Abstract
Both genetic and environmental factors contribute to individual differences in body weight regulation. The present study examined a possible role for the dendritic arbor of hypothalamic ventromedial nucleus (VMH) neurons in a model of diet-induced obesity (DIO) in male rats. Rats were screened and selectively bred for being either susceptible, i.e., exhibiting DIO, or diet resistant (DR) when exposed to a 31% fat diet. A 2×2 experimental design was used, based on these two strains of rats and exposure to rat chow versus the 31% fat diet for seven weeks. Golgi-impregnated neurons were measured for soma size and dendrite parameters, including number, length, and direction. As previously observed, each VMH neuron had a single long primary dendrite. Genetic background and diet did not affect soma size or the number of dendrites of VMH neurons. However, genetic background exerted a main effect on the length of the long primary dendrites. In particular, the long primary dendrites were approximately 12.5% shorter on the VMH neurons in the DIO rats compared with DR rats regardless of diet. This effect was isolated to the long primary dendrites extending in the dorsolateral direction, with these long primary dendrites 19% shorter for the DIO group compared with the DR group. This finding implicates the connectivity of the long primary dendrites on VMH neurons in the control of energy balance. The functional significance of these shortened dendrites and their afferents warrants further study.
Indexing terms: diet-induced obesity, energy balance, feeding behavior, neural plasticity, obesity
INTRODUCTION
Individual differences in body weight regulation are partially determined by genetic factors [1–5]. Nevertheless, a detailed account of the hereditary effects on adiposity has been elusive to date, in part because a large number of genes that may each play a minor role [6]. Furthermore, genetic influences are likely to interact with environmental effects, such as diet and lifestyle. Given the increasing incidence, earlier onset, and negative health consequences of obesity [7–11], there is great interest in discovering the neurological mechanisms through which genes can affect obesity.
A useful animal model for human obesity is the selective breeding of outbred Sprague-Dawley rats [12]. One strain has been selectively inbred to resist weight gain when placed on a 31% fat, high-energy (HE) diet, referred to as diet-resistant (DR) rats. In contrast, another strain has been inbred to select for offspring that increase body weight on this diet, referred to as diet-induced obesity (DIO) rats. Several physiological variables are correlated with these body weight phenotypes. These include decreased anorectic and thermogenic responses to the adipocyte hormone leptin [13], in association with reduced leptin receptor mRNA, leptin binding, and leptin signaling in the hypothalamus in the DIO rats compared with the DR rats [14, 15]. These obesity-strain differences were primarily localized to the hypothalamic ventromedial nucleus (VMH).
Discrete regions within the medulla, hypothalamus and limbic system are known to play a role in the control of food intake and body weight. Many studies have highlighted the roles of the arcuate nucleus, paraventricular nucleus, and lateral hypothalamic area. Several lines of evidence support the inclusion of the VMH in this distributed neural network. For example, animals with a disruption of the SF-1 gene, which manifests with a very selective deficit in VMH neuronal development, have marked dysregulation of energy balance [16]. SF-1 expression in the VMH is linked to leptin receptor-containing neurons in the VMH. These receptors are critical for normal energy balance, such that deletion of the leptin receptor from SF-1 neurons produces mice that become obese on a high fat diet [17]. Importantly, the VMH is one of the sites where DIO rats display deficient leptin receptors and leptin signaling. In fact, the expression of these receptors is selectively altered in the VMH by cross-fostering DR offspring to obese DIO dams or, conversely, cross-fostering DIO offspring with DR mothers [18]. Other energy balance-related genes expressed in the VMH include the ATP-sensitive potassium channel, glucose transporters, and glucokinase [19–21]. In addition, our laboratory recently has demonstrated that food deprivation is associated with structural plasticity of VMH dendrites [22]. It is unknown whether or not an inherited basis for altered food intake also might be associated with changes in VMH dendrites.
Acute and developmental effects of metabolic status have been shown to alter synaptic activity and hypothalamic connectivity [23–25]. Given that DIO rats have abnormal hypothalamic connections and reduced areal extent of the VMH [26], we postulated that DIO rats would exhibit specific remodeling within the dendritic arbor of VMH neurons. To test this hypothesis, the dendritic arbor of Golgi-impregnated VMH neurons was assessed in DR and DIO rats maintained on either rat chow or HE diet.
MATERIALS AND METHODS
Animals
Animal studies were in compliance with Animal Care Committee of the East Orange Veterans Affairs Medical Center. All rats were housed at 23–24 C on a 12:12-h light-dark cycle (lights off at 1800 h). Selectively bred male DIO and DR rats raised in our own vivarium were used [12], and all litters were culled to 10 pups per dam at birth. DIO and DR rats (n=16 per genotype) were weaned at 21 d of age and fed Purina rat chow ad libitum which contains 3.30 kcal/g with 23.4% as protein, 4.5% as fat and 72.1% as carbohydrate which is primarily in the form of complex polysaccharide [27].
