Abstract
This article reports a pilot study of the effect of tai chi (TC), a pharmacological adjunct and mild aerobic exercise, on osteoarthritic knee pain in elders with cognitive impairment (CI). The TC program included a warm-up, 12-form Sun-style TC, and a cool-down period, for a total of 20-40 minutes per session, twice a week for 15 weeks. The results showed no significant differences in knee pain after the TC intervention in 7 elders with CI. However, more minutes of TC attendance were related to improved pain scores (Spearman's rho = .78, P <.05). Greater accuracy in TC performance was also correlated with improvements in pain scores (Spearman's rho = .70, P = .08). Of 4 elders who participated in TC practice regularly (more than 20 sessions), 3 showed clinically important improvements, but 3 elders who participated in no sessions or only a few sessions showed no improvement.
Knee pain from osteoarthritis (OA) is among the leading causes of disability in elders.1 Approximately 16.3%–33% have knee OA2,3 and experience weakness in their quadriceps muscle and difficulty performing activities of daily living (ADLs) because of pain. Cognitive impairment (CI) further limits ADL functioning in elders with knee OA pain,4 because ADL performance involves attention to detail in addition to physically performing the tasks.5 Up to 15.3% of elders aged 65 and older have CI,6 and the prevalence doubles every 5 years after 65, with about half of elders aged 85 and over reported to have CI.7,8 Elders with CI include those with and without a specific diagnosis of dementia. The limited ability of elders with CI to communicate pain verbally makes it difficult for health care providers to offer appropriate interventions to manage their OA knee pain. With unrelieved pain, CI elders with OA of the knee or hip may avoid lower extremity activities, such as getting out of the bed or a chair, walking, doing household chores, shopping, or going out for social activities. As a result, elders with CI may become more sedentary and vulnerable to further physical disability and deconditioning.9
The effects of pharmacological interventions on pain are controversial10-12 and their side effects sometimes prevent elders from taking them.13 Nevertheless, 4 g of acetaminophen per day was found to be as safe and effectives as naproxen in the management of mild to moderate osteoarthritis when given for a year.14,15 Thus, it would seem that if we can alleviate elders' pain with pharmacological interventions such as acetaminophen, they may be more likely to engage in exercises. This may then decrease their use of pain medication and thus prevent some of the side effects of pharmacological interventions.
In a randomized clinical trial, persons (age range: 40–80) who had knee OA pain and participated in a 6-month home strengthening exercise program reported less pain than control subjects (P <.05).16 Another large-scale trial found that a 2-year home-based exercise program significantly lowered elders' OA pain scores on the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index (P <.01, effect size = .25).17 Recently, a Cochrane review of 17 studies that employed quadriceps muscle strengthening exercises, aerobic walking programs, or complex comprehensive rehabilitation concluded that exercise had a beneficial effect (standardized mean difference of .39 for knee OA pain; 95% confidence interval [CI]: 0.30–0.47]).18
Tai chi may be superior to other forms of exercise for elders with CI for several reasons. First, TC involves slow stretching of the limbs and trunk and gradually strengthens muscles and increases range of motion without exacerbating elders' OA pain as other exercises may do.19,20 Second, it requires less physical strength than other forms of exercise. Third, TC can be modified to allow elders who are physically frail to practice in a standing or sitting position, and they also can practice TC in small spaces, at any time, and individually or in groups, regardless of weather conditions. Finally, TC provides additional benefits, such as reduced risk of falls, improved depressive symptoms and sleep, increased cardiovascular function and social interaction, and enhanced quality of life.21-26 Because TC does not require any special equipment or space, it is a flexible and inexpensive form of exercise.
