Abstract
Purpose
Radical nephrectomy (RN), compared with partial nephrectomy (PN), increases the risk of chronic kidney disease, a significant risk factor for cardiovascular (CV) events and death. Given equivalent oncologic efficacy in patients with small renal tumors (RTs), RN may result in overtreatment. We analyzed a population-based cohort of patients to determine if RN is associated with an increase in CV events and mortality compared with PN.
Materials and Methods
Using Surveillance, Epidemiology, and End Results (SEER) cancer registry data linked with Medicare claims, we identified 2991 patients older than 65 years of age treated with RN or PN for RTs 4 cm or smaller between 1995 and 2002. Primary end points of CV events and overall survival were assessed using Kaplan-Meier survival estimation, Cox proportional hazards regression, and negative binomial regression.
Results
A total of 2547 (81%) patients underwent RN and 556 (19%) underwent PN. During a median follow-up of 4 years, 609 patients had a CV event and 892 patients died. Adjusting for preoperative demographic and comorbid variables, RN was associated with an increased risk of overall mortality (hazard ratio [HR] 1.38, P<0.01) and a 1.4 times greater number of CV events after surgery (P<0.05). RN, however, was not associated with an increased risk of time to first CV event (HR 1.21, P=0.10) or CV death (HR 0.95, P=0.84).
Conclusion
RN, currently the most common treatment for small RTs, may be associated with significant, adverse treatment effects compared with PN. PN should be considered for most patients with small RTs.
Keywords: Radical Nephrectomy, Partial Nephrectomy, Mortality, Cardiovascular Events
Introduction
In 2008 there will be approximately 54,400 newly diagnosed cases of renal tumors (RTs) in the United States.1 For nearly two decades, the incidence has been steadily rising, largely attributed to the incidental detection of small, localized tumors from widespread use of abdominal imaging for unrelated conditions.2 This has resulted in a downward stage migration, with small incidental RTs (≤4 cm, T1a) now accounting for the largest proportion of newly diagnosed renal masses.3
As expected, the increased incidence of RTs has been mirrored by an increase in the number of surgical procedures performed to remove these tumors.4 What has been unanticipated, however, is the continuing increase in mortality observed among these patients after surgery, including patients with RTs ≤ 4 cm.4 Assuming that complete surgical removal of small localized RTs is generally curative, the mortality rates of patients with small RTs should be declining, not rising.
This seemingly paradoxical trend has drawn attention to the significance of comorbid conditions and competing causes of mortality in patients undergoing surgical treatment for RTs, and has demonstrated a need to reassess the treatment effects from removal of these tumors.4 One mechanism by which the surgical treatment of RTs may adversely influence non-onocologic outcomes is through the development of chronic kidney disease (CKD) after surgery. CKD is associated with the development of kidney failure, as well as cardiovascular (CV) disease and premature death.5, 6
Although radical nephrectomy (RN) is a significant risk factor for the development of CKD in patients with RTs,7 it continues to be the most common procedure performed for patients with small RTs in the United States and abroad.8, 9 This widespread utilization of RN may result in overtreatment of many of these tumors, contributing to the rising overall mortality seen in these patients, particularly individuals with small RTs who are unlikely to die of renal cancer. To investigate this hypothesis, we examined a population-based cohort of patients with RTs ≤4 cm to determine whether RN is associated with increased risk of CV events and death compared with partial nephrectomy (PN).