Diets
At seven weeks of age, rats were weighed and randomized by genotype and body weight into two groups per genotype (n=8 per subgroup). One group continued on chow and the other was fed a high energy (HE) diet (Research Diets no. C11024F, New Brunswick, NJ) which contains 4.47 kcal/g with 21% of the metabolizable energy content as protein, 31% as fat, and 48% as carbohydrate, 50% of which is sucrose [27] for the next seven weeks. Body weight and food intake were measured every 3–4 d over the 7 wk on their respective diets. Seven weeks was chosen as the duration of exposure to the HE because effects on body weight would be apparent. Rats then were anesthetized with CO2 and the brains were quickly removed.
Golgi Impregnation and Morphological Analysis
The brains were prepared for Golgi impregnation using the FD Rapid Golgi Stain kit (FD Neurotechnologies; Ellicot City, MD). In brief, brains were incubated in a potassium dichromate, mercuric chloride, and potassium chromate solution for two weeks. After this incubation, hypothalamic sections including the VMH were obtained using a vibratome (200 μm, Vibratome Series 1000). The sections were mounted onto gelatinized slides and cover-slipped. Sections containing the VMH were viewed with a BX50 research microscope (Olympus; Central Valley, PA). Neurons were considered to be in the dmVMH vs. the vlVMH based on the borders of the subdivisions according to Paxinos and Watson (1986). For atlas plates in which the VMH was not subdivided, neurons were considered to be in the dmVMH or vlVMH if they were located in the dorsal or ventral portion of the VMH, respectively. VMH neurons then were visualized at 100 x and traced via camera lucida. The complete length of each dendrite was drawn, which often required adjusting the plane in the z axis. Next, the tracings were scanned with a Hewlett Packard Scanjet 3970, and dendrite length was measured in NIH Image v.1.62. Only neurons in which the dendritic arbor was clearly discernible in one 200••μm section were analyzed. Soma area and dendrite length were measured in triplicate using NIH Image 1.62. The location of the neurons within the VMH, i.e., within the dorsomedial versus ventrolateral subdivision, was charted.
Several additional features of the dendrites were noted, as described previously [28, 29]. First, the type of dendrite was categorized as either primary or secondary, based on whether it emerged from the cell body or another dendrite, respectively. The primary dendrites were further classified according to their length. For each neuron, the longest primary dendrite was assigned as the “long primary dendrite” (LPD) and all other primary dendrites were referred to as “short primary dendrites” (as noted in Figure 3). In the present study, the direction of the LPDs was classified as one of four mutually exclusive categories: dorsolateral, dorsomedial, ventromedial, or ventrolateral based on dorsal-ventral and medial-lateral axis bisections of each neuron in the coronal plane. An experimenter blind to the treatment groups conducted all morphological analyses.
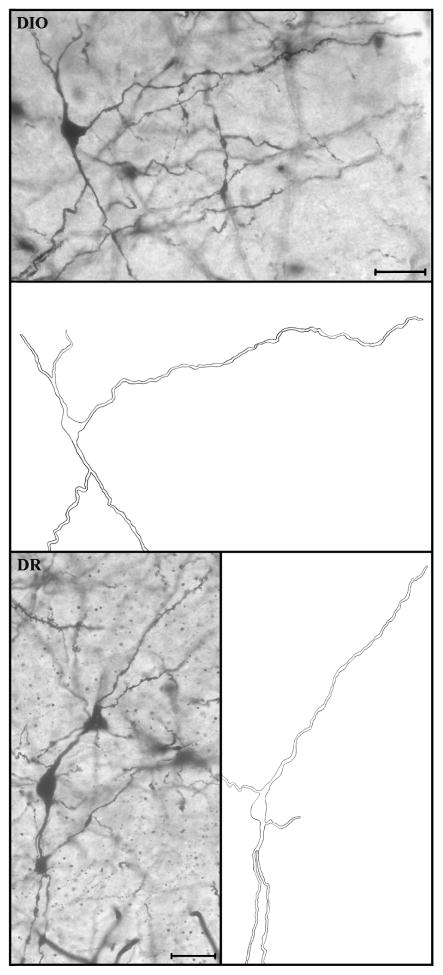
Figure 3.
Digital photomicrographs and corresponding camera lucida drawings of representative Golgi-impregnated VMH neurons from the DIO group (top) and the DR group (bottom). The entire length of the long primary dendrite (LPD) extending in the dorsolateral direction is shown, whereas secondary dendrites and short primary dendrites are cropped for presentation purposes. Note that the camera lucida drawings are based on multiple z-planes, thereby depicting the views of dendrites that are not well focused in photomicrograph. VMH neurons were photographed at 20x. Scale bar = 40 μm for both panels.
