Abstract
Hyper-homocysteinemia is an independent risk factor for stroke and neurological abnormalities. However the underlying cellular mechanisms by which elevated homocysteine can promote neuronal death is not clear. In the present study we have examined the role of NMDA receptor mediated activation of the extracellular-signal regulated mitogen activated protein (ERK MAP) kinase pathway in homocysteine-dependent neurotoxicity. The study demonstrates that in neurons L-homocysteine-induced cell death is mediated through activation of NMDA receptors. The study also shows that homocysteine-dependent NMDA receptor stimulation and resultant Ca2+ influx leads to rapid and sustained phosphorylation of ERK MAP kinase. Inhibition of ERK phosphorylation attenuates homocysteine mediated neuronal cell death thereby demonstrating that activation of ERK MAP kinase signaling pathway is an intermediate step that couples homocysteine mediated NMDA receptor stimulation to neuronal death. The findings also show that cAMP response-element binding protein (CREB), a pro-survival transcription factor and a downstream target of ERK, is only transiently activated following homocysteine exposure. The sustained activation of ERK but a transient activation of CREB together suggest that exposure to homocysteine initiates a feedback loop that shuts off CREB signaling without affecting ERK phosphorylation and thereby facilitates homocysteine mediated neurotoxicity.
Keywords: Homocysteine, NMDA receptors, ERK MAP kinase, CREB, Ca2+ influx, neuronal cell death
INTRODUCTION
Homocysteine, a sulfur-containing amino acid, is formed as an intermediate during methionine metabolism. Nutritional deficiency of the cofactors or genetic mutations and impaired activity of the key enzymes in the homocysteine metabolic pathway cause accumulation of plasma homocysteine resulting in hyper-homocysteinemia. Additionally, general health status of an individual that involves advanced age, insufficient renal and hepatic function, diabetes mellitus, hypothyroidism, as well as medications including folate and vitamin B6 antagonists may raise plasma homocysteine levels (Hankey and Eikelboom 2001; Austin et al. 2004; Obeid and Herrmann 2006; Zoccolella et al. 2006). Individuals with hyper-homocysteinemia are known to be at risk for neurological diseases including vascular dementia and stroke (Gottfries et al. 1998; Sacco et al. 1998). Hyper-homocysteinemia is also associated with neurological abnormalities such as mental retardation, age-associated dementia, Alzheimer’s disease, Parkinson’s disease, cerebral atrophy and seizures (Watkins and Rosenblatt 1989; Van den Berg et al. 1995; Gottfries et al. 1998; Miller 1999; Seshadri et al. 2002).
Recent reports indicate that hyper-homocysteinemia in otherwise healthy elderly people results in reduced size and volume of hippocampus and cortex (den Heijer et al. 2003). Studies in animal models of neurological disorders indicate that hyper-homocysteinemia sensitizes hippocampal neurons to excitotoxic insults (Kruman et al. 2000) and greatly enhances neuronal death in mouse models of cerebral stroke (Endres et al. 2005), Alzheimer’s and Parkinson’s diseases (Duan et al. 2002; Kruman et al. 2002). In vitro studies in cultured hippocampal neurons suggest that homocysteine induces a dose-dependent increase in apoptotic cell death (Kruman et al. 2000). However the underlying cellular mechanisms by which elevated homocysteine induce neuronal death or exacerbate the consequences of other insults are still unclear. It has been suggested that elevated homocysteine may induce brain damage by increasing cellular oxidative stress as well as by leading to hypo-methylation of DNA and proteins (Obeid and Herrmann 2006). Increased homocysteine levels may also play a role in neuronal death via stimulation of glutamate receptors (Kruman et al. 2000; Zieminska et al. 2003; Obeid and Herrmann 2006). Homocysteine is an agonist for metabotropic glutamate receptors as well as for NMDA (N-methyl-D-aspartate) and AMPA (amino-3-hydroxy-5-methyl-4-isoxazolepropionate)/Kainate ionotropic glutamate receptors (Lipton et al. 1997; Yuzaki and Connor 1999; Lazarewicz et al. 2003; Shi et al. 2003; Zieminska et al. 2003; Luchowska et al. 2005; Robert et al. 2005). Recent in vitro studies indicate that elevated homocysteine induces oxidative injury in nerve terminals and involves NMDA receptor stimulation, neuronal nitric oxide synthase activation and associated free radical formation (Jara-Prado et al. 2003). In addition, tau protein phosphorylation by elevated homocysteine also involves NMDA receptor stimulation (Ho et al. 2002). Furthermore, homocysteine sensitizes cultured neurons to excitotoxic or oxidative insults to allow larger Ca2+ influx (Kruman et al. 2000). The associated homocysteine-mediated cell death observed was also NMDA receptor-dependent (Kruman et al. 2000). Therefore homocysteine-NMDA receptor stimulation may be the initial key step for inducing neuronal damage.
Stimulation of NMDA receptors by various agonists triggers a multitude of signaling cascades that regulate a diverse array of neuronal functions. The extracellular-signal regulated (ERK) MAP kinase signaling cascade is one such target that may play a critical role in neuronal cell survival or death depending on its magnitude and duration of activation. Generally, ERK MAP kinase is transiently stimulated by neurotrophic factors and neurotransmitters such as glutamate and has been shown to be important in neuronal survival and long-term potentiation (Boulton et al. 1991; Xia et al. 1995; Segal and Greenberg 1996). A possible role for ERK MAP kinase in neuronal death is less clear but there is evidence that sustained activation of the ERK MAP kinase pathway is detrimental to cell survival (Marshall 1995; Herdegen and Leah 1998; Alessandrini et al. 1999; Irving et al. 2000; Stanciu et al. 2000; Stanciu and DeFranco 2002). Although homocysteine is an agonist for NMDA receptors very few studies have investigated the role of homocysteine in the regulation of ERK MAP kinase signaling pathways and its effect on neuronal cell survival and death. In this regard a recent study (Robert et al. 2005) has shown elevated levels of phosphorylated ERK in the hippocampus of hyper-homocysteinemic cystathionine beta synthase knockout mice and a transient phosphorylation of ERK by elevated homocysteine in an ex vivo model of mice hippocampal slice cultures. However the role of ERK MAP kinase in facilitating homocysteine-mediated neuronal cell death is unclear.
The aim of the present study is to examine the effect of a pathological level of homocysteine on NMDA receptor mediated neuronal cell death and activation of the ERK MAP kinase signaling pathway. Our results show that homocysteine-NMDA receptor mediated Ca2+ influx results in a rapid and relatively sustained ERK MAP kinase phosphorylation. Our findings also demonstrate that activation of the ERK MAP kinase-signaling pathway plays a critical role in mediating homocysteine dependent neuronal cell death.
MATERIALS AND METHODS
Materials and reagents
All tissue culture reagents were obtained from Invitrogen, Carlsbad. L-homocysteine thiolactone, L-Glutamate, NMDA, glycine, cytosine D-arabinofuranoside, DL-2-Amino-5-phosphonopentanoic acid (APV), 6-cyano-7-nitroquinoxaline-2, 3-dione (CNQX), (+) α-methyl-4-carboxyphenylglycine (MCPG), EGTA were from Sigma-Aldrich, USA. Nifedipine, Genistein, H89, Ro-32-0432, PD98059, NG-Propyl-L-arginine (N-PLA), 7-nitroindazole (7-Ni), were obtained from EMD biosciences. Anti-phospho-ERK monoclonal antibody recognizing phosphorylation at residues Thr202/Tyr204 of ERK 1 and 2 (TPEYP-ERK), anti-phospho-CREB (Ser133) polyclonal antibody, anti-total CREB polyclonal antibody, anti-phospho-p38 polyclonal antibody and anti-total p38 polyclonal antibody were obtained from Cell Signaling Technology. Anti-total ERK2 polyclonal antibody was purchased from Santa Cruz Biotechnology. Anti-rabbit and anti-mouse secondary antibodies conjugated to Alexa 488 and Cy3 were obtained from Molecular Probes, Invitrogen.
Primary cortical neuronal cultures
The Institutional Animal Care and Use Committee of the University of New Mexico approved all experimental protocols involving animals. Pregnant female Sprague Dawley rats (16-day gestation) were obtained from Harlem Laboratories. Primary neuronal cultures were established from rat embryos that were 16–17 day old as described earlier (Paul et al. 2003). Briefly, the cortex with the adjoining striatum were dissected out, dissociated mechanically and re-suspended in Dulbecco’s minimum essential medium/F12 (1:1) supplemented with 5% fetal calf serum. Cells (8 × 106 cells/60 mm dish) were plated on poly-D-lysine coated tissue culture dishes (BD Biocoat) and grown for 12–14 days in vitro (DIV) at 37°C in a humidified atmosphere consisting of 5% CO2. To inhibit proliferation of non-neuronal cells, we added 10μM cytosine D-arabinofuranoside to the cultures 72 hours after plating. Thereafter we placed neurons in Minimal Essential Medium containing 5% fetal calf serum until the day of experiment. The experiments were performed on cortical neuron cultures (14 DIV) that were ~95% pure as determined by immunocytochemical staining with antibodies to neuron specific enolase (neuronal marker) and Glial fibrillary acidic protein (astrocyte marker).