Materials and Methods
Data
Our sample was obtained from Surveillance, Epidemiology, and End Results (SEER) cancer registry data linked with Medicare claims. SEER, a consortium of population-based cancer registries sponsored by the National Cancer Institute, currently includes 17 registries covering approximately 26% of the population.2 For all incident cancers in their coverage areas, the SEER registries collect information regarding site and extent of disease, first course of treatment, and sociodemographic characteristics, with active follow-up for date and cause of death.2 For cancer patients aged 65 and older residing in SEER areas, Medicare claims have been linked to SEER files. Medicare is the primary health insurer for 97% of Americans 65 years and older, covering inpatient hospital care (Part A) and outpatient care and physician services (Part B). Compared with the US elderly population, the SEER-Medicare population has similar age and sex distributions, but has a smaller proportion of non-whites, and individuals in SEER-Medicare are more likely to live in urban areas and affluent areas.2
Patient Selection
In the linked SEER-Medicare database, we identified all first primary renal-cortical tumors (ICD-O-2 Topography codes C64, C64.9) diagnosed between 1995 and 2002. The cohort was restricted to patients aged 66 and older whose primary tumor was less than or equal to 4 cm (pT1a). We excluded patients whose diagnoses were made only at the time of death, patients who were in a managed care plan during the course of treatment, and patients who lacked Part A or Part B Medicare coverage.
Surgery
Although SEER records information on cancer-directed surgery, it does not identify procedure dates. Therefore we defined the type and date of renal surgery based on Medicare claims within the first 6 months after cancer diagnosis. Using Current Procedural Terminology (CPT) codes and ICD-9-Clinical Modification (ICD-9-CM) procedure codes, we classified definitive renal surgery as either RN (CPT codes 50220, 50225, 50230, 50545, 50546; ICD-9-CM codes: 5551, 5552, 5553, 5554) or PN (CPT codes: 50240, 50280, 50290, 50542, 50543; ICD-9-CM codes 5531 5539, 554). If a patient had a claim for RN within 30 days after a claim for PN, type of surgery was categorized as RN. Patients were excluded from the study if they had no claim for definitive renal surgery within 6 months of diagnosis, if they had a claim for RN more than 30 days after a claim for PN, or if they had a claim for PN preceded by a claim for RN.
Outcomes
Primary end points of the analysis were CV events, CV deaths, and all-cause mortality after renal surgery. CV events, based on claims for inpatient care, included myocardial infarction, ischemic stroke, transient ischemic attack, percutaneous coronary intervention, coronary artery bypass graft surgery, and hospitalization for a diagnosis of acute angina, congestive heart failure, coronary artery disease, or peripheral vascular disease. Diagnosis and procedure codes identifying these events were adapted from a prior study of CV events in patients with CKD.5
Date of death was identified from Medicare enrollment records, with follow-up for vital status through December 31, 2004. CV deaths, identified from the underlying cause of death reported on the state death certificate, included deaths attributed to diseases of the heart (ICD-9: 390-398, 402, 404, 410-429; ICD-10: I00-I09, I11, I13, I20-I51), hypertension without heart disease (ICD-9: 400-401, 403; ICD-10: I10, I12), or cerebrovascular diseases (ICD-9: 430-438; ICD-10: I60-I69).
Predictors of Surgery
We examined several variables hypothesized to predict type of surgery and potentially confound the relationship between surgery and the primary end points. Demographic characteristics, from SEER and Medicare records, included age at diagnosis, race, marital status, urban-rural location, and area-level socioeconomic status.
Comorbidity was defined in two ways. In one set of analyses we used the Romano modification of the Charlson comorbidity index,10, 11 based solely on inpatient claims during the year prior to surgery. In separate analyses we identified specific medical conditions based on diagnosis codes in inpatient, outpatient, and physician claims during the year prior to surgery.
Statistical Analysis
Unadjusted associations between type of renal surgery and patient characteristics were examined using chi-square statistics. We used multivariable logistic regression to estimate the adjusted effects of each characteristic on the likelihood of receiving RN versus PN. Odds ratios (OR), 95% confidence intervals, and two-sided P values were calculated for each predictor.
The relationship between type of surgery and each primary end point was evaluated in a time-to-event framework. In all analyses, the time origin was the date of surgery. In analysis of the time to first CV event, patients who died without experiencing an event were censored at the time of death. In analysis of the time until CV death, patients were censored if they died of non-CV causes. We constructed Kaplan-Meier survival curves stratified by type of surgery, and we used multivariable Cox proportional hazards regression to assess the effect of surgery type on the hazard of each end point, controlling for demographic characteristics and comorbidity. The effect of surgery type on the total number of CV events per person-time at risk was assessed using negative binomial regression. We also evaluated interactions between type of surgery and patient characteristics, including preexisting comorbid conditions. All analyses were performed using SAS software (Cary, NC).
