Abstract
Whether the contribution of inflammation to risk for chronic metabolic disease differs with ethnicity is not known. The objective of this study was to determine: (i) whether ethnic differences exist in markers of inflammation and (ii) whether lower insulin sensitivity among African Americans vs. whites is due to greater inflammatory status. Subjects were African-American (n = 108) and white (n = 105) women, BMI 27–30 kg/m2. Insulin sensitivity was assessed with intravenous glucose tolerance test and minimal modeling; fat distribution with computed tomography; body composition with dual-energy X-ray absorptiometry; markers of inflammation (tumor necrosis factor (TNF)-α, soluble tumor necrosis factor receptor (sTNFR)-1, sTNFR-2, C-reactive protein (CRP), and interleukin (IL)-6) with enzyme-linked immunosorbent assay (ELISA). Whites had greater intra-abdominal adipose tissue (IAAT), insulin sensitivity, and concentrations of TNF-α, sTNFR-1, and sTNFR-2 than African Americans. Greater TNF-α in whites vs. African Americans was attributed to greater IAAT in whites. Among whites, but not African Americans, CRP was independently and inversely associated with insulin sensitivity, after adjusting for IAAT (r = −0.29 P < 0.05, and r = −0.13 P = 0.53, respectively). Insulin sensitivity remained lower in African Americans after adjusting for CRP (P < 0.001). In conclusion, greater IAAT among whites may be associated with greater inflammation. Insulin sensitivity was lower among African Americans, independent of obesity, fat distribution, and inflammation.
INTRODUCTION
Ethnic differences exist in the prevalence of certain metabolic diseases. For example, African Americans, relative to whites, are at greater risk for obesity, type 2 diabetes, hypertension, stroke, and mortality related to cardiac arrest. However, whites have a higher prevalence of coronary artery calcification and atherosclerosis (1) when compared to African Americans and other ethnic groups (2), and in the presence of type 2 diabetes, have a greater risk for coronary artery disease (3). Ethnic difference in phenotypes, such as visceral adiposity, markers of inflammation, and insulin resistance, has been extensively documented, and may contribute to the unique disease profile displayed by various ethnic groups (4). It has been extensively documented that African Americans are relatively insulin resistant as compared to whites (5–8). The ethnic difference is significant even when controlling for body composition and fat distribution. Paradoxically, African Americans have lesser intra-abdominal adipose tissue (IAAT), the depot most closely associated with disease risk (9), than whites. This ethnic difference in fat distribution has been observed in numerous studies (10–13). The physiologic cause for greater insulin resistance among African Americans has not been identified.
One potential explanation for ethnic differences in disease risk is inflammation. Chronic subclinical inflammation is commonly associated with type 2 diabetes and cardiovascular disease (14,15). Although the cause-and-effect nature of these relationships is not clear, it has been suggested that inflammation affects disease processes in part by causing or exacerbating insulin resistance. Epidemiologic studies have demonstrated a positive association between markers of inflammation and insulin resistance (16,17). Both experiments with animal models (18) and prospective studies conducted in humans (19) have shown that elevated levels of acute-phase proteins predict the development of insulin resistance or type 2 diabetes. However, it is also possible that insulin resistance promotes production of acute-phase proteins (20). Further research is needed to fully understand the nature of the associations among markers of inflammation and the physiologic processes that regulate carbohydrate metabolism.
Few studies have determined whether chronic subclinical inflammation differs with ethnicity. Based on data from the NHANES and the Women’s Health Study, African-American women had higher concentrations of C-reactive protein (CRP) compared to whites (21,22). However, this ethnic difference was largely or entirely eliminated by adjustment for confounding factors. Results from a tri-ethnic population indicated that associations between CRP and insulin measures were weaker in African Americans as compared to whites (17). Further research is needed to determine the extent to which ethnicity independently affects inflammation, and the association of inflammation with insulin sensitivity.
To determine whether independent associations exist between inflammation and insulin sensitivity, the associations among obesity, inflammation, and insulin sensitivity must be teased apart (23). Obesity, particularly abdominal obesity, is associated with insulin resistance. Both IAAT and deep subcutaneous abdominal adipose tissue (SAAT) have been associated with reduced insulin sensitivity (24). IAAT also produces tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), which in turn stimulates secretion of the acute-phase protein CRP from the liver (25). Adiponectin, an insulin-sensitizing adipocyte-derived hormone, is more closely associated with subcutaneous adipose tissue than IAAT (26). Adiponectin is anti-inflammatory, inhibits synthesis/secretion of TNF-α and CRP, and interferes with NF-κβ activation (27). Preferential deposition of IAAT may exacerbate both inflammation and insulin resistance by reducing adiponectin synthesis. Thus, abdominal adiposity may be linked to insulin resistance both via inflammation, and through other mechanisms.
The objectives of this study were to determine: (i) whether ethnic differences exist in markers of inflammation, and (ii) whether lower insulin sensitivity among African Americans vs. whites is associated with greater inflammatory status, after accounting for total and regional adiposity, as assessed with dual-energy X-ray absorptiometry (total body fat) and computed tomography scanning (IAAT, and superficial and deep SAAT). Outcomes of interest were IL-6, CRP, TNF-α, and the soluble cell-surface receptors of TNF-α, sTNFR-1 and -2. These receptors are shed into circulation following TNF-α binding, and are thought to provide a circulating measure of systemic TNF-α activity, which often may occur at an autocrine/paracrine level (14,28,29). sTNFR-2 is thought to signal the metabolic actions of TNF-α (30). We also examined associations of insulin sensitivity and inflammation with circulating concentrations of adiponectin.
