Abstract
Objective
To compare the efficacy of recombinant human follicle stimulating hormone (rhFSH), to rhFSH with 4 additional O-linked carbohydrates (rhFSH-CTP), rhFSH with 4 additional N-linked carbohydrates (rhFSH-N4) and the current gold-standard for rodent ovarian stimulation, pregnant mare serum gonadotropin (PMSG), on fertility parameters in mice.
Design
Animal Study
Setting
Academic Research Center
Subjects
Adult C57Bl/6J female mice
Interventions
Ovarian stimulation with 5IUof rhFSH, rhFSH-CTP, rhFSH-N4 or PMSG. 46 hours later 5IU of hCG was injected to promote ovulation then females were mated overnight.
Main Outcome Measures
Eggs retrieved following ovulation, in vitro embryo development, delivery rate, litter size
Results
Hyperglycosylated FSH analogues, rhFSH-CTP and rhFSH-N4, enhanced egg ovulation and embryo maturation significantly better than rhFSH. RhFSH-N4 produced more eggs (28.5±1.9 per mouse) and embryos (17.8±1.6) compared to rhFSH-CTP (18.3±1.2 and 9.0±1.0, respectively). Treatment with rhFSH, rhFSH-N4 and PMSG produced statistically equivalent delivery rates and litter sizes. The delivery rate was surprisingly lower with rhFSH-CTP (14%) compared to PMSG (33%).
Conclusions
Compared to rhFSH, treatment with hyperglycosylated rhFSH-CTP and rhFSH-N4 led to superior rates of ovulated eggs and subsequent in vitro embryo development. RhFSH-N4 was equivalent to PMSG while rhFSH-CTP was significantly lower than PMSG therapy for all fertility parameters studied.
Keywords: fertility, glycoengineering, hyperglycosylated FSH, N-linked glycosylation, O-linked glycosylation, ovary
Introduction
Exogenous administration of follicle stimulating hormone (FSH) has become a mainstay of treatment for infertile women participating in assisted reproduction technology (ART). Although current FSH preparations are effective for hyperstimulation of the ovary they present several therapeutic limitations. First, current FSH medications require a subcutaneous injection on a daily or twice-daily schedule for as long as 10–12 days per assisted reproduction cycle. Even in this highly motivated patient population the frequent administration of these painful injections can reduce patient compliance. Second, if a patient has a poor response to 250IU of FSH twice a day, higher doses are unable to provide additional benefit to the patient. For these reasons, the production of a long-acting FSH analog to reduce the frequency of injections could improve patient compliance and the development of an FSH analogue with enhanced biological activity would potentially improve ovarian stimulation results in patients who demonstrated a poor response to current FSH therapies.
Several laboratories have produced long-acting, hyperglycosylated FSH analogues. Boime and colleagues determined that the 30 amino acid carboxy-terminal peptide (CTP) sequence located at 3′ end of the beta subunit of hCG was responsible for the extended half-life of the hCG protein in vivo (1). They further demonstrated that synthetic ligation of the CTP to the 3′ end of the FSH beta subunit (rhFSH-CTP) produced a biologically active protein with an extended half-life(2, 3). Additional studies with hCG confirmed the increased half-life of CTP was due to the presence of four O-linked carbohydrates and removal of the carbohydrates accelerated the clearance of the protein from circulation(1, 2, 4, 5). Perlman and colleagues used molecular methods to attach additional glycosylation sites to the common alpha subunit of the glycoprotein family which also extended the half-life of follicle stimulating hormone (6). The Lustbader laboratory utilized the CTP sequence as a linker for the alpha and beta subunits of FSH to generate a single-chain hyperglycosylated FSH protein (7). The single-chain protein exhibited a longer half-life and was proven to elevate serum estradiol for approximately 5 days in a non-human primate (7, 8).
