Abstract
Background and Purpose
While chemokines have been implicated in cardiovascular diseases, few studies have addressed the role of these inflammatory mediators in ischemic stroke. This study tested the hypothesis that RANTES (CCL5; regulated upon activation, normal T-cell expressed and secreted) mediates the cerebral microvascular dysfunction, inflammation and tissue injury induced by brain ischemia and reperfusion (I/R).
Methods
Following 60 minute middle cerebral artery occlusion and reperfusion (MCAO/R), the adhesion of leukocytes and platelets in cerebral venules, infarct volume and blood-brain barrier (BBB) permeability were measured in wild type mice (WT), RANTES-deficient mice (RANTES−/−), WT mice transplanted with RANTES−/− bone marrow (RANTES>WT) and control bone marrow chimeras (WT>WT). The concentration of RANTES and several cytokines was also measured by ELISA and a cytometric bead array.
Results
The enhanced leukocyte and platelet adhesion, increased BBB permeability, and tissue infarction elicited in WT and WT>WT mice after MCAO/R were significantly blunted in RANTES−/− mice. Similar attenuation of the MCAO/R-induced responses were noted in RANTES>WT chimeras. While RANTES deficiency did not alter the changes in tissue cytokine levels elicited by MCAO/R, plasma concentrations IL-6, IL-10 and IL-12 were all reduced.
Conclusions
These findings implicate blood cell-derived RANTES in the microvascular, inflammatory and tissue injury responses of the brain to ischemia and reperfusion.
Keywords: RANTES, cerebral infarct, platelets, cytokines, chemokines
Introduction
There is a growing body of evidence that implicates inflammatory cells and mediators in pathogenesis of ischemic stroke. A variety of cytokines and chemokines are produced by postischemic brain tissue and these mediators are thought to attract inflammatory cells to the site of injury through mechanisms that involve activation of endothelial cells and other circulating cells (e.g., platelets). Several cytokines, including TNF-α and IL-1β, have been shown to contribute to inflammatory cell recruitment, BBB dysfunction, and tissue injury in animal models of ischemic stroke.1 Chemokines, and their receptors, have also been implicated in pathogenesis of ischemic stroke. Patients suffering from a stroke exhibit elevated plasma levels of the chemokines MCP-1 and IL-8.2, 3 Studies in animal models have also revealed the same chemokines (e.g., MCP-1) contribute to the leukocyte recruitment, brain infarction and edema elicited by cerebral ischemia-reperfusion (I/R).4–6 While a role for other chemokines in stroke pathogenesis has been inferred based on the elevated plasma levels detected following a stroke, their potential contribution to this disease process has not been directly assessed.
RANTES (CCL5; regulated upon activation, normal T-cell expressed and secreted), a member of the CC-chemokine family, promotes the directed migration of leukocytes into damaged or inflamed tissue. Aggregates of RANTES that form on cell surfaces have been shown to act as powerful activators of leukocytes. While the chemotactic properties of RANTES are mediated through engagement with its receptor (CCR5), the leukocyte activation effect of the chemokine reflects an oligomerization-dependent interaction with cell surface glycosaminoglycans (GAGs).7, 8 RANTES is produced by a variety of cells, including Tlymphocytes, platelets, endothelial cells, smooth muscle cells, and glial cells. Although all of these cells likely contribute to the pathogenesis of ischemic stroke, it remains unclear whether RANTES produced by some or all of these cells mediates the inflammation, microvascular dysfunction and tissue injury induced by cerebral I/R.
The overall objective of this study was to assess the contribution of RANTES to brain inflammation and injury in an animal model of ischemic stroke. The recruitment of leukocytes and platelets, blood-brain barrier permeability, infarct size, and blood & tissue concentrations of different cytokines and chemokines were monitored and compared between wild type and RANTES-deficient mice. In order to assess the contribution of blood cells vs parenchymal cells to the RANTES-mediated injury responses, bone marrow chimera mice were created. Our findings strongly implicate this chemokine in the pathogenesis of experimental ischemic stroke and suggest that the RANTES that mediates the cerebral inflammation, blood-brain barrier dysfunction and tissue infarction is largely derived from circulating blood cells.
Methods
Animal preparation
Male C57BL/6J mice (wild type; WT) were obtained (n=20 for sham operation, n=31 for I/R) from Jackson Laboratories (Bar Harbor, ME). RANTES knockout mice (RANTES−/−) on a C57BL/6J background were originally generated by Danoff and coworkers.9 A breeding pair of RANTES−/− was obtained from Dr. Sally Sarawar in the Torrey Pines Institute for Molecular Studies, San Diego. A breeding colony was established in the animal resource facility of the Louisiana State University Health Sciences Center, Shreveport (n=28). The experimental procedures employed in this study were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC), and were in compliance with the guidelines of the National Institutes of Health.
Creating bone marrow chimera mice
Bone marrow (BM) cells, collected from the femurs and tibias of donor mice (WT and RANTES−/−), were injected (2 × 106 BM cells) via the femoral vein into recipient mice (congenic WT with same phenotype as C57BL/6J mice; B6.SJLPtprcaPep3b/BoyJ), following total-body irradiation sufficient to eliminate the recipients blood cells. The BM chimeras were kept in autoclaved cages, with 0.2% neomycin added to drinking water for the first 2 weeks. 6∼8weeks later, reconstitution of BM cells was verified by flow cytometry by testing for the % blood leukocytes positive for CD45.1 (recipient isoform of CD45) versus CD45.2 (donor isoform of CD45). Successful BM reconstitution was set as >90% of CD45.2-positive population in total leukocytes as previously described.10 Two BM chimeras were produced: (1) WT recipients receiving BM from RANTES−/− donor mice (RANTES>WT; n=24) and (2) WT recipients receiving BM from WT donor mice (WT>WT; n=18, as positive controls).
Middle cerebral artery occlusion (MCAO) and reperfusion (MCAO/R)
Mice were anesthetized with intraperitoneal ketamine (100mg/kg) and xylazine (10mg/kg). Transient (60 minutes) focal cerebral ischemia was induced by left MCAO using the intraluminal filament method. A 7–0 silicone coated nylon monofilament (Doccol Corporation, CA) was advanced via the external carotid artery. In the sham group, the arteries were visualized but not disturbed. After 60 min, the fiber was removed. MCAO/R was verified using a LASER Doppler flowmeter to monitor cerebral blood flow during ischemia and reperfusion. Core temperature was kept at 36°C. Percentage survival was calculated for mice allowed to reperfuse for 24 hours.