Several studies conducted with non-CI elderly samples have found that TC improved OA pain.27,28 On the basis of data from elders without CI, we expected that elders with OA knee pain would increase their activity level, and the weight-bearing aspects of TC would gradually strengthen the quadriceps muscles after repeated practice of TC forms. This in turn might reestablish normal mechanics around the knee joint, and the improved stability of the knee joint would protect the joint by reducing excessive stress and strain on the lax joint capsule, where the nociceptors are located. As a result, knee pain was expected to decline.29-32 This mechanism should not differ between elders with CI and without CI because other studies have shown that both elders groups have the same positive results in fitness, strength, pain, and physical function from exercise.18,33 None of these studies, however, assessed the effects of TC on OA knee pain in elders with CI. Therefore, the primary aim of this pilot study was to investigate the effect of 12-form Sun-style TC on osteoarthritic knee pain in 7 elders with CI. It was hypothesized that TC intervention would reduce the elders' OA knee pain. This study also tracked treatment fidelity by examining minute and session attendance and the accuracy of TC performance and examined the effect of the level of fidelity on outcomes. It was hypothesized that by attending more minutes or sessions or performing TC with a higher level of accuracy, elders would have less pain after the TC intervention. Finally, this study estimated the clinical significance of TC for pain reduction.
Method
Design
The study used a pretest–posttest 1-arm design to investigate TC's effects on OA knee pain in elders with CI.
Sample and Participants
Residents were recruited from 4 long-term care facilities located in central Arkansas. Inclusion criteria were 1) aged 60 years old, 2) English speaking, 3) self-report of knee OA pain, 4) moderate to mild CI (Mini-Mental Status Exam [MMSE] score 15–27), 5) no depressive symptoms (Geriatric Depression Scale [GDS-15] <5), 6) physician's permission to participate in TC, and 7) low activity levels (defined as noninvolvement in a regular exercise program in the month before participation in the study).
Exclusion criteria were 1) moderate or severe hearing deficits, 2) Parkinson's disease (defined by any 2 of these features: resting tremor, rigidity, bradykinesia, or impaired postural reflexes), 3) cancer pain, 4) diabetic neuropathy, 5) arthroscopic surgery or total knee or total hip replacement surgery in the previous 3 months, 6) a history of vertigo in the previous month, 7) a history of falls during the past 3 months, 8) any fractures in the past 6 months, or 9) use of cholinesterase inhibitors during the previous 3 months. After the Committee on the Conduct of Human Research at the University of Arkansas for Medical Sciences approved the study, nursing home administrators, and advanced practice nurses identified potential participants and obtained written permission for a research assistant (RA) to contact the elders and their families. The RA visited the elders and family members and explained the study. If they agreed to participate, the RA obtained consent and Health Insurance Portability and Accountability Act research authorization and screened for eligibility. The RA also obtained medical clearance from each elder's medical provider.
Intervention
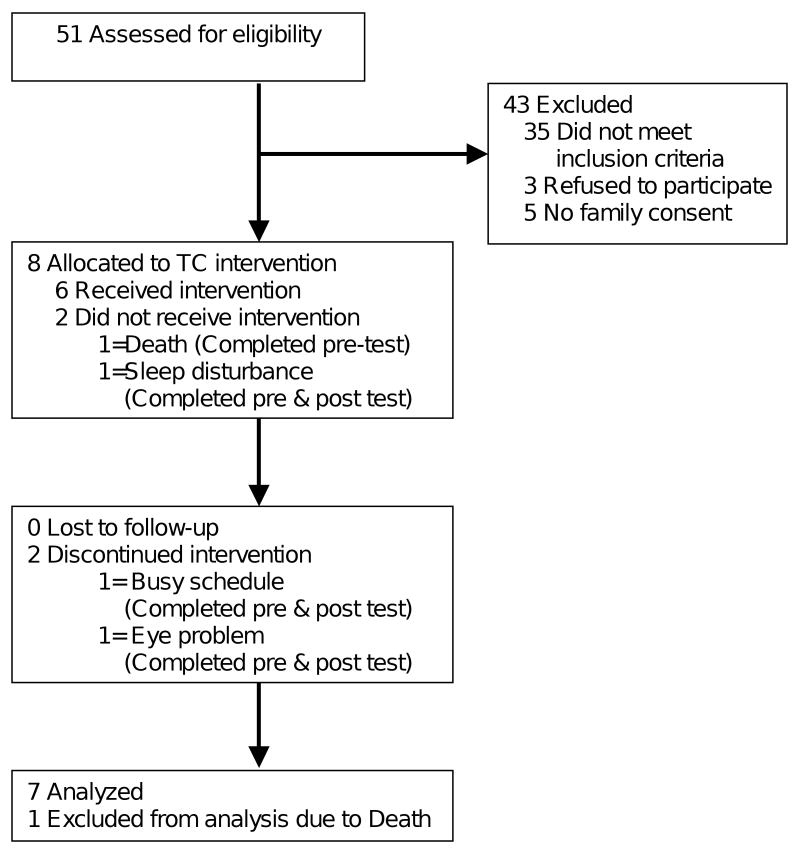
Participants received 12-form Sun TC for arthritis, developed by Lam34 and endorsed by the U.S. Arthritis Foundation, twice a week for 15 weeks. The intervention occurred in the nursing home's activity room with 1-2 elders in each group. The TC program included a warm-up, TC, and a cool-down period, for a total of 20-40 minutes per session. A certified TC instructor with 4 years of experience led participants in performing the TC forms. The instructor gradually increased the time that participants practiced from 20 to 40 minutes as participants gained physical strength and familiarity. The instructor started with Form 1 in Session 1 and gradually added 1 new form in each of the following sessions as participants made progress. The intervention was conducted in an activity room located in each facility. Figure 1 shows the flow of participants.
