Abstract
Technological advances in testing have led to the discovery of genetic variants that contribute to many illnesses including nicotine dependence. A multi-stage model of the development of nicotine dependence underlies these genetic studies, and it includes a progression through several stages of smoking behavior from never smoking to nicotine dependence. The final step in this model of dependence is the progression from established smoking behavior to the development of nicotine dependence. Contrasting individuals who smoke only a few cigarettes per day, or “chippers”, to heavy smoking, nicotine dependent subjects, focuses a genetic study on the transition from smoking to nicotine dependence. This approach has identified distinct genetic variants that contribute to nicotine dependence on chromosome 15 in the region of the α5-α3-β4 family of nicotinic receptor genes.
This region of association includes an amino acid change in the α5 nicotinic receptor protein, which is most likely a biological variant altering the risk of developing dependence. There is also evidence that other variants alter the α5 nicotinic receptor gene expression and potentially the risk of smoking. The discovery of these genetic variants and their contribution to the development of nicotine dependence highlight some of the many challenges in genetic studies. The first is that the prevalence of risk alleles can vary across populations so that a genetic risk factor can have a larger or small effect in a population depending on its frequency. The second challenge is that the risk that each genetic variant contributes in the development of a disorder is small and so it is many genes along with environmental risk factors that contribute to the development of a disorder. Interestingly, recent genetic studies of lung cancer and chronic obstructive pulmonary disease demonstrate that this same region has an important genetic influence on these disorders. Finally, there are differences in the risk of developing nicotine dependence based on gender and socioeconomic status. As our understanding of the genetic contributions of nicotine dependence increases, we may improve and personalize our treatments for smoking cessation and enhance our knowledge of other smoking related diseases in those who are at high risk for the many adverse consequences of smoking.
Keywords: Nicotine dependence, genetics, nicotinic receptor, chromosome 15
1.1 Introduction
Genetic studies uniquely provide important insights into biological pathways involved in the development of complex behavior and diseases in humans. The completion of the human genome project, coupled with rapid advances in genotyping technology, has led to a new generation of more powerful experiments that can survey the relationship between human genetic variation and disorders, and the genetic study of nicotine dependence has benefited from these advances.
2.1 Model of Nicotine Dependence for Genetic Studies
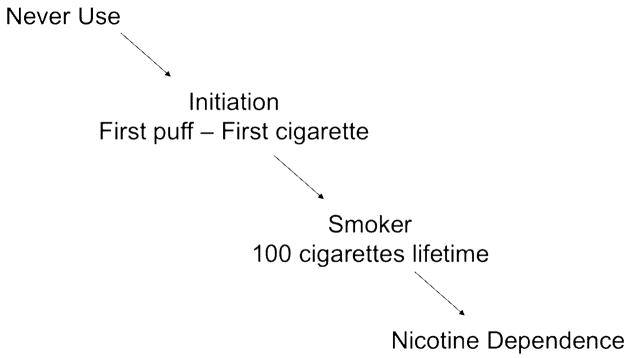
A model of stages of smoking behavior underlies genetic studies of nicotine dependence. The development of nicotine dependence is the last step in a sequence of behavioral events that starts with the initiation of cigarette use (See Figure 1). There are at least three steps in this process: the transition from never smoking to the initiation of cigarette use; the conversion from experimental smoking to the establishment of regular smoking behavior; and finally the development of nicotine dependence among smokers. Both gender and socioeconomic status can influence the probability of transitioning through the different stages to the development of dependence. In addition, current smoking is more concentrated in lower socioeconomic groups and it is more common in men than women (Centers for Disease Control, 2008a). Each step in this pathway of smoking represents a potential point for intervention to prevent the onset of nicotine dependence, and different genetic and environmental factors influence the progression through each stage.
Figure 1. Steps Involved in the Development of Nicotine Dependence.
Smoking begins with the experimentation of smoking a first cigarette, and it is the initial necessary step in the development of nicotine dependence. Many public health campaigns to reduce smoking focus on experimentation with cigarettes in adolescence, which is the greatest period of risk for initiation. Environmental factors that influence the start of smoking include cigarette pricing, peer smoking, and parental monitoring (Chaloupka et al, 2005, Chilcoat et al, 1996; Kandel et al., 2004). In addition, a general genetic predisposition that captures a risk taking and impulsive personality is thought to influence whether a person will take this first step of initiation.