We also reported the production of a series of single-chain, long-acting FSH proteins produced by the addition of novel N-linked carbohydrate sequences (9). The consensus sequence for the addition of N-linked carbohydrates is known (Asn-X-Ser/Thr, where X represents any amino acid except Pro) while no clear sequence has been identified for O-linked glycosylation. Therefore it is easier to manipulate N-linked glycosylation alterations. An additional benefit of N-linked carbohydrates is the increased number of sialic acid binding sites leading to reduced pI and longer in vivo half-life (9, 10). Furthermore, N-linked carbohydrates are more complex which has been suggested to enhance the biological activity of a protein beyond simply increasing the protein half-life(11). Previous evaluation of in vivo activity of rhFSH-CTP and rhFSH-N4 in rats demonstrated that both rhFSH-CTP and rhFSH-N4 enhanced protein half-life to a similar extent; but rhFSH-N4 increased biological activity as demonstrated by greater ovarian folliculogenesis as compared to rhFSH-CTP which could not be explained by increase of half-life alone(9). Furthermore, when rhFSH-N4 was injected into mice, it was found to have a similar bioactivity on ovarian folliculogenesis as the current gold standard for rodent hyperstimulation protocols PMSG(12).
Women undergoing ART are designated “good responders” based on the number of oocytes collected and the number of viable embryos available for transfer. The ultimate goal of ART is a pregnancy resulting in the live-birth of a healthy infant. Based on our previous data that demonstrated rhFSH-CTP and rhFSH-N4 were superior to rhFSH therapy for promoting large antral follicle production in rodents, we hypothesized that, compared to rhFSH, hyperglycosylated FSH proteins would also increase the number of eggs released during ovulation and enhance the number of embryos that develop in female mice. We also postulated that rFSH-N4 would be equivalent to PMSG during an evaluation of birth rates and pups per litter.
This study was designed to provide a direct comparison of rhFSH, rhFSH-CTP, rhFSH-N4 and PMSG on mouse female fertility including: the number of eggs released into the fallopian tubes following ovulation; the number of fertilized eggs that progress to the embryo stage in vitro; the delivery rate; and litter size. There was a surprising discrepancy between ovulation data and delivery data for rhFSH-CTP. In contrast, administration of rhFSH-N4 was statistically equivalent to the gold-standard PMSG for all parameters studied.
Methods
Materials
Hormones
Follitropin-beta (Follistim, Organon, Roseland, NJ, USA) and urinary hCG (American Pharmaceutical Partners, Inc., Schaumburg, IL, USA) were generously donated for research purposes by Drs. Husami and Vidali.
Hyperglycosylated rhFSH-CTP and rhFSH-N4 were produced and purified as previously described (7, 9). Briefly, CHO-K1 cells stably transfected with a hyperglycosyated FSH construct were grown in suspension cultures; with spinner bottles seeded at 105 cells/ml in CHO-S-SFM II media (Life Technologies, Rockville, MD, USA) containing 400μg/ml G418. When cultures reached a density of 1.5×106 cells/ml supernatants received 0.2mmol/l phenylmethylsulphonyl fluoride (PMSF) and were filtered through a 0.2μm membrane and stored at 4°C.
For affinity purification of protein analogues a sepharose column was prepared by coupling purified A103 (13), a monoclonal antibody specific for the alpha subunit of gonadotropins, to cyanogen bromide (CNBr)-Sepharose-4B according to the manufacturer’s instruction (Amersham Biosciences, Piscataway, NJ). The CHO cell supernatant was passed over the sepharose column. The column was washed with at least 10 bed volumes of PBS and then 2 bed volumes of distilled water. Protein was eluted with 1M acetic acid and immediately dried on a Speed-Vac concentrator (ThermoForma, Marietta, OH). The purified protein was then re-suspended in dH2O.
RhFSH-CTP and rhFSH-N4 protein concentrations were determined by an FSH RIA using an antibody to FSH β-subunit (Biodesign International, Saco, ME) and compared to a standard curve derived from recombinant FSH.
Experimental Animals
Approval was obtained from the IACUC at Columbia University in accordance with the NIH guide for the care and use of laboratory animals. C57Bl/6J mice were ordered from Jackson Laboratories (Bar Harbor, ME). All animal experiments were conducted in accord with accepted standards of humane animal care.
