Abstract
Background
Antiarrhythmic drugs are important in protecting against ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy (ARVC), but no studies have provided data in a group rigorously screened for the disease.
Objective
To examine the efficacy of empiric antiarrhythmic drugs in a rigorously characterized cohort of ARVC patients.
Methods
Antiarrhythmic medicines were examined in all subjects with implantable cardioverter-defibrillators (ICDs) enrolled in the North American ARVC/D Registry. A Cox proportional hazards model was used to account for time on each drug, and a hierarchical analysis was performed for repeated measures within individuals.
Results
Ninety-five patients were studied, with a mean follow-up of 480 ± 389 days. Fifty-eight (61%) received beta-blockers, and these medicines were not associated with an increased or decreased risk of ventricular arrhythmias. Sotalol was associated with a greater risk of any clinically relevant ventricular arrhythmia as defined by sustained ventricular tachycardia or ICD therapy (hazard ratio [HR] 2.55, 95% CI 1.02–6.39, p=0.045), but this was not statistically significant after adjusting for potential confounders. An increased risk of any ICD shock and first clinically relevant ventricular arrhythmia while on sotalol remained significant after multivariable adjustment. Those on amiodarone (n=10) had a significantly lower risk of any clinically relevant ventricular arrhythmia (HR 0.25, 95% CI 0.07–0.95, p=0.041), a finding that remained significant after multivariable adjustment.
Conclusion
In a cohort of well characterized ARVC subjects, neither beta-blockers nor sotalol appeared to be protective. Evidence from a small number of patients suggests that amiodarone has superior efficacy in preventing ventricular arrhythmias.
Keywords: ARVC, ARVD, Antiarrhythmic drugs, Ventricular arrhythmias, ICD
Arrhythmogenic right ventricular cardiomyopathy (ARVC; also known as arrhythmogenic right ventricular dysplasia or ARVD) is a genetically determined cardiomyopathy associated with fibrous and fatty replacement of the right ventricular myocardium, ventricular arrhythmias, and sudden cardiac death. While the prevalence of the disease is estimated to be approximately 0.02% to 0.1% in the general population (1), mortality rates range from 4% to 20% in the major studies (2). In fact, up to 5% of sudden deaths in young adults in the United States and up to 25% of exercise related deaths in the Veneto region in Italy are attributed to ARVC (2).
While an implantable cardioverter-defibrillator (ICD) is generally recommended as the best therapy to prevent death in the setting of ARVC in the US (3), antiarrhythmic drugs also play a major role in the treatment of the disease. Implantable defibrillators are not as widely available in many other countries and first line therapy even for the highest risk patients will often be antiarrhythmic medicines. Even in patients with ICDs, antiarrhythmic drugs are often required to reduce symptoms due to premature ventricular contractions or ventricular tachycardia (VT) and are crucial in reducing the incidence of ICD shocks.
Despite the potentially important role of antiarrhythmic medicine in ARVC, a prospective study regarding the efficacy of different agents in a population rigorously determined to have ARVC has not yet been reported. Current practice is based largely on anecdote, extrapolation from other conditions, and studies that did not necessarily differentiate between different agents (4). One of the most cited reports was a European study involving serial programmed electrical stimulation testing in ARVC subjects, suggesting that sotalol therapy associated with acute prevention of VT/VF induction may be particularly effective (5). In order to study the effectiveness of antiarrhythmic drugs in ARVC in a well characterized population, we assessed the efficacy of antiarrhythmic drugs prescribed to patients participating in the North American ARVC study.
Methods
The Multidisciplinary Study of Right Ventricular Dysplasia established the North American ARVC/D Registry that consists of 18 enrolling centers in the United States and Canada (see appendix), a clinical center at the University of Arizona, a data coordinating center at the University of Rochester, a genetic center at Baylor College of Medicine, six core laboratories in the United States and Europe, and an NIH appointed Data and Safety Monitoring Board (see appendix). Individuals with left-bundle branch morphology ventricular arrhythmias or those who exhibited repetitive premature ventricular contractions (at least 1000 during a twenty-four hour Holter study) who also met task force criteria for the diagnosis of ARVC (6) were included: diagnostic tests, including copies of 12 lead electrocardiograms, signal averaged electrocardiograms, magnetic resonance imaging, right ventricular angiograms, and right ventricular biopsies were sent to the study’s core laboratories for blinded assessment. The diagnostic test results were sent to the Data Coordination Center and entered into a secure web based data management system. The data was monitored online, with error notices for items that did not match field requirements. Based on the interpretation of each test as affected, borderline, or non-affected, the principal investigator (FM) performed final classification of the phenotype.