Results
Patient Characteristics and Predictors of Treatment
The study cohort included 2991 patients with definitive surgery for a RT of 4 cm or smaller diagnosed between 1995 and 2001. Five hundred fifty-six patients (18.6%) had PN and 2435 patients (81.4%) had RN. The demographic and clinical characteristics of all patients are shown in Table 1. Patients receiving PN were more likely to be younger, male, married, and treated more recently. No differences were noted in type of surgery based on urban vs. rural residence, area-level socioeconomic status, or race. In unadjusted analysis, comorbidities between the two cohorts were similar except for preoperative renal disease (P<0.01) and cerebrovascular disease (P<0.05) (Table 1).
Table 1. Characteristics of the Study Cohorts.
| Characteristic (N=2991) |
PN (N=556) |
RN (N=2435) |
Total | P-value |
|---|---|---|---|---|
| Age | <0.001 | |||
| 66-69 | 22.4% | 77.6% | 691 | |
| 70-74 | 20.2% | 79.8% | 936 | |
| 75-79 | 17.7% | 82.3% | 815 | |
| 80-84 | 13.9% | 86.1% | 423 | |
| 85+ | 7.1% | 92.9% | 126 | |
| Gender | <0.001 | |||
| Male | 20.5% | 79.5% | 1,713 | |
| Female | 16.0% | 84.0% | 1,278 | |
| Marital Status | <0.05 | |||
| Not Married | 16.6% | 83.4% | 1,136 | |
| Married | 19.8% | 80.2% | 1,855 | |
| Race | NS | |||
| White | 18.5% | 81.5% | 2,575 | |
| Black | 20.0% | 80.0% | 230 | |
| Other | 17.7% | 82.3% | 186 | |
| Urban vs. Rural Residence | NS | |||
| Urban | 18.9% | 81.1% | 2,594 | |
| Rural | 16.9% | 83.1% | 397 | |
| Year of Surgery | <0.001 | |||
| 1995 | 8.1% | 91.9% | 235 | |
| 1996 | 15.0% | 85.0% | 254 | |
| 1997 | 13.5% | 86.5% | 230 | |
| 1998 | 10.8% | 89.2% | 240 | |
| 1999 | 15.7% | 84.3% | 235 | |
| 2000 | 19.0% | 81.0% | 536 | |
| 2001 | 21.7% | 78.3% | 589 | |
| 2002 | 26.0% | 74.0% | 672 | |
| Census Tract % Poverty Level | NS | |||
| Lowest Tertile | 16.1% | 83.9% | 646 | |
| Second Tertile | 17.1% | 82.9% | 648 | |
| Third Tertile | 16.3% | 83.7% | 646 | |
| Charlson-Romano Index | NS | |||
| 0 | 18.8% | 81.3% | 2,320 | |
| 1 | 18.7% | 81.3% | 347 | |
| 2+ | 17.3% | 82.7% | 324 | |
| Diabetes | NS | |||
| No | 18.0% | 82.0% | 2,181 | |
| Yes | 20.1% | 79.9% | 810 | |
| Acute Myocardial Infarction | NS | |||
| No | 18.5% | 81.5% | 2,741 | |
| Yes | 20.0% | 80.0% | 250 | |
| Congestive Heart Failure | NS | |||
| No | 18.9% | 81.1% | 2,287 | |
| Yes | 17.5% | 82.5% | 704 | |
| Cerebrovascular Disease | <0.05 | |||
| No | 19.3% | 80.7% | 2,517 | |
| Yes | 14.8% | 85.2% | 474 | |
| Vascular Disease | NS | |||
| No | 18.6% | 81.4% | 2,358 | |
| Yes | 18.5% | 81.5% | 633 | |
| Renal Insufficiency | <0.01 | |||
| No | 17.9% | 82.1% | 2,717 | |
| Yes | 25.2% | 74.8% | 274 | |
| Hypertension | NS | |||
| No | 18.2% | 81.8% | 2,310 | |
| Yes | 19.8% | 80.2% | 681 | |
Abbreviation: NS, not significant
Adjusting for demographic characteristics and preexisting comorbid conditions, factors predictive of RN included age at surgery (OR 1.43, P<0.001), female gender (OR 1.31, P<0.05), and cerebrovascular disease (OR 1.41, P<0.05). Factors predictive of PN included a more-recent year of surgery (OR 0.84, P<0.0001) as well as preexisting renal disease (OR 0.66, P<0.01) (Table 2).