Ovarian hyperstimulation
Four week old C57Bl/6J females (n=20 per group) were injected with 5IU ip of one of the following treatments: 1) rhFSH; 2) rhFSH-CTP; 3) rhFSH-N4; or 4) PMSG. Forty-six hours later mice were injected with 5IU ip of hCG to promote ovulation. Females were then mated overnight with an adult C57Bl/6J male mouse of proven fertility. Sixteen hours later females were checked for the presence of a vaginal plug, separated from the males, anesthetized with isoflurane inhalation, blood collected by cardiac puncture, ovaries and uterus were extirpated and immediately placed in EmbryoMax M2 media (Millipore, Billerica, MA). Fallopian tubes were flushed to collect eggs. Cumulus cells were removed by treating the isolated eggs with hyaluronidase therapy (Sigma, St. Louis MO). Eggs were cultured in 1mL of EmbryoMax M16 media (Millipore, Billerica, MA) and an overlay of sterile mineral oil (Sigma, St. Louis MO) then incubated at 37C in 5%CO2. Seventy-two hours later eggs were evaluated for progression to 8 cell stage embryos. The following formula was applied:
Delivery Rates and Litter size
Adult reproductive age C57Bl/6J females (n=30 per group) of previously proven fertility were injected with 5IU ip of one of the following treatments: 1) rhFSH; 2) rhFSH-CTP; 3) rhFSH-N4; or 4) PMSG. Forty-six hours later mice were injected with 5IU ip of hCG to promote ovulation. Females were then mated overnight with an adult C57Bl/6J male mouse of proven fertility. The following morning females were checked for the presence of a vaginal plug, separated from males and then monitored for signs of pregnancy. Females identified as pregnant were separated into individual cages. Cages were checked every morning for the presence of pups and the number of pups delivered was recorded.
Statistics
Data are expressed as mean±standard error (SEM). Analysis of Variance between groups (ANOVA) was performed for multiple group comparisons. The Mann-Whitney test was used for analysis of non-parametric data. For evaluation of pregnancy rates, chi-square analysis was performed. A p value of less than 0.05 was considered significant. All analysis calculations were computed using the PRISM software (GraphPad Software, Inc., San Diego, CA, USA)
Results
Ovulation Rates
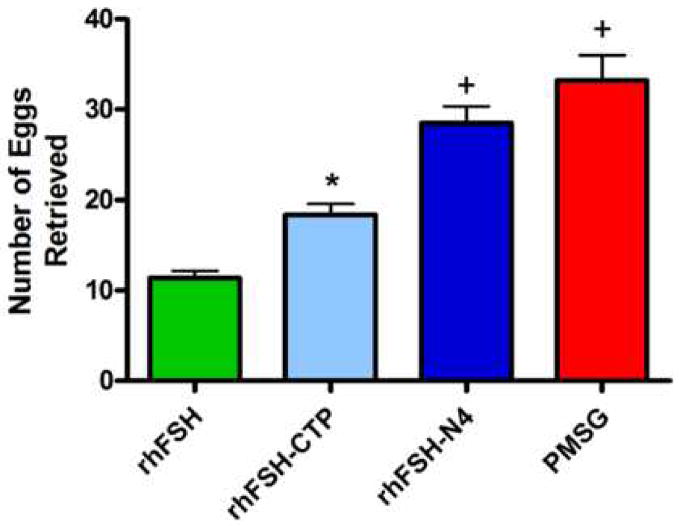
The number of eggs retrieved from the fallopian tubes following treatment with rhFSH-CTP was significantly higher compared to rhFSH (18.3±1.2 and 11.4±0.7, respectively) (p<0.05). Treatment with rhFSH-N4 produced a mean of 28.5±1.9 eggs per mouse. PMSG treated mice produced 32.2±2.7 eggs. The mean egg production of rhFSH-N4 was statistically equivalent to PMSG and significantly higher than both rhFSH (p<0.001) and rhFSH-CTP (p<0.01) (Figure 1).
Figure 1.
