Abstract
Studies of Parkinson’s disease (PD) suggest that cognitive deficits accompany the classically recognized motor symptoms, and that these cognitive deficits may result from damage to frontal-basal ganglia circuits. PD patients are impaired on ordering events and action components into coherent sequences. In this study, we examined early-stage, nondemented, medicated PD subjects and matched control subjects during a semantic event sequencing task using functional MRI (fMRI). The task required subjects to examine four pictures of meaningful events, determine the correct temporal relationship between each picture, and re-order the pictures into a coherent sequence. There were two main findings. First, we found abnormal activation within the prefrontal cortex (PFC) and the “default” network in the PD group. Distinct areas of the PFC showed both hypoactivation and hyperactivation, whereas the “default” network showed reduced levels of resting activation in PD. Secondly, we observed left caudate hyperactivation in the PD group. The findings are discussed in relationship to how more activation may be compensatory, but does not necessarily mean efficient and correlated brain function.
Keywords: dopamine, basal ganglia, dorsolateral prefrontal cortex, executive functions, fMRI
1. Introduction
Parkinson’s disease (PD) is an aging-related neurodegenerative disorder characterized by the classical motor symptoms of bradykinesia, rigidity, tremor, postural instability, and gait disturbances. A growing body of neuropsychological and neuroimaging evidence suggests that patients with PD also have diverse cognitive problems affecting spatial, memory, and executive abilities, even at relatively early stages of the disease [1, 14, 18]. Behavioral research on PD has demonstrated deficits in strategic control, attention shifting, planning, working memory, and perceptuomotor temporal sequencing. Yet, most neuroimaging studies focus on motor symptoms and few on cognitive problems, and so relatively little is known about the brain basis of high-level cognitive dysfunction in PD [7]. The present functional magnetic resonance imaging (fMRI) study sought to examine the functional integrity of frontal-basal ganglia circuits in early-stage, nondemented, medicated PD participants during a semantic event sequencing task, an executive function that is known to be impaired in PD and is central to many high-level activities of daily living, such as following a recipe to prepare a meal or organizing a daily schedule.
Neuropsychological findings suggest that damage to the fronto-striatal system in PD results in problems with sequencing meaningful events. PD patients have been shown to be impaired on picture arrangement tests in which scrambled picture sets must be reordered to tell a story [3, 12, 49] and on related tasks entailing ordering and organizing script information that is presented as action sequence components in a scrambled order [23, 52].
We designed a semantic event sequencing task that is an fMRI variant [50] of the Picture Arrangement subtest of the Wechsler Adult Intelligence Scale-III (WAIS-III) [51]. Similarly, our picture sequencing task required subjects to examine four pictures of meaningful events, determine the correct temporal relationship, and, finally, re-order the pictures into a coherent sequence [25, 33]. In a previous fMRI study using this sequencing task with young healthy subjects, we demonstrated that this task engages a distributed network of occipitotemporal, parietal, frontal and basal ganglia regions [50]. More important, we found that the crucial components of this network for accomplishing semantic event sequencing are the dorsolateral prefrontal cortex (DLPFC) and the globus pallidus internal part (GPi), especially in the left hemisphere.
The goal in the present study was to examine the functional integrity of these frontal-basal ganglia circuits in patients with PD. We predicted that PD patients would show abnormal brain activity relative to a matched control group, specifically in the prefrontal cortex and the basal ganglia.
2. Methods
2.1. Subjects
Thirteen volunteers with idiopathic PD (mean age: 57.6 ± 6.8 years (range: 46–67), mean education: 16.1 ± 2.4 years (range: 14–21), 3 males) and thirteen matched healthy control volunteers (mean age: 57 ± 8.6 years (range: 42–70), mean education: 16.4 ± 1.9 years (range: 13–19), 3 males) (Table 1) participated with informed consent and approval of Mass General Hospital and Boston University. Diagnoses were made by staff neurologists in the outpatient clinic of the Parkinson’s Disease Center in the Department of Neurology, Boston Medical Center. The PD and control participants were recruited through the Vision & Cognition Laboratory in the Department of Psychology at Boston University. Some of the control participants were also recruited through the Harvard Cooperative Program on Aging.
Table 1.
