Abstract
Biomaterials prepared from polyesters of lactic acid and glycolic acid, or a mixture of the two, degrade in the presence of water into the naturally occurring metabolites, lactic acid and glycolic acid. While the lactic acid degradation product that is released from biomaterials is well-tolerated by the body, lactic acid can influence the metabolic function of cells; it can serve as an energy substrate for cells, and has been shown to have antioxidant properties. Neural precursor cells, a cell population of considerable interest as a source of cells for neural tissue regeneration strategies, generate a high amount of reactive oxygen species, and when associated with a degradable biomaterial, may be impacted by released lactic acid. In this work, the effect of lactic acid on a neural cell population containing proliferative neural precursor cells was examined in monolayer culture. Lactic acid was found to scavenge exogenously added free radicals produced in the presence of either hydrogen peroxide or a photoinitiator (I2959) commonly utilized in the preparation of photopolymerizable biomaterials. In addition to its effect on exogenously added free radicals, lactic acid reduced intracellular redox state, increased the proliferation of the cell population, and modified the cell composition. The findings of this study provide insight into the role that lactic acid plays naturally on developing neural cells and are also of interest to biomaterials scientists that are focused on the development of degradable lactic-acid based polymers for cell culture devices. The effect of lactic acid on other cell populations may differ and should be characterized to best understand how cells function in degradable cell culture devices.
Keywords: Lactic acid, neural precursor cell, reactive oxygen species, biomaterial, biodegradable, redox
1. Introduction
A wide variety of materials are being developed for tissue engineering applications that involve the delivery of cells. Polyesters of lactic acid and glycolic acid, or a mixture of the two, are the most widely studied materials for this purpose. Various devices have been prepared from these materials including degradable sutures, drug releasing microparticles, nanoparticles, and porous scaffolds for cellular applications.
The interest in polyesters of lactic and glycolic acid is due in part to the fact that they degrade in the presence of water into the naturally occurring metabolites, lactic acid and glycolic acid. Lactic acid is a weak acid (pKa = 3.86) that reversibly dissociates in water to produce a hydrogen ion and the lactate ion. Under physiological conditions (pH = 7.4) the majority of lactic acid is present in dissociated form as lactate. Both lactic acid and lactate are capable of entering cells. Lactic acid is uncharged and small enough to permeate through the lipid membrane; lactate is capable of entering cells via the monocarboxylate transporter (MCT) protein shuttle system (Philp et al. 2005). Once inside of the cell, lactate can serve as a source of energy in the Cori cycle by conversion to glucose. Alternatively lactate can be oxidized to pyruvate (via lactate dehydrogenase) which is then oxidized to acetyl-CoA and fuels the TCA cycle in mitochondria producing the metabolic products carbon dioxide, water, and NADH. Lactate’s internalization and conversion is demonstrated by the dramatic increase in intracellular pyruvate that occurs when cells are exposed to exogenous lactate (Yanagida et al. 1995).
While biomaterials prepared from poly(lactic acid) are well-tolerated by the body, producing minimal inflammation upon implantation, the lactic acid degradation product that is released and can dissociate to lactate may influence the metabolic function of cells in close proximity to the biomaterial. In addition to its role as an energy substrate for cells, lactic acid has been shown to have antioxidant properties that may serve to protect cells from damage due to free radicals that are naturally produced throughout a cell’s life cycle. Lactate can scavenge superoxide radicals, hydroxyl radicals, and is capable of inhibiting lipid peroxidation (Groussard et al. 2000). When oxidized, pyruvate is produced, a molecule that has been shown to scavenge hydrogen peroxide and superoxide radicals (Herz et al. 1997; Yanagida et al. 1995).
Neural precursor cells isolated from the developing brain, a cell population of considerable interest as a source of cells for neural tissue regeneration strategies, may be particularly sensitive to these possible direct and indirect effects of lactic acid. Neural precursor cells proliferate rapidly and undergo a high rate of respiration, leading to the generation of a large amount of reactive oxygen species (Tsatmali et al. 2005). During periods of spiking reactive oxygen species levels, a cell’s redox state becomes more oxidized and damage to cellular proteins, nucleic acids, and lipids can occur. In addition, a shift in intracellular redox state has been shown to impact neural precursor cell fate where more oxidized cellular states lead to differentiation and more reduced states lead to self-renewal (Smith et al. 2000; Tsatmali et al. 2006). Thus, when exposed to lactic acid released from a biomaterial, neural precursor cells may be protected from damage due to naturally occurring reactive oxygen species; intracellular redox state may shift to one that is more reduced, thus maintaining cells in a proliferative state. As a first step towards understanding the impact of lactic acid on neural precursor cell function, the effect of lactic acid on monolayer cultures containing neural precursor cells was examined in this work. Studies were designed to answer four questions: (1) First, does lactic acid tend to protect cultured neural cells from free-radical induced injury? (2) Does lactic acid shift the intracellular redox state of cultured neural cells? (3) If so, is the proliferative capacity of the neural cell population enhanced, and finally (4) How does lactic acid impact the cellular composition of the culture?
To answer these questions, a primary cell population isolated from the developing rodent brain containing proliferative precursor cells was cultured as a monolayer. A protective antioxidant effect of lactic acid was identified by measuring cell viability in cultures exposed to hydrogen peroxide which generates free radicals that a cell would naturally encounter during respiration. Given the emerging interest in degradable lactic-acid containing photopolymerizable hydrogels for tissue engineering applications, cells were also exposed to free radicals generated by ultraviolet-light-initiated dissociation of Irgacure 2959 (1-[4-(2-hydroxyethoxy)-phenyl]-2-hydroxy-2-methyl-1-propane-1-one), a photoinitiator that is widely used to initiate the polymerization of macromers to form cross-linked hydrogels. Our goal in doing so was to identify a potential scavenging effect of lactic acid on the type of free radicals that are generated during a photopolymerization reaction (Bryant et al. 2000; Williams et al. 2005). In addition, the impact of lactic acid on intracellular redox state and the proliferation of the cell population was examined using a redox sensitive fluorescent dye and by monitoring total DNA content in cultures, respectively. Finally, both immunocytochemistry and qRT-PCR were used to examine the cellular composition of monolayer cultures exposed to differing concentrations of lactic acid for extended periods of time.
2. Materials and Methods
2.1 Cell harvest and dissociation
Embryonic day 14–15 (E14-E15) Sprague-Dawley rat embryos were isolated by Cesarean section. NIH guidelines for the care and use of laboratory animals were observed. Embryos were decapitated, the skull and dura were removed, and the forebrain dissected out. All dissections were carried out in ice cold dissection solution (Hank’s Balanced Salt Solution (HBSS, Gibco) supplemented with 2.5 mM HEPES buffer (Gibco), 6.5 mg/mL glucose (Fisher Scientific), 2 mM CaCl2 (Fisher Scientific), 1 mM MgSO4 (Mallinkrodt), and 4 mM NaHCO3 (Sigma). The dissected forebrain tissues were minced with scissors into small pieces ∼ 1 mm in diameter and enzymatically dissociated into a single-cell suspension using papain (Sigma) as a protein-cleaving agent. The dissociation was quenched by a solution of BSA and trypsin inhibitor (Sigma) in Dulbecco's Modified Eagle's Medium/F12 (DMEM/F12, Mediatech). All dissociation procedures were carried out at 37°C in a dissociation solution composed of 82 mM Na2SO4, 30 mM K2SO4, 5.8 mM MgCl2, 0.25 mM CaCl2, 1 mM HEPES, 20 mM Glucose, 1% phenol red, and 0.2 mM NaOH in diH20, final pH 7.4. Tissue pieces were transferred to 10 mL of DMEM/F12 medium containing 25 µg/mL DNAse and triturated to a single-cell suspension using a Pasteur pipette.