Graph of mean number of eggs ovulated per animal: rhFSH, rhFSH-CTP, rhFSH-N4 and PMSG. *p<0.05 compared to rhFSH; +p<0.001 compared to rhFSH; p<0.01 compared to rhFSH-CTP. Error bars represent ± SEM
In vitro embryo development
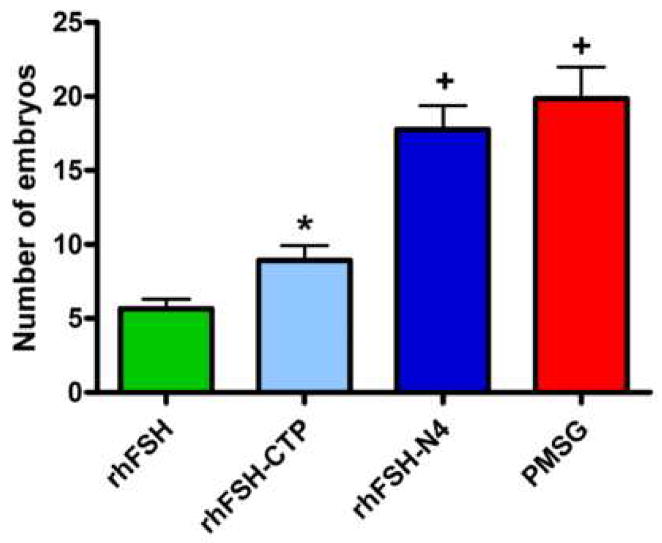
Following 72 hours of culture, the number of eggs progressing to the 8-cell embryo stage was 5.7±0.7 embryos per mouse from rhFSH-treated animals (Figure 2). Treatment with rhFSH-CTP significantly increased the number of in vitro maturated embryos (9.0±1.0 embryos per mouse) compared to rhFSH (p<0.05). The largest number of mature embryos in vitro occurred with rhFSH-N4 and PMSG therapies (17.8±1.6 and 19.9±2.2 embryos per mouse, respectively). The embryo rates for rhFSH-N4 and PMSG were statistically equivalent to each other but statistically higher compared to both rhFSH (p<0.001) and rhFSH-CTP (p<0.01).
Figure 2.
Graph of mean number of in vitro maturated embryos: rhFSH, rhFSH-CTP, rhFSH-N4, and PMSG. *p<0.05 compared to rhFSH. +p<0.001 compared to rhFSH; p<0.01 compared to rhFSH-CTP. Error bars represent ± SEM
When the fertilization rate of individual animals was calculated, the mean for rhFSH and rhFSH-CTP treated animals was equivalent with 47.1±4.13% and 50.0±3.63%, respectively. The fertilization rate for rhFSH-N4 was the highest at 64.0±3.05% which was statistically equivalent to PMSG at 58.7±4.0%. Fertilization rate of rhFSH-N4 was higher than rhFSH (p<0.05) and rhFSH-CTP (p<0.05).
Delivery Rates
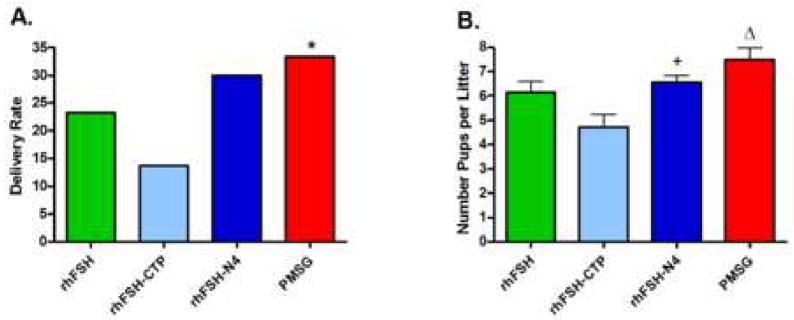
When adult C57Bl/6J female mice were randomized to receive rhFSH, rhFSH-CTP, rhFSH-N4 or PMSG the number of mice that became pregnant and delivered pups was 23% for rhFSH (7/30); 30% for rhFSH-N4 (9/30) and 33% for PMSG (10/30) (Figure 3A). Although there was a trend towards higher delivery rates with rhFSH-N4 and PMSG they were not statistically better after chi-square analysis. Surprisingly, rhFSH-CTP resulted in the lowest number of deliveries (17%; 5/30). Thus, to ensure that this finding was accurate rather than an artifact, we evaluated an additional 20 mice with FSH-CTP and confirmed the low delivery rate. The final delivery rate of 14% (7/50) mice was significantly lower than PMSG on chi-square analysis (p<0.05).
Figure 3.
Pregnancy studies. A. Bar graph of delivery rates in female mice treated with either rhFSH, rhFSH-CTP, rhFSH-N4 and PMSG. *p<0.05 compared to PMSG. B. Graph of Litter size following treatment with rhFSH, rhFSH-CTP, rhFSH-N4 and PMSG. +p<0.05 compared to rhFSH-CTP; Δp<0.001 compared to rhFSH-CTP. Error bars represent ± SEM
Litter Size
The mean number of pups delivered per litter was statistically equivalent for rhFSH, rhFSH-N4 and PMSG. The litter size for rhFSH-CTP treated mice (4.7±0.52) was significantly lower compared to rhFSH-N4 (6.6±0.29; p<0.05) and PMSG (7.5±0.48; p<0.001) (Figure 3B). All pups delivered were included in our evaluation. All treatment groups produced live pups that were capable of survival for up to one week at which time pups were euthanized by the investigators. Frequently several pups in the litter were either ignored or killed by the mother at the time of birth, especially with larger litters, resulting in a few dead pups at the time of tally the morning after delivery. On gross evaluation no identifiable defect was observed with the pups.
