Abstract
In the terrorist radiation exposure scenario, radiation victims are likely to suffer from additional injuries such as sepsis. Our previous studies have shown that ghrelin is protective in sepsis. However, it remains unknown whether ghrelin ameliorates sepsis-induced organ injury and mortality after radiation exposure. The purpose of this study is to determine whether human ghrelin attenuates organ injury and improves survival in a rat model of radiation combined injury (RCI) and, if so, the potential mechanism responsible for the benefit. To study this, adult male rats were exposed to 5-Gy whole body irradiation followed by cecal ligation and puncture (CLP, a model of sepsis) 48 h thereafter. Human ghrelin (30 nmol/rat) or vehicle (saline) was infused intravenously via an osmotic minipump immediately after radiation exposure. Blood and tissue samples were collected at 20 h after RCI (68 h after irradiation or 20 h after CLP) for various measurements. To determine the longterm effect of human ghrelin after RCI, the gangrenous cecum was removed at 5 h after CLP and 10-d survival was recorded. In addition, vagotomy or sham vagotomy was performed in sham and RCI animals immediately prior to ghrelin administration, and various measurements were performed at 20 h after RCI. Our results showed that serum levels of ghrelin and its gene expression in the stomach were decreased markedly at 20 h after RCI. Administration of human ghrelin attenuated tissue injury markedly, reduced proinflammatory cytokine levels, decreased tissue myeloperoxidase activity, and improved survival after RCI. Furthermore, elevated plasma levels of norepinephrine (NE) after RCI were reduced significantly by ghrelin. However, vagotomy prevented ghrelin’s beneficial effects after RCI. In conclusion, human ghrelin is beneficial in a rat model of RCI. The protective effect of human ghrelin appears to be attributed to re-balancing the dysregulated sympathetic/parasympathetic nervous systems.
INTRODUCTION
There is a growing concern in the world about the exposure of radiation due to the threat of nuclear terrorism. A radiological terrorist attack could involve the dispersal of radioactive materials by an attack on a nuclear facility, deployment of a radiation dispersal device, or, less likely, detonation of a nuclear weapon. In the terrorist radiation exposure scenario, the amount of radiation may not be enough to cause immediate illness or large scale casualties, but radiation victims likely will suffer from injuries, infection, and sepsis secondary to the bomb blast. Infection is a major cause of mortality in the irradiated host because of immunosuppressive effects that result from declining lymphohematopoietic elements secondary to radiation- induced bone marrow aplasia (1).
Infection and sepsis remain a critical problem with significant morbidity and mortality even in the modern era of critical care management (2–5). Severe sepsis is the second leading cause of death among patients in non-coronary intensive care units (ICU) and the tenth leading cause of death overall in this nation (6–9). Evidence indicates that in the United States alone, more than 750,000 people develop sepsis each year with an overall mortality of 28.6% (10). It is expected that both the morbidity and mortality of sepsis increase after radiation exposure. However, little information is available regarding radiation exposure followed by infection and sepsis.
Ghrelin, a novel gastrointestinal hormone, was first discovered by Kojima et al. in 1999 as an endogenous ligand for the growth hormone secretagogue receptor type 1a (GHSR-1a, ghrelin receptor) (11). Ghrelin originally was reported to induce growth hormone release through pituitary GHSR-1a stimulation (12–14). However, a large body of evidence has indicated other physiological functions of ghrelin mediated by the central and peripheral ghrelin receptors (15). It has been linked to the regulation of pituitary hormone secretion, feeding, energy homeostasis, gastrointestinal function, cardiovascular system, and immune system (16–18). Our recent studies have shown that circulating levels of ghrelin decreased significantly in a rat model of polymicrobial sepsis induced by cecal ligation and puncture (CLP), and ghrelin administration decreases inflammatory responses, improves organ blood flow, attenuates tissue injury, and reduces mortality under such conditions (18–21). However, it remains unknown whether ghrelin ameliorates sepsis-induced organ injury and mortality after radiation exposure. The purpose of this study was to determine whether human ghrelin attenuates organ injury and improves survival in a rat model of radiation combined injury (RCI, radiation exposure followed by CLP) and, if so, the potential mechanism responsible for its benefit.
MATERIALS AND METHODS
Experimental Animals
Male Sprague-Dawley rats (250 g to 300 g), purchased from Charles River Laboratories (Wilmington, MA, USA), were used in this study. The rats were housed in a temperature-controlled room on a 12-h light/dark cycle and fed on a standard Purina rat chow diet. Animal experimentation was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (22). This project was approved by the Institutional Animal Care and Use Committee (IACUC) of the Feinstein Institute for Medical Research.
Animal Model of Radiation Combined Injury
Our pilot study showed that rats died within 2 wks after whole-body exposure of 10-gray (Gy) γ radiation. We therefore decided to expose the animals to a sub-lethal 5-Gy radiation followed by sepsis 48 h later. Rats were anesthetized with intraperitoneal pentobarbital (40 mg/kg BW) and exposed to whole body irradiation performed with Gammacell 1000 irradiator (Atomic Energy of Canada Ltd) as recently described (23). The radioactive source used was Cesium-137 (Cs-137). The irradiator was set to deliver γ-irradiation at a dose rate of approximately 360 rad/min for 1.4 min, a total of 5 Gray per animal. During irradiation, the rats were restrained with plastic wrap and placed in a fitted container in upright position. During radiation, the container rotated continuously in front of the radiation source for even exposure. Upon completion, rats were allowed to recover and returned to their cages; 48 h later, polymicrobial sepsis was induced by CLP as described previously (24). Briefly, rats were fasted overnight prior to the induction of sepsis, but allowed water ad libitum. The animals were anesthetized by isoflurane inhalation, and a 2-cm ventral midline abdominal incision was made. The cecum was then exposed, ligated just distal to the ileocecal valve to avoid intestinal obstruction, punctured twice with an 18-gauge needle, and returned to the abdominal cavity. The incision was closed in layers. Sham operated animals underwent the same surgical procedure except that the cecum was neither ligated nor punctured. Animals were killed 20 h after CLP, with blood, tissue, and organs harvested for later analyses.