Demographic Data
| Control (n=13) | PD (n=13) | |
|---|---|---|
| Age (years) | 57 ± 2.4 | 57.6 ± 1.9 |
| Education (years) | 16.4 ± 0.5 | 16.1 ± 0.7 |
| Onset side | N/A | 7 LPD, 6 RPD |
| Disease duration (years) | N/A | 5.5 ± 0.5 |
| Hoehn & Yahr stage | N/A | 2.15 ± 0.09 |
| UPDRS score | N/A | 34.15 ± 2.7 |
| UPDRS motor score | N/A | 22.4 ± 2 |
| MMSE | 29.3 ± 0.5 (n=10) | 29.6 ± 0.2 (n=11) |
| DRS | 143.2 ± 0.2 (n=9) | 143.5 ± 0.15 (n=12) |
| ANART | 122.5 ± 1.7 | 121.6 ± 1.1 |
| Digit Symbol* | 74.5 ± 4.2 | 62.3 ± 3 (n=12) |
| Symbol Search | 32.4 ± 1.6 | 29.3 ± 1.7 (n=12) |
| Trails A (sec.)† | 29.2 ± 2.3 | 37.3 ± 3.1 (n=12) |
| Trails B (sec.) | 61.4 ± 3.7 | 82.3 ± 10.5 (n=12) |
| BDI-II^ | 2.2 ± 1.2 | 8.5 ± 2 |
| STAI-S | 27.9 ± 2.5 | 31.2 ± 1.7 (n=12) |
| STAI-T | 31.2 ± 2.9 | 34 ± 3.1 (n=12) |
Demographic data (mean ± SEM) for Parkinson’s disease (PD) and control subjects (N=13, unless noted otherwise).
UPDRS: Unified Parkinson’s Disease Rating Scale, MMSE: Mini Mental State Examination, DRS: Dementia Rating Scale, ANART: American National Adult Reading Test, BDI: Beck Depression Inventory, STAI: Spielberger State and Trait Anxiety Inventory, S: State, T: Trait.
All PD subjects and 10 control subjects had at least one dementia measure (either MMSE or DRS). 10 PD and 9 control subjects had both MMSE and DRS measures.
: p = 0.029,
: p = 0.045,
: p = 0.019
Exclusion criteria for all participants included neurological disease or medical disorders that impair central nervous system function, head trauma with more than a few minutes loss of consciousness or other complications, learning disability, psychiatric conditions, including schizophrenia, bipolar disorder, personality disorder, but not anxiety and depression because these conditions are often comorbid with PD, history of substance (drug, alcohol) dependence, or intravenous drug use, history of electro-shock treatment, English as non-native language, and specific MRI safety considerations.
All PD patients had unilateral symptom onset (left-onset in 7, and right-onset in 6 PD participants) and asymmetrical disease course. The average duration of disease was 5.5 ± 1.9 years. All patients were responsive to either levodopa-carbidopa or dopamine receptor agonists. Eleven patients were on a combination of up to 4 medications including levodopa-carbidopa, dopamine receptor agonists (pramipexole, ropinirole, pergolide), catechol-O-methyl-transferase (COMT) inhibitors (entacapone, tolcapone), monoamine oxidase B (MAO-B) inhibitors (selegiline), amantadine, and anticholinergics (trihexyphenidyl), and 2 were on dopamine receptor agonists only. Seven patients were on antidepressants, three on antianxiety medications as needed, and four were taking wakefulness-promoting drugs (modafinil). Only one patient was on anticholinergic medication. Three patients who were on anxiolytics on an as needed basis did not take their meds on the scanning day and the day before scanning.
Scanning started within 2 ± 1.5 hr after the first dose of dopaminergic medication for the day. Before scanning, patients underwent a neurological assessment while on dopaminergic medication, including Hoehn and Yahr staging [28] and the Unified Parkinson’s Disease Rating Scale (UPDRS) [19]. The mean UPDRS score was 34.1 ± 9.8 points, including the mentation, behavior, mood and activities of daily living components rated by interview, motor examination, and therapy-related complications (e.g., dyskinesia, dystonia, clinical fluctuations, anorexia/nausea/vomiting, sleep disturbances, symptomatic orthostasis). The mean score on the motor examination part was 22.4 ± 7.2. In addition to tremor, all patients had at least two more cardinal symptoms: bradykinesia, rigidity, or postural instability. The mean Hoehn and Yahr score was 2.15 ± 0.3 (10 subjects had a score of 2, 2 subjects had 2.5, and 1 subject had 3). A score of 2 refers to mild bilateral involvement without impairment of balance [28].
The two groups were about evenly matched on handedness. The control group included 3 left-handed subjects (one weakly, two strongly left-handed), and the PD group included 2 left-handed subjects (one moderately, one strongly left-handed) as assessed by the Edinburgh handedness questionnaire [39]. In 8 PD subjects, the side of the dominant hand was also the more affected side (6 right-handed subjects with right-onset and 2 left-handed subjects with left-onset PD).