The next step in the development of dependence is the transition from an experimental smoker to a “smoker”. In this model, a smoker is defined as an individual who has smoked 100 or more cigarettes, a threshold which is used in many large-scale epidemiological studies. Factors that influence this transition from experimentation to regular smoking involve some of the same environmental and genetic features as well as novel ones. Cost, peer influences, and parental characteristics continue to contribute to whether an experimenter with cigarettes will continue to smoke cigarettes and become a smoker. Also, initial responses to smoking, either positive or negative, predict whether a person smokes in the future (Pomerleau et al., 1998).
The progression from smoking to nicotine dependence is also influenced by genetic and environmental factors. A cluster of symptoms and behaviors define nicotine dependence including tolerance to nicotine, withdrawal symptoms, and use of cigarettes despite social restrictions and health consequences. Tolerance is demonstrated by using a larger amount of a substance to obtain the same effect, which is consistent with smoking 20 or more cigarettes a day. Withdrawal is a characteristic set of symptoms that occur when a substance is discontinued. To avoid withdrawal, smokers will smoke a first cigarette of the day minutes after awakening, and if a smoker quits smoking, a withdrawal syndrome will begin. Finally, there are several behaviors that typify the psychological aspects of dependence, which include the craving for cigarettes and continued smoking despite knowledge of the physical and social adverse consequences.
Not all smokers are nicotine dependent. About half of current smokers are dependent on cigarettes, and many others have some symptoms of dependence (Grant et al., 2004). There is a third group of smokers who have no symptoms of dependence, a group called “chippers” (Shiffman, 1989). In contrast to the nicotine dependent smokers who smoke daily and generally are heavier smokers, frequently smoking 20 cigarettes a day, chippers are smokers who have not developed dependence, smoke a few cigarettes a day, and may not smoke daily. In genetic studies, chippers represent a unique contrast sample to the smokers who develop nicotine dependence.
This multistep model for the development of nicotine dependence aids in the study of both genetic and environmental factors that influence dependence by identifying features that are common or specific to each transition. For instance, though cigarette pricing and parental monitoring are effective interventions to reduce smoking initiation, these factors appear to play a lesser role in whether a smoker progresses to dependence. Similarly, this model defines different potential contrast groups for genetic studies of nicotine dependence. The comparison of nicotine dependent smokers with individuals who have never smoked will identify common genetic factors that contribute to one or more steps in the development of nicotine dependence. On the other hand, the comparison of non-dependent smokers, chippers, to nicotine dependent smokers focuses on the unique genetic factors that contribute to the transition from smoking to the development of nicotine dependence.
The measurement of nicotine dependence is another important aspect in the study of cigarette smoking, and multiple distinct dependence criteria exist. One of the most widely used measures of nicotine dependence is the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991), which was developed for the purpose of predicting quitting success (Fagerström, 1989). The FTND incorporates cigarettes per day in its measurement, as well as queries about early morning smoking and difficulty refraining from smoking despite illness and social restrictions. Another commonly used measure of nicotine dependence is based on the Diagnostic and Statistical Manual, Version IV (DSM-IV) diagnosis (American Psychiatric Association, 2000). The DSM-IV criteria for dependence are similar across all substances of abuse and are primarily modeled on the alcohol dependence syndrome, without specific attention to nicotine dependence. The DSM-IV criteria include tolerance, withdrawal, use despite physical or psychological problems, as well as other facets of continued use despite adverse effects. In our recent genetic study of nicotine dependence, only 75% of our nicotine dependent cases defined by an FTND score of 4 or more were also nicotine dependent using the DSM-IV dependence criteria. Similarly, among our non-dependent smoking controls, defined by an FTND score of zero, 24% met criteria for DSM-IV nicotine dependence (Bierut et al., 2008). This is consistent with other studies that compare the overlap between these two criteria sets (Breslau and Johnson, 2000). Recently, new measures have been developed that are multidimensional and specific to nicotine dependence, such as the Nicotine Dependence Syndrome Scale (NDSS; Shiffman et al., 2004) and Wisconsin Inventory of Smoking Dependence Motives (WISDM; Piper et al., 2004). These different measures for nicotine dependence, though correlated, emphasize varying aspects of addiction to nicotine such as cigarettes smoked per day, craving, and withdrawal, and the underlying genetic components may contribute to one measure of dependence more strongly than another.