2.2 Cell culture
All cell culture was conducted using dissociated E14-E15 fetal forebrain cells with day of dissection equal to day 0 of culture. Immediately following isolation immunocytochemical staining verified that the cell population is composed of a mixture of post-mitotic neurons (66%) and the remainder proliferative nestin positive cells capable of proliferating and/or differentiating into neurons or glia. For monolayer cultures, dissociated cells were seeded at 2×105 cells/cm2 on polyornithine (1.5 µg/cm2, Sigma) and fibronectin (0.75 µg/cm2, Chemicon) coated tissue culture polystyrene plates. Culture media consisted of DMEM/F12 (50:50) supplemented with 1% P/S (Mediatech), 1% N2 (Invitrogen), 2.5 mM L-glutamine (Mediatech), 10ng/mL bFGF (Sigma), and in some cases lactic acid at the concentrations shown. Cultures were grown at 37°C in 100% humidity with 5% CO2. Media was replaced every 2–3 days.
2.3 Measurement of total DNA and ATP content in cell culture
To quantify total DNA or ATP content, media was aspirated from monolayer cultures and replaced with 400µL of cell lysis buffer (20 mM Tris (Bio-Rad), 2 mM EDTA (Bio-Rad), 150 mM NaCl, 0.5% Triton X-100 (Fisher Biotech) in diH2O). Cultures were stored at −20°C until the day of DNA and ATP quantification. This procedure allowed multiple assays to be performed on aliquots of individual samples.
DNA was measured using the Quant-iT PicoGreen dsDNA assay kit (Invitrogen) as directed by the manufacturer and ATP was quantified using an amended protocol for the CellTiter-Glo luminescent cell viability assay (Promega). Briefly, for each assay, sample in lysis buffer was combined with an equal volume of reagent in a 384-well assay plate and mixed well. Samples were kept on ice and only minimal ATP degradation occurred from cell lysis to signal quantification. After a 10 minute incubation period, fluorescence (DNA assay) or luminescence (ATP assay) was quantified using a Fluostar Optima plate reader (BMG Labtech). Data are presented as the average of 5 different experiments each completed with 4 replicates.
2.4 Free radical scavenging activity of lactic acid
Irgacure 2959 (1-[4-(2-hydroxyethoxy)-phenyl]-2-hydroxy-2-methyl-1-propane-1-one, Ciba), a common photoinitator used to initiate photopolymerization reactions, was exposed to ultraviolet light (365 nm) with the sole purpose of generating free radicals in solution. The effect that lactic acid has on the number of free radicals generated by this molecule in solution was then examined in the absence of cells or macromolecules. The goal in doing so was to identify a potential scavenging effect of lactic acid on free radicals in a simplified environment. Experiments here used photoinitiator at 0.25mg/mL as this was found to provide a maximum viability decrease from control levels (data not shown) and is in a range previously documented as toxic to susceptible cell types (Williams et al. 2005). A solution containing photoinitiator (0.25 mg/mL), lactic acid at a pH of 7.4 (0, 0.005, 0.01,0.05, 0.5 and 5 mg/mL) and luminol (0.25 mM) was added to a 96 well plate. In the presence of free radicals luminol is oxidized in a reaction that results in the production of the light-emitting species, 3-aminophthalate; emitted light, which is proportional to the number of free radical species in solution can then be measured with a luminometer. Each well was exposed to ultraviolet light for 10 minutes and luminescence was measured kinetically thereafter using a FluoStar Optima plate reader.
The potential of lactic acid to scavenge peroxide radicals, a type of radical that cells are naturally exposed to as they undergo respiration, was also evaluated. Lactic acid concentrations were chosen to encompass the range of concentrations that media would be expected to contain when cells are cultured in poly(lactic acid)-containing degradable hydrogels. A theoretical calculation showed that this range would be 0.02–0.5mg/mL depending on the culture conditions and polymer formulation used. A model hydrogel system confirmed this range as lactic acid was measured in the media at 0.06–0.18mg/mL throughout the lifetime of the hydrogels (unpublished observations).
A solution of PBS supplemented with hydrogen peroxide (1 mM), lactic acid at a pH of 7.4 (0, 0.005, 0.01,0.05, 0.5 and 5 mg/mL) and luminol (0.25 mM) was added to a 96 well plate. Identical to the conditions described above for free radicals generated via exposure of photoinitiator to ultraviolet light, the luminescence of each well was measured after 10 minutes of incubation using a FluoStar Optima plate reader.
In both cases, the effect of lactic acid (pH 7.4) on the number of free radicals generated in solution was estimated by normalizing the chemiluminescent signal in lactic-acid containing samples to that in control samples which contained no lactic acid.
2.5 The effect of lactic acid on cell viability in the presence of free radicals
Free radicals can cause damage to cell membranes, nucleic acids and proteins, effects which can ultimately lead to cell death (Fridovich 1978; Halliwell and Gutteridge 1999; McCord and Fridovich 1978; Williams et al. 2005). Here the study examined the extent to which free radicals produced from photoinitiator or hydrogen peroxide induce cell toxicity when applied to a mixed population of neural cells composed of both differentiated neurons and neural precursor cells. Lactic acid was evaluated for its ability to reduce the toxicity of these free radicals upon its addition to culture medium, presumably by serving as a free radical scavenger. Media was aspirated from monolayer cultures (after 5–7 days of growth) and a solution of PBS containing photoinitiator (0.25 mg/mL, 1.11mM) or hydrogen peroxide (1.11 mM, 0.038mg/mL) and lactic acid was added to each well. Lactic acid at a pH of 7.4 was applied at 0.1, 0.3, or 0.5 mg/mL. Doses less than 0.1 mg/mL were not tested as these lower doses minimally impacted free radical levels in the absence of cells; a dose of 5 mg/mL was found to reduce cell viability. Positive control culture media consisted of PBS and lactic acid at a pH of 7.4 (0, 0.1, 0.3, and 0.5 mg/mL). Cultures containing I2959 and different dosages of lactic acid were exposed to 10 minutes of ultraviolet light under ambient conditions and were then placed in an incubator at 37°C in 100% humidity with 5% CO2 for an additional 10 minutes prior to measuring cell viability. Cultures containing hydrogen peroxide and different dosages of lactic acid were incubated for 10 minutes under ambient conditions and then placed in an incubator at 37°C in 100% humidity and 5% CO2 for 10 minutes prior to measuring cell viability. Following this period of exposure to free radicals, solutions were aspirated from each well and were replaced with 400µl of cell lysis buffer. Thus the total exposure time of cells to radicals and/or lactic acid was 20 minutes. Total DNA and ATP were quantified as described in section 2.3. The viability of the population of cells in each well was assessed by dividing total ATP content by total DNA content in the culture. DNA content is directly proportional to the number of cells present in a sample and thus ATP/DNA provides a metabolic activity or viability assessment that is normalized to account for differences in cell number. Data is expressed as viability values normalized as a percent of control samples not containing radicals or lactic acid. Data is compiled from six different photoinitiator experiments and two hydrogen peroxide experiments, each completed with 4 replicates.
2.6 Measurement of redox state
To identify an effect of lactic acid on the intracellular redox state of a mixed population of neural cells containing neurons and precursor cells (cultured for 5–7 days), media was aspirated and monolayers cultures were incubated for 30 minutes in phenol red-free culture medium supplemented with lactic acid adjusted to a pH of 7.4 (0, 0.005, 0.1, 0.05, 0.5 or 5 mg/mL) or the well-studied antioxidant N-acetyl-L-cysteine (1mM) for comparison. Following this incubation, cultures were rinsed 2X with PBS and were then stained for 30 minutes in culture medium with CMTM-H2-Rosamine (Mitotracker Orange dihydroxytetramethyl rosamine, Molecular Probes) (Kweon et al. 2001; Smith et al. 2000). The dye used to determine redox state, Mitotracker Orange (M7511), is a reduced form that fluoresces upon oxidation. After incubation, cultures were rinsed 2X with PBS and fluorescence was measured (544 nm excitation and 576 emission wavelengths) using a Fluostar Optima plate reader. Fluorescence values in cultures treated with lactic acid or N-acetyl-L-cysteine were normalized to levels in control cultures treated with no lactic acid. Data is compiled from 9 experiments each completed with 4 replicates.
