Abstract
Background
The impact of overall dietary patterns that reflect actual eating behaviors on mortality due to cardiovascular or other chronic diseases is largely unknown.
Methods and Results
We prospectively evaluated the relation between dietary patterns and risk of cardiovascular, cancer, and all-cause mortality among 72,113 women who were free of myocardial infarction, angina, coronary artery surgery, stroke, diabetes, or cancer, and were followed from 1984 to 2002. Dietary patterns were derived by factor analysis based on validated food frequency questionnaires administrated every 2 to 4 years. Two major dietary patterns were identified: high prudent pattern scores represented high intakes of vegetables, fruit, legumes, fish, poultry, and whole grains, whereas high western pattern scores reflected high intakes of red meat, processed meat, refined grains, french fries, and sweets/desserts. During 18 years of follow-up, 6,011 deaths occured, including 1,154 cardiovascular deaths and 3,139 cancer deaths. After multivariable adjustment, the prudent diet was associated with a 28% lower risk of cardiovascular mortality (95% confidence interval 13 to 40%) and a 17% lower risk of all-cause mortality (10 to 24%) when comparing the highest to the lowest quintile. In contrast, the western pattern was associated with a higher risk of mortality from cardiovascular disease (22%, 1 to 48%), cancer (16%, 3 to 30%), and all-causes (21%, 12 to 32%).
Conclusion
Greater adherence to the prudent pattern may reduce the risk of cardiovascular and total mortality, whereas greater adherence to the western pattern may increase the risk among initially healthy women.
Keywords: diet, cardiovascular diseases, mortality, epidemiology
Different dietary components have been suggested as important modifiable risk factors for chronic diseases, especially for cardiovascular diseases (CVD)1 that are the leading causes of death in the United States and other westernized countries.2 While traditionally nutritional research has primarily focused on single nutrients or foods, there is currently a growing interest in dietary patterns that consider the complexity of overall diet.3,4
Two major approaches have been applied to derive dietary patterns.4 The hypothesis-oriented approach based on scientific evidence or prevailing dietary recommendations typically uses dietary indices or scores to reflect the quality of the diet or the degree of adherence to a particular, predefined diet. In contrast, the exploratory approach using factor or cluster analysis empirically identifies patterns that represent actual eating behaviors of the study population; typically these are two to six extracted patterns that reflect different dietary compositions.5
Recently, several studies have examined the impact of dietary indices on the risk of cardiovascular or total mortality. These indices were defined on the basis of general dietary recommendations or characteristics of the Mediterranean diet.6,7 To a lesser extent studies have investigated the relation of dietary patterns that reflect existing eating habits to mortality from CVD,8-10 other major chronic diseases,10,11 or all-causes.8,10,-18 The majority of these studies were small and inadequately powered. Therefore, the aim of the present study was to evaluate the potential impact of major dietary patterns derived by factor analysis on subsequent risk of mortality due to CVD, cancer, and all-causes in a large cohort study of women.
Methods
Study Population
The Nurses' Health Study (NHS) was established in 1976, when 121,700 female US nurses aged 30 to 55 years, reflecting the racial composition of women trained as registered nurses at that time (97% were Caucasian), responded to a questionnaire on health-related factors.19 Since 1976, this cohort has been followed using a biennially mailed questionnaire. The follow-up rate exceeds 90% of the potential person-time for every 2-year period.19 For the present analysis, we included women who had completed a food frequency questionaire (FFQ) in 1984 with less than 70 missing items and a range of total energy intake between 500 and 3500 kcal/d (n=81,757). From this sample we excluded all women with missing information on age (n=34) or BMI at baseline (n=98) and who had reported a history of cancer (n=4,451), myocardial infarction (n=824), angina (n=1,747), coronary artery bypass surgery (n=63), stroke (n=275), or diabetes (n=2,152) before 1984. The final analytical cohort comprised 72,113 women with a follow-up from 1984 to 2002. The study was approved by the Institutional Review Board of the Brigham and Women's Hospital, Boston, MA.
Assessment of Dietary Intake and Dietary Patterns
Dietary intake was assessed by validated, semi-quantitative FFQs administered in 1984, 1986, 1990, 1994, and 1998. Each FFQ assessed how often, on average, a specified portion size of at least 116 food items was consumed during the past year. Frequency of intake was measured using nine categories, ranging from “never or less than once per month” to “6 or more times per day”. Values for nutrients were derived from the U.S. Department of Agriculture sources20 and supplemented with information from manufactures. Intakes of dietary fiber, folate, and trans-fat were energy adjusted by the residual method.21
To identify dietary patterns, the items of the FFQs were first aggregated into 37 to 39 food groups (Table 1). The classification of food groups was based on similarities in nutrient profile and culinary preference following the classification of a previous study in these women.22 Second, we applied factor analysis (principal component analysis) with the orthogonal rotation procedure varimax to the predefined food groups; a method described in detail elsewhere.23 Briefly, each obtained dietary pattern (called factor) represents a linear combination of all food groups, which are weighted by their factor loadings, and explains as much interindividual variation of the food groups as possible. Each subject receives a score for each dietary pattern with a higher score indicating a higher adherence to the respective pattern. We determined the dietary patterns to retain based on the Scree test (a graphical presentation of eigenvalues, with eigenvalues >1 explaining more variance than an individual food group) and the interpretability of factors.23 The scree test allowed us to identify two major patterns with the largest eigenvalues (>2.75). Similar to our previous analyses,22 these patterns were labeled as the “prudent” and the “western” patterns. The reproducibility and relative validity of dietary patterns derived by factor analysis have been previously shown to be reasonably good.24
Table 1.