METHODS AND PROCEDURES
Subjects
Subjects were 213 healthy, overweight, premenopausal women who volunteered for, and enrolled in, an ongoing study designed to examine metabolic factors that predispose women to weight gain. Inclusion criteria for the parent study were BMI 27–30 kg/m2, premenopausal, age 20–41 years, sedentary (<2 h per week regular exercise), normal glucose tolerance (determined by oral glucose tolerance test), family history of overweight/obesity in at least one first-degree relative, and no use of medications that affect body composition or metabolism. All women were nonsmokers and reported experiencing menses at regular intervals. The study was approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham (UAB). All women provided informed consent before participating in the study.
Protocol
Subjects were evaluated in the overweight state (prior to any intervention). All testing was conducted after a 4-week weight stabilization period, and in the follicular phase of the menstrual cycle (within 10 days of menses). During the weight stabilization period, subjects’ body weights were measured 3–5 times per week at the General Clinical Research Center at UAB. A macronutrient-controlled diet was provided during the final 2 weeks of weight maintenance. The energy content was appropriately adjusted to ensure a stable body weight (<1% variation from initial body weight). All diets consisted of ~22% of energy from fat, 23% from protein, and 55% from carbohydrate.
Body composition and fat distribution
Total and regional body composition, including total fat mass, percent body fat, leg fat mass, and lean body mass, were measured by dual-energy X-ray absorptiometry in the Department of Nutrition Sciences at UAB with a Lunar Prodigy densitometer and Software Version 1.33 (GE-Lunar, Madison, WI). Subjects were scanned in light clothing while lying flat on their backs with arms at their sides. IAAT and SAAT were analyzed by computed tomography scanning (31,32) with a HiLight/Advantage Scanner (General Electric, Milwaukee, WI) located in the UAB Department of Radiology. SAAT was further subdivided into superficial and deep compartments (33). Subjects were scanned in the supine position with arms stretched above their heads. A 5 mm scan at the level of the umbilicus (approximately the L4–L5 intervertebral space) was taken. Scans were analyzed for cross-sectional area (cm2) of adipose tissue using the density contour program with Hounsfield units for adipose tissue set at −190 to −30. All scans were analyzed by the same individual. The CV for repeat cross-section analysis of scans among 40 subjects in our laboratory is <2% (32).
Insulin sensitivity
Whole-body insulin sensitivity was assessed on an in-patient basis in the General Clinical Research Center after an overnight fast with an insulin-modified, frequently sampled intravenous glucose tolerance test. Prior to testing, flexible intravenous catheters were placed in the antecubital spaces of both arms. Three, 2.0 ml blood samples were taken over a 20-min period for determination of basal glucose and insulin (the average of the values was used for basal “fasting” concentrations). At time “0,” glucose (50% dextrose; 11.4 g/m2) was administered intravenously. Insulin (0.02 U/kg, Humulin, Eli Lilly, Indianapolis, IN) was injected at 20 min post-glucose injection. Blood samples (2.0 ml) were then collected at the following times (min) relative to glucose administration: 2, 3, 4, 5, 6, 8, 10, 12, 15, 19, 20, 21, 22, 24, 26, 28, 30, 35, 40, 45, 50, 55, 60, 70, 80, 100, 120, 140, and 180. Sera were stored at −85 °C until analyzed. Glucose and insulin values were entered into the MINMOD computer program (ver. 3, Richard N. Bergman) for determination of the insulin sensitivity index (34–36).
Laboratory analyses
All analyses were conducted in the Core Laboratory of UAB’s General Clinical Research Center and Clinical Nutrition Research Center. Glucose was measured using an Ektachem DT II System (Johnson and Johnson Clinical Diagnostics, Rochester, NY). In the Core laboratory, this analysis has a mean intra-assay CV of 0.61%, and a mean interassay CV of 2.56%. Insulin was assayed in duplicate 100 μl aliquots using double-antibody radioimmunoassay (Linco Research, St Charles, MO). In the Core laboratory, this assay has a sensitivity of 3.35 μIU/ml, a mean intra-assay CV of 3.49%, and a mean interassay CV of 5.57%. Adiponectin was assayed in duplicate 100 μl aliquots using double-antibody radioimmunoassay (Linco Research, St Charles, MO). In the Core laboratory, this assay has a sensitivity of 1.0 ng/ml, a mean intra-assay CV of 5.4%, and a mean interassay CV of 6.3%.
