Abstract
Obesity is one of the most significant, and potentially most preventable, risk factors for the development of osteoarthritis, and numerous studies have shown a strong association between body mass index and osteoarthritis of the hip, knee, foot, and hand. However, the mechanism(s) by which obesity contributes to the onset and progression of osteoarthritis are not fully understood. The strong association between body mass index, altered limb alignment, and osteoarthritis of the knee—and the protective effects of weight loss—support the classic hypothesis that the effects of obesity on the joint are due to increased biomechanical loading and associated alterations in gait. However, obesity is now considered to be a low-grade systemic inflammatory disease, and recent studies suggest that metabolic factors associated with obesity alter systemic levels of pro-inflammatory cytokines that are also associated with osteoarthritis. Thus, the ultimate influence of obesity on osteoarthritis may involve a complex interaction of genetic, metabolic, and biomechanical factors. In this respect, mouse models of obesity can provide excellent systems in which to examine causal relationships among these factors. In recent years, there have been surprisingly few reports examining the effects of obesity on osteoarthritis using mouse models. In this paper, we review studies on mice and other animal models that provide both direct and indirect evidence on the role of obesity and altered diet in the development of osteoarthritis. We also examine the use of different body mass indices for characterizing “obesity” in mice by comparing these indices to typical adiposity levels observed in obese humans. Taken together, evidence from studies using mice suggest that a complex interaction of environmental and genetic factors associated with obesity contribute to the incidence and severity of osteoarthritis. The ability to control these factors, together with the development of methods to conduct more intricate measures of local biomechanical factors, make mouse models an excellent system to study obesity and osteoarthritis.
Keywords: Inflammation, Adiposity, Animal Models, High Fat Diet, Body Mass Index, Guinea pig, Adipokine, Leptin
1. Obesity and Osteoarthritis in Humans
Obesity is one of the largest, and perhaps most preventable, risk factors for the development of osteoarthritis (OA) [1]. Numerous studies have shown a positive association between body mass index (BMI – body mass divided by height squared) and OA in weight-bearing joints, such as the hip, knee, and foot [2–7], as well as in non-weight-bearing joints, such as those in the hand [6]. Because obesity precedes the development of OA [3, 8], obesity itself, or a factor associated with it, is causally implicated in the initiation of the degenerative changes. However, the mechanism(s) by which obesity contributes to the onset and progression of OA are not fully understood. Historically, it has been hypothesized that the effects of obesity on the joint are predominantly due to increased biomechanical loading and associated alterations in gait [9–12]. However, a growing collection of data suggest that metabolic factors associated with obesity may alter systemic levels of pro-inflammatory cytokines that are also associated with OA, and obesity is now considered to be a low-grade systemic inflammatory disease [13–17]. Thus, the ultimate influence of obesity on OA may involve a complex interaction of biomechanical and inflammatory factors [18, 19]
Efforts to identify the causal link between obesity and OA have tended to focus on the strong association between these factors in the knee joint. Prospective studies consistently show that obesity (defined as a BMI greater than 30 kg/m2) increases the relative risk of developing knee OA by 2–10 fold [3, 6, 7]. Obesity is associated with changes in local biomechanical factors that impair joint stability, shift load bearing to less frequently loaded regions, and increase loading magnitude in these regions that eventually develop OA. Quadriceps muscle weakness, which is associated with knee OA, instability, and disability [20], is also a risk factor for developing incident, but not progressive, knee OA in older obese women [21, 22]. Limb alignment—the varus-valgus angle formed by the mechanical axis of the femur and tibia in the coronal plane—influences medial-vs-lateral joint loading and is implicated as a mediating factor in the development of knee OA in obese individuals. Sharma et al. [23] showed that BMI was correlated with OA severity in patients with varus, but not valgus, aligned limbs. They also showed that BMI correlated with varus, but not valgus, malaligned limbs. Consequently, the partial correlation between BMI and OA severity was almost entirely accounted for by varus limb malalignment. Moreover, a recent prospective study by Felson et al. [24] showed that the effect of BMI on progression of OA is dependent on limb alignment. Obesity increased OA progression in people with moderate malalignment, but not in people with neutral alignment or severe malalignment. These studies suggest that local biomechanical factors mediate both the onset and progression of knee OA associated with obesity.
In contrast, evidence for a systemic metabolic or molecular correlate of obesity and OA is scant. Apart from Hart et al. [25], few population-based studies have identified an independent association between a systemic metabolic or molecular correlate of obesity and OA (e.g., serum cholesterol, glucose, lipids, uric acid, blood pressure, or body fat distribution) [26–32]. Recent studies, however, suggest that inflammatory molecules secreted from adipose tissue may provide a critical link between obesity and OA.
2. Obesity, Inflammation, and Osteoarthritis
Obesity is now characterized as a mild, chronic inflammatory disease with the discovery that activated macrophages within adipose tissue [15, 33, 34] produce cytokines and cytokine-like molecules termed adipokines that may act on other cells and tissues throughout the body. Recent studies have focused on one adipokine in particular—leptin—as an important molecular mediator of obesity and OA. In people with severe OA, synovial fluid leptin concentrations are greater than serum concentrations and are significantly correlated with BMI [35–37]. Similarly, leptin gene expression is correlated with BMI in severely arthritic cartilage [37]. Joint leptin levels are also greater in women, consistent with their higher risk of developing OA with increasing age [36]. Perhaps the most intriguing role of leptin in particular, and adipokines in general, are their ability to mediate both metabolic and inflammatory processes [38, 39].