Statistical Analysis
For each neuron, dendrite length was calculated for each of the three dendrite types, which required the averaging when there were multiple short primary dendrites and secondary dendrites. For each animal, mean dendrite length for each dendrite type was calculated as the averages for each for all its neurons. Statistical comparisons between the four treatment groups were performed by two-way ANOVA and Newman-Keul’s post-hoc comparisons. The distribution of dendrites extended in each of the four possible quadrants in the coronal plane was assessed with a chi-square analysis. Data are expressed as the mean and standard error. Significance was set at p<0.05. The statistical software used was Prism 4.0 (GraphPad, San Diego, CA).
RESULTS
Body Weight and Food Intake
At seven weeks of age, chow-fed DIO rats were heavier than DR rats (Table 1). After seven weeks on HE diet, DIO rats gained 17% more body weight than chow-fed DIO rats (Table 1, Figure 1). On the other hand, DR rats fed HE diet gained no more weight than DR rats fed chow (Table 1, Figure 1). Overall, chow-fed DIO and DR and DR rats fed HE diet gained weight at the same rate while DIO rats on HE diet gained weight more rapidly than the other groups and this difference became statistically significant by 23 d (Figure 1). Over the entire seven-week period, DIO rats ate 38% more calories than DR rats, but there were no significant differences in caloric intake between rats on chow vs. those on HE diet for either group (Table 1, Figure 1). Finally, there were no significant differences in feed efficiency (amount of weight gained/caloric intake) among the groups (Table 1).
Table 1.
DIO and DR rats were fed chow from weaning until seven weeks of age and then either fed chow or HE diet for an additional seven weeks. Data are mean + SEM. Groups with different superscripts differ significantly (P<0.05) after two-way ANOVA demonstrated a significant between-group difference.
| DIO Chow | DIO HE | DR Chow | DR HE | |
|---|---|---|---|---|
| BW initial, g | 267±7a | 266±6a | 218±8b | 209±13b |
| BW final, g | 492±10a | 527±10b | 417±15c | 428±13c |
| BW gain, g | 216±8a | 253±14b | 199±6a | 217±7a |
| Intake total, kcal | 4670±67a | 4884±95a | 3567±118b | 3394±95b |
| Feed efficiency, BW gain/intake | 0.04623±0.0053a | 0.0518±0.0055a | 0.0558±0.0058a | 0.0639±0.0065a |
Figure 1.
Left: Body weight gain in DIO and DR rats during seven weeks while being fed either chow or HE diet. Asterisk indicates p=0.05 or less when DIO on HE were compared to all other groups. Right: Average daily intake in DIO and DR rats over seven weeks on chow or HE diet. Asterisks indicate p=0.05 or less when DIO on HE were compared to all other groups. DIO rats consumed more calories than DR rats, regardless of diet, over the entire seven-week period.
Morphological Analysis
Of the 32 animals with body weight and food intake measurements, 28 animals reached criteria for adequate Golgi labeling to be included in the neural morphology analysis, with six to eight animals per group. The locations of these neurons included in this study (as shown in Figure 2) included both the dorsomedial subdivision (70 neurons) and the ventrolateral subdivision (95 neurons). Representative Golgi-stained neurons are shown in Figure 3. For each animal, neurons were assayed from at least three different brain sections, for a total of four to seven neurons per animal. There was an average of 5.9 neurons per animal, with 35 to 49 neurons per group, and a total of 165 neurons. In general, the morphological features of these neurons were similar to those observed in previous studies using Golgi impregnation, Lucifer yellow cell filling, or biolistic cell filling [22, 28, 30], including moderately spiny dendrites.
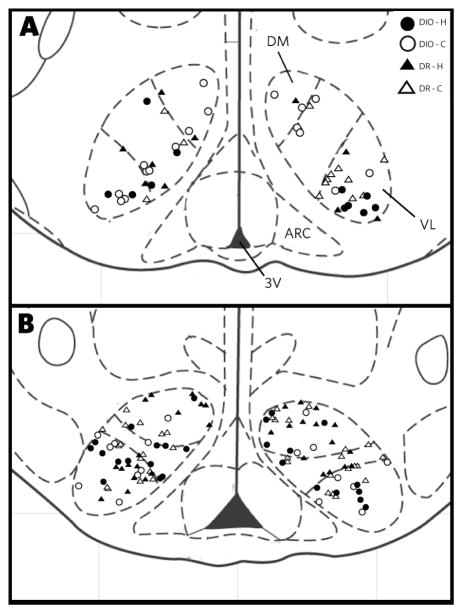
Figure 2.
