Abstract
The selected strain of rodent used in experimental models of traumatic brain injury is typically dependent upon the experimental questions asked and the familiarity of the investigator with a specific rodent strain. This archival study compares the injury responsiveness and recovery profiles of two popular outbred strains, the Long-Evans (LE) and the Sprague-Dawley (SD), after brain injury induced by lateral fluid percussion injury (LFPI). General findings include a significantly longer duration of unconsciousness in LE rats, but similar durations of apnea. Both strains displayed the same level of initial FPI-induced behavioral deficits, followed by a more rapid rate of functional recovery in SD rats. Cortical volume loss was not significantly different, but close inspection of the data suggests the possibility that LE rats may be more susceptible to damage in the hemisphere contralateral to the injury site than are SD rats. It is hoped that the information provided here encourages greater attention to the subtle differences and similarities between strains in future pre-clinical efficacy studies of traumatic brain injury.
Key words: beam walk, fluid percussion injury, loss of consciousness, Morris water maze, recovery of function, strain differences, traumatic brain injury
Introduction
Reproducibility and generalizability of results from different laboratories have been a constant challenge in the field of experimental neurotrauma. Several reviews have previously attempted to delineate the critical issues that need to be addressed (Statler et al., 2001; Narayan et al., 2002) so as to provide a stronger basis for successful clinical trials. Specific to this report, the heterogeneity of clinical neurotrauma is not well replicated in experimental models that generally strive for homogeneity in the samples tested. Despite the homogeneity of samples within experimental studies, there is still a tremendous amount of variability across different studies. For example, the use of the lateral fluid percussion injury (LFPI) model has been established as a clinically relevant experimental model of traumatic brain injury (TBI). However, due to variations in LFPI devices across laboratories, the standard reporting of atmospheres of pressure (atm) as an index of injury severity is not acceptable. Indeed, there has been a recent increase in published reports of experimental TBI in which the latency of loss of consciousness (LOC) has been used in place of the traditional atm index to more accurately represent the severity of the injury sustained following a fluid percussion brain injury (Giza et al., 2002; Li et al., 2004; Smith et al., 2005; Hoane et al., 2006). The duration of LOC has been positively correlated with fluid pulse pressure (atm) and functional outcome in adults as an index of severity of injury (Dixon et al., 1987; Delahunty et al., 1995). By using LOC in place of atm as an index of severity, differences in the placement of the injury, variations in different models of LFPI devices, and even differences in surgical techniques can be minimized, allowing for better generalizability of results.
Furthermore, a widely accepted corollary remains uninvestigated. Although strain differences in rats have been fairly well documented, there remains a paucity of literature specifically comparing the behavioral recovery profiles of different rodent strains following TBI. Indeed, while most researchers acknowledge that strain differences can significantly contribute to the variability of behavioral results obtained across different studies, a systematic investigation of behavioral recovery comparing different strains has not yet been reported.
Significant strain-dependent differences have been observed in the assessment of ischemic lesion volumes (Oliff et al., 1996; Takaba et al., 2004; Bardutzky et al., 2005; Walberer et al., 2006), and in forebrain activation before and after spinal cord injury (Paulson et al., 2005). Differences in strains have also been well documented in addictive behaviors and in the relation of physiological differences in the hypothalamic-pituitary-adrenal axis (Kosten and Ambrosio, 2002; Kearns et al., 2006). Inter-strain differences in cognitive function have also been systematically investigated by Whishaw and colleagues, who coined the term “strain drain” to describe the effects of domestication on different strains of rats, and in this regard LE rats appear to be least affected (Harker and Whishaw, 2002, 2004). Indeed, the LE rat's superior cognitive performance has been repeatedly confirmed (Lindner and Schallert, 1988; Tonkiss et al., 1992; Andrews et al., 1995). Strain differences in cognitive performance have also been observed in aging and in gender studies, and in experiments in which physiological, environmental, and/or pharmacological variables have been manipulated (Lindner and Schallert, 1988; Diana et al., 1994; Tâoth et al., 1996; van der Staay and Blokland, 1996; Gleason et al., 1999; Vorhees et al., 1999; Wyss et al., 2000; Vales et al., 2006). Variations in motor and sensorimotor integration as assessed by skilled forelimb reaching performance can also be strain-dependent (Nikkhah et al., 1998; Whishaw et al., 2003). Behavioral differences between strains in exploratory behaviors have also been observed (Meyerson et al., 2006), and have been associated with strain-related differences in EEG activity (Inoue et al., 1990; Sisson et al.,1991; Mayo-Michelson and Young, 1993; De Bruin et al., 2000; Van Lier et al., 2003). Inter-strain differences have been observed in differential stress responses both physiologically and behaviorally (Faraday, 2002; Pardon et al., 2002; Ferguson and Cada, 2004; Ma and Morilak, 2004; Zamudio et al., 2005), and in the development of neuropathic pain (Carr et al., 1992; Xu et al., 2001).
Moreover, the differences in the visual system of albino versus non-albino strains have been closely investigated. Non-albino rats have been reported to have greater visual acuity, better color detection, higher resolution, superior dark adaptation, and less light sensitivity compared to albino rat strains (Drèager, 1985; Grant et al., 2001; Prusky et al., 2002). The pigmented LE strain has been reported to perform better in Morris water maze tasks compared to albino strains; further, it has been suggested that this is due to better visual acuity in the non-albino animals (Lindner and Schallert, 1988; D'Hooge and De Deyn, 2001). Differences between albino and pigmented rats in inter-ocular transference were noted by Sheridan (Sheridan, 1965). Sheridan's findings were later supported by anatomical evidence showing reduced uncrossed visual pathways to the primary optic regions of the brain in albino rats compared to pigmented rats (Lund, 1965).
Clearly, the differences between the strains of rats can affect the ability to generalize results obtained across different laboratories. The purpose of this study is to present the behavioral similarities and differences between two strains of rodents that are frequently used in experimental models of TBI: Long-Evans (LE) and Sprague-Dawley (SD) rats. Rats were assessed using a battery of assessments commonly employed in pre-clinical investigations of TBI. We further propose that these similarities and differences should be taken into consideration when the rate of functional recovery is assessed in any pre-clinical study of neurotrauma. The intent of this report is to shed some light on the behavioral differences between two strains of rats subjected to the same procedures. An in-depth analysis of strain differences and the interaction with the pathophysiology and subsequent recovery are beyond the scope of this report. Hence this paper attempts to provide some insight into a comparison of the rate of recovery between LE and SD rats, as assessed by a variety of different behavioral tasks following LFPI.
Methods
Animals
The data used in the following analyses comprises archival data collected from control and sham animals that were used in four different studies (Smith et al., 2005; Hoane et al., 2006; Smith et al., 2006; Holland et al., 2008). During the course of conducting these various experiments, the authors noticed a possible difference in the durations of loss of consciousness between the different strains. In the studies by Smith and colleagues, only Long-Evans animals were used, while the studies by Hoane and Holland and associates used Sprague-Dawley rats. The surgeries were performed by the same surgeon using the same equipment in the same operating room. Further, the LFPI device was calibrated each time prior to injury to the same reading. In addition, the animals were tested and trained in the same facilities using the same apparatus by the same testers. Given the similarities across these four studies, the authors believed that there was sufficient control between tasks to allow comparison of the data obtained from the two different strains of animals. Unfortunately, due to the retrospective nature of this analysis, the numbers of animals in each group varied across the different tasks. Nonetheless, it was felt that each animal had received approximately the same level of pre- and post-injury handling and experience, and that the results in this study do not constitute differences in experimental control.