Administration of Ghrelin
Immediately after radiation exposure, human ghrelin (2 nmol, 6.74 μg, Phoenix Pharmaceuticals, Belmont, CA, USA) was given intravenously as a bolus, followed by continuous infusion by means of an Alzet mini-osmotic pump for 68 h (26 nmol, 87.64 μg). In addition, at the time of CLP (48 h post radiation), another intravenous bolus of human ghrelin (2 nmol, 6.74 μg) was given. Thus, each animal received 30 nmol human ghrelin (~33.71 μg/100 g BW) for the entire treatment period. The dosage of ghrelin was comparable to that used in the rat model of sepsis which produced significant protection (18,20,21). Vehicle treated animals received an equivalent volume of normal saline.
Determination of Plasma Levels of Ghrelin
Plasma concentrations of ghrelin were measured using enzyme immunoassay kits according to the instructions provided by the manufacturer (Peninsula Laboratories, San Carlos, CA, USA).
Determination of Serum Levels of Organ Injury Markers
Serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), lactate, and creatinine were determined by using assay kits according to the manufacturer’s instructions (Pointe Scientific, Canton, MI, USA).
Determination of Serum Levels of TNF-α and IL-6
The concentrations of tumor necrosis factor-α(TNF-α) and interleukin-6 (IL-6) in the serum were quantified by using commercially obtained enzyme-linked immunosorbent assay (ELISA) kits specific for rat-TNF-αand IL-6 (BD BioSciences, San Jose, CA, USA). The assay was carried out according to the instructions provided by the manufacturer.
Determination of Ghrelin, TNF-α, and IL-6 Gene Expression
Ghrelin gene expression in the stomach, and TNF-αand IL-6 gene expression in the small intestine, were determined by real-time quantitative polymerase chain reaction (PCR). Total RNA was extracted from the stomach and small intestine by Tri-Reagent (Molecular Research Center, Cincinnati, OH, USA). Real-time quantitative PCR was carried out on cDNA samples reverse transcribed from 2 μg RNA using murine leukemia virus reverse transcriptase (Applied Biosystems, Foster City, CA, USA). Using the QuantiTect SYBR Green PCR kit (Qiagen, Valencia, CA, USA), reactions were carried out in 24 μL final volume containing 2 pmol of forward and reverse primers, 12 μL QuantiTect Master Mix, and 1 μL cDNA. Amplification was performed according to Qiagen’s recommendations with an Applied Biosystems 7300 real-time PCR. Expression amount of rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used for normalization of each sample, and analysis of each specific mRNA was conducted in duplicate. Relative expression of mRNA was calculated by the 2–ΔΔCt method, and results expressed as fold change with respect to the corresponding experimental control. The following rat primers were used: GAPDH (AF106860): 5′-ATG ACT CTA CCC ACG GCA AG-3′ (forward), 5′-CTG GAA GAT GGT GAT GGG TT-3′ (reverse); ghrelin (NM_021669): 5′-GAG CCC AGA GCA CCA GAA AG-3′ (forward), 5′-GCA GTT TAG CTG GTG GCT TCT T-3′ (reverse); TNF-α(NM_012675): 5′-TGA TCG GTC CCA ACA AGG A-3′ (forward), 5′-GGG CCA TGG AAC TGA TGA GA-3′ (reverse); IL-6 (NM_012589): 5′-AGG GAG ATC TTG GAA ATG AGA AAA-3′ (forward), 5′-CAT CAT CGC TGT TCA TAC AAT CAG-3′ (reverse).
Granulocyte Myeloperoxidase Assessment
Neutrophil accumulation in the small intestine, lungs, and kidneys was estimated using the myeloperoxidase (MPO) activity assay as described previously (25). Briefly, samples of 100 mg were suspended in 1 mL of 0.5% hexadecyltrimethylammonium bromide in 50 mmol/L phosphate buffer (pH 6.0), and sonicated 90 s on ice. Homogenates were cleared by centrifuging at 13,400g for 10 min at 4°C, and the protein concentration of supernatants was determined by using Bio-Rad DC Protein Assay Kit (Bio-Rad, Hercules, CA, USA). The reaction was carried out in a 96-well plate by adding 290 μL of 50 mmol/L phosphate buffer with 3 μL substrate solution (containing 20 mg/mL o-dianisidine hydro chloride), and 3 μL H2O2 (20 mmol/L). Sample (10 μL) was added to each well to start the reaction. Plates were read spec-trophotometrically at 460 nm for 3 min on CERES UV 900C Microplate reader (Bio-Tek Inst Inc., Winooski, VT, USA). MPO activity (1 unit defined as change in absorbance of 1 per min) was expressed as units per gram of tissue.
Survival Study
A 10 d survival study was conducted to determine if human ghrelin can affect survival beneficially in an animal model. In additional groups of RCI rats, human ghrelin or vehicle was administered as described above. At 5 h after CLP, the necrotic cecum was excised and the abdominal cavity was washed twice with 40 mL of warm, sterilized normal saline solution. The abdominal incision was closed in layers. The procedure of cecal excision in CLP animals was performed to mimic the clinical situation in which septic focus is removed whenever possible. The animals were then returned to their cages and allowed food and water ad libitum. All surviving animals were killed on d 10.
Determination of Plasma Levels of Norepinephrine (NE)
The concentrations of NE in the plasma were quantified by the use of commercially obtained ELISA kit specific for NE (IBL-America Inc., Minneapolis, MN, USA). The assay was carried out according to the instructions provided by the manufacturer as we described previously (26).
Vagotomy and Sham Vagotomy
In additional groups of animals, the trunks of the subdiaphragmatic vagus were transected as described previously (18). Briefly, immediately after radiation exposure, prior to the administration of ghrelin, a 2-cm ventral midline abdominal incision was made. The dorsal and ventral branches of the vagus nerve were dissected from the esophagus. Each branch of the nerve was tied with surgical sutures at two points separated by approximately 1 cm, and then severed between the sutures. Sham- vagotomized animals underwent the same surgical procedure with the exception that their vagus nerves were neither tied nor severed. After the surgery, the animals from both vagotomy and sham vagotomy were allowed food and water ad libitum. Human ghrelin or vehicle was administrated immediately following vagotomy as described above in the vagus nerve intact animals; 48 h later, polymicrobial sepsis was induced by CLP. Serum concentrations of AST, ALT, LDH, lactate, creatinine, TNF-α, IL-6, as well as MPO activities in the small intestine, lungs, and kidneys were determined at 20 h after CLP as described above.
Statistical Analysis
All data were expressed as means ± SE and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method for multiple group analysis or Student t test for two group analysis. Survival study was estimated by the Kaplan–Meier method and compared by using the log-rank test. Differences in values were considered significant at P < 0.05.
