The Suzuki-Miyaura reaction has become one of the most valuable synthetic processes for the construction of carbon-carbon bonds,[1] and our laboratories has developed many highly active catalyst systems that efficiently process challenging combinations of aryl halides and boronic acids.[2] Recently, we have been able to extend our methodology to the cross-coupling of heteroaryl boronic acids and esters, which serve as important building blocks for the assembly of biologically active molecules.[3]-[4] However, 2-substituted nitrogen-containing heteroaryl organoboranes, which are of importance for the construction of numerous natural products and pharmaceutically interesting compounds,[5] were not effectively coupled using our standard conditions. Further examination of the literature indicated that only a few methods have been developed that allow for the Suzuki-Miyaura reaction of 2-pyridyl nucleophiles with aryl halides, and in these examples, only aryl iodides have been demonstrated as suitable coupling partners.[3],[6]-[10] The difficulty can be attributed to several factors: (1) electron-deficient heteroaryl boron derivatives undergo transmetallation at a relatively slow rate (2) these reagents rapidly decompose via a protodeboronation pathway. The lack of an efficient method to process this class of nucleophiles led us to develop a technique specifically designed to accomplish this transformation.
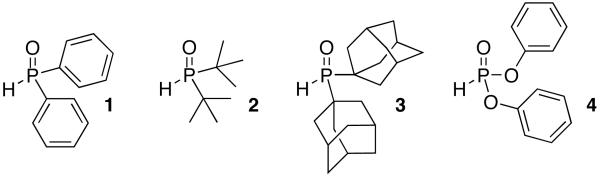
We found that catalysts based upon phosphite or phosphine oxide ligands (1-4) were highly active for the Suzuki-Miyaura reaction of 2-pyridyl boron derivatives with 1-bromo-4-butylbenzene (Scheme 1).[6] The use of these has been pioneered by the work of Li, and elegant applications by Ackermann and Wolf have appeared more recently. However, the reaction remained sensitive to the nature of the nucleophile and base. For example, the reaction of commercially-available reagents, such as 2-pyridyl boronic acid,[7] pinacol boronate ester[8] or N-phenyl diethanolamine boronate ester,[9] with 4-n-butylbromobenzene produced low yields of the desired biaryl product (Table 1, Entries 1-3). Similarly, attempts to use organotrifluoroborates resulted in a low conversion of the aryl bromide (Table 1, Entry 4).[10] Although 2-pyridylboronates have been employed in Suzuki-Miyaura reactions, the cross-coupling processes result in only poor to modest yields of the desired biaryl product.[11] However, when lithium triisopropyl 2-pyridyl boronate (A) was employed as the nucleophile, the desired product could be obtained in an 85% yield with 100% conversion of the aryl halide (Table 1, Entry 5). Although A is not yet commercially available, it is stable under an argon atmosphere for up to a month, and it can be prepared in near quantitative yield from 2-bromopyridine via lithium halogen exchange and immediate in situ quenching of the resulting anion with triisopropylborate. In addition, this reaction could be performed in multigram quantities to provide A in an  excellent yield. Lithium triisopropyl 2-(6-methoxypyridyl)boronate (B) and lithium triisopropyl 2-(5-fluoropyridyl)boronate (C) were also prepared employing this protocol in 90% and 96% yield, respectively. Similarly, under these conditions, 2-bromopyridines possessing a protected aldehyde (D) or a nitrile (E) could be efficiently transformed to the corresponding boronates.[12]
excellent yield. Lithium triisopropyl 2-(6-methoxypyridyl)boronate (B) and lithium triisopropyl 2-(5-fluoropyridyl)boronate (C) were also prepared employing this protocol in 90% and 96% yield, respectively. Similarly, under these conditions, 2-bromopyridines possessing a protected aldehyde (D) or a nitrile (E) could be efficiently transformed to the corresponding boronates.[12]
Scheme 1.
Effective Phosphite and Phosphine Oxide Ligands
Table 1.
The Effects of the Base and Nucleophile[a]
 | ||||
|---|---|---|---|---|
| Entry | Ar–BR3 | Base | GC Yield (%) | Conversion (%) |
| 1 |  |
KF NaOtBu |
0 8 |
<10 36 |
| 2 |  |
KF NaOtBu |
0 49 |
<10 73 |
| 3 |  |
KF NaOtBu |
6 15 |
43 100 |
| 4 |  |
KF NaOtBu |
0 10 |
<10 37 |
| 5 |  |
KF NaOtBu |
85 68 |
100 100 |
Reaction Conditions: 1 equiv of aryl or heteroaryl bromide, 1.5 equiv of 2-pyridylboronate, 3.0 equiv of base, Dioxane (3 mL/mmol halide), cat. Pd2dba3, L:Pd = 3:1.