Figure 1. Participant flow.
Instruments and Measures
Screening tools
The MMSE, a 30-item cognitive screen measuring orientation, registration, short-term memory, attention and concentration, language, and constructional capacity, assessed cognitive functioning. Test–retest reliability is 0.83,35 and criterion validity is 0.83 with the Short Portable Mental Status Questionnaire and .88 with the Cognitive Capacity Screening Examination.36,37 The 15-item GDS scale was used to identify depressive symptoms.38 The cutoff score of 5 has a sensitivity of 85% and a specificity of 74% in identifying depressive symptoms.39
Primary outcome
The Medical Outcomes Study short form (SF-36) includes 8 subscales: physical function, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health.40 Validity and reliability in the general population are established.41,42 We used only the bodily pain subscale, which measures the magnitude of pain and the level of pain's interference with daily life. Higher scores indicate more pain. The RA read the questions to the participant, obtained the responses, and recorded the answers. High scores indicate less pain with a possible range from 0% to 100%. The intraclass correlation coefficient with elders with mild to moderate CI is 0.65, indicating good reliability. Modest convergent validity was demonstrated by examining the correlations between the bodily pain subscale and the Geriatric Depression Scale (r = –.35).43
Other measures
Attendance sheets recorded the number of sessions of TC and the minutes attended. We also videotaped participants' performance of TC at posttest, and these tapes were coded to measure accuracy of performance.44 Elders watched the instructor performing TC twice and then followed the instructor performing TC twice while being videotaped. Watching the videotapes, the coder used the best trial for each form to rate the participants' TC performance on three 5-point rating scales (0, 0.5, 1, 1.5, and 2) assessing movement, stability of balance, and weight shifting, and on two 3-point rating scales (0, 1, and 2) assessing range of motion and coordination. Interrater reliability was .87 for 16 codings.44 The scores were summed across the forms and divided by the maximum score, 80, to create a score ranging from 0% to 100%, with higher scores indicating greater accuracy of TC performance.
Blinding
Because this was a 1-arm design without a control group, blinding participants, the interventionist (TC instructor) or the assessor to group assignment was not necessary. However, the procedure for measuring the primary outcome was standardized, which minimized the assessor's bias when assessing pain. Staff members who assessed TC performance by coding the videotapes did not have contact with the elders or view their records and thus remained blinded to elders' pain scores. The first author visited the sites to monitor the instructor's treatment fidelity and participants' treatment adherence. She and other team members reviewed only de-identified coded data.
Data Analysis
Univariate analysis, t test, and nonparametric correlations were used to examine the study aims.
Results
Seven elders with a mean age of 83 6 participated; 2 were African American and 1 was a man. The average MMSE score was 21.5 with a range from 15 to 26. Elders' pain scores averaged 71.1% ± 8.1% before the TC intervention with a range from 61% to 84%. Their pain scores averaged 74.4% ± 26.7% after the TC intervention with a range from 21% to 100%. The 7 participants' pain scores before and after the TC intervention were compared. The result showed no significant differences before and after the TC intervention [t(df) = –.35(6), P = .74].