Intravital videomicroscopy
During these experiments, the blood pressure was monitored via a femoral artery and the blood gas was analyzed at the end. The procedures used to monitor blood cell-vessel wall interactions in murine cerebral venules are described elsewhere in detail.11 Briefly, at 4 hours after reperfusion, a craniectomy (1 mm posterior, 4 mm lateral from the bregma) was made under controlled ventilation. The craniectomy was soaked with artificial cerebrospinal fluid, and a glass coverslip was placed over it. An upright fluorescent microscope with 3CCD video camera system was used to observe randomly selected 100 µm segments of pial venules (25–50 µm diameter). 100 × 106 platelets from donor mice were labeled ex vivo with carboxyfluorescein diacetate succinimudyl ester.12 These green fluorescent platelets were administered to recipient mice followed by continuous infusion of 0.02% Rhodamine 6G, which labeled leukocytes red. Adherent leukocytes and platelets were defined as cells bound to venules for ≥ 30 and 2 seconds, respectively. Cell adhesion data was expressed as number of cells per mm2 of venular surface, calculated from venular diameter and length, assuming cylindrical geometry.
Infarction volume
After 24 hours of reperfusion, 1mm-thick coronal sections of the brain were immersed in 0.05% 2,3,5-triphenyltetrazolium chloride (TTC) solution for 30 minutes. The total areas of each brain section and the infarcted region were quantified with the software program, NIH image. Infarct volume was corrected for edema as previously described.13
Blood-brain barrier (BBB) dysfunction
A 2% solution of Evans blue (EB; Sigma-Aldrich, MO) was injected (4ml/kg) intravenously immediately following MCAO/R, or after sham operation. 24 hours later, the blood was obtained for plasma collection and the brain was sampled after transcardial perfusion with phosphate buffered saline (PBS; 100mmHg, 5 minutes). The cerebral hemispheres and the plasma were homogenized in 50% trichloroacetic acid, sonicated and then centrifuged. The supernatant was diluted with ethanol and the concentrations of EB in brain tissue and plasma were measured using a fluorescence spectrophotometer (FLUOstar Optima, BMG LABTECH, Inc., NC). BBB permeability was normalized by dividing tissue EB concentration (µg/g brain weight) by the plasma concentration (µg/ml).
RANTES in plasma and brain tissue
24 hours after reperfusion, the blood was sampled with citrate to prevent platelet activation, and plasma was obtained. Thereafter, the mice were transcardially perfused with PBS (without heparin) for 5 minutes. The brain samples were homogenized and sonicated in PBS containing protease inhibitors (Protease inhibitor cocktail, Sigma-Aldrich, MO), followed by centrifugation for collection of the supernatant. RANTES levels in brain tissue supernatant and plasma were measured using an ELISA kit (Quantikine for mouse RANTES, R&D systems, MN). In addition to the groups outlined above, a group of recombinase activating gene-1 deficient mice (Rag-1−/−; B6.129S7-Rag-1<tm1Mom>/J), which are deficient in both T- and B-lymphocytes, was added for this parameter (n=6). RANTES is produced by T-cells but not B-cells. Therefore, Rag1−/− mice were used to address whether Tcells could be a source of RANTES following MCAO/R.
Cytokines in plasma and brain tissue
24 hours after reperfusion, the blood was collected into heparin-coated syringe, and plasma was obtained. After a 5-minute transcardial perfusion of PBS, the brain hemispheres were homogenized, sonicated and centrifuged in PBS containing protease inhibitors. A cytometric bead array (Mouse Inflammation Kit, BD Biosciences, CA) was used to measure the concentration of six cytokines (IL-12, TNF-α, IFN-γ, MCP-1, IL-10, IL-6) and the cytokine concentrations were expressed as either pg/ml (plasma) or pg/g brain weight (brain).
Statistical analysis
All data were expressed as mean ± SE. Statistical difference between the different groups was determined by ANOVA with Fisher’s post-hoc test. A paired t-test was used to compare responses between right and left hemispheres. Evaluation of survival rate was performed with chi-squared analysis. Statistical significance was set as p<0.05.
Results
Physiological parameters
Table 1 shows the values of body weight, mean arterial blood pressure, blood pH, PaCO2, O2 saturation, and survival rate. There was no significant difference between groups for any parameter except survival rate, which was significantly lower in WT-I/R mice when compared to WT-sham and RANTES−/−-I/R groups.
Table 1.
Values for different physiological variables in sham-operated wild type mice (WT-sham), and wild type mice (WT-I/R), RANTES knockout mice (RANTES−/−), WT mice transplanted with WT bone marrow cells (WT>WT), and WT mice transplanted with RANTES−/− bone marrow cells (RANTES>WT) subjected to I/R.
| WT-sham | WT-I/R | RANTES−/− | WT>WT | RANTES>WT | |
|---|---|---|---|---|---|
| Body weight (g) | 23.7 ± 0.4 | 23.4 ± 0.5 | 25.9 ± 0.6 | 24.7 ± 0.6 | 23.5 ± 0.5 |
| Mean arterial blood pressure (mmHg) | 79.4 ± 3.2 | 82.1 ± 1.5 | 82.1 ± 6.4 | 77.4 ± 3.5 | 79.0 ± 4.6 |
| pH | 7.30 ± 0.05 | 7.35 ± 0.03 | 7.29 ± 0.06 | 7.29 ± 0.02 | 7.33 ± 0.03 |
| pCO2 (mmHg) | 38.0 ± 7.2 | 42.2 ± 3.8 | 41.3 ± 6.9 | 41.9 ± 1.2 | 43.0 ± 3.9 |
| O2 saturation (%) | 99.3 ± 0.1 | 99.4 ± 0.1 | 99.3 ± 0.3 | 99.2 ± 0.2 | 99.4 ± 0.1 |
| Survival rate (%) | 100 | 78.7* | 96.8† | 88.9 | 89.7 |
Data are expressed as mean±SE.
p<0.05 vs WT-sham;
p<0.05 vs WT-I/R by chi-squared analysis.