Markers of inflammation were assessed using enzyme-linked immunosorbent assays (ELISAs). All samples were analyzed in duplicate. TNF-α was analyzed using the high-sensitivity ELISA kit (Quantikine HSTA00C, R&D Systems, Minneapolis, MN). Four TNF-α values were below the minimum detectable concentration (0.50 pg/ml); these samples were assigned the value of the minimum detectable concentration. sTNFR-1 was measured with the EASIA ELISA kit (KAC1761, Invitrogen, Carlsbad, CA). sTNFR-2 was measured with the EASIA ELISA kit (KAC1771, Invitrogen). IL-6 was assayed using the high-sensitivity ELISA kit (Quantikine HS600B, R&D Systems). CRP was assayed with the high-sensitivity ELISA kit (030–9710s, ALPCO, Windham, NH). CRP concentrations >10 mg/l represent an acute state of inflammation (37); therefore all values of CRP >10 mg/l were omitted from analyses (this affected 16 values).
Statistical analysis
Descriptive statistics were computed for all study variables of interest, and distributions were examined. Insulin sensitivity and markers of inflammation were log-10 transformed prior to statistical analysis to ensure a normal distribution. All statistical tests were two-sided and were performed using a type I error rate of 0.05. All statistical analyses were performed using SAS (version 9.1; SAS Institute, Cary, NC).
Overall comparisons between the two ethnic groups were performed using the two-group t-test. Analysis of covariance was used to examine potential ethnic differences in markers of inflammation after sequentially adjusting for total body fat mass, percent body fat mass, total body fat and lean mass, and IAAT. Pearson’s correlation coefficients were calculated to examine associations of markers of inflammation with total and regional adipose tissue, with adiponectin, and with insulin sensitivity. Preliminary correlation analysis also was used to examine the association between individual adipose tissue depots and insulin sensitivity. Based on the results of these analyses, partial correlation analysis was used to determine if markers of inflammation were independently associated with insulin sensitivity after accounting for IAAT. Because fat distribution and insulin sensitivity differed with ethnicity, analyses were conducted within each ethnic group. Multiple linear regression analysis was used to identify independent contributions of ethnicity and inflammation (CRP) to the dependent variable insulin sensitivity, after adjusting for IAAT and adiponectin.
RESULTS
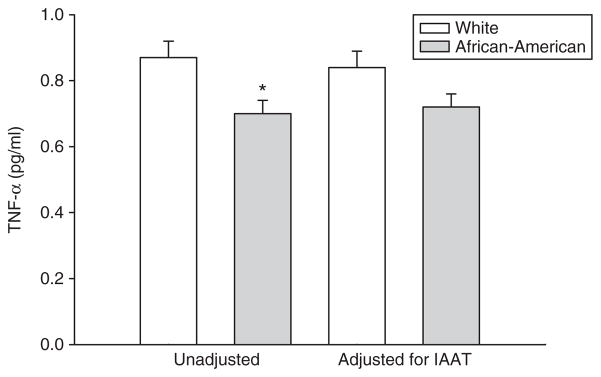
Descriptive statistics by ethnicity are shown in Table 1. By design, BMI did not differ between African-American and white women. Similarly, total body fat, percent body fat, and total body lean mass did not differ with ethnicity. Whites had significantly greater IAAT and insulin sensitivity than African Americans. Concentrations of TNF-α, sTNFR-1, and sTNFR-2 were higher in whites than African Americans; no other marker of inflammation differed with ethnicity, either before or after adjustment for body composition or fat distribution. Analysis of covariance revealed that adjustment for IAAT reduced the ethnic difference in TNF-α (P = 0.054; Figure 1), but did not affect that of the receptors, which remained greater in whites vs. African Americans (P < 0.05 for both; data not shown). Similar adjustment for total body fat or percent body fat did not alter the results.
Table 1.
descriptive statistics by ethnicity (mean ± s.d.)
| Whites (n = 105) | African Americans (n = 108) | P | |
|---|---|---|---|
| Age (years) | 34.3 ± 6.4 | 34.2 ± 6.0 | 0.842 |
| Body weight (kg) | 77.5 ± 7.8 | 76.8 ± 6.4 | 0.483 |
| BMI (kg/m2) | 28.2 ± 1.4 | 28.3 ± 1.3 | 0.424 |
| Fat mass (kg) | 34 ± 5 | 33 ± 4 | 0.072 |
| Body fat (%) | 46 ± 4 | 45 ± 4 | 0.066 |
| Lean mass (kg) | 40 ± 4 | 40 ± 4 | 0.471 |
| IAAT (cm2)a | 94 ± 30 | 64 ± 26 | <0.001 |
| SSAAT (cm2)a | 188 ± 50 | 194 ± 48 | 0.350 |
| DSAAT (cm2)a | 141 ± 54 | 143 ± 51 | 0.794 |
| Fasting glucose (mg/dl) | 88 ± 6 | 86 ± 6 | 0.019 |
| Fasting insulin (μIU/ml) | 11 ± 3 | 12 ± 4 | 0.144 |
| Adiponectin (μg/ml) | 9.88 ± 3.78 | 8.97 ± 4.27 | 0.027 |
| Insulin sensitivity (×10−4 min−1/(μIU/ml))b | 3.48 ± 1.82 | 2.50 ± 1.89 | <0.001 |
| TNF-α (pg/ml)c | 1.10 ± 1.15 | 0.80 ± 0.57 | 0.003 |
| sTNFR-1 (ng/ml)d | 1.89 ± 0.37 | 1.71 ± 0.35 | <0.001 |
| sTNFR-2 (ng/ml)e | 4.33 ± 1.21 | 3.72 ± 1.03 | <0.001 |
| IL-6 (pg/ml)f | 1.79 ± 1.32 | 1.66 ± 1.20 | 0.458 |
| CRP (mg/l)g | 1.97 ± 1.70 | 2.28 ± 2.09 | 0.451 |
Boldface values indicate P < 0.05.