A total of 54 adult male rats approximately 4 months old were used in this study. Two outbred strains, LE (Charles Rivers Laboratories, Inc., Chicago, IL) and SD (Harlan Sprague Dawley, Inc., Indianapolis, IN) rats, were purchased directly from their respective vendors and housed in the institutional facilities for 1 month prior to brain injury. All animals were housed singly in standard cages on a 12/12-h light-dark cycle. Standard rat chow and water were provided ad libitum. Each animal was handled and provided with training on the beam walk prior to brain injury. The amount of handling and training were equivalent for both strains of rats, as were all environmental conditions. A total of 17 rats of each strain were subjected to brain injury, while the remaining 10 per strain were treated as shams. All experimental procedures were reviewed and approved by the institutional animal care and use committee and were in accordance with National Institutes of Health guidelines.
Traumatic brain injury
Each rat underwent an aseptic surgical procedure to induce an injury of moderate severity using the LFPI model. The methods and procedures for this surgical protocol have been previously published (Smith et al., 2005; Hoane et al., 2006; Holland et al., 2008). On the day of the surgery, each animal was weighed and placed into an induction chamber for 3–4 min with a gas anesthetic mixture of 4% isoflurane, 0.2 L/min nitrous oxide, and 0.8 L/min oxygen. When the animal was non-responsive to a toe pinch, the isoflurane was turned down to 2% and redirected to a nose cone. The animal was shaved and placed in a stereotaxic frame with the attached nose cone. A surgical plane of anesthesia, as indicated by respiratory rate and depth, was maintained by titrating the amount of isoflurane (2–4%) throughout the 30-min surgery. Following application of povidone-iodine and a mid-line incision, a 4.0-mm craniotomy was created over the left hemisphere (epicenter at anteroposterior: −4.4 mm from the bregma; medio-lateral: 2.4 mm) keeping the dura intact. A female Luer-Loc connector was affixed to the skull over the craniotomy using dental acrylic and filled with sterile saline. Anesthesia was turned off and the animal was immediately attached to the LFPI device (VCU Biomedical Engineering, Richmond, VA) in prone position. A fluid bolus (mean = 1.80 atm; SD = 0.09) was delivered upon the first positive withdrawal response to a toe pinch. Duration of LOC and apnea were determined by recording the latency from the moment of impact to the time a positive withdrawal response and voluntary respiration was initiated. Anesthesia was then reinstated and the incision was sutured. The animal was placed on a warming bed and observed for a period of 1 h prior to returning to the colony room. Sham-treated animals underwent the same surgical procedure including a craniotomy, but did not receive LFPI.
Behavioral assessments
All the rats were pre-trained to criterion on the beam walk and were subsequently tested after TBI. Of the total group, different subsets of rats also underwent additional testing on locomotor placing, forelimb flexion, vibrissae-forelimb placing, and reference and working memory tasks in the Morris water maze. With the exception of the Morris water maze, all performance evaluations were conducted on days 2, 4, 6, 8, 10, 12, and 14, post-LFPI. Reference memory testing occurred on days 11, 12, 13, and 14 post-LFPI, and was followed immediately by working memory testing on days 15, 16, and 17. The behavioral measures reported in this study constitute a standard battery of tests assessing sensorimotor and cognitive performance. These tasks are commonly used in pre-clinical TBI investigations involving rats to assess the level of brain injury–induced deficits, and subsequent recovery as an index of treatment efficacy (Hamm, 2001; Smith et al., 2005; Hoane et al., 2006).
Beam walk
The beam walk task is a sensorimotor task that measures sensorimotor coordination and balance (Feeney et al., 1982; Hoane et al., 2006). Each animal was trained to criterion to traverse a 120-cm-long elevated beam (2.5 cm wide). Criterion performance was assessed as the ability to traverse the beam with a maximum of two footslips. Generally, the animals are trained to criterion in 3 days and are able to traverse the beam with no footslips. Post-injury performance was evaluated on a scale of 1 to 7. A score of 7 was considered as performance equivalent to criterion performance, while a score of 1 indicated that the animal was unable to maintain its balance on the beam and did not attempt to traverse the length of the beam.
Locomotor placing
The locomotor placing task is also a sensorimotor task (Hoane et al., 1997; Schallert and Woodlee, 2005) wherein the animal's activity in freely traversing a grid is observed and foot-faults that occur during locomotion are recorded. The grid (85 × 55 cm with 3 × 3-cm openings) was composed of 15 test tube racks that were bound together. Each animal was assessed for one 3-min period on each post-TBI test day. All animals were exposed to this task and baseline performance was recorded prior to injury.
Forelimb flexion
The forelimb flexion task is a neurological test (McIntosh et al., 1989). The animal is placed on a flat surface and lifted into the air by grasping the base of the tail. The amount of flexion and/or adduction of the forelimbs are rated on a 4-point scale (4 = normal, no flexion; 3 = less than 50% of flexion of the contralateral forelimb with adduction clearly present; 2 = more than 50% of flexion; and 1 = complete adduction of the forelimb). Baseline performance prior to injury was recorded.
Vibrissae-forelimb placing
The vibrissae-forelimb placing task (Schallert and Woodlee, 2005; Hoane et al., 2006; Hoane et al., 2007) is a sensorimotor test that evaluates the reflexive placing of the ipsilateral forelimb when the vibrissae on the same side make contact with a surface. In intact rats, automatic placing of the forelimb is elicited each time the vibrissae on the same side are touched to a horizontal acrylic glass surface. Each animal was given 10 trials per side during each post-TBI testing day. If the animal did not respond within 5 sec of vibrissae stimulation, the trial was recorded as unsuccessful. All animals were exposed to this task and baseline performance was recorded prior to injury.
Morris water maze
The Morris water maze is a common test of cognitive performance used with rodents in studies of neurotrauma (Hoane et al., 2003; Smith et al., 2005; Hoane et al., 2006). Two standard tests evaluating reference and working memory were employed. In the reference memory task, the animals are placed into a pool for 4 trials per day for 4 days. The latency and path length to attain the submerged fixed platform was recorded for each trial. Following the reference memory task, the animals were tested on the working memory version of the test in which they were given 4 trials per day for 3 days. A 15-min intra-trial interval was maintained each day. The submerged platform was moved to a different location in the pool each day. A trial was terminated when the animal acquired the platform, after which it was allowed to remain on the platform for 15 sec. In the event the animal did not find the platform within the 90-sec time limit, it was guided to the platform and placed on it for 15 sec. This test was conducted post-LFPI and the animals were not exposed to the task prior to TBI. A visual platform was not employed in this study, which is standard in our behavioral paradigms (Hoane et al., 2003, 2006; Smith et al., 2005).