Drawing of the mediobasal hypothalamus summarizing the location of all VMH neurons in this study, including neurons from both the DIO (open symbols) and DR (filled symbols) animals. The drawings are based on Paxinos and Watson [49]; Panel A is bregma −2.30 and Panel B is bregma −3.80. Abbreviations: f, fornix; 3V, third ventricle.
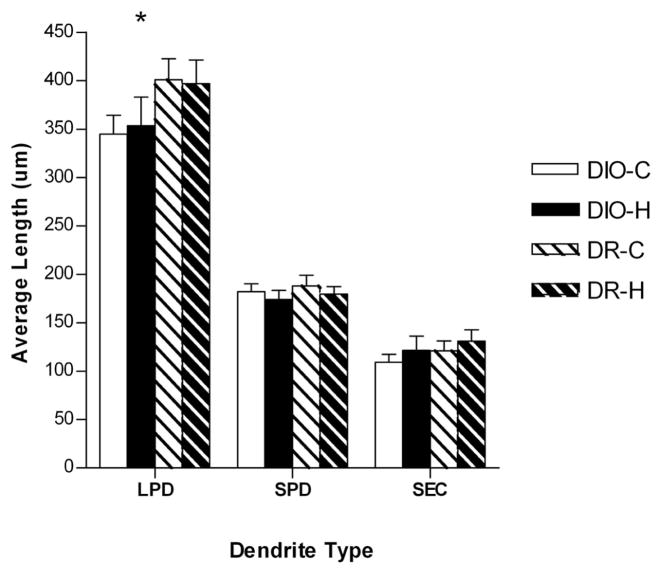
There were no main effects of either genotype or diet on the soma size or the number of dendrites per VMH neuron (data not shown). As shown in Figure 4, the type of diet and the genetic background did not affect the length of either short primary dendrites or secondary dendrites. However, there was a main effect of genotype on the length of the LPDs (F=4.40, p=0.047). In particular, whether animals were fed chow or the HE diet, LPDs were approximately 50 μm (12.5%) shorter for the DIO rats compared with the DR rats (349 ± 16 μm versus 399 ± 16 μm, respectively). There was no main effect of diet or interactions on the length of the LPDs.
Figure 4.
Bar graph depicting the length of long primary dendrites (LPDs), short primary dendrites (SPD), and secondary dendrites (SEC) in DIO and DR rats fed either chow (C) or high energy (H) diet (DIO-C, n=7; DIO-H, n=6; DR-C, n=8; DR-H n=7). There was a main effect for LPDs to be significantly shorter in the DIO rats compared with the DR rats. There was no effect of genetic background on another dendrite type, and no effect of diet on any dendrite type. Asterisk indicates a significant main effect (p = 0.047).
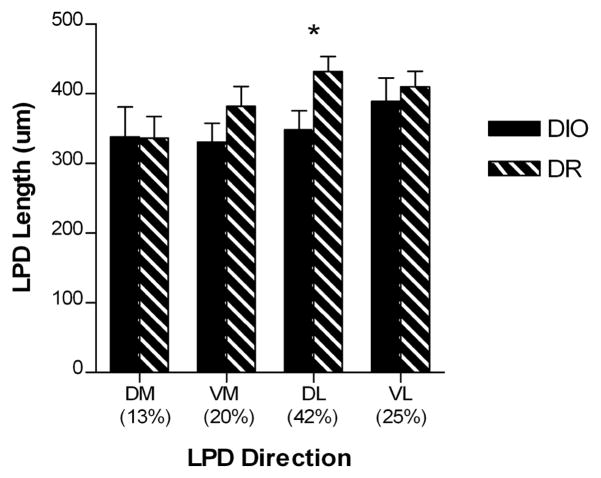
The LPDs extended in each of the four main directions, but predominantly dorsolaterally (42%) and ventrolaterally (25%), with dorsomedial and ventromedial being less common (13% and 20%, respectively). There was no main effect of diet for the LPDs extending in any direction, nor an interaction between diet and genetic background. Given the lack of diet effect, the HE and chow groups were combined within DIO and DR. When neurons were classified based on the direction of the LPD, there was a main effect of genotype on LPD length, but only for the neurons with the LPD extending in the dorsolateral direction (F=5.52, p=0.028, Figure 5). Specifically, on neurons with a dorsolateral LPD, these dendrites were approximately 83 μm (19%) shorter for the DIO rats compared with the DR rats (349 ± 27 μm versus 432 ± 22 μm, respectively).
Figure 5.
Bar graph depicting the length of VMH long primary dendrites (LPDs) in DIO and DR rats fed either chow or HE diet, according to direction (DIO, n=13; DR, n=15). There was a main effect for dorsolateral LPDs to be significantly shorter in DIO rats compared with DR rats. There was no effect of genetic background on another LPD direction, and no effect of diet on any dendrite direction. Asterisk indicates a significant main effect (p = 0.028).