The integrated nature of metabolic and inflammatory signaling pathways is well illustrated by the altered metabolic phenotypes observed in cytokine-deficient transgenic mice. Targeted deletions of a number of pro-inflammatory cytokines that are secreted by adipose tissue and over-expressed in obesity, including interleukin-6 (IL-6), IL-6/IL-1, IL-18 (an IL-1 family member), and leptin (an IL-6 family member), lead to adult obesity phenotypes [40–44]. Central or peripheral administration of IL-6, IL-1β, IL-18, or leptin reduce feeding and induce weight-loss, consistent with a pro-anorectic role for these cytokines [42, 45–47]. Moreover, targeted deletions of the receptors to IL-1 and leptin produce obese phenotypes [48, 49] while deletion of IL-1 receptor antagonist (IL-1Ra) produces a lean, low body fat phenotype [50, 51]. The IL-1 and IL-6 cytokine families signal through signal transducer and activator of transcription 3 (STAT3) and/or Toll-like receptor/myeloid differentiation factor 88 (MyD88) [52, 53]. Mice with inactive STAT3, but not those with inactive MyD88 [54], are hyperphagic and develop obesity [55], suggesting that the STAT3 signaling pathway may mediate the integrated metabolic and inflammatory effects of these cytokines. These transgenic mouse models offer an exciting opportunity to investigate the role of adipose-secreted inflammation in the development of OA with obesity. In particular, a recent study of IL-6 knockout mice showed that male, but not female, knockout mice developed greater OA with age compared to wild-type mice [56]. Surprisingly, though, relatively few studies have utilized mouse models of obesity to study the etiopathogenesis of OA.
3. Obese mouse models of OA: Missing in action?
Despite obesity being one of the strongest and most well-established non-traumatic risk factors for the development of OA, the use of obese mouse models to study the etiopathogenesis of OA is nearly absent. Since 1950, there have been approximately 17,000 research articles published on OA, many of which were published within the past 20 years (Fig. 1; see figure legend for search details). A similar trend is also evident for studies of obesity and OA. The two most highly-cited papers on obesity and OA [3, 57], which together have been cited over 600 times as of September 2007, have ushered in an era of exponential growth in the number of articles published on obesity and OA (Fig. 1). This era witnessed a similar growth in the number of articles using mice to study OA (Fig. 1). However, of the approximated 600 articles on obesity and OA or mouse studies of OA, fewer than 10 articles are returned with search topics on obesity, mice, and OA (Fig. 1).
Figure 1.
Research on obese mouse models of OA remains flat despite a dramatic increase in research on obesity and OA and mouse models of OA. The main graph shows the number of research articles retrieved by a Web of Science® (ISI Web of KnowledgeSM, v.4.0) search for the following sets of terms: osteoarthritis=“osteoarthritis OR osteoarthrosis OR ‘degenerative joint disease’”, obesity=“obesity OR fat OR lard”, and mice=“mouse OR mice OR murine”. The inset graph displays the number of research articles retrieved for the previously described osteoarthritis search terms compared to the osteoarthritis plus obesity search terms. These data show that research in OA has taken off dramatically since 1990. Databases=Science Citation Index Expanded (SCI-EXPANDED); Social Sciences Citation Index (SSCI); Arts & Humanities Citation Index (A&HCI). Search conducted in September 2007 by TMG.
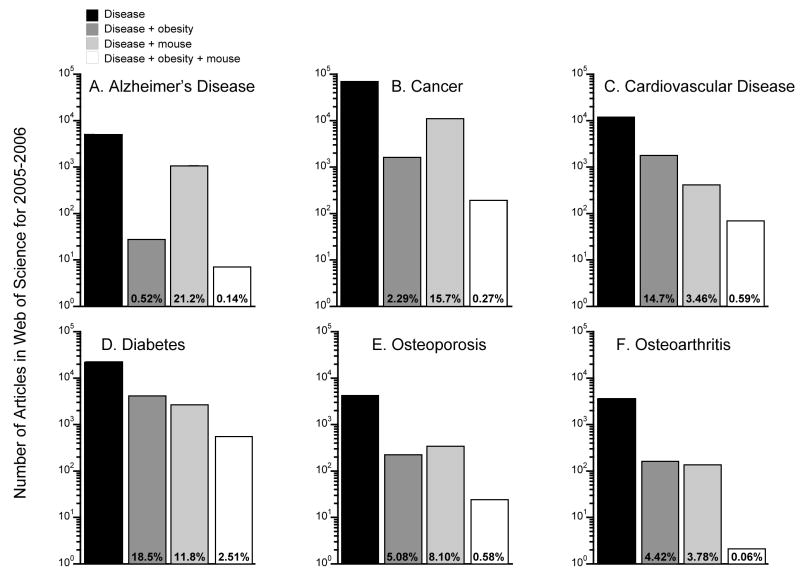
The dearth of articles on mouse models of obesity and OA is also evident when the results for OA are compared to other diseases. Figure 2 shows the number of citations for research articles published from 2005–2006 on a range of disease topics. Results are tabulated according to the total number of articles on the disease alone or in combination with keywords for obesity, mouse, or obesity plus mouse (see Figure 2 legend for details). Among the various diseases shown—Alzheimer’s, cancer, cardiovascular disease, diabetes, osteoporosis, and OA—OA had the fewest articles retrieved by the combined obesity, mouse, and OA search; only 2 articles were retrieved with the combined search, which equaled 0.056% of the total OA articles retrieved during this time period. For comparison, the percent of total studies on mouse models and obesity for the other diseases ranged from 0.14% (Alzheimer’s) to 2.5% (diabetes). Even osteoporosis, which is negatively associated with obesity, had more than 10-times as many articles on mouse models and factors associated with obesity compared to OA. Why are there so few studies using obese mouse models of OA? It may be due to several studies from the 1960’s and 1970’s showing negative results relating body weight and OA in mice.
