Abstract
The hypothalamic ventromedial nucleus (VMH) displays sexual dichotomies in its overall size, neurochemistry, and neuronal morphology. These differences may underlie the sex differences observed in functions mediated by the VMH, such as reproductive behaviors and energy balance. A previous Golgi impregnation analysis of VMH dendrites reported sex differences in total dendrite length the ventrolateral region of the VMH [15]. The present study tested the hypothesis that this sex difference is localized to a specific dendrite type. VMH neurons were visualized with Golgi impregnation. Overall, male rats displayed significantly longer dendrites than females for VMH neurons. This sex difference was apparent in both the dorsomedial and the ventrolateral subdivisions of the VMH. When neurons were classified based on dendrite type, namely long primary, short primary and secondary dendrites, the increased length for males was observed for all dendrite types. Furthermore, when long primary dendrites were categorized according to whether they extended in the dorsomedial, ventrolateral, ventromedial or dorsolateral direction, the sex difference in length occurred for all directions. These results indicate that the previously identified dendrite categories for VMH neurons are integral to VMH circuitry for both males and females. Given that the sex difference in dendrite length applied to all dendrite types, the elongated male VMH dendrites may provide additional sites to process input from both local interneurons and extranuclear afferents.
Keywords: dendrites, lordosis, reproductive behavior, sexual dimorphism, VMH
INTRODUCTION
Various behaviors and brain functions manifest sexual dichotomies and estrous cycle fluctuations, including ingestive behaviors, emotion, and cognitive functions [1]. A well-studied example is female rodent sexual behavior, in which striking effects of both gender and phase of the estrous cycle are seen. During the hours before and after ovulation, a female rodent becomes sexually receptive. At this time, courting males trigger the receptive posture in females, termed the lordosis reflex, which is gated by the ovarian hormone estrogen as ovulation approaches. Ovariectomy prevents the display of the lordosis reflex, and this effect is reversed by estrogen replacement. The lordosis response is not exhibited by intact males or even by adult castrated males treated with ovarian hormones [22]. Therefore, this behavior has provided a model system to examine the cellular mechanisms underlying sex-specific behaviors. The brain region targeted by estrogen to control this behavior is the ventromedial nucleus of the hypothalamus (VMH). Interestingly, the VMH has also been implicated in male mating behavior [10, 11, 27]. Therefore, a better understanding of VMH neural circuitry would assist in explaining the neural basis of sexually dimorphic, hormone-gated behaviors.
Structural sex differences in the adult VMH have been observed, based on a variety of methodological approaches. Based on analysis of Nissl stain, the volume of the adult VMH is approximately 25% larger in males compared with females [4, 5, 15, 18]. This difference in volume cannot be explained by differences in the total number of neurons [15]. Instead, neuronal soma size contributes to differential VMH size, with males exhibiting larger neurons than females [5, 15]. In addition, the amount of neuropil in the VMH is greater in males than females [15]. At the ultrastructural level, the literature is less consistent. In some cases, a higher density of spine and shaft synapses have been seen in males than females [14, 17, 23]. However, it also has been reported that females have a greater density of dendritic synapses and spines than males [15, 24]. This discrepancy may relate to the sex-specific synaptic connectivity on different dendrite types, which was not accounted for in prior studies.
The synaptic organization of the VMH is not well understood compared with some other brain regions, making it difficult to interpret changes in synapse number or spine density. Previous work in our lab has categorized VMH dendrites as follows: a single very long primary dendrite, a few much shorter primary dendrites, and a few secondary dendrites. We have proposed that the long primary dendrite may be uniquely positioned to contact afferents terminating in the fiber plexus surrounding the VMH. We have shown that the length of these long primary dendrites can change, based on ovarian hormone conditions and food availability [6, 9]. The short primary dendrites, in contrast, may be situated to receive input from local interneurons. The spine density on these dendrites, but not other dendrite types, is affected by estradiol treatment, possibly via afferent input from nearby estradiol receptor-containing interneurons [2]. Given the notion that VMH dendrites are differentiated to process different classes of input, the goal of the present analysis was to test the hypothesis that sex differences in the dendritic arbor of VMH neurons pertain to specific elements of the dendritic tree. Golgi-impregnated VMH neurons from intact male and hormone-treated ovariectomized females were compared for dendrite number, type, length, and direction.