Table 1 shows attendance at sessions and minutes attended, TC performance, and pain scores before and after the TC intervention. Elders attended an average of 17 ± 14 sessions, with a range from 0 to 29. Attendance in minutes, taking into consideration no attendance, late attendance, minutes of unplanned rest, and early termination of a session, averaged 618 ± 503, with a range from 0 to 1130 minutes. Performance scores were between 0% and 53% with an average of 19% ± 18%, indicating that as a group, these elders performed approximately 1 of 5 of the forms accurately after the TC intervention. The Spearman's rho correlation between the change in pain scores and the number of minutes attended was .78 (P < .05), indicating that with increasing minutes of TC practice, there was a corresponding improvement in pain scores. The correlation between the change in pain score and the number of sessions attended was not significant (Spearman's rho correlation = .64, P = .12). The correlation between the change in pain score and accuracy of performance was .70 (P = .08), suggesting that a higher level of accuracy in TC performance was associated with improvements in pain scores.
Table 1. Changes in pain scores and attendance.
| Group* | ID | MMSE | Accuracy of Performance | Attendance | Pain score | MCID for Improvement or Worsening44 | ||
|---|---|---|---|---|---|---|---|---|
| Session | Minute | Pretest (%) |
Posttest (%) |
|||||
| Regular dose group | 1 | 15 | 25% | 27 | 1130 | 72 | 100 | Improved |
| 2 | 16 | 53% | 29 | 1065 | 72 | 100 | Improved | |
| 3 | 22 | 21% | 27 | 975 | 61 | 84 | Improved | |
| 4 | 25 | 19% | 27 | 888 | 84 | 72 | Worsened | |
| Low dose group | 5 | 25 | 0% | 4 | 190 | 74 | 72 | No change |
| 6 | 26 | 15% | 2 | 77 | 61 | 21 | Worsened | |
| 7 | 22 | 0% | 0 | 0 | 74 | 72 | No change | |
All participants completed the assessments. Regular dose group participated in 21 or more sessions of TC. Low dose group participated in 20 or less sessions of TC
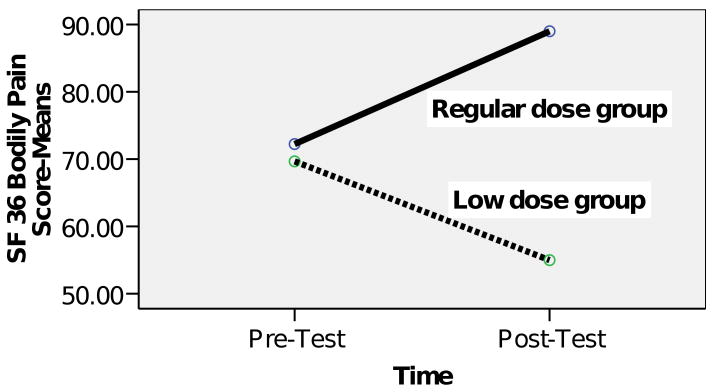
Participants required 16 to 20 sessions to learn the complete set of TC forms. The 4 elders (case numbers 1-4) who participated in 21 or more sessions formed the regular dose group. The 3 elders (case numbers 5-7) who participated in fewer than 21 sessions formed the low-dose group. The regular-dose group showed an improvement in pain score (mean: pretest 72.3 ± 9.4 vs. posttest 89.0 ± 13.6), whereas the low-dose group showed a deterioration in pain score (mean: pretest 69.7 ± 7.5 vs. posttest 55.0 ± 29.4.0) [t(df) = 2.0(5), P = .100], although the difference was not significant (Figure 2).
Figure 2. Changes in bodily pain scores.
According to Angst and colleagues,45 a minimal clinically important difference (MCID) for improvement is defined as an improvement of at least 7.8% from pretest on the SF-36 bodily pain subscale; the MCID for worsening is defined as deterioration of at least 7.2% from pretest on the SF-36 bodily pain subscale.45 Three of four participants in the regular-dose group showed MCID for improvement, compared with none of the participants in the low-dose group (see the last column of Table 1).