2.2. Behavioral Tests
None of the participants were demented as assessed by the Mini Mental State Examination (MMSE) (mean: 29.6 points for PD, 29.3 points for control) [20] or the Dementia Rating Scale (DRS) (mean: 143.5 points for PD, 143.2 points for control) [37] (Table 1). To characterize the cognitive and behavioral profiles of both groups, participants were tested on standard clinical neuropsychological tests: American National Adult Reading Test (ANART) [24] for premorbid intellectual functioning; Digit Symbol and Symbol Search subtests of WAIS-III [51] for psychomotor speed, and Trail Making A and B tests [44] for complex attention and executive function. We also collected scores on the Digit Span WAIS-III and FAS letter fluency tests for most subjects, further assessing working memory and executive function. Emotional status was evaluated using the Beck Depression Inventory-II (BDI-II) [4] and Spielberger State Trait Anxiety Inventory (STAI) [48]. A repeated measures ANOVA was performed to assess the group effect on neuropsychological performance and group × test interactions. Statistical threshold was set at p < 0.05 (Geisser-Greenhouse corrected). Independent-sample t-tests were performed to detect subtle group differences. Statistical threshold was set at p < 0.05, uncorrected for the t-tests. All statistical tests were performed using SPSS 11.0.2 for Macintosh.
2.3. Design
As in our prior semantic event sequencing study, a block design was used [50]. Each block in both picture sequencing (PS) and object discrimination control (CON) tasks included 4 trials. In each run, 3 blocks each of the PS and CON tasks alternated with each other. A white cross at the center of the computer screen indicated a baseline fixation period of 30 s at the start and 40 s at the end of each run; participants were instructed to fixate the cross and rest during this time. Each subject performed 4 runs in one experimental session (one run of one PD subject was lost due to technical problems). The order of PS and CON blocks in each run was randomized and counterbalanced across subjects.
2.4. Procedure
The PS task required subjects to order a series of four pictures (e.g., airplane lifting off, bird building a nest). The CON task controlled for the visuospatial, semantic, and motor components of the PS task, and did not include a semantic sequencing component. The CON task required subjects to find the living item among a set of four objects (see Figure 1).
Figure 1.
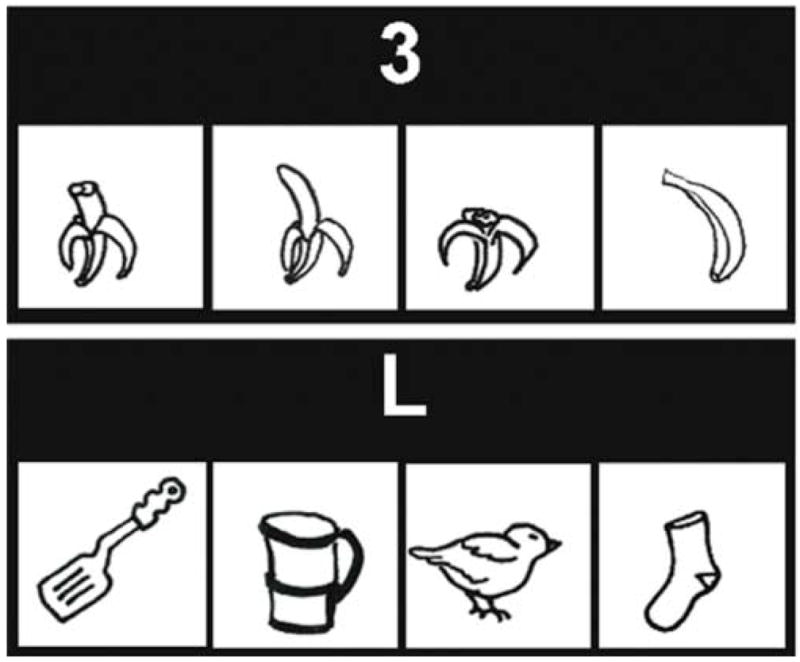
Top. Picture Sequencing (PS) task. On each trial, four pictures were shown that were temporally related. In the example, a banana is being peeled and eaten. The pictures were black and white line drawings (3.4 cm × 3.4 cm, resolution 59 pixels/cm, eye-to-screen distance 57 cm) presented simultaneously in a scrambled order at the center of the computer screen. A number cue (2, 3 or 4) centered above the pictures cued subjects to find a specific picture in the sequence. Subjects were given 10 s to order the pictures and identify the target picture. The number cue remained illuminated throughout the 10 s period. Subjects were told not to respond until they saw the “GO!” signal displayed immediately after the 10 s period above the pictures for another 2 s. Subjects indicated the location of the target picture by pressing one of four keys on a response box that had the same spatial array as the pictures.
Bottom. Object Discrimination Control (CON) task. Four black and white line drawings of living and nonliving objects were presented simultaneously. Three out of four line drawings were from the nonliving category. The cue “L” above the pictures instructed the subjects to identify the object from the living category. In the example, the bird is the living object. All other procedures in the CON task were the same as the PS task.