4. Discussion
This study tested the hypothesis that genetic differences in the propensity to develop DIO are associated with intrinsic differences in VMH connectivity. The dendritic arbor of VMH neurons was assessed in DR and DIO rats maintained on either rat chow or HE diet, a diet on which only the DIO rats become obese (Table 1; [31]). There were several striking findings. First, within the dendritic arbor of the VMH neurons, LPDs were selectively affected by genetic background, with significantly shorter LPDs in the DIO group compared with the DR group. Second, the effect of genetic background was confined to LPDs that extended in the dorsolateral direction, implicating extrinsic input arriving in the VMH lateral shell. Finally, the type of ad libitum diet did not modify this group difference, suggesting that shorter LPDs precedes the excessive weight gain for the DIO group given access to the HE diet. Each of these aspects of the results will be discussed in turn.
4a. Energy balance and LPDs
The first major finding, that heritable factors that control energy balance also influence the arbor of VMH dendrites, is notable given that an acute challenge to energy balance, namely food deprivation, also remodels dendrites in the VMH [22]. There are some similarities and differences between these paradigms. In both cases, the length of the LPD was selectively affected, without changes in the number of dendrites or the length of other dendrite types. Across these very different metabolic conditions, the shortened LPD length was correlated with increased drive for food intake, which is latent in the DIO rats fed chow, but not correlated with actual body weight. During fasting, leptin levels fall precipitously [22], whereas in the chow-fed DIO rat peripheral levels of leptin and nutrients are comparable to those in chow-fed DR, [12–15, 25, 31, 32]. The magnitude of change was more striking after food restriction (a 33% reduction in LPD length) compared with the DIO genotype (approximately 12% reduction), although in both cases, the affected dendrites extended in the lateral direction (more specifically resolved to the dorsolateral direction in the present study).
As noted above, both groups of DIO rats, fed either chow or HE diet, had shorter LPDs than the DR rats, yet only the DIO rats given the HE diet over-consumed their diet and gained excessive weight. If LPD length is important in determining food consumption, this prompts the question of why the DIO rats fed rat chow do not overeat and gain excessive weight. Thus, there may be an interaction between VMH circuitry, in particular the LPDs, and diet-based factors.
As the genetic differences between DR and DIO rats are elucidated, an important question will be how the genomic differences between these strains are manifested at the level of hypothalamic wiring. The neurophysiological consequence of the shortened LDPs in the DIO strain of rats may be reduced synaptic input that normally restrains feeding. A logical candidate in the regulation of dendrite length in the VMH is brain-derived neurotrophic factor (BDNF), which has been shown to regulate feeding behavior in the VMH [33–35]. However, no differences in BDNF mRNA levels in the VMH have been found between DIO versus DR rats [32].
The present finding of shorter LPDs in the DIO strain of rats corresponds well to the 15% reduced areal extent of the VMH, as assessed using Nissl stain for cell bodies, was observed in the DIO rats compared with DR rats [26]. This effect on regional size was selective, with no change observed in the arcuate, dorsomedial or paraventricular nuclei. We recently have reported on the sex difference in LPD length for VMH neurons [36]. As in the present results, the sex with the larger VMH, namely males, also has longer LPDs. This raises the question of whether LPD length is determined secondarily to the distance that must be traveled to reach afferents in the VMH shell. Further studies are needed to identify other factors that contribute to the areal extent of the VMH in DIO versus DR rats, such as the number of neurons and area of neuropil. The current results suggest that soma size does not contribute to the strain difference in VMH area between DIO and DR rats (data not shown). Given that a large (45%) reduction in adrenergic receptor binding in the VMH has been observed in DIO compared with DR rats [26], the reduced LPD length seems likely to correspond to reduced extranuclear synaptic input.
The number of neurons in present study was not adequate to rigorously compare dorsomedial and ventrolateral regions of the VMH. Leptin receptors and signaling are localized to the dorsomedial VMH [14, 37], a finding which has supported the notion of functionally distinct domains within the VMH. Likewise, tract-tracing evidence to date suggests somewhat different amygdaloid and hypothalamic afferent innervation and periaqueductal gray efferent targets of these two regions [38–41]. However, our previous study suggested that the effect of food deprivation on LPD length was localized to the ventrolateral VMH [22]. Given that selective ablation of estrogen receptor-α expression in the VMH, which are predominantly expressed in the ventrolateral VMH, has striking effects on energy balance [42], the role of ventrolateral subdivision in energy balance may be greater than previously expected. Additional studies are needed to clarify the respective roles of the VMH sub-regions in the control of energy homeostasis.