CRP, C-reactive protein; DSAAT, deep subcutaneous abdominal adipose tissue; IAAT, intra-abdominal adipose tissue; IL-6, interleukin-6; SSAAT, superficial subcutaneous abdominal adipose tissue; sTNFR-1, soluble tumor necrosis factor receptor-1; sTNFR-2, soluble tumor necrosis factor receptor-2; TNF-α, tumor necrosis factor-α.
n = 211 for CT scan data.
n = 204 for insulin sensitivity.
n = 212 for TNF-α.
n = 213 for sTNFR-1.
n = 212 for sTNFR-2.
n = 213 for IL-6.
n = 198 for CRP.
Figure 1.
TNF-α (pg/ml) in white and African-American women. Left side: unadjusted (*significantly lower among African Americans). Right side: adjusted for IAAT (no difference between groups).
Correlation analyses showed significant associations between total and regional adipose tissue, and markers of inflammation (Table 2). In general, among all women combined, IAAT was positively associated with the TNF system and IL-6. The major ethnic difference detected was that, among whites, all depots measured were associated with IL-6, including deep and superficial SAAT. In contrast, among African Americans, no depot was associated with IL-6. Also, among African Americans but not whites, DSAAT was associated with sTNFR-1 and sTNFR-2.
Table 2.
Pearson product moment correlations for markers of inflammation with total and regional adipose depots for all women combined and by ethnicity
| TNF-α | sTNFR-1 | sTNFR-2 | IL-6 | CRP | |
|---|---|---|---|---|---|
| All | |||||
| Fat mass | −0.00 | 0.25 | 0.16 | 0.17 | 0.01 |
| IAAT | 0.18 | 0.20 | 0.22 | 0.17 | 0.07 |
| SSAAT | −0.08 | 0.16 | 0.10 | 0.08 | −0.01 |
| DSAAT | −0.14 | 0.14 | 0.09 | 0.15 | −0.03 |
| Leg fat | −0.02 | 0.14 | 0.11 | 0.09 | −0.02 |
| Whites | |||||
| Fat mass | 0.07 | 0.28 | 0.14 | 0.27 | −0.06 |
| IAAT | 0.15 | 0.14 | 0.11 | 0.23 | 0.15 |
| SSAAT | −0.01 | 0.24 | 0.17 | 0.21 | −0.02 |
| DSAAT | −0.15 | −0.06 | −0.03 | 0.23 | −0.03 |
| Leg fat | 0.07 | 0.26 | 0.15 | 0.21 | −0.08 |
| African Americans | |||||
| Fat Mass | −0.17 | 0.17 | 0.12 | 0.03 | 0.10 |
| IAAT | 0.03 | 0.07 | 0.10 | 0.06 | 0.05 |
| SSAAT | −0.15 | 0.13 | 0.06 | −0.07 | 0.01 |
| DSAAT | −0.13 | 0.31 | 0.23 | 0.06 | −0.02 |
| Leg fat | −0.07 | 0.10 | 0.15 | 0.01 | 0.03 |
Boldface values indicate P < 0.05.
CRP, C-reactive protein; DSAAT, deep subcutaneous abdominal adipose tissue; IAAT, intra-abdominal adipose tissue; IL-6, interleukin-6; SSAAT, superficial subcutaneous abdominal adipose tissue; sTNFR-1, soluble tumor necrosis factor receptor-1; sTNFR-2, soluble tumor necrosis factor receptor-2; TNF-α, tumor necrosis factor-α.
Adiponectin was not significantly associated with CRP or sTNFR-2 in either group. In whites, the association of adiponectin with IL-6 was significant (r = −0.35, P < 0.001), as was that with TNF-α (r = −0.22, P = 0.026); the association with sTNFR-1 approached significance (r = -0.17, P = 0.080). Adiponectin was associated with insulin sensitivity in all women combined (r = 0.40, P < 0.001) and within each ethnic group (r = 0.44, P < 0.001 for whites, and r = 0.33, P < 0.001 for African Americans).
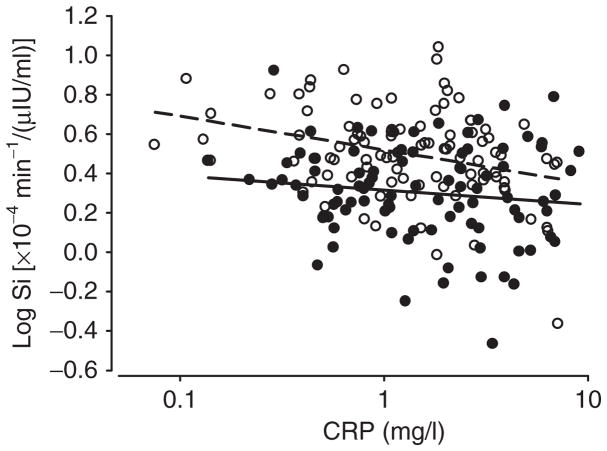
Within whites, CRP was inversely associated with insulin sensitivity both before and after adjusting for IAAT (Table 3, Figure 2). Within whites, IL-6 was associated with unadjusted insulin sensitivity, but the association was not significant after statistical adjustment for IAAT (Table 3) or adiponectin (not shown). Markers of inflammation were not associated with insulin sensitivity in African Americans. Insulin sensitivity remained lower in African Americans vs. whites after adjusting for CRP (Table 4, Figure 2).
