Abstract
Hematophagous arthropods such as Triatoma infestans, the vector of Trypanosoma cruzi, elicit host-immune responses during feeding. Characterization of antibody responses to salivary antigens offers the potential to develop immunologically based monitoring techniques for exposure to re-emergent triatomine bug populations in peridomestic animals. IgG-antibody responses to the salivary antigens of T. infestans have been detected in chickens as soon as 2 days after the first exposure to five adult bugs. Chickens and guinea pigs regularly exposed to this number of triatomines showed a significantly lower anti-saliva antibody titre than animals exposed to 25 adults and fifth instars of four different T. infestans strains originating from Bolivia and from Northern Chile. Highly immunogenic salivary antigens of 14 and 21 kDa were recognised by all chicken sera and of 79 kDa by all guinea pig sera. Cross-reactivity studies using saliva or salivary gland extracts from different hematophagous species, e.g. different triatomines, bed bugs, mosquitoes, sand flies and ticks, as well as chicken sera exposed to triatomines and mosquitoes, demonstrated that the 14 and 21 kDa salivary antigens were only found in triatomines. Sera from peridomestic chickens and guinea pigs in sites of known T. infestans challenge in Bolivia also recognised the 14 and 21 kDa antigens. These represent promising epidemiological markers for the detection of small numbers of feeding bugs and hence may be a new tool for vector surveillance in Chagas disease control programs.
Keywords: Antibody responses, Chagas disease, Chickens, Guinea pigs, Salivary proteins, Surveillance, Triatoma infestans, Cross-reactivity
1. Introduction
Chagas disease is caused by the protozoan parasite Trypanosoma cruzi and is transmitted by triatomine bugs (Hemiptera), of which Triatoma infestans is the most effective vector (Schaub, 2008). Triatoma infestans is well adapted to the human environment and feeds as an obligate hematophagous insect mainly on peridomestic and domestic hosts such as chickens, guinea pigs, dogs and cats, as well as humans. The control strategy has mainly been based on vector control using insecticides in houses and nearby animal holdings as well as the control of transmission of T. cruzi by blood transfusion (Dias and Schofield, 1999; Ramsey and Schofield, 2003). As a result of extensive control programs such as the “Southern Cone Initiative” in endemic Latin American countries, the incidence of Chagas disease has been reduced from an estimated 20 million people in the 1980s to a recent estimate of less than 8 million (WHO, 2007). Countries such as Brazil, Uruguay, Chile, Paraguay, Southern Bolivia, parts of Argentina and Southern Peru have been formally certified free of human Chagas disease transmission by T. infestans (Schofield et al., 2006).
Nevertheless, Chagas disease is not fully controlled and re-emergence is a continuous threat. High rates of T. cruzi transmission are still apparent in many endemic and newly populated areas such as Bolivia with infection rates of up to 90%, and several of the endemic countries have yet to develop serious large-scale surveillance and intervention programs (Dias et al., 2002; Ramsey and Schofield, 2003; Chippaux et al., 2008). In previously controlled and Chagas disease-free areas of Argentina and Uruguay, there is still a risk of recrudescence due to a dramatic reduction in surveillance activities (Gürtler et al., 2007). Moreover, after elimination of domestic species, related peridomestic or sylvatic bug populations will still exist. These conspecific populations may replace the previous domestic populations due to changes in the ecological balance (Cecere et al., 2002). Additionally, a new challenge is presented by pyrethroid-resistant T. infestans bugs which persisted after insecticide spraying in Argentina (Gürtler et al., 2007; Toloza et al., 2008). However, the main challenge is the rapid re-infestation of triatomines after insecticide spraying (Gürtler et al., 2007; Toloza et al., 2008). New methodologies are required to detect re-emerging T. infestans populations at an early stage and for sustained, long-term monitoring of previously endemic Chagas disease regions (Schofield et al., 2006).
The saliva of hematophagous arthropods contains a complex mixture of proteins with biological activity. These include modification of the humoral and cell-mediated host immune response, as well as hemostatic responses such as vasoconstriction, blood coagulation and platelet aggregation (Ribeiro, 1995; Kalvachova et al., 1999; Nascimento et al., 2001; Champagne, 2005; Rohousova et al., 2005b; Billingsley et al., 2006). Salivary proteins also elicit an antibody response in their hosts, and this has been used as an epidemiological tool and biological marker of exposure to disease vectors including mosquitoes, ticks, tsetse flies and sand flies (Schwartz et al., 1991, 1993; Sanders et al., 1998; Lane et al., 1999; Barral et al., 2000; Inokuma et al., 2000; Gomes et al., 2002; Rohousova et al., 2005a; Cornelie et al., 2007; Poinsignon et al., 2007; Hostomska et al., 2008; Volf et al., 2008). Furthermore, these antibody responses to the saliva can be also used as markers for transmission risk of infectious disease agents (Schwartz et al., 1991; Drakeley et al., 2005; Remoue et al., 2006; Rohousova and Volf, 2006). As shown previously, an antibody response is detectable in mice after exposure to low numbers of triatomines (Volf et al., 1993). We therefore predict that a similar anti-salivary antigen-specific response in the peridomestic hosts of triatomines, such as chickens and guinea pigs, may indicate a recent exposure to triatomine bites and be a potential measure of transmission risk of Chagas disease.
In this study, we analyze the IgG antibody response of chickens and guinea pigs to the saliva of T. infestans. In particular, we aim to identify salivary antigens which induce strong antibody responses in chickens and guinea pigs after a few feeding events of a low number of T. infestans. These antigens will be compared with salivary gland proteins recognised by sera samples from hosts collected in endemic Chagas disease areas and will be evaluated for their usefulness as epidemiological markers to detect low-level infestations of T. infestans.
2. Materials and methods
2.1. Triatomines, bed bugs, mosquitoes, sand flies and ticks: Origin, maintenance, saliva and salivary gland collection
Four different strains of T. infestans were used for this study. Three strains originated from Bolivia: one domestic strain from the city of Cochabamba (Coch), department of Cochabamba, collected in 2003; one sylvatic strain from Pasacaya (Pasa), province of Mizque, department of Cochabamba, from 2003 and one sylvatic strain from the Pampa Soyco (PaSo), province Esteban Arze, of the department of Cochabamba, collected in 2004. The fourth domestic strain originated from Northern Chile (Chile), the Cachiyuyo village at the border of the provinces Atacama and Coquimbo (Schaub and Schottelius, 1984; Kollien and Schaub, 1998). Triatoma brasiliensis and Triatoma sordida were originally collected from a chicken house from Síeo do Cleniro and Bairro Sao Sosó in the state of Piauí, Brazil (by G.A. Schaub). Rhodnius prolixus originated from Colombia and Panstrongylus megistus were obtained from colonies from the Instituto Oswaldo Cruz from Brazil (J. Jurberg, Departamento de Entomologia, Instituto Oswaldo Cruz, Rio de Janeiro, Brazil). All triatomines were reared at 26 ± 1 °C, 60–70% relative humidity with a 16/8 h light/dark cycle and regularly fed on chickens (Schaub, 1989). Pooled saliva was obtained from about 300 starved fifth instars and adults using capillary pipettes (Amino et al., 2001). Typically volumes from 0.5 to 1 μl were obtained from each bug. The protein concentration was determined using a BCA Protein Assay Kit (Perbio Science) according to the manufacturer’s instructions and the saliva was stored at −80 °C until use.