Blood cell-vessel wall interactions and blood cell-blood cell interactions
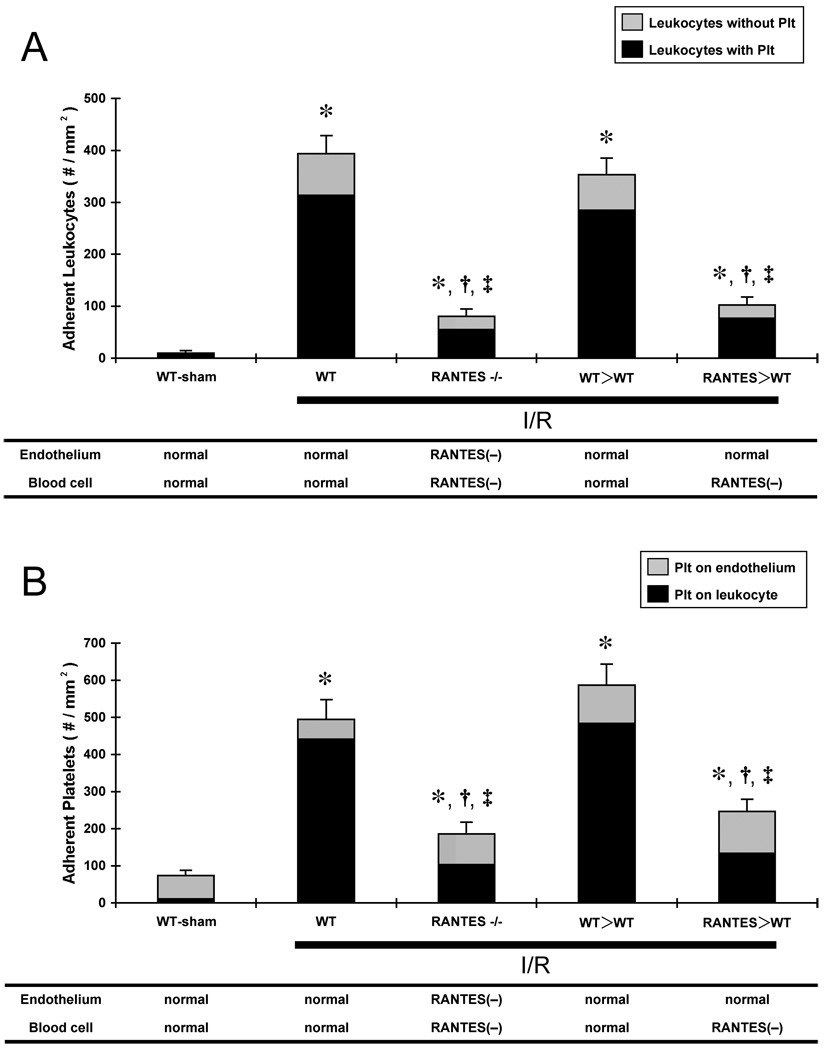
Figure 1 summarizes the blood cell-vessel wall interactions induced in cerebral venules following sham surgery (WT-sham), and at 4 hours of reperfusion following a 60 min ischemic challenge in WTI/R, RANTES−/−, WT>WT, and RANTES>WT mice. RANTES−/− and RANTES>WT mice exposed to MCAO/R exhibited significant reductions in the number of adherent leukocytes (panel A) and platelets (panel B) compared to WT-I/R and WT>WT-I/R mice. The magnitude of the reductions in adherent leukocytes and platelets were very similar for the RANTES−/− and RANTES>WT mice.
Figure 1.
Effects of ischemia-reperfusion (I/R) on the adhesion of leukocytes (panel A) and platelets (Plt) (panel B) in cerebral venules in wild type mice (WT), RANTES knockout mice (RANTES−/−), WT mice transplanted with WT bone marrow cells (WT>WT), and WT mice transplanted with RANTES−/− bone marrow cells (RANTES>WT). The black area in each bar reflects the proportion of leukocytes and platelets that are involved in leukocyte-platelet aggregation. The standard errors represent the total number of adherent leukocytes (A) or platelets (B). The tables beneath the panels show the predicted ability of vascular endothelium and blood cells to produce RANTES. (*: p<0.05 vs WT-sham, †: p<0.05 vs WT-I/R, ‡: p<0.05 vs WT>WT)
Infarct volume
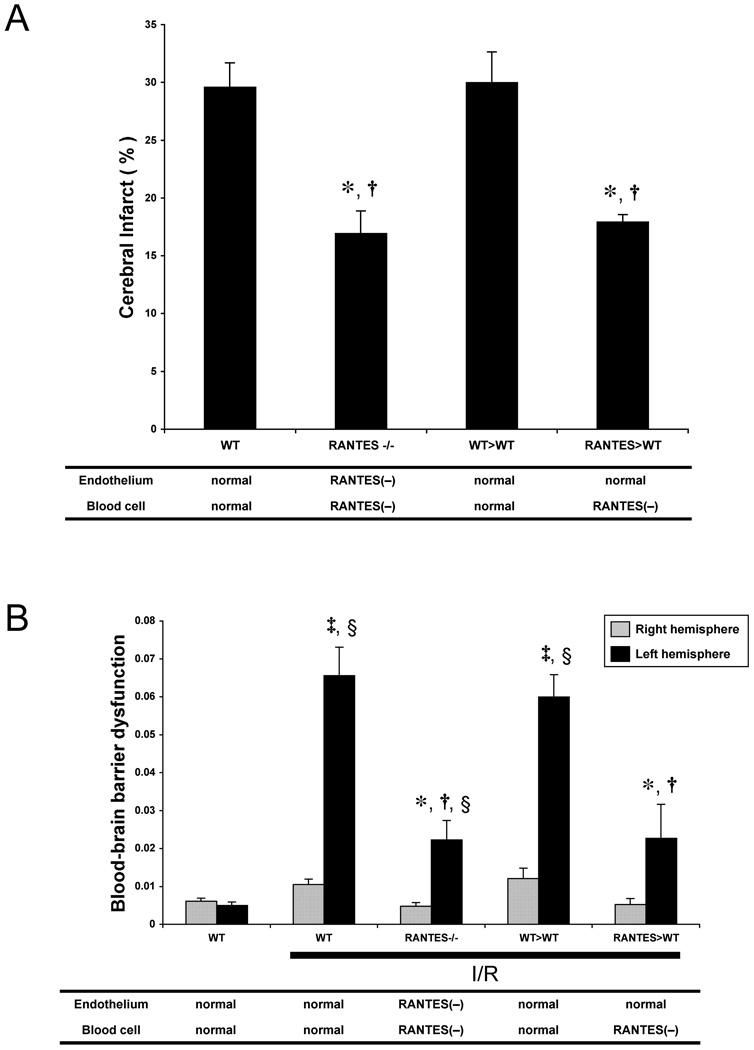
The brain infarct volumes in WT, RANTES−/−, WT>WT and RANTES>WT mice subjected to 60 min of MCAO and 24 hours of reperfusion are presented in Figure 2A. The infarct volumes in RANTES−/− (17.0±1.9%) and RANTES>WT (18.0±0.6%) were significantly smaller than in WT (29.7±2.0%) and WT>WT (30.0±2.6%) mice. RANTES−/− and RANTES>WT mice showed nearly identical reductions in infarct volume compared to control (WT & WT>WT) mice.
Figure 2.