4b. LPD direction
The second notable finding in this experiment was that LPDs extending in the dorsolateral direction were selectively affected by genetic background. This finding is in accord with our previous study in which food deprivation affected laterally extending, but not medially extending, LPDs [22]. A shell of extranuclear afferents surrounds the neurons in the core of the VMH [43]. Dorsolaterally extending LPDs are likely to receive input from axons arriving in this fiber plexus. Additional studies are needed to ascertain the source, chemical coding, and physiological significance of the input to these dorsolateral LPDs. Some candidate sources of input are the anterior hypothalamic area and dorsomedial nucleus of the hypothalamus [40]. Knowledge of the diminished chemical signals that impinge on these VMH LPDs in the DIO rats may suggest a “replacement” therapy that could restrain their feeding and allow body weight maintenance in the face of a HE diet.
4c. Diet and LPD
The third finding in this study was that dendrite length was not affected by the HE diet. Thus, the weight gain in DIO rats maintained on the HE diet did not cause a change in dendrite length. Although we did not directly assess adiposity here, we have previously demonstrated that the excessive body weight gain of the DIO rats on HE diet is always associated with increased adiposity [12–15, 25, 31, 32]. Thus, obesity per se was also not responsible for the findings here. In contrast to the marked effect of food deprivation, which reduced LPD length in the VMH by 125 μm [22], the present study revealed no effect of the HE diet compared with rat chow on the length of LPDs in the VMH, even in the DR rats. One possible explanation is that as long as animals are well fed, the LPDs are at maximal length, when genetic effects are held constant. If so, other paradigms of over-feeding may not produce changes in the length of LPDs in the VMH.
Recent studies have refined our understanding of the energy balance functions mediated by the VMH. In SF-1 knockout mice, the abnormal VMH development is associated with a delayed onset of obesity [16]. Weight gain in these animals depends on both increased food intake and reduced activity. A similar pattern was observed when the leptin receptor was selectively disrupted in the VMH SF-1 neurons [17], with both energy intake and expenditure being affected. The expression of glucokinase in the VMH has been linked to a role in the counter-regulatory responses during hypoglycemia [44]. Tracing studies and classic lesion studies have suggested a role for the VMH in inhibiting insulin secretion [45, 46]. In the present model system, rather than destruction of the nucleus, disruption of its development, or deletion of an important receptor, a more subtle change in dendrite length is manifested in DIO rats, even in the absence of a HE diet, along with a reduced hypothalamic sensitivity to leptin [47]. The relationship between leptin action and dendrite arborization in this nucleus warrants further investigation.
As mentioned above, the VMH is part of a broader hypothalamic network that includes the arcuate nucleus, paraventricular nucleus and lateral hypothalamic area, which together regulate energy balance. It is unknown whether these other regions also exhibit changes in dendritic arbor. The VMH was a logical region for the focus of the present study because the dendritic tree is well described [22, 28, 48]. Furthermore, in previous studies certain differences between DIO and DR rats were most striking in the VMH [26]. Nevertheless, similar studies in other brain regions are warranted to more fully understand behaviorally relevant strain differences in hypothalamic connectivity.
The present study was not designed to address the developmental time course of the effect of genetic strain on dendritic arbor. Therefore, possible environmental effects, such as in utero or neonatal nutrition, hormones and/or maternal care, cannot be ruled out. However, previous studies have demonstrated that DIO rats exhibit stable leptin resistant from the early postnatal to the adult period [14, 31]. This altered hormone sensitivity is reflected in an aberrant patterning of the arcuate nucleus projections containing neuropeptide Y, agouti-related protein, and α-melanocyte stimulating hormone to the PVN, without significant effects of maternal diet [25]. Taken together, these previous findings have not indicated experiential effects on this hypothalamic circuitry.
In summary, the present results add to our understanding of the neurological basis of energy balance, using the clinically relevant model system of a genetic predisposition to gain weight when exposed to a HE diet. In particular, these findings implicate the LPDs in the VMH as being a site of altered neuronal wiring in rats prone to become obese. These results fit well with previous evidence for variability in this dendrite type in the VMH, as well as previous evidence that the VMH plays an important role in energy balance. Future studies are warranted to investigate the connectivity of the VMH LPDs.
Acknowledgments
The authors thank Song-I Yang, Neelam Shah, and Sarah Ferri-Kolwicz for their assistance in data analysis and presentation. These results were presented in preliminary format the Annual Meeting of the Society for Neuroscience, San Diego, California, 2007.