L-Homocysteine preparation, treatment and inhibitor studies
L-homocysteine (200 mM stock) was prepared by alkali hydrolysis followed by neutralization as described earlier (Poddar et al. 2001). For homocysteine treatment, cells were washed twice with Hank’s balanced salt solution (HBSS) followed by treatment in HBSS containing 50 μM glycine (Lipton et al. 1997). Cells were stimulated with various concentrations of L-homocysteine as specified in experiments for the indicated times at 37°C. For inhibitor studies, all pharmacological compounds were added 20 minutes before addition of L-homocysteine. After treatment, the cells were washed with phosphate buffered saline (PBS, pH 7.4) containing 50 mM NaF; 10 mM Na4P2O7; 1 mM Na3VO4 and lysed in SDS sample buffer followed by SDS-PAGE and immunoblot analysis.
Immunoblotting procedure
Equal amounts of protein lysates, as estimated using a BCA protein assay kit (Pierce), were resolved by 8% SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane. The membranes were blocked for 1 h at room temperature with 5% non-fat dry milk, then incubated overnight at 4°C or for 1 h at room temperature with the appropriate rabbit polyclonal or mouse monoclonal antibodies as indicated in each figure according to manufacturer’s protocol. Horseradish peroxidase coupled to anti rabbit or anti mouse IgG that are raised in goat were used as secondary antibodies. Immune complexes were detected on X-ray films after treatment with the West Pico supersignal chemiluminescence reagents (Pierce Biotechnology, USA). Densitometric analysis of the images obtained from X-ray films was performed using the Image J software. Statistical comparison was done using One-way analysis of variance (ANOVA, Bonferroni’s multiple comparison test) and differences were considered significant when p < 0.05.
Immunocytochemical and Hoechst DNA-staining procedures
Neuronal cultures were grown on 2-well glass chamber slides coated with poly-d-lysine (BD BioCoat) for 14 days in vitro. After stimulation, samples were fixed with 4% paraformaldehyde for 10 minutes. For immuno-cytochemistry the cells were permeabilized with 0.1% Triton X-100 in PBS (pH 7.4) for 10 minutes, blocked with 10% normal goat serum and 1% BSA in PBS for 1 hour followed by incubation with a mixture of anti-TPEYP-ERK and anti-ERK antibodies overnight at 4°C. Samples were then incubated with a mixture of anti-rabbit and anti-mouse secondary antibodies conjugated to Cy3 and Alexa 488, respectively, washed with PBS and mounted with Vectashield mounting fluid (Vector laboratories). For assessment of nuclear damage cells were incubated with Hoechst 33342 dye for 15 minutes, washed with PBS (pH 7.4) and mounted with Vectashield mounting medium. The percentage of pyknotic nuclei was quantitatively assessed under a fluorescent microscope. A total of 1500 cells were counted for each set of experiments. Mean ± s.e.m. (n = 3) were used for statistical comparison using ANOVA (Bonferroni’s multiple comparison test). Differences were considered significant when p < 0.05.
RESULTS
Homocysteine mediated regulation of ERK MAP kinase
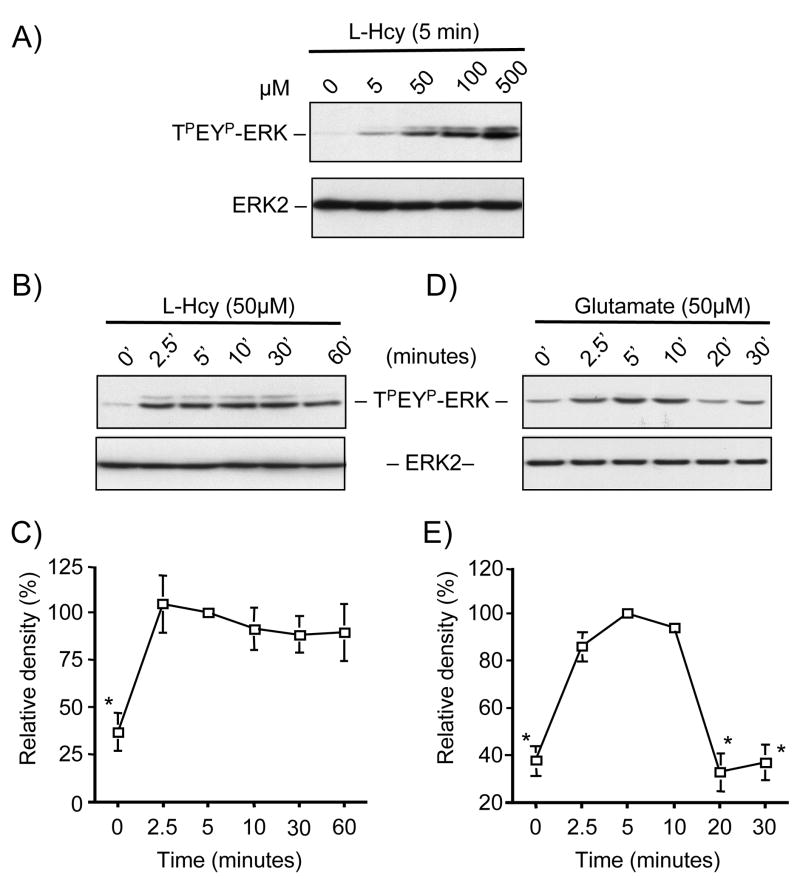
Initial studies examined whether homocysteine can lead to the phosphorylation and subsequent activation of ERK MAP kinase in neurons. For these experiments cortical neuronal cultures were treated with increasing concentrations of L-homocysteine (0, 5, 50, 100, and 500 μM) for 5 minutes and analyzed by immunoblotting with anti-TPEYP-ERK. We observed a dose-dependent increase in ERK2 phosphorylation, which was significantly higher than the basal amount with 50 μM and greater concentrations of homocysteine (Figure 1A, upper panel). Total ERK2 amounts remained unaltered by this treatment (Figure 1A, lower panel).
Figure 1.
Homocysteine stimulates phosphorylation of ERK2 in neurons. (A) Immunoblot analysis of neurons treated with (A) different concentrations of L-homocysteine (L-Hcy) for 5 minutes, (B, C) L-homocysteine (L-Hcy, 50 μM) or (D, E) glutamate (100 μM) for the specified times. Total ERK2 was also analyzed to indicate total protein loading. (C, E) Quantification of phosphorylated ERK2 by computer assisted densitometry. Values are mean ± s.e.m. (n=3). Asterisk * indicates significant difference from 5 minutes L-homocysteine or glutamate treatment (p < 0.05).
We next treated neuronal cultures with L-homocysteine (50 μM) for various lengths of time (0, 2.5, 5, 10, 30 and 60 minutes) to examine the temporal profile of ERK2 phosphorylation. A significant increase in ERK2 phosphorylation was observed within 2.5 minutes of stimulation that remained elevated throughout the time course examined (Figure 1B and 1C). It has been previously demonstrated that ERK MAP kinase is phosphorylated in response to the neurotransmitter glutamate, which plays a key role in neuronal survival, long-term potentiation as well as in neuronal cell death. To investigate the temporal profile of ERK2 phosphorylation following exposure to glutamate we treated neurons with 50 μM of glutamate for various time periods (0, 2.5, 5, 10, 20 and 30 minutes). As shown in Figure 1D and 1E, phosphorylation of ERK2 increased within 2.5 minutes of glutamate stimulation followed by a time-dependent decrease to basal levels by 30 minutes. These findings suggest that homocysteine-mediated regulation of ERK MAP kinase signaling pathway may have varied consequences on gene expression as compared to that with glutamate.