Discussion
FSH is encoded by a single gene, but the pituitary is capable of making more than 17 different isoforms of FSH through post-translational modifications(14). Alteration of the carbohydrate sulfonation and sialylation patterns can lower the pH of the protein and increase the half-life of the isoform. Remodeling of carbohydrate complexity can significantly affect the biological activity of the hormones(15). When investigators added an additional N-linked glycosylation site onto erythropoietin, a novel analogue was produced, darbepoetin alfa, which resulted in a longer half-life and a reduction in the frequency of administration from daily to weekly (11). More importantly, darbepoetin alfa produced strong biological effects at a lower dose with a more consistent serum level of the medication. Our laboratory used similar glycoengineering techniques to produce a variety of hyperglycosylated, long-acting, FSH hormones.
The goal of this report was to compare the efficacy of two single-chain hyperglycosylated FSH proteins on ovulatory and pregnancy parameters in mice. We previously reported both rhFSH-N4 and rhFSH-CTP were superior to rhFSH for ovarian stimulation in rodents and rhFSH-N4 produced more large antral follicles with significantly higher inhibin A levels compared to rhFSH-CTP (9, 12). Furthermore, rhFSH-N4 was statistically equivalent to the current gold standard in rodent ovarian stimulation, PMSG. We were unsuccessful in our attempt to surpass the biological effects of PMSG on ovarian follicle development by either increasing the dose of rhFSH-N4 injected or co-administering hCG to provide additional LH activity (12).
Based on the previous analysis of large antral follicle production we hypothesized that treatment with hyperglycosylated FSH analogues would produce more eggs in response to a standard hyperstimulation protocol and that rhFSH-N4 would be superior to rhFSH-CTP but statistically equivalent to PMSG. The results from the ovulation studies proceeded as expected with PMSG producing the highest number of eggs, then rhFSH-N4 followed by rhFSH-CTP and finally rhFSH producing the fewest number of ovulated eggs.
With the collection of a higher number of eggs with hyperglycosylated FSH therapy, we predicted rhFSH-CTP and rhFSH-N4 treatment would also result in a higher number of embryos per mouse that developed in vitro. Granulosa cells play an integral role in oocyte growth and development to create a competent oocyte capable of re-entering the meiotic cell cycle. There was minimal concern that hyperglycosylation of FSH would adversely effect egg quality because a recent report noted that granulosa cells exposed to either rhFSH or rhFSH-CTP were found to have similar gene expression profiles(16). Surprisingly rhFSH-N4 therapy resulted in the highest fertilization rate, defined here as the percentage of oocytes collected that progressed to the 8 cell embryo stage. This would suggest that rhFSH-N4 therapy produced a larger percentage of healthy, high-quality eggs relative to rhFSH and rhFSH-CTP which is consistent with our previous data demonstrating that rhFSH-N4 stimulated a significantly higher production of inhibin A, a marker of healthy granulosa cells(9). Accordingly, in evaluation of mean number of total embryos per mouse that developed in vitro, rhFSH-N4 was significantly higher than rhFSH-CTP and rhFSH.
We had originally hypothesized that the pregnancy rates and mouse litter size would be equivalent for all gonadotropins analyzed for two reasons. First, both rhFSH and PMSG therapies adversely effect the uterine environment in mice resulting in a reduced pregnancy rate compared to randomly cycling adult females (17–19). Second, despite a significant increase in the number of developing embryos present following ovarian hyperstimulation, the litter size is physiologically restrained by the physical limitations of the mouse uterus (20).