Adult Cimex lectularius were provided by Bayer CropScience, Monheim, Germany and were reared at 27 °C with 80% relative humidity and fed on anesthetised guinea pigs. Mosquitoes and sand flies were reared at the insectaries of the Laboratory of Malaria and Vector Research, National Institutes of Health, Rockville, MD, USA. Anopheles freeborni, Aedes aegypti and Culex quinquefasciatus were reared at 28 °C, 75% relative humidity and a 12/12 h light/dark cycle. Adult mosquitoes were fed with 10% sucrose solution for maintenance and blood-fed on anesthetised BALB/c mice (Dimopoulos et al., 1997, 1998). Phlebotomus dubosqui (Baraoueli Health District, Segou Region, Mali, Africa) and Lutzomyia longipalpis (Jacobina strain, Brazil) were reared according to Modi and Tesh (1983) with modifications. Briefly, adult sand flies were maintained at 26 °C and 70% relative humidity with a 14/10 h light/dark cycle, and either fed with 30% fructose solution or on anesthetised BALB/c mice (used for P. dubosqui) or C57Bl/6 mice (used for L. longipalpis).
Pathogen-free Dermacentor variabilis and Amblyomma americanum adults as well as Ixodes scapularis nymphs were obtained from tick colonies maintained at Oklahoma State University, Stillwater, OK, USA. Ticks were maintained at 24 °C and 90% relative humidity under a 14/10 h light/dark cycle and fed for 3 days on 6-week-old outbred female albino Hartley guinea pigs (Charles River Laboratories, Wilmington, MA, USA).
Ten pairs of salivary glands from starved adult bed bugs, 40 pairs from starved adult female mosquitoes aged 6 days, 35 gland pairs of 5–7 days old female sand flies after emergence, three salivary gland pairs of adult ticks (D. variabilis, A. americanum) and 20 pairs of nymphal ticks (I. scapularis) after 3 days of feeding were transferred to PBS. Salivary glands were disrupted by sonication and centrifuged at 12,000g for 5 min. The protein concentration of the supernatant was measured using the NanoDrop™ spectrophotometer (Thermo Scientific) and the samples stored at −80 °C until used.
2.2. Exposure of chickens and guinea pigs to triatomines
To determine the earliest IgG response to salivary proteins of T. infestans, five chickens (6 months old) were exposed to five starved adult T. infestans (Chile strain) for 1 h. Animals were then bled daily for 5 days. Blood samples were processed as described below.
For the long-term study of the antibody response of animals exposed to a low or high number of T. infestans, 12 chickens (6 months old) and 10 guinea pigs (4 months old) were subject to blood-feeding by four different strains of T. infestans according to the regimen outlined in Table 1. Feeding took place every 2 weeks over a period of 1 h or 30 min for the chickens and guinea pigs, respectively. All four T. infestans strains were represented in challenges and each experimental animal was always challenged with the same strain of bugs. Prior to the first triatomine feeding, pre-exposure serum was taken from each animal as a negative control. For the positive control pooled chicken serum was obtained from animals used for the maintenance of different triatomine species in the Zoology/Parasitology Group, Ruhr-University of Bochum, Germany. These chickens had been subject to a minimum of 6 months exposure to bugs.
Table 1.
Allocation of chickens and guinea pigs to different Triatoma infestans strains during the serial feeding experiment. Twelve chickens and 10 guinea pigs were each split into two groups of the same number of animals which were either exposed to a small number of bugs (five adult T. infestans; low exposure group) or a high number of bugs (five adults and 20 fourth and fifth instars; high exposure group) every 2 weeks for 24 weeks (chickens) or 20 weeks (guinea pigs). Four different strains of T. infestans, three Bolivian (Coch, Pasa, PaSo) and one Chilean strain (Chile), were used and the same strain was fed on the same animal throughout the experiment.
| Animal exposed to T. infestans | Strain of T. infestans used for feeding | Number of animals in the low exposure group | Number of animals in the high exposure group |
|---|---|---|---|
| Chicken | Coch | 1 | 1 |
| Pasa | 1 | 2 | |
| PaSo | 3 | 1 | |
| Chile | 1 | 2 | |
| Guinea pig | Coch | 1 | 1 |
| Pasa | 1 | 1 | |
| PaSo | 2 | 1 | |
| Chile | 1 | 2 |
Additionally, groups of three chickens (age 6 months) were exposed to five adults of T. brasiliensis, T. sordida, R. prolixus and P. megistus per chicken, respectively, over a period of 1 month following the schedule of the long-term challenge of T. infestans as described above.
Blood samples were taken from all chickens (from the brachial vein) and guinea pigs (from the ear vein after isofluorane anaesthesia) 5 days after each triatomine feeding, centrifuged at 10,000g for 10 min at room temperature and the sera were stored at −20 °C until used. All animal procedures at the Ruhr-University of Bochum were licensed by the Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, Recklinghausen, Germany. All animal procedures at the University of Aberdeen, UK, were approved by the University of Aberdeen Ethical Review Committee and licensed by the UK Home Office, and all animal studies at the National Institutes of Allergy and Infectious Diseases (NIAID) were approved by the Animal Care and Use Committee at NIAID, Bethesda, MD, USA.
2.3. Exposure of chickens to mosquito species
An. freeborni, Ae. aegypti and Cx. quinquefasciatus were fed once weekly on chickens for a period of 1 month until all insects were fully engorged (approximately 500 insects/animal). After the last exposure, the animals were bled and the blood processed as described above (Section 2.2.).
2.4. Collection of serum samples from chickens and guinea pigs from Bolivia
Blood samples from peridomestic animals, 18 chickens and 26 guinea pigs, were collected from houses with known T. infestans infestation in rural villages in the department of Cochabamba, Bolivia; Sipe Sipe (17°27′2.784″S, 66°21′38.914″W, 2555m): five chickens, 10 guinea pigs; Lipez (17°33′47.127″S, 66°15′27.643″W, 2542m): five chickens, nine guinea pigs and Arpita (17°33′51.622″S, 66°4′15.049″W, 718 m): eight chickens, seven guinea pigs. Blood was obtained and processed as described above (Section 2.2.).
2.5. ELISA
Concentrations of anti-saliva IgG in chicken and guinea pig sera were measured by ELISA. Ninety-six well plates (Immunolon, Nunc) were coated with 0.5 μg salivary protein of T. infestans per well in 50 μl carbonate buffer (35 mM NaHCO3, 9mM Na2CO3, pH 9.6) overnight at 4 °C. After three washes in 200 μl PBST (PBS (137 mM NaCl, 8.1 mM Na2HPO4, 2.68 mM KCl, 1.47 mM KH2PO4, pH 7.3) with 0.05% Tween 20), the plates were blocked with 100 μl 2% dried skimmed milk in PBS for 1 h at 37 °C. The plates were washed again three times with PBST and incubated with 100 μl guinea pig or chicken serum diluted 1:100 in PBST with 2% dried skimmed milk for 1 h at 37 °C. Each serum sample was analyzed in triplicate and each plate included a positive and negative control (see Section 2.2.) and a secondary antibody control (2% dried skimmed milk in PBST was added instead of sera, allowing assessment of non-specific secondary antibody binding). After washing three times with PBST, the wells were incubated with either 100 μl peroxidase-conjugated rabbit anti-chicken IgY or rabbit anti-guinea pig IgG (Sigma–Aldrich) diluted 1:20,000 in PBST with 2% dried skimmed milk for 1 h at 37 °C. After a further three washes, plates were developed using 200 μl o-phenylenediamine dihydrochloride solution (Sigma fast™, Sigma–Aldrich). The reaction was stopped after 30 min incubation in the dark at room temperature with 50 μl 3M H2SO4, and the OD of absorbance at 492 nm was read using a VersaMax microplate spectrophotometer (Molecular Devices). Every experiment was repeated and the final OD492nm was determined by calculating the mean OD492nm of the triplicate wells in two experiments, respectively, and subtracting the OD492nm of the negative control.