Infarct volume (panel A) and blood-brain barrier permeability to Evans blue (panel B) induced by ischemia-reperfusion (I/R) in wild type mice (WT), RANTES knockout mice (RANTES−/−), WT mice transplanted with WT bone marrow cells (WT>WT), and WT mice transplanted with RANTES−/− bone marrow cells (RANTES>WT). The tables beneath the panels show the predicted ability of vascular endothelium and blood cells to produce RANTES. (*: p<0.05 vs WT-I/R, †: p<0.05 vs WT>WT, ‡: p<0.05 vs WT-sham, §: p<0.05 vs contralateral hemisphere in the same group)
Blood-brain barrier dysfunction
Figure 2B summarizes the changes in blood-brain barrier permeability to Evans blue (EB) induced by 60 min ischemia and 24 hours reperfusion in the different experimental groups. BBB permeability in the left (injured) hemisphere of WT mice (0.066±0.007) and WT>WT chimeras (0.060±0.006) after I/R was significantly elevated compared to sham-WT mice (0.005±0.001). RANTES−/− mice (0.022±0.005) and RANTES>WT chimeras (0.023±0.009) exhibited marked reductions in BBB permeability, compared to WT-I/R and WT>WT-I/R groups.
RANTES levels in brain tissue and plasma
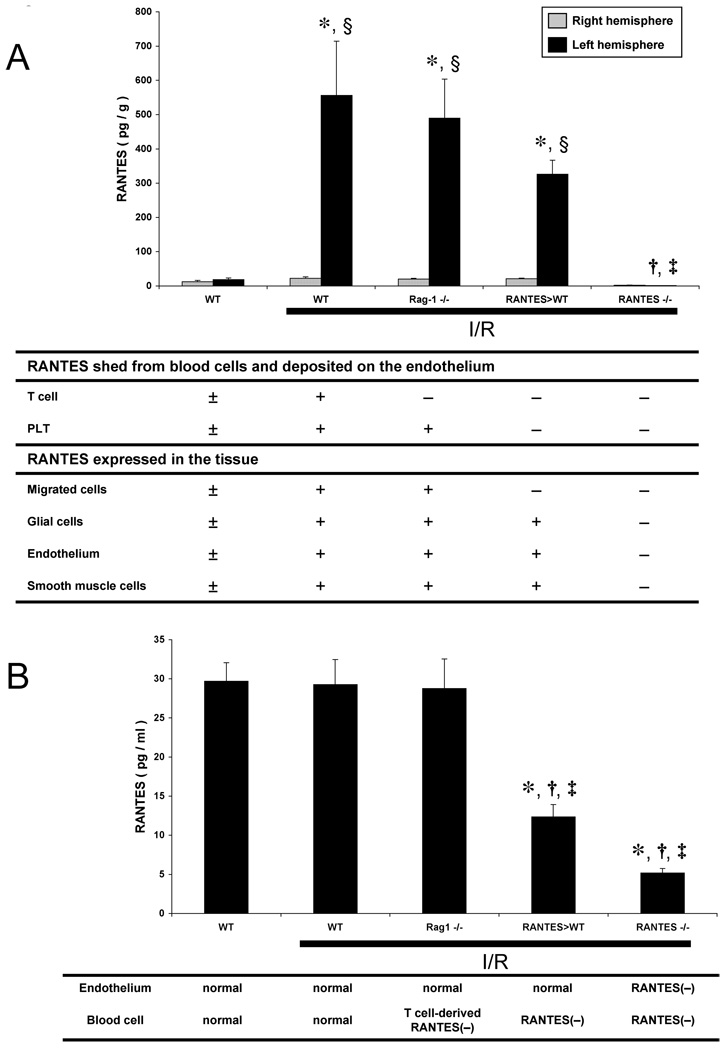
The objective of this series of experiments was to determine if RANTES levels in postischemic brain tissue (Figure 3A) and plasma (Figure 3B) are elevated following MCAO/R and whether blood cells are the major source of the RANTES accumulation elicited by I/R. To address the possibility that T-cells, a well-known source of RANTES, contribute to the I/R-induced chemokine accumulation, Rag-1 knockout mice (Rag-1−/−) were included in the analysis. Transcardial PBS perfusion of the brain under physiological pressure was applied to minimize the contribution of trapped blood to the tissue RANTES estimates without eliminating RANTES bound to the vascular endothelial surface. The tissue RANTES measurements reveal a significant accumulation of the chemokine after I/R, which did not differ statistically between WT, Rag-1−/−, and RANTES>WT groups. However, RANTES was not detected in brain tissue after I/R in RANTES−/− mice, suggesting that the tissue levels are largely derived from parenchymal cells, rather than circulating blood cells. However, estimates of RANTES concentration in plasma indicates that much of the chemokine appearing in this compartment is derived from circulating blood cells, since RANTES>WT mice exhibited a plasma concentration that was 42% of WT and Rag-1−/− mice. The comparable values for RANTES in Rag-1−/− and WT mice suggest that lymphocytes are an unlikely source of the chemokine in plasma. RANTES−/− mice exhibited an 82% reduction in plasma RANTES compared to WT mice.
Figure 3.