Factor-loadings for Food Groups of the Two Major Dietary Patterns Identified from Food Frequency Questionnaires in 1984, 1986, 1990, 1994, and 1998 in Women of the NHS*
| Food group | 1984 |
1986 |
1990 |
1994 |
1998 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prudent | Western | Prudent | Western | Prudent | Western | Prudent | Western | Prudent | Western | |
| Other vegetables | 0.68 | - | 0.72 | - | 0.69 | - | 0.71 | - | 0.71 | - |
| Green, leafy vegetables | 0.65 | - | 0.64 | - | 0.64 | - | 0.64 | - | 0.63 | - |
| Cruciferous vegetables | 0.61 | - | 0.60 | - | 0.61 | - | 0.63 | - | 0.62 | - |
| Legumes | 0.59 | - | 0.56 | - | 0.58 | - | 0.59 | - | 0.57 | - |
| Dark-yellow vegetables | 0.58 | - | 0.63 | - | 0.65 | - | 0.64 | - | 0.62 | - |
| Fruit | 0.58 | - | 0.59 | - | 0.57 | - | 0.58 | - | 0.59 | - |
| Fish | 0.51 | - | 0.53 | - | 0.51 | - | 0.47 | - | 0.50 | - |
| Tomatoes | 0.46 | 0.18 | 0.55 | 0.19 | 0.49 | 0.17 | 0.52 | - | 0.53 | - |
| Poultry | 0.44 | - | 0.41 | - | 0.42 | - | 0.34 | - | 0.40 | 0.17 |
| Whole grains | 0.39 | - | 0.39 | - | 0.41 | - | 0.40 | - | 0.40 | - |
| Salad dressing | 0.36 | - | 0.38 | - | 0.34 | - | 0.31 | - | 0.33 | - |
| Low-fat dairy | 0.32 | - | 0.32 | - | 0.32 | - | 0.33 | -0.20 | 0.28 | - |
| Fruit juice | 0.24 | - | 0.25 | - | 0.22 | - | 0.23 | - | 0.22 | - |
| Nuts | 0.18 | - | 0.22 | 0.20 | 0.18 | 0.20 | - | 0.26 | 0.19 | 0.21 |
| Egg white | NA | NA | NA | NA | NA | NA | NA | NA | 0.22 | - |
| Olive oil | NA | NA | NA | NA | 0.31 | - | 0.32 | - | 0.39 | - |
| Organ meats | 0.23 | - | - | - | - | - | - | - | - | - |
| Tea | - | - | - | - | - | - | - | - | 0.19 | - |
| Wine | - | - | - | - | - | - | - | - | - | - |
| Low-sugar beverages | - | - | - | - | - | - | - | 0.15 | - | 0.21 |
| Refined grains | - | 0.58 | - | 0.58 | - | 0.52 | - | 0.46 | - | 0.50 |
| Processed meat | - | 0.57 | - | 0.58 | - | 0.60 | - | 0.58 | - | 0.58 |
| Red meat | - | 0.55 | - | 0.57 | - | 0.60 | - | 0.61 | - | 0.62 |
| French fries | - | 0.47 | - | 0.48 | - | 0.47 | - | 0.47 | - | 0.48 |
| Condiments | - | 0.45 | - | 0.32 | - | 0.35 | - | 0.32 | - | 0.36 |
| Sweets and desserts | - | 0.43 | - | 0.49 | - | 0.43 | - | 0.41 | - | 0.36 |
| Potatoes | - | 0.39 | - | 0.36 | 0.23 | 0.30 | 0.24 | 0.29 | 0.21 | 0.33 |
| High-fat diary | - | 0.37 | - | 0.42 | - | 0.48 | - | 0.47 | - | 0.45 |
| Pizza | - | 0.35 | - | 0.35 | - | 0.36 | - | 0.34 | - | 0.39 |
| Mayonnaise | 0.19 | 0.34 | 0.19 | 0.35 | - | 0.35 | 0.21 | 0.33 | 0.23 | 0.33 |
| High-sugar beverages | -0.16 | 0.32 | - | 0.32 | - | 0.32 | - | 0.29 | - | 0.30 |
| Eggs | 0.16 | 0.30 | - | 0.33 | - | 0.42 | - | 0.44 | - | 0.40 |
| Margarine | - | 0.29 | - | 0.27 | - | 0.28 | - | 0.31 | - | 0.25 |
| Snacks | - | 0.28 | - | 0.32 | - | 0.28 | - | 0.32 | 0.16 | 0.32 |
| Butter | - | 0.27 | - | 0.29 | - | 0.33 | - | 0.33 | - | 0.31 |
| Soups | - | 0.22 | 0.27 | 0.29 | - | 0.31 | - | 0.34 | - | 0.32 |
| Coffee | - | - | - | - | - | 0.15 | - | 0.16 | - | 0.19 |
| Beer | - | - | - | - | - | - | - | - | - | - |
| Liquor | - | - | - | - | - | - | - | - | - | - |
Factor loadings are identical to Pearson correlation coefficients. Factor loadings with absolute values <0.15 are not shown for simplicity. NA, not applicable because the particular item was not on that year's questionnaire.