2.7 Measurement of cell composition by qRT-PCR
To identify an effect of lactic acid on the cell composition of a mixed population of neural cells containing neurons and precursor cells, monolayer cultures were grown with or without lactic acid as described in Section 2.2 for five-six days. Selected lactic acid concentrations were identified for PCR analysis. Standard phenol:chloform RNA extraction was performed on monolayer cultures using TriReagent (Sigma). After isolation, RNA was treated with DNAse (Ambion) and quantified using the Quant-iT RiboGreen RNA Assay Kit (Invitrogen). Equal amounts of RNA (100 ng) were used in each reverse transcription reaction to prevent the need for housekeeping genes (Hashimoto et al. 2004). cDNA synthesis and SYBRgreen polymerase chain reaction (PCR) were carried out according to the manufacturer’s recommendations (Biorad). Neurons were identified by β-tubulin-III and neural precursors were identified by nestin expression. Primers sequences are as follows (5’–3’): β-tubulin-III (fwd-gtttgtgatgggtgtgaacc, rev-tcttctgagtggcagtgatg); nestin (fwd-gtggcctctgggatgatg, rev-ttgaccttcctccccctc) (Invitrogen). Data represent the average fold change in gene expression relative to the control condition cultured without lactic acid.
2.7 Measurement of cell composition by immunocytochemistry
Further analysis of cell composition was performed using immunocytochemistry. Neurons were identified by β-tubulin-III and neural precursors were identified by nestin positive labeling. No cells stained positive for GFAP (a marker for glial cells) at time points examined in this study. Monolayers on coated glass coverslips were cultured for five days with or without lactic acid. After five days, cultures were rinsed and fixed in paraformaldehyde overnight at 4°C. Cultures were rinsed with PBS and incubated at room temperature with immunoblock (10% Normal Goat Serum (Invitrogen), 2% Bovine serum albumin (Sigma), 0.25% Triton-X-100 (Sigma)). β-tubulin-III (Promega) and nestin (Chemicon) primary antibodies were diluted 1:800 in immunoblock and applied to cultures overnight at 4°C. Cultures were rinsed 4X with immunoblock and the Alexaflour 546 secondary antibody (Invitrogen) was applied at 1:400 dilution for 4 hours at room temperature with gentle mixing. Cultures were rinsed 4X with PBS. Nuclei were labeled with 4’,6-diamidino-2-phenylindole, dihydrochloride (DAPI, Invitrogen) for 5 minutes at 1:1000 dilution in PBS. Cultures were rinsed 3X with PBS, mounted with Flouromount-G (Southern Biotech), and coverslipped before imaging.
All images were collected using a Ziess LSM Pascal confocal microscope. Fluorescent images were captured with a 40X oil immersion objective (Zeiss Achroplan 40X). Two to three images were collected every 1µm to create a projection. Ziess Axiovision software was used for image analysis and cell counting. Images contained a minimum of 115 cells with all clearly distinguishable nuclei in an image being counted as antibody positive or negative. Cell counts were performed on four images per condition.
2.9 Data analysis
Results are reported as mean +/− the standard error of the mean (SEM). Statistics were compiled using a single-factor ANOVA with an alpha value of 0.05. Statistical differences were determined by p-values less than 0.05, 0.01 or 0.001 as reported.
3. Results
3.1 Lactic acid scavenges free radicals in solution
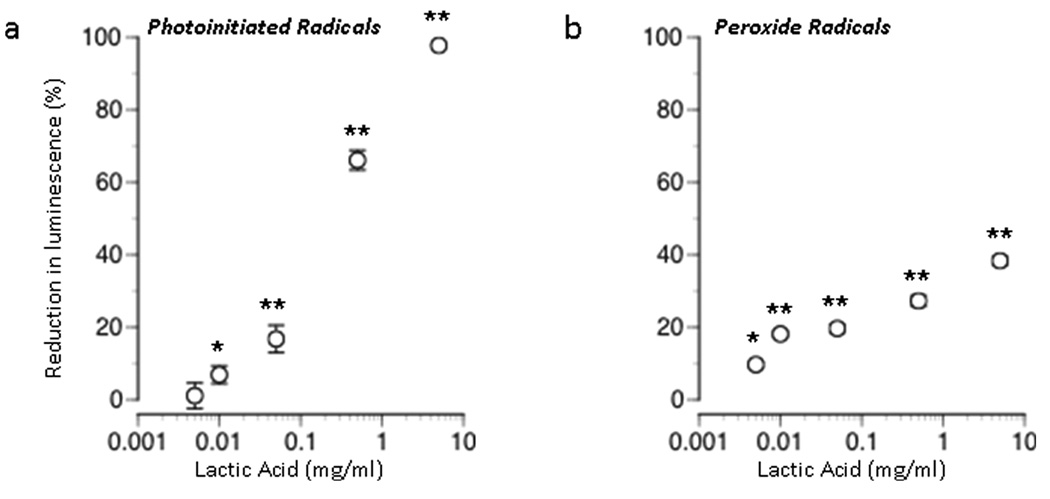
The number of free radicals generated in solution after exposing photoinitiator to ultraviolet light was reduced in a dose-dependent manner when lactic acid (pH 7.4) was applied at dosages ranging in between 0.005 – 5 mg/mL (Figure 1a). This reduction in the number of free radicals present in solution suggests a free radical scavenging effect of lactic acid.
Figure 1. Scavenging of free radicals by lactic acid.
Effect of lactic acid concentration on free radical induced luminescence when added at different dosages to solutions of PBS (a) in the presence of photoinitiator-generated free radicals or (b) free radicals generated from H2O2. The 100% reference value corresponds to complete reduction of the number of free radicals created in the absence of lactic acid. Results are expressed as mean ± SEM. Values significantly different from control values are indicated as follows: (*P < 0.01, ** P < 0.001).
In the presence of H2O2, lactic acid (pH 7.4) also reduced the number of free radicals present in solution in a dose-dependent manner (Figure 1b). A maximum reduction of 38 ± 1 % was observed at the highest concentration of lactic acid tested (5 mg/mL). Although less potent at scavenging peroxide radicals, this finding indicated that lactic acid can act as a free radical scavenger for naturally occurring radicals, in addition to those generated by photoinitiator molecules.