RESULTS
Ghrelin Levels Decrease after RCI
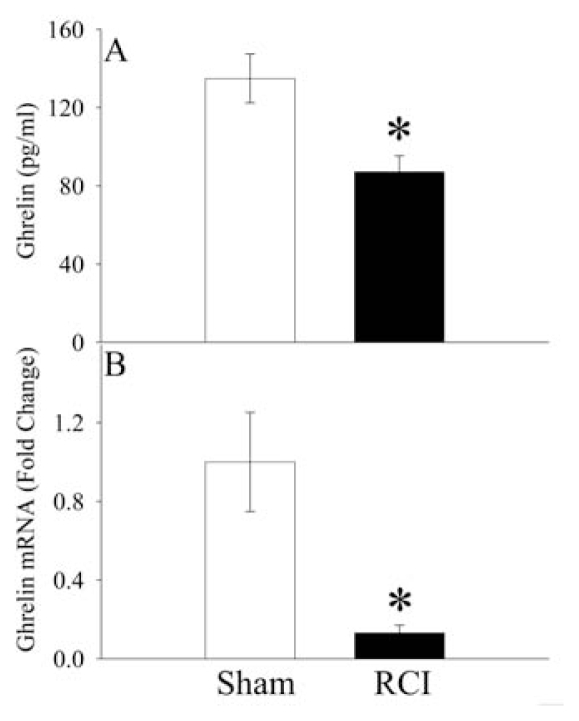
As shown in Figure 1A, serum levels of ghrelin in sham operated animals were 134 ± 31 pg/mL. Similar to CLP alone animals (19), serum levels of ghrelin in RCI animals decreased to 87 ± 20 pg/mL (P < 0.05), representing a 35% reduction at 20 h after RCI (68 h after irradiation or 20 h after CLP). And ghrelin gene expression in the stomach (the major source of ghrelin in the circulation [11]) was decreased by 87% at 20 h after RCI (P < 0.05, Figure 1B).
Figure 1.
Alterations in serum levels of ghrelin (A) and its gene expression in the stomach (B) at 20 h after radiation combined injury (RCI) or sham operation (Sham). Data are presented as means ± SE (n = 6) and compared by Student t test: *P < 0.05 versus Sham group.
Human Ghrelin Attenuates Organ Injury after RCI
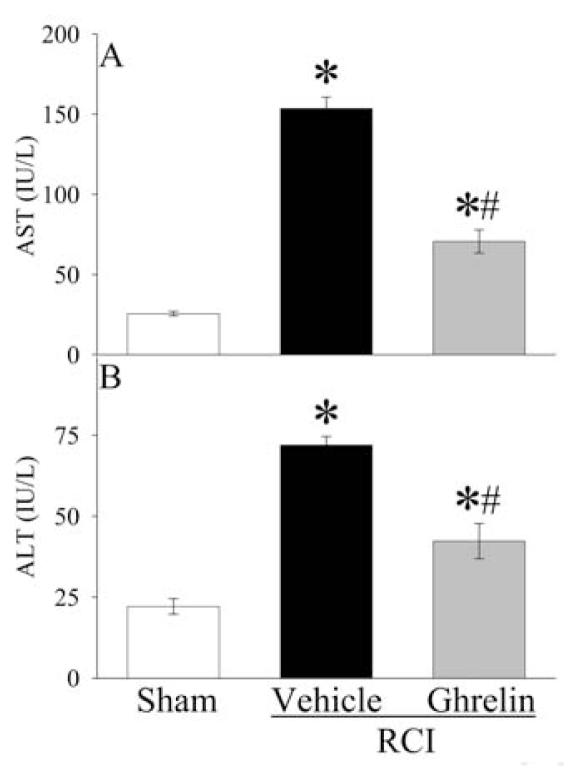
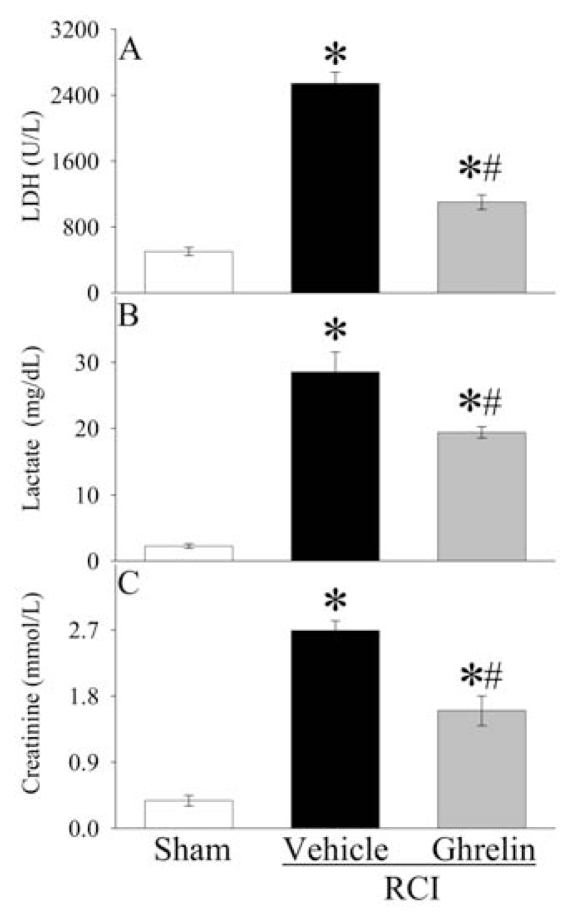
Compared with sham operated animals, marked increase in liver enzymes (AST, ALT) was observed at 20 h after RCI (Figure 2A, B), indicating hepatic injury. Human ghrelin treatment significantly reduced serum levels of AST and ALT by 54% and 41%, respectively. Similarly, serum levels of LDH increased by 407% at 20 h after RCI in vehicle treated animals (Figure 3A). Human ghrelin treatment decreased serum LDH levels by 57% (P < 0.05). Serum levels of lactate, a marker for systemic hypoxia, increased dramatically at 20 h after RCI, and administration of human ghrelin decreased lactate levels significantly by 32% (P < 0.05, Figure 3B). As shown in Figure 3C, serum creatinine levels, a marker of renal dysfunction, increased by 608% at 20 h after RCI in vehicle treated animals (P < 0.05). Treatment with human ghrelin decreased serum creatinine levels by 41% (P < 0.05).
Figure 2.