Table 3.
Pearson correlations (r) for markers of inflammation and adiponectin with insulin sensitivity by ethnicity; unadjusted, and adjusted for IAAT
| Whites |
African Americans |
|||
|---|---|---|---|---|
| Unadjusted | Adjusted for IAAT | Unadjusted | Adjusted for IAAT | |
| TNF-α | −0.15 | −0.09 | 0.03 | 0.08 |
| sTNFR-1 | −0.04 | 0.02 | −0.01 | −0.10 |
| sTNFR-2 | −0.07 | −0.02 | 0.13 | 0.11 |
| IL-6 | −0.24* | −0.19 | −0.04 | −0.14 |
| CRP | −0.30* | −0.29* | −0.15 | −0.13 |
| Adiponectin | 0.44* | 0.40* | 0.33* | 0.29* |
Boldface values indicate correlation significant P < 0.05.
CRP, C-reactive protein; IAAT, intra-abdominal adipose tissue; IL-6, interleukin-6; sTNFR-1, soluble tumor necrosis factor receptor-1; sTNFR-2, soluble tumor necrosis factor receptor-2; TNF-α, tumor necrosis factor-α.
P < 0.05.
Figure 2.
CRP vs. insulin sensitivity in white (open circles, r = −0.29; P < 0.001) and African-American (closed circles, r = −0.13; P = 0.53) women; adjusted for IAAT. Insulin sensitivity remained lower in African Americans vs. whites (P < 0.001) after accounting for the effect of CRP. The P value for the (ethnicity × CRP) interaction term was 0.31.
Table 4.
Multiple linear regression model for insulin sensitivity
| Independent variable | Parameter estimate ± SEE | P |
|---|---|---|
| Intercept | 0.25 ± 0.10 | 0.019 |
| Ethnicity | −0.20 ± 0.04 | <0.001 |
| CRP | −0.10 ± 0.04 | 0.009 |
| Adiponectin | 0.41 ± 0.08 | <0.001 |
| IAAT | −0.001 ± 5.6 × 10−4 | 0.012 |
Model R2 = 0.34. Ethnicity coded 0 = white and 1= African American.
CRP, C-reactive protein; IAAT, intra-abdominal adipose tissue.
DISCUSSION
It is becoming increasingly clear that chronic, low-grade, systemic inflammation is associated with obesity and metabolic disease (38,39). However, whether ethnicity affects the production of pro-inflammatory cytokines, or the association of inflammation with disease risk, has not been extensively examined. This study was conducted to address these gaps. Specifically, objectives of this study were to determine, (i) whether ethnic differences exist in markers of inflammation, and (ii) whether lower insulin sensitivity among African Americans vs. whites is associated with greater inflammatory status, after accounting for total and regional adiposity.
Previous reports have indicated ethnic differences in inflammation (21,22). In NHANES, age-adjusted CRP was higher among African-American (3.1 mg/l) compared to white (2.3 mg/l) women (P < 0.01), but the difference disappeared after adjusting for confounding factors (education, waist circumference, systolic blood pressure, smoking status, etc.) (21). Likewise in the Women’s Health Study, significantly higher median CRP among African Americans (2.96 mg/l) vs. whites (2.02 m/l) was attenuated after adjustment for BMI (22). In this study, unadjusted CRP values did not differ with ethnicity (2.28 ± 2.09 and 1.97 ± 1.70 mg/l for African Americans and whites, respectively; P = 0.451), perhaps due to the homogeneous nature of our cohort regarding age, obesity status, and lack of hormone use, or to the careful dietary control prior to testing.
We are the first to report that ethnic differences exit in the TNF system. Specifically, whites had greater TNF-α, sTNFR-1, and sTNFR-2 than African Americans. The ethnic difference in TNF-α was attenuated (P = 0.054) after adjusting for IAAT (Figure 1), suggesting that white women have greater concentrations of TNF-α in circulation due at least in part to greater IAAT. The ethnic differences in sTNFR-1 and sTNFR-2 remained significant even when adjusted for total and regional adipose tissue. It is likely that these receptors emanate from various tissue types (liver, skin, lung, skeletal muscle, and adipose tissue) (40–42), as well as various adipose tissue depots. Thus, it is reasonable to hypothesize that statistical adjustment for any one tissue type or region may not explain greater TNF receptor activity among whites vs. African Americans. Rather, greater TNF-α activity among whites vs. African Americans may be responsible for the greater number of circulating receptors.
In this study, associations between markers of inflammation and insulin sensitivity were examined. Inflammation has been implicated in the etiology of insulin resistance (23), although the cause-and-effect nature of the association is not clear, and alternative scenarios could be postulated (20,43). We found that, among the markers of inflammation examined, only CRP was inversely associated with insulin sensitivity, as has been reported in a large, ethnically diverse sample (17). In our population, African Americans had lower insulin sensitivity than whites, and this difference remained after accounting for CRP (Figure 2). Further, when examined by ethnic group, we found that CRP was associated with insulin sensitivity in whites, but not in African Americans. Although this study may not have been powered to detect a significant (ethnicity × CRP) interaction, this observation, even if preliminary, may have important implications for understanding ethnic differences in the etiology of chronic metabolic disease, and warrants further study in a larger sample.