Brain tissue (panel A) and plasma (panel B) concentrations of RANTES following ischemia-reperfusion (I/R) in wild type mice (WT), Rag-1 knockout mice (Rag-1−/−: lymphocytedeficient mice), WT mice transplanted with RANTES−/− bone marrow cells (RANTES>WT), and RANTES knockout mice (RANTES−/−). The table beneath each panel lists the potential sources of RANTES in each group for either brain tissue or plasma. (*: p<0.05 vs WT-sham, †: p<0.05 vs WT-I/R, ‡: p<0.05 vs Rag-1−/−, §: p<0.05 vs contralateral hemisphere in the same group)
Plasma cytokine levels
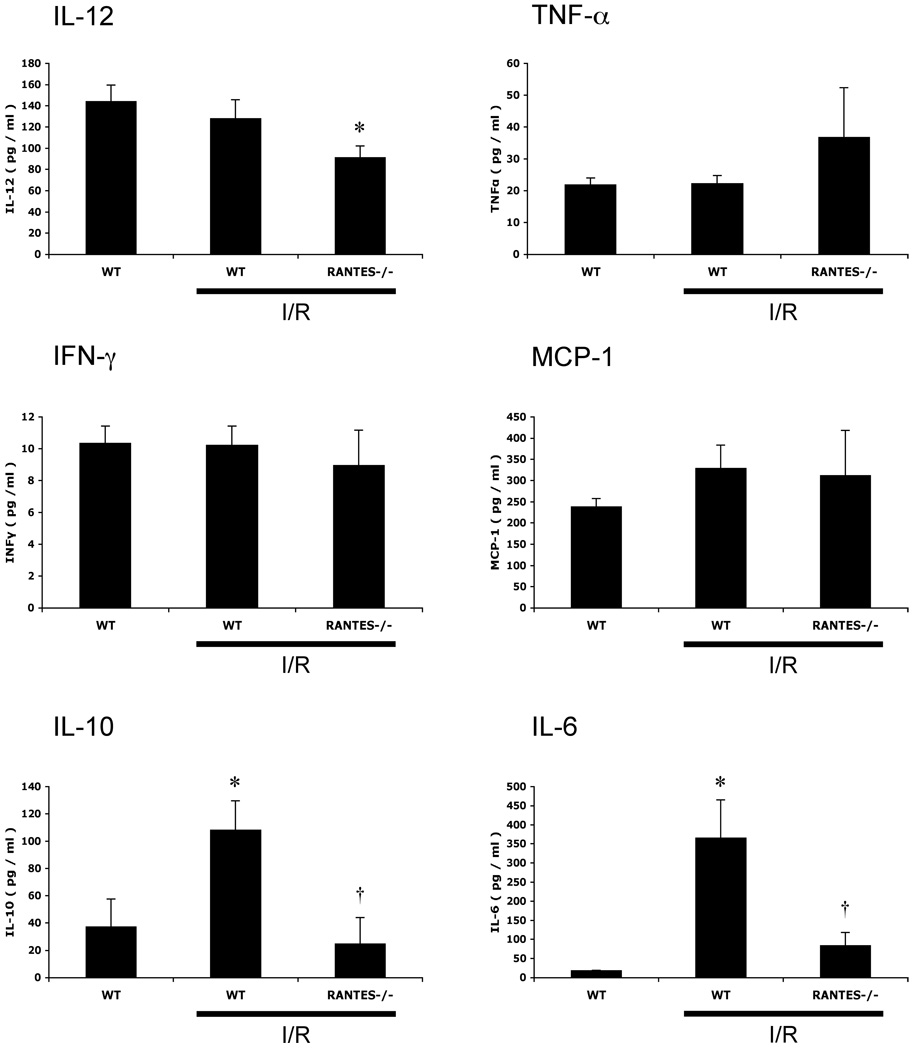
Figure 4 summarizes the changes in plasma concentrations of different cytokines (IL-12, TNF-α, IFN-γ, MCP-1, IL-10, IL-6) in WT-sham, WT-I/R, and RANTES−/− mice. MCAO/R elicited significant increases in plasma IL-10 (108.1±21.4 pg/ml) and IL-6 (365.1±100.4 pg/ml) concentration in WT mice, and these changes were significantly blunted (24.6±19.3, 83.7±21.5 pg/ml, respectively) in the RANTES−/− mice subjected to I/R. Although plasma IL-12 concentration was unaffected by MCAO/R in WT mice, RANTES−/− mice exhibited a small but significant reduction (63%) compared to WT-sham mice.
Figure 4.
Plasma concentrations of interleukin-12 (IL-12), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), monocyte chemotattractant protein-1 (MCP-1), interleukin-10 (IL-10), and interleukin-6 (IL-6) following brain ischemia-reperfusion (I/R) in wild type mice (WT) and RANTES knockout mice (RANTES−/−). (*: p<0.05 vs WT-sham, †: p<0.05 vs WT-I/R)
Brain cytokine levels
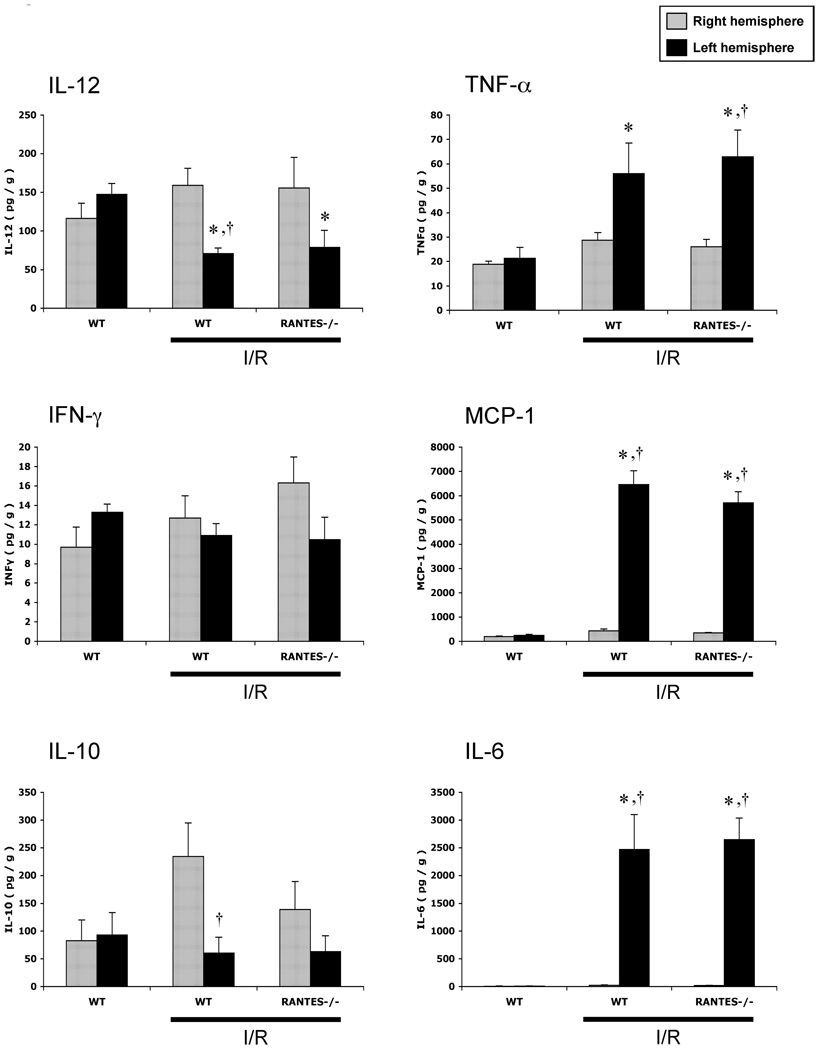
The changes in brain tissue cytokine concentrations after MCAO/R are summarized in Figure 5. MCAO/R elicited significant reductions in IL-12 and IL-10 in the left (injured) hemisphere of WT mice, while corresponding increases in TNF-α, MCP-1, and IL-6 were detected. However, these responses were unaffected by RANTES deficiency.
Figure 5.