Alterations in serum levels of aspartate aminotransferase (AST) (A) and alanine aminotransferase (ALT) (B) at 20 h after radiation combined injury (RCI) with treatment by normal saline (Vehicle) or human ghrelin (Ghrelin) or sham operation (Sham). Data are presented as means ± SE (n = 6/group) and compared by ANOVA and Student-Newman-Keuls method: *P < 0.05 versus Sham group; #P < 0.05 versus Vehicle group.
Figure 3.
Alterations in serum levels of lactate dehydragenese (LDH) (A), lactate (B), and creatinine (C) at 20 h after radiation combined injury (RCI) with treatment by normal saline (Vehicle) or human ghrelin (Ghrelin) or sham operation (Sham). Data are presented as means ± SE (n = 6/group) and compared by ANOVA and Student-Newman-Keuls method: *P < 0.05 versus Sham group; #P < 0.05 versus Vehicle group.
Human Ghrelin Inhibits Proinflammatory Responses after RCI
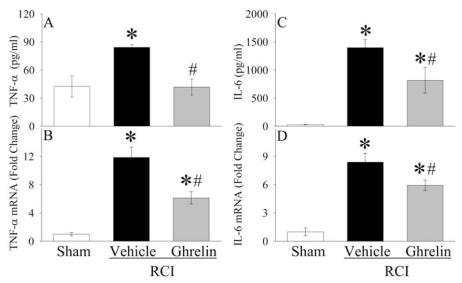
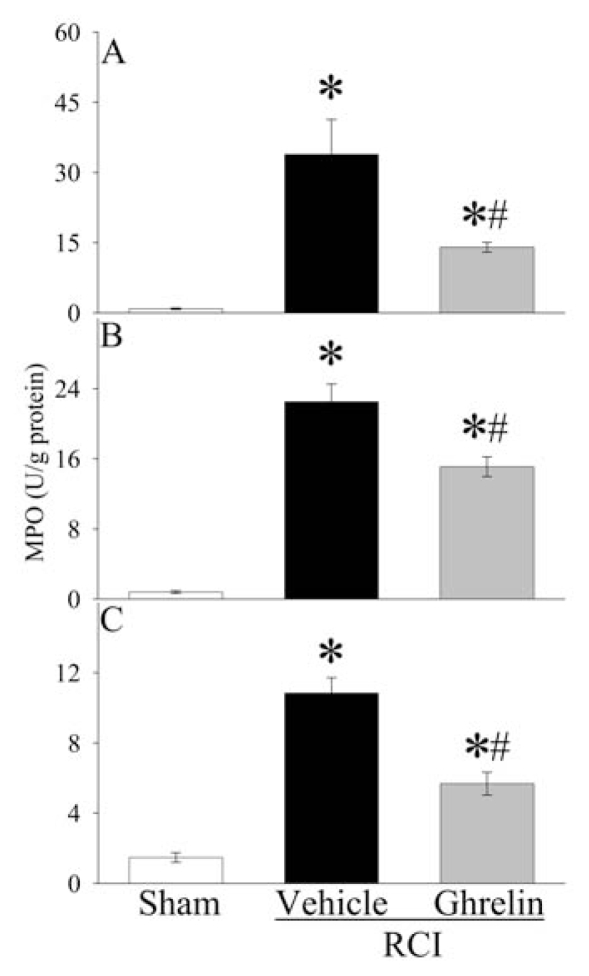
As indicated in Figure 4A, serum levels of TNF-αwere doubled at 20 h after RCI (P < 0.05). Treatment with human ghrelin reduced serum TNF-αlevels by 50% in RCI animals (P < 0.05), and there was no significant difference in serum TNF-αlevels between sham operated and human ghrelin treated RCI animals. Similarly, TNF-αgene expression in the small intestine increased by eleven-fold at 20 h after RCI, and human ghrelin treatment decreased it by 48% (P < 0.05, Figure 4B). As shown in Figure 4C, serum levels of IL-6 increased by fifty-two-fold 20 h after RCI in vehicle treated animals (P < 0.05). Human ghrelin treatment decreased them by 42% (P < 0.05). IL-6 gene expression in the small intestine increased by more than seven-fold at 20 h after RCI, and human ghrelin treatment decreased it by 29% (P < 0.05, Figure 4D). The level of myeloperoxidase (MPO) activity is an indicator of neutrophil infiltration. As demonstrated in Figures 5A–C, significant increases in intestinal, pulmonary, and renal MPO activities were observed in vehicle treated RCI rats as compared with sham animals. Treatment with human ghrelin significantly inhibited the increases in MPO activities in the small intestine (Figure 5A), lungs (Figure 5B), and kidneys (Figure 5C) after RCI (P < 0.05). These results show that ghrelin downregulates proinflammatory cytokines and attenuates the influx of neutrophils into the small intestine, lungs, and kidneys after RCI.
Figure 4.
Alterations in serum levels of TNF-α(A), TNF-αgene expression in the small intestine (B), serum levels of IL-6 (C), and IL-6 gene expression in the small intestine (D) at 20 h after radiation combined injury (RCI) with treatment by normal saline (Vehicle) or human ghrelin (Ghrelin) or sham operation (Sham). Data are presented as means ± SE (n = 4–6/group) and compared by ANOVA and Student-Newman-Keuls method: *P < 0.05 versus Sham group; #P < 0.05 versus Vehicle group.
Figure 5.
Alterations in myeloperoxidase (MPO) activities in the intestine (A), lungs (B) and kidneys (C) at 20 h after radiation combined injury (RCI) with treatment by normal saline (Vehicle) or human ghrelin (Ghrelin) or sham operation (Sham). Data are presented as means ± SE (n = 4–6/group) and compared by ANOVA and Student-Newman-Keuls method: *P < 0.05 versus Sham group; #P < 0.05 versus Vehicle group.