For the purposes of this analysis, only individuals who met the ARVC task force criteria for ARVC and who had an ICD placed were included. Task force major criteria include severe global or regional dysfunction and/or structural abnormality of the right ventricle such as dilation or aneurysm, fibrofatty replacement of the myocardium on biopsy, an epsilon wave on 12 lead electrocardiogram, and autopsy or biopsy confirmed family history of ARVC; minor criteria include minor regional or global dysfunction and/or structural abnormality of the right ventricle, inverted T waves in the right precordial leads in the absence of right bundle branch block, late potentials on signal averaged electrocardiogram, left bundle branch block ventricular tachycardia or more than 1000 premature ventricular contractions in twenty-four hours, and a family history of premature sudden death or ARVC; two major or one major and two minor or four minor criteria are required for diagnosis (6). Each patient was assigned an “ARVC score” based on the diagnostic tests used to make the diagnosis, with 2 points for fulfilling a major criteria of a category and 1 point for meeting the minor criteria of a category. Those without an ICD were not included since follow-up data regarding medicine changes and arrhythmias were insufficiently complete. Also, the ICD subjects provided a uniform group with the ability to detect serial arrhythmias. Initially, patients were excluded if they had an ICD implanted prior to enrollment. Since patients and their personal physicians often became aware of this study after ICD implant, this severely hampered referral for enrollment. During the second year of enrollment, the Data Safety Monitoring Board agreed to allow enrollment of patients whose ICDs were implanted within 6 months. In the last two years of the study, patients were permitted to be enrolled if they had an ICD implanted for less than two years.
Single- or dual-chamber ICDs were implanted according to customary practices at the discretion of the enrolling center, and referring electrophysiologists made all programming decisions regarding rate cutoff criteria for antitachycardia and shock therapy for ventricular tachyarrhythmias. Ultimately, 45% of the devices were dual chamber, and the remainder were single chamber ICDs. All patients received defibrillators capable of recording and storing electrogram data for future collection and interpretation. Patients received routine ICD follow-up every 3–6 months as well as with any ICD shocks or symptomatic arrhythmias. Stored electrograms were reviewed following any device therapy and with each scheduled follow-up, and electrograms for all ICD therapies were sent to the ICD core laboratory (Tufts-New England Medical Center, Boston, MA) where all therapies were reviewed by expert electrophysiologists and classified as appropriate or inappropriate for sustained ventricular arrhythmias. All ICD therapies were considered, including anti-tachycardia pacing and shock therapy.
Each patient was contacted at least once yearly and interim data related to medications, symptoms, documented arrhythmias, events determined by interrogation of ICDs, and medication changes were entered into the database. Medication prescriptions were not standardized and were determined by the treating physician.
All subjects signed institutional review board approved informed consent.
Statistical Analysis
Normally distributed continuous variables are presented as means ± SD and were compared using t-tests. Continuous variables that were not normally distributed are expressed as medians and interquartile ranges (IQR) and were compared using the Wilcoxon rank sum test. Categorical variables were compared using the χ2 test. Medicines and potential confounders were examined as time dependent covariates in a Cox proportional hazard model (7) to examine one of four different outcomes: any clinically relevant arrhythmia (defined as sustained ventricular tachycardia or VT/ventricular fibrillation [VF] requiring ICD anti-tachycardia pacing therapy or ICD shock), any ICD shock (defined as an appropriate ICD discharge for a ventricular arrhythmia), first clinically relevant arrhythmia, or first ICD shock. Using outcomes of “any clinically relevant arrhythmia” or “any ICD shock”, the same patient remained in the model and could contribute repeated data and potentially multiple outcomes- in these circumstances, a Cox model with a robust standard error was used to account for clustering within individuals (8). The comparisons were primarily between time on drug versus time not on drug; for example, for amiodarone, the comparison would be between all subjects during the time they were receiving amiodarone versus all subjects during the time they were not receiving amiodarone. Therefore, the same subject could be in both groups. Potential confounders (see results) were added to the regression model based on previously demonstrated associations or those typically deemed to be clinically important, or having associations with both the predictor and outcome with p values < 0.1, or changing the regression coefficient by > 10%
Analyses were performed using Stata 9.2 (College Station, Texas). Two-sided p values < 0.05 were considered statistically significant.