3.2 Lactic acid reduces free radical induced damage to cells
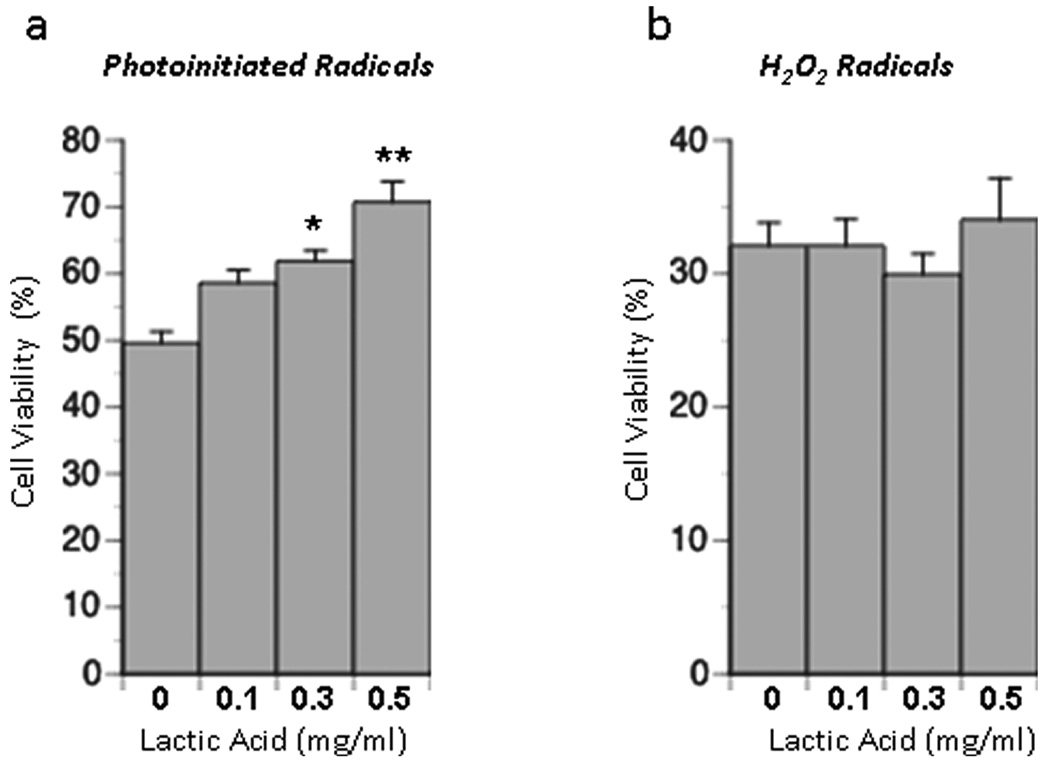
In the absence of reactive polymer groups, free radicals generated when the photoinitiator I2959 is exposed to ultraviolet light are toxic to a variety of cells when applied at dosages near that used in this study (0.25 mg/ml) (Williams et al. 2005). In this work we show that when exposed to cultures of primary neural cells which contain neurons and neural precursor cells, I2959-generated free radicals induce damage that leads to cell death. This cell death is reflected by a decrease in cell viability (ATP/DNA content) to 49.6% of control when cultures are exposed to activated photoinitiator. Addition of lactic acid to cultures improved cell viability in the presence of photoinitiator-generated free radicals in a dose-dependent manner. Viability was improved by 9.0, 12.3, and 21.1% by adding 0.1, 0.3, and 0.5 mg/mL of lactic acid (pH 7.4) respectively to culture medium (Figure 2a). Values for the 0.3 and 0.5 mg/mL conditions were statistically different from one another and from levels in control cultures.
Figure 2. Effect of lactic acid on cell viability in the presence of free radicals.
Neural cell cultures were incubated for 10 minutes with different concentrations of lactic acid and either (a) photoinitiated radicals or (b) radicals generated from H2O2. Data are expressed as the % increase in cell viability above levels in the absence of lactic acid. Results are expressed as mean ± SEM. Values significantly different from control values are indicated as follows: (*P < 0.01).
When applied at a dose equimolar to that of photoinitiator (1.11 mM), H2O2 reduced the viability of cells to 32.1% of control. When added to cultures exposed to H2O2, however, the addition of 0.1, 0.3, and 0.5 mg/mL lactic acid (pH 7.4) had no measurable effect on cell viability (Figure 2b). In both of these studies control cultures consisted of monolayer cultures treated with PBS or PBS + lactic acid.
In the absence of exogenously generated free radicals, viability was not statistically different when cultures were treated with different doses of lactic acid (data not shown). This finding suggests that short-term exposure to lactic acid does not acutely influence ATP production within the cell. Thus the increase in ATP content observed in the presence of exogenously generated free radicals was likely due to a free radical scavenging effect which in turn results in an improvement in cell viability.
3.3. Lactic acid impacts cellular redox state
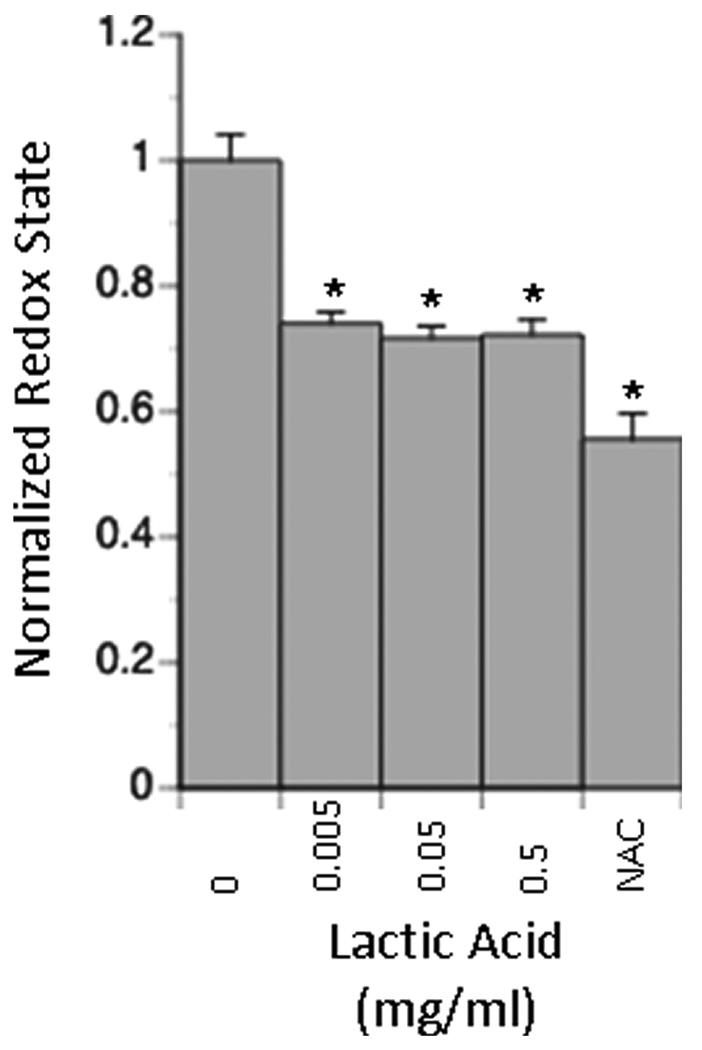
In the absence of exogenously generated free radicals, cultures exposed to lactic acid (pH 7.4) exhibited a more reduced intracellular redox state than cultures exposed to control media (Figure 3). The average fluorescence of the entire population of cells in lactic-acid exposed cultures dropped to 70% of control levels. This reduction in fluorescence suggests that more cells within this population exhibited a reduced intracellular redox state. The extent to which intracellular redox state is reduced is comparable to the reduction in redox state observed when N-acetyl-L-cysteine, a potent antioxidant is added to the culture medium at its maximally effective dosage.
Figure 3. Effect of lactic acid on intracellular redox state in neural cell culture.
Neural cells were cultured as a monolayer and then exposed to different dosages of lactic acid. Intracellular redox state was measured using a reduced rosamine dye. Reduction in intracellular redox state is comparable to that observed when cells are grown in the presence of NAC, a potent antioxidant. Data are presented normalized to intracellular redox state in the absence of lactic acid and represent the mean +/− SEM. All conditions are statistically different from the 0 mg/ml LA control (*P < 0.01).