Because under- or overreporting of food items may result in an increased extraneous variation, dietary pattern scores were energy adjusted using the residual method.21 To additionally reduce random within-person variation and best reflect long-term dietary intake, we calculated cumulative averages of the dietary pattern scores as described previously.25 Therefore, the mortality risk for each follow-up cycle was related to the average of dietary pattern scores derived from all preceding FFQs. For example, the dietary pattern score from the FFQ in 1984 was used to predict mortality risk from 1984 to 1986, whereas the average of dietary pattern scores from the FFQs in 1984 and 1986 was used to predict risk from 1986 to 1990, and the average of dietary pattern scores from the FFQs in 1984, 1986, and 1990 was used to predict risk from 1990 to 1994, and so on. We stopped updating a woman's dietary pattern scores at the beginning of the follow-up period during which she reported a diagnosis of CVD, diabetes, or cancer because changes in dietary habits following these diagnoses could confound the association between diet and mortality.25 We also conducted a secondary analysis in which we only used the baseline (1984) dietary pattern scores.
Assessment of Nondietary Variables
Information on age, body weight, cigarette smoking, menopausal status, use of hormone replacement therapy, history of hypertension, and use of multivitamin supplements was provided biennially by self-report of participants. Body mass index (BMI) was calculated as the ratio of weight (in kg) to squared height (in m2), with the latter being assessed at baseline of the NHS only. The reported body weights of the participants have been shown to be highly correlated with technician-measured weights (r=0.96).19 Physical activity was assessed every 2 to 4 years and was expressed as average time spent on physical activities of at least moderate intensity per week.26
End Point Ascertainment
Deaths were reported by family members or postal authorities or, for persistent nonresponders, were ascertained through searches of the National Death Index. The cause of death was assigned by physician reviewers primarily based on medical records if both medical records and death certificates were available. For the present analysis, all deaths due to CVD (International Classification of Diseases, Eighth Revision [ICD-8] codes 390 through 458), cancer (ICD-8 codes 140 through 207) or other causes (deaths excluding cardiovascular and cancer deaths) that occurred between the return date of the 1984 questionnaire and June 2002 were included in the analysis. The follow-up for death in the NHS has been estimated to be 98% complete.27
Statistical Analysis
Relative risks (RRs) for each quintile of the dietary pattern scores were estimated by Cox proportional hazards regression using the lowest quintile as the reference category. Person-time of follow-up was defined as period from the return date of the questionnaire mailed to participants in 1984 until the date of death or the end of follow-up (June 30, 2002). All analyses were stratified by age and follow-up period. In multivariable analyses, RRs were adjusted for BMI, physical activity, smoking, hormone replacement therapy, history of hypertension, use of multivitamin supplements, missing FFQ during follow-up, and total energy intake. In our primary analysis, when associations between cumulatively averaged dietary patterns and mortality were examined, cumulatively averaged values of energy intake and physical activity were included. We also updated information on all other covariates (except for history of hypertension) using the most recent data for each 2-year cycle of follow-up. Trend tests were conducted by including the median score of each pattern quintile as a continuous variable into the models. Additionally, we examined the possible non-linear relation of dietary pattern scores to the risk of mortality non-parametrically using restricted cubic spline regression with 4 knots.28 Tests for non-linearity were conducted using the likelihood ratio test,29 comparing the model including only the linear term with the model including the linear and cubic spline terms.
Further, we conducted stratified analyses to investigate whether the observed association between dietary patterns and risk of mortality was modified by age (<60 vs. ≥60 years), physical activity level (≤3.5 vs. >3.5 hours/week), smoking status (current smokers vs. nonsmokers), or overweight status (<25 vs. ≥25 kg/m2). Interaction tests were performed by including a product term with the respective stratification variable and the median score of the pattern quintile as a continuous variable in the model, using the likelihood ratio test.29 All statistical analyses were performed using SAS software 9.1 (SAS Institute Inc., Cary, NC).
The authors had full access to and take responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Two major dietary patterns, each comprising at least 37 food groups, were identified for each cycle of the follow-up including an FFQ (Table 1). High factor loading scores for the pattern labeled as “prudent” represented a high consumption of vegetables, fruit, legumes, fish, poultry, and whole grains, whereas high factor loading scores for the pattern labeled as “western” corresponded to a high consumption of red meat, processed meat, refined grains, french fries, and sweets and desserts.
Table 2 shows the characteristics of the 72,113 women eligible for this study according to quintiles of the dietary patterns scores at baseline. Women with higher prudent pattern scores were slightly older, exercised more, were less likely to be smokers and more likely to use hormone replacement therapy and multivitamin supplements, and had a more advantageous nutrient profile than those with lower sores for this pattern. In contrast, women with higher western pattern scores were younger, less physically active, more likely to smoke, less likely to use hormone replacement therapy and multivitamin supplements, and had a more unfavorable nutrient profile than those who scored low on this pattern.
Table 2.