Results
Ninety-five ARVC patients with ICDs were included in the analysis and were followed for a mean 480 ± 389 days. The median ARVC score (2 points for fulfilling a major criterion and 1 point for fulfilling a minor criterion) was 4, with an inter-quartile range of 4 to 5. Over the follow-up period, 235 clinically relevant arrhythmias (either sustained VT or ICD therapy for VT/VF) were observed in 32 patients, with a mean tachycardia cycle length of 302 ± 45 ms; 49 of these events resulted in an appropriate ICD shock. The mean tachycardia cycle length of ventricular arrhythmias resulting in an ICD shock was 256 ± 43 ms. There were no deaths. Patient characteristics of those with and without clinically relevant ventricular arrhythmias are shown in Table 1. Those with a previous history of sustained VT, aborted sudden death, or syncope (when examined as a group) more commonly exhibited a clinically relevant ventricular arrhythmias during the study period (p=0.022).
Table 1.
Baseline characteristics of those with and without malignant ventricular arrhythmias
| Clinically Relevant Ventricular Arrhythmia (n=32) | No Clinically Relevant Ventricular Arrhythmia (n=63) | p value | |
|---|---|---|---|
| Mean Age | 37 ± 15 | 39 ± 14 | 0.62 |
| Male | 13 (41%) | 22 (36%) | 0.63 |
| Race | 0.58 | ||
| White | 26 (81%) | 56 (90%) | |
| Black | 1 (3%) | 1 (2%) | |
| Asian/Pacific Islander | 2 (6%) | 3 (5%) | |
| Hispanic | 2 (6%) | 2 (3%) | |
| Other | 1 (3%) | 0 | |
| NYHA Class* | 0.38 | ||
| Class I | 26 (90%) | 50 (94%) | |
| Class II | 2 (7%) | 3 (6%) | |
| Class III | 1 (4%) | 0 | |
| Class IV | 0 | 0 | |
| Left ventricular ejection fraction (%)† | 60 ± 8 | 60 ± 10 | 0.96 |
| Right ventricular ejection fraction (%)‡ | 44 ± 9 | 42 ± 14 | 0.73 |
| Maximum 12 lead QRS duration (ms) | 101 ± 18 | 103 ± 22 | 0.77 |
| Body mass index (kg/m2) | 26 ± 5 | 25 ± 5 | 0.53 |
| Family history of sudden death§ | 11 (39%) | 21 (38%) | 0.92 |
| Previous history of sustained VT or aborted sudden death | 28 (88%) | 44 (71%) | 0.073 |
| Syncope | 10 (31%) | 13 (21%) | 0.27 |
| Previous history of sustained VT, aborted sudden death, or syncope | 30 (94%) | 46 (74%) | 0.022 |
NYHA (New York Heart Association Class) was available for 82 participants
Left ventricular ejection fraction by echocardiography or magnetic resonance imaging was available in 76 participants
Right ventricular ejection fraction by magnetic resonance imaging was available in 41 articipants
Information regarding a family history of sudden death was available in 82 participants
Beta-blockers
Fifty-eight participants took a beta-blocker at some point during the study for a median duration of 591 days (IQR 378–942). The beta-blockers included atenolol, metoprolol, bisoprolol, and carvedilol. Although sotalol has beta-blocking properties, it was not included in the beta-blocker group for the purposes of these analyses. While taking a beta-blocker, subjects were not significantly more or less likely to experience a clinically relevant arrhythmia when compared to those not taking a beta-blocker (HR 1.75, 95% CI 0.48–6.37, p=0.40) or when compared to participants not taking any antiarrhythmic medicines or beta-blockers (HR 0.63, 95% CI 0.26–1.60, p=0.34). Although the hazard ratio favored a reduction in any ICD shocks with beta-blocker therapy, this did not reach statistical significance (HR 0.54, 95% CI 0.25–1.18, p=0.12). Beta-blockers were also not associated with risk of first clinically relevant arrhythmia or first ICD shock. Adjustment for potentially important confounders, including age, gender, New York Heart Association (NYHA) class (modeled as an ordinal variable), left ventricular ejection fraction, a family history of sudden death, a previous history of sustained VT or aborted sudden death, or ARVC score did not meaningfully change any of these results.