Brain tissue concentrations of interleukin-12 (IL-12), tumor necrosis factor-α (TNF- α), interferon-γ (IFN-γ), monocyte chemotattractant protein-1 (MCP-1), interleukin-10 (IL-10), and interleukin-6 (IL-6) following ischemia-reperfusion (I/R) in wild type mice (WT) and RANTES knockout mice (RANTES−/−). (*: p<0.05 vs WT-sham, †: p<0.05 vs contralateral hemisphere in the same group)
Discussion
Relatively little attention has been devoted to the contribution of chemokines to the pathogenesis of ischemic stroke. Although recent work has implicated CCL2 (MCP-1) and CCR2 in cerebral ischemia-reperfusion (I/R) injury in mice,4, 5 a role for other members of the CC-chemokine family, such as RANTES (CCL5), in stroke has not been previously addressed. RANTES has been implicated in variety of pathological conditions, including atherosclerosis, asthma, AIDS, cancer, transplantation and inflammatory bowel disease.14, 15 Like other chemokines, RANTES mediates chemotaxis or cell migration through G-protein coupled receptors (GPCR) at nanomolar concentrations, and aggregates of RANTES can induce cell activation (proliferation, apoptosis, cytokine release) via a GPCR-independent pathway at micromolar concentrations.7, 8 Inasmuch as inflammatory cell recruitment into the postischemic brain is considered an important pathophysiological component of this disease process, RANTES appears to be a worthy candidate mediator of the inflammatory responses to ischemic stroke.
The results of our study provide two major lines of evidence that implicate RANTES in the inflammation, microvascular dysfunction, and tissue injury elicited in a murine model of focal cerebral ischemia. First, we demonstrate that brain tissue, not plasma, levels of RANTES are significantly elevated in wild type mice subjected to focal cerebral ischemia and reperfusion. Second, significant protection against the I/R-induced inflammatory and tissue injury responses was noted in RANTES−/− mice, as well as in RANTES>WT chimeras.
The results of this study suggest that RANTES plays a major role in the recruitment of both leukocytes and platelets into the cerebral microvasculature after I/R. This effect on leukocyte recruitment is consistent with the well-documented effects of RANTES on leukocyte migration. Indeed, RANTES has been implicated in the trafficking and retention of leukocytes in atherosclerotic plaques,16 in the leukocyte adhesion in cerebral venules of mice with experimental autoimmune encephalitis,17 as well as in monocyte arrest on monolayers of cultured endothelial cells.18 It has been previously shown that RANTES liberated from α-granules of activated platelets or platelet microparticles binds to GAGs on the endothelial cell surface where it promotes leukocyte adhesion.19 The avidity of RANTES for GAGs would ensure that blood cell-derived RANTES is concentrated on the endothelial cell surface in the inflamed or damaged tissue. Although platelets are known to express CCR1, 3 and 4, all of which can interact with RANTES20, the results of a previous in vitro study indicate that RANTES per se does not promote the activation or aggregation of platelets, or the adhesion of platelets to endothelium21. Thus, the effect of RANTES on I/R-induced platelet adhesion observed in our study may reflect the binding of platelets to leukocytes, rather than direct binding to vascular endothelium, as supported by our finding that 89% of I/R-induced platelet adhesion in WT-I/R mice could be attributed to platelet interactions with leukocytes rather than directly with the vascular wall. Hence, a reduction of leukocyte adhesion due to RANTES deficiency would be expected to elicit a comparable reduction in platelet adhesion.
Our observation that the BBB dysfunction induced by cerebral I/R was greatly attenuated in RANTES−/− mice suggests that this chemokine either directly or indirectly increases BBB permeability. Studies in lung have failed to demonstrate a role for RANTES in the increased vascular permeability induced by I/R.22 There is evidence, however, that RANTES can promote the migration of leukocytes across monolayers of brain endothelial cells.23 This observation, coupled to reports linking leukocyte transmigration to BBB permeability,24 suggests that the directed movement of leukocytes across the BBB may contribute to the increased BBB permeability observed in our study. However, it remains unclear whether such trafficking of leukocytes does occur to a significant extent at 24 hour reperfusion and whether this could account for the large increment in BBB permeability. Another possible explanation is that RANTES activates adherent leukocytes, which in turn release mediators that disrupt the BBB.25,26
A variety of cells are known to produce RANTES, including T-lymphocytes, platelets, endothelial cells, smooth muscle cells, and glial cells etc. In order to address whether the source of RANTES that mediates the I/R-induced inflammation, microvascular dysfunction, and tissue injury is circulating blood cells or cells lining the vasculature or glial cells, we employed bone marrow chimera mice. Our findings indicate that RANTES>WT chimeras exhibit attenuated blood cell adhesion, BBB permeability, and tissue infarction responses similar to those observed in RANTES−/− mice. This suggests that a circulating blood cell is the likely source of RANTES that mediates the I/R-induced cerebral responses. The persistent elevation of brain tissue RANTES in RANTES>WT chimeras (Figure 3A) suggests that non-blood cells (endothelial cells, vascular smooth muscle cells and / or glial cells) are likely to account for the majority of the I/R-induced elevation of brain tissue RANTES. Of the total RANTES detected in brain tissue of WT-I/R, it may be estimated from Figure 3A that approximately 40% is derived from blood cells while 60% is derived from non-blood cells. Nonetheless, RANTES>WT chimeras clearly exhibited protective responses comparable to RANTES−/− mice, suggesting that deposition of blood cell-derived RANTES onto the endothelial cell surface is of equal or greater importance to RANTES generated from parenchymal cells in mediating the inflammatory and injury responses to I/R. While parenchymal cell-derived RANTES can move to the endothelial surface by transcytosis,27 the liberation of RANTES from blood cells is a much faster response and is more effective for the initiation of the pathogenesis.
The bone marrow (BM) chimeras developed from RANTES−/− mice provide useful insights into the potential contribution of blood vs vascular/extravascular cells to the RANTESmediated responses. However, there is some evidence in literature suggesting that transplanted BM cells may gain access to the extravascular compartment when chimerization is achieved. For example, it has been shown that, at 3 months following BM transplantation, a significant number (over 10,000 cells/brain) of transplanted cells are detected in the brain but most of the cells are distributed in the areas of the brain that lack a blood-brain barrier, like the leptomeninges and circumventricular organs.28 These cells preserved their hematopoietic identity (macrophage) and were able to replace the resident population of leptomeningeal macrophages. The transdifferentiation of donor cells into neurons was rarely seen except in the cerebellum. In another study,29 it was shown that donor-derived cells start to appear at perivascular/leptomeningeal sites as early as 2 weeks after BM transplantation. These reports suggest that we cannot exclude the possibility that our RANTES>WT chimeras may have included donor-origin (RANTES−/−) macrophages in circumventricular organs and perivascular regions, which could have offered a more protection against I/R injury.