Grant sponsor: National Institutes of Health Grants MH64371 and DK52018 (LMF-C) and the Research Service of the Veterans Administration and DK30066 (BEL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bouchard C. Inheritance of fat distribution and adipose tissue metabolism. In: Vague J, Bjorntopr P, Guy-Grand B, Rebuffe-Scrive M, Vague P, editors. Metabolic complications of human obesities. Amsterdam: Elsevier; 1985. pp. 87–97. [Google Scholar]
- 2.Garn SM. Continuities and changes in fatness from infancy to adulthood. Current Problems in Pediatrics. 1985;15:1–47. doi: 10.1016/0045-9380(85)90015-5. [DOI] [PubMed] [Google Scholar]
- 3.Price RA, et al. Genetic contributions to human fatness: An adoption study. Am J Psychiatry. 1987;144:1003–1008. doi: 10.1176/ajp.144.8.1003. [DOI] [PubMed] [Google Scholar]
- 4.Stunkard AJ, Foch TT, Hrubec A. A twin study of human obesity. JAMA. 1986;256:51–54. [PubMed] [Google Scholar]
- 5.Stunkard AJ, et al. An adoption study of human obesity. NEJM. 1986;314:193–198. doi: 10.1056/NEJM198601233140401. [DOI] [PubMed] [Google Scholar]
- 6.Rasche A, Al-Hasani H, Herwig R. Meta-analysis approach identifies candidate genes and associated molecular networks for type-2 diabetes mellitus. BMC Genomics. 2008;9:310–316. doi: 10.1186/1471-2164-9-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jasik CB, Lustig RH. Adolescent obesity and puberty: the “perfect storm”. Annals of the New York Academy of Sciences. 2008;1135:265–279. doi: 10.1196/annals.1429.009. [DOI] [PubMed] [Google Scholar]
- 8.Adair LS. Child and adolescent obesity: epidemiology and developmental perspectives. Physiology & Behavior. 2008;94:8–16. doi: 10.1016/j.physbeh.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Pischon T, Nothlings U, Boeing H. Obesity and cancer. Proceedings of the Nutrition Society. 2008;67:128–145. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]
- 10.Morris MJ. Cardiovascular and metabolic effects of obesity. Clinical & Experimental Pharmacology & Physiology. 2008;35:416–419. doi: 10.1111/j.1440-1681.2008.04912.x. [DOI] [PubMed] [Google Scholar]
- 11.Griffin KAK, Holly, Bidani, Anil K. Adverse renal consequences of obesity. American Journal of Physiology - Renal Physiology. 2008;294:F685–F696. doi: 10.1152/ajprenal.00324.2007. [DOI] [PubMed] [Google Scholar]
- 12.Levin BE, et al. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273:R725–R730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 13.Gorski JN, Dunn-Meynell AA, Levin BE. Maternal obesity increases hypothalamic leptin receptor expression and sensitivity in juvenile obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1782–R1791. doi: 10.1152/ajpregu.00749.2006. [DOI] [PubMed] [Google Scholar]
- 14.Levin BE, et al. Abnormalities of leptin and ghrelin regulation in obesity-prone juvenile rats. Am J Physiol. 2003;285:E949–E957. doi: 10.1152/ajpendo.00186.2003. [DOI] [PubMed] [Google Scholar]
- 15.Irani BG, Dunn-Meynell AA, Levin BE. Altered hypothalamic leptin, insulin and melanocortin binding associated with moderate fat diet and predisposition to obesity. Endocrinology. 2007;148:310–316. doi: 10.1210/en.2006-1126. [DOI] [PubMed] [Google Scholar]
- 16.Majdic G, et al. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- 17.Dhillon J, et al. Leptin directly activates SF1 neurons in the VMH and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Gorski JN, et al. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistence. Am J Physiol Regul Integr Comp Physiol. 2006;291:R768–R778. doi: 10.1152/ajpregu.00138.2006. [DOI] [PubMed] [Google Scholar]
- 19.Miki T, et al. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nature Neurosci. 2001;4:507–512. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- 20.Ngarmukos C, Baur EL, Kumagai AK. Co-localization of GLUT1 and GLUT4 in the blood-brain barrier of the rat ventromedial hypothalamus. Brain Res. 2001;900:1–8. doi: 10.1016/s0006-8993(01)02184-9. [DOI] [PubMed] [Google Scholar]
- 21.Kang L, et al. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes. 2006;55:412–420. doi: 10.2337/diabetes.55.02.06.db05-1229. [DOI] [PubMed] [Google Scholar]
- 22.Flanagan-Cato LM, et al. Food restriction alters neuronal morphology in the hypothalamic ventromedial nucleus of male rats. Endocrinology. 2008 doi: 10.1210/en.2007-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinto S, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 24.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 25.Bouret SG, et al. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab. 2008;7:179–185. doi: 10.1016/j.cmet.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin BE. Reduced paraventricular nucleus norepinephrine responsiveness in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 1996;270:R456–R461. doi: 10.1152/ajpregu.1996.270.2.R456. [DOI] [PubMed] [Google Scholar]