3.1 Genetic Association Findings on Chromosome 15
Genetic factors play a prominent role in nicotine dependence, and twin studies estimate that the heritability of dependence is approximately 50% (Lessov et al., 2004). Consistently, twin studies support a strong genetic contribution to the development of nicotine dependence in both men and women and across multiple cultures (Madden et al., 1999).
Recently several large-scale studies have identified the region on chromosome 15 that includes the family of α5α3β4 nicotinic receptor genes as associated with the risk of whether a smoker becomes nicotine dependent. The first study to identify this region was a case control candidate gene study that contrasted nicotine dependent smokers with smokers who never reported any symptoms of nicotine dependence using the FTND (Saccone et al., 2007). Most of the nicotine dependent subjects smoked 20 or more cigarettes per day. Using non-dependent smokers who smoked no more than 10 cigarettes per day as comparison subjects to nicotine dependent cases focused the genetic study on the variants that influence the biological predisposition of whether a smoker progressed to nicotine dependence defined by the FTND.
Once a genetic association is discovered, replication in independent populations is critical. Using a measure of cigarettes smoked per day that contrasts light smokers with heavy smokers, Berrettini and colleagues independently identified the same genetic association on chromosome 15 (Berrettini et al., 2008). This finding of association on chromosome 15 has been subsequently confirmed by additional groups using correlated clinical characteristics such as nicotine dependence, smoking quantity, and heavy versus light smoking groups in different populations including community based populations, lung cancer patients, chronic obstructive pulmonary disease patients, and alcohol dependent subjects (Amos et al., 2008; Bierut et al., 2008; Pillai et al., 2009; Stevens et al., 2008; Thorgeirsson et al., 2008; Weiss et al., 2008). The consistency of this genetic association across various populations using correlated clinical characterizations of smoking behavior supports the robustness of this genetic result.
4.1 Understanding an Association and Search of Biological Function
The replication of genetic association increases the validity of a finding and justifies the further search for the specific genetic variants that change biological function. When a genetic association is found, it represents not only an association with the tested genetic variants, or single nucleotide polymorphisms (SNPs), but also an association with untested, highly correlated SNPs that can span across many genes on the same chromosome. To move from an association to an understanding of biological function, a study of the underlying genetic correlation between SNPs, which is also called linkage disequilibrium, is needed.
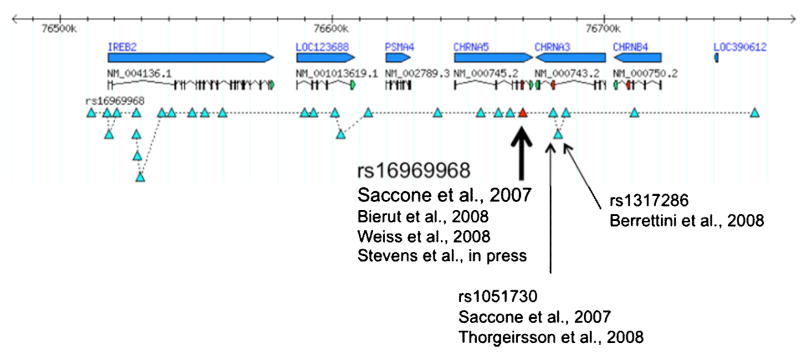
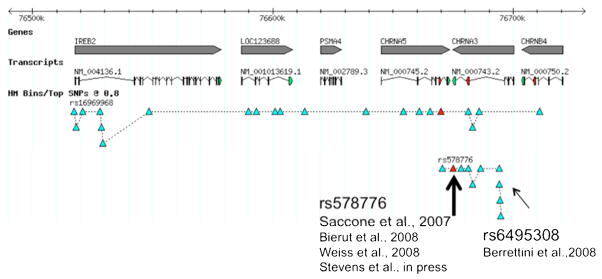
A further examination of the associated region on chromosome 15 demonstrates that there are distinct groups of correlated SNPs that are associated with nicotine dependence and smoking quantity, and these findings can be represented by two SNPs: rs16969968 and rs578776 (Saccone et al., 2009). See Figures 2 and 3. Both genetic variants are associated with nicotine dependence, but the correlation between these SNPs is relatively low (r2 < 0.2), which means that the association of each SNP with nicotine dependence cannot be statistically explained by the other. Both discrete findings have been identified in multiple studies (Berrettini et al., 2008; Bierut et al., 2008; Saccone et al., 2007; Stevens et al., 2008; Weiss et al., 2008).