Table 2. Predictors of Surgery.
| Characteristic | Adjusted Odds Ratio | 95% C I | P-value |
|---|---|---|---|
| Age (per year) | 1.04 | 1.02 to 1.06 | <0.0001 |
| Gender | |||
| Male | Reference | ||
| Female | 1.31 | 1.07 to 1.61 | <0.05 |
| Year of Surgery (per year) | 0.84 | 0.8 to 0.89 | <0.0001 |
| Race | |||
| White | Reference | ||
| Black | 1.02 | 0.71 to 1.47 | NS |
| Other | 1.07 | 0.72 to 1.60 | NS |
| Urban vs. Rural Residence | |||
| Urban | Reference | ||
| Rural | 1.24 | 0.92 to 1.67 | NS |
| Marital Status | |||
| Not Married | Reference | to | |
| Married | 0.89 | 0.72 to 1.11 | NS |
| Census Tract % Poverty Level | |||
| Lowest | Reference | ||
| Second Tertile | 0.81 | 0.6 to 1.09 | NS |
| Third Tertile | 0.84 | 0.61 to 1.15 | NS |
| Co-morbid Condition | |||
| Diabetes | 0.93 | 0.75 to 1.16 | NS |
| Acute Myocardial Infarction | 0.91 | 0.64 to 1.29 | NS |
| Congestive Heart Failure | 1.23 | 0.96 to 1.58 | NS |
| Cerebrovascular Disease | 1.41 | 1.06 to 1.89 | <0.05 |
| Vascular Disease | 1.03 | 0.81 to 1.30 | NS |
| Kidney Insufficiency | 0.66 | 0.48 to 0.90 | <0.01 |
| Hypertension | 0.87 | 0.68 to 1.10 | NS |
Note: Odds ratios are adjusted for all variable in table and for SEER registry
Abbreviation: NS, not significant
Primary End Point Analysis
Cardiovascular events
A total of 609 patients had at least one CV event after surgery, including 84 patients (15.1%) in the PN group and 525 patients (21.6%) in the RN group. Median follow-up time was 43 months overall and 48 months among patients who were alive at the end of follow-up. In the PN group, the 3-year and 5-year probabilities of freedom from a CV event were 86% and 82%, respectively. In the RN group, the 3-year and 5-year probabilities of freedom from a CV event were 82% and 75%. CV event-free survival, stratified by type of surgery, is depicted in Figure 1A. In unadjusted analysis, RN was associated with an increased risk of a CV event [hazard ratio (HR) 1.37, P<0.01]. Controlling for demographic characteristics and preexisting comorbid conditions, the risk of a CV event was greater in the RN cohort than the PN cohort, but did not reach statistical significance (HR 1.21, P=0.1). (Table 3) In the interest of parsimony, urban-rural residence and census-tract poverty were excluded from this and subsequent multivariable models, as they did not confound the relationship between type of surgery and the primary end points. Interactions between type of surgery and patient characteristics were not statistically significant.
Figure 1. Kaplan Meier Estimates According to Surgery Type.