METHODS
Animals
The Institutional Animal Care and Use Committee of the University of Pennsylvania pre-approved all procedures with animals. Seven adult male and 38 adult female Sprague-Dawley rats (Taconic, Hudson, NY) were housed in a temperature-controlled room (22°C), with a 12:12 light/dark cycle, with lights on at 12 am. Food (Purina rat chow) and tap water were continuously available. Animals were allowed at least one week to acclimate to the colony, and during the subsequent 17 days, males were weighed daily, and their food intake was monitored in individual wire mesh cages.
Females were group-housed in plastic tubs (41mm x 21mm x 22mm) with standard bedding. After the one-week acclimation period, females were bilaterally ovariectomized. The ovariectomy was performed under general anesthesia (90mg/kg ketamine and 9mg/kg xylazine, both i.p.). After the ovariectomy was completed, rats were given yohimbine (2.1mg/kg, i.p.) to counteract the anesthesia produced by xylazine. One week after surgery, animals were assigned to a vehicle control group or one of two hormone treatment groups. The hormones, 17-β estradiol benzoate (EB; 10 μg in 100 μL sesame oil, s.c.) and progesterone (P; 500 μg in 100 μL propylene glycol, s.c.), were given in a specific regimen across four days. The vehicle control group (n=8) received injections of sesame oil (100 μL, s.c.) on Days 1 and 2 followed by propylene glycol (100 μL, s.c.) on Day 4 between 0800–9000 hours. Animals in the vehicle group were sacrificed four hours (1200–1300 hours) after the propylene glycol administration. The two other treatment groups were primed with EB on Days 1 and 2. On Day 4, they received either vehicle (EB group, n=10) or P (EBP group, n=5). Animals in the EB group were sacrificed 4 or 24 hours after the vehicle injection (i.e., 48 or 96 hours after the first administration of estradiol). Animals in the EBP group were sacrificed four hours after the progesterone injection of Day 4.
Golgi Impregnation and Morphological Analysis
Animals were deeply anesthetized with ketamine and xylazine (100mg/kg and 9mg/kg, respectively, both i.p.). Brains were quickly removed and prepared for Golgi impregnation using the FD Rapid Golgi Stain kit (FD Neurotechnologies; Ellicot City, MD). In brief, brains were incubated in a potassium dichromate, mercuric chloride, and potassium chromate solution for two weeks. After this incubation, hypothalamic sections including the VMH were obtained using a vibratome (200 μm, Vibratome Series 1000). The sections were mounted onto gelatinized slides and cover-slipped. Individual neurons (n=4–7 per animal) and their processes were visualized at 1000x using camera lucida. Soma area and dendrite length were measured in triplicate using NIH Image 1.62. The location of the neurons within the VMH, i.e., within the dorsomedial versus ventrolateral subdivision, was charted.
Several additional features of the dendrites were noted, as described previously [2, 3]. First, the type of dendrite was categorized as either primary or secondary, based on whether it emerged from the cell body or another dendrite, respectively. The primary dendrites were further classified according to their length. For each neuron, the longest primary dendrite was assigned as the “long primary dendrite” and all other primary dendrites were referred to as “short primary dendrites”. In the present study, the direction of the long primary dendrites were classified as one of four mutually exclusive categories: dorsolateral, dorsomedial, ventromedial, or ventrolateral based on dorsal-ventral and medial-lateral axis bisections of each neuron in the coronal plane. An experimenter blind to the treatment groups conducted all morphological analyses.
Statistical Analysis
For each neuron, dendrite length was calculated for each of the three dendrite types, which required the averaging when there were multiple short primary dendrites and secondary dendrites. For each animal, mean dendrite length for each dendrite type was calculated as the averages for each for all its neurons. Statistical comparisons between the two sexes, across the dorsomedial versus ventroalteral VMH subdivisions, were performed by two-way ANOVA and Bonferroni-corrected t-test post-hoc comparisons. The distribution of dendrites extending in each of the four possible quadrants in the coronal plane was assessed with a chi-square analysis. Data are expressed as the mean and standard error (SE). Significance was set at p<0.05. The statistical software used was Prism 4.0 (GraphPad, San Diego, CA).
