.1038/nm1403. [DOI] [PubMed] [Google Scholar]
- 107.Schneider D, Berrouschot J, Brandt T, et al. Safety, pharmacokinetics and biological activity of enlimomab (anti-ICAM-1 antibody): an open-label, dose escalation study in patients hospitalized for acute stroke. Eur Neurol. 1998;40:78–83. doi: 10.1159/000007962. [DOI] [PubMed] [Google Scholar]
- 108.Enlimomab Acute Stroke Trial Investigators. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57:1428–1434. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- 109.Vuorte J, Lindsberg PJ, Kaste M, et al. Anti-ICAM-1 monoclonal antibody R6.5 (Enlimomab) promotes activation of neutrophils in whole blood. J of Immun. 1999;162:2353–2357. [PubMed] [Google Scholar]
- 110.Zhang R, Powers C, Zhang Z, et al. Infusion of intercellular adhesion molecule 1 antibody (18h) upregulates E-and P-selectin expression during focal embolic cerebral ischemia in rats [abstract] Stroke. 1998;29:282. [Google Scholar]
- 111.Furuya K, Takeda H, Azhar S, et al. Examination of several potential mechanisms for the negative outcome in a clinical stroke trial of enlimomab, a murine anti-human intercellular adhesion molecule-1 antibody: a bedside-to-bench study. Stroke. 2001;32:2665–2674. doi: 10.1161/hs3211.098535. [DOI] [PubMed] [Google Scholar]
- 112.Becker KJ. Anti-leukocyte antibodies: LeukArrest (Hu23F2G) and Enlimomab (R6.5) in acute stroke. Curr Med Res Opin. 2002;18(Suppl 2):18–22. doi: 10.1185/030079902125000688. [DOI] [PubMed] [Google Scholar]
- 113.Jiang N, Chopp M, Chahwala S. Neutrophil inhibitory factor treatment of focal cerebral ischemia in the rat. Brain Res. 1998;788:25–34. doi: 10.1016/s0006-8993(97)01503-5. [DOI] [PubMed] [Google Scholar]
- 114.Krams M, Lees KR, Hacke W, et al. Acute Stroke Therapy by Inhibition of Neutrophils (ASTIN). An Adaptive Dose-Response Study of UK-279, 276 in Acute Ischemic Stroke. Stroke. 2003;34:2543–2548. doi: 10.1161/01.STR.0000092527.33910.89. [DOI] [PubMed] [Google Scholar]
- 115.Yenari MA, Kunis D, Sun GH, et al. Hu23F2G, an antibody recognizing the leukocyte CD11/CD18 integrin, reduces injury in a rabbit model of transient focal cerebral ischemia. Exp Neurol. 1998;153:223–233. doi: 10.1006/exnr.1998.6876. [DOI] [PubMed] [Google Scholar]
- 116.Sughrue ME, Mehra A, Connolly ES, Jr, et al. Anti-adhesion molecule strategies as potential neuroprotective agents in cerebral ischemia: a critical review of the literature. Inflamm Res. 2004;53:497–508. doi: 10.1007/s00011-004-1282-0. [DOI] [PubMed] [Google Scholar]
- 117.Iadecola C, Alexander M. Cerebral ischemia and inflammation. Curr Opin Neurol. 2001;14:89–94. doi: 10.1097/00019052-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 118.Ziv Y, Finkelstein A, Geffen Y, et al. A Novel Immune-Based Therapy for Stroke Induces Neuroprotection and Supports Neurogenesis. Stroke. 2007;38:774–782. doi: 10.1161/01.STR.0000255784.27298.23. [DOI] [PubMed] [Google Scholar]
- 119.Yilmaz G, Vital S, Stokes KY, et al. Recruitment of bone marrow stromal cells in the cerebral microvasculature after focal ischemia-reperfusion is mediated by selectins. FASEB J. 2007;21:913.5. [Meeting Abstract] [Google Scholar]