Baseline Characteristics by Quintile of Dietary Patterns Among Women of the NHS (1984)
| Characteristic | Quintile of the Prudent Pattern |
P for Trend | Quintile of the Western Pattern |
P for Trend | ||||
|---|---|---|---|---|---|---|---|---|
| 1 (Lowest) | 3 | 5 (Highest) | 1 (Lowest) | 3 | 5 (Highest) | |||
| Age, y | 48.3 | 50.9 | 52.5 | <0.001 | 52.9 | 50.5 | 48.6 | <0.001 |
| BMI, kg/m2 | 24.8 | 24.9 | 25.0 | <0.001 | 24.3 | 24.7 | 25.5 | <0.001 |
| Physical activity, h/wk | 2.1 | 2.4 | 2.8 | 0.005 | 2.7 | 2.4 | 2.2 | <0.001 |
| Current smoking, % | 33.3 | 22.7 | 18.1 | <0.001 | 13.9 | 23.6 | 36.2 | <0.001 |
| Current hormone therapy, % | 9.5 | 11.0 | 12.3 | <0.001 | 12.7 | 11.1 | 9.3 | <0.001 |
| History of hypertension, % | 19.5 | 19.9 | 21.9 | <0.001 | 20.3 | 19.4 | 20.9 | 0.08 |
| Multivitamin supplements, % | 30.3 | 37.2 | 43.9 | <0.001 | 46.8 | 36.3 | 28.3 | <0.001 |
| Dietary intake | ||||||||
| Alcohol, g/d | 7.4 | 7.0 | 6.8 | <0.001 | 7.0 | 7.1 | 6.9 | 0.18 |
| PUFA:SFA ratio | 0.48 | 0.55 | 0.64 | <0.001 | 0.59 | 0.54 | 0.52 | <0.001 |
| MUFA:SFA ratio | 1.01 | 1.03 | 1.04 | <0.001 | 1.01 | 1.03 | 1.03 | <0.001 |
| Trans-fatty acids, g/d | 3.8 | 3.5 | 2.8 | <0.001 | 2.6 | 3.5 | 4.0 | <0.001 |
| Fiber, g/d | 12.3 | 16.0 | 21.3 | <0.001 | 19.8 | 15.8 | 13.9 | <0.001 |
| Folate, μg/d | 207 | 268 | 356 | <0.001 | 330 | 268 | 229 | <0.001 |
Values are means unless otherwise indicated. Data, except age, were directly standardized to the age distribution of the cohort. PUFA, polyunsaturated fatty acids. SFA, saturated fatty acids. MUFA, monounsaturated fatty acids.
During 1,249,469 person-years, we ascertained 6,011 deaths, including 1,154 deaths from CVD, 3,139 deaths from cancer, and 1,718 deaths from other causes. After adjustment for age, the cumulatively averaged prudent pattern was significantly and inversely associated with mortality due to CVD, cancer, and other causes as well as with all-cause mortality (Table 3). After adjusting for potential confounders, the observed associations were attenuated. However, the decrease in risk for women in the highest compared with the lowest quintile of the prudent diet remained significant for cardiovascular mortality (28%, 95% confidence interval 13 to 40%), mortality from other causes (30%, 19 to 40%), and total mortality (17%, 10 to 24%). The association between the prudent pattern and mortality from cancer was no longer significant. For the cumulatively averaged western pattern, significantly positive associations with cause-specific and all-cause mortality were observed after adjustment for age. Again, these associations were attenuated after accounting for potential confounders. However, the increase in risk for participants comparing extreme quintiles of the western diet remained significant for cardiovascular mortality (22%, 1 to 48%), cancer mortality (16%, 3 to 30%), mortality due to other causes (31%, 12 to 52%), and total mortality (21%, 12 to 32%). The attenuation of risk in the multivariable compared to the age-adjusted model was most apparent after adjustment for physical activity and smoking. The major causes of other mortality were diseases of the respiratory system (463 deaths), which substantially contributed to the observed association between the patterns and the risk of other mortality.
Table 3.
RRs (95% CIs) of Mortality by Quintile of Dietary Patterns among women of the NHS (1984-2002)
| Variable | Quintile of Dietary Pattern |
|||||
|---|---|---|---|---|---|---|
| 1 (Lowest) | 2 | 3 | 4 | 5 (Highest) | P for Trend | |
| Prudent pattern | ||||||
| Cardiovascular mortality | ||||||
| Deaths, n/person-years | 278/252,727 | 225/254,676 | 240/251,645 | 201/249,771 | 210/240,650 | |
| Age-adjusted | 1.0 | 0.67 (0.56-0.80) | 0.65 (0.55-0.77) | 0.49 (0.41-0.59) | 0.50 (0.42-0.60) | <0.001 |
| Multivariable* | 1.0 | 0.78 (0.65-0.93) | 0.85 (0.71-1.01) | 0.69 (0.57-0.83) | 0.72 (0.60-0.87) | <0.001 |
| Cancer mortality | ||||||
| Deaths, n/person-years | 632/252,727 | 605/254,676 | 610/251,645 | 644/249,771 | 648/240,650 | |