2.6. Protein electrophoresis and Western blotting
Salivary proteins were separated by SDS–PAGE on 15% acrylamide gels under reducing conditions using the Hoefer SE 600 apparatus (GE Healthcare) (Laemmli, 1970). For the triatomine species, each lane contained 80 μg protein from crude saliva. For the other arthropod species 80 μg of salivary gland extract was applied. Proteins were denatured for 5 min at 95 °C in loading buffer and resolved at 150 V for 50 min followed by 300 V for 1.5 h. After electrophoresis, silver staining was carried out as described by Heukeshoven and Dernick (1985). Molecular weights were calculated using ImageMaster™ 1D Elite software, version 4.0 (GE Healthcare) with reference to the mobility of standard proteins (Prestained Protein Marker, New England Biolabs) included in one lane of the gel.
The proteins of a duplicate gel were transferred for Western blotting onto a nitrocellulose membrane using a Mini Trans-Blot cell (Bio-Rad Laboratories) at 200 mA for 1.5 h. After the transfer, the membrane was incubated with 5% dried skimmed milk in PBS overnight at 4 °C, was washed three times in PBST and placed on a Mini-Protean II Multiscreen apparatus (Bio-Rad Laboratories). The membrane was incubated with appropriate chicken or guinea pig sera diluted 1:100 in PBST with 5% dried skimmed milk for 2 h at room temperature in each channel of the Multiscreen apparatus. Sera of chickens and guinea pigs prior to exposure to bug bites were used as negative controls. The membrane was removed from the apparatus after three washes with PBST and again washed in PBST. Afterwards, the blot was incubated with peroxidase-conjugated rabbit anti-chicken IgY or rabbit anti-guinea pig IgG as secondary antibody (Sigma–Aldrich) diluted 1:20,000 in PBST with 5% dried skimmed milk for 1 h at room temperature followed by three washes in PBST. The proteins were visualised using Roti-Lumin chemiluminescence substrate (Roth). The membranes were exposed onto X-ray film in a dark room and developed using a Konica SRX-101A Medical Film Processor (Fischer–Peinemann).
Five different experiments were carried out by Western blotting. (1) Serum samples from 12 chickens and 10 guinea pigs exposed to T. infestans (low and high exposures) were tested individually per feeding event to track the recognition of salivary proteins over time and per exposure group (Section 2.2.). (2) To test the degree of cross-reactivity between the different T. infestans strains individual serum samples of the last exposures of 12 chickens (week 24) or 10 guinea pigs (week 20, low and high exposure to the four T. infestans strains) were used. (3) Salivary proteins or salivary gland extracts of all arthropods except T. infestans were transferred onto nitrocellulose membranes and incubated with pooled sera from the last exposure of 12 chickens or 10 guinea pigs to T. infestans. (4) Salivary proteins of T. infestans (Chile strain) were separated by electrophoresis and the membranes with the transferred proteins incubated with pooled chicken sera from the last exposure (4 weeks; Section 2.2.) to the triatomines T. brasiliensis, T. sordida, R. prolixus and P. megistus (Section 2.2.) or to the mosquitoes An. freeborni, Ae. aegypti and Cx. quinquefasciatus (Section 2.3.). (5) Chicken and guinea pig sera from different villages of Bolivia were tested for their reactivity to salivary proteins of T. infestans (Section 2.4.).
2.7. Data analysis
Analysis of data was performed using SigmaStat 3.1 (Systat Software). An ANOVA with a Pairwise Multiple Comparison Procedure (Holm–Sidak test) was carried out to determine the effect of feeding different numbers of bugs (low and high exposure) on chickens and guinea pigs in repeated experiments over time. The level of significance was P ≤ 0.05. For group comparison (low and high exposure groups) of the antibody responses of the chickens and guinea pigs by ELISA, the unpaired Student’s t-test was used, analysing significant differences among means of data with normal distribution. Variables of non-normal distribution were compared using the Mann–Whitney Rank Sum test. P-values of 0.05 or less were considered statistically significant.
3. Results
3.1. Development of immune response to salivary antigens during exposure to T. infestans
Antibodies to total salivary protein of T. infestans were detectable in all five chickens as early as 2 days after a single challenge with five bugs (mean OD of 0.015). In the long-term study, after 5 days an IgG response was measured in sera from the low and high exposure chicken groups (mean OD of 0.055 and 0.065, respectively, Fig. 1a). At this time point a weak response was also detected in guinea pig sera (mean OD of 0.008 and 0.009 for the low and high exposure group, respectively, Fig. 1b). In chickens and guinea pigs antibody responses increased with serial exposure to T. infestans, but the antibody response of the guinea pigs (maximum mean OD of 1.008 and 2.045 for the low and high exposure group, respectively, Fig. 1b) increased more rapidly and reached a higher level than in chickens (maximum mean OD of 0.6194 and 1.8576 for the low and high exposure group, respectively, Fig. 1a). Animals exposed to a low number of T. infestans bugs showed a significantly lower antibody response than animals exposed to a high number of bugs (One Way Repeated Measures ANOVA: P < 0.001 for both chickens and guinea pigs).
Fig. 1.

IgG response of 12 chickens (a) and 10 guinea pigs (b) to the saliva of Triatoma infestans. The results are presented as mean ELISA O.D.s from the low (open squares, six chickens or five guinea pigs were each exposed to five adult T. infestans per feeding event) and the high (closed squares, six chickens or five guinea pigs were each exposed to 25 T. infestans (five adults and 20 fourth and fifth instars) per feeding event) exposure group. Animals were exposed to T. infestans every 2 weeks and always monitored 5 days later until 24 weeks (chickens) or 20 weeks (guinea pigs) of exposure. After the last feeding event the antibody response of the animals was monitored every 4 weeks (post-exposure time) until no antibody response was detectable.
The decay of anti-saliva antibody response after the last exposure of the animals to T. infestans differed between chickens and guinea pigs. In chickens, the post-challenge period began after 24 weeks of serial challenge and a significant antibody response was detectable for 16 (low exposure group) or 20 weeks (high exposure group) of the post-challenge period (Fig. 1a). In comparison, in guinea pigs the antibody response remained detectable for 24 (low exposure group) to 28 weeks (high exposure group) of the post-challenge period.