- 27.Levin BE, Hogan S, Sullivan AC. Initiation and perpetuation of obesity and obesity resistance in rats. Am J Physiol. 1989;256:R766–R771. doi: 10.1152/ajpregu.1989.256.3.R766. [DOI] [PubMed] [Google Scholar]
- 28.Calizo LH, Flanagan-Cato LM. Estrogen selectively induces dendritic spines within the dendritic arbor of rat ventromedial hypothalamic neurons. J Neurosci. 2000;20:1589–1596. doi: 10.1523/JNEUROSCI.20-04-01589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calizo LH, Flanagan-Cato LM. Estrogen-induced dendritic spine elimination on female rat ventromedial hypothalamic neurons that project to the periaqueductal gray. J Comp Neurol. 2002;447:234–248. doi: 10.1002/cne.10223. [DOI] [PubMed] [Google Scholar]
- 30.Flanagan-Cato LM, et al. Sexual behavior induces the expression of activity-regulated cytoskeletal protein (Arc) and modifies neuronal morphology in the female rat ventromedial nucleus. J Neuroendocrinology. 2006;18:857–864. doi: 10.1111/j.1365-2826.2006.01483.x. [DOI] [PubMed] [Google Scholar]
- 31.Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2002;283:R941–R948. doi: 10.1152/ajpregu.00245.2002. [DOI] [PubMed] [Google Scholar]
- 32.Patterson CM, Dunn-Meynell AA, Levin BE. Three weeks of early onset exercise prolongs obesity resistence in DIO rats after exercise cessation. Am J Physiol. 2008;294:R290–R301. doi: 10.1152/ajpregu.00661.2007. [DOI] [PubMed] [Google Scholar]
- 33.Rios M, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity an hyperactivity. Mol Endocrinology. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 34.Komori T, et al. Induction of brain-derived neurotrophic factor by leptin in the ventromedial hypothalamus. Neuroscience. 2006;139:1107–1115. doi: 10.1016/j.neuroscience.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 35.Wang C, et al. Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1037–R1045. doi: 10.1152/ajpregu.00125.2007. [DOI] [PubMed] [Google Scholar]
- 36.Griffin GD, Flanagan-Cato LM. Sex differences in the dendritic arbors of neurons in the hypothalamic ventromedial nucleus. Physiology and Behavior. 2009 doi: 10.1016/j.physbeh.2009.02.019. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elias CF, et al. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol. 2000;423:261–281. [PubMed] [Google Scholar]
- 38.Luiten PGM, et al. Differential input from the amygdaloid body to the ventromedial hypothalamic nucleus in the rat. Neurosci Lett. 1983;35:253–258. doi: 10.1016/0304-3940(83)90326-9. [DOI] [PubMed] [Google Scholar]
- 39.Fahrbach SE, Morrell JI, Pfaff DW. Studies of ventromedial hypothalamic afferents in the rat using three methods of HRP application. Exp Brain Res. 1989;77:221–233. doi: 10.1007/BF00274980. [DOI] [PubMed] [Google Scholar]
- 40.Ter Horst GJ, Luiten PGM. Phaseolus vulgaris leuco-agglutinin tracing of intrahypothalamic connections of the lateral, ventromedial, dorsomedial and paraventricular hypothalamic nuclei in the rat. Brain Res Bull. 1987;18:191–203. doi: 10.1016/0361-9230(87)90190-0. [DOI] [PubMed] [Google Scholar]
- 41.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- 42.Musatov S, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Nat Acad Sci (USA) 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Millhouse OE. Certain ventromedial hypothalamic afferents. Brain Res. 1973;55:89–105. doi: 10.1016/0006-8993(73)90490-3. [DOI] [PubMed] [Google Scholar]
- 44.Levin BE, et al. Ventromedial hypothalamic glucokinase is an important mediator of the counterregulatory response to insulin-induced hypoglycemia. Diabetes. 2008;57:1371–1379. doi: 10.2337/db07-1755. [DOI] [PubMed] [Google Scholar]
- 45.Powley TL. The ventromedial hypothalamic syndrome, satiety, and a cephalic phase hypothesis. Psychol Rev. 1977;84:89–126. [PubMed] [Google Scholar]
- 46.Buijs RM, et al. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol. 2001;431:405–423. doi: 10.1002/1096-9861(20010319)431:4<405::aid-cne1079>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 47.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signalling before obesity onset. Am J Physiol Regul Integr Comp Physiol. 2004;286:R143–R150. doi: 10.1152/ajpregu.00393.2003. [DOI] [PubMed] [Google Scholar]
- 48.Griffin GD, Flanagan-Cato LM. Estradiol and progesterone differentially regulate the dendritic arbor of neurons in the hypothalamic ventromedial nucleus of the female rat (Rattus norvegicus) J Comp Neurol. 2008;510:631–640. doi: 10.1002/cne.21816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. London: Academic Press, Ltd; 1986. [DOI] [PubMed] [Google Scholar]