In an exploratory analysis, effects of each individual beta-blocker were examined. Only atenolol demonstrated a statistically significant association: 20 subjects received atenolol for a median 665 days (IQR 188–930) throughout the study; while on atenolol, subjects were 75% less likely to have a clinically relevant ventricular arrhythmia throughout the duration of the study (95% CI 37%–90% less likely, p=0.003), a finding that remained significant after adjusting for the same confounders listed in the above paragraph (adjusted HR 0.25, 95% CI 0.08–0.80, p=0.018). This did not appear to be due to an especially high dose of atenolol since the median dose was 25 mg daily (IQR 25–50 mg). However, none of the individual beta-blockers (including atenolol) were significantly associated with any ICD shock (i.e., ICD shock throughout the study duration), first clinically relevant arrhythmia, or first ICD shock.
Sotalol
Thirty-eight patients were treated with sotalol at some point during study follow-up for a median 644 days (IQR 464–1091). Association between sotalol use and the outcomes of any clinically relevant arrhythmia, any ICD shock, first clinically relevant arrhythmia, and first ICD shock are shown in Table 2. The median daily dose of sotalol was 240 mg (IQR 160–320 mg). The mean tachycardia cycle length of those with ventricular arrhythmias while taking sotalol was significantly slower: 311 ± 41 ms versus 292 ± 46 ms in those not taking sotalol, p=0.0009. Although not all associations were statistically significant, the hazard ratios consistently demonstrated no effect or favored a detrimental effect of sotalol, whether compared to no sotalol or compared to no other antiarrhythmic agents and whether or not potential confounders are taken into account (Table 2). Adjusting for ARVC score did not meaningfully change any of these results.
Table 2.
Associations between sotalol and ventricular arrhythmias in ARVC
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
| Any Clinically Relevant Ventricular Arrhythmia | HR | 95% CI | p value | HR | 95% CI | p value |
| On sotalol versus not | 2.55 | 1.02–6.39 | 0.045 | 1.29 | 0.86–1.92 | 0.22 |
| On sotalol versus no antiarrhythmic agents | 4.10 | 1.09–15.2 | 0.037 | 6.50 | 2.34–18.02 | <0.001 |
| Any ICD Shock | ||||||
| On sotalol versus not | 2.16 | 1.15–4.07 | 0.017 | 2.36 | 1.09–5.12 | 0.03 |
| On sotalol versus no antiarrhythmic agents | 1.97 | 0.73–5.3 | 0.18 | 3.15 | 0.88–11.3 | 0.077 |
| First Clinically Relevant Ventricular Arrhythmia | ||||||
| On sotalol versus not | 2.52 | 1.24–5.07 | 0.010 | 2.46 | 1.02–5.96 | 0.046 |
| On sotalol versus no antiarrhythmic agents | 3.49 | 1.24–9.82 | 0.018 | 7.54 | 1.54–36.8 | 0.012 |
| First ICD Shock | ||||||
| On sotalol versus not | 1.59 | 0.69–3.63 | 0.28 | 1.90 | 0.68–5.36 | 0.22 |
| On sotalol versus no antiarrhythmic agents | 1.69 | 0.60–4.78 | 0.32 | 3.83 | 0.85–17.4 | 0.81 |
Adjusted for age, gender, history of sustained VT or aborted sudden death, family history of sudden death, and NYHA class
In order to assess if a dose effect was present, individuals that received the upper quartile dose of sotalol (320 mg a day) were analyzed alone. Six patients received at least 320 mg of sotalol during the study period, and patients had a worse outcome on this dose compared to everyone not receiving 320 mg of sotalol (i.e., compared to all participants not receiving 320 mg of sotalol): before adjustment for potential confounders, HR for any clinically relevant ventricular arrhythmia was 3.0, 95% CI 1.1–7.9, p=0.032; after adjustment for potential confounders, the HR was 14.0, 95% CI 1.6–125.1, p=0.018).