| Age-adjusted | 1.0 | 0.83 (0.75-0.93) | 0.78 (0.70-0.87) | 0.78 (0.70-0.87) | 0.77 (0.69-0.86) | <0.001 |
| Multivariable* | 1.0 | 0.92 (0.82-1.03) | 0.93 (0.83-1.05) | 0.96 (0.85-1.07) | 0.98 (0.87-1.10) | 0.97 |
| Other mortality | ||||||
| Deaths, n/person-years | 436/252,727 | 349/254,676 | 309/251,645 | 300/249,771 | 324/240,650 | |
| Age-adjusted | 1.0 | 0.67 (0.58-0.77) | 0.54 (0.47-0.63) | 0.49 (0.42-0.57) | 0.52 (0.45-0.60) | <0.001 |
| Multivariable* | 1.0 | 0.79 (0.68-0.91) | 0.71 (0.61-0.83) | 0.67 (0.58-0.78) | 0.70 (0.60-0.81) | <0.001 |
| Total mortality | ||||||
| Deaths, n/person-years | 1,346/252,727 | 1,179/254,676 | 1,159/251,645 | 1,145/249,771 | 1,182/240,650 | |
| Age-adjusted | 1.0 | 0.75 (0.69-0.81) | 0.68 (0.62-0.73) | 0.62 (0.58-0.67) | 0.63 (0.58-0.68) | <0.001 |
| Multivariable* | 1.0 | 0.85 (0.78-0.92) | 0.84 (0.78-0.91) | 0.81 (0.74-0.88) | 0.83 (0.76-0.90) | <0.001 |
| Western pattern | ||||||
| Cardiovascular mortality | ||||||
| Deaths, n/person-years | 216/244,089 | 208/250,155 | 230/252,459 | 246/253,060 | 254/249,706 | |
| Age-adjusted | 1.0 | 1.10 (0.91-1.33) | 1.37 (1.14-1.65) | 1.66 (1.38-2.00) | 1.95 (1.62-2.34) | <0.001 |
| Multivariable* | 1.0 | 0.98 (0.81-1.19) | 1.13 (0.93-1.36) | 1.20 (0.99-1.45) | 1.22 (1.01-1.48) | 0.009 |
| Cancer mortality | ||||||
| Deaths, n/person-years | 620/244,089 | 597/250,155 | 649/252,459 | 637/253,060 | 636/249,706 | |
| Age-adjusted | 1.0 | 1.04 (0.93-1.17) | 1.23 (1.10-1.37) | 1.31 (1.17-1.47) | 1.46 (1.30-1.63) | <0.001 |
| Multivariable* | 1.0 | 0.99 (0.88-1.11) | 1.11 (0.99-1.25) | 1.11 (0.99-1.24) | 1.16 (1.03-1.30) | 0.004 |
| Other mortality | ||||||
| Deaths, n/person-years | 343/244,089 | 318/250,155 | 315/252,459 | 353/253,060 | 389/249,706 | |
| Age-adjusted | 1.0 | 1.04 (0.89-1.21) | 1.13 (0.97-1.32) | 1.42 (1.22-1.65) | 1.77 (1.53-2.05) | <0.001 |
| Multivariable* | 1.0 | 1.02 (0.87-1.19) | 1.05 (0.90-1.23) | 1.21 (1.03-1.41) | 1.31 (1.12-1.52) | <0.001 |
| Total mortality | ||||||
| Deaths, n/person-years | 1,179/244,089 | 1,123/250,155 | 1,194/252,459 | 1,236/253,060 | 1,279/249,706 | |
| Age-adjusted | 1.0 | 1.05 (0.97-1.14) | 1.23 (1.13-1.33) | 1.41 (1.30-1.53) | 1.64 (1.51-1.77) | <0.001 |
| Multivariable* | 1.0 | 1.00 (0.92-1.08) | 1.10 (1.02-1.20) | 1.16 (1.06-1.26) | 1.21 (1.12-1.32) | <0.001 |
Adjusted for age (months), follow-up period (2-y intervals), BMI (<23, 23-24.9, 25-26.9, 27-29.9, 30-34.9, ≥35 kg/m2), physical activity (≤0.5, 0.6-2, 2.1-3.5, 3.6-5, >5 h/wk), smoking (never, past, 1-14, ≥15 cigarettes/d, missing information), hormone replacement therapy (premenopause, never, current, past, missing information), history of hypertension (yes/no), use of multivitamin supplements (yes/no), missing food frequency questionnaire during follow-up (yes/no), and total energy intake (quintiles).
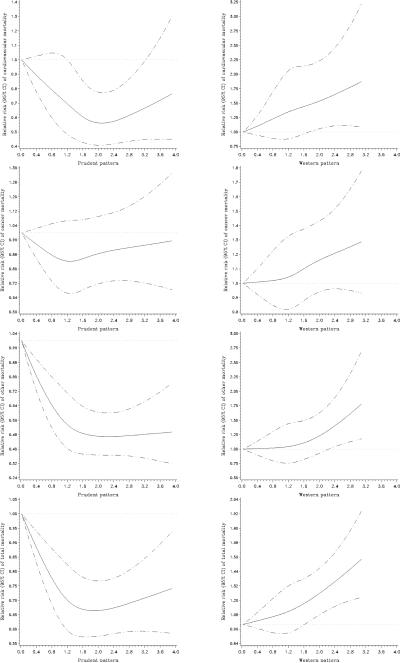
Secondary analyses using baseline dietary pattern scores yielded similar results, although the associations were somewhat weaker (data not shown). Spline regression models showed a linear relation of the western pattern to cause-specific mortality as well as to total mortality (P for non-linearity all > 0.05) (Figure). For the prudent pattern, as already suggested by the quintile-specific RRs, a deviation from linearity was revealed for cardiovascular, other, and total mortality, (P for non-linearity all < 0.05) and no association was observed with cancer mortality. Additional analyses showed no significant interaction between patterns and either age, physical activity level, smoking status, or overweight status in terms of cause-specific and all-cause mortality (P for interaction all > 0.05) (data not shown).
Figure.
RRs (95% CIs) of continuous dietary pattern scores for mortality among women of the NHS (1984-2002).
RRs (solid black lines) and 95% CIs (dotted lines) were derived from spline regression models to examine the possible non-linear relation of dietary pattern scores to mortality (adjusted for variables in the multivariable model in Table 3). For simplicity of presentation, the reference values of dietary pattern scores (z-scores) were set to zero.