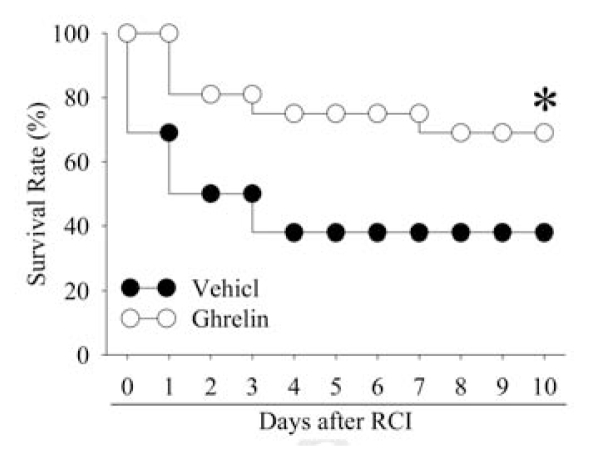
Human Ghrelin Improves Survival after RCI
To use mortality as an important endpoint to test the efficacy of human ghrelin after RCI, the time to excise the necrotic cecum was determined first. Our preliminary study showed that excising the necrotic cecum at 2 and 10 h after CLP resulted in survival rates of 83% and 17%, respectively. In this regard, we have chosen to excise the necrotic cecum at 5 h after CLP. As shown in Figure 6, the survival rate after RCI and cecal excision at 5 h after CLP with vehicle (saline) administration was 69% at d 1, 50% at d 2 and d 3, and decreased to 38% at d 4 to d 10. Treatment with human ghrelin, however, improved the survival rate to 69%, which was significantly higher than that in RCI vehicle treated animals (P < 0.05, Figure 6).
Figure 6.
Alterations in the survival rate during 10 d after radiation combined injury (RCI) treatment by normal saline treatment (Vehicle) or human ghrelin treatment (Ghrelin). There were 16 animals in each group. The survival rate was estimated by the Kaplan–Meier method and compared by using the log-rank test. *P < 0.05 versus Vehicle.
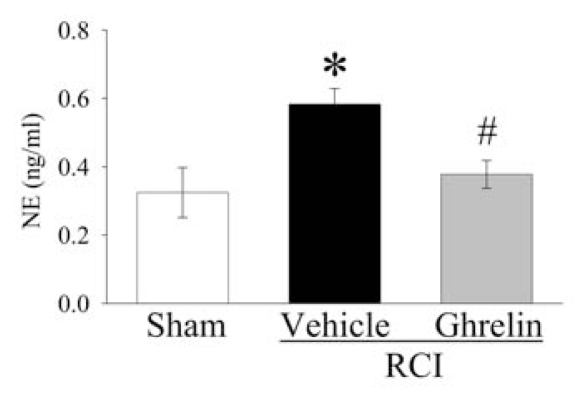
Effects of Human Ghrelin Administration on Plasma Levels of Norepinephrine (NE) after RCI
The interaction between the central nervous system and immune system under various inflammatory diseases has found considerable interest in the past several decades. Our previous studies have shown that NE, the sympathetic neurotransmitter, upregulates proinflammatory cytokines by activating α2-adrenoceptor (27–29). To determine whether ghrelin inhibits sympathetic activity, plasma levels of NE were measured at 20 h after RCI. As shown in Figure 7, plasma levels of NE increased significantly at 20 h after RCI as compared with those in sham operated animals (P < 0.05). Intravenous administration of human ghrelin decreased plasma levels of NE by 35% in RCI animals (P < 0.05).
Figure 7.
Alterations in plasma levels of nor-epinephrine (NE) at 20 h after radiation combined injury (RCI) with treatment by normal saline (Vehicle) or human ghrelin (Ghrelin) or sham operation (Sham). Data are presented as means ± SE (n = 6/group) and compared by ANOVA and Student-Newman-Keuls method: *P < 0.05 versus Sham group; #P < 0.05 versus Vehicle group.
Effects of Vagotomy on Human Ghrelin’s Effects on Organ Injury after RCI
To determine whether ghrelin’s beneficial effects after RCI are mediated via activation of the vagus nerve, sham vagotomy or vagotomy was performed in sham and RCI animals immediately prior to the administration of human ghrelin. Serum levels of AST, ALT, LDH, lactate, and creatinine were measured at 20 h after RCI in sham-vagotomized or vagotomized animals. As shown in Table 1, both sham-vagotomized and vagotomized rats subjected to RCI had significant increases in circulating levels of AST, ALT, LDH, lactate, and creatinine compared with sham operated animals (P < 0.05). These results are similar to the vagus nerve–intact animals (see Figure 2, see Figure 3). When sham-vagotomized septic animals were treated with ghrelin, the levels of AST, ALT, LDH, lactate, and creatinine were reduced markedly (P < 0.05, Table 1). However, ghrelin treatment has no measurable effects on serum levels of AST, ALT, LDH, lactate, and creatinine in vagotomized RCI animals.
Table 1.
Effects of sham vagotomy or vagotomy on serum levels of AST, ALT, LDH, lactate, and creatinine in sham operated animals (Sham) and septic animals treated with normal saline (Vehicle) or ghrelin (Ghrelin) at 20 h after radiation combined injury (RCI).
| Sham | RCI-Vehicle | RCI-Ghrelin | ||
|---|---|---|---|---|
| AST (IU/L) | Sham vagotomy | 30 ± 2.4a | 139 ± 6.0b | 70 ± 5.0bc |
| Vagotomy | 35 ± 1.4 | 166 ± 8.0b | 166 ± 7.0b | |
| ALT (IU/L) | Sham vagotomy | 18 ± 0.9 | 79 ± 3.3b | 42 ± 2.1bc |
| Vagotomy | 18 ± 1.0 | 78 ± 2.9b | 75 ± 2.9b | |
| LDH (U/L) | Sham vagotomy | 561±60.3 | 2526 ± 145.3b | 1092 ± 88.5bc |
| Vagotomy | 601 ± 36.7 | 2403 ± 176.6b | 2198 ± 172.0b | |
| Lactate (mg/dl) | Sham vagotomy | 14 ± 1.4 | 30 ± 2.8b | 22 ± 1.2bc |
| Vagotomy | 16 ± 1.0 | 30 ± 2.4b | 29 ± 2.3b | |
| Creatinine (mmol/L) | Sham vagotomy | 0.5 ± 0.03 | 2.5 ± 0.28b | 1.6 ± 0.18bc |
| Vagotomy | 0.6 ± 0.07 | 2.6 ± 0.21b | 2.7 ± 0.09b |
Data are presented as means ± SE (n = 6/group) and compared by ANOVA and Student-Newman-Keuls method.
P < 0.05 versus respective Sham group.
P < 0.05 versus respective Vehicle group.