We did not observe an association between TNF-α and insulin sensitivity. Although animal model studies have suggested a causal role for TNF-α and insulin resistance (44), data from clinical studies have not lead to a clear picture regarding this association, with some studies suggesting a positive relationship (45,46), and others no relationship (47,48). These inconsistent observations suggest that circulating TNF-α may not be causally related to insulin sensitivity, or that insulin resistance may result in elevated TNF-α. It is also possible that the main actions of TNF-α occur at the autocrine/paracrine level, and may not be reflected in circulating concentrations (49,50). We also did not observe an independent association between IL-6 and insulin sensitivity. Thus, of all of the markers of inflammation evaluated in this study, CRP alone was independently related to insulin sensitivity, suggesting that this protein is a useful marker for global inflammatory status, as it related to metabolic outcomes, among healthy subjects.
Adiponectin may reduce the secretion of pro-inflammatory cytokines. In this study, adiponectin was higher among whites vs. African Americans. It is therefore unlikely that ethnic differences in adiponectin were causally related to higher TNF-α among whites. Adiponectin was inversely associated with IL-6 and TNF-α in whites, but not African Americans. This observation supports the role for adiponectin in decreasing secretion of pro-inflammatory cytokines, but indicates that this action may be ethnicity-specific. Whether reported ethnic differences in adiponectin sub-fraction distribution (51) are related to this observation remains to be determined. In this population, adiponectin was independently associated with insulin sensitivity, along with ethnicity and CRP (Table 4).
This study is the first to determine the association among regional adipose tissue depots and circulating markers of inflammation in healthy premenopausal women, and if ethnic differences exist in these relationships. We observed that ethnic differences existed in the nature of these associations, and that associations in general were stronger among white vs. African-American women. In our population, TNF-α was positively associated only with IAAT, a relationship that was driven by the association among white women. This may reflect a direct contribution of resident macrophages in IAAT to circulating TNF-α, and may suggest that macrophage infiltration of IAAT differs with ethnicity. Both TNF-α message and protein have been localized to IAAT (23). Resident macrophages within IAAT produce the majority of TNF-α; very little is produced by adipocytes or stromal tissue. Expression levels are higher in tissue from obese individuals, increase under conditions of IAAT accrual, (52), and decrease with weight loss (23). Thus, the degree to which IAAT produces TNF-α is influenced by the metabolic state of the individual. In vitro secretion studies suggest that human IAAT secretes TNF-α (53). The association observed in this study between IAAT and circulating TNF-α may suggest that IAAT is a relevant physiologic source of TNF-α, at least in certain populations. However, we can not exclude the possibility that factors related to or emanating from IAAT affect the secretion of TNF-α from cells localized outside of IAAT.
The soluble TNF receptors were associated with several adipose tissue depots in an ethnicity-specific manner. Associations with IAAT were stronger among whites, whereas associations with deep SAAT were observed within African-American women. We observed that IL-6 was associated with both SAAT and IAAT, but these associations were observed only within white women. CRP was not associated with any adipose tissue depot. This is not surprising, as CRP is produced primarily by the liver in response to stimulation from IL-6 (14). However, CRP was most closely correlated with IAAT in white women (r = 0.15; P = 0.13), perhaps reflecting the contribution of IAAT to circulating IL-6 in this group. Taken together, positive associations among regional adipose tissue depots and markers of inflammation in general were stronger among white women when compared to African-American women. This observation may suggest that adiposity-mediated inflammation, as a health risk factor, is more relevant a concern among white vs. African-American women.
Strengths of this study included robust measures of insulin sensitivity, body composition, and body fat distribution. A limitation of this study is its cross-sectional nature; longitudinal data will be required to determine if ethnic background affects the relationship between inflammation and insulin sensitivity, and the associated progression of metabolic disease. Further, we did not examine all potentially relevant lipid depots, such as that within skeletal muscle. Our preliminary data indicated that TNF-α was associated with intramyocellular lipid in white, but not African-American women; however intramyocellular lipid did not differ with ethnicity (54). In addition, results are limited to a population of healthy, overweight, premenopausal women. Similar studies in other populations (men, obese individuals, and postmenopausal women) would be of interest.
In conclusion, within this population of healthy, overweight, premenopausal African-American and white women carefully matched for BMI, serum concentrations of TNF system markers were higher in whites, a difference that appeared due in part to their greater IAAT. Insulin sensitivity was lower among African Americans, a difference that was independent of obesity, fat distribution, and inflammation. Further study is needed to examine possible ethnic differences in obesity-related low-grade, systemic inflammation, and its progression to insulin resistance and disease.
Acknowledgments
This work was supported by R01DK51684, R01DK49779, M01-RR-00032, and P30-DK56336. Stouffer’s Lean Cuisine and Weight Watchers Smart Ones kindly provided food used during the weight-maintenance periods. We acknowledge David Bryan and Robert Petri for technical assistance; Maryellen Williams and Cindy Zeng for laboratory analyses; Paul Zuckerman for project coordination.