RESULTS
The females in this study were included in a previous report that focused on the effects of ovarian hormone treatments [9]. Effects of ovarian hormones on the dendritic arbor in the female VMH will not be discussed in the current report, although they are apparent in the figures. The present study focuses on sex differences, and therefore, the females are combined in a single group for statistical comparison with males.
Dendrite number
Dendrite number was compared based on the two factors of sex and VMH subdivision (Figure 1). There was a main effect of sex (F=81.57 (df 1, 46), p<0.0001), but not a main effect of VMH region, nor an interaction between these two factors. When the dorsomedial and ventrolateral VMH were pooled, males exhibited more dendrites per neuron than females. In particular, male VMH neurons had approximately 5.0 dendrites per neuron, whereas for females the average dendrite per neuron was approximately 3.5 dendrite per neuron. This sex difference was based on more short primary dendrites and secondary dendrites in males [F=41.71 (df 1, 46), p<0.0001 and F=29.46 (df 1, 46), p<0.0001, respectively].
Figure 1.
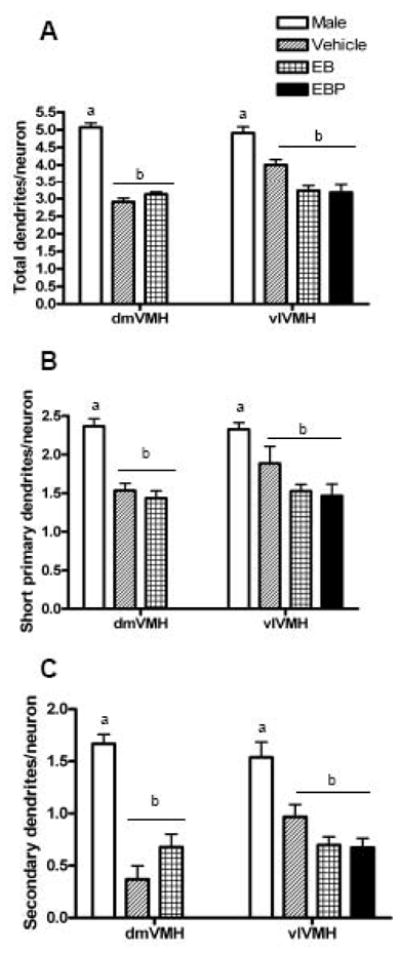
Bar graph comparing the number of dendrites on hypothalamic ventromedial nucleus (VMH) neurons in the dorsomedial and ventromedial VMH in male and female rats. All females were ovariectomized and treated with either vehicle, estradiol benzoate (EB), or estradiol plus progesterone (EBP). In the present analysis, females were combined in a single group. Groups that a significantly different are denoted with different letters above the bars. Panel A: The total number of dendrites per neuron. Panel B: The number of short primary dendrites per neuron. Panel C: The number of secondary dendrites per neuron.
Dendrite length
Dendrite length was compared across two factors, namely, sex and VMH subdivision, for each of the three dendrite categories (Figure 2). For the long primary dendrites, there was a main effect of sex [F=111.22 (df 1, 46), p<0.0001], but no main effect of VMH region, and no interaction between these two factors. For the short primary dendrites, there was a main effect of sex [F=62.20 (df 1, 45), p<0.0001], and no main effect of VMH region, but an interaction between these two factors [F=7.18 (df 1, 43), p<0.0103]. For the secondary dendrites, there was a main effect of sex [F=38.73 (df 1, 45), p<0.0001], and borderline main effect of VMH region [F=3.77 (df 1, 43), p<0.0588], and an interaction between these two factors [F=4.22 (df 1, 43), p<0.0461)]. However, post-hoc comparisons did not indicate any significant group differences except the sex differences within each VMH.
Figure 2.