Figure 2.
Research utilizing obese mouse models is much less common in the field of OA compared to other chronic diseases. The graphs show the number of articles retrieved by the Web of Science® (ISI Web of KnowledgeSM, v.4.0) using the following search terms: “Alzheimer’s”, “Cancer”, “Cardiovascular disease OR CVD”, “Diabetes”, “Osteoporosis”, and “Osteoarthritis.” These disease search terms were also combined with the search terms for “obesity” and “mouse” described in the legend for Figure 1. Search databases and search dates were the same as reported in Figure 1. Note: the Y-axis is a log-scale.
4. Experimental mouse studies of obesity and OA
Sokoloff and colleagues investigated the relationship between experimental obesity and OA in mice by altering diet. In one study, three strains of mice (DBA/2JN, C57L/HeN, and STR/N) and one strain of rat (Osborne-Mendel) were fed several different high-fat diets [58]. They found that the highest percent fat diet—60% vegetable fat by weight—caused OA in 2 strains of mice and in the rat. Weight-gain induced with lower fat diets—a 37.4% fat diet or a diet with 18% fat that produces overeating by restricting water consumption to water provided in the diet—did not increase the incidence of OA. DBA/2JN mice, which did not develop OA when fed the 60% vegetable fat diet [58], were similarly unaffected by diets high in saturated fats (e.g., lard, safflower oil, and cottonseed oil), despite developing significant increases in body fat and body [59]. It is not known why this strain of mice is resistant to developing OA when fed a high-fat diet. In a “reverse” experiment to the obesity-inducing diets, Sokoloff and colleagues investigated how reducing the caloric density of the standard chow diet affected the development of OA in spontaneous OA and obesity prone STR/N mice. The altered diet reduced body mass by approximately 12%, but it did not reduce the incidence of OA [58].
STR/ort mice, an inbred sub-strain of STR/N mice, continue to be a valuable animal model of spontaneous OA [60]. The strain’s spontaneous obesity suggests that the excess body weight, or a factor associated with it, may play a role in the accelerated development of OA in this strain. However, the lack of correlation between OA and naturally varying or diet-altered body weight suggests that body weight itself is not a causal factor in the strain’s spontaneous development of OA [58, 60, 61]. The presence of degenerative changes in the temporomandibular joint of STR/ort mice suggests that the basis of the disease is systemic [62]. Future studies of systemic inflammatory changes in STR/ort mice may benefit from considering the role of adipokines in the development of spontaneous OA.
5. High-fat diet-induced mouse models of obesity and OA in C57BL mice
In a series of studies extending from the 1940’s-1970’s, Drs. Martin and Ruth Silberberg examined the effects of a high-fat diet on the development of OA in mice. Their choice of mouse strain—C57BL—could not have been more fortuitous as it has become a widely used inbred mouse strain for studying mouse models of human disease. C57BL/6 mice are susceptible to high-fat diet-induced obesity, and as a result of high-fat feeding they develop many pathological changes associated with human metabolic syndrome, such as abdominal obesity, hyperglycemia, hyperinsulinemia, and hypertension [63]. When fed a high-fat diet (30% lard by weight), C57BL mice develop approximately a 2-fold increase in the incidence of knee OA [64–66]. The increased incidence of OA is due to an acceleration of the onset of degenerative changes rather than an increase in the rate of progression (Fig. 3). Low-fat and high-fat diet fed animals that developed OA had significantly increased body weights compared to diet-matched mice, supporting a link between body weight and OA. However, low-fat fed animals that developed OA and high-fat fed animals that did not develop OA had similar body weights, indicating that weight alone was not the causal factor in etiology of high-fat diet-induced OA [64].
Figure 3.
Feeding male C57BL mice a high-fat diet increases the incidence of knee OA. The similar slopes of OA incidence versus age between the high-fat and normal chow fed mice indicate that a high-fat diet accelerates the onset, but not the progression, of OA. The age at which ~50% of the mice developed OA was shifted earlier by about 8 months in the high-fat fed mice. Data shown are from papers by the Silberbergs [64–66]. OA was defined as a histological grade of either a 2 or 3 on a 0–3 scale. A grade of 2 indicates “advanced age changes with marked degeneration and liquefaction of cartilage cells and matrix and roughening of the surface of the joint” [65]. A grade of 3 indicates “proliferation and hypertrophy of the articular tissues, ulceration of the cartilage and eburnation of the bone at the floor of such ulcers, fibrosis and cyst formation in the bone marrow with involvement of ligaments and synovialis” [65].