Discussion
This feasibility which pilot tested the effects of TC on OA pain in elders with CI and looked specifically at the relationships among change in pain score and sessions attended, minutes attended, and accuracy of TC performance. A significant relationship was observed between the change in pain scores and the minutes of TC attended, but the relationship between the change in pain score and the number of sessions attended did not achieve significance. Among the elders who came to the TC sessions, some required unplanned rest during the practice, came late, or left early for various reasons. Thus the number of sessions attended may not be an appropriate measure of attendance. Calculating the number of minutes of participation, a more precise measure, may be better for exploring the dose–response relationship between attendance and outcomes.
According to the treatment implementation model, if a treatment is properly delivered and a patient understands it and practices as prescribed, the desired outcome should be expected.46 On the basis of this model, the more accurate the performance of these TC forms, the less pain should be reported. However, there was only a borderline significant relationship between the change in pain score and the accuracy of TC performance. In the regular-dose group, the accuracy of TC performance was not always associated with the number of minutes attended. This suggests that minutes attended is a better indicator of the benefit of TC than the accuracy of TC performance. However, a full-scale study is required to verify this.
We used 2 approaches to examine the impact of TC on pain: statistical significance and clinical significance. By comparing the low-dose and regular-dose groups, the study was able to show a trend toward reduced pain after the TC intervention. Examining clinical significance allowed us to see the impact of the intervention on individuals' perception of pain or to see changes that were clinically meaningful.47,48
Although the SF-36 is a popular tool in social science, using a disease-specific pain measure, an observational method or a pain tool that is validated for elders with CI might have been a more valid measure of pain in elders with CI and OA of the knee. Further, although we collected information to decide participants' eligibility, we failed to collect information on medication intake and comorbidities. This information might be important to examine the comparability of the groups and should be collected in a future study.
Despite limitations, this study suggested that TC may have some benefit in reducing OA knee pain in elders with CI. Elders with moderate CI performed with enough accuracy to receive a benefit from TC, indicating that a TC intervention may be feasible with this population. However, few elders in long-term care settings were eligible to participate in the TC intervention because they had participated in a regular exercise program over the previous month, were too ill, did not have enough physical strength to walk or stand, or had severe dementia. Thus, implementing the TC intervention in long-term care facilities may not be cost-effective. Community-dwelling elders with CI may be better participants for a TC intervention, but working with this population involves another set of obstacles. For example, practicing TC over time is key to perfecting the TC forms. However, given elders' forgetfulness and their need for visual and verbal cues from the instructor while practicing TC, prescribing home TC for them to practice alone is not realistic. Thus, the TC intervention may need to be modified to ease its physical and cognitive requirements and extend its length to give elders with CI more time to learn and practice with the instructor or a partner, such as a family caregiver.
The small sample in this study prevents generalization. Future goals include replicating this study with a larger sample of community-dwelling elders with CI randomized to treatment and control groups. If TC shows efficacy and effectiveness in reducing pain in elders with CI in larger studies, it can be promoted as an adjunctive intervention for this population. We will also test the sustainability of the TC intervention in elders with CI and the effect of the involvement of caregivers on outcomes. This may include examining the length of time elders with CI continue to practice TC after a study and examining ways to facilitate caregiver involvement. With information on the sustainability of the TC intervention and the effect of caregiver involvement, we can assess the feasibility of implementing a TC program at home that may achieve the desired outcomes and promote quality of life for both the elders and their family members.
Acknowledgments
This study was supported by the Beverly HealthCare Corporation and the National Institute on Aging funded Alzheimer's Disease Center (Grant No. 5 P30 AG019606-05). It was also supported in part by the John A. Hartford Foundation under the Building Academic Geriatric Nursing Capacity Scholars Program. Without the research participants, nursing home staff, Marye Ann Boyd, and Nola Ballinger, this study could not have been completed.