Histology
The animals in the study were sacrificed on day 40 post-LFPI. Each received an overdose of urethane (3.0 g/kg; 0.5 g/mL IP) followed by transcardial perfusion with 0.9% phosphate-buffered saline (PBS) and 10% phosphate-buffered formalin (PBF). The brains were removed from the skulls and post-fixed in PBF for 48 h and 30% sucrose for an additional 3–5 days prior to frozen sectioning. The brains were blocked and 40-μm serial coronal sections were obtained using a sliding microtome and collected in PBS. After being allowed to rest in PBS for a minimum of 24 h, the sections were mounted on gelatin-subbed microscope slides, stained with cresyl violet, and cover-slipped.
Lesion analysis
A series of coronal sections were stained with cresyl violet, dehydrated, and cover-slipped. The extent of the lesion was analyzed with an Olympus microscope (BX-51; Olympus America, Center Valley, PA) and an Olympus 13.5 megapixel digital camera (DP-70). Images of the sections throughout the extent of the injury [at bregma coordinates 3.80, 4.30, and 5.30 mm (Paxinos and Watson, 2005)] were captured at 0.44 × (1.25 × objective and 0.35 × c-mount) using the digital capturing system, and area measurements of the ipsilateral and contralateral cortices were determined using ImageTool software (ImageTool, Roswell, GA). Area measurements were transformed to a volume measurement (Coggeshall, 1992). Consistent with previous studies the extent of cortical injury was measured by calculating the percent reduction in the ipsilateral cortex compared to the contralateral cortex using the formula (1 − (ipsi/contra) × 100) (Hoane et al., 2006; Holland et al., 2008).
Statistical analysis
All statistical analyses were conducted using SPSS version 15 for Windows (SPSS, Inc., Chicago, IL). Behavioral tests were analyzed using GLM procedures with repeated measures followed by Tukey's HSD post-hoc tests where applicable. Injury parameters (weight, apnea, and loss of consciousness) and lesion volume were analyzed using an independent samples t-test. A significance level of p < 0.05 was employed in the interpretation of the results.
Results
Severity of injury
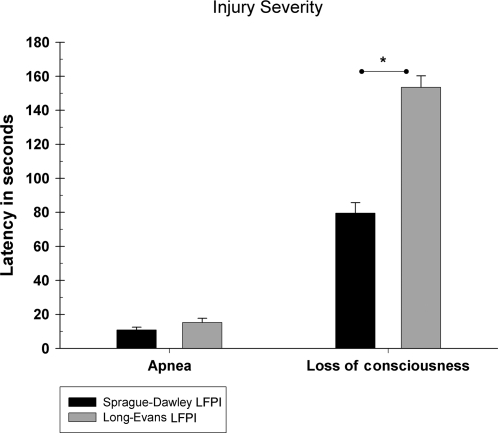
There were no significant weight differences between the strains [t (32) = .029, p = 0.98]. Weight at the time of LFPI was equivalent across both the LE and SD rats (mean = 404.09 g, SEM = 7.03). Similarly, there were no significant differences in the duration of apnea following TBI [t (32) = −1.444, p = 0.16]. However, the LE rats did display slightly greater durations of apnea (mean = 15.18 sec, SEM = 2.52) compared to the SD animals (mean = 10.82 sec, SEM = 1.64). Further, the duration of LOC was significantly different [t (32) = −4.133, p < 0.001]. As shown in Figure 1, the LE rats required a greater length of time to regain a positive hindlimb withdrawal reflex following LFPI (mean = 153.47 sec, SEM = 6.81) compared to the SD animals (mean = 79.47 sec, SEM = 6.15).
FIG. 1.
Latency of apnea and loss of consciousness following TBI by LFPI (mean ± SEM). SD rats (n = 17) displayed a significantly shorter duration of unconsciousness compared to LE (n = 17) rats as indicated by the asterisk (*p < 0.05).
Beam walk
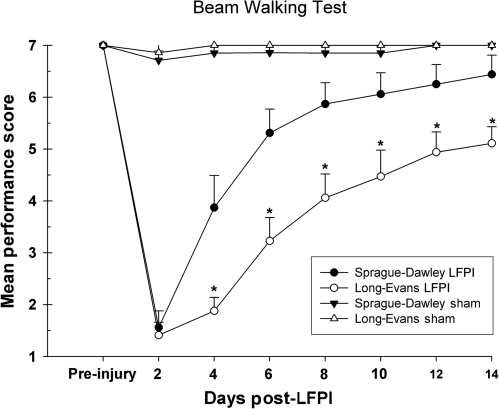
Beam walk performance was evaluated in sham-operated and brain-injured LE and SD rats (LFPI: SD n = 16, LE n = 17; sham: SD n = 7, LE n = 7). There were no significant differences in the performance of the shams for either strain. However, the injured rats displayed a significant difference in the rate of recovery following LFPI [F (3,43) = 21.69, p =0.001]. Post-hoc comparisons revealed a significant injury effect for both strains of LFPI rats when compared to shams (p < 0.001). It is also evident, as shown in Figure 2, that the injured SD rats showed a quicker rate of recovery over time compared to the LE animals (p < 0.001). With the exception of the first test point on day 2 post-LFPI, the injured SD rats performed significantly better on the beam walk compared to the injured LE rats on all post-LFPI test days (days 4, 6, 8, 10, 12, and 14) (p < 0.05).
FIG. 2.
Graph of beam walk performance. SD LFPI rats displayed a significantly faster rate of recovery to pre-injury criterion levels compared to LE rats, as indicated by the asterisk (*p < 0.05). Shams did not show any impairment. LE rats continued to show a deficit and did not attain criterion performance after LFPI.
Locomotor placing
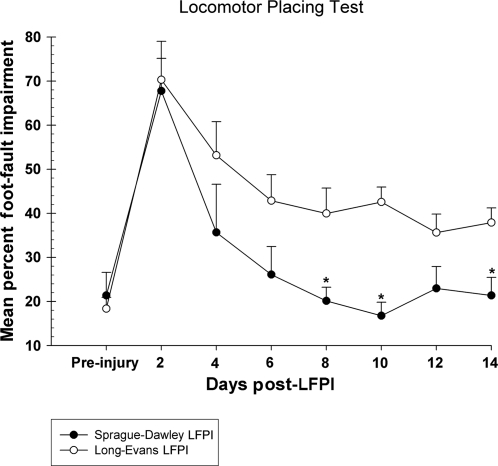
Performance on this task was evaluated in LE and SD injured rats. Overall performance was significantly different between the groups [F (1,11) = 7.07, p = 0.02]. Figure 3 shows the difference in the rate of recovery between the LE and SD rats. The SD rats recovered to pre-injury baseline levels by day 8, making fewer foot-faults during locomotion compared to the LE animals (p < 0.05). On the contrary, the LE rats showed little continued recovery after day 6 post-LFPI. Compared to their pre-injury baseline performance, the LE rats displayed approximately a 20% increase in the number of foot-faults per quadrant for the last 5 test points ending on day 14 post-LFPI.