Homocysteine mediated phosphorylation of ERK2 is NMDA receptor dependent
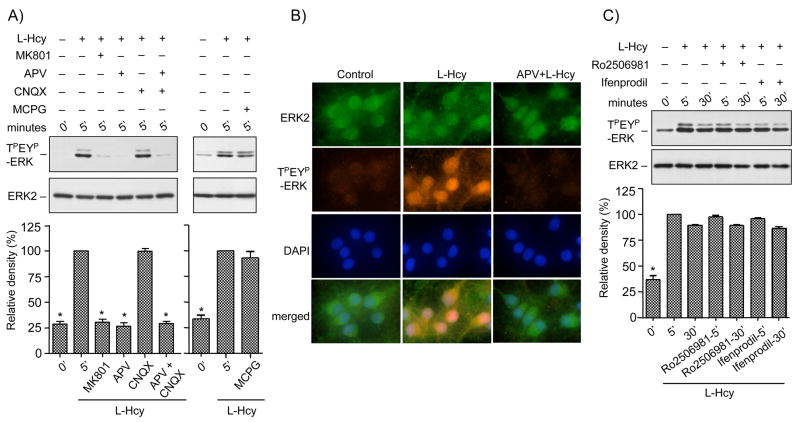
We next examined the possible role of ionotropic (NMDA and AMPA/kainate) and metabotropic glutamate receptors in homocysteine-mediated ERK2 phosphorylation. Immunoblot analysis in Figure 2A shows that incubation of neurons with the NMDA receptor antagonists, MK801 or APV before treatment with L-homocysteine (50 μM, 5 minutes) blocked the phosphorylation of ERK2. In contrast, the AMPA/kainate receptor antagonist, CNQX and the metabotropic glutamate receptor antagonist, MCPG had no effect on the homocysteine-mediated phosphorylation of ERK2. Treatment with both APV and CNQX blocked homocysteine-mediated ERK2 phosphorylation. Immunocytochemical studies further demonstrated that under basal conditions ERK2 was primarily in the dephosphorylated form and retained in the cytoplasm (Figure 2B, left panel). Treatment with L-homocysteine (50 μM, 5 minutes) resulted in the phosphorylation and nuclear translocation of the phosphorylated ERK2 (TPEYP-ERK) by 5 minutes (Figure 2B, center panel). Pre-incubation with APV, inhibited the phosphorylation and nuclear translocation of ERK2. Taken together these results indicate that NMDA receptors play a role in homocysteine-mediated phosphorylation and subsequent nuclear translocation of ERK2.
Figure 2.
Homocysteine mediated NMDA receptor stimulation leads to phosphorylation and nuclear translocation of ERK2 in neurons. (A) Immunoblot analysis of cells treated with control buffer, MK810 (2.5 μM), APV (200 μM), CNQX (50 μM), APV and CNQX or MCPG (500 μM) before treatment with L-homocysteine (L-Hcy, 50 μM) for 5 minutes. Quantification of phosphorylated ERK2 by computer-assisted densitometry is shown below. Values are mean ± s.e.m. (n=3). Asterisk * indicates significant difference from 5 minutes L-homocysteine treatment (p < 0.001). (B) Immunocytochemical staining of cells with anti-TPEYP-ERK antibody (orange) and anti-ERK2 antibody (green) demonstrating nuclear translocation of phosphorylated ERK2 in neurons. Nucleus is counterstained with DAPI (blue). (C) Immunoblot analysis of the effect of Ro-2506981 and Ifenprodil on homocysteine mediated ERK2 phosphorylation. Quantification of phosphorylated ERK2 by computer-assisted densitometry is shown below. Values are mean ± s.e.m. (n=3). Asterisk * indicates significant difference from 5 minutes L-homocysteine treatment (p < 0.001).
Recent findings suggest the existence of two functionally distinct pools of NMDA receptors (Luo et al. 1997), NR1/NR2A containing NMDA receptors (NR2A-NMDAR) and NR1/NR2B containing NMDA receptors (NR2B-NMDAR). We next examined the effect of NR2B-antagonists, Ro 256981 and Ifenprodil on homocysteine-mediated phosphorylation of ERK2. Figure 2C shows that addition of either of the NR2B antagonists before treatment with homocysteine for 5 or 30 minutes had no effect on ERK2 phosphorylation, suggesting that homocysteine-induced phosphorylation of ERK2 is mediated primarily by NR2A-NMDAR. At present there is no selective NR2A-NMDAR antagonist available to provide a better indication for the involvement of NR2A-NMDAR activation.
Homocysteine-NMDA receptor mediated phosphorylation of ERK2 is Ca2+-dependent
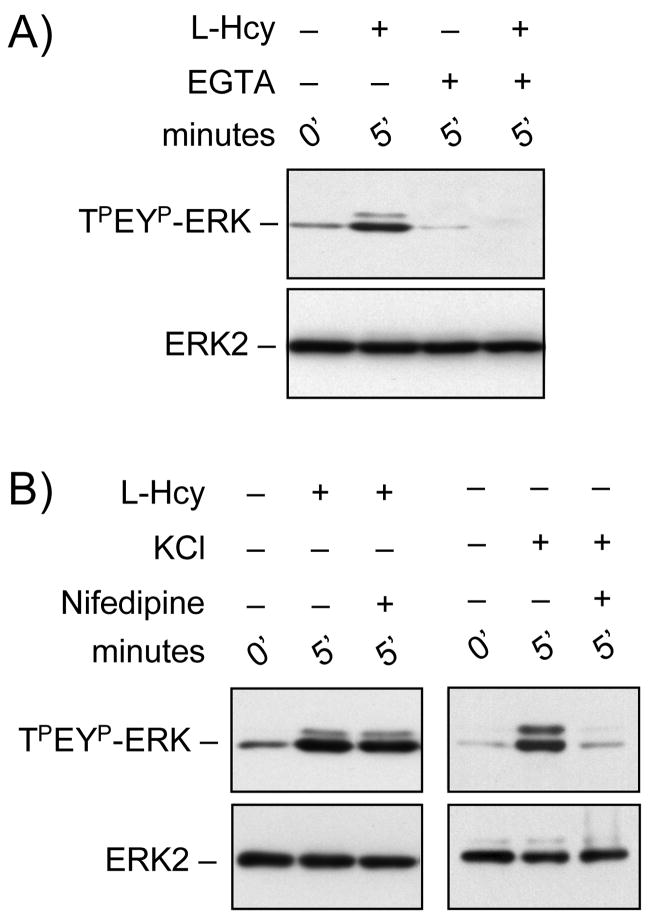
We next determined the role of extracellular Ca2+ in homocysteine-NMDA receptor mediated phosphorylation of ERK2. Removal of extracellular Ca2+ present in the media by chelating with EGTA prior to treatment with L-homocysteine (50 μM, 5 minutes) blocked homocysteine-dependent ERK2 phosphorylation (Figure 3A). EGTA alone had no effect on the phosphorylation of ERK2 (Figure 3A). Another principal mediator of Ca2+ entry involved in the activation of ERK MAP kinase in neurons is the L-type voltage-gated Ca2+ channel (Dolmetsch et al. 2001). Next we wanted to determine if, in addition to NMDA receptors, L-type voltage-gated Ca2+ channels also play a role in homocysteine-mediated activation of ERK2. Treatment with Nifedipine, a L-type voltage-gated Ca2+ channel blocker prior to stimulation with L-homocysteine (50 μM, 5 minutes) shows that blocking L-type voltage-gated Ca2+ channels did not have any effect on homocysteine-induced phosphorylation of ERK2 (Figure 3B). To ensure the efficacy of nifedipine in the above experiment, in control studies neurons were treated with KCl (60 mM, 5 minutes) in the absence or presence of Nifedipine and analyzed for phosphorylation of ERK2 (Figure 3B). Consistent with earlier findings (Lenz and Avruch 2005), our result shows that nifedipine can effectively block KCl-mediated phosphorylation of ERK2, demonstrating the involvement of L-type voltage-gated Ca2+ channels.
Figure 3.
Homocysteine-NMDA receptor mediated ERK2 phosphorylation is Ca2+-dependent. Immunoblot analysis of cells incubated with either (A) EGTA (2 mM) or (B) Nifedipine (15 μM) prior to treatment with 50 μM of L-homocysteine (L-Hcy) or 60 mM of KCl for 5 minutes.