Our RANTES measurements in Rag-1−/− mice suggest that lymphocytes are an unlikely source of the chemokine. However, this does not exclude the possibility that T-lymphocytes, which have been implicated in the pathogenesis of ischemic stroke,30 could influence the release of RANTES from other cells. Platelets are a more likely source, since they are known to release large quantities of the chemokine from α-granules when activated.31 Although we made an effort to directly address the role of platelets in the RANTES-mediated brain responses to I/R by rendering mice thrombocytopenic using anti-platelet serum (rabbit anti-mouse thrombocyte antiserum, Accurate Chemicals, NY), the animals (n=7) could not tolerate the I/R protocol due to excessive brain hemorrhage after the reperfusion. The absence of an elevated RANTES concentration in plasma after I/R (Figure 4B) is consistent with clinical data showing that serum RANTES concentration does not differ between stroke patients and normal subjects,32 but this may reflect the efficient binding of locally released RANTES to GAGs on cerebrovascular endothelial cells. Indeed, RANTES is reported to have a higher affinity to heparin than other chemokines (RANTES > MCP-1 > IL-8 > MIP1α).27
The mechanisms underlying the inflammatory and microvascular alterations mediated by RANTES in cerebral I/R remain unclear. Since the MCAO/R model is associated with a significant increase in tissue and plasma levels of cytokines, we addressed whether the protection observed in RANTES−/− mice is associated with a blunted cytokine response. Our findings revealed that the enhanced cytokine levels elicited by cerebral I/R largely do not differ between WT and RANTES−/− mice especially in brain tissue, where increases in TNF-α, MCP-1, IL-6 and reductions in IL-12 were detected in the injured hemisphere of both WT and RANTES−/− mice. However, significant reductions in plasma IL-6 and IL-10 concentrations were noted in RANTES−/− compared to WT-I/R mice.
While it is well recognized that cerebral I/R is associated with increases in both plasma and tissue IL-6 concentrations, the pathophysiological importance of these changes remains controversial, with some reports proposing IL-6 as a mediator of injury33, 34 and others suggesting that the cytokine is protective against ischemic stroke.35, 36 IL-10, on the other hand, is more universally considered to be an anti-inflammatory cytokine with neuroprotective effects37. RANTES is a potent inducer of IL-10 production by peripheral monocytes,38 therefore RANTES deficiency might be expected to lower plasma IL-10 levels, as noted in our study. Nonetheless, it remains unclear whether the changes noted in plasma cytokines in RANTES−/− mice account for the protection afforded in this mutant.
In conclusion, our findings implicate RANTES as a mediator of I/R-induced BBB disruption, tissue injury, and the inflammatory and prothrombogenic phenotype assumed by the cerebral microvasculature after focal ischemia-reperfusion. Blood cells, probably platelets, are the likely source of RANTES that mediates these responses. The protective effect of RANTES deficiency may be linked to changes in the plasma concentrations of certain cytokines, namely IL-6, IL-10 and IL-12. RANTES-directed interventions may prove useful in the treatment of ischemic stroke.
Acknowledgments and Funding
We thank Dr. Sally R. Sarawar from the Torrey Pines Institute for Molecular Studies, 3550 General Anatomics Ct., San Diego, CA 92121 for providing a breeding pair of RANTES−/− mice.
This work was supported by a grant from the National Heart Lung and Blood Institute (HL26441).
Footnotes
Author Disclosures
Satoshi Terao: No disclosures
Gokhan Yilmaz: No disclosures
Karen Y Stokes: No disclosures
Janice Russell: No disclosures
Mami Ishikawa: No disclosures
Takeshi Kawase: No disclosures
D.Neil Granger:
Research Grant: NHLBI 26441, Amount: >= $10,000, NIDDK 65649, Amount: >= $10,000, NIDDK 43785, Amount: >= $10,000
References
- 1.Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 2.Arakelyan A, Petrkova J, Hermanova Z, Boyajyan A, Lukl J, Petrek M. Serum levels of the MCP-1 chemokine in patients with ischemic stroke and myocardial infarction. Mediators Inflamm. 2005;2005:175–179. doi: 10.1155/MI.2005.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kostulas N, Pelidou SH, Kivisakk P, Kostulas V, Link H. Increased IL-1beta, IL-8, and IL-17 mRNA expression in blood mononuclear cells observed in a prospective ischemic stroke study. Stroke. 1999;30:2174–2179. doi: 10.1161/01.str.30.10.2174. [DOI] [PubMed] [Google Scholar]
- 4.Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Effects of the chemokine CCL2 on blood-brain barrier permeability during ischemia-reperfusion injury. J Cereb Blood Flow Metab. 2006;26:797–810. doi: 10.1038/sj.jcbfm.9600229. [DOI] [PubMed] [Google Scholar]
- 5.Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007;38:1345–1353. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto T, Ikeda K, Mukaida N, Harada A, Matsumoto Y, Yamashita J, Matsushima K. Prevention of cerebral edema and infarct in cerebral reperfusion injury by an antibody to interleukin-8. Lab Invest. 1997;77:119–125. [PubMed] [Google Scholar]
- 7.Appay V, Rowland-Jones SL. RANTES: A versatile and controversial chemokine. Trends Immunol. 2001;22:83–87. doi: 10.1016/s1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- 8.Bacon KB, Premack BA, Gardner P, Schall TJ. Activation of dual T cell signaling pathways by the chemokine RANTES. Science. 1995;269:1727–1730. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- 9.Makino Y, Cook DN, Smithies O, Hwang OY, Neilson EG, Turka LA, Sato H, Wells AD, Danoff TM. Impaired T cell function in RANTES-deficient mice. Clin Immunol. 2002;102:302–309. doi: 10.1006/clim.2001.5178. [DOI] [PubMed] [Google Scholar]
- 10.Petnehazy T, Stokes KY, Wood KC, Russell J, Granger DN. Role of blood cellassociated AT1 receptors in the microvascular responses to hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2006;26:313–318. doi: 10.1161/01.ATV.0000193625.32499.71. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa M, Vowinkel T, Stokes KY, Arumugam TV, Yilmaz G, Nanda A, Granger DN. CD40/CD40 ligand signaling in mouse cerebral microvasculature after focal ischemia/reperfusion. Circulation. 2005;111:1690–1696. doi: 10.1161/01.CIR.0000160349.42665.0C. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa M, Sekizuka E, Yamaguchi N, Nakadate H, Terao S, Granger DN, Minamitani H. Angiotensin II type 1 receptor signaling contributes to platelet-leukocyte-endothelial cell interactions in the cerebral microvasculature. Am J Physiol Heart Circ Physiol. 2007;292:H2306–H2315. doi: 10.1152/ajpheart.00601.2006. [DOI] [PubMed] [Google Scholar]