Figure 2. Genetic Association on Chromosome 15 with Nicotine Dependence.
The top line represents the region on chromosome 15 from 76,510,000 through 76,730,000 base pairs. The second and third lines signify the genes in this chromosomal location (IREB2, LOC123688, PSMA4, CHRNA5, CHRNA3, and CHRNB4) and gene transcripts. The fourth line represents the genetic variants associated with nicotine dependence identified by rs16969968 and the correlated variants (r2 > 0.8). Studies that have identified as association with nicotine dependence and correlated smoking characteristics are noted.
Figure 3. Genetic Association on Chromosome 15 with Nicotine Dependence.
The top line represents the region on chromosome 15 from 76,510,000 through 76,730,000 base pairs. The second and third lines signify the genes in this chromosomal location (IREB2, LOC123688, PSMA4, CHRNA5, CHRNA3, and CHRNB4) and gene transcripts. The fourth line represents the genetic variants associated with nicotine dependence identified by rs16969968 and the correlated variants (r2 > 0.8). The fifth line identifies the genetic variants associated with nicotine dependence identified by rs578776 and correlated variants (r2 > 0.8). Studies that have identified as association with nicotine dependence and correlated smoking characteristics are noted.
The first finding tagged by rs16969968 is correlated (r2 > 0.8) with many SNPs that span 6 genes in populations of European descent. These genes include the α5-α3-β4 nicotinic receptor gene cluster (CHRNA5-CHRNA3-CHRNB4), and correlated SNPs are also located in the following genes: iron-responsive element binding protein 2 (IREB2), an iron regulatory protein; LOC123688, a putative protein of unknown function; and α4 proteasome subunit protein (PSMA4), a protein that cleaves other proteins.
To understand which variant (or variants) leads to biological changes that alter the risk for developing nicotine dependence requires further investigation. The most plausible genes that may influence smoking behavior in this region are the family of nicotinic receptor genes. The nicotinic receptor genes are a group of proteins that form pentameric receptors that bind nicotine, and so are involved in physiologic responses related to smoking. The variant that appears most promising to biologically contribute to nicotine dependence is rs16969968, a polymorphism that changes an amino acid in the α5 nicotinic receptor protein. This amino acid is highly conserved across species, and in vitro models that insert this single amino acid change demonstrate a change in receptor function (Bierut et al., 2008). This evidence supports the hypothesis that the genetic variant rs16969968 most likely causes biological changes that alter the risk of developing nicotine dependence and heavy smoking. However, the contribution of other correlated variants cannot be ruled out.
The second association finding is identified by rs578776 on chromosome 15. There are several SNPs correlated with rs578776 (r2 > 0.8) in populations of European descent, and these variants are clustered in the CHRNA5-CHRNA3 region. None of these SNPs alter the amino acid structure of the nicotinic receptor subunit proteins. Though a recent study has demonstrated that other genetic variants in this region alter mRNA levels of the α5 nicotinic receptor gene (Wang et al., 2009) the relationship with rs58776 is not definitive. Understanding the biological regulation of these genes requires further study.
At this point, there is robust evidence that distinct genetic variants in the region of the nicotinic receptor genes contribute to smoking behavior of cigarettes smoked per day and nicotine dependence. These association results and biological studies provide evidence that there may be two (or more) biological functions contributing to differences in smoking behaviors. One risk mechanism appears to result from an amino acid change in the α5 nicotinic receptor gene and the second biologic change may be an alteration in this gene’s expression. Another interpretation is that these distinct markers tag other underlying (but not genotyped) genetic variants and alternative biological roles exist. Additional animal studies are needed to fully explicate the genetic and functional mechanisms.
5.1 Implications for Men and Women and Other Populations
These strong and most robust findings of association of rs16969968 and rs578776 with nicotine dependence and smoking quantity can have different implications for varying populations. These SNPs contribute similarly to the risk of developing nicotine dependence in men and women (Saccone et al., 2007). The A allele of rs16969968 (allele frequency 38% in nicotine dependent cases and 32% in non-dependent smokers) increases the risk of becoming nicotine dependent compared to non-dependent by 30% for both men and women. The A allele of rs578776 (allele frequency 22% in nicotine dependence cases and 28% in non-dependent smokers) decreases the risk of developing nicotine dependence by more than 30% (Saccone et al., 2007; Saccone et al., 2009).