Role of protein kinases and neuronal nitric oxide synthase in homocysteine-NMDA receptor mediated ERK2 phosphorylation
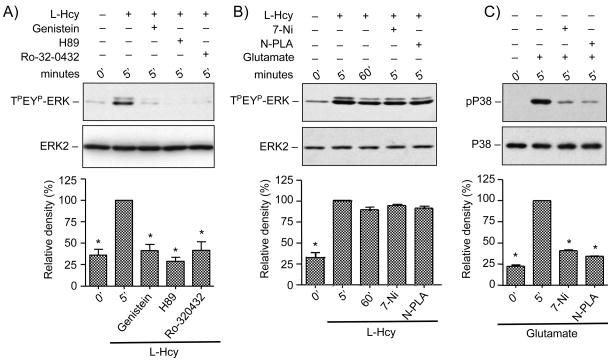
Cyclic-AMP dependent protein kinase A (PKA), protein kinase C (PKC), several tyrosine kinase family members (PTKs) and neuronal nitric oxide synthase (nNOS) are known to play a critical role in transducing intracellular signals in response to NMDA receptor stimulation (Durkin et al. 1996; Yun et al. 1999; Banko et al. 2004; Salter and Kalia 2004). To evaluate the role of these kinases in the phosphorylation of ERK2, neuronal cultures were treated with L-homocysteine for 5 minutes in the presence or absence of, H89, Ro-32-0432 or genistein, specific inhibitors of PKA, PKC and PTK respectively. Figure 4A shows that inhibition of PKA, PKC or PTK resulted in attenuation of ERK2 MAP kinase phosphorylation. Next cells were incubated with the selective nNOS inhibitors N-PLA or 7-Ni prior to treatment with L-homocysteine. Figure 4B shows that nNOS inhibitors had no significant effect on homocysteine-mediated phosphorylation of ERK2 demonstrating that NO is not involved in this phenomenon. As a positive control, for the pharmacological inhibition of nitric oxide, in some experiments neurons were treated with glutamate (50 μM, 5 minutes) in the presence or absence of N-PLA or 7-Ni and phosphorylation of p38 MAP kinase was analyzed by immunoblotting. Confirming previous reports (Cao et al. 2005) our data showed that both N-PLA and 7Ni effectively blocked glutamate-mediated phosphorylation of p38 MAP kinase (Figure 4C).
Figure 4.
Effects of protein kinase and nNOS inhibitors on homocysteine mediated ERK2 phosphorylation in neurons. Cells were pre-incubated with (A) protein kinase inhibitors Genistein (100 μM), H89 (40 μM), Ro-32-0432 (2 μM) or (B) nNOS inhibitors N-PLA (1 μM) or 7-Ni (3 μM) before exposure to 50 μm of L-homocysteine (L-Hcy) and (C) nNOS inhibitors N-PLA (1 μM) or 7-Ni (3 μM) before treatment with glutamate. Immunoblot analysis was performed with anti-TPEYP-ERK antibody and anti-ERK2 antibody (A and B); and anti-phospho-p38 (p-p38) antibody and anti p38 antibody (C). Quantification of phosphorylated ERK2 by computer-assisted densitometry is shown below. Values are mean ± s.e.m. (n=3). Asterisk * indicates significant difference from 5 minutes L-homocysteine or glutamate treatment (p < 0.001).
Inhibition of ERK2 attenuates homocysteine-NMDA receptor mediated neuronal cell death
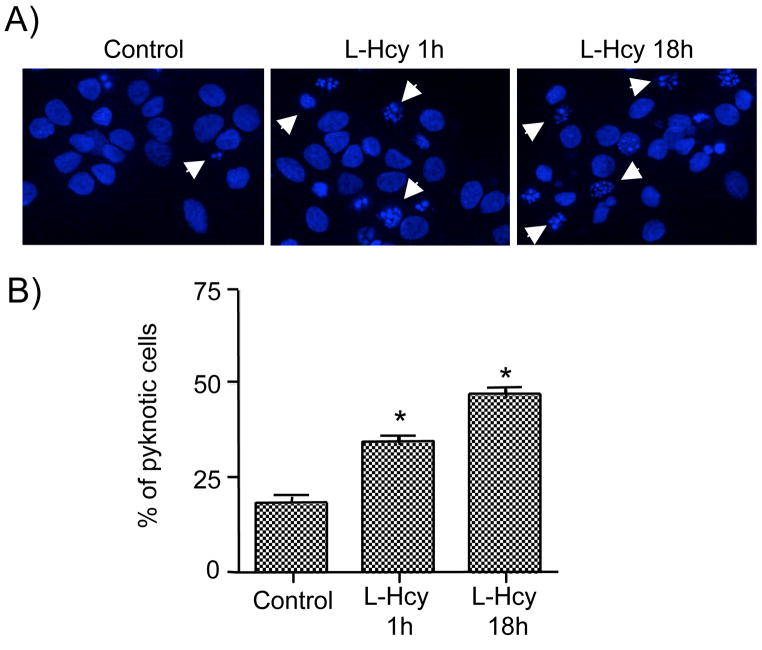
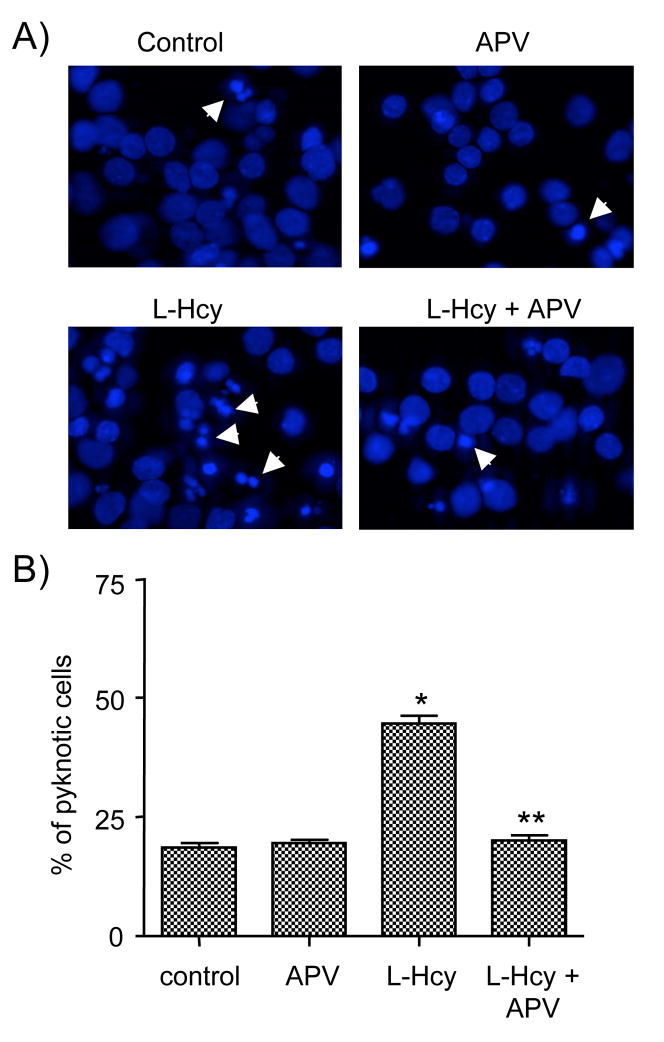
To determine the effect of homocysteine on cell death, neurons were exposed to L-homocysteine (50 μM) either for 1 hour (short term exposure) or 18 hours (long term exposure). Neurons that were treated for 1 hour were maintained in the experimental medium without homocysteine for another 17 hours. Cells were then processed for Hoechst DNA staining, an early indicator of apoptosis (Daxhelet et al. 1989; Kruman et al. 2000). Figure 5A and 5B show that under basal condition 17.4 ± 1.32% of the cells were apoptotic. Following exposure to L-homocysteine for 1 hour 30.4 ± 1.6% of the cells became apoptotic whereas exposure for 18 hours resulted in 44.7 ± 1.57% of the cells to become apoptotic. Immunoblot analysis also showed that ERK2 remained phosphorylated for 18 hours (data not shown). To determine a role for NMDA receptors in homocysteine-mediated neuronal cell death APV was added to the cells prior to treatment with L-homocysteine for 18 hours and analyzed for death by Hoechst DNA staining. Figure 6A and 6B show that pretreatment with APV leads to a significant reduction in the percentage of apoptotic cells (16.6 ± 1.4%).
Figure 5.
Homocysteine induces cell death in neurons. Neuron cultures were treated with L-homocysteine (L-Hcy, 50 μM) for either 1 hr and then maintained in the experimental medium for another 18 hours or continuously exposed to homocysteine for 18 hours. (A) Representative photomicrographs of Hoechst DNA staining. Examples of pyknotic DNA are indicated with arrows. (B) Quantitative analysis of the percentages of neurons with pyknotic nuclei is represented as mean ± s.e.m. (n = 1500 cells/condition from 3 experiments). Asterisk * indicates significant difference from control (p < 0.05).
Figure 6.
Homocysteine induced neuronal cell death is mediated by NMDA receptor. Neuronal cultures were treated with 50 μM of L-homocysteine (L-Hcy) for 18 hours in the presence or absence of APV (200 μM). (A) Representative photomicrographs of Hoechst DNA staining. Examples of pyknotic DNA are indicated with arrows. (B) Quantitative analysis of the percentages of neurons with pyknotic nuclei is represented as mean ± s.e.m. (n = 1500 cells/condition from 3 experiments). Asterisk * indicates significant difference from control (p < 0.001), and ** indicates significant difference from L-Hcy treatment (p < 0.001).