3.2. Salivary gland protein profile of T. infestans
SDS–PAGE of pooled saliva from about 300 bugs belonging to each of the four different T. infestans strains revealed a complex protein profile with relative molecular weights ranging from approximately 4 to 85 kDa (Fig. 2). The most distinct bands in all strains were at 25 and 31 kDa. While several prominent bands were shared between strains there were also variations in the intensity of bands between strains, e.g. the 14 kDa protein band was highly intense in the Pasa and PaSo strain, moderately intense in the Chile strain, and not visible in the Coch strain (Fig. 2, grey arrowhead). The band at 17 kDa was very distinct in all strains and less intense in the Coch strain (Fig. 2, black arrowhead). Additionally, strain-specific protein bands were observed such as the 7 kDa band of the Coch strain or the 5 kDa band of the Pasa strain. These results were consistently reproducible in SDS-PAGE analysis of 10 independently collected batches of saliva.
Fig. 2.
Salivary gland protein profile of four different Triatoma infestans strains: a domestic strain from the city of Cochabamba, Bolivia (Coch), two sylvatic strains from the department of Cochabamba, Bolivia (Pasa and PaSo) and a domestic strain which originated from Northern Chile (Chile). The 14 and 17 kDa salivary proteins are indicated with grey and black arrowheads, respectively.
3.3. Salivary gland antigens of T. infestans
The serum samples from the exposure time course experiment were also used to probe Western blots of T. infestans salivary proteins in order to observe the development of antigen-specific immune responses of animals exposed to low and high numbers of T. infestans. Sera from the low- and high-exposure chicken groups recognised antigenic bands of 10–79 kDa with some strain-specific variation (Table 2). As an example, Fig. 3 presents the results of a probed Western blot of SDS–PAGE resolved Chile strain salivary proteins with individual serum samples from a Chile strain challenged chicken over the time course of challenge. Responses were detected from week 1 of exposure against antigens of 14, 21 and 26 kDa. A 79-kDa protein was detected from the third exposure (week 5) until the end of the challenge. In addition, a 12-kDa polypeptide was recognised from week 13 onwards. Serum from the animal prior to exposure to T. infestans bites confirmed the specificity of the anti-saliva recognition (lane NC, Fig. 3).
Table 2.
Cross-reactions between host serum and salivary antigens of different Triatoma infestans strains.
| Triatoma infestans strain (donor of saliva) | Antigens recognised in saliva (kDa) | Detection with chicken sera exposed to the T. infestans strain |
Detection with guinea pig sera exposed to the T. infestans strain |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Coch | Pasa | PaSo | Chile | Coch | Pasa | PaSo | Chile | ||
| Coch | 82 | x | x | ||||||
| 79 | x | x | x | x | |||||
| 62 | x | x | x | x | |||||
| 59 | x | x | x | x | |||||
| 21 | x | x | x | x | |||||
| 18 | x | x | x | x | |||||
| 14 | x | x | x | x | x | x | |||
| 12 | x | x | x | x | |||||
| 10 | x | x | x | x | |||||
| Pasa | 79 | x | x | x | x | ||||
| 62 | x | x | |||||||
| 21 | x | x | x | x | |||||
| 18 | x | x | x | ||||||
| 16 | x | x | x | ||||||
| 14 | x | x | x | x | x | x | x | x | |
| PaSo | 79 | x | x | x | x | ||||
| 62 | x | x | x | x | |||||
| 26 | x | x | x | x | |||||
| 21 | x | x | x | x | |||||
| 18 | x | x | |||||||
| 15 | x | x | x | ||||||
| 14 | x | x | x | x | x | x | x | x | |
| Chile | 79 | x | x | x | x | x | x | x | x |
| 62 | x | x | x | x | |||||
| 26 | x | x | x | x | |||||
| 21 | x | x | x | x | |||||
| 14 | x | x | x | x | x | x | x | ||
| 12 | x | x | x | x | |||||
‘x’ indicates specific antibody binding to the respective salivary antigen in pairwise combinations of chicken/guinea pig sera elicited by each strain and salivary proteins of each strain.
Fig. 3.
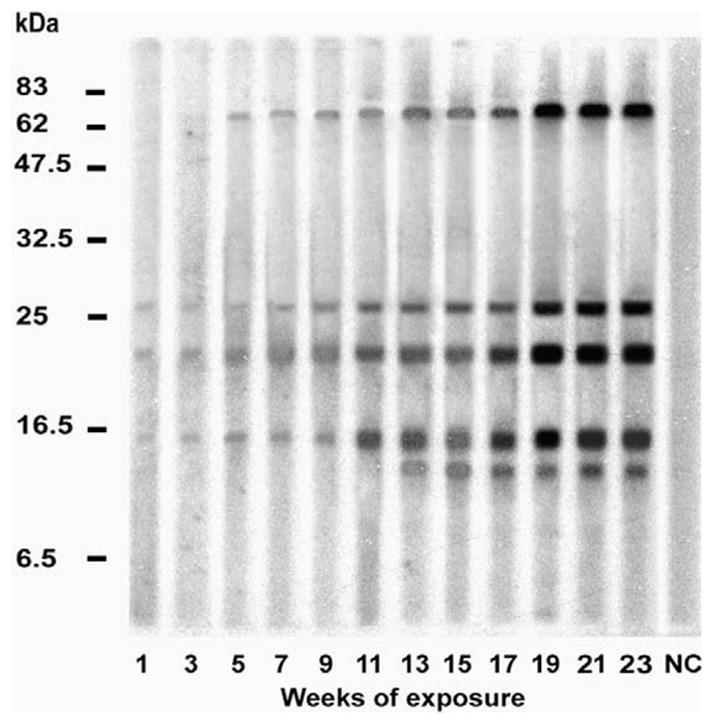
Salivary proteins of Triatoma infestans recognised by chicken sera. The figure represents a typical immunoblot using 12 sera samples from one out of six chickens from the low exposure group (five adult T. infestans exposed to each animal per feeding event). Triatoma infestans Chile strain salivary proteins were separated by electrophoresis and the immunoblot was incubated with chicken serum challenged with the Chile strain. The numbers at the foot of each lane indicate the weeks of exposure of the chickens to T. infestans. Serum from chickens prior to exposure to bug bites was used as a negative control (NC) in the experiments to demonstrate the specificity of the IgG-anti-saliva T. infestans responses.
The high and low-exposure guinea pig sera reacted with salivary antigens of 14–79 kDa with some strain-specific variation (Table 2). As an example, the time course of the evolution of Western blot detectable serum responses in Chile strain challenged guinea pigs is presented in Fig. 4, revealing bands of 14, 62, 79 and 120 kDa. Of these, the response to the 79 kDa antigen was evident from the seventh week of exposure (Fig. 4, black arrowhead), while the other antigens took a longer period of exposure, from weeks 11 and/or 13, before eliciting detectable responses.
Fig. 4.

Salivary proteins of Triatoma infestans recognised by guinea pig sera from the low exposure group. The figure represents a typical immunoblot of one out of five guinea pigs from the low exposure group (exposed to five adult T. infestans per feeding event). Triatoma infestans Chile strain saliva and guinea pig sera exposed to the Chile strain were used for the Western blot. The numbers at the foot of each lane indicate the weeks of exposure of the guinea pigs to T. infestans. Serum from guinea pigs prior to exposure to bug bites was used as a negative control (NC) in the experiments to demonstrate the specificity of the IgG-anti-saliva T. infestans responses. Arrowhead marks the 79 kDa antigen.