To further elucidate the role of excess body weight versus a high-fat diet in initiating the development of knee OA in mice, Silberberg [67] induced hyperphagia by administering aurothioglucose to chemically-lesion the satiety center of the hypothalamus. Overeating the standard chow diet led to about a 65% increase in body weight by 6–9 months of age. By 15 months, however, there was no evidence of increased OA, suggesting that a high-fat diet, not excessive caloric intake, is critical for the development of diet-induced OA in C57BL mice. Further studies showed that lard-based high-fat diets induced greater degenerative changes in knees compared to vegetable-oil based diets (e.g., cottonseed oil; [68]). Supplementing the lard-based diets with linoleic acid, a polyunsaturated fatty acid, reduced the severity of degenerative knee changes [69]. These results suggest that diets high in saturated fatty acids, and in particular lard, mediate the diet-induced susceptibility to obesity and OA observed in C57BL mice.
6. Obesity and body composition in mice
Obesity is typically defined in humans based on a BMI ≥30. For mice, however, there is no typical metric used to define obesity. We investigated the relationship between various body mass indices and body adiposity using body composition and anatomical data collected during several of our studies using diet-induced and leptin impaired transgenic mouse models of obesity. We then sought to identify values for these indices that are comparable to a typical body adiposity observed in humans with a BMI of 30.
Female C57BL/6J, ob/ob (B6.V-Lepob/J), and db/db (B6.Cg-m +/+ Leprdb/J) mice were purchased from the Jackson Laboratories and housed in the Duke University Vivarium according to an approved IACUC protocol. All C57BL/6J mice were fed either a 10% kcal fat or 45% kcal fat diet for at least 36 wks before body composition and anatomical measurements were made at 44–54 wks of age. ob/ob and db/db mice (i.e., leptin deficient and leptin receptor deficient, respectively) were fed a 10% kcal fat diet, and body composition and anatomical measurements were made at 44 wks. Body fat content was measured in mice using a dual-energy X-ray absorptiometry system (PIXImus2 DEXA, Faxitron X-ray Corp.). Percent body fat was calculated as body fat mass, excluding the head, divided by total body mass. Femur length was measured three times using digital calipers, and the average femur length was used as the characteristic length value for calculating the various body mass indices.
Percent body fat was compared to four body mass indices: 1) body mass (g), 2) body mass divided by femur length (g/mm), 3) body mass divided by femur length squared (g/mm2), and 4) body mass divided by femur length cubed (g/mm3) (Figure 4). ob/ob and db/db mice have shorter femurs compared to wild-type controls [70]; therefore, regression analyses for C57BL/6J mice were performed separately from leptin impaired mice. For C57BL/6J mice, body mass divided by femur length cubed had the highest correlation with percent body fat (R=0.93) (Figure 4D). However, even body mass, the index with the poorest correlation, still had a correlation coefficient >0.90. For leptin impaired mice, body mass divided by femur length squared or cubed was also significantly correlated to percent body fat, although the correlation coefficients were lower than those for C57BL/6J mice (Figures 4C and 4D). These data suggest that of the various body mass indices tested, body mass divided by femur length cubed provides the best index of estimating percent body fat in C57BL/6J or leptin impaired transgenic mice.
Figure 4.
Percent body fat versus four different body mass indices in female C57BL/6J and leptin impaired mice that were 44–54 wks old. A. Percent body fat (%Fb) versus body mass (Mb). B. Percent body fat versus body mass divided by femur length (Lf). C. Percent body fat versus body mass divided by femur length squared. D. Percent body fat versus body mass divided by femur length cubed. The horizontal dashed lines are the percent body fat estimated for a 50 year old Caucasian woman with a BMI of 30. The vertical dashed lines indicate the index value for this percent body fat. Solid lines are least squares regressions, and statistics were calculated using JMP (v. 6.0).
We then determined how these four different body mass indices in mice compared to the BMI-based classification of human obesity. Within a large population of humans, body fat varies significantly at a given BMI depending on sex, age, and ethnicity [71, 72]. Using the Caucasian prediction equation presented in Table 2 from Deurenberg et al. [72], we estimated that a 50 year old woman with a BMI of 30 would have approximately 41% body fat. All of the leptin impaired obese mice had body fat levels greater than 41%, and some of the C57BL/6J mice fed a high-fat diet also had body fat levels greater than 41%. The indices corresponding to this level of body fat (41%) are noted by the vertical dotted lines in Figure 4. For 44–54 wk-old female C57BL/6J mice fed either a normal or high-fat diet, body mass is nearly equivalent to percent body fat (Figure 4A).
The data presented in Figure 4 provide a preliminary approach for quantifying and characterizing “obesity” in mice using an adaptation of BMI for mice. Ultimately, however, to develop a more relevant definition of obesity, there needs to be a better understanding of the relationship between body composition and impaired metabolic, motor, and/or immune function. Such a definition was recently proposed by Corvera and colleagues in a recent meeting summary of the 2006 Keystone conference on ‘Adipogenesis, obesity, and inflammation’ and ‘Diabetes mellitus and the control of cellular energy metabolism,’:
“Obesity is a degree of adiposity that causes impairment in normal motor or metabolic function… including (but not limited to) deregulation of adipokines and other products of adipocytes, a deregulation of inflammation in adipose tissue and other organs, involving the recruitment of macrophages and other immune cells, that ultimately leads to an accumulation of lipids in non-adipose tissue [73].”