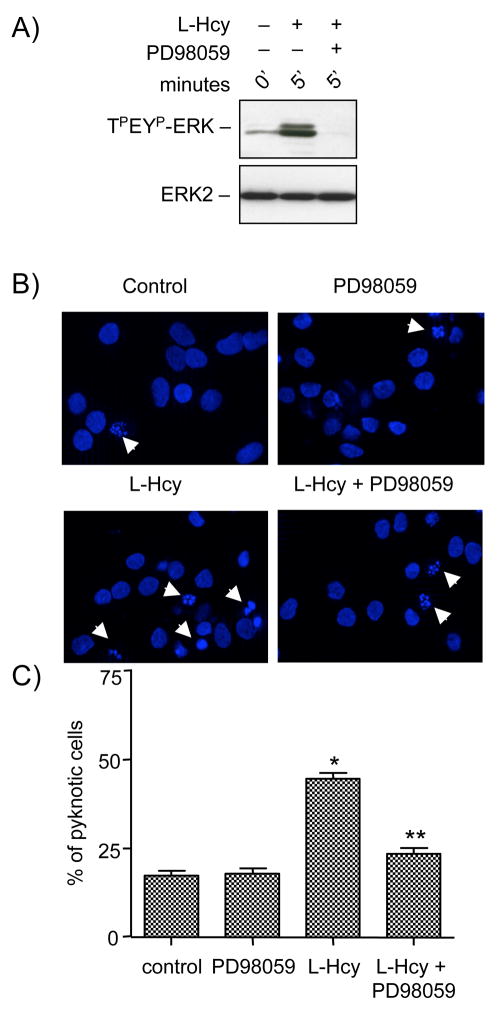
To further clarify the role of ERK2 in homocysteine-NMDA receptor mediated neuronal cell death neurons were treated with L-homocysteine (50 μM, 5 minutes) in the absence or presence of PD98059, a selective inhibitor of MEK1/2, an upstream kinase of ERK MAP kinase. Immunoblot analysis showed that PD98059 completely prevented homocysteine induced ERK2 phosphorylation without affecting total ERK2 levels (Figure 7A). In a parallel series of experiments neurons were treated with homocysteine (50 μM) in the absence or presence of PD98059 and 18 hours later cell death was assessed by Hoechst DNA staining. Figure 7B and 7C show that inhibition of ERK2 phosphorylation significantly attenuated homocysteine-induced neuronal cell death (23.3 ± 1.6%). Incubation with PD98059 alone had no effect on cell death (17.89 ± 1.58%) in these experiments. These findings indicate that phosphorylation and subsequent activation of ERK MAP kinase pathway plays a critical role in homocysteine-NMDA receptor mediated neuronal cell death.
Figure 7.
Homocysteine-NMDA receptor induced neuronal cell death is regulated by ERK MAP kinase. (A) Immunoblot analysis of neurons pre-incubated with PD98059 (15 μM) before treatment with 50 μM of L- homocysteine (L-Hcy) for 5 minute. (B, C) Neuronal cultures were treated with 50 μM of L-homocysteine (L-Hcy) for 18 hours in the presence or absence of PD98059 (15 μM). (B) Representative photomicrographs of Hoechst DNA staining. Examples of pyknotic DNA are indicated with arrows. (C) Quantitative analysis of the percentages of neurons with pyknotic nuclei is represented as mean ± s.e.m. (n = 1500 cells/condition from 3 experiments). Asterisk * indicates significant difference from control (p < 0.001), and ** indicates significant difference from L-Hcy treatment (p < 0.001).
Homocysteine leads to transient phosphorylation of cAMP response-element binding (CREB) protein
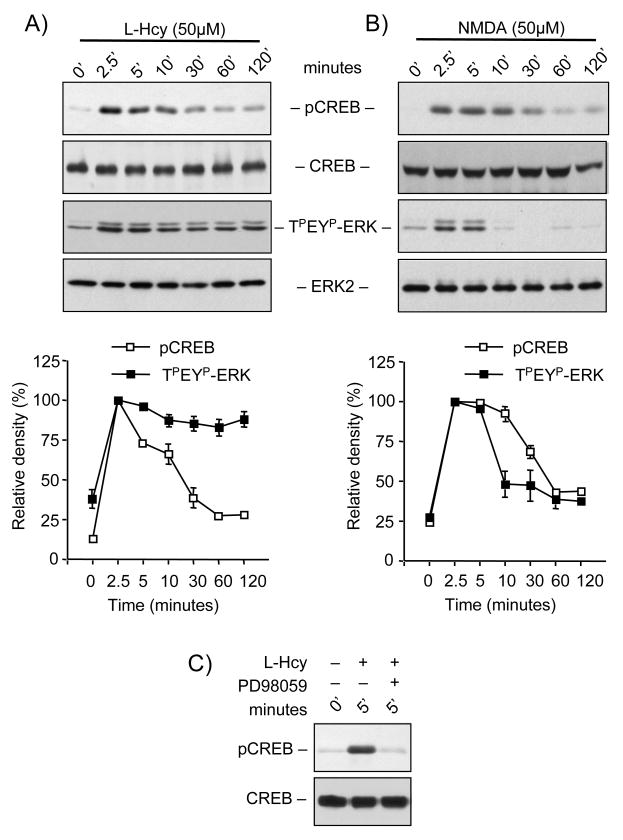
An important downstream target of ERK-dependent signaling is the transcription factor CREB, which is known to promote cell survival (Vanhoutte et al. 1999). To determine the temporal profile of CREB phosphorylation neurons were treated with L-homocysteine (50 μM) for different time periods (0, 2.5, 5, 10, 30, 60 and 120 minutes) followed by immunoblot analysis with anti phospho-CREB antibody. Figure 8A shows that phosphorylation of CREB increased within 2.5 minutes of stimulation and this gradually declined to basal levels within 30 minutes. When compared with the kinetics of homocysteine induced ERK2 phosphorylation (Figure 8A) the onset of CREB phosphorylation coincided with the phosphorylation of ERK2. However unlike the sustained nature of ERK2 phosphorylation, the phosphorylation of CREB was transient. On the other hand, Figure 8B shows that in neurons treated with NMDA (50 μM) for specified time periods (0, 2.5, 5, 10, 30, 60 and 120 minutes) phosphorylation of both CREB and ERK2 followed the same temporal profile. This later result is consistent with previous findings, which showed that treatment with glutamate or NMDA resulted in transient increase in phosphorylation of both ERK2 and CREB within 2.5 minutes followed by a time-dependent decrease to basal levels by 30 minutes (Chandler et al. 2001; Paul et al. 2003). Finally, to confirm that homocysteine-mediated phosphorylation of CREB is downstream of ERK MAP kinase neurons were pre-incubated with PD98059 prior to treatment with L-homocysteine (50 μM, 5 minutes). Figure 8C shows that treatment with PD98059 blocked homocysteine-mediated phosphorylation of CREB.
Figure 8.
Homocysteine leads to transient phosphorylation of CREB in neurons. Neuronal cultures were either treated with (A) 50 μM of L- homocysteine (L-Hcy) or (B) 50 μM NMDA for specific time periods as indicated. Immunoblot analysis was performed with an antibody that recognizes CREB phosphorylated at ser133 (pCREB), anti-CREB, anti-TPEYP-ERK and anti-ERK2 antibodies as indicated. Time-dependent phosphorylation of CREB and ERK2 by computer-assisted densitometry is quantitated. Values are mean ± s.e.m. (n=3). Asterisk * indicates significant difference from 2.5 minutes L-Hcy or NMDA treatment (p<0.05). (C) Immunoblot analysis with anti pCREB and anti-CREB antibody, of cells pre-incubated with PD98059 (15 μM) before treatment with 50 μM of L-Hcy for 5 minutes.
DISCUSSION
A key finding of the present study is that prolonged exposure to homocysteine leads to significant increases in neuronal cell death over basal levels that is dependent on NMDA receptor activation. Most importantly, the study demonstrates a role of ERK MAP kinase in neuronal cell death. Stimulation of NMDA receptors by homocysteine lead to a rapid and sustained activation of ERK2 and the inhibition of ERK2 protected the neurons against homocysteine-induced cell death. In contrast, CREB a downstream target of ERK and a pro-survival factor is only transiently activated following treatment with homocysteine. This indicates that homocysteine not only leads to the activation of ERK but also stimulates a negative feed back loop that involves dephosphorylation and inactivation of CREB. As a consequence certain pro-apototic targets of ERK2 (Zhuang and Schnellmann 2006) may be activated that eventually leads to neuronal cell death.