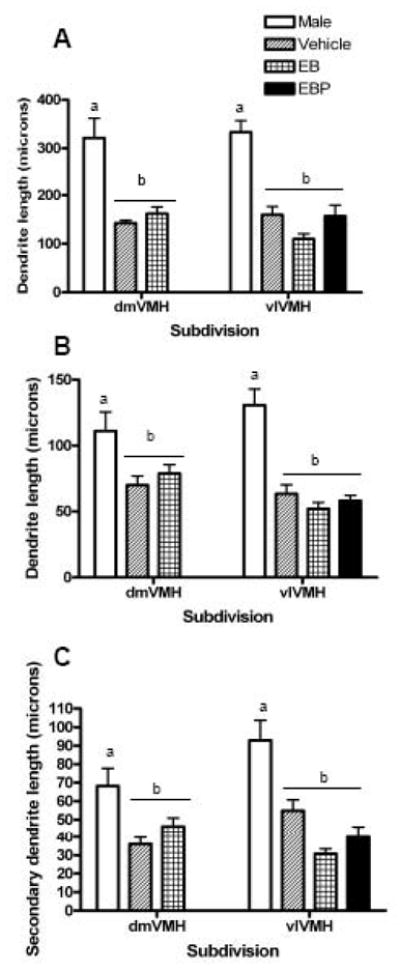
Bar graph comparing the length of dendrites of hypothalamic ventromedial nucleus (VMH) neurons in the dorsomedial and ventromedial VMH in male and female rats. All females were ovariectomized and treated with either vehicle, estradiol benzoate (EB), or estradiol plus progesterone (EBP). In the present analysis, females were combined in a single group. Groups that a significantly different are denoted with different letters above the bars. Panel A: The length of long primary dendrites. Panel B: The length of short primary dendrites. Panel C: The length of secondary dendrites.
Thus, males exhibited strikingly longer dendrites than females for all three dendrite types. The difference was most marked for the long primary dendrites, which were approximately 150 μm longer in the males compared with the females. However, both the short primary and secondary dendrites were also significantly longer in males, approximately 50 μm and 35 μm longer, respectively.
Dendrite direction
The proportion of long primary dendrites extending in each of four possible directions was compared between the two sexs in both VMH subdivisions (Figure 3). A regional difference was noted in both sexes. Specifically, in the dorsomedial subdivision of both sexes, the proportion of long primary dendrites extending in each of the possible four directions not significantly different than chance (chi-square test, p>0.05). However, in the ventrolateral subdivision of both sexes, dendrites were significantly more likely to extend in the ventrolateral direction than the other three possible directions (chi-square, p<0.0001 for both sexes).
Figure 3.
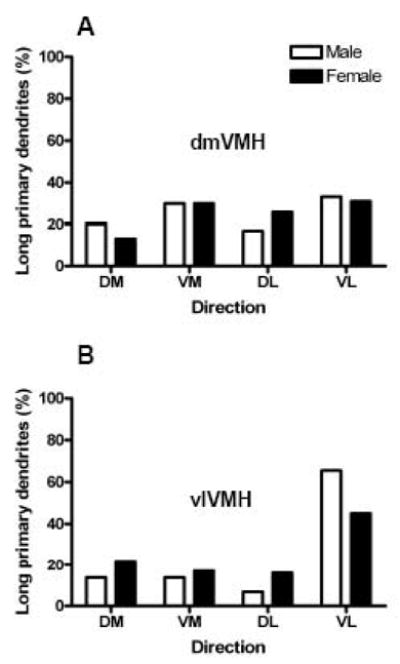
Bar graph comparing the percentage of long primary dendrites that extended in each quadrant for hypothalamic ventromedial nucleus (VMH) neurons in the dorsomedial and ventromedial VMH in male and female rats. All females were ovariectomized and treated with either vehicle, estradiol benzoate (EB), or estradiol plus progesterone (EBP). In the present analysis, females were combined in a single group. Panel A: The percent of long primary dendrites extending in each direction in the dorsomedial VMH. Panel B: The percent of long primary dendrites extending in each direction in the ventromedial VMH. Abbreviations: DM, dorsomedial; VM, ventromedial; DL dorsolateral; VL, ventrolateral.
Lastly, the length of long primary dendrites was compared across two factors, namely, sex and the quadrant in which that dendrite extended (Figure 4). In the dorsomedial VMH, a main effect of sex was observed (p<0.0001), but no main effect of dendrite direction or interaction between these two factors was apparent. In post-hoc comparisons, males had significantly elongated long primary dendrites in the dorsomedial VMH for all directions except the dorsomedial direction (Bonferroni-corrected t-tests, all p < 0.01). In the ventrolateral VMH, a main effect of sex was observed (p<0.0001), but no main effect of dendrite direction or interaction was apparent. In post-hoc comparisons, males had significantly elongated long primary dendrites in the ventrolateral VMH for all dendrite directions (Bonferroni-corrected t-tests, all p < 0.01).
Figure 4.