FIG. 3.
Graph of locomotor placing (footslips/quadrants). SD rats displayed a significantly faster rate of recovery to baseline levels compared to LE rats. The performance of the LE rats remained significantly more impaired, as indicated by the asterisk (*p < 0.05). Sham animals are not shown.
Forelimb flexion
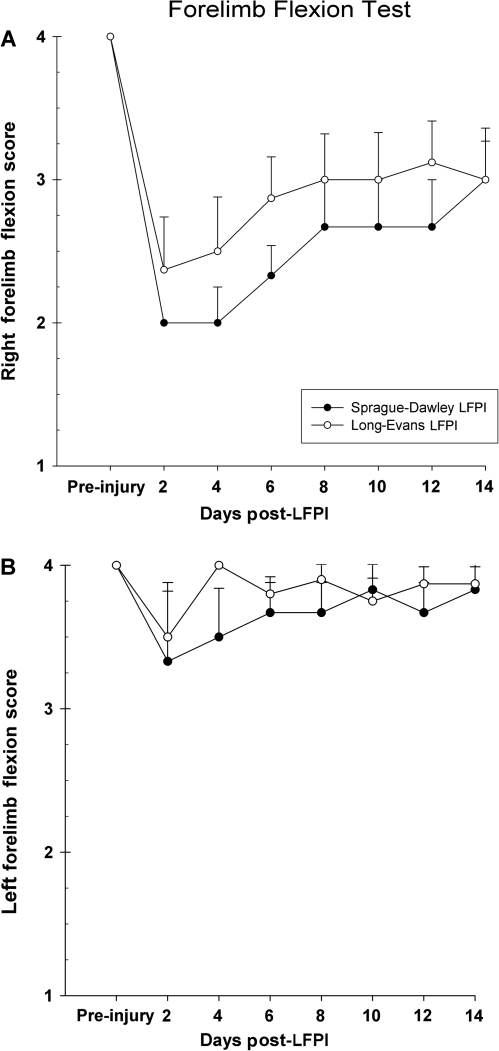
Forelimb flexion was assessed in brain-injured LE and SD rats. Both groups of rats displayed a similar degree of impairment in the extension of the right forelimb contralateral to injury [F (1,12) = 0.90, p = 0.36] (Fig. 4A). Although both strains showed some recovery during the 2-week testing period, neither regained normal extension of the right limb by the last testing day. There were no significant differences in the reflex extension of the left forelimb between the groups. Further, the left forelimb did not appear to show any significant impairment due to brain injury (Fig. 4B).
FIG. 4.
(A) Graph of right side forelimb flexion. Normal reflexive extension of the forelimb contralateral to the brain injury is impaired in both SD and LE rats. There were no significant differences in recovery. (B) Graph of left side forelimb flexion. LFPI did not significantly impair the reflexive extension of the forelimb ipsilateral to the brain lesion. Both SD and LE rats performed in a similar manner. Sham animals are not shown.
Vibrissae-forelimb placing
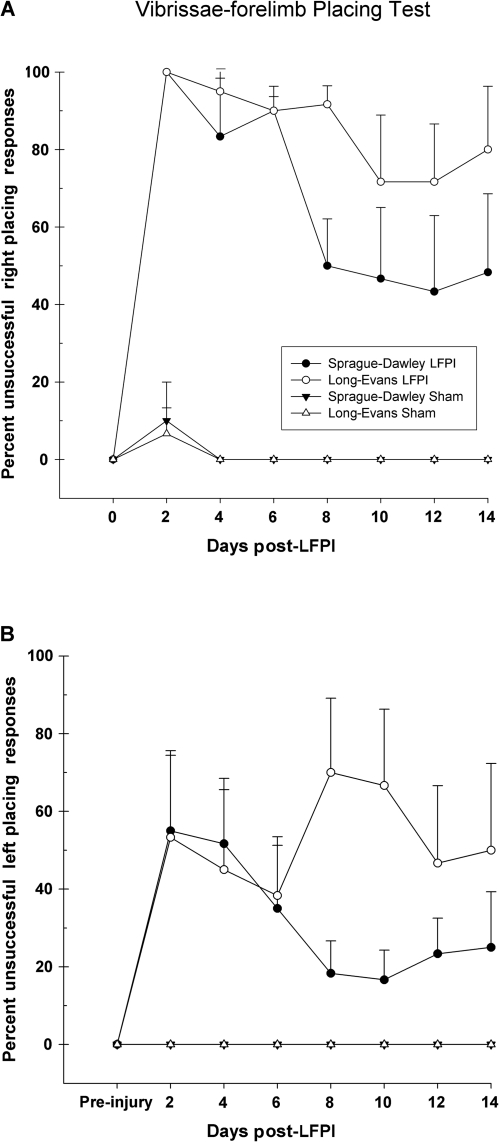
Sham-operated (LE n = 6, SD n = 7) and LFPI (LE n = 6, SD n = 6) rats were included in this assessment. Shams successfully placed on all 10 trials at every assessment period with the exception of the first test day (day 2 post-LFPI). An injury effect was observed [F (3,21) = 48.93, p = 0.001] in which both injured groups were significantly impaired in the ability to successfully place the right forelimb in response to stimulation of the right side vibrissae, as compared to shams (p < 0.001). LFPI induced a severe deficit in the first week post-LFPI for both LE and SD rats. Neither group was able to successfully place more than once in the acute time points. Although post-hoc comparisons did not yield a statistically significant difference between the two injured groups (p = 0.159), inspection of Figure 5A shows that on day 8 post-LFPI the SD rats improved to approximately 50% placing, but did not show any further improvement through the last day of testing. On the contrary, the LE rats displayed a slightly slower rate of recovery and never improved beyond 20% of successful placing throughout the entire testing period.
FIG. 5.
(A) Graph of right side vibrissae-forelimb placing. LFPI significantly affected the ability of both SD and LE rats to successfully place the right forelimb in response to stimulation of the right vibrissae. The impairment was not statistically significantly different between the two strains, although the SD rats showed a trend toward faster and greater recovery. (B) Graph of left side vibrissae-forelimb placing. The successful placing of the left forelimb in response to left-sided vibrissae stimulation is detrimentally affected by LFPI compared with shams. LFPI had a greater detrimental effect on LE rats in the placing of the limb ipsilateral to injury compared to SD rats.
Interestingly, the assessment of left side vibrissae-forelimb placing (ipsilateral to injury) yielded a significant injury effect [F (3,21) = 7.65, p = 0.001] compared to shams (Fig. 5B). Post-hoc tests revealed that the performance of the injured SD rats, although somewhat deficient, was not statistically significant compared to shams (p > 0.05). In contrast, the LE rats displayed significantly greater deficits (p < 0.001), and their ability to successfully place on the left side appeared to be more variable. By the second week of testing, the injured SD rats showed a trend toward recovery, placing at approximately 80%, while the LE rats remained at only 50%.