Mild to moderate hyper-homocysteinemic conditions exist in individuals whose plasma homocysteine concentration ranges between 15–100 μM and these levels are considered to be a risk factor for neurodegenerative diseases (Hankey and Eikelboom 2001; Austin et al. 2004; Obeid and Herrmann 2006; Zoccolella et al. 2006). Severe hyper-homocysteinemic conditions prevail when plasma levels of total homocysteine are >100 μM and may be found in individuals with inherited genetic disorders (Hankey and Eikelboom 2001; Austin et al. 2004; Obeid and Herrmann 2006; Zoccolella et al. 2006). In mammalian brain, under normal conditions total homocysteine levels as high as 10 μM has been reported in certain regions (Broch and Ueland 1984). During hyper-homocysteinemia, elevated homocysteine concentrations in brain may either result from cellular metabolism within the brain itself or by diffusion and carrier/receptor mediated transport mechanisms across the blood brain barrier (Obeid and Herrmann 2006). In animal models of hyper-homocysteinemia it has been shown that plasma homocysteine levels of 100 μM compromises the integrity of the blood-brain barrier resulting in leakage and exposure of brain cells to relatively higher homocysteine levels (Kamath et al. 2006). Additionally, disruption of the blood-brain barrier following cerebral stroke (Phillis et al. 1994) or head trauma (Palmer et al. 1994) in animals results in exposure of the brain to near plasma levels of amino acids including homocysteine (Lipton et al. 1997). In stroke patients the brain may remain exposed to high homocysteine levels for prolonged periods (Lindgren et al. 1995; Perry et al. 1995; Selhub et al. 1995). This implies that even moderate plasma homocysteine levels, resulting from common vitamin cofactor deficiency, medications or diseases (Obeid and Herrmann 2006), may contribute to neuronal damage in vivo (Lipton et al. 1997). In this context a recent in vitro study has shown that treatment with DL-homocysteine (0.5 – 250 μM) results in a dose-dependent increase in apoptosis in hippocampal neurons (Kruman et al. 2000). Our results also demonstrate that 50 μM L-homocysteine induces significant cell death in cortical neurons.
ERK 1 and ERK 2 are versatile protein kinases that are ubiquitiously expressed in the central nervous system. They have been implicated in numerous signaling cascades within neurons. Functions assigned to ERK activity include neuronal maturation and survival during development (Dolmetsch et al. 2001), changes in synaptic plasticity underlying learning and memory (English and Sweatt 1997; Kornhauser and Greenberg 1997; Atkins et al. 1998; Blum et al. 1999; Coogan et al. 1999) and regulation of immediate early genes CREB and Elk-1 (Pende et al. 1997; Vanhoutte et al. 1999; Mabuchi et al. 2001; Lee et al. 2005). Recent studies indicate that ERK MAP kinase may also play a role in promoting neuronal cell death both in development and in neurodegenerative disorders. Bhat and Zhang (1999) first reported that inhibition of ERK MAP kinase using PD98059 rescues oligodendrocytes from H2O2-induced cell death and this observation was subsequently confirmed in neurons (Lesuisse and Martin 2002). Inhibition of ERK also improved cell survival of neurons subjected to oxidative stress and seizure like activity (Murray et al. 1998). Evidence for the involvement of ERK MAP kinase in neurodegeneration in vivo was obtained from an animal model of stroke induced by transient occlusion of the middle cerebral artery. An increased phosphorylation of ERK was detected following stroke and pretreatment with inhibitors that blocked phosphorylation of ERK resulted in a significant decrease in infarct volume (Alessandrini et al. 1999; Namura et al. 2001; Wang et al. 2004). ERK MAP kinase was also activated in the damaged brain tissue obtained from a mice model of traumatic brain injury and pre-treatment with PD98059 prior to trauma resulted in a significant reduction in cortical lesion (Mori et al. 2002; Clausen et al. 2004).
It has been proposed that transient activation of ERK MAP kinase has different consequences as compared with sustained activation (Colucci-D’Amato et al. 2003; Zhuang and Schnellmann 2006). Transient activation of ERK plays a pivotal role in neuronal maturation, survival and long-term potentiation (Boulton et al. 1991; Xia et al. 1995; Segal and Greenberg 1996). Whereas sustained activation of ERK may play a critical role in triggering pro-apoptotic signals and neuronal cell death. For example, sustained activation of ERK by inhibition of protein phosphatases has been shown to induce neuronal cell death in hippocampal slices (Runden et al. 1998). Again sustained activation of ERK following a relatively prolonged (6–9 hours) treatment with glutamate (5 mM) leads to caspase-dependent neuronal cell death (Stanciu et al. 2000). Inhibition of ERK protected the neurons against this glutamate-induced cell death even if the inhibition was initiated 1 hour after addition of glutamate. However the underlying signaling mechanisms that lead to the transient or sustained phosphorylation of ERK is not completely understood. As evident from earlier studies as well as our findings, NMDA receptor stimulation by glutamate or NMDA leads to transient increase in ERK2 phosphorylation (Jiang et al. 2000a, b; Chandler et al. 2001; Paul et al. 2003). This is consistent with the suggestion that glutamate mediated NMDA receptor stimulation leads to activation of both stimulatory and inhibitory pathways involved in the modulation of ERK signaling (Marshall 1995; Vincent et al. 1998; Vanhoutte et al. 1999; Chandler et al. 2001; Paul et al. 2003; Waxman and Lynch 2005a, b). Supporting this hypothesis recent findings demonstrated that glutamate-mediated NMDA receptor stimulation not only activates the phosphatidylinositol 3-kinase pathway leading to phosphorylation of ERK but also initiates a negative feedback loop that involves inhibition of the phosphatidylinositol 3-kinase pathway (Chandler et al. 2001; Kim et al. 2005) as well as the activation of the tyrosine phosphatase STEP (Paul et al. 2003). The later in turn dephosphorylates ERK and limits the duration of its activation. Furthermore it has been shown that activation of NR2A-NMDARs promotes ERK MAP kinase activation whereas NR2B-NMDARs inhibits ERK activation (Kim et al. 2005; Waxman and Lynch 2005b; Ivanov et al. 2006). Our findings now demonstrate that homocysteine-mediated NMDA receptor stimulation leads to sustained activation of ERK2, which is mediated through NR2A-NMDARs. Taken together these findings suggest that homocysteine may lead to a lower level of receptor stimulation that is sufficient to activate NR2A-NMDARs and promote ERK2 activation. While glutamate or NMDA may lead to a higher-level of receptor stimulation that can activate both NR2A- and NR2B-NMDA receptors resulting in sequential activation of both the stimulatory and inhibitory pathways to reduce the duration of ERK2 activation. Thus the duration of ERK MAP kinase activation following NMDA receptor stimulation depends on the nature of the extracellular stimulus and may have different consequences on intracellular signaling pathways eventually leading to different cellular responses.
The dual role of ERK MAP kinase in cell survival and death suggests that a unique profile of gene expression may be elicited depending on the duration and/or magnitude of ERK MAP kinase activation (Marshall 1995; Herdegen and Leah 1998). One downstream target of ERK-dependent signaling is the transcription factor CREB (Pende et al. 1997) and is a potentially important component of the survival pathway (Vanhoutte et al. 1999; Mabuchi et al. 2001; Hara et al. 2003; Lee et al. 2005). Our findings show that homocysteine induced phosphorylation of CREB (serine133) is mediated through ERK MAP kinase but the duration of CREB’s phosphorylation is transient as compared to phosphorylation of ERK. Persistent phosphorylation of CREB at serine133 has been correlated with cell survival and neuroprotection (Mabuchi et al. 2001; Hara et al. 2003; Lee et al. 2005). This suggests that in spite of the initial ERK mediated phosphorylation of CREB, homocysteine-dependent NMDA receptor stimulation may activate a feedback loop that shuts off the CREB signaling without affecting phosphorylation of ERK. This in turn facilitates the activation of ERK dependent pro-apoptotic pathways that may eventually lead to neuronal cell death. In conclusion the present study provides new insights into the signaling mechanisms underlying homocysteine mediated NMDA receptor stimulation that may be associated with the pathology of hyper-homocysteinemia. Further studies are necessary to elucidate the signaling mechanisms downstream of homocysteine-NMDA receptor stimulation of ERK MAP kinase that may lead to neuronal cell death.
Acknowledgments
We would like to thank CW Shuttleworth and JA Connor for their helpful comments. This work was supported by the National Institutes of Health grants P20 RR15636 (Okada, Y.) and New Mexico Trust Fund, UNM HSC RAC (Poddar, R.).