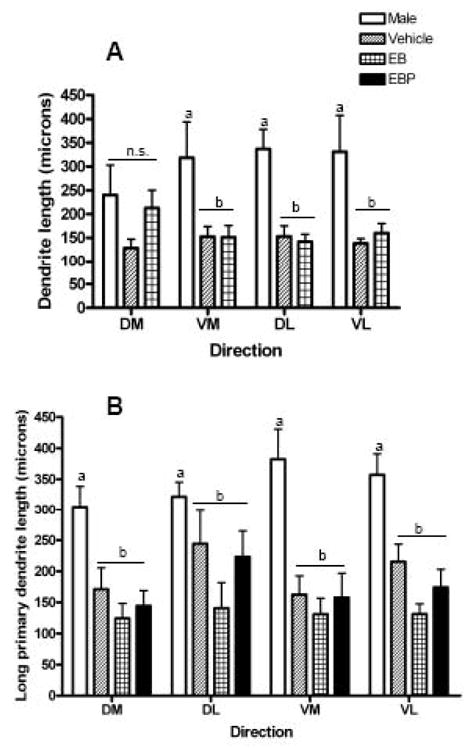
Bar graph comparing the length of long primary dendrites that extend in each quadrant for hypothalamic ventromedial nucleus (VMH) neurons in the dorsomedial and ventromedial VMH in male and female rats. All females were ovariectomized and treated with either vehicle, estradiol benzoate (EB), or estradiol plus progesterone (EBP). In the present analysis, females were combined in a single group. Groups that are significantly different are denoted with different letters above the bars. Panel A: The length of long primary dendrites extending in each direction in the dorsomedial VMH. Panel B: The length of long primary dendrites extending in each direction in the ventromedial VMH. Abbreviations: DM, dorsomedial; VM, ventromedial; DL dorsolateral; VL, ventrolateral.
DISCUSSION
This study tested the hypothesis that the previously observed sex difference in dendrite length in the VMH was localized to specific dendrite types of VMH neurons. The present results are consistent with previous evidence that males have more dendrite surface than females, as measured here by the number and length of VMH dendrites [23]. A trend for longer dendrites in males also has been reported in neonatal rats [26]. In addition, these results make the novel observation that the lengthening of dendrites in males is generalized to all dendrite types, including long primary dendrites, regardless of the directionality of the long primary dendrites. Thus, the hypothesis that a sex difference in VMH dendrites is dendrite-specific was not supported. The differences observed here represent a true dichotomy between males and females, and not simply an ovarian hormone modulation that results in females oscillating around the values obtained from males [19].
As mentioned in the Introduction, previous studies have found that males have a larger volume of the VMH than females [5, 15, 18]. This sex difference is based on larger neurons and more neuropil [5, 15]; however, the number of neurons in the VMH is not sexually dimorphic [15]. A functional significance for this larger soma may be to provide biosynthetic and metabolic support for the larger dendritic tree in males. The larger dendritic arbor in males, in turn, may contribute to the sex difference in neuropil volume within the VMH. An expanded dendritic arbor in males is also consistent with the finding that males have more dendritic synapses, which may be a consequence of having additional dendrite surface [23].
The present results run counter to a previous report, also based on Golgi impregnation, which did not find shorter dendrites in the VMH for females compared with males [15]. The female groups differ somewhat between the two studies, with the present study having used ovariectomized females and hormone replacement, whereas Madiera et al. used intact cycling females. A possible temporally dynamic effect of estradiol on dendrite length, obscured by the time points used in each study, may account for the disparate results. Another methodological difference between these two studies was that in the present report brains were obtained during the dark phase of the light/dark cycle, whereas Madiera et al. collected brains in the light phase. A possible sex-specific effect of circadian rhythm on VMH dendrite length has not been studied. Other studies in the literature do not fully clarify the discrepancy. For example, one study [24] used electron microscopy to compare synapse density in male and female VMH. The results, although not directly assessing dendrite length, suggest a modest cycle-dependent difference, with females displaying somewhat more dendritic synapses per neuron than males. These animals were also killed in the light portion of the light/dark cycle. In contrast, another study [23], also using electron microscopy, found that males exhibit nearly twice as many dendrite synapses as intact females in the VMH. That study is strikingly consistent with the present results. Here, we explored the hypothesis that the discrepancies may be accounted for based on dendrite-specific sex effects. However, the pattern of sex differences was similar in all dendrite types in this study.