7. Non-murine animal models of obesity and OA
Outside these early studies of obesity in mice, there have been few direct investigations of the relationship between obesity and OA using animal models. The Hartley guinea pig is a well-studied animal model of spontaneous OA [74]. They grow to be heavier than other guinea pig strains, suggesting that they could be an obese animal model of OA [58, 75]. In comparison to age-matched Strain 13 guinea pigs, which represent an OA-resistant strain, Hartley guinea pigs weigh significantly more and exhibit greater histologic joint damage as well as increased concentrations of OA biomarkers in the serum and knee synovial fluid [76, 77]. Importantly, caloric restriction greatly reduces body weight and also reduces the severity of OA in the knee joints [78].
Caloric restriction also mitigates the development of spontaneous OA in other species, such as dogs. Dogs fed a restricted diet equivalent to 75% of the ad libitum caloric intake had a lower prevalence and a later onset of hip joint OA [79, 80]. The restricted diet doubled the median age for the onset of OA from 6 yrs to 12 yrs of age compared to ad libitum fed dogs [80]. Caloric restriction also delayed and reduced the development of OA in broiler strain fowls [81, 82]. These broad inter-species findings that caloric restriction reduces the incidence of OA suggest that caloric restriction may be a generalized model of reducing spontaneous age-associated development of OA.
8. Future Directions
Taken together, these findings suggest that a complex interaction of environmental and genetic factors associated with obesity may also contribute to the incidence and severity of OA. The ability to manipulate genetic factors through use of genetically modified mice provides important model systems for studying these interactions in vivo. It is now apparent that OA is not simply related to body weight in mice, but may be influenced by other factors such as diets high in saturated fats. The effects of diet and genetic factors on the development of obesity and OA may also be reflected in systemic concentrations of cytokines or OA biomarkers that are altered with weight gain and weight loss. The ability to control systemic and genetic factors, together with advancing methods to conduct more intricate measures of local biomechanical factors, make mouse models an excellent system to study obesity and OA.
Acknowledgments
Supported by the Arthritis Foundation and NIH grants AR51672, EB01630, AR50245, AG15768, AR48852 and AR48182. The authors thank Stephen Johnson and Chelsea He for assistance with data collection and analysis. They also thank Drs. Beverley Fermor, Virginia Kraus, William Kraus, Frank Keefe, Deborah Muoio, Lori Setton, Dan Schmitt, William Wetsel, and Zhen Yan for many insightful discussions.
References
- 1.Powell A, Teichtahl AJ, Wluka AE, Cicuttini FM. Obesity: a preventable risk factor for large joint osteoarthritis which may act through biomechanical factors. Br J Sports Med. 2005;39:4–5. doi: 10.1136/bjsm.2004.011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper C, Inskip H, Croft P, Campbell L, Smith G, McLaren M, Coggon D. Individual risk factors for hip osteoarthritis: obesity, hip injury, and physical activity. Am J Epidemiol. 1998;147:516–522. doi: 10.1093/oxfordjournals.aje.a009482. [DOI] [PubMed] [Google Scholar]
- 3.Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 4.Kellgren JH, Lawrence JS. Osteo-arthrosis and disk degeneration in an urban population. Ann Rheum Dis. 1958;17:388–397. doi: 10.1136/ard.17.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leveille SG, Guralnik JM, Ferrucci L, Hirsch R, Simonsick E, Hochberg MC. Foot pain and disability in older women. Am J Epidemiol. 1998;148:657–665. doi: 10.1093/aje/148.7.657. [DOI] [PubMed] [Google Scholar]
- 6.Oliveria SA, Felson DT, Cirillo PA, Reed JI, Walker AM. Body weight, body mass index, and incident symptomatic osteoarthritis of the hand, hip, and knee. Epidemiology. 1999;10:161–166. [PubMed] [Google Scholar]
- 7.Spector TD, Hart DJ, Doyle DV. Incidence and progression of osteoarthritis in women with unilateral knee disease in the general population: the effect of obesity. Ann Rheum Dis. 1994;53:565–568. doi: 10.1136/ard.53.9.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Body mass index in young men and the risk of subsequent knee and hip osteoarthritis. The American journal of medicine. 1999;107:542–548. doi: 10.1016/s0002-9343(99)00292-2. [DOI] [PubMed] [Google Scholar]
- 9.Gushue DL, Houck J, Lerner AL. Effects of childhood obesity on three-dimensional knee joint biomechanics during walking. J Pediatr Orthop. 2005;25:763–768. doi: 10.1097/01.bpo.0000176163.17098.f4. [DOI] [PubMed] [Google Scholar]