- 13.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 14.Krensky AM, Ahn YT. Mechanisms of disease: Regulation of RANTES (CCL5) in renal disease. Nat Clin Pract Nephrol. 2007;3:164–170. doi: 10.1038/ncpneph0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fagerstam JP, Whiss PA, Strom M, Andersson RG. Expression of platelet P-selectin and detection of soluble P-selectin, NPY and RANTES in patients with inflammatory bowel disease. Inflamm Res. 2000;49:466–472. doi: 10.1007/s000110050618. [DOI] [PubMed] [Google Scholar]
- 16.Sheikine Y, Hansson GK. Chemokines and atherosclerosis. Ann Med. 2004;36:98–118. doi: 10.1080/07853890310019961. [DOI] [PubMed] [Google Scholar]
- 17.dos Santos AC, Barsante MM, Arantes RM, Bernard CC, Teixeira MM, Carvalho-Tavares J. CCL2 and CCL5 mediate leukocyte adhesion in experimental autoimmune encephalomyelitis--an intravital microscopy study. J Neuroimmunol. 2005;162:122–129. doi: 10.1016/j.jneuroim.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Baltus T, Weber KS, Johnson Z, Proudfoot AE, Weber C. Oligomerization of RANTES is required for CCR1-mediated arrest but not CCR5-mediated transmigration of leukocytes on inflamed endothelium. Blood. 2003;102:1985–1988. doi: 10.1182/blood-2003-04-1175. [DOI] [PubMed] [Google Scholar]
- 19.von Hundelshausen P, Weber KS, Huo Y, Proudfoot AE, Nelson PJ, Ley K, Weber C. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001;103:1772–1777. doi: 10.1161/01.cir.103.13.1772. [DOI] [PubMed] [Google Scholar]
- 20.Clemetson KJ, Clemetson JM, Proudfoot AE, Power CA, Baggiolini M, Wells TN. Functional expression of CCR1, CCR3, CCR4, and CXCR4 chemokine receptors on human platelets. Blood. 2000;96:4046–4054. [PubMed] [Google Scholar]
- 21.Shenkman B, Brill A, Brill G, Lider O, Savion N, Varon D. Differential response of platelets to chemokines: RANTES non-competitively inhibits stimulatory effect of SDF-1 alpha. J Thromb Haemost. 2004;2:154–160. doi: 10.1111/j.1538-7836.2004.00527.x. [DOI] [PubMed] [Google Scholar]
- 22.Krishnadasan B, Farivar AS, Naidu BV, Woolley SM, Byrne K, Fraga CH, Mulligan MS. Beta-chemokine function in experimental lung ischemia-reperfusion injury. Ann Thorac Surg. 2004;77:1056–1062. doi: 10.1016/S0003-4975(03)01600-X. [DOI] [PubMed] [Google Scholar]
- 23.Ubogu EE, Callahan MK, Tucky BH, Ransohoff RM. Determinants of CCL5-driven mononuclear cell migration across the blood-brain barrier. Implications for therapeutically modulating neuroinflammation. J Neuroimmunol. 2006;179:132–144. doi: 10.1016/j.jneuroim.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Inglis VI, Jones MP, Tse AD, Easton AS. Neutrophils both reduce and increase permeability in a cell culture model of the blood-brain barrier. Brain Res. 2004;998:218–229. doi: 10.1016/j.brainres.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 25.Merrill JE, Murphy SP. Inflammatory events at the blood brain barrier: Regulation of adhesion molecules, cytokines, and chemokines by reactive nitrogen and oxygen species. Brain Behav Immun. 1997;11:245–263. doi: 10.1006/brbi.1997.0496. [DOI] [PubMed] [Google Scholar]
- 26.Wong D, Dorovini-Zis K, Vincent SR. Cytokines, nitric oxide, and cGMP modulate the permeability of an in vitro model of the human blood-brain barrier. Exp Neurol. 2004;190:446–455. doi: 10.1016/j.expneurol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Middleton J, Patterson AM, Gardner L, Schmutz C, Ashton BA. Leukocyte extravasation: Chemokine transport and presentation by the endothelium. Blood. 2002;100:3853–3860. doi: 10.1182/blood.V100.12.3853. [DOI] [PubMed] [Google Scholar]
- 28.Vallieres L, Sawchenko PE. Bone marrow-derived cells that populate the adult mouse brain preserve their hematopoietic identity. J Neurosci. 2003;23:5197–5207. doi: 10.1523/JNEUROSCI.23-12-05197.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, de Boer BA, Frotscher M, Kreutzberg GW, Persons DA, Dirnagl U. Targeting gene-modified hematopoietic cells to the central nervous system: Use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- 30.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 31.Tan KT, Watson SP, Lip GY. The endothelium and platelets in cardiovascular disease: Potential targets for therapeutic intervention. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:169–178. doi: 10.2174/1568016043477260. [DOI] [PubMed] [Google Scholar]
- 32.Zaremba J, Ilkowski J, Losy J. Serial measurements of levels of the chemokines CCL2, CCL3 and CCL5 in serum of patients with acute ischaemic stroke. Folia Neuropathol. 2006;44:282–289. [PubMed] [Google Scholar]
- 33.Smith CJ, Emsley HC, Gavin CM, Georgiou RF, Vail A, Barberan EM, del Zoppo GJ, Hallenbeck JM, Rothwell NJ, Hopkins SJ, Tyrrell PJ. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4:2. doi: 10.1186/1471-2377-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark WM, Beamer NB, Wynn M, Coull BM. The initial acute phase response predicts long-term stroke recovery. J Stroke Cerebrovasc Dis. 1998;7:128–131. doi: 10.1016/s1052-3057(98)80139-0. [DOI] [PubMed] [Google Scholar]
- 35.Ali C, Nicole O, Docagne F, Lesne S, MacKenzie ET, Nouvelot A, Buisson A, Vivien D. Ischemia-induced interleukin-6 as a potential endogenous neuroprotective cytokine against NMDA receptor-mediated excitotoxicity in the brain. J Cereb Blood Flow Metab. 2000;20:956–966. doi: 10.1097/00004647-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Sotgiu S, Zanda B, Marchetti B, Fois ML, Arru G, Pes GM, Salaris FS, Arru A, Pirisi A, Rosati G. Inflammatory biomarkers in blood of patients with acute brain ischemia. Eur J Neurol. 2006;13:505–513. doi: 10.1111/j.1468-1331.2006.01280.x. [DOI] [PubMed] [Google Scholar]
- 37.Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, Dantzer R, Kelley KW. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–449. [PubMed] [Google Scholar]
- 38.Shahrara S, Park CC, Temkin V, Jarvis JW, Volin MV, Pope RM. RANTES modulates TLR4-induced cytokine secretion in human peripheral blood monocytes. J Immunol. 2006;177:5077–5087. doi: 10.4049/jimmunol.177.8.5077. [DOI] [PubMed] [Google Scholar]