3.4. Cross-reactivity of antibodies against strain-specific T. infestans salivary gland proteins
In order to be of value as an infestation indicator, candidate salivary antigens must be present in all strains of T. infestans. Therefore, a series of cross-reaction experiments were carried out in which Western blots of salivary proteins from each of the four strains of T. infestans were incubated with sera from animals challenged with each strain. The 14 and 21 kDa protein bands were consistently recognised in all four strains of T. infestans by chicken sera (Table 2). Similarly, antibodies to the 79 kDa protein were present in sera of guinea pigs in all T. infestans strain combination experiments. However, the main salivary proteins of 14, 62 and 82 kDa were only recognised in particular combinations of the challenge strain and guinea pig serum, e.g. the 62 kDa protein of all strains was recognised by all guinea pig sera apart from the Pasa strain.
3.5. Cross-reactivity of antibodies to T. infestans saliva with salivary proteins of other species
Salivary proteins from other hematophagous arthropods were analyzed in Western blot experiments using pooled sera from chickens and guinea pigs challenged with T. infestans in the long-term study (Fig. 5a and b). Triatomine saliva contained the most cross-reacting proteins. Immune-chicken sera recognised nine and 15 protein bands in T. brasiliensis and T. sordida saliva, respectively, followed by seven cross-reacting salivary proteins in the hemipteran C. lectularius (Fig. 5a). The other insects shared up to three cross-reacting proteins with the triatomines. In particular, chicken antibodies to 14 and 21 kDa T. infestans salivary proteins cross-reacted with salivary proteins from the other triatomine species and in particular the 14 kDa protein was triatomine-specific (Fig. 5a, grey and black arrowhead). Similarly, guinea pig antibodies to the 79 kDa protein of T. infestans cross-reacted with three out of four triatomine species (T. brasiliensis, T. sordida and P. megistus, Fig. 5b). However, this protein may not be triatomine-specific as immune chicken sera recognise a 79 kDa salivary gland protein in C. lectularius and An. freeborni (Fig. 5a, white arrowhead).
Fig. 5.
Cross-reactivity of salivary gland proteins between different blood-sucking arthropods. Pooled animal sera from chickens (a) and guinea pigs (b) after 24 and 20 weeks of exposure to Triatoma infestans bites, respectively, were tested with either crude saliva from different triatomines (Triatoma brasiliensis (Tb), Triatoma sordida (Ts), Rhodnius prolixus (Rp) and Panstrongylus megistus (Pm)), or salivary gland protein extract from different blood-sucking arthropods, in particular Cimex lectularius (Cl), Anopheles freeborni (Af), Aedes aegypti (Aa), Culex quinquefasciatus (Cq) and Phlebotomus dubosqui (Pd). All Western blots included a negative control for each animal species. Negative Western blot results from Lutzomyia longipalpis, Dermacentor variabilis, Amblyomma americanum and Ixodes scapularis as well as negative controls are not shown. The cross-reacting 14, 21 and 79 kDa antigens are marked with grey, black and white arrowheads, respectively.
In addition, the specificity of T. infestans salivary proteins as triatomine exposure markers was evaluated in Western blots probed with sera from chickens challenged with four different triatomines (T. brasiliensis, T. sordida, R. prolixus, P. megistus) and three mosquito species (An. freeborni, Ae. aegypti, Cx. quinquefasciatus). Sera from chickens exposed to the other triatomines all gave a similar pattern of binding with recognition of T. infestans salivary proteins mainly between 30 and 14 kDa (Fig. 6). Of these, a 14 kDa protein was recognised by chicken sera from all triatomine challenges and a 21 kDa protein was also detected in all challenges with the exception of P. megistus (Fig. 6, grey and black arrowheads). Additionally, a 17 kDa protein reacted with all triatomines and An. freeborni. A 79 kDa protein was only detected by T. sordida-challenged chicken sera and by An. freeborni and Ae. aegypti (Fig. 6, white arrowhead). Mosquito-challenged chicken sera only weakly recognised T. infestans salivary proteins not cross-reacting with the triatomine-specific antigens.
Fig. 6.

Cross-reactivity of salivary proteins between Triatoma infestans, different triatomines and mosquito species. Pooled chicken sera after the last exposure (4 weeks) of three chickens exposed weekly to triatomines (Triatoma brasiliensis (Tb), Triatoma sordida (Ts), Rhodnius prolixus (Rp), Panstrongylus megistus (Pm)) or mosquitoes (Anopheles freeborni (Af), Aedes aegypti (Aa), Culex quinquefasciatus (Cq)) were tested in Western blots with salivary proteins of T. infestans (Chile strain). All Western blots included a negative control of chicken serum prior to bug exposure (NC). The cross reacting 14, 21 and 79 kDa antigens are marked with grey, black and white arrowheads, respectively.
3.6. Anti-salivary gland antibody responses in chickens and guinea pigs under natural challenge by T. infestans
To further evaluate the potential of salivary antigens as markers of T. infestans exposure, we carried out Western blot analyses of the anti-salivary responses in peridomestic animals exposed to triatomines in three different Bolivian villages. Fig. 7 presents an example of Western blots probed with a subset of four chicken and five guinea pig samples collected in four different Bolivian villages. The 14 and 12 kDa protein (Fig. 7, 14 kDa: grey arrowhead) were recognised by chicken sera from all villages and the 79, 28, 26, 21 and 6 kDa antigens were only detected in sera from a subset of villages, e.g. the 21 kDa protein (Fig. 7, black arrowhead) only by sera from chickens from Lipez and Sipe Sipe. Similarly, a 79 kDa protein (Fig. 7, white arrowhead) was detected in Western blots by guinea pig sera from all villages, while responses to the 14 and 12 kDa proteins were only detected in animals from a subset of villages.
Fig. 7.

Immunoblots of Triatoma infestans (Chile strain) salivary proteins recognised by chicken and guinea pig sera from Bolivia. Chicken (18) and guinea pig (26) sera were collected from households in rural villages in the department of Cochabamba in Bolivia. A representative subset of four individual chicken sera and five guinea pig sera is shown. The village origins were Lipez (Li), Arpita (Ar) and Sipe Sipe (Si). Laboratory sera of chickens and guinea pigs prior to exposure to bug bites were used as negative controls (NC). Grey, black and white arrowheads mark the 14, 21 and 79 kDa antigens, respectively.
4. Discussion
In addition to its multiple pharmacophysiological effects in facilitating blood-feeding, the saliva of hematophagous arthropods elicits antibody responses which have potential as epidemiological indicators of exposure. These approaches depend on the identification of salivary antigens which cause a predictable and non-variable response within the host population, and ultimately will benefit from the development of highly immunogenic recombinant antigens rather than the use of the entire crude saliva of insects (Sanders et al., 1998; Orlandi-Pradines et al., 2007).
Antibody responses to salivary proteins to triatomines were studied in previous investigations to address different immunological issues (Fox and Bayona, 1968; Marshall, 1982; Chapman et al., 1986; Pinnas et al., 1986; Volf et al., 1993; Nascimento et al., 2001). In this investigation we present data on specific antibody responses of two typical peridomestic hosts of the triatomine bug T. infestans, chickens and guinea pigs, under high and low exposure conditions. We identified the main immunogenic salivary antigens and evaluated their potential as exposure markers using naturally exposed hosts from Bolivia.