Footnotes
Disclosure
The authors declared no conflict of interest.
References
- 1.Grundy SM. Obesity, metabolic syndrome, and coronary atherosclerosis. Circulation. 2002;105:2696–2698. doi: 10.1161/01.cir.0000020650.86137.84. [DOI] [PubMed] [Google Scholar]
- 2.Orakzai SH, Orakzai RH, Nasir K, et al. Subclinical coronary atherosclerosis: racial profiling is necessary! Am Heart J. 2006;152:819–827. doi: 10.1016/j.ahj.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Freedman BI, Hsu FC, Langefeld CD, et al. The impact of ethnicity and sex on subclinical cardiovascular disease: the Diabetes Heart Study. Diabetologia. 2005;48:2511–2518. doi: 10.1007/s00125-005-0017-2. [DOI] [PubMed] [Google Scholar]
- 4.Forouhi NG, Sattar N. CVD risk factors and ethnicity—a homogeneous relationship? Atheroscler Suppl. 2006;7:11–19. doi: 10.1016/j.atherosclerosissup.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Gower BA, Nagy TR, Goran MI. Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes. 1999;48:1515–1521. doi: 10.2337/diabetes.48.8.1515. [DOI] [PubMed] [Google Scholar]
- 6.Gower BA, Ard JD, Hunter GR, Fernandez JR, Ovalle F. Elements of the metabolic syndrome: association with insulin sensitivity, and effects of ethnicity. Metab Syndr Rel Disord. 2007;5:77–86. doi: 10.1089/met.2006.0027. [DOI] [PubMed] [Google Scholar]
- 7.Haffner SM, D’Agostino R, Jr, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45:742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 8.Osei K, Schuster DP. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabetic Med. 1994;11:755–762. doi: 10.1111/j.1464-5491.1994.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 9.Despres JP. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition. 1993;9:452–459. [PubMed] [Google Scholar]
- 10.Conway JM, Yanovski SZ, Avila NA, Hubbard VS. Visceral adipose tissue differences in black and white women. Am J Clin Nutr. 1995;61:765–771. doi: 10.1093/ajcn/61.4.765. [DOI] [PubMed] [Google Scholar]
- 11.Weinsier RL, Hunter GR, Gower BA, Schutz Y, Darnell BE, Zuckerman PA. Body fat distribution in white and black women: different patterns of intra-abdominal and subcutaneous abdominal adipose tissue utilization with weight loss. Am J Clin Nutr. 2001;74:631–636. doi: 10.1093/ajcn/74.5.631. [DOI] [PubMed] [Google Scholar]
- 12.Albu JB, Murphy L, Frager DH, Johnson JA, Pi-Sunyer FX. Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes. 1997;46:456–462. doi: 10.2337/diab.46.3.456. [DOI] [PubMed] [Google Scholar]
- 13.Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996;45:1119–1124. doi: 10.1016/s0026-0495(96)90011-6. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland JP, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Metab Syndr Rel Disord. 2004;2:104. doi: 10.1089/met.2004.2.82. [DOI] [PubMed] [Google Scholar]
- 15.Nesto R. C-reactive protein, its role in inflammation, type 2 diabetes, and cardiovascular disease, and the effects of insulin-sensitizing treatment with thiazolidinediones. Diabetic Med. 2004;21:810–817. doi: 10.1111/j.1464-5491.2004.01296.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Wildman RP, Hamm LL, et al. Association between inflammation and insulin resistance in US nondiabetic adults. Diabetes Care. 2004;27:2960–2968. doi: 10.2337/diacare.27.12.2960. [DOI] [PubMed] [Google Scholar]
- 17.Festa A, D’Augostino RJ, Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRIS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 18.Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κβ. Nature Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Festa A, D’Agostino R, Jr, Tracy RP, Haffner SM. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the Insulin Resistance and Atherosclerosis Study (IRAS) Diabetes. 2002;51:1131–1137. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 20.Campos SP, Baumann H. Insulin is a prominent modulator of the cytokine-stimulated expression of acute-phase plasma protein genes. Mol Cell Biol. 1992;12:1789–1797. doi: 10.1128/mcb.12.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ford ES, Giles WH, Mokdad AH, Myers GL. Distribution and correlates of C-reactive protein concentrations among adult US women. Clin Chem. 2004;50:574–581. doi: 10.1373/clinchem.2003.027359. [DOI] [PubMed] [Google Scholar]
- 22.Albert MA, Glynn RJ, Buring J, Ridker PM. C-reactive protein levels among women of various ethnic groups living in the United States (from the Women’s Health Study) Am J Cardiol. 2004;93:1238–1242. doi: 10.1016/j.amjcard.2004.01.067. [DOI] [PubMed] [Google Scholar]
- 23.Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 24.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941–E948. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 25.Pepys MB, Hirshfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bush N, Darnell BE, Oster R, Goran M, Gower BA. Adiponectin is lower among African-Americans, and is independently related to insulin sensitivity in children and adolescents. Diabetes. 2005;54:2772–2778. doi: 10.2337/diabetes.54.9.2772. [DOI] [PubMed] [Google Scholar]