Abbreviations
- NMDA
N-methyl-D-aspartate
- AMPA
amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- ERK
Extracellular-signal regulated kinase
- CREB
cAMP response-element binding protein
- NO
nitric oxide
References
- Alessandrini A, Namura S, Moskowitz MA, Bonventre JV. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12866–12869. doi: 10.1073/pnas.96.22.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Austin RC, Lentz SR, Werstuck GH. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ. 2004;11(Suppl 1):S56–64. doi: 10.1038/sj.cdd.4401451. [DOI] [PubMed] [Google Scholar]
- Banko JL, Hou L, Klann E. NMDA receptor activation results in PKA- and ERK-dependent Mnk1 activation and increased eIF4E phosphorylation in hippocampal area CA1. Journal of neurochemistry. 2004;91:462–470. doi: 10.1111/j.1471-4159.2004.02734.x. [DOI] [PubMed] [Google Scholar]
- Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Broch OJ, Ueland PM. Regional distribution of homocysteine in the mammalian brain. Journal of neurochemistry. 1984;43:1755–1757. doi: 10.1111/j.1471-4159.1984.tb06105.x. [DOI] [PubMed] [Google Scholar]
- Cao J, Viholainen JI, Dart C, Warwick HK, Leyland ML, Courtney MJ. The PSD95-nNOS interface: a target for inhibition of excitotoxic p38 stress-activated protein kinase activation and cell death. The Journal of cell biology. 2005;168:117–126. doi: 10.1083/jcb.200407024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LJ, Sutton G, Dorairaj NR, Norwood D. N-methyl D-aspartate receptor-mediated bidirectional control of extracellular signal-regulated kinase activity in cortical neuronal cultures. The Journal of biological chemistry. 2001;276:2627–2636. doi: 10.1074/jbc.M003390200. [DOI] [PubMed] [Google Scholar]
- Clausen F, Lundqvist H, Ekmark S, Lewen A, Ebendal T, Hillered L. Oxygen free radical-dependent activation of extracellular signal-regulated kinase mediates apoptosis-like cell death after traumatic brain injury. J Neurotrauma. 2004;21:1168–1182. doi: 10.1089/neu.2004.21.1168. [DOI] [PubMed] [Google Scholar]
- Colucci-D’Amato L, Perrone-Capano C, di Porzio U. Chronic activation of ERK and neurodegenerative diseases. Bioessays. 2003;25:1085–1095. doi: 10.1002/bies.10355. [DOI] [PubMed] [Google Scholar]
- Coogan AN, O’Leary DM, O’Connor JJ. P42/44 MAP kinase inhibitor PD98059 attenuates multiple forms of synaptic plasticity in rat dentate gyrus in vitro. JNeurophysiol. 1999;81:103–110. doi: 10.1152/jn.1999.81.1.103. [DOI] [PubMed] [Google Scholar]
- Daxhelet GA, Coene MM, Hoet PP, Cocito CG. Spectrofluorometry of dyes with DNAs of different base composition and conformation. Anal Biochem. 1989;179:401–403. doi: 10.1016/0003-2697(89)90152-8. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Vermeer SE, Clarke R, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MM. Homocysteine and brain atrophy on MRI of non-demented elderly. Brain. 2003;126:170–175. doi: 10.1093/brain/awg006. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. Journal of neurochemistry. 2002;80:101–110. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- Durkin JP, Tremblay R, Buchan A, Blosser J, Chakravarthy B, Mealing G, Morley P, Song D. An early loss in membrane protein kinase C activity precedes the excitatory amino acid-induced death of primary cortical neurons. Journal of neurochemistry. 1996;66:951–962. doi: 10.1046/j.1471-4159.1996.66030951.x. [DOI] [PubMed] [Google Scholar]
- Endres M, Ahmadi M, Kruman I, Biniszkiewicz D, Meisel A, Gertz K. Folate deficiency increases postischemic brain injury. Stroke. 2005;36:321–325. doi: 10.1161/01.STR.0000153008.60517.ab. [DOI] [PubMed] [Google Scholar]
- English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. The Journal of biological chemistry. 1997;272:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- Gottfries CG, Lehmann W, Regland B. Early diagnosis of cognitive impairment in the elderly with the focus on Alzheimer’s disease. J Neural Transm. 1998;105:773–786. doi: 10.1007/s007020050094. [DOI] [PubMed] [Google Scholar]
- Hankey GJ, Eikelboom JW. Homocysteine and stroke. Curr Opin Neurol. 2001;14:95–102. doi: 10.1097/00019052-200102000-00015. [DOI] [PubMed] [Google Scholar]
- Hara T, Hamada J, Yano S, Morioka M, Kai Y, Ushio Y. CREB is required for acquisition of ischemic tolerance in gerbil hippocampal CA1 region. Journal of neurochemistry. 2003;86:805–814. doi: 10.1046/j.1471-4159.2003.01847.x. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Ho PI, Ortiz D, Rogers E, Shea TB. Multiple aspects of homocysteine neurotoxicity: glutamate excitotoxicity, kinase hyperactivation and DNA damage. J Neurosci Res. 2002;70:694–702. doi: 10.1002/jnr.10416. [DOI] [PubMed] [Google Scholar]
- Irving EA, Barone FC, Reith AD, Hadingham SJ, Parsons AA. Differential activation of MAPK/ERK and p38/SAPK in neurones and glia following focal cerebral ischaemia in the rat. Brain Res Mol Brain Res. 2000;77:65–75. doi: 10.1016/s0169-328x(00)00043-7. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. The Journal of physiology. 2006;572:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jara-Prado A, Ortega-Vazquez A, Martinez-Ruano L, Rios C, Santamaria A. Homocysteine-induced brain lipid peroxidation: effects of NMDA receptor blockade, antioxidant treatment, and nitric oxide synthase inhibition. Neurotox Res. 2003;5:237–243. doi: 10.1007/BF03033381. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Gu Z, Zhang G, Jing G. N-methyl-D-aspartate receptor activation results in regulation of extracellular signal-regulated kinases by protein kinases and phosphatases in glutamate-induced neuronal apototic-like death. Brain Res. 2000a;887:285–292. doi: 10.1016/s0006-8993(00)03003-1. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Gu Z, Zhang G, Jing G. Diphosphorylation and involvement of extracellular signal-regulated kinases (ERK1/2) in glutamate-induced apoptotic-like death in cultured rat cortical neurons. Brain Res. 2000b;857:71–77. doi: 10.1016/s0006-8993(99)02364-1. [DOI] [PubMed] [Google Scholar]
- Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, Wagner DD. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. 2006;107:591–593. doi: 10.1182/blood-2005-06-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Kornhauser JM, Greenberg ME. A kinase to remember: dual roles for MAP kinase in long-term memory. Neuron. 1997;18:839–842. doi: 10.1016/s0896-6273(00)80322-0. [DOI] [PubMed] [Google Scholar]
- Kruman II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L, Mattson MP. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci. 2000;20:6920–6926. doi: 10.1523/JNEUROSCI.20-18-06920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, Haughey N, Lee J, Evans M, Mattson MP. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci. 2002;22:1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarewicz JW, Ziembowicz A, Matyja E, Stafiej A, Zieminska E. Homocysteine-evoked 45Ca release in the rabbit hippocampus is mediated by both NMDA and group I metabotropic glutamate receptors: in vivo microdialysis study. Neurochem Res. 2003;28:259–269. doi: 10.1023/a:1022329317218. [DOI] [PubMed] [Google Scholar]
- Lee B, Butcher GQ, Hoyt KR, Impey S, Obrietan K. Activity-dependent neuroprotection and cAMP response element-binding protein (CREB): kinase coupling, stimulus intensity, and temporal regulation of CREB phosphorylation at serine 133. J Neurosci. 2005;25:1137–1148. doi: 10.1523/JNEUROSCI.4288-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz G, Avruch J. Glutamatergic regulation of the p70S6 kinase in primary mouse neurons. The Journal of biological chemistry. 2005;280:38121–38124. doi: 10.1074/jbc.C500363200. [DOI] [PubMed] [Google Scholar]
- Lesuisse C, Martin LJ. Immature and mature cortical neurons engage different apoptotic mechanisms involving caspase-3 and the mitogen-activated protein kinase pathway. J Cereb Blood Flow Metab. 2002;22:935–950. doi: 10.1097/00004647-200208000-00005. [DOI] [PubMed] [Google Scholar]
- Lindgren A, Brattstrom L, Norrving B, Hultberg B, Andersson A, Johansson BB. Plasma homocysteine in the acute and convalescent phases after stroke. Stroke. 1995;26:795–800. doi: 10.1161/01.str.26.5.795. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM, Rayudu PV, Arnelle DR, Stamler JS. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5923–5928. doi: 10.1073/pnas.94.11.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchowska E, Luchowski P, Paczek R, Ziembowicz A, Kocki T, Turski WA, Wielosz M, Lazarewicz J, Urbanska EM. Dual effect of DL-homocysteine and S-adenosylhomocysteine on brain synthesis of the glutamate receptor antagonist, kynurenic acid. J Neurosci Res. 2005;79:375–382. doi: 10.1002/jnr.20359. [DOI] [PubMed] [Google Scholar]