Effects of Vagotomy on Human Ghrelin’s Effects on Inflammatory Responses after RCI
To determine whether vagotomy also eliminates ghrelin’s effects on inflammatory responses after RCI, proinflammatory cytokines (TNF-αand IL-6) in the serum and MPO activities in the small intestine, lungs, and kidneys were measured at 20 h after RCI in sham-vagotomized or vagotomized animals. As indicated in Table 2, sham vagotomy did not alter the antiinflammatory effect of ghrelin at 20 h after RCI. In contrast, vagotomy immediately prior to the administration of ghrelin abolished the inhibitory effects of ghrelin on the circulating levels of TNF-αand IL-6 (Table 2). Similarly, vagotomy completely prevented the inhibitory effect of this agent on MPO activities in the small intestine, lungs, and kidneys at 20 h after RCI (Table 2).
Table 2.
Effects of sham vagotomy or vagotomy on serum levels of TNF-α, IL-6, as well as myeloperoxidase (MPO) activities in the intestine, lungs and kidneys in sham operated animals (Sham) and septic animals treated with normal saline (Vehicle) or ghrelin (Ghrelin) at 20 h after radiation combined injury (RCI).
| Sham | RCI-Vehicle | RCI-Ghrelin | ||
|---|---|---|---|---|
| TNF-α (pg/mL) | Sham vagotomy | 46 ± 9.0a | 83 ± 5.6b | 53 ± 4.2bc |
| Vagotomy | 51 ± 6.7 | 83 ± 4.4b | 78 ± 4.5b | |
| IL-6 (pg/mL) | Sham vagotomy | 41 ± 1.9 | 1507 ± 120.1b | 670 ± 64.0bc |
| Vagotomy | 37 ± 4.2 | 1335 ± 85.3b | 1417 ± 109.6b | |
| Intestinal MPO (μU/mg) | Sham vagotomy | 1.2 ± 0.08 | 28.1 ± 3.27b | 14.6 ± 1.08bc |
| Vagotomy | 1.2 ± 0.13 | 29.9 ± 2.90b | 31.0 ± 1.96b | |
| Pulmonary MPO (μU/mg) | Sham vagotomy | 1.0 ± 0.14 | 23.3 ± 2.03b | 15.1 ± 1.34bc |
| Vagotomy | 1.3 ± 0.14 | 24.9 ± 2.49b | 23.9 ± 2.41b | |
| Renal MPO (μU/mg) | Sham vagotomy | 1.8 ± 0.21 | 11.4 ± 0.86b | 5.5 ± 0.58bc |
| Vagotomy | 1.6 ± 0.27 | 10.3 ± 0.95b | 11.5 ± 1.39b |
Data are presented as means ± SE (n = 6/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method.
P ± 0.05 versus respective Sham group.
P < 0.05 versus respective Vehicle group.
DISCUSSION
The terrorist radiation exposure scenario presents a very real and dangerous possibility. It is well recognized that radiation injury is most often accompanied by trauma, burn, infection, and sepsis. In fact, sepsis was the primary cause of death in several recent radiation accidents (30). Therefore, there is a dire need for therapy and treatments geared directly toward sepsis in radiation victims. However, little information is available regarding the pathophysiology of sepsis in radiation victims and its treatment. Using a rat model of RCI (radiation exposure followed by cecal ligation and puncture), the current study shows that ghrelin levels were downregulated significantly after radiation injury combined with severe sepsis, and administration of human ghrelin reduced sepsis-induced organ injury and mortality in irradiated rats.
Ghrelin is a novel gastrointestinal hormone. The biological effects of ghrelin are mediated through the ghrelin receptor (GHSR-1a), a 7 transmembrane domain Gq protein coupled receptor (11,31). Ghrelin is the only identified endogenous ligand for this receptor. Ghrelin originally was reported to induce growth hormone release through pituitary GHSR-1a stimulation (12–14). However, recent studies have indicated multiple paracrine, autocrine, and endocrine roles of ghrelin, reflecting the wide distribution of GHSR-1a (32–35). In addition to the established effects on food intake and GH production, ghrelin has emerged as a potent immunoregulatory and antiinflammatory agent (16,17,36). An appropriate inflammatory response eliminates the invading microorganisms without causing damage to tissues and organs. However, excessive and sustained inflammatory responses can lead to severe tissue injury. Elevated production/release of proinflammatory cytokines (for example, TNF-αand IL-6) can cause tissue injury in sepsis (37,38). It has been shown that radiation-induced production of proinflammatory cytokines in the blood, peripheral lymphoid tissues, and lungs contributed to the disorders associated with radiation injury (39–41). Ran et al. showed that serum levels of TNF-αand IL-6 were increased significantly after 12-Gy γ radiation followed by 30% body surface burn in the rat (42). Similarly, the current study also shows that the production of TNF-αand IL-6 was elevated significantly after radiation injury combined with severe sepsis. Since administration of a specific ghrelin receptor antagonist increased proinflammatory cytokine release in normal animals (21,26), the acute ghrelin deficiency after RCI may have contributed to local and systemic inflammatory derangements after RCI.
Our previous studies have shown that rat ghrelin is beneficial in rat models of polymicrobial sepsis (20,21). Human ghrelin is a 28 amino acid peptide with a fatty acid chain modification on the N-terminal third amino acid (17,43). Rat ghrelin differs from human ghrelin by two amino acids (17). The antigenicity of rat proteins in humans prevents the use of rat ghrelin in humans. Therefore, human ghrelin must be tested in rats before these findings can be verified in humans. Our current results clearly demonstrated that human ghrelin administration inhibited proinflammatory cytokine release, reduced neutrophil infiltration, attenuated organ injury, and improved survival after RCI in rats. In this regard, we have overcome one major obstacle hampering the development of ghrelin as a therapeutic agent for sepsis in human radiation victims.