Footnotes
This feasibility study pilot tested the effects of tai chi on knee osteoarthritis pain in cognitively impaired elders and looked specifically at the relationships among change in pain score and sessions attended, minutes attended, and accuracy of tai chi performance.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Pao-Feng Tsai, Associate professor in the College of Nursing, University of Arkansas for Medical Sciences, Little Rock.
Cornelia Beck, Professor in the Department of Geriatrics, College of Medicine, University of Arkansas for Medical Sciences, Little Rock.
Jason Y. Chang, Associate professor in the Department of Neurobiology and Developmental Sciences, College of Medicine, University of Arkansas for Medical Sciences, Little Rock.
Jody Hagen, Adjunct assistant professor in the Department of Geriatrics, College of Medicine, University of Arkansas for Medical Sciences, Little Rock; and a clinical neuropsychologist in private practice at Living Well, PLLC.
Kuo Yong-Fang, Associate professor at the Sealy Center on Aging, Department of Preventive Medicine and Community Health, University of Texas Medical Branch.
Paula K. Roberson, Professor in and the chair of the Department of Biostatistics, Colleges of Medicine and Public Health, University of Arkansas for Medical Sciences, Little Rock.
Karl Rosengren, Professor in the Departments of Psychology and of Kinesiology & Community Health, University of Illinois at Urbana-Champaign.
Linda Beuscher, Assistant professor in the School of Nursing, Vanderbilt University, Nashville, Tennesee.
Cathy Doan, Affiliated with the College of Nursing, University of Arkansas for Medical Sciences, Little Rock.
K. J. S. Anand, Morris & Hettie Oakley Endowed Chair of Critical Care Medicine and professor of pediatrics, anesthesiology, pharmacology, neurobiology & developmental sciences, College of Medicine, University of Arkansas for Medical Sciences, Little Rock, and director of the Pain Neurobiology Laboratory, Arkansas Children's Hospital Research Institute.
References
- 1.Campbell AJ, Busby WJ, Robertson MC, et al. Disease, impairment, disability and social handicap: a community based study of people aged 70 years and over. Disabil Rehabil. 1994;16:72–9. doi: 10.3109/09638289409166015. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Naimark A, Anderson J, et al. The prevalence of knee osteoarthritis in the elderly: the Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30:914–8. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Xu L, Nevitt MC, et al. Comparison of the prevalence of knee osteoarthritis between the elderly Chinese population in Beijing and whites in the United States: the Beijing Osteoarthritis Study. Arthritis Rheum. 2001;44:2065–71. doi: 10.1002/1529-0131(200109)44:9<2065::AID-ART356>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 4.Jordan J, Luta G, Renner J, et al. Knee pain and knee osteoarthritis severity in self-reported task specific disability: the Johnston County Osteoarthritis Project. J Rheumatol. 1997;24:1344–9. [PubMed] [Google Scholar]
- 5.Shah KR, Carr D, Roe CM, et al. Impaired physical performance and the assessment of dementia of the Alzheimer type. Alzheimer Dis Assoc Disord. 2004;18:112–9. doi: 10.1097/01.wad.0000127441.77570.f3. [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer RI, Afifi AA, Chance JM. Prevalence of Alzheimer's disease in a retirement community. Am J Epidemiol. 1987;125:420–36. doi: 10.1093/oxfordjournals.aje.a114548. [DOI] [PubMed] [Google Scholar]
- 7.Evans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer's disease in a community population of older persons: higher than previously reported. JAMA. 1989;262:2551–6. [PubMed] [Google Scholar]
- 8.Hebert LE, Scherr PA, Bienias JL, et al. State-specific projections through 2025 of Alzheimer disease prevalence. Neurology. 2004;62:1645. doi: 10.1212/01.wnl.0000123018.01306.10. [DOI] [PubMed] [Google Scholar]