As anticipated, ovarian stimulation with rhFSH, rhFSH-N4 and PMSG produced statistically equivalent birth rates and litter sizes although there was a trend of higher delivery rates with rhFSH-N4 and PMSG. Surprisingly, rhFSH-CTP treatment resulted in a delivery rate below rhFSH which was discordant from our previous results demonstrating that rhFSH-CTP produced more large antral follicles, more ovulated eggs and more embryos per mouse compared to rhFSH. Although the delivery rate of rhFSH-CTP was not statistically lower that rhFSH, it was significantly below PMSG. Previous studies of rhFSH in mice demonstrated that high doses of rhFSH (10–20IU), but not low doses of rhFSH, adversely affected embryo development (21). One concern with the extended half-life of rhFSH-CTP was that if a high serum FSH level persisted into the luteal phase the normal stages of embryo implantation and development might be disrupted. However, if this was the cause then we would expect a similar reduction of delivery rate with the rhFSH-N4 hyperglycosylated analogue. Yet, the delivery rate of rhFSH-N4 was not adversely affected and was in fact statistically equivalent to PMSG.
Mice treated with rhFSH-CTP were also found to have a smaller litter size compared to rhFSH-N4 and PMSG, although this was statistically equivalent to rhFSH. Since mice are multiparous animals, the reduced number of deliveries and reduced litter size are consistent with disruption in normal fetal development leading to in utero resorption of the pups. Unfortunately, unlike humans, mice do not produce chorionic gonadotropin or any other pregnancy-specific hormone; thus, there is no assay to diagnose early pregnancy in mice making it difficult to screen for subsequent miscarriage. Therefore, further studies are required to carefully elucidate the stage where embryo or fetal development is disrupted following rhFSH-CTP therapy.
While these studies certainly raise concerns about the use of rhFSH-CTP to promote pregnancy in mice, the importance of this information for patients is as yet unclear. A non-single chain version of rhFSH-CTP known as corifollitropin alfa (Organon 6286, NV Organon, The Netherlands) has been developed and as in currently in Phase III clinical trials for use in female infertility. Consistent with our findings that rhFSH-CTP exhibits a longer half-life and an enhanced biological activity in rats, early clinical studies reported that a single injection of corifollitropin alfa could enhance testosterone production in hypogonadotropic males, enhance estradiol production in normally cycling women, and promote folliculogenesis in anovulatory women seeking fertility (22–24). In the mouse studies reported here we found rhFSH-CTP was superior to rhFSH for egg retrieval and in vitro embryo maturation. Clinical studies also reported that rhFSH-CTP was able to promote follicle growth in females undergoing IVF resulting in an equivalent number of eggs retrieved and equivalent number of high-quality embryos ready to transfer compared to standard rhFSH therapy. The benefit of rhFSH-CTP, however, was the reduced number of hormone injections required to achieve ovarian stimulation. Finally, Beckers and colleagues previously reported a live birth following administration of rhFSH-CTP to a woman who had previously exhibited a poor response to standard rhFSH therapy(25). Similarly, mice treated with rhFSH-CTP were able to achieve and maintain pregnancy. Treatment with rhFSH-CTP, however, revealed a trend of lower birth rates and smaller litter sizes compared to rhFSH therapy, but this did not reach statistical significance. Interestingly, the use of N-linked glycosylation to extend the half-life and increase biological activity of FSH was capable of enhancing ovarian stimulation in mice without the unfavorable reduction of live birth rate and litter size associated with O-linked glycosylation.
In conclusion, in mice, the use of the hyperglycosylated FSH analogues rhFSH-CTP and rhFSH-N4 stimulated a larger number of eggs following ovulation and a higher number of 8-cell embryos with in vitro culturing compared to rhFSH. Therefore, the use of hyperglycosylated FSH analogues potentially provides an additional novel therapy for ovarian stimulation in infertile women. One concern that arose from these studies was that the delivery rate associated with rhFSH-CTP was lower than expected in comparison to rhFSH, rhFSH-N4, and PMSG, the gold standard for mouse ovarian stimulation. Further studies are necessary to elucidate the source of the discrepancy between the antral follicle, egg production and in vitro embryo data relative to the delivery and litter size data seen with rhFSH-CTP therapy. Use of the N-linked hyperglycosylated rhFSH-N4 was not associated with a reduction of live birth rates or smaller litter sizes and was statistically equivalent to PMSG for all fertility parameters evaluated.
Acknowledgments
This research supported by the New York Metropolitan Endometriosis Center for Patient Care and Research as well as National Institute of Health grant R01DK063224 (JWL).
Footnotes
A portion of this research was presented at the American Society for Reproductive Medicine Annual Conference in Washington, DC, October 2007.