- 27.Ouchi N, Walsh K. A novel role for adiponectin in the regulation of inflammation. Arterioscler Thromb Vasc Biol. 2008;28:1219–1221. doi: 10.1161/ATVBAHA.108.165068. [DOI] [PubMed] [Google Scholar]
- 28.Higuchi M, Aggarwal BB. TNF induces internalization of the p60 receptor and shedding of the p80 receptor. J Immunol. 1994;152:3550–3558. [PubMed] [Google Scholar]
- 29.Fernandez-Real JM, Lainez B, Vendrell J, et al. Shedding of TNF-α receptors, blood pressure, and insulin sensitivity in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2002;282:E952–E959. doi: 10.1152/ajpendo.00444.2001. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Real JM, Broch M, Ricart W, et al. Plasma levels of the soluble fraction of tumor necrosis factor receptor 2 and insulin resistance. Diabetes. 1998;47:1757–1762. doi: 10.2337/diabetes.47.11.1757. [DOI] [PubMed] [Google Scholar]
- 31.Kekes-Szabo T, Hunter GR, Nyikos I, et al. Development and validation of computed tomography derived anthropometric regression equations for estimating abdominal adipose tissue distribution. Obesity Res. 1994;2:450–457. doi: 10.1002/j.1550-8528.1994.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 32.Goran M, Kaskoun MC, Shuman WP. Intra-abdominal adipose tissue in young children. Int J Obesity. 1995;19:279–283. [PubMed] [Google Scholar]
- 33.Smith SR, Lovejoy JC, Greenway F, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 34.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man. Measurement of insulin sensitivity and β-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed. 1986;23:113–122. doi: 10.1016/0169-2607(86)90106-9. [DOI] [PubMed] [Google Scholar]
- 36.Yang YJ, Youn JH, Bergman RN. Modified protocols improve insulin sensitivity estimation using the minimal model. Am J Physiol Endocrinol Metab. 1987;253:E595–E602. doi: 10.1152/ajpendo.1987.253.6.E595. [DOI] [PubMed] [Google Scholar]
- 37.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 39.Pradhan AD, Manson JE, Rifai N, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 40.Bazzoni F, Beutler B. How do tumor necrosis factor receptors work? J Inflamm. 1995;45:221–238. [PubMed] [Google Scholar]
- 41.Rothe J, Gehr G, Loetscher H, Lesslauer W. Tumor necrosis factor receptors—structure and function. Immunol Res. 1992;11:81–90. doi: 10.1007/BF02918612. [DOI] [PubMed] [Google Scholar]
- 42.Vandenabeele P, Declercq W, Beyaert R, Fiers W. Two tumor necrosis factor receptors: structure and function. Trends Cell Biol. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- 43.Hooper PL, Hooper PL. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones. doi: 10.1007/s12192-008-0073-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 45.Katsuki A, Sumida Y, Murashima S, et al. Serum levels of tumor necrosis factor-alpha are increased in obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83:859–862. doi: 10.1210/jcem.83.3.4618. [DOI] [PubMed] [Google Scholar]
- 46.Zinman B, Hanley AJG, Stewart B, Harris JK, Fantus IG. Circulating tumor necrosis factor-α concentrations in a native Canadian population with high rates of type 2 diabetes mellitus. J Clin Endocrinol Metab. 1999;84:272–278. doi: 10.1210/jcem.84.1.5405. [DOI] [PubMed] [Google Scholar]
- 47.Plomgaard P, Nielsen AR, Fischer CP, et al. Associations between insulin resistance and TNF-á in plasma, skeletal muscle and adipose tissue in humans with and without type 2 diabetes. Diabetologia. 2007;50:2562–2571. doi: 10.1007/s00125-007-0834-6. [DOI] [PubMed] [Google Scholar]
- 48.Zavaroni I, Numeroso F, Dongiovanni P, et al. What is the contribution of differences in three measures of tumor necrosis factor-alpha activity to insulin resistance in healthy volunteers? Metabolism. 2003;52:1593–1596. doi: 10.1016/s0026-0495(03)00329-9. [DOI] [PubMed] [Google Scholar]
- 49.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet-induced weight loss decreases skeletal muscle inflammation gene expression in frail obese elderly. J Appl Cell Physiol. 2008;105:473–478. doi: 10.1152/japplphysiol.00006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lara-Castro C, Doud EC, Tapia PC, et al. Adiponectin multimers and metabolic syndrome traits: relative adiponectin resistance in African-Americans. Obesity. doi: 10.1038/oby.2008.411. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishikawa K, Takahashi K, Bujo H, et al. Subcutaneous fat modulates insulin sensitivity in mice by regulating TNFa expression in visceral fat. Horm Metab Res. 2006;38:631–638. doi: 10.1055/s-2006-954580. [DOI] [PubMed] [Google Scholar]
- 53.Fain JN, Bahouth SW, Madan AK. TNFα release by the nonfat cells of human adipose tissue. Int J Obesity. 2004;28:616–622. doi: 10.1038/sj.ijo.0802594. [DOI] [PubMed] [Google Scholar]
- 54.Lawrence JC, Hyatt TC, Hunter GR, et al. Associations between markers of inflammation and regional intramuscular fat vary with ethnicity. Obesity. 2007;15(Suppl):A205. [Google Scholar]