- 9.Steultjens MP, Dekker J, Bijlsma JW. Avoidance of activity and disability in patients with osteoarthritis of the knee: the mediating role of muscle strength. Arthritis Rheum. 2002;46:1784–8. doi: 10.1002/art.10383. [DOI] [PubMed] [Google Scholar]
- 10.Turk DC, Loeser JD, Monarch ES. Chronic pain: purposes and costs of interdisciplinary pain rehabilitation programs. Trend Evidence-Based Neuropsych. 2002;4:64–9. [Google Scholar]
- 11.Turk DC. Clinical effectiveness and cost-effectiveness of treatments for patients with chronic pain. Clin J Pain. 2002;18:355–65. doi: 10.1097/00002508-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Buffum MD, Sands L, Miaskowski C, et al. A clinical trial of the effectiveness of regularly scheduled versus as-needed administration of acetaminophen in the management of discomfort in older adults with dementia. J Am Geriatr Soc. 2004;52:1093–7. doi: 10.1111/j.1532-5415.2004.52305.x. [DOI] [PubMed] [Google Scholar]
- 13.Lansbury G. Chronic pain management: a qualitative study of elderly people's preferred coping strategies and barriers to management. Disabil Rehabil. 2000;22:2–14. doi: 10.1080/096382800297079-1. [DOI] [PubMed] [Google Scholar]
- 14.Temple AR, Benson GD, Zinsenheim JR, et al. Multicenter, randomized, double-blind, active-controlled, parallel-group trial of the long-term (6-12 months) safety of acetaminophen in adult patients with osteoarthritis. Clin Ther. 2006;28:222–35. doi: 10.1016/j.clinthera.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Whelton A. Clinical implications of nonopioid analgesia for relief of mild-to-moderate pain in patients with or at risk for cardiovascular disease. Am J Cardiol. 2006;97:3–9. doi: 10.1016/j.amjcard.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 16.O'Reilly SC, Muir KR, Doherty M. Effectiveness of home exercise on pain and disability from osteoarthritis of the knee: a randomised controlled trial. Ann Rheum Dis. 1999;58:15–9. doi: 10.1136/ard.58.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas KS, Muir KR, Doherty M, et al. Home based exercise programme for knee pain and knee osteoarthritis: randomised controlled trial. BMJ. 2002;325:752. doi: 10.1136/bmj.325.7367.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fransen M, McConnell S, Bell M. Exercise for osteoarthritis of the hip or knee. Cochrane Database Syst Rev. 2005;2 doi: 10.1002/14651858.CD004286. [DOI] [PubMed] [Google Scholar]
- 19.Taylor SK. Tai Chi for chronic pain and arthritis. Tech Orthopaedics. 2003;18:110–4. [Google Scholar]
- 20.Kirsteins AE, Dietz F, Hwang SM. Evaluating the safety and potential use of a weight-bearing exercise, tai-chi chuan, for rheumatoid arthritis patients. Am J Phys Med Rehabil. 1991;70:136–41. doi: 10.1097/00002060-199106000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Li F, Harmer P, Fisher KJ, et al. Tai Chi and fall reductions in older adults: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2005;60:187–94. doi: 10.1093/gerona/60.2.187. [DOI] [PubMed] [Google Scholar]
- 22.Chou KL, Lee PW, Yu EC, et al. Effect of tai chi on depressive symptoms amongst Chinese older patients with depressive disorders: a randomized clinical trial. Int J Geriatr Psychiatry. 2004;19:1105–7. doi: 10.1002/gps.1178. [DOI] [PubMed] [Google Scholar]
- 23.Li F, Fisher KJ, Harmer P, et al. Tai chi and self-rated quality of sleep and daytime sleepiness in older adults: a randomized controlled trial. J Am Geriatr Soc. 2004;52:892–900. doi: 10.1111/j.1532-5415.2004.52255.x. [DOI] [PubMed] [Google Scholar]
- 24.Taylor-Piliae RE, Froelicher ES. Effectiveness of tai chi exercise in improving aerobic capacity: a meta-analysis. J Cardiovasc Nurs. 2004;19:48–57. doi: 10.1097/00005082-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Hartman CA, Manos TM, Winter C, et al. Effects of tai chi training on function and quality of life indicators in older adults with osteoarthritis. J Am Geriatr Soc. 2000;48:1553–9. doi: 10.1111/j.1532-5415.2000.tb03863.x. [DOI] [PubMed] [Google Scholar]