Amiodarone
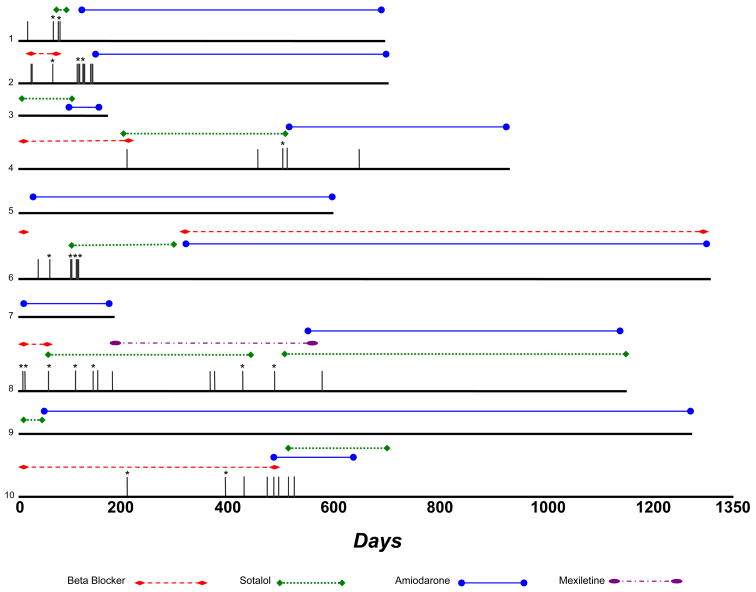
Ten participants took amiodarone for a median 545 days (IQR 190–583). The primary predictor in the amiodarone analyses was time receiving amiodarone, without special consideration (such as blanking of outcomes) during either amiodarone loading or withdrawal. While taking amiodarone, participants had a 75% lower risk of any clinically relevant ventricular arrhythmia (HR 0.25, 95% CI 0.07–0.95, p=0.041); after adjustment for age, gender, NYHA class, left ventricular ejection fraction, a family history of sudden death and a previous history of sustained VT or aborted sudden death, participants had a 97% decreased risk (HR 0.03, 95% CI 0.01–0.64, p=0.025). Comparing only to those not taking other antiarrhythmic drugs, amiodarone was also significantly associated with a lower risk of any clinically relevant ventricular arrhythmia (HR 0, p<0.001); after adjustment for potential confounders, this remained significant. Those taking amiodarone prior to a first event had a hazard ratio of 0 for either any ICD shock, first clinically relevant arrhythmia, or first ICD shock (of note, a hazard ratio of 0 means that all the first events occurred in those not taking amiodarone before they occurred in those taking amiodarone). Figure 1 provides a timeline of the 10 patients receiving amiodarone, with data on each antiarrhythmic medicine used for those patients included.
Figure 1. Timeline of antiarrhtyhmic medicine use in the 10 ARVC patients that received amiodarone at any time during study follow-up.
Each patient is represented by a different number (1–10). Vertical grey lines represent episodes of either sustained ventricular tachycardia or ICD therapy; an asterix (*) denotes an ICD shock.
Other Medications
Sixteen participants received angiotensin converting enzyme inhibitors or angiotensin receptor blockers during the study and there was no apparent relationship between the use of these medicines and ventricular arrhythmias. Three patients received statins, and there was also no consistent association with an increased or decreased risk of ventricular arrhythmias.