- 10.Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52:2026–2032. doi: 10.1002/art.21139. [DOI] [PubMed] [Google Scholar]
- 11.DeVita P, Hortobagyi T. Obesity is not associated with increased knee joint torque and power during level walking. J Biomech. 2003;36:1355–1362. doi: 10.1016/s0021-9290(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 12.Messier SP. Osteoarthritis of the knee and associated factors of age and obesity: effects on gait. Med Sci Sports Exerc. 1994;26:1446–1452. [PubMed] [Google Scholar]
- 13.Das UN. Obesity, metabolic syndrome X, and inflammation. Nutrition. 2002;18:430–432. doi: 10.1016/s0899-9007(01)00747-x. [DOI] [PubMed] [Google Scholar]
- 14.Lago F, Dieguez C, Gomez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine & growth factor reviews. 2007;18:313–325. doi: 10.1016/j.cytogfr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visser M. Higher levels of inflammation in obese children. Nutrition. 2001;17:480–481. doi: 10.1016/s0899-9007(01)00509-3. [DOI] [PubMed] [Google Scholar]
- 17.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 18.Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33:195–200. doi: 10.1097/00003677-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, Setton LA, Weinberg JB. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004:17–26. doi: 10.1097/01.blo.0000131233.83640.91. [DOI] [PubMed] [Google Scholar]
- 20.Hurley MV, Scott DL, Rees J, Newham DJ. Sensorimotor changes and functional performance in patients with knee osteoarthritis. Ann Rheum Dis. 1997;56:641–648. doi: 10.1136/ard.56.11.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandt KD, Heilman DK, Slemenda C, Katz BP, Mazzuca SA, Braunstein EM, Byrd D. Quadriceps strength in women with radiographically progressive osteoarthritis of the knee and those with stable radiographic changes. J Rheumatol. 1999;26:2431–2437. [PubMed] [Google Scholar]
- 22.Slemenda C, Heilman DK, Brandt KD, Katz BP, Mazzuca SA, Braunstein EM, Byrd D. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41:1951–1959. doi: 10.1002/1529-0131(199811)41:11<1951::AID-ART9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Sharma L, Lou C, Cahue S, Dunlop DD. The mechanism of the effect of obesity in knee osteoarthritis: the mediating role of malalignment. Arthritis Rheum. 2000;43:568–575. doi: 10.1002/1529-0131(200003)43:3<568::AID-ANR13>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 24.Felson DT, Goggins J, Niu J, Zhang Y, Hunter DJ. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum. 2004;50:3904–3909. doi: 10.1002/art.20726. [DOI] [PubMed] [Google Scholar]
- 25.Hart DJ, Doyle DV, Spector TD. Association between metabolic factors and knee osteoarthritis in women: the Chingford Study. J Rheumatol. 1995;22:1118–1123. [PubMed] [Google Scholar]
- 26.Abbate LM, Stevens J, Schwartz TA, Renner JB, Helmick CG, Jordan JM. Anthropometric measures, body composition, body fat distribution, and knee osteoarthritis in women. Obesity (Silver Spring) 2006;14:1274–1281. doi: 10.1038/oby.2006.145. [DOI] [PubMed] [Google Scholar]
- 27.Bagge E, Bjelle A, Eden S, Svanborg A. Factors associated with radiographic osteoarthritis: results from the population study 70-year-old people in Goteborg. J Rheumatol. 1991;18:1218–1222. [PubMed] [Google Scholar]
- 28.Dahaghin S, Bierma-Zeinstra SM, Koes BW, Hazes JM, Pols HA. Do metabolic factors add to the effect of overweight on hand osteoarthritis? The Rotterdam Study. Ann Rheum Dis. 2007;66:916–920. doi: 10.1136/ard.2005.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis MA, Ettinger WH, Neuhaus JM. The role of metabolic factors and blood pressure in the association of obesity with osteoarthritis of the knee. J Rheumatol. 1988;15:1827–1832. [PubMed] [Google Scholar]
- 30.Davis MA, Neuhaus JM, Ettinger WH, Mueller WH. Body fat distribution and osteoarthritis. Am J Epidemiol. 1990;132:701–707. doi: 10.1093/oxfordjournals.aje.a115711. [DOI] [PubMed] [Google Scholar]
- 31.Hochberg MC, Lethbridge-Cejku M, Scott WW, Jr, Reichle R, Plato CC, Tobin JD. The association of body weight, body fatness and body fat distribution with osteoarthritis of the knee: data from the Baltimore Longitudinal Study of Aging. J Rheumatol. 1995;22:488–493. [PubMed] [Google Scholar]
- 32.Martin K, Lethbridge-Cejku M, Muller DC, Elahi D, Andres R, Tobin JD, Hochberg MC. Metabolic correlates of obesity and radiographic features of knee osteoarthritis: data from the Baltimore Longitudinal Study of Aging. J Rheumatol. 1997;24:702–707. [PubMed] [Google Scholar]
- 33.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dumond H, Presle N, Terlain B, Mainard D, Loeuille D, Netter P, Pottie P. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003;48:3118–3129. doi: 10.1002/art.11303. [DOI] [PubMed] [Google Scholar]