3.4. Chronic exposure to lactic acid enhances neural cell proliferation
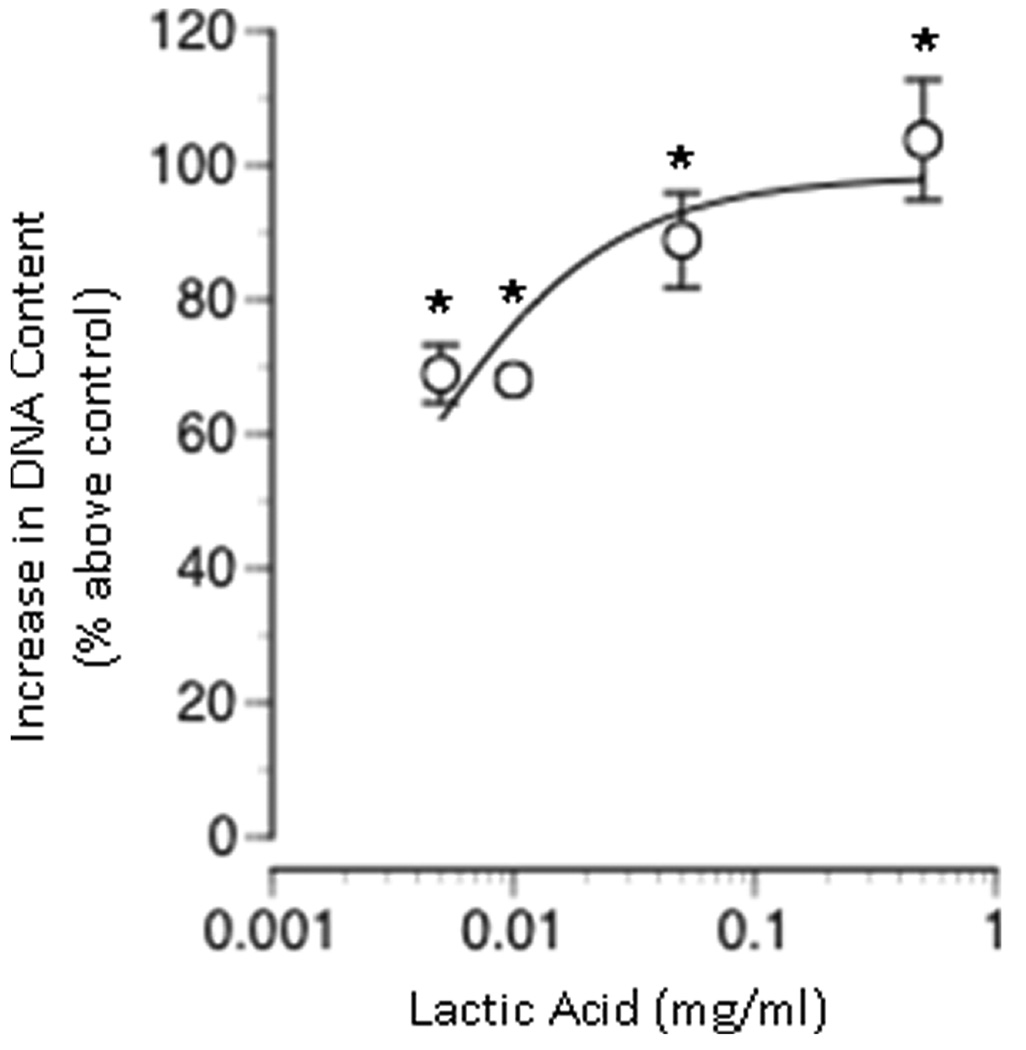
When cultured for 5 days in the presence of lactic acid (0, 0.005, 0.01, 0.05, or 0.5 mg/mL), a dose-dependent increase in total DNA content was observed (Figure 4). Total DNA content increased with increasing lactic acid concentration; a maximum increase in DNA content was achieved with a lactic acid concentration of 0.5 mg/mL. Total DNA content was significantly elevated above that of control for the lowest dosage of lactic acid tested 0.005 mg/mL.
Figure 4. Effect of lactic acid on total DNA content in monolayer cultures.
Neural cells were seeded in monolayer and grown in the presence of varying concentrations of lactic acid. All cultures were assayed for total DNA content after 5 days of culture. Total DNA content is expressed as the percent above the control condition (0 mg/ml lactic acid). Data represents the mean +/− SEM. Values significantly different from control values are indicated as follows: (*P < 0.01).
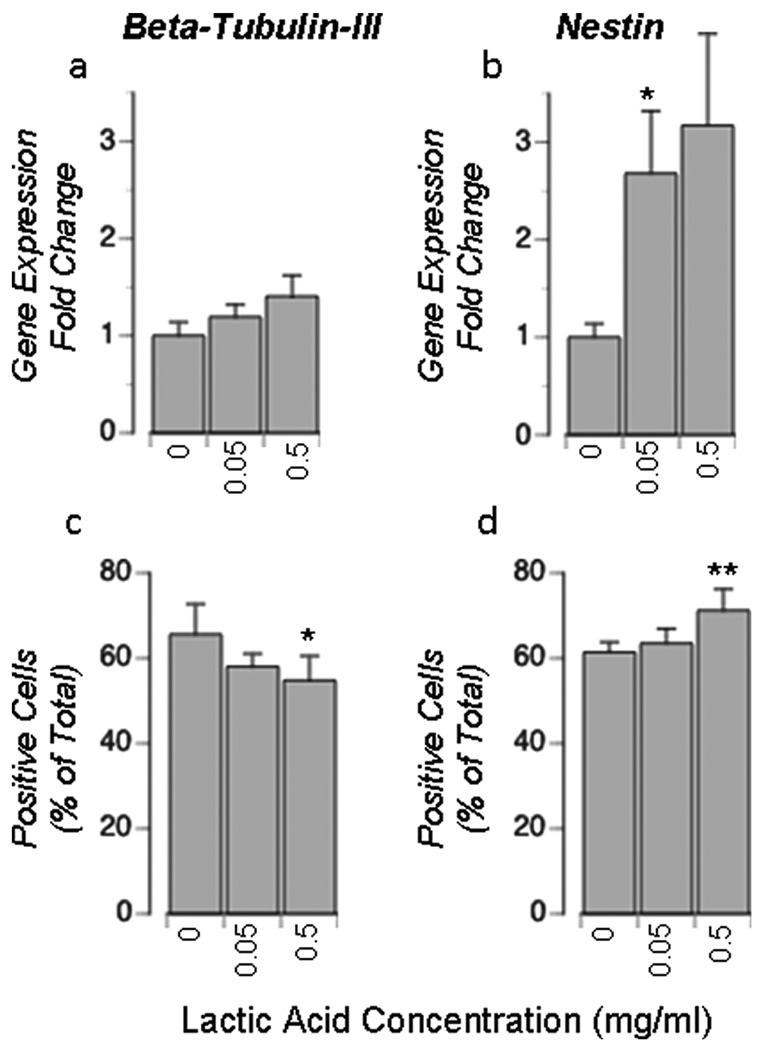
3.5. Chronic exposure to lactic acid modifies monolayer cell composition
When cultured for 5 days in the presence of lactic acid (0, 0.05, or 0.5 mg/mL), cultures present a different cellular composition than control cultures (Figure 5). RT-PCR showed that β-tubulin gene expression is statistically unchanged while nestin gene expression increases with increasing lactic acid media concentration. Immunocytochemistry showed a similar increase in nestin-positive cells, but a small decrease (statistically insignificiant) in β-tubulin-positive cells.
Figure 5. Effect of lactic acid on cellular composition in monolayer cultures.
Neural cells were seeded in monolayer and grown in the presence of varying concentrations of lactic acid for 5–6 days. Cell composition was assessed by RT-PCR (a, b) or immunocytochemistry (c, d). qRT-PCR data is expressed as fold change in a gene relative to the 0mg/mL lactic acid condition (a, b). Immunocytochemistry was used to determine the percentage of cells labeled positive for respective cell-specific proteins (c,d). Data represents the mean +/− SEM (a, b) or +/− Std Dev (c, d). Values significantly different from control values are indicated as follows: (*P < 0.05, **P < 0.01).