Discussion
In this evaluation of antiarrhythmic agents in a cohort of rigorously characterized ARVC patients, we found that beta-blockers were neither harmful nor protective against clinically relevant ventricular arrhythmias, that sotalol was not effective, and that amiodarone, although only received by a relatively small number of patients, had the greatest efficacy.
As exemplified by this study and the fact that no deaths occurred in these high risk patients over an average of more than 1 year of follow-up, ICDs appear to be effective in preventing death in ARVC patients.
Despite the importance of pharmacologic therapy in ARVC, a study of antiarrhythmic efficacy in a rigorously characterized ARVC population has not previously been reported. The only other study to previously examine specific antiarrhythmic agents first determined acute efficacy of drugs by response to serial testing with programmed ventricular stimulation (5). This European study of originally 81 patients and more recently updated to 191 patients (9) suggested that sotalol was the most effective agent, and amiodarone did not appear to be particularly useful— however, a critical difference is that this study determined selection of antiarrhythmic therapy based on suppression of VT in the electrophysiology laboratory. In contrast, ours is the first study to describe the outcome of different antiarrhythmic drugs chosen empirically by practicing physicians. The acute nature of assessing drug efficacy in the previous study may have been insufficient to allow for full amiodarone loading and consequently full antiarrhythmic effect of the drug. One limitation of this previous study was that subjects were included if they had either “proven or highly suspected” ARVC. They did prospectively follow these patients, again finding that sotalol was the most effective agent, but the predictor was the antiarrhythmic they were on when discharged and did not take into account duration of therapy or the possibility that medicines may have been changed. Finally, ascertainment of the outcome depended largely on clinical presentation as very few of these subjects had ICDs, making the detection of ventricular tachycardia incomplete. Despite this limitation and because it was previously the only study that evaluated antiarrhythmic drugs in ARVC, this study is frequently referenced as an indication for sotalol as a first line antiarrhythmic agent in ARVC (2,9). In fact, it may be that electrophysiology guided antiarrhythmic therapy is useful in identifying ARVC patients that are good candidates for sotalol.
Because the same ARVC patient can have multiple events (i.e., multiple episodes of ventricular arrhythmias and/or ICD therapies) and because medicines can be changed in the same patient, assessment of individual drug efficacy is difficult and complex. By studying prospectively collected data in this well characterized cohort of ARVC patients with ICDs using a Cox proportional hazards model in a hierarchical structure, we were able to report on the efficacy of a given drug while accounting for the time patients were taking a particular drug and the fact that there were repeated measures within individuals.
Beta-blockers are generally recommended for ARVC patients (3, 10). While these agents are likely safe, we found no evidence that they were protective. Because more than half of all participants were taking a beta-blocker at some point during follow-up (61%), it is unlikely that insufficient power to detect a meaningful beta-blocker effect was responsible for this negative finding. However, there may be differences between beta-blockers. Notably, atenolol was significantly associated with a reduced risk of any clinically relevant arrhythmia. While multiple hypothesis testing could be responsible for this positive finding, it is unlikely to fully explain a robust p value of 0.003: even using the conservative Bonferroni correction for testing 4 beta-blockers, the threshold p value for statistical significance would be 0.05 divided by 4 (or 0.0125). Finally, we can not exclude confounding by indication that was not sufficiently addressed with our measured potential confounders in our regression model; in other words, perhaps healthier or lower risk individuals (as determined by some unknown/unmeasured factor) were more likely to receive atenolol.