Discussion
In this large cohort study of women, we derived two major dietary patterns. Greater adherence to the prudent pattern, characterized by a high intake of vegetables, fruit, legumes, fish, poultry, and whole grains, was related to a lower risk of cardiovascular and total mortality. In contrast, greater adherence to the western pattern, reflecting a high intake of red and processed meat, refined grains, french fries, and sweets and desserts, was linked to a higher risk of cardiovascular, cancer, and total mortality.
The relation of overall dietary patterns with mortality due to CVD or other chronic diseases has not been widely examined. Similar to our study, the “prudent” pattern (characterized by a frequent intake of fruits, vegetables, and wholemeal bread) was associated with a decreased risk of cardiovascular mortality in Danish women.8 In recent studies including Asian populations, a “vegetable-rich” pattern10 and a pattern characterized by a frequent consumption of vegetables, fruit, soy products, seaweeds, and fish9 were inversely related to cardiovascular mortality, whereas a pattern characterized by a frequent consumption of meat and butter was directly related to this outcome.9 The results of the present study for the association between patterns and the major cause of other mortality, i.e. diseases of the respiratory system, are in line with previous findings on dietary patterns and nonmalignant respiratory outcomes.30,31
Further, several previous studies have examined the relation between overall dietary patterns and all-cause mortality. In a Japanese cohort study, a dietary factor reflecting a frequent intake of plant foods such as green-yellow vegetables, fruit, soybean products, seaweeds and potatoes was inversely related to all-cause mortality.14 Among Danish men and women, the “prudent” pattern was associated with a decreased risk of total mortality.8 In an U.S. study, a lower risk of total mortality was observed for the “fruit-vegetables-whole grain” pattern among men.12 In different European elderly populations, a plant-based dietary pattern was linked to a reduced risk of all-cause mortality13,17,18 and in German elderly men and women, a pattern reflecting high intakes of all types of meat, condiments, butter, and eggs was related to an increased risk of all-cause mortality.15 Among British women, a reduced risk of total mortality was observed for a pattern defined by frequent intakes of fruit, salad, vegetables, and brown bread, whereas an increased risk was found for a pattern characterized by frequent intakes of chips, crisps, fried food, processed meat, and soft drinks.16 Despite these positive results, other findings did not indicate a significant association between dietary patterns and all-cause mortality.8,11,32 These inconsistent results may be due to specific population characteristics such as gender or prevalent diseases. Further, inconsistencies may be explainable by differences in dietary assessment methods. For example, in a Danish study,8 the “western” pattern was based on 28 assessed food items compared to at least 116 items that were classified into 37 to 39 food groups in our study.
The results of the present study are supported by previous analyses of the association between dietary patterns and chronic diseases and biomarkers among women of our or a similar cohort, respectively. In particular, the prudent pattern was favorably associated with the risk of coronary heart disease,33 weight maintenance,34 and plasma concentrations of markers of inflammation and endothelial dysfunction,35 which could have contributed to the observed inverse relation of the prudent pattern to cardiovascular mortality in the present study. Conversely, the western pattern showed a positive association with the risk of coronary heart disease,33 stroke,36 type 2 diabetes,37 weight gain,34 and concentrations of inflammatory and endothelial markers.35 However, the prudent diet was not significantly related to the risk of postmenopausal breast cancer,22 colorectal cancer,38 or pancreatic cancer,39 which are among the main causes of mortality from cancer in women. Thus, it is not surprising that we found no significant association between the prudent pattern and cancer mortality after accounting for potential confounders in the present study. However, the western pattern was directly related to the risk of colon cancer in a previous study38 and directly associated with cancer mortality in the present study. The different associations between the patterns and specific diseases and causes of death may be due to different effects of characteristic pattern components on specific outcomes. Thus, a high consumption of fruit and vegetables, two main components of the prudent pattern, has been shown to be linked to a decreased risk of CVD,40,41 whereas the evidence from prospective studies for a reduced risk is limited for most cancer sites.42 The results of our study are further strengthened by distinctive nutrient compositions of the prudent and the western pattern. For example, the intake of trans-fatty acids - a recognized risk factor for CVD1 - was inversely associated with the prudent pattern and the intake of fiber and folate - which have been shown to be associated with lower CVD risk1 - was directly related to the prudent pattern, whereas an opposite trend in nutrient intake was evident for the western pattern (see Table 2).
The large size of the cohort and long duration of follow-up provided adequate power for the analyses of cause-specific deaths and for the stratified analyses. The prospective design and the high rate of follow-up minimized the possibility of recall and selection biases. Another unique feature of this study is the existence of repeated measures of diet, which allowed us to calculate cumulative averages of dietary intakes to best represent long-term diet and reduce measurement errors.
Several limitations of this study need to be acknowledged. The dietary patterns identified by factor analysis represent existing eating habits of the study population, but do not necessarily reflect optimal diets with the greatest impact on mortality. In addition, factor analysis involves the subjectivity in selecting and grouping the food items, choosing the method of factor rotation, and determining the numbers of patterns to be retained.43 Variations in these criteria may induce variations in the composition of identified patterns and in the observed diet-disease associations. However, we defined the food groups and patterns using a standard method applied in numerous previous studies. Further, dietary patterns may represent a lifestyle in general43 and even though we carefully adjusted for known and suspected confounder variables, residual confounding cannot be ruled out due to the observational nature of this study. Finally, our study population was rather homogenous in terms of occupational class, ethnic group, and gender, which reduces residual confounding, but limits the generalizability of results.