In the present study, the sex difference in dendritic tree was apparent in both the dorsomedial and ventrolateral VMH. This is consistent with previous reports that found sex differences in soma size and overall volume in both the dorsomedial and ventrolateral regions [5, 15]. Although sex differences are apparent in both of these regions of the VMH, some neuronal morphological distinctions exist between them. For example, the dendritic arbor is more complex in the ventrolateral compared with the dorsomedial region [15]. Our laboratory previously reported that there are more and longer secondary dendrites in the ventrolateral region of the VMH than the dorsomedial in females [9]. However, in males, the length of the long primary dendrites was equivalent in the dorsomedial and ventrolateral VMH.
Previous studies have explored the developmental mechanisms that give rise to various levels of sex differences in the VMH. Tobet and colleagues have described the embryonic timeline for VMH differentiation in the developing hypothalamus [20]. Neonatal manipulation of testosterone and estrogen exposure can reverse sex differences in the VMH [18, 23]. Recent work has sought to distinguish the roles of estrogen receptor subtypes in VMH sexual differentiation [7, 12, 28]. However, in addition to a role for estrogen receptors [29], androgen action also contributes to the sexual differentiation of the VMH, perhaps by controlling the expression of aromatase, and in turn, estradiol production [5, 16]. A postnatal time course analysis has indicated that the adult pattern of sex-specific connectivity is established by postnatal day 45, but not at postnatal day 20 [23]. A very recent study employed cultured slices of neonatal VMH to demonstrate that the masculinizing effect of estradiol on dendritic spines is dependent on glutamate activity [25]. Consistent with the present findings that males have longer VMH dendrites than females, that study also observed that estradiol treatment was associated with longer dendrites. However, the neurochemical mechanisms the mediate the organization of sex differences in dendrite length in the VMH were not explored.
Our laboratory has investigated the regulation of VMH dendrites in a variety of conditions. The length of the long primary dendrites appears to be particularly variable. In adult females, ovarian hormone treatment causes rapid and reversible changes in long primary dendrite length, without affecting short primary dendrites [9]. Likewise, in adult males, long primary dendrites are shortened by food restriction [6], although they are not changed by maintenance on a high-energy diet [13]. In addition to their regulation by hormonal and energy balance cues, the long primary dendrites are affected by genotype. In particular, rats with a genetic propensity to gain weight on a high-energy diet exhibit shorter long primary dendrites than rats bred to resist weight gain [13]. Based on their extreme length, long primary dendrites may be positioned to receive extranuclear inputs. Thus, male rats, having a larger VMH to traverse, may require added length on the long primary dendrites to perform the function of receiving extranuclear input [21]. Figure 5 illustrates the dendritic arbors observed for VMH neurons across a variety of conditions.
Figure 5.
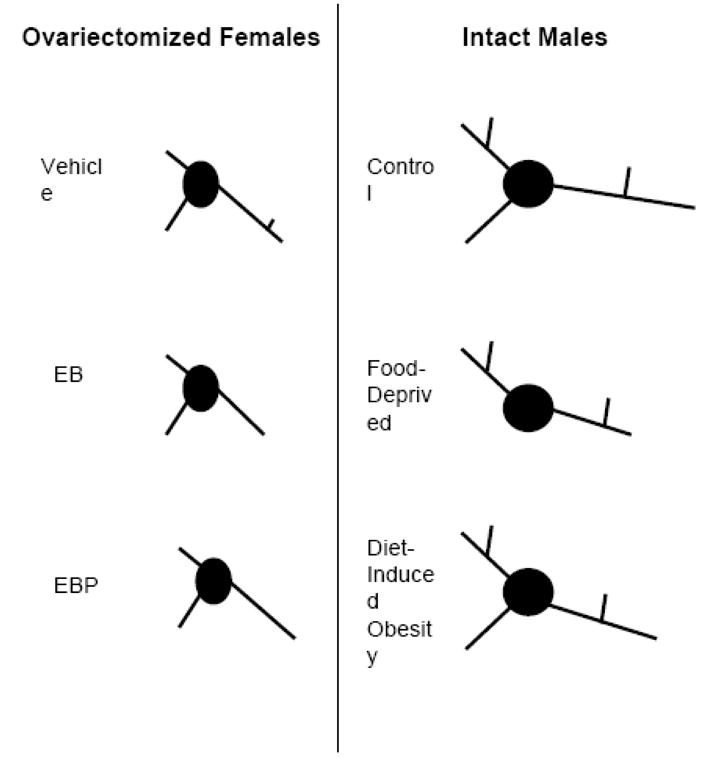
Schematic summarizing the pattern of dendritic branching for hypothalamic ventromedial nucleus (VMH) neurons during different conditions, based on this and previous studies [6, 9, 13]. The left panel illustrates the typical dendritic arbor in ovariectomized females treated with either vehicle, estradiol benzoate (EB), or EB and progesterone (EBP). The right panel depicts the typical dendritic arbor in intact males fed ad libitum diet, food deprived, or selectively bred to exhibit diet-induced obesity. Overall, the difference between the sexes appears more striking than the differences produced within each sex caused by ovarian hormones or energy balance signals.