While previous data suggested that sotalol might be especially effective for ARVC patients (5), our data suggest that it may have no significant protective effect and may even be harmful. In general, even after adjusting for potential confounders, those on sotalol were at higher risk for any clinically relevant ventricular arrhythmia or ICD shock throughout the study and at higher risk for the first clinically relevant arrhythmia (whether compared to those not on sotalol or those on no antiarrhythmic drug). Although the point estimate also suggested a higher risk of first ICD shock, this association was not statistically significant; however, as first ICD shock was the least common outcome, we may have had insufficient power to detect a statistically significant difference for this particular outcome. Those taking sotalol had a statistically significantly slower ventricular tachycardia cycle length, suggesting that any proarrhythmia (if present) was unlikely to be due to Torsades de Pointes, which would be expected to be faster than the mean 192 beats per minute observed. In fact, this slower rate demonstrates that the sotalol had some effect on the ventricular myocardium and suggests that it might make arrhythmias more tolerable. While serial QTc measurements may also have helped to determine the true potassium-blocking effect of sotalol, these measurements were not available for each patient every time a medicine or dose was changed. Once again, there is the possibility that confounding by indication played a role: in order for confounding to explain an apparent harmful effect of sotalol, one would have to posit that those who were more likely to have arrhythmias were more likely to receive sotalol. While this is possible, it would appear unlikely to explain the finding that those receiving sotalol were at higher risk for their first clinically relevant ventricular arrhythmia. It is also possible that the therapies other than sotalol (such as amiodarone) were simply more efficacious, however even when compared to no therapy, sotalol did not appear to be protective. Finally, even though we could not detect a protective effect of the higher doses of sotalol in this study (320 mg or above, based on the highest quartile), the doses of sotalol given (median 240 mg daily, IQR 160–320 mg) may have been insufficient, particularly as the successful sotalol experience described in Europe involved doses of 320–480 mg daily and up to 640 mg/day in some cases (9).
Although amiodarone was only taken by 10 patients during the study, it appeared to have a consistent protective effect, regardless of the outcome examined or whether or not potential confounders were taken into account. As those most likely to be at risk for sustained VT or VF would more likely be treated with amiodarone, confounding by indication would not appear to be responsible for these positive findings, particularly since amiodarone reduced the risk of any clinically relevant arrhythmia (i.e., if anything, confounding by indication would have been expected to make amiodarone appear less effective than it actually was). For example, it might generally be thought that amiodarone would be reserved for those at the highest risk of requiring ICD therapy or sustained VT, therefore resulting in a false association between amiodarone and higher risk of arrhythmias. However, even when examining all significant ventricular arrhythmias (including those who had a change in therapy after an arrhythmic event), amiodarone was still significantly associated with a lower risk. Notably, we do not have data on medicine related toxicities other than proarrhythmia. Given the relatively small number of patients on amiodarone, the superior efficacy observed should be viewed with some caution; in particular, when considering applying these findings to clinical practice, the possibility of long-term toxicities, particularly when treating younger and otherwise healthy individuals, must still be guided by clinical judgment.
Our study has several limitations. As eluded to above, confounding is certainly a possibility when dealing with this observational data, particularly as the choice of antiarrhythmic agents was not standardized and was determined by the discretion of the treating physician. Confounding by indication may have played an important role- however, as above, it is unlikely that this is sufficient to explain all of our findings related to either sotalol or amiodarone. Other unmeasured confounders may be present, and only randomization in a prospective experimental study can adequately address this. While the use of ICDs to detect arrhythmias increased our sensitivity in ascertainment of the outcome, we can not exclude the possibility that some of the arrhythmias resulting in ICD therapy may ultimately have spontaneously terminated and therefore may not have led to lethal or potentially even symptomatic tachycardias if the ICD had not been in place. However, in individuals with ICDs, the most clinically relevant outcome is often avoidance of therapies, particularly avoidance of ICD shocks. As ICD programming was determined by the treating providers rather than a uniformly adopted protocol, it is possible that some ICD therapies may have differed between participants receiving different drugs primarily as a result of differences in ICD programming. Although all rhythms resulting in ICD therapies were reviewed by the ICD core laboratory, we also can not exclude the possibility that some arrhythmias may have been misclassified, particularly in subjects with single chamber devices. While our exclusion of ARVC subjects without ICDs allowed for study of a uniform group and a sensitive and specific method of detecting ventricular arrhythmia, this may have limited generalizability to lower risk ARVC patients without ICDs; importantly, as all comparisons were performed within this group, this limitation should not have caused any bias (i.e., there is no limitation to internal validity). Finally, some of the subgroup analyses (such as outcomes involving first occurrence events) involved smaller sample sizes that may have been prone to over-fitting in the multivariate models; however, the consistent findings for each drug across adjusted and unadjusted analyses as well as across different outcomes (any event or first event) suggests that the smaller sample sizes likely did not cause substantial error. Despite these limitations, the data from this study is uniquely robust in that it included only well characterized ARVC patients and sensitive and specific outcome data obtained from continuous monitoring via implantable devices.