- Luo J, Wang Y, Yasuda RP, Dunah AW, Wolfe BB. The majority of N-methyl-D-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B) Molecular pharmacology. 1997;51:79–86. doi: 10.1124/mol.51.1.79. [DOI] [PubMed] [Google Scholar]
- Mabuchi T, Kitagawa K, Kuwabara K, Takasawa K, Ohtsuki T, Xia Z, Storm D, Yanagihara T, Hori M, Matsumoto M. Phosphorylation of cAMP response element-binding protein in hippocampal neurons as a protective response after exposure to glutamate in vitro and ischemia in vivo. J Neurosci. 2001;21:9204–9213. doi: 10.1523/JNEUROSCI.21-23-09204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Miller JW. Homocysteine and Alzheimer’s disease. Nutr Rev. 1999;57:126–129. doi: 10.1111/j.1753-4887.1999.tb06936.x. [DOI] [PubMed] [Google Scholar]
- Mori T, Wang X, Jung JC, Sumii T, Singhal AB, Fini ME, Dixon CE, Alessandrini A, Lo EH. Mitogen-activated protein kinase inhibition in traumatic brain injury: in vitro and in vivo effects. J Cereb Blood Flow Metab. 2002;22:444–452. doi: 10.1097/00004647-200204000-00008. [DOI] [PubMed] [Google Scholar]
- Murray B, Alessandrini A, Cole AJ, Yee AG, Furshpan EJ. Inhibition of the p44/42 MAP kinase pathway protects hippocampal neurons in a cell-culture model of seizure activity. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:11975–11980. doi: 10.1073/pnas.95.20.11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namura S, Iihara K, Takami S, Nagata I, Kikuchi H, Matsushita K, Moskowitz MA, Bonventre JV, Alessandrini A. Intravenous administration of MEK inhibitor U0126 affords brain protection against forebrain ischemia and focal cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11569–11574. doi: 10.1073/pnas.181213498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid R, Herrmann W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 2006;580:2994–3005. doi: 10.1016/j.febslet.2006.04.088. [DOI] [PubMed] [Google Scholar]
- Palmer AM, Marion DW, Botscheller ML, Bowen DM, DeKosky ST. Increased transmitter amino acid concentration in human ventricular CSF after brain trauma. Neuroreport. 1994;6:153–156. doi: 10.1097/00001756-199412300-00039. [DOI] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- Pende M, Fisher TL, Simpson PB, Russell JT, Blenis J, Gallo V. Neurotransmitter- and growth factor-induced cAMP response element binding protein phosphorylation in glial cell progenitors: role of calcium ions, protein kinase C, and mitogen-activated protein kinase/ribosomal S6 kinase pathway. J Neurosci. 1997;17:1291–1301. doi: 10.1523/JNEUROSCI.17-04-01291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry IJ, Refsum H, Morris RW, Ebrahim SB, Ueland PM, Shaper AG. Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet. 1995;346:1395–1398. doi: 10.1016/s0140-6736(95)92407-8. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Smith-Barbour M, O’Regan MH, Perkins LM. Amino acid and purine release in rat brain following temporary middle cerebral artery occlusion. Neurochem Res. 1994;19:1125–1130. doi: 10.1007/BF00965145. [DOI] [PubMed] [Google Scholar]
- Poddar R, Sivasubramanian N, DiBello PM, Robinson K, Jacobsen DW. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular disease. Circulation. 2001;103:2717–2723. doi: 10.1161/01.cir.103.22.2717. [DOI] [PubMed] [Google Scholar]
- Robert K, Pages C, Ledru A, Delabar J, Caboche J, Janel N. Regulation of extracellular signal-regulated kinase by homocysteine in hippocampus. Neuroscience. 2005;133:925–935. doi: 10.1016/j.neuroscience.2005.03.034. [DOI] [PubMed] [Google Scholar]
- Runden E, Seglen PO, Haug FM, Ottersen OP, Wieloch T, Shamloo M, Laake JH. Regional selective neuronal degeneration after protein phosphatase inhibition in hippocampal slice cultures: evidence for a MAP kinase-dependent mechanism. J Neurosci. 1998;18:7296–7305. doi: 10.1523/JNEUROSCI.18-18-07296.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco RL, Roberts JK, Jacobs BS. Homocysteine as a risk factor for ischemic stroke: an epidemiological story in evolution. Neuroepidemiology. 1998;17:167–173. doi: 10.1159/000026169. [DOI] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nature reviews. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- Selhub J, Jacques PF, Bostom AG, D’Agostino RB, Wilson PW, Belanger AJ, O’Leary DH, Wolf PA, Schaefer EJ, Rosenberg IH. Association between plasma homocysteine concentrations and extracranial carotid-artery stenosis. N Engl J Med. 1995;332:286–291. doi: 10.1056/NEJM199502023320502. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- Shi Q, Savage JE, Hufeisen SJ, Rauser L, Grajkowska E, Ernsberger P, Wroblewski JT, Nadeau JH, Roth BL. L-homocysteine sulfinic acid and other acidic homocysteine derivatives are potent and selective metabotropic glutamate receptor agonists. J Pharmacol Exp Ther. 2003;305:131–142. doi: 10.1124/jpet.102.047092. [DOI] [PubMed] [Google Scholar]
- Stanciu M, DeFranco DB. Prolonged nuclear retention of activated extracellular signal-regulated protein kinase promotes cell death generated by oxidative toxicity or proteasome inhibition in a neuronal cell line. The Journal of biological chemistry. 2002;277:4010–4017. doi: 10.1074/jbc.M104479200. [DOI] [PubMed] [Google Scholar]
- Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, Reynolds I, Klann E, Angiolieri MR, Johnson JW, DeFranco DB. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. The Journal of biological chemistry. 2000;275:12200–12206. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- Van den Berg M, Boers GH, Franken DG, Blom HJ, Van Kamp GJ, Jakobs C, Rauwerda JA, Kluft C, Stehouwert CD. Hyperhomocysteinaemia and endothelial dysfunction in young patients with peripheral arterial occlusive disease. Eur J Clin Invest. 1995;25:176–181. doi: 10.1111/j.1365-2362.1995.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P, Barnier JV, Guibert B, Pages C, Besson MJ, Hipskind RA, Caboche J. Glutamate induces phosphorylation of Elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Mol Cell Biol. 1999;19:136–146. doi: 10.1128/mcb.19.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SR, Sebben M, Dumuis A, Bockaert J. Neurotransmitter regulation of MAP kinase signaling in striatal neurons in primary culture. Synapse. 1998;29:29–36. doi: 10.1002/(SICI)1098-2396(199805)29:1<29::AID-SYN3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Wu DC, Huang FP, Yang GY. Inhibition of MEK/ERK 1/2 pathway reduces pro-inflammatory cytokine interleukin-1 expression in focal cerebral ischemia. Brain Res. 2004;996:55–66. doi: 10.1016/j.brainres.2003.09.074. [DOI] [PubMed] [Google Scholar]
- Watkins D, Rosenblatt DS. Functional methionine synthase deficiency (cblE and cblG): clinical and biochemical heterogeneity. Am J Med Genet. 1989;34:427–434. doi: 10.1002/ajmg.1320340320. [DOI] [PubMed] [Google Scholar]
- Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtype mediated bidirectional control of p38 mitogen-activated protein kinase. The Journal of biological chemistry. 2005a;280:29322–29333. doi: 10.1074/jbc.M502080200. [DOI] [PubMed] [Google Scholar]
- Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005b;11:37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yun HY, Dawson VL, Dawson TM. Glutamate-stimulated calcium activation of Ras/Erk pathway mediated by nitric oxide. Diabetes Res Clin Pract. 1999;45:113–115. doi: 10.1016/s0168-8227(99)00039-x. [DOI] [PubMed] [Google Scholar]
- Yuzaki M, Connor JA. Characterization of L-homocysteate-induced currents in Purkinje cells from wild-type and NMDA receptor knockout mice. J Neurophysiol. 1999;82:2820–2826. doi: 10.1152/jn.1999.82.5.2820. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Schnellmann RG. A death-promoting role for extracellular signal-regulated kinase. J Pharmacol Exp Ther. 2006;319:991–997. doi: 10.1124/jpet.106.107367. [DOI] [PubMed] [Google Scholar]
- Zieminska E, Stafiej A, Lazarewicz JW. Role of group I metabotropic glutamate receptors and NMDA receptors in homocysteine-evoked acute neurodegeneration of cultured cerebellar granule neurones. Neurochem Int. 2003;43:481–492. doi: 10.1016/s0197-0186(03)00038-x. [DOI] [PubMed] [Google Scholar]
- Zoccolella S, Martino D, Defazio G, Lamberti P, Livrea P. Hyperhomocysteinemia in movement disorders: Current evidence and hypotheses. Curr Vasc Pharmacol. 2006;4:237–243. doi: 10.2174/157016106777698414. [DOI] [PubMed] [Google Scholar]