In conclusion, in this large cohort study we found that women with higher prudent pattern scores had a lower long-term risk of cardiovascular and all-cause mortality, whereas women with higher western pattern scores had a higher long-term risk of cardiovascular, cancer, and all-cause mortality. These data highlight the importance of health professionals' and public health efforts that help to adopt healthy overall dietary patterns including high intakes of plant foods such as vegetables, fruit, legumes, and whole grains, high intakes of fish and poultry, and low intakes of red and processed meat, refined grains, french fries, and sweets.
Acknowledgments
Funding Sources This study was supported by NIH grants CA87969, HL60712, and CA95589. C.H. was supported by fellowships of the German Academic Exchange Service and the Hans & Eugenia Juetting-Foundation. C.M. is supported by a discretionary grant by Beth Israel Deaconess Medical Center.
Footnotes
Disclosures None.
References
- 1.Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech Rep Ser. 2003;916:i–viii. 1–149. [PubMed] [Google Scholar]
- 2.WHO WHO Mortality Database. Available at: http://www.who.int/healthinfo/morttables/en/index.html. Accessed February, 2008.
- 3.Zarraga IG, Schwarz ER. Impact of dietary patterns and interventions on cardiovascular health. Circulation. 2006;114:961–73. doi: 10.1161/CIRCULATIONAHA.105.603910. [DOI] [PubMed] [Google Scholar]
- 4.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev. 2004;62:177–203. doi: 10.1301/nr.2004.may.177-203. [DOI] [PubMed] [Google Scholar]
- 6.Kant AK. Dietary patterns and health outcomes. J Am Diet Assoc. 2004;104:615–35. doi: 10.1016/j.jada.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Trichopoulou A. Traditional Mediterranean diet and longevity in the elderly: a review. Public Health Nutr. 2004;7:943–7. doi: 10.1079/phn2004558. [DOI] [PubMed] [Google Scholar]
- 8.Osler M, Heitmann BL, Gerdes LU, Jorgensen LM, Schroll M. Dietary patterns and mortality in Danish men and women: a prospective observational study. Br J Nutr. 2001;85:219–25. doi: 10.1079/bjn2000240. [DOI] [PubMed] [Google Scholar]
- 9.Shimazu T, Kuriyama S, Hozawa A, Ohmori K, Sato Y, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Dietary patterns and cardiovascular disease mortality in Japan: a prospective cohort study. Int J Epidemiol. 2007 doi: 10.1093/ije/dym005. [DOI] [PubMed] [Google Scholar]
- 10.Cai H, Shu XO, Gao YT, Li H, Yang G, Zheng W. A prospective study of dietary patterns and mortality in Chinese women. Epidemiology. 2007;18:393–401. doi: 10.1097/01.ede.0000259967.21114.45. [DOI] [PubMed] [Google Scholar]
- 11.Kroenke CH, Fung TT, Hu FB, Holmes MD. Dietary patterns and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:9295–303. doi: 10.1200/JCO.2005.02.0198. [DOI] [PubMed] [Google Scholar]
- 12.Kant AK, Graubard BI, Schatzkin A. Dietary patterns predict mortality in a national cohort: the National Health Interview Surveys, 1987 and 1992. J Nutr. 2004;134:1793–9. doi: 10.1093/jn/134.7.1793. [DOI] [PubMed] [Google Scholar]
- 13.Waijers PM, Ocke MC, van Rossum CT, Peeters PH, Bamia C, Chloptsios Y, van der Schouw YT, Slimani N, Bueno-de-Mesquita HB. Dietary patterns and survival in older Dutch women. Am J Clin Nutr. 2006;83:1170–6. doi: 10.1093/ajcn/83.5.1170. [DOI] [PubMed] [Google Scholar]
- 14.Kumagai S, Shibata H, Watanabe S, Suzuki T, Haga H. Effect of food intake pattern on all-cause mortality in the community elderly: a 7-year longitudinal study. J Nutr Health Aging. 1999;3:29–33. [PubMed] [Google Scholar]
- 15.Hoffmann K, Boeing H, Boffetta P, Nagel G, Orfanos P, Ferrari P, Bamia C. Comparison of two statistical approaches to predict all-cause mortality by dietary patterns in German elderly subjects. Br J Nutr. 2005;93:709–16. doi: 10.1079/bjn20051399. [DOI] [PubMed] [Google Scholar]
- 16.Whichelow MJ, Prevost AT. Dietary patterns and their associations with demographic, lifestyle and health variables in a random sample of British adults. Br J Nutr. 1996;76:17–30. doi: 10.1079/bjn19960006. [DOI] [PubMed] [Google Scholar]
- 17.Bamia C, Trichopoulos D, Ferrari P, Overvad K, Bjerregaard L, Tjonneland A, Halkjaer J, Clavel-Chapelon F, Kesse E, Boutron-Ruault MC, Boffetta P, Nagel G, Linseisen J, Boeing H, Hoffmann K, Kasapa C, Orfanou A, Travezea C, Slimani N, Norat T, Palli D, Pala V, Panico S, Tumino R, Sacerdote C, Bueno-de-Mesquita HB, Waijers PM, Peeters PH, van der Schouw YT, Berenguer A, Martinez-Garcia C, Navarro C, Barricarte A, Dorronsoro M, Berglund G, Wirfalt E, Johansson I, Johansson G, Bingham S, Khaw KT, Spencer EA, Key T, Riboli E, Trichopoulou A. Dietary patterns and survival of older Europeans: the EPIC-Elderly Study (European Prospective Investigation into Cancer and Nutrition) Public Health Nutr. 2007;10:590–8. doi: 10.1017/S1368980007382487. [DOI] [PubMed] [Google Scholar]