While a larger VMH in males may explain the need for elongated long primary dendrites to traverse to the afferent shell, it does not necessarily explain the increase in the number and length of short primary dendrites. Furthermore, the sex difference in VMH connectivity may be more than a quantitative difference in the number of synaptic connections, but also qualitative difference in the types of synaptic wiring. Qualitative differences may be based on excitatory versus inhibitory activity [8].
In conclusion, the VMH has been implicated in variety of functions, most notably sexually dichotomous mating behavior. The present study examined structural differences in the neuronal tree of VMH neurons, and although sex differences were observed, the basic characteristics of the VMH dendritic arbor were similar between the two sexes. Thus, sex differences in VMH function may be largely determined by differences in its sources of afferents and/or projection targets [27], with males having more dendrite surface to integrate a wider array of local and extranuclear input.
Acknowledgments
The authors thank Denise LaBelle and Elena Weinreb for their excellent technical support. This work was supported by the National Institutes of Health Grants MH64371, DK52018, and training grant MH017168.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cahill L. Why sex matters for neuroscience. Nature Rev Neurosci. 2006;7:477–84. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 2.Calizo LH, Flanagan-Cato LM. Estrogen selectively induces dendritic spines within the dendritic arbor of rat ventromedial hypothalamic neurons. J Neurosci. 2000;20:1589–96. doi: 10.1523/JNEUROSCI.20-04-01589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calizo LH, Flanagan-Cato LM. Estrogen-induced dendritic spine elimination on female rat ventromedial hypothalamic neurons that project to the periaqueductal gray. J Comp Neurol. 2002;447:234–48. doi: 10.1002/cne.10223. [DOI] [PubMed] [Google Scholar]
- 4.Dorner G, Staudt J. Structural changes in the hypothalamic ventromedial nucleus of the male rat, following neonatal castration and androgen treatment. Neuroendocrinology. 1969;4:278–81. doi: 10.1159/000121758. [DOI] [PubMed] [Google Scholar]
- 5.Dugger BN, Morris JA, Jordan CL, Breedlove SM. Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Hormones and Behavior. 2007;51:195–201. doi: 10.1016/j.yhbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flanagan-Cato LM, Fluharty SJ, Weinreb EB, LaBelle DR. Food restriction alters neuronal morphology in the hypothalamic ventromedial nucleus of male rats. Endocrinology. 2008 doi: 10.1210/en.2007-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzales KL, Tetel MJ, Wagner CK. Estrogen receptor (ER) beta modulates ERalpha responses to estrogens in the developing rat ventromedial nucleus of the hypothalamus. Endocrinology. 2008;149:4615–21. doi: 10.1210/en.2008-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gratten DR, Selmanoff M. Sex differences in the activity of γ-aminobutyeric acidergic neurons in the rat hypothalamus. Brain Research. 1997;775:244–9. doi: 10.1016/s0006-8993(97)01069-x. [DOI] [PubMed] [Google Scholar]
- 9.Griffin GD, Flanagan-Cato LM. Estradiol and progesterone differentially regulate the dendritic arbor of neurons in the hypothalamic ventromedial nucleus of the female rat (Rattus norvegicus) J Comp Neurol. 2008;510:631–40. doi: 10.1002/cne.21816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harding SM, McGinnis MY. Androgen receptor blockade in the MPOA or VMN: effects on male sociosexual behaviors. Physiology & Behavior. 2004;81:671–80. doi: 10.1016/j.physbeh.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Harding SM, McGinnis MY. Microlesions of the ventromedial nucleus of the hypothalamus: effects on sociosexual behaviors in male rats. Behavioral Neuroscience. 2005;119:1227–34. doi: 10.1037/0735-7044.119.5.1227. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda Y, Nagai A, Ikeda MA, Hayashi S. Sexually dimorphic and estrogen-dependent expression of estrogen receptor beta in the ventromedial hypothalamus during rat postnatal development. Endocrinology. 2003;144:5098–104. doi: 10.1210/en.2003-0267. [DOI] [PubMed] [Google Scholar]