While protein gel electrophoresis demonstrated a complex mixture of proteins in T. infestans saliva, the Western blot experiments indicate that only a subset of proteins is immunogenic, and the major proteins such as the 17, 25 and 31 kDa proteins did not react with serum from T. infestans challenged chickens or guinea pigs, even after 20 weeks of exposure. Although it is not known why the less distinct salivary proteins appear to be more immunogenic, similar results were described for mice exposed to T. infestans and for human responses to salivary proteins of An. gambiae (Volf et al., 1993; Cornelie et al., 2007).
Antigens recognised by chicken sera were typically of low molecular weight (10–26 kDa) compared with those which reacted with guinea pig sera (62–82 kDa). In comparison, Hecht et al. (2006) detected 27 proteins for chickens with molecular masses ranging from 21 to 104 kDa but these antigens were determined under different experimental conditions. The higher molecular weight range of antigens recognised by guinea pig sera appears to be consistent with those described in other mammalian experimental triatomine hosts such as mice (80, 100, 120 kDa), rabbits (68–79 kDa) and humans (47, 58, 77, 79 kDa) (Volf et al., 1993; Nascimento et al., 2001; Barbosa et al., 2004). Thus, the specific antigens recognised after triatomine challenge vary with host species. However, some antigens are shared between different host species such as the 79 kDa protein which is recognised by rabbits, guinea pigs and humans (Nascimento et al., 2001; Barbosa et al., 2004). It is possible that this is also recognised by mice and accounts for the 80 kDa protein found by Volf et al. (1993). While this protein also reacted with chicken and guinea pig sera from Bolivia, the response of chickens in controlled laboratory challenges was limited to animals exposed to the Chilean T. infestans strain. Differences in the saliva composition appear not only between species but also between populations as demonstrated in studies with other triatomine species (Barbosa et al., 1999, 2004; Pineda et al., 2008). In order to control for strain variation, we used four different strains of T. infestans in challenge experiments. For example, the 14 kDa protein of the Coch strain reacted with sera of guinea pigs exposed to the Coch and Pasa strains but not with sera of guinea pigs exposed to the PaSo and Chile strains, even though the same protein from the Pasa, PaSo and Chile strains of bugs were detected by nearly all guinea pig sera irrespective of the triatomine challenge strain. This complex pattern of responses is consistent with studies of rabbit sera exposed to different P. megistus strains which recognised different salivary proteins and of salivary protein maxadilan in L. longipalpis which demonstrated high levels of allelic variation and thus antigenic polymorphism in sand fly populations (Lanzaro et al., 1999; Barbosa et al., 2004; Milleron et al., 2004).
Because of this variability in immunogenicity of salivary antigens in certain strain-host combinations, it was important to identify salivary antigens which elicit an antibody response regardless of the challenge strain of T. infestans. In our study these criteria are met by the 14 and 21 kDa salivary antigens which were present in all T. infestans strains from Bolivia and Chile and were detected in sera from all of 12 independently exposed chickens in laboratory experiments. The 79 kDa antigen present in all T. infestans strains was recognised by the sera of 10 independently exposed guinea pigs.
Salivary antigens may be shared between different species of triatomines and with other hematophagous arthropods. An ideal surveillance tool would recognise the other triatomines such as T. brasiliensis, T. sordida, R. prolixus and P. megistus, as these are also vectors of Chagas disease and may invade houses after eradication of T. infestans. In cross-reaction experiments with T. infestans challenged chicken serum, only proteins of 14 and 21 kDa were detected in the saliva of the triatomines and were not recognised in the bed bugs, mosquito, sand fly or tick saliva. In a characterization of these antigens using immune serum from chickens challenged with T. brasiliensis, T. sordida, R. prolixus and P. megistus or other hematophagous arthropods, no antibodies to these antigens were elicited in mosquito challenged chickens, but all species of triatomines elicited a response to the 14 kDa antigen. The 79 kDa protein was not only recognised by guinea pig sera challenged with triatomines but also by animal sera either reacting with C. lectularius and An. freeborni salivary gland proteins or challenged with mosquito species such as An. freeborni and Ae. aegypti. Thus, it cannot be excluded that recognition of this salivary protein by guinea pigs in Bolivia results from the challenge by another species of blood-sucking arthropods even if the laboratory challenged guinea pigs were kept under controlled conditions without any exposure to insect bites.
An evaluation of the efficacy of these antigens as biological markers of exposure was performed using chicken and guinea pig sera from peridomestic sites of known bug infestation in Bolivia. All chicken sera (18) reacted with the 14 kDa protein, which, interestingly, was also recognised by the serum of guinea pigs from two out of three locations. The 21 kDa salivary protein also reacted with chicken sera from two out of three locations. The 79 kDa antigen was detected by all guinea pig sera (26) and chicken sera from two out of three locations. Thus, sera from animals under natural challenge in Bolivia indicate that antibodies to the 14, 21 and 79 kDa T. infestans salivary proteins are potential exposure markers; however, the 79 kDa protein has to be excluded as a potential candidate due to its cross-reactivity with other hematophagous arthropods.
In conclusion, this study has demonstrated the potential of salivary antigens of T. infestans for the early detection of exposure to triatomines bites even with small numbers of T. infestans using anti-saliva IgG responses of relevant peridomestic hosts. In surveillance campaigns using such antigens, re-infestation of T. infestans can be already detected 2 days after the first exposure to bug bites as demonstrated in this study. However, surveillance should not be launched within 4 (chickens used for surveillance) and 6 months (guinea pigs used for surveillance) after insecticide spraying due to the persistence of anti-saliva antibodies.
Acknowledgments
The authors are very grateful to Sabine Kindermann, Holger Schlierenkamp, Charles Soukou, Sonja Ortmann, Dr. Witold Mütze and Van My Pham for their technical assistance and to Christian Meiser for the introduction to the technology to obtain saliva of Triatoma infestans. We thank Dr. Reiner Pospischil (Bayer Crop-Science, Monheim, Germany) for providing Cimex lectularius and Dr. José Jurberg (Departamento de Entomologia, Instituto Oswaldo Cruz, Rio de Janeiro, Brazil) for providing the original stock of Panstrongylus megistus. A.S. was funded by the University of Aberdeen, UK (Sixth Century Scholarship), the Boehringer Ingelheim Fonds (BIF), Heidesheim, Germany (Travel award) and the German Academic Exchange Service (DAAD), Bonn, Germany (Short term scholarship). The support of the ‘Deutsche Forschungsgemeinschaft’ (project Scha 339/13-1) and the Humboldt Foundation to N.M.-M. is gratefully acknowledged.