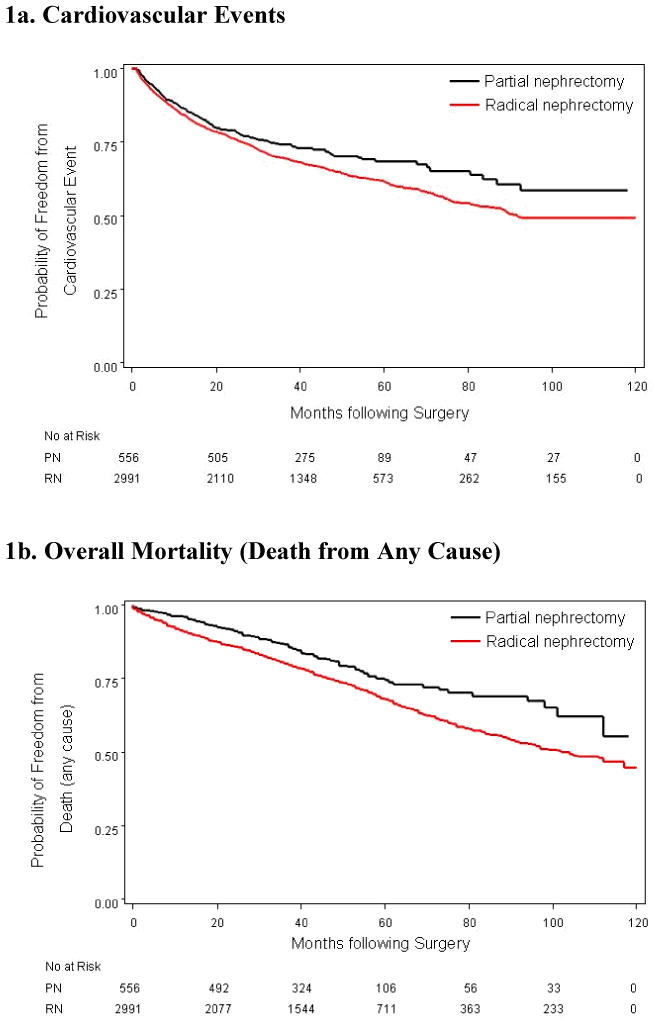
Table 3. Characteristics Associated with Time to 1st CV event.
| Time to 1st CV Event | Hazard Ratio | 95% C.I. | P-value |
|---|---|---|---|
| RN vs. PN | 1.21 | 0.96 to 1.53 | NS |
| Age (per year) | 1.04 | 1.03 to 1.06 | <0.001 |
| Female vs. Male | 0.77 | 0.64 to 0.91 | <0.01 |
| Year of Diagnosis (per year) | 0.90 | 0.86 to 0.93 | <0.001 |
| Race (Black vs. White) | 1.47 | 1.13 to 1.92 | <0.01 |
| Race (Other vs. White) | 0.77 | 0.54 to 1.10 | NS |
| Rural vs. Urban | 1.18 | 0.94 to 1.48 | NS |
| Married vs. Not Married | 1.04 | 0.87 to 1.24 | NS |
| Diabetes | 1.47 | 1.23 to 1.75 | <0.001 |
| Acute Myocardial Infarction | 1.42 | 1.11 to 1.81 | <0.05 |
| Congestive Heart Failure | 1.71 | 1.42 to 2.06 | <0.001 |
| Cerebrovascular Disease | 1.57 | 1.29 to 1.91 | <0.001 |
| Vascular disease | 1.28 | 1.06 to 1.54 | <0.05 |
| Kidney Disease | 1.17 | 0.90 to 1.52 | NS |
| Hypertension | 1.42 | 1.18 to 1.70 | <0.001 |
Abbreviation: NS, not significant
Using negative binomial regression to estimate the effect of surgery type on the total number of CV events, accounting for per person-time at risk, we found a significant difference between groups. The incidence of CV events in the patients who received RN was 1.4 times greater than in the patients who received PN (P<0.05), controlling for demographic characteristics and comorbidity.
Cardiovascular deaths
Of the 892 deaths, 173 were attributed to CV causes. Twenty-seven patients (4.9%) in the PN group and 146 patients (6.0%) in the RN group died secondary to CV causes. Controlling for patient characteristics, type of surgery was not statistically significantly associated with the risk of CV death. Predictors of CV-related deaths included increased age, black race, and history of congestive heart disease (data not shown).