However, there can be different implications of the risk that these genetic variants contribute to ethnic/racial populations because of the varying allele frequencies. Though the underlying biological mechanisms that lead to the development of nicotine dependence are most likely the same across populations, differing allele frequencies can alter the relative importance of genetic risk factors in various populations. The two findings in the chromosome 15 region have very different allele frequencies across world populations. The risk variant at rs16969968, which causes the amino acid change, is common in a population of European descent (minor allele frequency (MAF)= 0.42), but it is rare in an Asian sample (MAF= 0.01–0.03), and it is not seen in Sub Saharan African populations (MAF=0) (Bierut et al., 2008). Thus, rs16969968, a strong genetic risk factor for nicotine dependence in a population of European descent, will play only a small role as a genetic risk factor in an African American population because of the low prevalence of the risk allele.
The second association, tagged by rs578776, also has varying allele frequencies in the different populations. The minor allele in a European population is the common allele in African and Asian populations (European descent T allele = 0.24 and Sub-Saharan African population T allele = 0.65; Asian population T allele = 0.80) (NCBI Build 130). The T allele of rs578776, which reduces the risk of developing nicotine dependence, may have a greater protective influence on the African American and Asian populations because of its increased frequency.
This demonstration of strikingly different allele frequencies across populations underscores the importance of extending genetic studies of nicotinic dependence into diverse populations. Various allele frequencies in racial groups mean that genetic factors can have different effects across world populations.
6.1 Implications of Genetic Studies
These studies highlight robust findings of genetic contributions to the development of nicotine dependence. The discovery that genetic variation in the nicotinic receptors contributes to the risk of developing nicotine dependence supports the hypothesis that biological factors related to the pharmacologic response to nicotine contribute to this predisposition. Though the genetic findings in the α5α3β4 nicotinic receptor gene cluster alter the risk by 30% in a population of European descent, these results explain only a small proportion of the genetic risk of developing nicotine dependence (Saccone et al., 2009). It is unlikely that other common variants of a similar or larger effect will be discovered in European populations, which implies that many more genes are involved in the development of dependence. The genetic architecture of nicotine dependence probably requires the contribution of hundreds of genes of small effect, the interaction between genes, and the interplay of genes with environment to explain the risk of developing the complex behavior of smoking. Genetic studies of tens to hundreds of thousands of people will be needed to detect these genetic effects.
Though this is a daunting task, an understanding of the genetic contributions to nicotine dependence is important because cigarette smoking is the greatest contributor to preventable morbidity and mortality in the United States (Mokdad et al., 2004). Over 400,000 people in the U.S die each year from tobacco related illnesses such as lung cancer, chronic obstructive pulmonary disease, and ischemic heart disease (Centers for Disease Control, 2008b). The economic burden of smoking is similarly high and costs the U.S. almost $100 billion in medical expenses and a similar sum in lost productivity (Centers for Disease Control, 2005). Primary prevention to stop smoking initiation must remain the key intervention to reduce smoking related illnesses, and public health campaigns and anti-smoking messages have successfully reduced the population prevalence of smoking in the U.S. Yet, over 43 million adults continue to smoke (Centers for Disease Control, 2008a).
Lung cancer is the most striking disease associated with smoking, and its prevalence over time directly reflects changes in smoking behavior. Though there has been a reduction in smoking and recent decline in the prevalence of lung cancer, smoking remains the single largest contributor to cancer related mortality (American Cancer Society, 2008). The genetic locus confirmed to affect risk for nicotine dependence and smoking quantity on chromosome 15 and tagged by rs16969968, the variant that results in an amino acid change, is now recognized as one of the strongest genetic determinants of lung cancer risk in several high-profile lung cancer genome wide association studies (Amos et al., 2008; Hung et al., 2008; Thorgeirsson et al., 2008). This represents an exciting convergence of genetic findings for nicotine dependence risk and for lung cancer susceptibility. It has been shown that this same genetic region also contributes to chronic obstructive pulmonary disease, another smoking related illness (Pillai et al., 2009). These overlapping findings demonstrate that genetic research on smoking is very important not only to addiction research, but also to research on lung cancer and the many other diseases for which smoking is a major risk factor. Further insights into the genetic basis of nicotine dependence and smoking thus have strong potential to impact public health and disease prevention for many smoking related diseases.