Although the detailed mechanism of ghrelin’s beneficial effect after RCI warrants further investigation, our results have indicated it might involve ghrelin receptors in the central nervous system. It is now widely accepted that the nervous system reflexively regulates the inflammatory response in real time (44,45). Our previous studies have indicated that the release of the sympathetic neurotransmitter, norepinephrine (NE), from the small intestine is increased in sepsis, and that NE potentiates endotoxin-induced TNF-αupregulation via the A subtype of α2-adrenoceptors expressed on the surface of Kupffer cells (44). Ghrelin has sympathoinhibitory properties. Recent studies have shown a decrease in the sympathetic nerve activity in brown adipose tissues and kidneys after intravenous or intracerebroventricular injection of ghrelin (46–48). Ghrelin decreases plasma NE levels by 42% in patients with chronic heart failure (48). Our recent study has shown that ghrelin reduces NE levels and the downregulatory effect of ghrelin on proinflammatory cytokines can be reversed by coadministration of NE (21,26). In the current study, we also show that administration of human ghrelin decreased plasma NE levels by 35%. In this regard, human ghrelin’s beneficial effect after RCI may be due partially to its modulation of the overstimulated sympathetic nerve activation. Moreover, ghrelin activates the vagus nerve and vagal blockade abolishes ghrelin-induced feeding and growth hormone secretion (49). Stimulation of the vagus nerve can attenuate systemic inflammatory responses rapidly through inhibiting the activation of macrophages and endothelial cells (44,45). Our previous studies have shown that the antiinflammatory effect of ghrelin requires the intact vagus nerve, as vagotomy prevents its beneficial effects in sepsis and gut ischemia reperfusion injury (18,50). Similarly, our present study also indicates that the intact vagus nerve is required for human ghrelin’s beneficial effects after RCI. In this regard, the protective effect of human ghrelin after radiation combined injury might be attributed to rebalancing the dysregulated sympathetic/parasympathetic nervous systems. Since ghrelin has been shown to increase neuronal activity dose-dependently (51–53), we believe that the protective effect of ghrelin after RCI should be in a dose-dependent manner.
In conclusion, our current results demonstrate that human ghrelin has many beneficial effects, such as reduction of organ injury markers, attenuation of cytokine release, inhibition of neutrophils infiltration, and decrease in the mortality rate in animals after RCI. This information is promising, showing that the early administration of human ghrelin has the potential to be a safe and effective treatment in individuals exposed to radiation combined injury. The protective effect of human ghrelin appears to be attributed to rebalancing the dysregulated sympathetic/ parasympathetic nervous systems. With the results we have obtained, we hope to further pursue human ghrelin as a safe and effective treatment against radiation combined injury. In our future studies, we will determine how radiation itself affects the consequence of a septic insult and the optimal dose(s) of ghrelin after radiation combined injury.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health (NIH) grants R21 AI080536, R01 GM053008, and R01 AG028352 (PW). Some of the results of this study were presented previously at the 38th Critical Care Congress, January 31 through February 4, 2009 in Nashville, Tennessee, USA.
Footnotes
DISCLOSURES
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Flynn DF, Goans RE. Nuclear terrorism: triage and medical management of radiation and combined-injury casualties. Surg Clin North Am. 2006;86:601–36. doi: 10.1016/j.suc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170:1435–44. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howell G, Tisherman SA. Management of sepsis. Surg Clin North Am. 2006;86:1523–39. doi: 10.1016/j.suc.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Powers J, Jacobi J. Pharmacologic treatment related to severe sepsis. AACN Adv Crit Care. 2006;17:423–32. doi: 10.4037/15597768-2006-4007. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins I. Evidence-based sepsis therapy: a hospitalist perspective. J Hosp Med. 2006;1:285–95. doi: 10.1002/jhm.116. [DOI] [PubMed] [Google Scholar]
- 6.Bernard GR, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 7.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 8.Danai PA, Sinha S, Moss M, Haber MJ, Martin GS. Seasonal variation in the epidemiology of sepsis. Crit Care Med. 2007;35:410–5. doi: 10.1097/01.CCM.0000253405.17038.43. [DOI] [PubMed] [Google Scholar]
- 9.Strehlow MC, Emond SD, Shapiro NI, Pelletier AJ, Camargo CA., Jr National study of emergency department visits for sepsis, 1992 to 2001. Ann Emerg Med. 2006;48:326–31. doi: 10.1016/j.annemergmed.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Angus DC, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Kojima M, et al. Ghrelin is a growth- hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 12.Arvat E, et al. Preliminary evidence that Ghrelin, the natural GH secretagogue (GHS)- receptor ligand, strongly stimulates GH secretion in humans. J Endocrinol Invest. 2000;23:493–5. doi: 10.1007/BF03343763. [DOI] [PubMed] [Google Scholar]
- 13.Date Y, et al. Central effects of a novel acylated peptide, ghrelin, on growth hormone release in rats. Biochem Biophys Res Commun. 2000;275:477–80. doi: 10.1006/bbrc.2000.3342. [DOI] [PubMed] [Google Scholar]
- 14.Nass R, et al. Intracerebroventricular administration of the rat growth hormone (GH) receptor antagonist G118R stimulates GH secretion: evidence for the existence of short loop negative feedback of GH. J Neuroendocrinol. 2000;12:1194–9. doi: 10.1046/j.1365-2826.2000.00586.x. [DOI] [PubMed] [Google Scholar]
- 15.Cowley MA, Grove KL. Ghrelin—Satisfying a hunger for the mechanism. Endocrinol. 2004;145:2604–6. doi: 10.1210/en.2004-0346. [DOI] [PubMed] [Google Scholar]
- 16.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 17.Wang G, Lee HM, Englander E, Greeley GH., Jr Ghrelin—not just another stomach hormone. Regul Pept. 2002;105:75–81. doi: 10.1016/s0167-0115(02)00012-5. [DOI] [PubMed] [Google Scholar]
- 18.Wu R, et al. Ghrelin down-regulates proinflammatory cytokines in sepsis through activation of the vagus nerve. Ann Surg. 2007;245:480–6. doi: 10.1097/01.sla.0000251614.42290.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu R, Zhou M, Cui X, Simms HH, Wang P. Upregulation of cardiovascular ghrelin receptor occurs in the hyperdynamic phase of sepsis. Am J Physiol Heart Circ Physiol. 2004;287:H1296–302. doi: 10.1152/ajpheart.00852.2003. [DOI] [PubMed] [Google Scholar]