- 18.Masala G, Ceroti M, Pala V, Krogh V, Vineis P, Sacerdote C, Saieva C, Salvini S, Sieri S, Berrino F, Panico S, Mattiello A, Tumino R, Giurdanella MC, Bamia C, Trichopoulou A, Riboli E, Palli D. A dietary pattern rich in olive oil and raw vegetables is associated with lower mortality in Italian elderly subjects. Br J Nutr. 2007;98:406–15. doi: 10.1017/S0007114507704981. [DOI] [PubMed] [Google Scholar]
- 19.Colditz GA, Hankinson SE. The Nurses' Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–96. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Department of Agriculture . Composition of Foods: Raw, Processed, Prepared, 1963-1991. U.S. Government Printing Office; Washington, DC: 1992. [Google Scholar]
- 21.Willett WC, Stampfer MJ. Nutritional epidemiology. Oxford University Press; New York/Oxford: 1998. Implications of total energy intake for epidemiologic analysis; pp. 273–301. [Google Scholar]
- 22.Fung TT, Hu FB, Holmes MD, Rosner BA, Hunter DJ, Colditz GA, Willett WC. Dietary patterns and the risk of postmenopausal breast cancer. Int J Cancer. 2005;116:116–21. doi: 10.1002/ijc.20999. [DOI] [PubMed] [Google Scholar]
- 23.Hatcher L. A step-by-step approach to using SAS® for factor analysis and structural equation modeling. SAS Institute Inc.; Cary, NC: 1994. [Google Scholar]
- 24.Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69:243–9. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 25.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–40. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 26.Li TY, Rana JS, Manson JE, Willett WC, Stampfer MJ, Colditz GA, Rexrode KM, Hu FB. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation. 2006;113:499–506. doi: 10.1161/CIRCULATIONAHA.105.574087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140:1016–9. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 28.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6:356–65. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Rothman KJ, Greenland S. Modern epidemiology. Lippincott Williams & Wilkins; Philadelphia: 1998. Fundamentals of epidemiologic data analysis; pp. 201–229. [Google Scholar]
- 30.Varraso R, Fung TT, Barr RG, Hu FB, Willett W, Camargo CA., Jr Prospective study of dietary patterns and chronic obstructive pulmonary disease among US women. Am J Clin Nutr. 2007;86:488–95. doi: 10.1093/ajcn/86.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler LM, Koh WP, Lee HP, Tseng M, Yu MC, London SJ. Prospective study of dietary patterns and persistent cough with phlegm among Chinese Singaporeans. Am J Respir Crit Care Med. 2006;173:264–70. doi: 10.1164/rccm.200506-901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, Rock CL, Kealey S, Al-Delaimy WK, Bardwell WA, Carlson RW, Emond JA, Faerber S, Gold EB, Hajek RA, Hollenbach K, Jones LA, Karanja N, Madlensky L, Marshall J, Newman VA, Ritenbaugh C, Thomson CA, Wasserman L, Stefanick ML. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298:289–98. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fung TT, Willett WC, Stampfer MJ, Manson JE, Hu FB. Dietary patterns and the risk of coronary heart disease in women. Arch Intern Med. 2001;161:1857–62. doi: 10.1001/archinte.161.15.1857. [DOI] [PubMed] [Google Scholar]
- 34.Schulze MB, Fung TT, Manson JE, Willett WC, Hu FB. Dietary patterns and changes in body weight in women. Obesity. 2006;14:1444–53. doi: 10.1038/oby.2006.164. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80:1029–35. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- 36.Fung TT, Stampfer MJ, Manson JE, Rexrode KM, Willett WC, Hu FB. Prospective study of major dietary patterns and stroke risk in women. Stroke. 2004;35:2014–9. doi: 10.1161/01.STR.0000135762.89154.92. [DOI] [PubMed] [Google Scholar]
- 37.Fung TT, Schulze M, Manson JE, Willett WC, Hu FB. Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med. 2004;164:2235–40. doi: 10.1001/archinte.164.20.2235. [DOI] [PubMed] [Google Scholar]
- 38.Fung T, Hu FB, Fuchs C, Giovannucci E, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC. Major dietary patterns and the risk of colorectal cancer in women. Arch Intern Med. 2003;163:309–14. doi: 10.1001/archinte.163.3.309. [DOI] [PubMed] [Google Scholar]
- 39.Michaud DS, Skinner HG, Wu K, Hu F, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Dietary patterns and pancreatic cancer risk in men and women. J Natl Cancer Inst. 2005;97:518–24. doi: 10.1093/jnci/dji094. [DOI] [PubMed] [Google Scholar]
- 40.Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr. 2006;136:2588–93. doi: 10.1093/jn/136.10.2588. [DOI] [PubMed] [Google Scholar]
- 41.He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet. 2006;367:320–6. doi: 10.1016/S0140-6736(06)68069-0. [DOI] [PubMed] [Google Scholar]
- 42.Vainio H, Weiderpass E. Fruit and vegetables in cancer prevention. Nutr Cancer. 2006;54:111–42. doi: 10.1207/s15327914nc5401_13. [DOI] [PubMed] [Google Scholar]
- 43.Martinez ME, Marshall JR, Sechrest L. Invited commentary: Factor analysis and the search for objectivity. Am J Epidemiol. 1998;148:17–9. doi: 10.1093/oxfordjournals.aje.a009552. [DOI] [PubMed] [Google Scholar]