Morris water maze
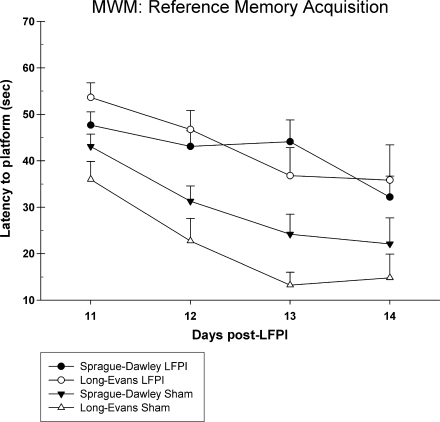
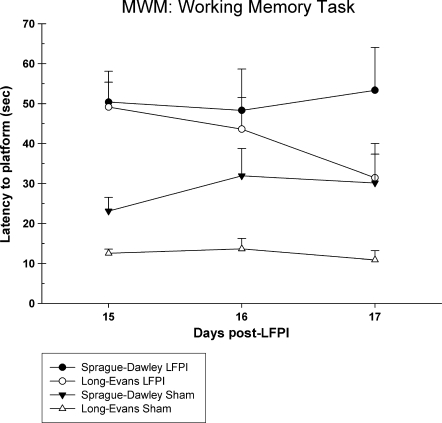
A total of 34 rats were tested in the Morris water maze (shams: LE n = 7, SD n = 9; LFPI: LE n = 9, SD n = 9). A statistically significant injury effect was observed in the reference memory version of the test [F (3,30) = 9.41, p = 0.001]. Rats that received an LFPI were significantly slower at acquiring the platform compared to sham-operated rats. However, there were no statistically significant differences between the two strains of rats (Fig. 6). Overall swim speed did not differ between the groups [F (3,33) = 1.02, p = 0.40]. Examination of the working memory data revealed a similar effect (Fig. 7). There was a significant injury effect [F (3,30) = 8.28, p = 0.001] in the working memory version of the test, and there were no significant latency differences between the LE and SD rats for either the sham-operated (p > 0.05) or the brain-injured groups (p > 0.05). Although the strain difference in both acquisition of reference memory and working memory between sham groups was not significant, it was found that the LE rats performed at a higher level than the SD rats.
FIG. 6.
Graph of latency to acquire the platform in the reference memory version of the Morris water maze task. SD and LE rats that were brain injured were slower at learning the task compared to shams. Sham-injured LE rats showed a trend toward faster acquisition of the task compared to sham-injured SD rats.
FIG. 7.
Graph of latency to acquire the platform in the working memory version of the Morris water maze task. Both SD and LE brain-injured groups were significantly impaired on this task compared to shams. The LE LFPI group showed a trend toward faster acquisition of the task compared to the SD LFPI rats. Similarly, the LE sham-injured rats demonstrated faster latencies in finding the platform compared to sham-injured SD rats.
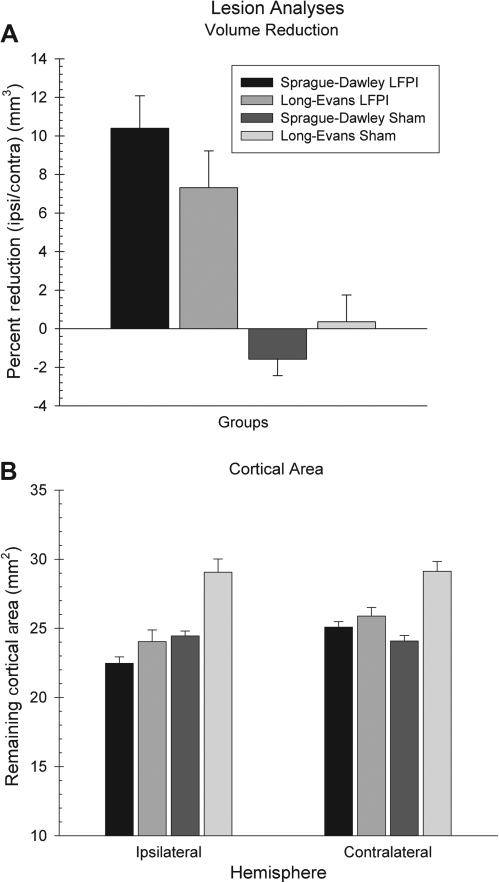
Lesion analysis
A significant injury effect was obtained in the percentage of cortical volume reduction [F (3,53) = 10.13, p = 0.001] wherein the animals receiving LFPI showed a greater loss of cortical volume compared to shams (Fig. 8A). Post-hoc analyses revealed that for the brain-injured rats there were no significant differences in the cortical volume lost between the LE and SD rats (p > 0.05). However, the SD rats showed a greater loss in volume compared to LE rats. Interestingly enough, when the mean area of the cortex for each hemisphere was compared, it was found that sham-operated LE rats showed significantly greater mean cortical area compared to sham-operated SD rats for both the left [F (3,53) = 13.33, p = 0.001] and right hemispheres [F (3,53) = 12.13, p = 0.001]. In addition, there was a greater difference between injured and sham LE rats on the contralateral side compared to SD rats (Fig. 8B). Thus, there appears to be more substantial loss of cortical area in the contralateral hemisphere of the LE rats compared to the SD rats.
FIG. 8.
(A) There were no significant differences in the mean percentage of cortical volume lost due to LFPI between the SD and LE rats. However, both LFPI groups showed significant loss of tissue volume due to brain injury compared to shams. (B) For both the left and right hemispheres, the LE shams showed significantly greater mean cortical area compared to all other groups.
Discussion
This archival investigation brings a new consideration into the interpretation of the results concerning recovery of function after LFPI. When subjected to the same injury via surgery performed by the same surgeon in a similar fashion, the latency of apnea was not significantly different, but the SD rats responded with significantly shorter durations of LOC compared to the LE rats. It is interesting to note that both strains displayed the same level of impairment across all tasks when initially tested at day 2 post-LFPI. However, on the beam walk and locomotor placing tasks, a statistically significant difference in the rate of recovery was noted with the SD rats, showing greater and faster improvement in their performance on the tasks. By the end of the testing period at 14 days post-LFPI, the SD rats were traversing the beam and the locomotor placing grid at levels that were not significantly different from their pre-injury performance. On the contrary, the LE rats were still significantly impaired on these tasks and did not show the same accelerated rate of recovery. A similar trend was observed in the vibrissae-forelimb placing task. For both the left and right side, the LE rats showed a greater and more persistent deficit in placing than the SD rats, despite having the same level of initial deficit. The level of impairment in the forelimb flexion task was similar for both the SD and LE rats. Both strains displayed a similar deficit and recovery rate in the right forelimb, while the left forelimb showed relatively little impairment.
The tests of cognitive performance in the reference and working memory versions of the Morris water maze demonstrated that both the LE and SD groups were similarly impaired compared to shams. The injured LE rats showed a slight improvement on the final day of testing in the working memory version compared to the SD rats. There were no significant differences in the rate of acquisition of the reference memory task between the two groups of injured rats. It is also interesting to note that when comparing both sham-operated groups, the LE rats displayed a higher level of cognitive performance compared to the SD rats. Although not statistically significant, the LE rats showed a strong trend toward faster acquisition of the submerged platform across the 4 days of testing in the reference memory version of the task. Moreover, the LE rats appeared to carry over the knowledge of the task into the working memory version in which the location of the platform was changed daily. On the other hand, the SD rats performed more poorly on the second day of the working memory version, with a slight increase in latency which did not improve on the following and final day. This matches the effects seen in LE rats in previous studies investigating their cognitive/spatial ability (Lindner and Schallert, 1988).