- 36.Presle N, Pottie P, Dumond H, Guillaume C, Lapicque F, Pallu S, Mainard D, Netter P, Terlain B. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthritis Cartilage. 2006;14:690–695. doi: 10.1016/j.joca.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Simopoulou T, Malizos KN, Iliopoulos D, Stefanou N, Papatheodorou L, Ioannou M, Tsezou A. Differential expression of leptin and leptin’s receptor isoform (Ob-Rb) mRNA between advanced and minimally affected osteoarthritic cartilage; effect on cartilage metabolism. Osteoarthritis Cartilage. 2007 doi: 10.1016/j.joca.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 38.Otero M, Lago R, Gomez R, Lago F, Gomez-Reino JJ, Gualillo O. Leptin: a metabolic hormone that functions like a proinflammatory adipokine. Drug News Perspect. 2006;19:21–26. doi: 10.1358/dnp.2006.19.1.966243. [DOI] [PubMed] [Google Scholar]
- 39.Otero M, Gomez Reino JJ, Gualillo O. Synergistic induction of nitric oxide synthase type II: in vitro effect of leptin and interferon-gamma in human chondrocytes and ATDC5 chondrogenic cells. Arthritis Rheum. 2003;48:404–409. doi: 10.1002/art.10811. [DOI] [PubMed] [Google Scholar]
- 40.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nature medicine. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 41.Chida D, Osaka T, Hashimoto O, Iwakura Y. Combined interleukin-6 and interleukin-1 deficiency causes obesity in young mice. Diabetes. 2006;55:971–977. doi: 10.2337/diabetes.55.04.06.db05-1250. [DOI] [PubMed] [Google Scholar]
- 42.Zorrilla EP, Sanchez-Alavez M, Sugama S, Brennan M, Fernandez R, Bartfai T, Conti B. Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc Natl Acad Sci U S A. 2007;104:11097–11102. doi: 10.1073/pnas.0611523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, Tack CJ, van Krieken H, Kim SH, Stalenhoef AF, van de Loo FA, Verschueren I, Pulawa L, Akira S, Eckel RH, Dinarello CA, van den Berg W, van der Meer JW. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nature medicine. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 45.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 46.Hellerstein MK, Meydani SN, Meydani M, Wu K, Dinarello CA. Interleukin-1-induced anorexia in the rat. Influence of prostaglandins. J Clin Invest. 1989;84:228–235. doi: 10.1172/JCI114145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jansson JO, Wallenius K, Wernstedt I, Ohlsson C, Dickson SL, Wallenius V. On the site and mechanism of action of the anti-obesity effects of interleukin- 6. Growth Horm IGF Res, 13 Suppl A. 2003:S28–32. doi: 10.1016/s1096-6374(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 48.Garcia MC, Wernstedt I, Berndtsson A, Enge M, Bell M, Hultgren O, Horn M, Ahren B, Enerback S, Ohlsson C, Wallenius V, Jansson JO. Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes. 2006;55:1205–1213. doi: 10.2337/db05-1304. [DOI] [PubMed] [Google Scholar]
- 49.Chua SC, Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 50.Hirsch E, Irikura VM, Paul SM, Hirsh D. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc Natl Acad Sci U S A. 1996;93:11008–11013. doi: 10.1073/pnas.93.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Somm E, Henrichot E, Pernin A, Juge-Aubry CE, Muzzin P, Dayer JM, Nicklin MJ, Meier CA. Decreased fat mass in interleukin-1 receptor antagonist-deficient mice: impact on adipogenesis, food intake, and energy expenditure. Diabetes. 2005;54:3503–3509. doi: 10.2337/diabetes.54.12.3503. [DOI] [PubMed] [Google Scholar]
- 52.Li X, Qin J. Modulation of Toll-interleukin 1 receptor mediated signaling. Journal of molecular medicine (Berlin, Germany) 2005;83:258–266. doi: 10.1007/s00109-004-0622-4. [DOI] [PubMed] [Google Scholar]
- 53.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 54.Ogimoto K, Harris MK, Jr, Wisse BE. MyD88 is a key mediator of anorexia, but not weight loss, induced by lipopolysaccharide and interleukin-1 beta. Endocrinology. 2006;147:4445–4453. doi: 10.1210/en.2006-0465. [DOI] [PubMed] [Google Scholar]
- 55.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 56.de Hooge AS, van de Loo FA, Bennink MB, Arntz OJ, de Hooge P, van den Berg WB. Male IL-6 gene knock out mice developed more advanced osteoarthritis upon aging. Osteoarthritis Cartilage. 2005;13:66–73. doi: 10.1016/j.joca.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 57.Felson DT, Zhang Y, Anthony JM, Naimark A, Anderson JJ. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann Intern Med. 1992;116:535–539. doi: 10.7326/0003-4819-116-7-535. [DOI] [PubMed] [Google Scholar]
- 58.Sokoloff L, Mickelsen O, Silverstein E, Jay GE, Jr, Yamamoto RS. Experimental obesity and osteoarthritis. Am J Physiol. 1960;198:765–770. doi: 10.1152/ajplegacy.1960.198.4.765. [DOI] [PubMed] [Google Scholar]
- 59.Sokoloff L, Mickelsen O. Dietary Fat Supplements, Body Weight and Osteoarthritis in Dba-2jn Mice. J Nutr. 1965;85:117–121. doi: 10.1093/jn/85.1.117. [DOI] [PubMed] [Google Scholar]