Authors have no conflict of interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matzuk MM, Hsueh AJ, Lapolt P, Tsafriri A, Keene JL, Boime I. The biological role of the carboxyl-terminal extension of human chorionic gonadotropin [corrected] beta-subunit. Endocrinology. 1990;126:376–83. doi: 10.1210/endo-126-1-376. [DOI] [PubMed] [Google Scholar]
- 2.Fares FA, Suganuma N, Nishimori K, LaPolt PS, Hsueh AJ, Boime I. Design of a long-acting follitropin agonist by fusing the C-terminal sequence of the chorionic gonadotropin beta subunit to the follitropin beta subunit. Proc Natl Acad Sci U S A. 1992;89:4304–8. doi: 10.1073/pnas.89.10.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaPolt PS, Nishimori K, Fares FA, Perlas E, Boime I, Hsueh AJ. Enhanced stimulation of follicle maturation and ovulatory potential by long acting follicle-stimulating hormone agonists with extended carboxyl-terminal peptides. Endocrinology. 1992;131:2514–20. doi: 10.1210/endo.131.6.1446593. [DOI] [PubMed] [Google Scholar]
- 4.deKretser D, Atkins R, Paulsen C. Role of the kidney in the metabolism of luteinizing hormone. J Endocrinol. 1973;58:425–34. doi: 10.1677/joe.0.0580425. [DOI] [PubMed] [Google Scholar]
- 5.Sowers J, Pekary A, Hershman J, Kanter MJJ, DiStefano I. Metabolism of exogenous human chorionic gonadotropin in men. J Endocrinol. 1979;80:83–9. doi: 10.1677/joe.0.0800083. [DOI] [PubMed] [Google Scholar]
- 6.Perlman S, Hazel Bvd, Christiansen J, Gram-Nielsen S, Jeppesen C, Andersen K, et al. Glycosylation of an N-terminal extension prolongs the half-life and increases in vivo activity of follicle stimulating hormone. J Clin Endocrinol Met. 2003;88:3227–35. doi: 10.1210/jc.2002-021201. [DOI] [PubMed] [Google Scholar]
- 7.Klein J, Lobel L, Pollak S, Ferin M, Xiao E, Sauer M, et al. Pharmacokinetics and pharmacodynamics of single-chain recombinant human follicle-stimulating hormone containing the human chorionic gonadotropin carboxyterminal peptide in the rhesus monkey. Fertil Steril. 2002;77:1248–55. doi: 10.1016/s0015-0282(02)03113-8. [DOI] [PubMed] [Google Scholar]
- 8.Klein J, Lobel L, Pollak S, Lustbader B, Ogden RT, Sauer MV, et al. Development and characterization of a long-acting recombinant hFSH agonist. Hum Reprod. 2003;18:50–6. doi: 10.1093/humrep/deg024. [DOI] [PubMed] [Google Scholar]
- 9.Weenen C, Pena JE, Pollak SV, Klein J, Lobel L, Trousdale RK, et al. Long-acting follicle-stimulating hormone analogs containing N-linked glycosylation exhibited increased bioactivity compared with o-linked analogs in female rats. J Clin Endocrinol Metab. 2004;89:5204–12. doi: 10.1210/jc.2004-0425. [DOI] [PubMed] [Google Scholar]
- 10.Hricovini M. Structural aspects of carbohydrates and the relation with their biological properties. Curr Med Chem. 2004;11:2565–83. doi: 10.2174/0929867043364414. [DOI] [PubMed] [Google Scholar]
- 11.Sinclair A, Elliott S. Glycoengineering : the effect of glycosylation on the properties of therapeutic proteins. J Pharm Sci. 2005;94:1626–35. doi: 10.1002/jps.20319. [DOI] [PubMed] [Google Scholar]
- 12.Ruman J, Pollak S, Trousdale R, Klein J, Lustbader J. Effects of long-acting recombinant human follicle stimulating hormone (rhFSH) analogues containing N-linked glycosylation on murine folliculogenesis. Fertil Steril. 2005;83(suppl 1):1303–9. doi: 10.1016/j.fertnstert.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 13.O'Connor JF, Birken S, Lustbader JW, Krichevsky A, Chen Y, Canfield RE. Recent advances in the chemistry and immunochemistry of human chorionic gonadotropin: impact on clinical measurements. Endocr Rev. 1994;15:650–83. doi: 10.1210/edrv-15-5-650. [DOI] [PubMed] [Google Scholar]