All-cause mortality
About 30% of the patients died during the study period, including 110 (19.8%) in the PN group and 782 patients (32.1%) in the RN group. The 3-year and 5-year survival probabilities were 87% and 74% after PN and 80% and 68% after RN. (Figure 1B) The median time to death after RN was 102 months (not reached in the PN group). In unadjusted analysis, RN was associated with a significantly increased risk of death from any cause (HR 1.46, P<0.001) Controlling for patient characteristics, RN remained a significant predictor of death from any cause (HR 1.38, P<0.001) (Table 4). Interactions between type of surgery and preexisting comorbid conditions were not statistically significant. Overall, 107 deaths (3.5%) were attributed to kidney cancer, including 8 (1.4%) in the PN group and 99 (4.0%) in the RN group.
Table 4. Characteristics Associated with Time to Death (any cause).
| Death (any cause) | Hazard Ratio | 95% C.I. | P-value |
|---|---|---|---|
| RN vs. PN | 1.38 | 1.13 to 1.69 | <0.001 |
| Age (per year) | 1.06 | 1.05 to 1.08 | <0.001 |
| Female vs. Male | 0.77 | 0.67 to 0.89 | <0.001 |
| Year of Diagnosis (per year) | 0.97 | 0.94 to 1.00 | NS |
| Race (Black vs. White) | 1.20 | 0.95 to 1.51 | NS |
| Race (Other vs. White) | 0.63 | 0.46 to 0.87 | <0.01 |
| Rural vs. Urban | 1.01 | 0.83 to 1.24 | NS |
| Married vs. Not Married | 0.81 | 0.70 to 0.93 | <0.001 |
| Diabetes | 1.16 | 1.00 to 1.35 | NS |
| Acute Myocardial Infarction | 1.41 | 1.15 to 1.74 | <0.001 |
| Congestive Heart Failure | 1.45 | 1.25 to 1.70 | <0.001 |
| Cerebrovascular Disease | 1.14 | 0.96 to 1.35 | NS |
| Vascular disease | 1.19 | 1.02 to 1.40 | <0.05 |
| Kidney Disease | 1.59 | 1.30 to 1.95 | <0.001 |
| Hypertension | 1.23 | 1.05 to 1.43 | <0.05 |
Abbreviation: NS, not significant
Discussion
Despite many studies demonstrating equivalent oncology efficacy between RN and PN in treating RTs ≤ 4cm and select tumors ≤ 7 cm,12-14 RN remains the most common form of treatment for newly diagnosed small RTs.8, 9 In this study as well as other population-based studies, fewer than 1 in 5 patients with RTs ≤ 4 cm are treated with PN.8
During the last decade there has been a paradigm shift at specialized medical centers in the United States, where elective PNs now account for up to 60% of all nephrectomies.15, 16 This trend toward organ preservation, similar to other solid malignancies such as breast cancer and soft-tissue sarcomas, is a result of improvements in surgical techniques, advances in the understanding of the biology of RTs, and an increasing awareness of the importance of preserving long-term kidney function.
Several studies have demonstrated poor kidney functional outcomes in patients treated with RN rather than PN.17, 18 Recently, we demonstrated that even in the setting of a normal baseline serum creatinine and two normally functioning kidneys, patients undergoing RN for a solitary small RT (≤4 cm) had a statistically significant greater risk of developing CKD after surgery (HR 3.8, P<0.0001). The 3-year probability of freedom from new onset of CKD was only 35% in patients who received RN compared with 80% in patients who received PN (P<0.0001).7
The clinical significance of iatrogenic CKD has been poorly studied and remains an investigational topic of great importance. Emerging evidence suggests that the type of treatment for small RTs may have a significant effect on morbidity and mortality. In a recent series from the Mayo Clinic, younger patients (<65 years of age) treated with RN instead of PN for pT1a tumors had a significantly increased risk of mortality after adjusting for preoperative variables associated with mortality.16 In our study, we examined a population-based cohort of patients aged 65 years and older. Patients treated with RN instead of PN for small RTs developed significantly more CV events over time and had a significantly greater risk of death from any cause. No significant differences, however, were observed between the two groups in the hazard of either a first CV event or CV-related death.