- 60.Mason RM, Chambers MG, Flannelly J, Gaffen JD, Dudhia J, Bayliss MT. The STR/ort mouse and its use as a model of osteoarthritis. Osteoarthritis Cartilage. 2001;9:85–91. doi: 10.1053/joca.2000.0363. [DOI] [PubMed] [Google Scholar]
- 61.Walton M. Obesity as an aetiological factor in the development of osteoarthrosis. Gerontology. 1979;25:36–41. doi: 10.1159/000212318. [DOI] [PubMed] [Google Scholar]
- 62.Dreessen D, Halata Z. Age-related osteo-arthrotic degeneration of the temporomandibular joint in the mouse. Acta Anat (Basel) 1990;139:91–96. doi: 10.1159/000146984. [DOI] [PubMed] [Google Scholar]
- 63.Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004;81:243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 64.Silberberg M, Silberberg R. Effects of a high fat diet on the joints of aging mice. AMA Arch Pathol. 1950;50:828–846. [PubMed] [Google Scholar]
- 65.Silberberg M, Silberberg R. Degenerative joint disease in mice fed a high-fat diet at various ages. Exp Med Surg. 1952;10:76–87. [PubMed] [Google Scholar]
- 66.Silberberg M, Silberberg R. Age factor and high-fat diets in the evolution of osteoarthritis in mice. J Gerontol. 1957;12:9–13. doi: 10.1093/geronj/12.1.9. [DOI] [PubMed] [Google Scholar]
- 67.Silberberg R. Obesity and joint disease. Gerontology. 1976;22:135–140. doi: 10.1159/000212130. [DOI] [PubMed] [Google Scholar]
- 68.Silberberg M, Silberberg R. Osteoarthrosis in mice fed diets enriched with animal or vegetable fat. Arch Pathol. 1960;70:385–390. [PubMed] [Google Scholar]
- 69.Silberberg M, Silberberg R, Orcutt B. Modifying effect of linoleic acid on articular aging and osteoarthrosis in lard-fed mice. Gerontologia. 1965;11:179–187. doi: 10.1159/000211491. [DOI] [PubMed] [Google Scholar]
- 70.Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34:376–383. doi: 10.1016/j.bone.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 71.Benn RT. Some mathematical properties of weight-for-height indices used as measures of adiposity. British journal of preventive & social medicine. 1971;25:42–50. doi: 10.1136/jech.25.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22:1164–1171. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 73.Corvera S, Burkart A, Kim JY, Christianson J, Wang Z, Scherer PE. Keystone meeting summary: ‘Adipogenesis, obesity, and inflammation’ and ‘Diabetes mellitus and the control of cellular energy metabolism, ‘ January 21–26, 2006, Vancouver, Canada. Genes Dev. 2006;20:2193–2201. doi: 10.1101/gad.1447506. [DOI] [PubMed] [Google Scholar]
- 74.Bendele AM, Hulman JF. Spontaneous cartilage degeneration in guinea pigs. Arthritis Rheum. 1988;31:561–565. doi: 10.1002/art.1780310416. [DOI] [PubMed] [Google Scholar]
- 75.Jimenez PA, Glasson SS, Trubetskoy OV, Haimes HB. Spontaneous osteoarthritis in Dunkin Hartley guinea pigs: histologic, radiologic, and biochemical changes. Lab Anim Sci. 1997;47:598–601. [PubMed] [Google Scholar]
- 76.Huebner JL, Hanes MA, Beekman B, TeKoppele JM, Kraus VB. A comparative analysis of bone and cartilage metabolism in two strains of guinea-pig with varying degrees of naturally occurring osteoarthritis. Osteoarthritis Cartilage. 2002;10:758–767. doi: 10.1053/joca.2002.0821. [DOI] [PubMed] [Google Scholar]
- 77.Huebner JL, Kraus VB. Assessment of the utility of biomarkers of osteoarthritis in the guinea pig. Osteoarthritis Cartilage. 2006;14:923–930. doi: 10.1016/j.joca.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 78.Bendele AM, Hulman JF. Effects of body weight restriction on the development and progression of spontaneous osteoarthritis in guinea pigs. Arthritis Rheum. 1991;34:1180–1184. doi: 10.1002/art.1780340916. [DOI] [PubMed] [Google Scholar]
- 79.Kealy RD, Lawler DF, Ballam JM, Lust G, Smith GK, Biery DN, Olsson SE. Five-year longitudinal study on limited food consumption and development of osteoarthritis in coxofemoral joints of dogs. J Am Vet Med Assoc. 1997;210:222–225. [PubMed] [Google Scholar]
- 80.Smith GK, Paster ER, Powers MY, Lawler DF, Biery DN, Shofer FS, McKelvie PJ, Kealy RD. Lifelong diet restriction and radiographic evidence of osteoarthritis of the hip joint in dogs. J Am Vet Med Assoc. 2006;229:690–693. doi: 10.2460/javma.229.5.690. [DOI] [PubMed] [Google Scholar]
- 81.Anderson-MacKenzie JM, Hulmes DJ, Thorp BH. Effects of body mass and genotype on avian degenerative joint disease pathology and articular cartilage proteoglycan distribution. Clin Exp Rheumatol. 1998;16:403–408. [PubMed] [Google Scholar]
- 82.Venkatesan N, Thorp BH, Hulmes DJ. Articular cartilage proteoglycan metabolism in avian degenerative joint disease: effects of strain selection and body weight. Connect Tissue Res. 1999;40:199–208. doi: 10.3109/03008209909005283. [DOI] [PubMed] [Google Scholar]