A catalyst based upon Pd2dba3 and 1 proved to be highly effective for the Suzuki-Miyaura reactions of A with aryl and heteroaryl bromides. For example, this system efficiently combined 3,5-(bis-trifluoromethyl)bromobenzene (Table 2, Entry 2) and 4-bromoanisole (Table 2, Entry 3) with A to furnish the desired biaryl in 82% and 74% yield, respectively. In addition, ortho-substituted aryl bromides were coupled in good to excellent yields (Table 2, Entries 4-5). Heteroaryl bromides were also suitable coupling partners as seen in the reactions of A with 5-bromopyrimidine (Table 2, Entry 6) and 4-bromoisoquinoline (Table 2, Entry 7) which smoothly resulted in a 91% and 82% yield, respectively, of the desired heterobiaryl compound. Utilizing a Pd2dba3/2 catalyst, a range of lithium triisopropyl 2-pyridylboronates possessing functional groups were successfully cross-coupled with aryl bromides. Indeed, this catalyst system allowed for the reaction of B and C with a variety of electron-poor, -neutral, -rich and ortho-substituted aryl bromides (Table 2, Entries 9-12). In addition, the reaction of 4-bromobenzonitrile and D furnished the desired biaryl in a 63% yield (Table 2, Entry 13). However, the cross-coupling reactions utilizing E resulted in incomplete conversion in its reaction with a variety of aryl bromides. We attributed this difficulty to the relatively slow rate of transmetallation of the highly electron deficient 2-pyridylboronate. Overall, however, this protocol still represents the most general method for the Suzuki-Miyaura reaction of 2-pyridyl nucleophiles with aryl or heteroaryl bromides.
Table 2.
The Reaction of A-D with Aryl Bromides[a]
 | ||||
|---|---|---|---|---|
| Entry | Boronate | Ligand | Product | Yield (%)[b] |
| 1 | A | 1 | 85 | |
| 2 | A | 1 |  |
82 |
| 3 | A | 1 | 74 | |
| 4 | A | 1 |  |
87 |
| 5 | A | 1 |  |
90 |
| 6 | A | 1 |  |
91 |
| 7 | A | 1 |  |
82 |
| 8 | A | 1 |  |
73 |
| 9 | B | 2 |  |
90[c] |
| 10 | B | 2 |  |
61[c] |
| 11 | C | 2 | 65[c] | |
| 12 | C | 2 |  |
40[c] |
| 13 | D | 1 |  |
63[c] |
Reaction Conditions: 1 equiv of aryl or heteroaryl bromide, 1.5 equiv of 2-pyridylboronate, 3.0 equiv of KF, Dioxane (3 mL/mmol halide), cat. Pd2dba3, L:Pd = 3:1.
Isolated yield based upon an average of two runs.
1.5% Pd2dba3 used instead of 1.0%.
Despite the efficacy of the Pd2dba3/1 catalyst system for the reactions of lithium triisopropyl 2-pyridylboronates with aryl bromides, more modest yields of the desired biaryls were obtained in the reactions of the corresponding aryl or heteroaryl chlorides. Employing 2 as the supporting ligand, however, provided a more active catalyst for this transformation. For example, the reaction of A with 4-chlorobenzonitrile furnished the desired product in 73% yield (Table 3, Entry 1). In addition, unactivated aryl chlorides were efficiently coupled as the reactions of 4-n-butylchlorobenzene (Table 3, Entry 2) and 4-chloroanisole (Table 3, Entry 4) with A resulted in a 76% and 78% yield, respectively, of the desired product. Similarly, under these conditions, ortho-substituted aryl chlorides were suitable substrates as the reaction of 2-chloro-p-xylene and A proceeded in a 70% yield (Table 3, Entry 3). In addition, a heteroaryl chloride, 3-chloropyridine, was coupled with A in an excellent yield to give o,m-bipyridine (Table 3, Entry 6).
Table 3.
The Reaction of A and B with Aryl Chlorides[a]
Reaction Conditions: 1 equiv of aryl or heteroaryl bromide, 1.5 equiv of 2-pyridylboronate, 3.0 equiv of KF, Dioxane (3 mL/mmol halide), cat. Pd2dba3, L:Pd = 3:1.