4. Discussion
4.1 Effect of free radicals on neural cell viability
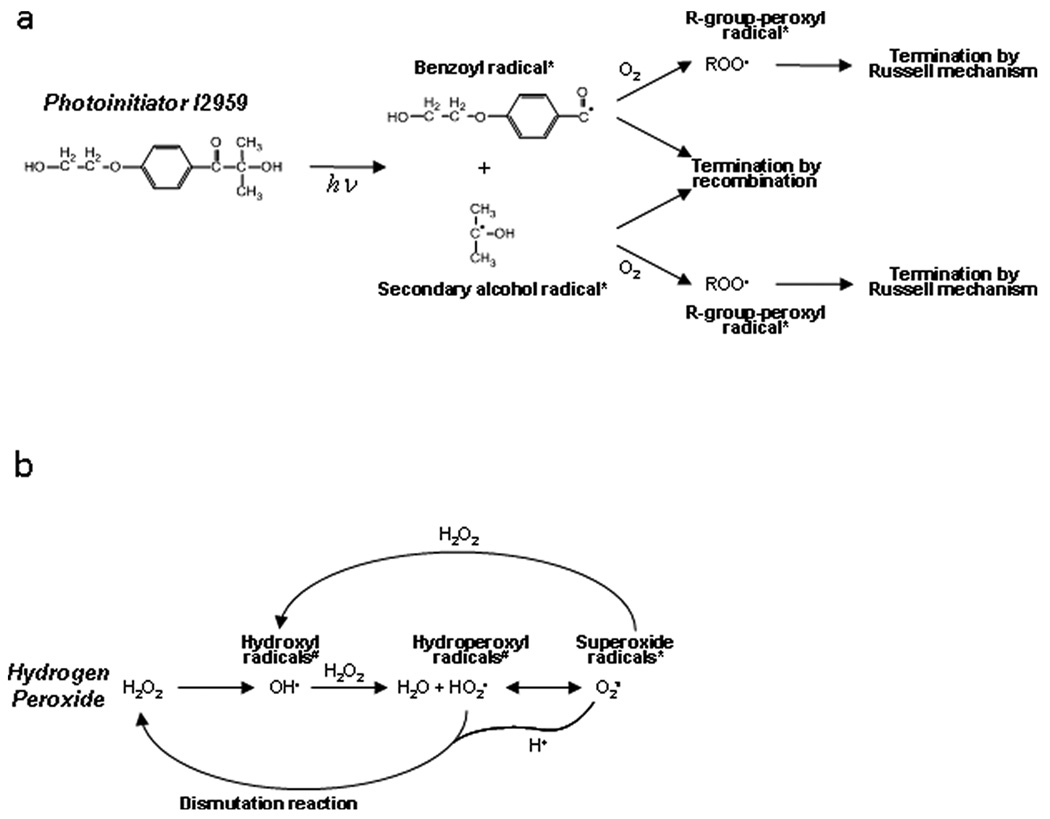
Upon activation I2959 dissociates into a benzoyl radical and a secondary alcohol radical (Bryant et al. 2000). These radicals are capable of a number of secondary reactions including: recombining in a termination reaction, reacting with oxygen by carbon radical transfer to produce peroxyl radicals, directly attacking lipids in the cell membrane, or reacting with other readily reactive groups to form secondary radicals (see Figure 6a for summary). In the case of photopolymerized hydrogel systems common to tissue engineering, the reactive groups are likely to be acrylate or methacrylate groups. In the absence of cells and reactive groups highly susceptible to radical attack, the initial carbon-centered radicals generated in this system will primarily react with dissolved oxygen in solution to produce peroxyl radicals (O'Brien and Bowman 2006). This reaction will continue until all initial photoinitiator-generated radicals are gone as our system is open to the air and oxygen can readily diffuse into our solution. Oxygen then serves essentially as a radical sink as it leads to the formation of R-group peroxyl radicals which are rather stable (Kloosterboer 1988) and ultimately terminate by the Russell mechanism (Halliwell and Gutteridge 1999).
Figure 6. Simplified schematic of radical initiation and propagation.
This figure highlights the most important and prevalent molecules present in the radical reaction pathways for both photoinitiator (a) and hydrogen peroxide (b) systems. R-groups may represent virtually any cellular component and may undergo carbon radical transfer or proceed to R-group-peroxyl radicals (depending on the reactivity). * indicates membrane impermeable radicals and # indicates membrane permeable radicals.
Hydrogen peroxide works in a different manner as it initially dissociates into hydroxyl radicals (Figure 6b). Hydroxyl radicals are typically short-lived as they are extremely reactive (greatest standard reduction potential of any radical species) (Buettner 1993). In the absence of carbon compounds, they primarily react with hydrogen peroxide to form hydroperoxyl (hydrated superoxide) radicals and water. Through de-protonation, hydroperoxyl radicals become superoxide radicals that can react with hydrogen peroxide to produce more hydroxyl radicals. Additionally, superoxide radicals (and their hydrated counterparts) may undergo a dismutation reaction, thus reforming hydrogen peroxide. In the absence of cells, the continued flux between hydroxyl radicals, hydroperoxyl radicals, superoxide radicals, and hydrogen peroxide gives rise to a persistent radical presence until ultimate decay into water and other unreactive byproducts (Halliwell and Gutteridge 1999).
When exposed to cells, the negative physiological effects of high concentrations of free radicals are widely understood; they have been shown to initiate lipid peroxidation, and damage to nucleic acids and proteins which, when extensive, can result in cell death (Fridovich 1978). While I2959 photoinitiated radicals penetrate poorly through the cell membrane (Williams et al. 2005) and are thus unlikely to target intracellular components, they will quickly react with extracellular substrates. Lipids of the cell membrane are a likely substrate for attack by benzoyl and secondary alcohol radicals leading to lipid peroxidation. Oxygen dissolved in the culture medium is another likely substrate which leads to the production of peroxyl radicals. Although peroxyl radicals penetrate through the cell membrane poorly, they are capable of initiating lipid-peroxidation, albeit to a lesser extent then carbon-centered radicals (Buettner 1993; Halliwell and Gutteridge 1999). In this work, cell viability in neural cell monolayer cultures was reduced to 49.6% of control levels following exposure to photoinitiator generated free radicals for a total of 20 minutes (10 minutes of initiation followed by 10 minutes of incubation) (Figure 2a). This reduction in viability was most likely the result of lipid peroxidation by I2959-generated free radicals (benzoyl radicals and secondary alcohol radicals) and to a lesser extent peroxyl radicals (Buettner 1993).
Radicals produced from hydrogen peroxide, including hydroperoxyl and hydroxyl radicals are capable of directly entering the cell (Halliwell and Gutteridge 1999). These radicals can initiate lipid peroxidation. They can also react with intracellular targets (such as esters, alcohols, and carbon-carbon double bonds) to form other carbon-centered radicals (Fridovich 1978). Lipid peroxidation, as well as damage to intracellular proteins and nucleic acids can alter cell function, including the balance between the reduced and oxidized forms of important redox pairs within the cell (NAD+/NADH and GSH/GSSG). When exposed to radicals generated from hydrogen peroxide (1.11 mM=0.038 mg/mL) for a total of 20 minutes, neural cell viability was reduced to 32.1% of control levels, an effect likely due to cell membrane damage as well as damage to intracellular targets (Figure 2b).