Another interesting observation is the fact that there were no significant differences in the percent of cortical volume lost due to injury between the LE and SD rats, although the LE rats possessed greater mean cortical areas compared to the SD rats. Further, it should be noted that there were no significant differences in the mean cortical area of the right hemisphere (contralateral to injury) between the SD shams and the SD LFPI rats. Conversely, the LE LFPI rats showed a significant decrement in the mean cortical area of this supposed “uninjured” hemisphere compared to the LE shams. The extent of histology employed in this study was insufficient to verify if indeed the LE rats sustained greater cortical degeneration (if at all) in the hemisphere contralateral to injury than the SD rats. Nonetheless, this observation deserves future investigation.
This study also brings further supporting evidence to the nature of the injury induced by LFPI. The common assumption of the hemisphere contralateral to the injury as the “intact” hemisphere is challenged by the present data. While the neurological assessment of the amount of reflexive flexion and adduction in the forelimbs indicated that the left forelimb (ipsilateral to injury) was relatively unimpaired, the data from the vibrissae-forelimb placing test showed the contrary. Figure 5 shows that both the SD and LE injured groups were impaired in both right and left forelimb placing. As expected, the impairment in the limb contralateral to LFPI was significant and did not return to pre-injury levels over the 2-week testing period. More interesting is the evidence that the LFPI animals showed a significant injury-induced impairment in the vibrissae-forelimb placing of the left forelimb (ipsilateral to injury). Moreover, the SD rats rebounded from this effect and performed at nearly sham levels. On the contrary, the injured LE rats showed the same level of impairment in the first week following injury, but performed significantly more poorly in the next week. While is it possible that the LE rats may experience greater secondary injury compared to the SD rats, the results of the present study cannot reliably support this statement. In addition, the extent of histology conducted in this study was insufficient to verify the possibility that perhaps the LE rats experienced some level of neurodegeneration in the hemisphere contralateral to the LFPI while the SD rats did not. However, in a recent study, acute degeneration of neurons was found in the contralateral “non-injured” hemisphere at 24 h after LFPI (Holland et al., 2008).
To conclude, the current findings are presented as a cursory, archival assessment of differences in the recovery and injury profiles of the LE and SD strains of outbred rodents. Clearly, a more in-depth study comparing the pathophysiology and subsequent recovery of these different strains is warranted. Nonetheless, the present authors hope that the results of this study may assist in raising awareness when making cross-study comparisons and generalizations of results involving different strains of rats in pre-clinical neurotrauma investigations. While similar in many respects, SD rats appeared to recover motor and sensorimotor function more quickly than LE rats. Further, with the level of injury induced in this study, the SD rats generally regained pre-injury or sham level performance while the LE rats remained impaired. Additional evidence gleaned from this study indicates that tasks involving motor coordination that are sensitive to practice such as the beam walk and locomotor placing tasks present the best recovery profile in both SD and LE rats. Tasks less sensitive to practice such as the forelimb flexion and vibrissae-forelimb placing tasks present as more persistent deficits after LFPI, even when some recovery is noted, especially in the LE rats. Results from the cognitive assessments support previously published reports indicating that LE rats generally perform better at cognitive tasks. The current data demonstrate that following LFPI, both LE and SD rats sustained the same level of impairment and were not significantly different in the rate at which they acquired the tasks; however, testing them longer may have allowed demonstration of a strain difference. Future investigations further delineating the similarities and differences in recovery and injury profiles between strains using other experimental models of TBI are necessary and encouraged, and may aid in facilitating more successful pre-clinical drug efficacy studies.
Acknowledgments
The authors would like to thank Bryan McConomy, Keith Gregory, Mustafa Aladin, and Nicholas Kuypers for their assistance in data collection. Thanks also to Jason Beare and Nicholas Kaufmann for their assistance in lesion analysis. Special thanks to Dr. Douglas C. Smith for the use of laboratory facilities. Funding for this project was provided by National Institute of Neurological Disorders and Stroke grant NS045647 to M.R.H.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Andrews J.S. Jansen J.H. Linders S. Princen A. Broekkamp C.L. Performance of four different rat strains in the autoshaping, two-object discrimination, and swim maze tests of learning and memory. Physiol. Behav. 1995;57:785–790. doi: 10.1016/0031-9384(94)00336-x. [DOI] [PubMed] [Google Scholar]
- Bardutzky J. Shen Q. Henninger N. Bouley J. Duong T.Q. Fisher M. Differences in ischemic lesion evolution in different rat strains using diffusion and perfusion imaging. Stroke. 2005;36:2000–2005. doi: 10.1161/01.STR.0000177486.85508.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr M.M. Best T.J. MacKinnon S.E. Evans P.J. Strain differences in autotomy in rats undergoing sciatic nerve transection or repair. Ann. Plast. Surg. 1992;28:538–544. [PubMed] [Google Scholar]
- Coggeshall R.E. A consideration of neural counting methods. TINS. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- De Bruin N.M. Van Luijtelaar E.L. Jansen S.J. Cools A.R. Ellenbroek B.A. Dopamine characteristics in different rat genotypes: the relation to absence epilepsy. Neurosci. Res. 2000;38:165–173. doi: 10.1016/s0168-0102(00)00154-1. [DOI] [PubMed] [Google Scholar]
- Delahunty T.M. Jiang J.Y. Black R.T. Lyeth B.G. Differential modulation of carbachol and trans-ACPD-stimulated phosphoinositide turnover following traumatic brain injury. Neurochem. Res. 1995;20:405–411. doi: 10.1007/BF00973095. [DOI] [PubMed] [Google Scholar]