- 26.Jong SY, Fang YY, Chao YF. The effect of tai-chi-qui-gong exercises on patients' pulmonary function, exercise capacity, and quality of life after lobectomy. Hu Li Za Zhi. 2004;51:46–54. in Chinese. [PubMed] [Google Scholar]
- 27.Lee EO, Song R, Bae SC. Effects of 12-week tai chi exercise on pain, balance, muscle strength, and physical functioning in older patients with osteoarthritis: randomized trial. J Am Geriatr Soc. 2001;44:s393. [PubMed] [Google Scholar]
- 28.Song R, Lee EO, Lam P, et al. Effects of tai chi exercise on pain, balance, muscle strength, and perceived difficulties in physical functioning in older women with osteoarthritis: a randomized clinical trial. J Rheumatol. 2003;30:2039–44. [PubMed] [Google Scholar]
- 29.Qin L, Choy W, Leung K, et al. Beneficial effects of regular tai chi exercise on musculoskeletal system. J Bone Miner Metab. 2005;23:186–90. doi: 10.1007/s00774-004-0559-2. [DOI] [PubMed] [Google Scholar]
- 30.Cheing GL, Hui-Chan CW, Chan KM. Does four weeks of TENS and/or isometric exercise produce cumulative reduction of osteoarthritic knee pain? Clin Rehabil. 2002;16:749–60. doi: 10.1191/0269215502cr549oa. [DOI] [PubMed] [Google Scholar]
- 31.Gerber LH. Exercise and arthritis. Bull Rheum Dis. 1990;39:1–9. [PubMed] [Google Scholar]
- 32.Fisher NM, Pendergast DR, Gresham GE, et al. Muscle rehabilitation: its effect on muscular and functional performance of patients with knee osteoarthritis. Arch Phys Med Rehabil. 1991;72:367–74. [PubMed] [Google Scholar]
- 33.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85:1694–704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Lam P. Tai chi for arthritis. Narwee, Australia: East Action Publishing; 2004. [Google Scholar]
- 35.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 36.Foreman MD. Reliability and validity of mental status questionnaires in elderly hospitalized patients. Nurs Res. 1987;36:216–20. [PubMed] [Google Scholar]
- 37.Anthony JC, LeResche L, Niaz U, et al. Limits of the “Mini-Mental State” as a screening test for dementia and delirium among hospital patients. Psychol Med. 1982;12:397–408. doi: 10.1017/s0033291700046730. [DOI] [PubMed] [Google Scholar]
- 38.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–73. [Google Scholar]
- 39.Herrmann N, Mittman N, Silver IL, et al. A validation study of the Geriatric Depression Scale short form. Int J Geriatr Psychiatry. 1996;11:457–60. [Google Scholar]
- 40.Ware JE., Jr SF-36 health survey update. Spine. 2000;25:3130–9. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 41.McHorney CA, Ware JE, Jr, Lu JF, et al. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 42.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Andresen EM, Gravitt GW, Aydelotte ME, et al. Limitations of the SF-36 in a sample of nursing home residents. Age Ageing. 1999;28:562–6. doi: 10.1093/ageing/28.6.562. [DOI] [PubMed] [Google Scholar]
- 44.Rosengren KS, Christou E, Yang Y, et al. Quantification of taiji learning in older adults. J Am Geriatr Soc. 2003;51:1186–7. doi: 10.1046/j.1532-5415.2003.51376.x. [DOI] [PubMed] [Google Scholar]
- 45.Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384–91. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 46.Lichstein KL, Riedel BW, Grieve R. Fair tests of clinical trials: a treatment implementation model. Adv Behav Res Ther. 1994;16:1–29. [Google Scholar]
- 47.Lee JS, Hobden E, Stiell IG, et al. Clinically important change in the visual analog scale after adequate pain control. Acad Emerg Med. 2003;10:1128–30. doi: 10.1111/j.1553-2712.2003.tb00586.x. [DOI] [PubMed] [Google Scholar]
- 48.Todd KH, Funk KG, Funk JP, et al. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27:485–9. doi: 10.1016/s0196-0644(96)70238-x. [DOI] [PubMed] [Google Scholar]