An understanding of the development of nicotine dependence may also contribute to our knowledge of smoking cessation. A study of smoking cessation in pregnancy has shown that this same genetic locus on chromosome 15 that increases the risk of developing nicotine dependence, heavy smoking, lung cancer, and other lung diseases, is also correlated with the success or failure of smoking cessation (Freathy et al., 2009). Among current smokers, almost half are nicotine dependent, and dependence is the strongest predictor of difficulty quitting. Though the majority of smokers (70%) want to quit and 41% try to quit smoking for at least one day, only 4% of smokers successfully quit in a year (Center for Disease Control, 2002). Smoking is now more concentrated in lower income groups and those with more social disadvantage such as minority populations. These remaining smokers in the U.S. may constitute a group that is especially refractory to prevention and treatment (Warner and Burns, 2003). In addition, 50% of adolescents experiment with smoking, and 20% of high school students report smoking in the last month (Centers for Disease Control, 2008c). Improved smoking cessation interventions are needed for these smokers. A major goal of understanding the genetic contributions to nicotine dependence is to identify new targets for smoking cessation therapies and to personalize treatment by matching cessation aids to individuals so that we can better help smokers quit and reduce the health burden of smoking.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Cancer Society. [accessed on 9/17/08];Cancer facts and figures, 2008. 2008 http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 2000. text revision. [Google Scholar]
- Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, Dong Q, Zhang Q, Gu X, Vijayakrishnan J, Sullivan K, Matakidou A, Wang Y, Mills G, Doheny K, Tsai YY, Chen WV, Shete S, Spitz MR, Houlston RS. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PA, Mayo K, Nurnberger J, Jr, Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan G, Tischfield JA, Edenberg HJ, Rice JP, Goate AM. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Johnson EO. Predicting smoking cessation and major depression in nicotine-dependent smokers. Am J Public Health. 2000;90:1122–1127. doi: 10.2105/ajph.90.7.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control. Tobacco use among adults--United States, 2000. Morbidity & Mortality Weekly Report. 2002;51:642–645. [PubMed] [Google Scholar]
- Centers for Disease Control. Annual smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 1997–2001. Morbidity & Mortality Weekly Report. 2005;54:625–628. [PubMed] [Google Scholar]
- Centers for Disease Control. Tobacco use among adults--United States, 2007. Morbidity & Mortality Weekly Report. 2008a;57:1221–1226. [PubMed] [Google Scholar]
- Centers for Disease Control. [accessed on 8/27/08];Targeting tobacco use: the nation’s leading cause of preventable death. 2008b http://www.cdc.gov/nccdphp/publications/aag/pdf/osh.pdf.
- Centers for Disease Control. Cigarette use among high school students - United States, 1991–2007. Morbidity & Mortality Weekly Report. 2008c;57:689–691. [PubMed] [Google Scholar]
- Chaloupka FJ, Pacula RL. The impact of price on youth tobacco use. Changing Adolescent Smoking Prevalence. Smoking and Tobacco Control Monograph. 2005;14:193–199. [Google Scholar]
- Chicoat HD, Anthony JC. Impact of parental monitoring on initiation of drug use through late childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:91–100. doi: 10.1097/00004583-199601000-00017. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12:159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- Freathy RM, Ring SM, Shields B, Galobardes B, Knight B, Weedon MN, Smith GD, Frayling TM, Hattersley AT. A common genetic variant in the 15q24 nicotnic acetylcholine receptor gene cluster (CHRNA5-CHRNA4-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet. 2009 doi: 10.1093/hmg/ddp216. Advance access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Chen C, Goodman G, Field JK, Liloglou T, Xinarianos G, Cassidy A, McLaughlin J, Liu G, Narod S, Krokan HE, Skorpen F, Elvestad MB, Hveem K, Vatten L, Linseisen J, Clavel-Chapelon F, Vineis P, Bueno-de-Mesquita HB, Lund E, Martinez C, Bingham S, Rasmuson T, Hainaut P, Riboli E, Ahrens W, Benhamou S, Lagiou P, Trichopoulos D, Holcatova I, Merletti F, Kjaerheim K, Agudo A, Macfarlane G, Talamini R, Simonato L, Lowry R, Conway DI, Znaor A, Healy C, Zelenika D, Boland A, Delepine M, Foglio M, Lechner D, Matsuda F, Blanche H, Gut I, Heath S, Lathrop M, Brennan P. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Kiros GE, Schaffran C, Hu MC. Racial/ethnic differences in cigarette smoking initiation and progression to daily smoking: a multilevel analysis. Am J Public Health. 2004;94:128–135. doi: 10.2105/ajph.94.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessov CN, Martin NG, Statham DJ, Todorov AA, Slutske WS, Bucholz KK, Madden PA. Defining nicotine dependence for genetic research: evidence for Australian twins. Psychol Med. 2004;34:865–879. doi: 10.1017/s0033291703001582. [DOI] [PubMed] [Google Scholar]
- Madden PA, Heath AC, Pedersen NL, Kaprio J, Koskenvuo MJ, Martin NG. The genetics of smoking persistence in men and women: a multicultural study. Behav Genet. 1999;29:423–431. doi: 10.1023/a:1021674804714. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. Jama. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Pillai SG, Ge D, Zhu G, Kong X, Shianna KV, Need AC, Feng S, Hersh CP, Bakke P, Gulsvik A, Ruppert A, Lødrup Carlsen KC, Roses A, Anderson W, Rennard SI, Lomas DA, Silverman EK, Goldstein DB ICGN Investigators. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet. 2009;5:e1000421. doi: 10.1371/journal.pgen.1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: The Wisconsin inventory of smoking dependence motives (WISDM-68) Journal of Consulting and Clinical Psychology. 2004;72:139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Namenek RJ. Early experiences with tobacco among women smokers, ex-smokers, and never-smokers. Addiction. 1998;93:595–599. doi: 10.1046/j.1360-0443.1998.93459515.x. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, Agrawal A, Breslau N, Grucza RA, Hatsukami D, Johnson EO, Madden PA, Swan GE, Wang JC, Goate AM, Rice JP, Bierut LJ. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet. 2009 doi: 10.1002/ajmg.b.30828. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Tobacco “chippers”--individual differences in tobacco dependence. Psychopharmacology (Berl) 1989;97:539–547. doi: 10.1007/BF00439561. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Water AJ, Hickcox M. The Nicotine Dependence Syndrome Scale: A multidimensional measure of nicotine dependence. Nicotine & Tobacco Research. 2004;6:327–349. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, Thun MJ, Goate A, Calle EE. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiology, Biomarkers & Prevention. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey SN, Bergthorsson JT, Thorlacius S, Gudmundsson J, Jonsson T, Jakobsdottir M, Saemundsdottir J, Olafsdottir O, Gudmundsson LJ, Bjornsdottir G, Kristjansson K, Skuladottir H, Isaksson HJ, Gudbjartsson T, Jones GT, Mueller T, Gottsater A, Flex A, Aben KK, de Vegt F, Mulders PF, Isla D, Vidal MJ, Asin L, Saez B, Murillo L, Blondal T, Kolbeinsson H, Stefansson JG, Hansdottir I, Runarsdottir V, Pola R, Lindblad B, van Rij AM, Dieplinger B, Haltmayer M, Mayordomo JI, Kiemeney LA, Matthiasson SE, Oskarsson H, Tyrfingsson T, Gudbjartsson DF, Gulcher JR, Jonsson S, Thorsteinsdottir U, Kong A, Stefansson K. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Grucza R, Cruchaga C, Hinrichs AL, Bertelsen S, Budde JP, Fox L, Goldstein E, Reyes O, Saccone N, Saccone S, Xuei X, Bucholz K, Kuperman S, Nurnberger J, Jr, Rice JP, Schuckit M, Tischfield J, Hesselbrock V, Porjesz B, Edenberg HJ, Bierut LJ, Goate AM. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol Psychiatry. 2009;14:501–510. doi: 10.1038/mp.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner KE, Burns DM. Hardening and the hard-core smoker: concepts, evidence, and implications. Nicotine Tob Res. 2003;5:37–48. doi: 10.1080/1462220021000060428. [DOI] [PubMed] [Google Scholar]
- Weiss RB, Baker TB, Cannon DS, von Niederhausern A, Dunn DM, Matsunami N, Singh NA, Baird L, Coon H, McMahon WM, Piper ME, Fiore MC, Scholand MB, Connett JE, Kanner RE, Gahring LC, Rogers SW, Hoidal JR, Leppert MF. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet. 2008;4:e1000125. doi: 10.1371/journal.pgen.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]