- D'Hooge R. De Deyn P.P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Brain Res. Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Diana G. Domenici M.R. Loizzo A. Scotti De Carolis A. Sagratella S. Age and strain differences in rat place learning and hippocampal dentate gyrus frequency-potentiation. Neurosci. Lett. 1994;171:113–116. doi: 10.1016/0304-3940(94)90618-1. [DOI] [PubMed] [Google Scholar]
- Dixon C.E. Lyeth B.G. Povlishock J.T. Findling R.L. Hamm R.J. Marmarou A. Young H.F. Hayes R.L. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- Drèager U.C. Calcium binding in pigmented and albino eyes. Proc. Natl. Acad. Sci. USA. 1985;82:6716–6720. doi: 10.1073/pnas.82.19.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraday M.M. Rat sex and strain differences in responses to stress. Physiol. Behav. 2002;75:507–522. doi: 10.1016/s0031-9384(02)00645-5. [DOI] [PubMed] [Google Scholar]
- Feeney D.M. Gonzalez A. Law W.A. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science (New York, N.Y.) 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- Ferguson S.A. Cada A.M. Spatial learning/memory and social and nonsocial behaviors in the spontaneously hypertensive, Wistar-Kyoto and Sprague-Dawley rat strains. Pharmacol. Biochem. Behav. 2004;77:583–394. doi: 10.1016/j.pbb.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Giza C.C. Prins M.L. Hovda D.A. Herschman H.R. Feldman J.D. Genes preferentially induced by depolarization after concussive brain injury: effects of age and injury severity. J. Neurotrauma. 2002;19:387–402. doi: 10.1089/08977150252932352. [DOI] [PubMed] [Google Scholar]
- Gleason T.C. Dreiling J.L. Crawley J.N. Rat strain differences in response to galanin on the Morris water task. Neuropeptides. 1999;33:265–270. doi: 10.1054/npep.1999.0044. [DOI] [PubMed] [Google Scholar]
- Grant S. Patel N.N. Philp A.R. Grey C.N. Lucas R.D. Foster R.G. Bowmaker J.K. Jeffery G. Rod photopigment deficits in albinos are specific to mammals and arise during retinal development. Vis. Neurosci. 2001;18:245–251. doi: 10.1017/s095252380118209x. [DOI] [PubMed] [Google Scholar]
- Hamm R.J. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J. Neurotrauma. 2001;18:1207–1216. doi: 10.1089/089771501317095241. [DOI] [PubMed] [Google Scholar]
- Harker K.T. Whishaw I.Q. A reaffirmation of the retrosplenial contribution to rodent navigation: reviewing the influences of lesion, strain, and task. Neurosci. Biobehav. Rev. 2004;28:485–496. doi: 10.1016/j.neubiorev.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Harker K.T. Whishaw I.Q. Place and matching-to-place spatial learning affected by rat inbreeding (Dark-Agouti, Fischer 344) and albinism (Wistar, Sprague-Dawley) but not domestication (wild rat vs. Long-Evans, Fischer-Norway) Behav. Brain Res. 2002;134:467–477. doi: 10.1016/s0166-4328(02)00083-9. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Akstulewicz S.L. Toppen J. Treatment with vitamin B3 improves functional recovery and reduces GFAP expression following traumatic brain injury in the rat. J. Neurotrauma. 2003;20:1189–1198. doi: 10.1089/089771503770802871. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Pierce J.L. Holland M.A. Birky N.D. Dang T. Vitek M.P. McKenna S.E. The novel apolipoprotein E-based peptide COG1410 improves sensorimotor performance and reduces injury magnitude following cortical contusion injury. J. Neurotrauma. 2007;24:1108–1118. doi: 10.1089/neu.2006.0254. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Raad C. Barth T.M. Non-competitive NMDA antagonists and anti-oxidant drugs reduce striatal atrophy and facilitate recovery of function following lesions of the rat cortex. Restor. Neurol. Neurosci. 1997;11:71–82. doi: 10.3233/RNN-1997-111208. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Tan A.A. Pierce J.L. Anderson G.D. Smith D.C. Nicotinamide treatment reduces behavioral impairments and provides cortical protection after fluid percussion injury in the rat. J. Neurotrauma. 2006;23:1535–1548. doi: 10.1089/neu.2006.23.1535. [DOI] [PubMed] [Google Scholar]
- Holland M.A. Tan A.A. Smith D.C. Hoane M.R. Nicotinamide treatment provides acute neuroprotection and GFAP regulation following fluid percussion injury. J. Neurotrauma. 2008;25:140–152. doi: 10.1089/neu.2007.0312. [DOI] [PubMed] [Google Scholar]
- Inoue M. Peeters B.W. Van Luijtelaar E.L. Vossen J.M. Coenen A.M. Spontaneous occurrence of spike-wave discharges in five inbred strains of rats. Physiol. Behav. 1990;48:199–201. doi: 10.1016/0031-9384(90)90285-c. [DOI] [PubMed] [Google Scholar]
- Kearns D.N. Gomez-Serrano M.A. Weiss S.J. Riley A.L. A comparison of Lewis and Fischer rat strains on autoshaping (sign-tracking), discrimination reversal learning and negative auto-maintenance. Behav. Brain Res. 2006;169:193–200. doi: 10.1016/j.bbr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Kosten T.A. Ambrosio E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- Li H.H. Lee S.M. Cai Y. Sutton R.L. Hovda D.A. Differential gene expression in hippocampus following experimental brain trauma reveals distinct features of moderate and severe injuries. J. Neurotrauma. 2004;21:1141–1153. doi: 10.1089/neu.2004.21.1141. [DOI] [PubMed] [Google Scholar]
- Lindner M.D. Schallert T. Aging and atropine effects on spatial navigation in the Morris water task. Behav. Neurosci. 1988;102:621–634. doi: 10.1037//0735-7044.102.5.621. [DOI] [PubMed] [Google Scholar]
- Lund R.D. Uncrossed visual pathways of hooded and albino rats. Science. 1965;149:1506–1507. doi: 10.1126/science.149.3691.1506. [DOI] [PubMed] [Google Scholar]
- Ma S. Morilak D.A. Induction of FOS expression by acute immobilization stress is reduced in locus coeruleus and medial amygdala of Wistar-Kyoto rats compared to Sprague-Dawley rats. Neuroscience. 2004;124:963–972. doi: 10.1016/j.neuroscience.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Mayo-Michelson L. Young G.A. EEG, EEG power spectral, and behavioral differences in response to acute ethylketocyclazocine administration in two inbred rat strains. Brain Res. Bull. 1993;31:345–351. doi: 10.1016/0361-9230(93)90226-2. [DOI] [PubMed] [Google Scholar]
- McIntosh T.K. Vink R. Noble L. Yamakami I. Fernyak S. Soares H. Faden A.L. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- Meyerson B.J. Augustsson H. Berg M. Roman E. The concentric square field: a multivariate test arena for analysis of explorative strategies. Behav. Brain Res. 2006;168:100–113. doi: 10.1016/j.bbr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Narayan R.K. Michel M.E. Ansell B. Baethmann A. Biegon A. Bracken M.B. Bullock M.R. Choi S.C. Clifton G.L. Contant C.F. Coplin W.M. Dietrich W.D. Ghajar J. Grady S.M. Grossman R.G. Hall E.D. Heetderks W. Hovda D.A. Jallo J. Katz R.L. Knoller N. Kochanek P.M. Maas A.I. Majde J. Marion D.W. Marmarou A. Marshall L.F. McIntosh T.K. Miller E. Mohberg N. Muizelaar J.P. Pitts L.H. Quinn P. Riesenfeld G. Robertson C.S. Strauss K.I. Teasdale G. Temkin N. Tuma R. Wade C. Walker M.D. Weinrich M. Whyte J. Wilberger J. Young A.B. Yurkewicz L. Clinical trials in head injury. J. Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkhah G. Rosenthal C. Hedrich H.J. Samii M. Differences in acquisition and full performance in skilled forelimb use as measured by the “staircase test” in five rat strains. Behav. Brain Res. 1998;92:85–95. doi: 10.1016/s0166-4328(97)00128-9. [DOI] [PubMed] [Google Scholar]