- 20.Wu R, et al. Ghrelin improves tissue perfusion in severe sepsis via downregulation of endothelin-1. Cardiovasc Res. 2005;68:318–26. doi: 10.1016/j.cardiores.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Wu R, et al. Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. Am J Respir Crit Care Med. 2007;176:805–13. doi: 10.1164/rccm.200604-511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington (DC): National Academy Press; 1996. p. 118. [Google Scholar]
- 23.Katanyutanon S, Wu R, Wang P. The effect of whole-body radiation on blood levels of gastrointestinal peptides in the rat. Int J Clin Exp Med. 2008;1:332–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Wu R, et al. Reversing established sepsis in rats with human vasoactive hormone adrenomedullin and its binding protein. Mol Med. 2009;15:28–33. doi: 10.2119/molmed.2008.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dwivedi AJ, et al. Adrenomedullin and adrenomedullin binding protein-1 prevent acute lung injury after gut ischemia-reperfusion. J Am Coll Surg. 2007;205:284–93. doi: 10.1016/j.jamcollsurg.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Wu R, et al. Ghrelin inhibits sympathetic nervous activity in sepsis. Am J Physiol Endocrinol Metab. 2007;293:E1697–702. doi: 10.1152/ajpendo.00098.2007. [DOI] [PubMed] [Google Scholar]
- 27.Yang S, Zhou M, Chaudry IH, Wang P. Norepinephrine-induced hepatocellular dysfunction in early sepsis is mediated by activation of alpha2-adrenoceptors. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1014–21. doi: 10.1152/ajpgi.2001.281.4.G1014. [DOI] [PubMed] [Google Scholar]
- 28.Zhou M, et al. The role of Kupffer cell alpha(2)-adrenoceptors in norepinephrine-induced TNF-alpha production. Biochim Biophys Acta. 2001;1537:49–57. doi: 10.1016/s0925-4439(01)00055-2. [DOI] [PubMed] [Google Scholar]
- 29.Zhou M, Das P, Simms HH, Wang P. Gut-derived norepinephrine plays an important role in up-regulating IL-1beta and IL-10. Biochim Biophys Acta. 2005;1740:446–52. doi: 10.1016/j.bbadis.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Turai I, Veress K, Gunalp B, Souchkevitch G. Medical response to radiation incidents and radionuclear threats. BMJ. 2004;328:568–72. doi: 10.1136/bmj.328.7439.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25:426–57. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 32.Hattori N, et al. GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab. 2001;86:4284–91. doi: 10.1210/jcem.86.9.7866. [DOI] [PubMed] [Google Scholar]
- 33.Sakata I, et al. Growth hormone secretagogue receptor expression in the cells of the stomach-projected afferent nerve in the rat nodose ganglion. Neurosci Lett. 2003;342:183–6. doi: 10.1016/s0304-3940(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 34.Papotti M, et al. Growth hormone secretagogue binding sites in peripheral human tissues. J Clin Endocrinol Metab. 2000;85:3803–7. doi: 10.1210/jcem.85.10.6846. [DOI] [PubMed] [Google Scholar]
- 35.Shuto Y, et al. Generation of polyclonal antiserum against the growth hormone secretagogue receptor (GHS-R): evidence that the GHS-R exists in the hypothalamus, pituitary and stomach of rats. Life Sci. 2001;68:991–6. doi: 10.1016/s0024-3205(00)01001-8. [DOI] [PubMed] [Google Scholar]
- 36.Taub DD. Novel connections between the neuroendocrine and immune systems: the ghrelin immunoregulatory network. Vitam Horm. 2008;77:325–46. doi: 10.1016/S0083-6729(06)77014-5. [DOI] [PubMed] [Google Scholar]
- 37.Koo DJ, Chaudry IH, Wang P. Kupffer cells are responsible for producing inflammatory cytokines and hepatocellular dysfunction during early sepsis. J Surg Res. 1999;83:151–7. doi: 10.1006/jsre.1999.5584. [DOI] [PubMed] [Google Scholar]
- 38.Hotchkiss RS, Karl IE. The Pathophysiology and Treatment of Sepsis. N Engl J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 39.Holler E, et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood. 1990;75:1011–6. [PubMed] [Google Scholar]
- 40.Hong JH, et al. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int J Radiat Biol. 1999;75:1421–7. doi: 10.1080/095530099139287. [DOI] [PubMed] [Google Scholar]
- 41.Linard C, et al. Acute induction of inflammatory cytokine expression after gamma- irradiation in the rat: effect of an NF-kappaB inhibitor. Int J Radiat Oncol Biol Phys. 2004;58:427–34. doi: 10.1016/j.ijrobp.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 42.Ran XZ, et al. Effects of serum from rats with combined radiation-burn injury on the growth of hematopoietic progenitor cells. J Trauma. 2007;62:193–8. doi: 10.1097/01.ta.0000215434.24726.72. [DOI] [PubMed] [Google Scholar]
- 43.Bednarek MA, et al. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem. 2000;43:4370–6. doi: 10.1021/jm0001727. [DOI] [PubMed] [Google Scholar]
- 44.Miksa M, Wu R, Zhou M, Wang P. Sympathetic excitotoxicity in sepsis: pro-inflammatory priming of macrophages by norepinephrine. Front Biosci. 2005;10:2217–29. doi: 10.2741/1691. [DOI] [PubMed] [Google Scholar]
- 45.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–96. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumura K, Tsuchihashi T, Fujii K, Abe I, Iida M. Central ghrelin modulates sympathetic activity in conscious rabbits. Hypertension. 2002;40:694–9. doi: 10.1161/01.hyp.0000035395.51441.10. [DOI] [PubMed] [Google Scholar]
- 47.Yasuda T, Masaki T, Kakuma T, Yoshimatsu H. Centrally administered ghrelin suppresses sympathetic nerve activity in brown adipose tissue of rats. Neurosci Lett. 2003;349:75–8. doi: 10.1016/s0304-3940(03)00789-4. [DOI] [PubMed] [Google Scholar]
- 48.Nagaya N, et al. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110:3674–9. doi: 10.1161/01.CIR.0000149746.62908.BB. [DOI] [PubMed] [Google Scholar]
- 49.Date Y, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–8. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 50.Wu R, et al. Orexigenic hormone ghrelin attenuates local and remote organ injury after intestinal ischemia-reperfusion. PLoS ONE. 2008;3:e2026. doi: 10.1371/journal.pone.0002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yanagida H, et al. Effects of ghrelin on neuronal activity in the ventromedial nucleus of the hypothalamus in infantile rats: an in vitro study. Peptides. 2008;29:912–8. doi: 10.1016/j.peptides.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 52.Takayama K, et al. Expression of c-Fos protein in the brain after intravenous injection of ghrelin in rats. Neurosci Lett. 2007;417:292–6. doi: 10.1016/j.neulet.2007.02.089. [DOI] [PubMed] [Google Scholar]
- 53.Obay BD, Tasdemir E, Tumer C, Bilgin HM, At-maca M. Dose dependent effects of ghrelin on pentylenetetrazole-induced oxidative stress in a rat seizure model. Peptides. 2008;29:448–55. doi: 10.1016/j.peptides.2007.11.020. [DOI] [PubMed] [Google Scholar]