References
- Amino R, Tanaka AS, Schenkman S. Triapsin, an unusual activatable serine protease from the saliva of the hematophagous vector of Chagas disease Triatoma infestans (Hemiptera: Reduviidae) Insect Biochem Mol Biol. 2001;31:465– 472. doi: 10.1016/s0965-1748(00)00151-x. [DOI] [PubMed] [Google Scholar]
- Barbosa SE, Diotaiuti L, Soares RP, Pereira MH. Differences in saliva composition among three Brazilian populations of Panstrongylus megistus (Hemiptera, Reduviidae) Acta Trop. 1999;72:91–98. doi: 10.1016/s0001-706x(98)00073-4. [DOI] [PubMed] [Google Scholar]
- Barbosa SE, Diotaiuti L, Braga EM, Pereira MH. Variability of the salivary proteins of 20 Brazilian populations of Panstrongylus megistus (Hemiptera: Reduviidae: Triatominae) Acta Trop. 2004;92:25–33. doi: 10.1016/j.actatropica.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Barral A, Honda E, Caldas A, Costa J, Vinhas V, Rowton ED, Valenzuela JG, Charlab R, Barral-Netto M, Ribeiro JM. Human immune response to sand fly salivary gland antigens: a useful epidemiological marker? Am J Trop Med Hyg. 2000;62:740–745. doi: 10.4269/ajtmh.2000.62.740. [DOI] [PubMed] [Google Scholar]
- Billingsley PF, Baird J, Mitchell JA, Drakeley C. Immune interactions between mosquitoes and their hosts. Parasite Immunol. 2006;28:143–153. doi: 10.1111/j.1365-3024.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- Cecere MC, Gürtler RE, Canale DM, Chuit R, Cohen JE. Effects of partial housing improvement and insecticide spraying on the reinfestation dynamics of Triatoma infestans in rural northwestern Argentina. Acta Trop. 2002;84:101–116. doi: 10.1016/s0001-706x(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Champagne DE. Antihemostatic molecules from saliva of blood-feeding arthropods. Pathophysiol Haemost Thromb. 2005;34:221–227. doi: 10.1159/000092428. [DOI] [PubMed] [Google Scholar]
- Chapman MD, Marshall NA, Saxon A. Identification and partial purification of species-specific allergens from Triatoma protracta (Heteroptera, Reduviidae) J Allergy Clin Immun. 1986;78:436–442. doi: 10.1016/0091-6749(86)90030-8. [DOI] [PubMed] [Google Scholar]
- Chippaux JP, Postigo JR, Santalla JA, Schneider D, Brutus L. Epidemiological evaluation of Chagas disease in a rural area of southern Bolivia. Trans R Soc Trop Med Hyg. 2008;102:578–584. doi: 10.1016/j.trstmh.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Cornelie S, Remoue F, Doucoure S, Ndiaye T, Sauvage FX, Boulanger D, Simondon F. An insight into immunogenic salivary proteins of Anopheles gambiae in African children. Malar J. 2007;6:75. doi: 10.1186/1475-2875-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias JCP, Schofield CJ. The evolution of Chagas disease (American trypanosomiasis) control after 90 years since Carlos Chagas discovery. Mem Inst Oswaldo Cruz. 1999;94 (Suppl 1):103–121. doi: 10.1590/S0074-02761999000700011. [DOI] [PubMed] [Google Scholar]
- Dias JCP, Silveira AC, Schofield CJ. The impact of Chagas disease control in Latin America: a review. Mem Inst Oswaldo Cruz. 2002;97:603–612. doi: 10.1590/s0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- Dimopoulos G, Richman A, Müller HM, Kafatos FC. Molecular immune responses of the mosquito Anopheles gambiae to bacteria and malaria parasites. Proc Natl Acad Sci USA. 1997;94:11508–11513. doi: 10.1073/pnas.94.21.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos G, Seeley D, Wolf A, Kafatos FC. Malaria infection of the mosquito Anopheles gambiae activates immune-responsive genes during critical transition stages of the parasite life cycle. EMBO J. 1998;17:6115–6123. doi: 10.1093/emboj/17.21.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakeley CJ, Corran PH, Coleman PG, Tongren JE, McDonald SLR, Carneiro I, Malima R, Lusingu J, Manjurano A, Nkya WMM, Lemnge MM, Cox J, Reyburn H, Riley EM. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci USA. 2005;102:5108–5113. doi: 10.1073/pnas.0408725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox I, Bayona IG. Circulating precipitating antibodies in the rabbit from the bites of Rhodnius prolixus as shown by agar-gel tests. J Parasitol. 1968;54:1239–1240. [PubMed] [Google Scholar]
- Gomes RB, Brodskyn C, de Oliveira CI, Costa J, Miranda JC, Caldas A, Valenzuela JG, Barral-Netto M, Barral A. Seroconversion against Lutzomyia longipalpis saliva concurrent with the development of anti-Leishmania chagasi delayed-type hypersensitivity. J Infect Dis. 2002;186:1530–1534. doi: 10.1086/344733. [DOI] [PubMed] [Google Scholar]
- Gürtler RE, Kitron U, Cecere MC, Segura EL, Cohen JE. Sustainable vector control and management of Chagas disease in the Gran Chaco, Argentina. Proc Natl Acad Sci USA. 2007;104:16194–16199. doi: 10.1073/pnas.0700863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht MM, Bussacos AC, Lozzi SP, Santana JM, Teixeira AR. Triatoma infestans chooses to feed upon immune prey. Am J Trop Med Hyg. 2006;75:893– 900. [PubMed] [Google Scholar]
- Heukeshoven J, Dernick R. Characterization of a solvent system for separation of water-insoluble poliovirus proteins by reversed-phase high-performance liquid chromatography. J Chromatogr. 1985;326:91–101. doi: 10.1016/s0021-9673(01)87434-3. [DOI] [PubMed] [Google Scholar]
- Hostomska J, Rohousova I, Volfova V, Stanneck D, Mencke N, Volf P. Kinetics of canine antibody response to saliva of the sand fly Lutzomyia longipalpis. Vector Borne Zoonotic Dis. 2008;8:443–450. doi: 10.1089/vbz.2007.0214. [DOI] [PubMed] [Google Scholar]
- Inokuma H, Ohno K, Onishi T. Is the detection of anti-Rhipicephalus sanguineus (Rs24p) antibodies a valuable epidemiological tool of tick infestation in dogs? Vet Res. 2000;31:365–369. doi: 10.1051/vetres:2000126. [DOI] [PubMed] [Google Scholar]
- Kalvachova P, Hribalova V, Kodym P, Volf P. Modulation of murine lymphocyte responsiveness by the saliva of Rhodnius prolixus (Hemiptera: Reduviidae) J Med Entomol. 1999;36:341–344. doi: 10.1093/jmedent/36.3.341. [DOI] [PubMed] [Google Scholar]
- Kollien AH, Schaub GA. The development of Trypanosoma cruzi (Trypanosomatidae) in the reduviid bug Triatoma infestans (Insecta): influence of starvation. J Eukaryot Microbiol. 1998;45:59–63. doi: 10.1111/j.1550-7408.1998.tb05070.x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane RS, Moss RB, Hsu YP, Wei T, Mesirow ML, Kuo MM. Anti-arthropod saliva antibodies among residents of a community at high risk for Lyme disease in California. Am J Trop Med Hyg. 1999;61:850–859. doi: 10.4269/ajtmh.1999.61.850. [DOI] [PubMed] [Google Scholar]