- Oliff H.S. Marek P. Miyazaki B. Weber E. The neuroprotective efficacy of MK-801 in focal cerebral ischemia varies with rat strain and vendor. Brain Res. 1996;731:208–212. doi: 10.1016/0006-8993(96)00582-3. [DOI] [PubMed] [Google Scholar]
- Pardon M.C. Gould G.G. Garcia A. Phillips L. Cook M.C. Miller S.A. Mason P.A. Morilak D.A. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience. 2002;115:229–242. doi: 10.1016/s0306-4522(02)00364-0. [DOI] [PubMed] [Google Scholar]
- Paulson P.E. Gorman A.L. Yezierski R.P. Casey K.L. Morrow T.J. Differences in forebrain activation in two strains of rat at rest and after spinal cord injury. Exp. Neurol. 2005;196:413–421. doi: 10.1016/j.expneurol.2005.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier; New York: 2005. [DOI] [PubMed] [Google Scholar]
- Prusky G.T. Harker K.T. Douglas R.M. Whishaw I.Q. Variation in visual acuity within pigmented, and between pigmented and albino rat strains. Behav. Brain Res. 2002;136:339–348. doi: 10.1016/s0166-4328(02)00126-2. [DOI] [PubMed] [Google Scholar]
- Schallert T. Woodlee M.T. Orienting and placing. In: Whishaw I.Q., editor; Kolb B., editor. The Behavior of the Laboratory Rat. Oxford University Press; New York: 2005. pp. 129–140. [Google Scholar]
- Sheridan C.L. Interocular transfer of brightness and pattern discriminations in normal and corpus callosum-sectioned rats. J. Comp. Physiol. Psychol. 1965;59:292–294. doi: 10.1037/h0021816. [DOI] [PubMed] [Google Scholar]
- Sisson D.F. Siegel J. Westenberg I.S. Are the differential effects of chloral hydrate on hooded rats vs. albino rats due to pigmentation or strain differences? Pharmacol. Biochem. Behav. 1991;39:665–670. doi: 10.1016/0091-3057(91)90144-q. [DOI] [PubMed] [Google Scholar]
- Smith D.C. Modglin A.A. Roosevelt R.W. Neese S.L. Jensen R.A. Browning R.A. Clough R.W. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J. Neurotrauma. 2005;22:1485–1502. doi: 10.1089/neu.2005.22.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.C. Tan A.A. Duke A. Neese S.L. Clough R.W. Browning R.A. Jensen R.A. Recovery of function after vagus nerve stimulation initiated 24 hours after fluid percussion brain injury. J. Neurotrauma. 2006;23:1549–1560. doi: 10.1089/neu.2006.23.1549. [DOI] [PubMed] [Google Scholar]
- Statler K.D. Jenkins L.W. Dixon C.E. Clark R.S. Marion D.W. Kochanek P.M. The simple model versus the super model: translating experimental traumatic brain injury research to the bedside. J. Neurotrauma. 2001;18:1195–1206. doi: 10.1089/089771501317095232. [DOI] [PubMed] [Google Scholar]
- Takaba H. Fukuda K. Yao H. Substrain differences, gender, and age of spontaneously hypertensive rats critically determine infarct size produced by distal middle cerebral artery occlusion. Cell. Mol. Neurobiol. 2004;24:589–598. doi: 10.1023/B:CEMN.0000036400.55503.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tâoth E. Bruin J.P. Heinsbroek R.P. Joosten R.N. Spatial learning and memory in calpastatin-deficient rats. Neurobiol. Learn. Mem. 1996;66:230–235. doi: 10.1006/nlme.1996.0063. [DOI] [PubMed] [Google Scholar]
- Tonkiss J. Shultz P. Galler J.R. Long-Evans and Sprague-Dawley rats differ in their spatial navigation performance during ontogeny and at maturity. Dev. Psychobiol. 1992;25:567–579. doi: 10.1002/dev.420250804. [DOI] [PubMed] [Google Scholar]
- Vales K. Bubenikova-Valesova V. Klement D. Stuchlik A. Analysis of sensitivity to MK-801 treatment in a novel active allothetic place avoidance task and in the working memory version of the Morris water maze reveals differences between Long-Evans and Wistar rats. Neurosci. Res. 2006;55:383–388. doi: 10.1016/j.neures.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Van Der Staay F.J. Blokland A. Behavioral differences between outbred Wistar, inbred Fischer 344, brown Norway, and hybrid Fischer 344 × brown Norway rats. Physiol. Behav. 1996;60:97–109. doi: 10.1016/0031-9384(95)02274-0. [DOI] [PubMed] [Google Scholar]
- Van Lier H. Drinkenburg W.H. Coenen A.M. Strain differences in hippocampal EEG are related to strain differences in behaviour in rats. Physiol. Behav. 2003;78:91–97. doi: 10.1016/s0031-9384(02)00893-4. [DOI] [PubMed] [Google Scholar]
- Vorhees C.V. Morford L.L. Inman S.L. Reed T.M. Schilling M.A. Cappon G.D. Moran M.S. Nebert D.W. Genetic differences in spatial learning between Dark Agouti and Sprague-Dawley strains: possible correlation with the CYP2D2 polymorphism in rats treated neonatally with methamphetamine. Pharmacogenetics. 1999;9:171–1781. [PubMed] [Google Scholar]
- Walberer M. Stolz E. Mèuller C. Friedrich C. Rottger C. Blaes F. Kaps M. Fisher M. Bachmann G. Gerriets T. Experimental stroke: ischaemic lesion volume and oedema formation differ among rat strains (a comparison between Wistar and Sprague-Dawley rats using MRI) Lab. Anim. 2006;40:1–8. doi: 10.1258/002367706775404426. [DOI] [PubMed] [Google Scholar]
- Whishaw I.Q. Gorny B. Foroud A. Kleim J.A. Long-Evans and Sprague-Dawley rats have similar skilled reaching success and limb representations in motor cortex but different movements: some cautionary insights into the selection of rat strains for neurobiological motor research. Behav. Brain Res. 2003;145:221–232. doi: 10.1016/s0166-4328(03)00143-8. [DOI] [PubMed] [Google Scholar]
- Wyss J.M. Chambless B.D. Kadish I. Van Groen T. Age-related decline in water maze learning and memory in rats: strain differences. Neurobiol. Aging. 2000;21:671–681. doi: 10.1016/s0197-4580(00)00132-9. [DOI] [PubMed] [Google Scholar]
- Xu X.J. Plesan A. Yu W. Hao J.X. Wiesenfeld-Hallin Z. Possible impact of genetic differences on the development of neuropathic pain-like behaviors after unilateral sciatic nerve ischemic injury in rats. Pain. 2001;89:135–145. doi: 10.1016/s0304-3959(00)00356-0. [DOI] [PubMed] [Google Scholar]
- Zamudio S. Fregoso T. Miranda A. De La Cruz F. Flores G. Strain differences of dopamine receptor levels and dopamine related behaviors in rats. Brain Res. Bull. 2005;65:339–347. doi: 10.1016/j.brainresbull.2005.01.009. [DOI] [PubMed] [Google Scholar]