Although these results seem incongruent, they are consistent with data from other studies examining the relationships between CKD, CV disease, and mortality. CKD may be a greater risk factor for recurrent CV events than for the onset of a first CV event.19 In addition, CKD and CV disease appear to act as strong independent risk factors, as opposed to synergistic risk factors, for mortality.20 Thus, it is not surprising to find that patients undergoing RN have an increase in the cumulative number of CV events and in all-cause mortality, but no increase in the risk of a first CV event or CV death.
Several limitations of this study warrant mention. The largest is the lack of randomization and the possible influence of selection bias on the observed differences in survival and CV events between the cohorts. To minimize the effect of selection bias, we controlled for demographic and comorbid factors that would be expected to influence both treatment choice and outcomes. Unfortunately, the only way to eliminate this bias would be to prospectively randomize patients to RN or RN, a study many would consider unethical today.
Another limitation is the lack of information on pre- and postoperative kidney function. Although Medicare claims provided data on important and relevant comorbidities, serum creatinine levels for these patients were not available to determine which patients had preexisting CKD and which patients developed CKD after surgery. Without such data, the proposed pathway by which the type of surgery influences the risk of CKD and subsequently the risk of CV events and mortality can be inferred but not directly observed.
Despite these limitations, our results have important ramifications for the treatment of small RTs. It is becoming increasingly evident that goals of treatment for RTs, particularly small incidental RTs, extend well beyond tumor control. Surgeons must carefully consider each patient on an individual basis and understand not only the biology of the disease but also the consequences that the treatment may have on the survivorship of the patient. Given the potentially serious, detrimental long-term effects of RN, it is worth reassessing the current treatment standards for RTs, making PN the preferred treatment for many newly diagnosed small tumors.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. Jama. 1999;281:1628. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen MM, Gill IS, Ellison LM. The evolving presentation of renal carcinoma in the United States: trends from the Surveillance, Epidemiology, and End Results program. J Urol. 2006;176:2397. doi: 10.1016/j.juro.2006.07.144. [DOI] [PubMed] [Google Scholar]
- 4.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 6.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 7.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Wei JT. National utilization trends of partial nephrectomy for renal cell carcinoma: a case of underutilization? Urology. 2006;67:254. doi: 10.1016/j.urology.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 9.Nuttall M, Cathcart P, van der Meulen J, Gillatt D, McIntosh G, Emberton M. A description of radical nephrectomy practice and outcomes in England: 1995-2002. BJU Int. 2005;96:58. doi: 10.1111/j.1464-410X.2005.05567.x. [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 11.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 12.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000;163:442. [PubMed] [Google Scholar]
- 13.Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol. 2000;163:730. [PubMed] [Google Scholar]
- 14.Leibovich BC, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004;171:1066. doi: 10.1097/01.ju.0000113274.40885.db. [DOI] [PubMed] [Google Scholar]
- 15.Scherr DS, Ng C, Munver R, Sosa RE, Vaughan ED, Jr, Del Pizzo J. Practice patterns among urologic surgeons treating localized renal cell carcinoma in the laparoscopic age: technology versus oncology. Urology. 2003;62:1007. doi: 10.1016/s0090-4295(03)00773-8. [DOI] [PubMed] [Google Scholar]
- 16.Thompson RH, Leibovich BC, Lohse CM, Zincke H, Blute ML. Complications of contemporary open nephron sparing surgery: a single institution experience. J Urol. 2005;174:855. doi: 10.1097/01.ju.0000169453.29706.42. [DOI] [PubMed] [Google Scholar]
- 17.McKiernan J, Simmons R, Katz J, Russo P. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology. 2002;59:816. doi: 10.1016/s0090-4295(02)01501-7. [DOI] [PubMed] [Google Scholar]
- 18.Lau WK, Blute ML, Weaver AL, Torres VE, Zincke H. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc. 2000;75:1236. doi: 10.4065/75.12.1236. [DOI] [PubMed] [Google Scholar]
- 19.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 20.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]