4.2 Effect of lactic acid on free radicals in the presence and absence of cells
In this work we show that lactic acid can scavenge free-radicals in the absence of cells. Lactic acid scavenged both photoinitiator generated free radicals and hydrogen peroxide generated radicals in a dose-dependent manner when applied at concentrations ranging from 0.1 to 5 mg/mL (Figure 1). Lactic acid can directly react with radicals generated by activated photoinitiator and those generated from hydrogen peroxide. The molecular geometry of lactic acid makes its 2-hydroxy hydrogen available for attack by a radical which results in the formation of a less-reactive R-group peroxyl radical by hydrogen abstraction (Herz et al. 1997). Thus the free radical scavenging capacity of lactic acid observed in the absence of cells was likely because lactic acid reacted with reactive radicals (in this case benzoyl radicals and secondary alcohol radicals or hydroxyl radicals, hydroperoxyl radicals, and superoxide radicals) to increase the rate at which they are converted to less reactive peroxyl radicals, which terminate by the Russell mechanism. As the pH of the lactic acid solution was adjusted to 7.4 before application, the observed scavenging effect cannot be simply due to a surplus of hydrogen ions.
In the presence of cells and photoinitiator-generated radicals, addition of lactic acid improved cell viability by 9–21% depending on dose (Figure 2a). While capable of reacting with oxygen to produce R-group peroxy radicals, in the presence of cells, benzoyl radicals and secondary alcohol radicals are also likely to propagate by reacting with lipids present in the cell membrane to form additional propagating carbon-centered radicals (lipid peroxidation). The addition of lactic acid may limit the number of propagating radicals formed by converting some of the benzoyl radicals and secondary alcohol radicals to less reactive peroxyl radicals before they are capable of reacting with and damaging lipids of the cell membrane. The radical reaction with lactic acid occurs more quickly than the reaction with other potential targets, including membrane-derived components (thus its antioxidant capacity) (Anbar and Neta 1967). A reduction in the number of reactive radical species would decrease the level of damage to cells, and thus improve cell viability relative to that which occurred in the absence of lactic acid as observed in this study.
While a scavenging effect of lactic acid on hydrogen peroxide radicals was observed in absence of cells, no protective effect on viability was observed in the presence of cells (Figure 2b). In the presence of cells, hydrogen peroxide generated radicals react rapidly with both intracellular and extracellular components to damage cells. Lactic acid reaction with hydrogen peroxide-derived radicals occurs at a rate equal in magnitude to that with reactive cellular components (Buettner 1993; Halliwell and Gutteridge 1999). While lactic acid is capable of scavenging some damaging free radicals, the extent of scavenging may not be sufficient to protect cells. Instead enough reactive radicals may remain in the culture system to damage, and ultimately kill cells. Contrary to our cell-free hydrogen peroxide system in which lactic acid can serve as a radical sink, lactic acid cannot effectively compete with cellular molecules such as nucleic acids, lipids, and proteins as a substrate for reaction with hydrogen peroxide generated radicals, and thus no scavenging effect is observed.
4.3 Lactic acid reduces intracellular redox state, stimulates cell proliferation, and changes the cellular composition of monolayer cultures
In the absence of exogenously applied free radicals, lactic acid was also found to impact the basal function of neural cells. In this study lactic acid was found to reduce intracellular redox state (Figure 3), a finding which suggests a role for lactic acid as a direct scavenger of endogenously present reactive oxygen species (including hydroxyl, hydroperoxyl, superoxide, and nitric oxide radicals) in addition to its role in scavenging exogenously generated free radicals. Lactic acid may also contribute to a more reduced intracellular redox state by increasing intracellular levels of pyruvate, an antioxidant that has been shown to increase levels of glutathione, a component of the cell’s natural antioxidant system (Degroot et al. 1995; Mallet 2000).
When exposed to populations of cells consisting in part of neural precursor cells as in this study, the shift in intracellular redox state that is observed in the presence of lactic acid may have significant implications on the type of cell that precursor cells decide to adopt. A recent study showed that a shift in the intracellular redox state to a more reduced state tends to promote the self-renewal of precursor cells while a shift towards a more oxidized intracellular redox state tends to promote their differentiation. The mechanism by which redox state and the choice to self-renew or differentiate are linked together has not been elucidated, but intracellular redox state has been shown to impact several signaling pathways (Kamata and Hirata 1999). Consistent with previous findings, here we show that when cellular redox state is reduced, an increase in cell proliferation is observed; effects mediated by lactic acid. A similar mechanism may link the change in redox state to a change in precursor cell fate, and ultimately to an increase in cell proliferation as both these results were observed in this study. The possibility that lactic acid directly impacts cell proliferation must also be considered from a metabolic perspective as lactic acid is a participant in multiple energy generation pathways (Alberts et al. 1998; Vicario and Medina 1992). In addition, entry into the S phase of the cell cycle (DNA replication) is facilitated by the presence of lactate (Rutz and Little 1995).
The findings of this study provide basic insight into the role that lactic acid plays naturally on developing neural cells. Neural precursor cells isolated from the forebrain were utilized in part due to their demonstrated potential for the treatment of degenerative disorders affecting the central nervous system (Armstrong et al. 2000; Gage et al. 1995; Lindvall et al. 1990; Oka et al. 2004; Pluchino et al. 2003; Studer et al. 1998). Lactic acid effects are also of interest to biomaterials scientists that are focused on the development of degradable lactic-acid based polymers for cell culture devices and/or implantation. The continued release of lactic acid from such devices as they degrade may serve as a continuous supply of lactic acid to cells in contact with the device. Depending on the rate of degradation of the material and cell culture conditions, lactic acid may be available to cells at bioactive levels that can impact cell function. In this study, lactic acid positively impacted neural precursor cell function: lactic acid scavenged endogenous and exogenous radical types, reduced intracellular redox state, and enhanced cell proliferation. Neural precursor cells are proliferative by nature and thus the cell division observed was viewed as a positive result. However, lactic acid may have varied affects on different cell types, and its effect should be characterized prior to implantation of degradable devices in vivo. While many degradable materials have been tested and deemed safe for in vivo applications, it is possible, particularly when considering the use of highly proliferative cell types like embryonic stem cells, that lactic acid released from an implanted material may contribute to tumorigenesis that is sometimes observed.
Since lactic acid was shown to scavenge photoinitiated free radicals, the results of this study also have implications for the development of photopolymerized hydrogels, particularly degradable PEG hydrogels prepared from macromers containing lactic acid and photopolymerizable methacrylate or acrylate groups in which I2959 is utilized as an initiator. Depending on the extent to which lactic acid is released from the degradable hydrogel and the cell culture conditions, lactic acid may be present within the hydrogel culture at levels that are sufficient to exert a protective effect on encapsulated cells during encapsulation and/or during extended culture. During encapsulation radicals are most likely to attack reactive acrylate or methacrylate groups, but also may initiate lipid peroxidation. Thus the protective effect of lactic acid on cells would likely result from direct radical scavenging as well as entry of lactic acid into the cell and subsequent conversion to pyruvate, an antioxidant capable of increasing intracellular reduced glutathione levels and boosting the antioxidant capacity of cell populations (Mallet 2000), an effect which is particularly important to developing and mature neural cells (Dringen et al. 2000; Schulz et al. 2000; Studer et al. 2000; Tsatmali et al. 2005; Tsatmali et al. 2006). A similar effect on glutathione levels may be observed during long term neural culture as well.
To summarize, in this study several findings were made. First, lactic acid was shown to scavenge both photoinitiator-generated free radicals and hydrogen peroxide generated radicals in the absence of cells. In the presence of cells, lactic acid protected cells from exposure to photoinitator generated radicals, but not to hydrogen peroxide generated radicals which are more reactive and are capable of directly entering the cell and reacting with membrane-based and intracellular components. Third, in addition to its effect on exogenously applied free radicals, lactic acid reduced intracellular redox state, a finding that may be due to an increased capacity to scavenge free radicals and/or augmented glutathione levels within the cells. The reduction in intracellular redox state observed after exposure to lactic acid may be in part related to the increase in proliferation and shift in cell type of the cell population that was cultured in the presence of lactic acid.