Isolated yield based upon an average of two runs.
1.5% Pd2dba3 used instead of 1.0%.
In summary, we have developed an efficient method for the Suzuki-Miyaura reaction of lithium triisopropyl 2-pyridylboronates. The boronates can be readily prepared in one step from the corresponding 2-bromo or 2-iodopyridine. This represents the first relatively general Suzuki-Miyaura cross-coupling reaction of these substrates with aryl and heteroaryl bromides and chlorides.
Experimental Section
General procedure for the Pd-catalyzed Suzuki-Miyaura reaction of lithium triisopropyl 2-pyridylboronates with aryl bromides: An oven-dried resealable Schlenk tube possessing a Teflon screw valve was charged with Pd2dba3 (2.0-3.0%), 1 (6.0-9.0%), lithium triisopropyl 2-pyridylboronate (0.375 mmol) and anhydrous KF (43.5 mg, 0.75 mmol). The Schlenk tube was capped with a rubber septum and then evacuated and backfilled with argon (this sequence was carried out two times). 1,4-Dioxane (0.75 mL) was added via syringe, through the septum, followed by the addition of the aryl halide (0.25 mmol) in a like manner (aryl halides that were solids were added with the other solid reagents). The septum was then replaced with a Teflon screw valve and the Schlenk tube was sealed. The reaction mixture was heated to 110 °C until the aryl halide had been completely consumed as determined by gas chromatography and was allowed to cool to room temperature. The reaction solution was then filtered through a thin pad of silica gel (eluting with ethyl acetate) and the eluent was concentrated under reduced pressure. The crude material so obtained was purified via flash chromatography on silica gel.
Scheme 2.
Synthesis of Lithium Triisopropyl 2-Pyridylboronates
Footnotes
We thank the National Institute of Health (GM 46059) for support of this work. We are grateful to Merck, Amgen and Boehringer Ingelheim for additional support. The Varian NMR instruments used in this work were purchased with funding from the NSF (CHE 9808061 and DBI 9729592).
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Recent Reviews: Miyaura N. Top. Curr. Chem. 2002;219:11–59. Bellina F, Carpita A, Rossi R. Synthesis. 2004;15:2419. Miyaura N. In: Metal-Catalyzed Cross-Coupling Reaction. Diederich F, de Meijere A, editors. Wiley-VCH; New York: 2004. Chapter 2.
- 2.Barder TE, Walker SD, Martinelli JR, Buchwald SL. J. Am. Chem. Soc. 2005;127:4685–4696. doi: 10.1021/ja042491j. [DOI] [PubMed] [Google Scholar]
- 3.For a review of the Suzuki-Miyaura reaction of heteroaryl nucleophiles, see: Tyrell E, Brookes P. Synthesis. 2004;4:469–483.
- 4.Billingsley KL, Buchwald SL. J. Am. Chem. Soc. 2007;129:3358–3366. doi: 10.1021/ja068577p. [DOI] [PubMed] [Google Scholar]
- 5.a) Richardson CM, Gillespie RJ, Williamson DS, Jordan AM, Fink A, Knight AR, Sellwood DM, Misra A. Bioorg. Med. Chem. Lett. 2006;16:5993–5997. doi: 10.1016/j.bmcl.2006.08.116. [DOI] [PubMed] [Google Scholar]; b) Gellibert F, De Gouville A-C, Woolven J, Mathews N, Nguyen V-L, Bertho-Ruault C, Patikis A, Grygielko ET, Laping NJ, Huet S. J. Med. Chem. 2006;49:2210–2221. doi: 10.1021/jm0509905. [DOI] [PubMed] [Google Scholar]; c) Salama I, Hocke C, Utz W, Prante O, Boeckler F, Huebner H, Kuwert T, Gmeiner P. J. Med. Chem. 2007;50:489–500. doi: 10.1021/jm0611152. [DOI] [PubMed] [Google Scholar]; d) Meyers KM, Kim N, Mendez-Andino JL, Hu XE, Mumin RN, Klopfenstein SR, Wos JA, Mitchell MC, Paris JL, Ackley DC, Holbert JK, Mittelstadt SW, Reizes O. Bioorg. Med. Chem. Lett. 2007;17:814–818. doi: 10.1016/j.bmcl.2006.10.053. [DOI] [PubMed] [Google Scholar]; e) Anderson DR, Meyers MJ, Vernier WF, Mahoney MW, Kurumbail RG, Caspers N, Poda GI, Schindler JF, Reitz DB, Mourey RJ. J. Med. Chem. 2007;50:2647–2654. doi: 10.1021/jm0611004. [DOI] [PubMed] [Google Scholar]