- Lanzaro GC, Lopes AH, Ribeiro JM, Shoemaker CB, Warburg A, Soares M, Titus RG. Variation in the salivary peptide, maxadilan, from species in the Lutzomyia longipalpis complex. Insect Mol Biol. 1999;8:267–275. doi: 10.1046/j.1365-2583.1999.820267.x. [DOI] [PubMed] [Google Scholar]
- Marshall N. Allergy to Triatoma protracta (Heteroptera: Reduviidae). II Antigen production in vitro. J Med Entomol. 1982;19:253–254. doi: 10.1093/jmedent/19.3.253. [DOI] [PubMed] [Google Scholar]
- Milleron RS, Mutebi JP, Valle S, Montoya A, Yin H, Soong L, Lanzaro GC. Antigenic diversity in maxadilan, a salivary protein from the sand fly vector of American visceral leishmaniasis. Am J Trop Med Hyg. 2004;70:286–293. [PubMed] [Google Scholar]
- Modi GB, Tesh RB. A simple technique for mass rearing Lutzomyia longipalpis and Phlebotomus papatasi (Diptera: Psychodidae) in the laboratory. J Med Entomol. 1983;20:568–569. doi: 10.1093/jmedent/20.5.568. [DOI] [PubMed] [Google Scholar]
- Nascimento RJ, Santana JM, Lozzi SP, Araujo CN, Teixeira AR. Human IgG1 and IgG4: the main antibodies against Triatoma infestans (Hemiptera: Reduviidae) salivary gland proteins. Am J Trop Med Hyg. 2001;65:219–226. doi: 10.4269/ajtmh.2001.65.219. [DOI] [PubMed] [Google Scholar]
- Orlandi-Pradines E, Almeras L, Denis de Senneville L, Barbe S, Remoue F, Villard C, Cornelie S, Penhoat K, Pascual A, Bourgouin C, Fontenille D, Bonnet J, Corre-Catelin N, Reiter P, Pages F, Laffite D, Boulanger D, Simondon F, Pradines B, Fusai T, Rogier C. Antibody response against saliva antigens of Anopheles gambiae and Aedes aegypti in travellers in tropical Africa. Microbes Infect. 2007;9:1454–1462. doi: 10.1016/j.micinf.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Pineda V, Montalvo E, Alvarez D, Santamaria AM, Calzada JE, Saldana A. Feeding sources and trypanosome infection index of Rhodnius pallescens in a Chagas disease endemic area of Amador County, Panama. Rev Inst Med Trop Sao Paulo. 2008;50:113–116. doi: 10.1590/s0036-46652008000200009. [DOI] [PubMed] [Google Scholar]
- Pinnas JL, Lindberg RE, Chen TMW, Meinke GC. Studies of kissing bug sensitive patients - Evidence for the lack of cross-reactivity between Triatoma protracta and Triatoma rubida salivary-gland extracts. J Allergy Clin Immun. 1986;77:364–370. doi: 10.1016/s0091-6749(86)80119-1. [DOI] [PubMed] [Google Scholar]
- Poinsignon A, Cornelie S, Remoue F, Grebaut P, Courtin D, Garcia A, Simondon F. Human/vector relationships during human African trypanosomiasis: initial screening of immunogenic salivary proteins of Glossina species. Am J Trop Med Hyg. 2007;76:327–333. [PubMed] [Google Scholar]
- Ramsey JM, Schofield CJ. Control of Chagas disease vectors. Salud Publica Mex. 2003;45:123–128. doi: 10.1590/s0036-36342003000200010. [DOI] [PubMed] [Google Scholar]
- Remoue F, Cisse B, Ba F, Sokhna C, Herve JP, Boulanger D, Simondon F. Evaluation of the antibody response to Anopheles salivary antigens as a potential marker of risk of malaria. Trans R Soc Trop Med Hyg. 2006;100:363–370. doi: 10.1016/j.trstmh.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect Agents Dis. 1995;4:143–152. [PubMed] [Google Scholar]
- Rohousova I, Ozensoy S, Ozbel Y, Volf P. Detection of species-specific antibody response of humans and mice bitten by sand flies. Parasitology. 2005a;130:493–499. doi: 10.1017/s003118200400681x. [DOI] [PubMed] [Google Scholar]
- Rohousova I, Volf P, Lipoldova M. Modulation of murine cellular immune response and cytokine production by salivary gland lysate of three sand fly species. Parasite Immunol. 2005b;27:469–473. doi: 10.1111/j.1365-3024.2005.00787.x. [DOI] [PubMed] [Google Scholar]
- Rohousova I, Volf P. Sand fly saliva: effects on host immune response and Leishmania transmission. Folia Parasit. 2006;53:161–171. [PubMed] [Google Scholar]
- Sanders ML, Jaworski DC, Sanchez JL, DeFraites RF, Glass GE, Scott AL, Raha S, Ritchie BC, Needham GR, Schwartz BS. Antibody to a cDNA-derived calreticulin protein from Amblyomma americanum as a biomarker of tick exposure in humans. Am J Trop Med Hyg. 1998;59:279–285. doi: 10.4269/ajtmh.1998.59.279. [DOI] [PubMed] [Google Scholar]
- Schaub GA. Trypanosoma cruzi: quantitative studies of the development of two strains in small intestine and rectum of the vector Triatoma infestans. Exp Parasitol. 1989;68:260–273. doi: 10.1016/0014-4894(89)90108-2. [DOI] [PubMed] [Google Scholar]
- Schaub GA. Kissing bugs. In: Mehlhorn H, editor. Encyclopedia of Parasitology. Vol. 1. Springer-Verlag; Heidelberg: 2008. pp. 684–686. [Google Scholar]
- Schaub GA, Schottelius J. Identification of trypanosomes isolated from Reduviidae from North Chile. Z Parasitenkd. 1984;70:3–9. doi: 10.1007/BF00929569. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Jannin J, Salvatella R. The future of Chagas disease control. Trends Parasitol. 2006;22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Schwartz BS, Ford DP, Childs JE, Rothman N, Thomas RJ. Anti-tick saliva antibody: a biologic marker of tick exposure that is a risk factor for Lyme disease seropositivity. Am J Epidemiol. 1991;134:86–95. doi: 10.1093/oxfordjournals.aje.a115996. [DOI] [PubMed] [Google Scholar]
- Schwartz BS, Nadelman RB, Fish D, Childs JE, Forseter G, Wormser GP. Entomologic and demographic correlates of anti-tick saliva antibody in a prospective study of tick bite subjects in Westchester County, New York. Am J Trop Med Hyg. 1993;48:50–57. doi: 10.4269/ajtmh.1993.48.50. [DOI] [PubMed] [Google Scholar]
- Toloza AC, Germano M, Cueto GM, Vassena C, Zerba E, Picollo MI. Differential patterns of insecticide resistance in eggs and first instars of Triatoma infestans (Hemiptera: Reduviidae) from Argentina and Bolivia. J Med Entomol. 2008;45:421–426. doi: 10.1603/0022-2585(2008)45[421:dpoiri]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Volf P, Grubhoffer L, Hosek P. Characterisation of salivary gland antigens of Triatoma infestans and antigen-specific serum antibody response in mice exposed to bites of T. Infestans. Vet Parasitol. 1993;47:327–337. doi: 10.1016/0304-4017(93)90033-j. [DOI] [PubMed] [Google Scholar]
- Volf P, Hostomska J, Rohousova I. Molecular crosstalks in Leishmania-sand fly-host relationships. Parasite. 2008;15:237–243. doi: 10.1051/parasite/2008153237. [DOI] [PubMed] [Google Scholar]
- WHO. New global effort to eliminate Chagas disease. Wkly Epidemiol Rec. 2007;82:259–260. [PubMed] [Google Scholar]