ACKNOWLEDGEMENTS
The authors would like to thank NIH for their support (R01 NS052597-02) and the U.S Department of Education’s Graduate Assistantships in Areas of National Need Program for fellowship to KJL. They would also like to thank Dr. Linda Watkins for providing the qRT-PCR primer sequences.
REFERENCES
- Alberts B, Bray D, Johnson A, Lewis J, Raff M, Roberts K, Walter P. New York: Garland Publishing, Inc; 1998. Essential Cell Biology. [Google Scholar]
- Anbar M, Neta P. A Compilation Of Specific Bimolecular Rate Constants For Reactions Of Hydrated Electrons Hydrogen Atoms And Hydroxyl Radicals With Inorganic And Organic Compounds In Aqueous Solution. International Journal Of Applied Radiation And Isotopes. 1967;18(7) 493-&. [Google Scholar]
- Armstrong RJE, Watts C, Svendsen CN, Dunnett SB, Rosser AE. Survival, neuronal differentiation, and fiber outgrowth of propagated human neural precursor grafts in an animal model of Huntington's disease. Cell Transplantation. 2000;9(1):55–64. doi: 10.1177/096368970000900108. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Nuttelman CR, Anseth KS. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. Journal Of Biomaterials Science-Polymer Edition. 2000;11(5):439–457. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- Buettner GR. The Pecking Order Of Free-Radicals And Antioxidants - Lipid-Peroxidation, Alpha-Tocopherol, And Ascorbate. Archives Of Biochemistry And Biophysics. 1993;300(2):535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- Degroot MJM, Vanhelden MAB, Dejong YF, Coumans WA, Vandervusse GJ. The Influence Of Lactate, Pyruvate And Glucose As Exogenous Substrates On Free-Radical Defense-Mechanisms In Isolated Rat Hearts During Ischemia And Reperfusion. Molecular And Cellular Biochemistry. 1995;146(2):147–155. doi: 10.1007/BF00944607. [DOI] [PubMed] [Google Scholar]
- Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain - Metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. European Journal Of Biochemistry. 2000;267(16):4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Biology Of Oxygen Radicals. Science. 1978;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, Suhr ST, Ray J. Survival And Differentiation Of Adult Neuronal Progenitor Cells Transplanted To The Adult Brain. Proceedings Of The National Academy Of Sciences Of The United States Of America. 1995;92(25):11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groussard C, Morel I, Chevanne M, Monnier M, Cillard J, Delamarche A. Free radical scavenging and antioxidant effects of lactate ion: an in vitro study. Journal Of Applied Physiology. 2000;89(1):169–175. doi: 10.1152/jappl.2000.89.1.169. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. New York: Oxford University Press; 1999. Free Radicals in Biology and Medicine. [Google Scholar]
- Hashimoto JG, Beadles-Bohling AS, Wiren KM. Comparison of RiboGreen (R) and 18S rRNA quantitation for normalizing real-time RT-PCR expression analysis. Biotechniques. 2004;36(1) doi: 10.2144/04361BM06. 54-+ [DOI] [PubMed] [Google Scholar]
- Herz H, Blake DR, Grootveld M. Multicomponent investigations of the hydrogen peroxide- and hydroxyl radical-scavenging antioxidant capacities of biofluids: The roles of endogenous pyruvate and lactate - Relevance to inflammatory joint diseases. Free Radical Research. 1997;26(1):19–35. doi: 10.3109/10715769709097781. [DOI] [PubMed] [Google Scholar]
- Kamata H, Hirata H. Redox regulation of cellular signalling. Cellular Signalling. 1999;11(1):1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Kloosterboer JG. Network Formation By Chain Crosslinking Photopolymerization And Its Applications In Electronics. Advances In Polymer Science. 1988;84:1–61. [Google Scholar]
- Kweon SM, Kim HJ, Lee ZW, Kim SJ, Kim SI, Paik SG, Ha KS. Real-time measurement of intracellular reactive oxygen species using mito tracker orange (CMH(2)TMRos) Bioscience Reports. 2001;21(3):341–352. doi: 10.1023/a:1013290316939. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Brundin P, Widner H, Rehncrona S, Gustavii B, Frackowiak R, Leenders KL, Sawle G, Rothwell JC, Marsden CD others. Grafts Of Fetal Dopamine Neurons Survive And Improve Motor Function In Parkinsons-Disease. Science. 1990;247(4942):574–577. doi: 10.1126/science.2105529. [DOI] [PubMed] [Google Scholar]
- Mallet RT. Pyruvate: Metabolic protector of cardiac performance. Proceedings Of The Society For Experimental Biology And Medicine. 2000;223(2):136–148. doi: 10.1046/j.1525-1373.2000.22319.x. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Biology And Pathology Of Oxygen Radicals. Annals Of Internal Medicine. 1978;89(1):122–127. doi: 10.7326/0003-4819-89-1-122. [DOI] [PubMed] [Google Scholar]
- O’Brien AK, Bowman CN. Impact of oxygen on photopolymerization kinetics and polymer structure. Macromolecules. 2006;39(7):2501–2506. [Google Scholar]
- Oka S, Honmou O, Akiyama Y, Sasaki M, Houkin K, Hashi K, Kocsis JD. Autologous transplantation of expanded neural precursor cells into the demyelinated monkey spinal cord. Brain Research. 2004;1030(1):94–102. doi: 10.1016/j.brainres.2004.09.062. [DOI] [PubMed] [Google Scholar]
- Philp A, Macdonald AL, Watt PW. Lactate - a signal coordinating cell and systemic function. Journal Of Experimental Biology. 2005;208(24):4561–4575. doi: 10.1242/jeb.01961. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del CarroU, Amadio S, Bergami A. other Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422(6933):688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Rutz HP, Little JB. Exogenous Lactate Interferes With Cell-Cycle Control In Balb/3t3 Mouse Fibroblasts. International Journal Of Radiation Oncology Biology Physics. 1995;31(3):525–528. doi: 10.1016/0360-3016(94)00362-O. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. European Journal Of Biochemistry. 2000;267(16):4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- Smith J, Ladi E, Mayer-Proschel M, Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2000;97(18):10032–10037. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. Journal Of Neuroscience. 2000;20(19):7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer L, Tabar V, McKay RDG. Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nature Neuroscience. 1998;1(4):290–295. doi: 10.1038/1105. [DOI] [PubMed] [Google Scholar]
- Tsatmali M, Walcott EC, Crossin KL. Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors. Brain Research. 2005;1040(1–2):137–150. doi: 10.1016/j.brainres.2005.01.087. [DOI] [PubMed] [Google Scholar]
- Tsatmali M, Walcott EC, Makarenkova H, Crossin KL. Reactive oxygen species modulate the differentiation of neurons in clonal cortical cultures. Molecular And Cellular Neuroscience. 2006;33(4):345–357. doi: 10.1016/j.mcn.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario C, Medina JM. Metabolism Of Lactate In The Rat-Brain During The Early Neonatal-Period. Journal Of Neurochemistry. 1992;59(1):32–40. doi: 10.1111/j.1471-4159.1992.tb08872.x. [DOI] [PubMed] [Google Scholar]
- Williams CG, Malik AN, Kim TK, Manson PN, Elisseeff JH. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials. 2005;26(11):1211–1218. doi: 10.1016/j.biomaterials.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Yanagida S, Luo CS, Doyle M, Pohost GM, Pike MM. Nuclear-Magnetic-Resonance Studies Of Cationic And Energetic Alterations With Oxidant Stress In The Perfused Heart - Modulation With Pyruvate And Lactate. Circulation Research. 1995;77(4):773–783. doi: 10.1161/01.res.77.4.773. [DOI] [PubMed] [Google Scholar]