- 14.Creus S, Pellizzari E, Cigorraga S, Campo S. FSH isoforms: bio and immuno-activities in post-menopausal and normal menstruating women. Clin Endocrinol. 1996;44:181–9. doi: 10.1046/j.1365-2265.1996.646467.x. [DOI] [PubMed] [Google Scholar]
- 15.Lambert A, Talbot J, Anobile C, Robertson W. Gonadotropin heterogeneity and biopotency: implications for assisted reproduction. Mol Hum Reprod. 1998;4:619–29. doi: 10.1093/molehr/4.7.619. [DOI] [PubMed] [Google Scholar]
- 16.Perlman S, Bouquin T, Hazel Bvd, Jensen T, Schambye H, Knudsen S, et al. Transcriptome analysis of FSH and FSH variant stimulation in granulosa cells from IVM patients reveals novel regulated genes. Mol Hum Reprod. 2006;12:135–44. doi: 10.1093/molehr/gah247. [DOI] [PubMed] [Google Scholar]
- 17.VanderAuwera I, D'Hooghe T. Superovulation of female mice delays embryonic and fetal development. Hum Reprod. 2001;16:1237–43. doi: 10.1093/humrep/16.6.1237. [DOI] [PubMed] [Google Scholar]
- 18.Ertzeid G, Storeng R. The impact of ovarian stimulation on implantation and fetal development in mice. Hum Reprod. 2001;16:221–5. doi: 10.1093/humrep/16.2.221. [DOI] [PubMed] [Google Scholar]
- 19.Kelley R, Kind K, Lane M, Robker R, Thompson J, Edwards L. Recombinant human follicle-stimulating hormone alters maternal ovarian hormone concentrations and the uterus and perturbs fetal development in mice. Am J Physiol Endocrinol Metab. 2006;291:E761–70. doi: 10.1152/ajpendo.00079.2006. [DOI] [PubMed] [Google Scholar]
- 20.Long C, Lamberson W, Bates R. Genetic correlations among reproductive traits and uterine dimensions in mice. J Anim Sci. 1991;69:99–103. doi: 10.2527/1991.69199x. [DOI] [PubMed] [Google Scholar]
- 21.Edwards L, Kind K, Armstrong D, Thompson J. Effects of recombinant human follicle-stimulating hormone on embryo development in mice. Am J Physiol Endocrinol Metab. 2005;288:E845–E51. doi: 10.1152/ajpendo.00398.2004. [DOI] [PubMed] [Google Scholar]
- 22.Bouloux P, Handelsman D, Jockenhovel F, Nieschlag E, Rabinovici J, Frasa W, et al. First human exposure to FSH-CTP in hypogonadotrophic hypogonadal males. Hum Reprod. 2001;16:1592–7. doi: 10.1093/humrep/16.8.1592. [DOI] [PubMed] [Google Scholar]
- 23.Duijkers IJ, Klipping C, Boerrigter PJ, Machielsen CS, De Bie JJ, Voortman G. Single dose pharmacokinetics and effects on follicular growth and serum hormones of a long-acting recombinant FSH preparation (FSH-CTP) in healthy pituitary-suppressed females. Hum Reprod. 2002;17:1987–93. doi: 10.1093/humrep/17.8.1987. [DOI] [PubMed] [Google Scholar]
- 24.Balen A, Mulders A, Fauser B, Schoot B, Renier M, Devroey P, et al. Pharmacodynamics of a single low dose of long-acting recombinant follicle-stimulating hormone (FSH-carboxy terminal peptide, corifollitropin alfa) in women with World Health Organization group II anovulatory infertility. J Clin Endocrinol Metab. 2004;89:6297–62304. doi: 10.1210/jc.2004-0668. [DOI] [PubMed] [Google Scholar]
- 25.Beckers N, Macklon N, Devroey P, Platteau P, Boerrigter P, Fauser B. First live birth after ovarian stimulation using a chimeric long-acting human recombinant follicle-stimulating hormone (FSH) agonist (recFSH-CTP) for in vitro fertilization. Fertil Steril. 2003;79:621–3. doi: 10.1016/s0015-0282(02)04804-5. [DOI] [PubMed] [Google Scholar]