- 6.For selected examples of phosphine oxides ligands in carbon-carbon bond forming cross-coupling processes, see: Li GY. Angew. Chem. 2001;113:1561. Angew. Chem., Int. Ed. 2001;40:1513–1516. doi: 10.1002/1521-3773(20010417)40:8<1513::aid-anie1513>3.0.co;2-c. Li GY, Marshall WJ. Organometallics. 2002;21:590–591. Wolf C, Lerebours R. J. Org. Chem. 2003;68:7551–7554. doi: 10.1021/jo0347056. Wolf C, Lerebours R. Org. Lett. 2004;6:1147–1150. doi: 10.1021/ol049851s. Lerebours R, Camacho-Soto A, Wolf C. J. Org. Chem. 2005;70:8601–8604. doi: 10.1021/jo051257o. Ackermann L, Born R, Spatz JH, Meyer D. Angew. Chem. 2005;117:7382. doi: 10.1002/anie.200501860. Angew. Chem., Int. Ed. 2005;44:7216–7219. doi: 10.1002/anie.200501860. Ackermann L, Althammer A. Org. Lett. 2006;8:3457–3460. doi: 10.1021/ol061116o. Wolf C, Ekoue-Kovi K. Eur. J. Org. Chem. 2006:1917–1925.
- 7.For examples of Suzuki-Miyaura reactions of 2-pyridyl boronic acids, see: Deshayes K, Broene RD, Chao I, Knobler CB, Diederich F. J. Org. Chem. 1991;56:6887–6895. Sindkhedkar MD, Mulla HR, Wirth MA, Cammers-Goodwin A. Tetrahedron. 2001;57:2991–2996. Mandolesi SD, Vaillard SE, Podestá JC, Rossi RA. Organometalllics. 2002;21:4886–4888. Bouillon A, Lancelot J-C, Sopkova de Oliveira Santos J, Collot V, Bovy PR, Rault S. Tetrahedron. 2003;59:10043–10049. Shinozuka T, Shimada K, Matsui S, Tamane T, Ama M, Fukuda T, Taki M, Takeda Y, Otsuka E, Yamato M, Naito S. Bioorg. Med. Chem. 2006;14:6807–6819. doi: 10.1016/j.bmc.2006.06.031.
- 8.During the development of this work, we discovered unpublished results from CombiPhos Catalysts, Inc. utilizing 2-pyridyl pinacol boronate esters. Chlorodialkyl phosphines and dialkyl phosphine oxides were used as supporting ligands. However, we found that 2-pyridyl pinacol boronate ester was not effectively coupled under our conditions as seen in Table 1.
- 9.For examples of Suzuki-Miyaura reactions of 2-pyridyl N,N-diethanolamine boronate esters, see: Hodgson PB, Salingue FH. Tetrahedron Lett. 2004;45:685–687. Gros P, Doudouh A, Fort Y. Tetrahedron Lett. 2004;45:6239–6241.
- 10.For work with 2-pyridyltrifluoroborates, see: Molander GA, Biolatto B. J. Org. Chem. 2003;68:4302–4314. doi: 10.1021/jo0342368.
- 11.a) Dube D, Fortin R, Friesen R, Wang Z, Gauthier J, Merck Frost Canada Inc. Substituted Pyridines as Selective Cyclooxygenase-2 Inhibitors. 1998 WO 98/03484. [Google Scholar]; b) O'Neill BT, Yohannes D, Bundesmann MW, Arnold EP. Org. Lett. 2000;2:4201–4204. doi: 10.1021/ol0067538. [DOI] [PubMed] [Google Scholar]; c) Ahman B, Hodgson P, Lewandowski S, Walton R, Pfizer Inc. Process for the Production of Quinazolines. 2002 WO 02/094815. [Google Scholar]
- 12.For a related protocol for the synthesis of 3-pyridine boronic acid, see: Li W, Nelson DP, Jensen MS, Hoerrner RS, Cai D, Larsen RD, Reider PJ. J. Org. Chem. 2002;67:5394–5397. doi: 10.1021/jo025792p.