- 13.LaBelle DR, Cox JM, Dunn-Meynell AA, Levin BE, Flanagan-Cato LM. Genetic and Dietary Effects on Dendrites in the Hypothalamic Ventromedial Nucleus. Am J Physiol Regul Integr Comp Physiol. 2009 doi: 10.1016/j.physbeh.2009.08.005. manuscript submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larriva-Sahd J, Rondan-Zarate A, Ramirez-Degollado M. Sexually dimorphic contribution from the fornix to the ventromedial hypothalamic nucleus: a quantitative electron microscopic study. Neurosci Lett. 1995;200:147–50. doi: 10.1016/0304-3940(95)12129-r. [DOI] [PubMed] [Google Scholar]
- 15.Madiera MD, Ferreira-Silva L, Paula-Barbosa MM. Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and Golgi study. J Comp Neurol. 2001;432:329–45. doi: 10.1002/cne.1106. [DOI] [PubMed] [Google Scholar]
- 16.Martini M, Di Sante G, Collado P, Pinos H, Guillamon A, Panzica GC. Androgen receptors are required for full masculinization of nitric oxide synthase system in rat limbic-hypothalamic region. Hormones & Behavior. 2008;54:557–64. doi: 10.1016/j.yhbeh.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto A, Arai Y. Male-female differences in synaptic organization of the ventromedial nucleus of the hypothalamus in the rat. Neuroendocrinology. 1986;42:232–6. doi: 10.1159/000124445. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto A, Arai Y. Sex difference in volume of the ventromedial nucleus of the hypothalamus in the rat. Endocrinol Japon. 1983;30:277–80. doi: 10.1507/endocrj1954.30.277. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy MM, Konkle ATM. When is a sex difference not a sex difference? Frontiers in Neuroendocrinology. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 20.McClellan KM, Parker KL, Tobet S. Development of the ventromedial nucleus of the hypothalamus. Frontiers in Neuroendocrinology. 2006;27:193–209. doi: 10.1016/j.yfrne.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Millhouse OE. Certain ventromedial hypothalamic afferents. Brain Res. 1973;55:89–105. doi: 10.1016/0006-8993(73)90490-3. [DOI] [PubMed] [Google Scholar]
- 22.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–82. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 23.Pozzo Miller LD, Aoki A. Stereological analysis of the hypothalamic ventromedial nucleus II. Hormone-induced changes in the synaptigenic pattern. Dev Brain Research. 1991;61:189–96. doi: 10.1016/0165-3806(91)90131-2. [DOI] [PubMed] [Google Scholar]
- 24.Sa SI, Madiera MD. Estrogen modulates the sexually dimorphic synaptic connectivity of the ventromedial nucleus. J Comp Neurol. 2005;484:68–79. doi: 10.1002/cne.20451. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz JM, Liang S-L, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58:584–98. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Todd BJ, Schwarz JM, Mong JA, McCarthy MM. Glutamate AMPA/kainate receptors, not GABA-A receptors, mediate estradiol-induced sex differences in the hypothalamus. Developmental Neurobiology. 2006;67:304–15. doi: 10.1002/dneu.20337. [DOI] [PubMed] [Google Scholar]
- 27.Wersinger SR, Baum MJ, Erskine MS. Mating -induced FOS-like immunoreactivity in the rat forebrain: a sex comparison and a dimorphic effect of pelvic nerve transection. J Neuroendocrinology. 1993;5:557–68. doi: 10.1111/j.1365-2826.1993.tb00522.x. [DOI] [PubMed] [Google Scholar]
- 28.Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. Journal of Comparative Neurology. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 29.Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. Journal of Comparative Neurology. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]