Conclusions
In this rigorously characterized North American ARVC population in which individual empirically prescribed antiarrhythmic agents were analyzed as time dependent covariates, we found that beta-blockers were neither protective nor harmful, that sotalol (at doses generally lower than those given in Europe) was not effective, and that amiodarone, although only given to 10 patients, exhibited superior efficacy in preventing sustained VT and ICD therapies.
Supplementary Material
Acknowledgments
Funding Sources
This work was made possible by grant number KL2 RR024130 (G.M.M.) from the National Center for Research Resources (NCRR), a component of the NIH, and the American Heart Association Western States Affiliate Beginning Grant-in-Aid Award (G. M. M.); this work was also supported in part by grants UO1-HL65594 and HL65691 from the National Heart, Lung and Blood Institute.
Abbreviations
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- ICD
implantable cardioverter-defibrillator
- VT
ventricular tachycardia
- IQR
interquartile range
- HR
hazard ratio
- NYHA
New York Heart Association
Footnotes
Author disclosures/potential conflicts of interest: Dr. Scheinman: Speakers fees from Medtronic, Boston Scientific, St. Jude
All other authors: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thiene G, Basso C. Arrhythmogenic right ventricular cardiomyopathy: An update. Cardiovasc Pathol. 2001;10(3):109–117. doi: 10.1016/s1054-8807(01)00067-9. [DOI] [PubMed] [Google Scholar]
- 2.Kies P, Bootsma M, Bax J, Schalij MJ, van der Wall EE. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: screening, diagnosis, and treatment. Heart Rhythm. 2006;3(2):225–234. doi: 10.1016/j.hrthm.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Calkins H. Arrhythmogenic right-ventricular dysplasia/cardiomyopathy. Curr Opin Cardiol. 2006;21(1):55–63. doi: 10.1097/01.hco.0000198984.70884.4d. [DOI] [PubMed] [Google Scholar]
- 4.Marcus FI, Fontaine GH, Frank R, Gallagher JJ, Reiter MJ. Long-term follow-up in patients with arrhythmogenic right ventricular disease. Eur Heart J. 1989;10 (Suppl D):68–73. doi: 10.1093/eurheartj/10.suppl_d.68. [DOI] [PubMed] [Google Scholar]
- 5.Wichter T, Borggrefe M, Haverkamp W, Chen X, Breithardt G. Efficacy of antiarrhythmic drugs in patients with arrhythmogenic right ventricular disease. Results in patients with inducible and noninducible ventricular tachycardia. Circulation. 1992;86(1):29–37. doi: 10.1161/01.cir.86.1.29. [DOI] [PubMed] [Google Scholar]
- 6.McKenna WJ, Thiene G, Nava A, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994;71(3):215–218. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145–157. doi: 10.1146/annurev.publhealth.20.1.145. [DOI] [PubMed] [Google Scholar]
- 8.Cook RJ, Lawless JF. Analysis of repeated events. Stat Methods Med Res. 2002;11(2):141–166. doi: 10.1191/0962280202sm278ra. [DOI] [PubMed] [Google Scholar]
- 9.Wichter T, Paul TM, Eckardt L, et al. Arrhythmogenic right ventricular cardiomyopathy. Antiarrhythmic drugs, catheter ablation, or ICD? Herz. 2005;30(2):91–101. doi: 10.1007/s00059-005-2677-6. [DOI] [PubMed] [Google Scholar]
- 10.Sen-Chowdhry S, Lowe MD, Sporton SC, McKenna WJ. Arrhythmogenic right ventricular cardiomyopathy: clinical presentation, diagnosis, and management. Am J Med. 2004;117(9):685–695. doi: 10.1016/j.amjmed.2004.04.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.