Subjects completed 48 trials of both tasks (PsyScope Version 1.2.5) [9]. The ordinal position of the pictures in the horizontal array was randomized and counterbalanced across trials. Trial timing was modified slightly from our prior study with a young population [50] to accommodate the slower processing of the older normal and PD populations in this study. Based on the results from a pilot behavioral study with different groups of PD and control participants, subjects were given 10 s to arrange the pictures and find the answer, and 2 s to respond. They were instructed not to respond until they saw the “GO!” signal that was illuminated after the 10 s sequencing period. This allowed us to synchronize the motor response component in both PS and CON tasks. By displaying the “GO!” cue on top of the pictures and always having the four pictures available to the subject, we intended to minimize the working memory requirement during the response period in both tasks. Subjects responded using both hands. Left-hand and right-hand responses were counterbalanced. Each subject practiced both tasks just before functional imaging outside and also inside the scanner during the acquisition of the structural MRI scans.
2.5. Performance Data Analysis
Response time (RT) and accuracy was assessed in a repeated measures ANOVA with group as the between-subject factor, and task condition as the within-subjects factor using SPSS 11.0.2 for Macintosh. Statistical threshold was set at p < 0.05 (Geisser-Greenhouse corrected).
2.6. FMRI Acquisition and Design
Scanning was performed on a 3T Siemens Allegra MRI system using a whole-head coil. High-resolution T1-weighted anatomical scans (MP-RAGE; FOV=256×256 mm, matrix=192×256, TR=6.6ms, TI=300 ms, TE=2.9 ms, flip angle=8° thickness=1.33 mm) and four T2*-weighted functional BOLD scans (179 images per scan, gradient-echo, echo-planar pulse sequence, 21 AC-PC slices, slice thickness = 5 mm, 1 mm skip between slices, TR = 2 s, TE = 30 ms, flip angle = 90°, 64×64, 3×3×5 mm3 voxels) were collected.
2.7. FMRI Data Analysis
BOLD data were analyzed using SPM2 (Welcome Dept. of Cognitive Neurology). All scans were realigned, unwarped [2], then normalized to MNI305 stereotactic space (interpolating to 2 mm3 voxels; neurological convention), and spatially smoothed with a 4 mm3 Gaussian kernel. For between-group comparisons, an 8 mm3 Gaussian kernel was used for smoothing in order to account for the intersubject variability of the cortical and subcortical structures. Statistical analyses employed the general linear model. Design matrices were modeled in scans convolved with a canonical hemodynamic response function with time derivative. High-pass filtering with a cutoff period of 128 s was applied, but global signal scaling was not used to avoid spurious deactivations.
Task-related activation was assessed by linear contrasts of PS blocks relative to fixation, and CON blocks relative to fixation. The 2 s response period was included in the analysis. Sequencing-related activation was assessed in linear contrasts of PS relative to CON blocks. Task-induced decreases in activation were also assessed in linear contrasts of fixation relative to PS and fixation relative to CON blocks, and sequencing-related decreases in activation were assessed in linear contrasts of CON relative to PS blocks. Contrast images were first created for each subject and were subsequently used in a second-level analysis treating subjects as a random effect (one-sample t-test). These group averaged statistical parametric maps (SPMs) were corrected across the whole brain for multiple voxel-wise comparisons using the false discovery rate (FDR) procedure (p < 0.05) [22]. Between-group comparisons of each contrast were made using two-sample t-tests. In these between-group comparisons, we ensured that the direction of signal change would be the same as for the within-group contrast by using masking. For example, the PS > CON contrast from the PD group was used as an inclusive mask for evaluating the target, namely the PD > control group comparison in the PS > CON contrast. The significance level of the mask was p < 0.05, uncorrected; inclusive masking at this liberal threshold removes all voxels from the target contrast that do not reach the significance level in the masking contrast. The resulting SPM shows only those voxels that are shared both by the target and masking contrasts. Between the two groups, we aimed to detect subtle differences in signal intensity primarily within the 6 regions of interest (ROIs: prefrontal cortex, caudate, and globus pallidus internal part, bilaterally). So, the statistical threshold for between-group comparisons was set to p < 0.008 (0.05 divided by 6), uncorrected. Extent threshold was always 5 voxels.
2.7.1. Functional Connectivity Analysis
To assess the functional connectivity between the basal ganglia and other brain regions, especially frontal lobes, in the PS task, we performed a correlation analysis. We selected the globus pallidus internal part (GPi) and caudate bilaterally as the regions of interest (ROIs) because both areas are critical for sequencing [16, 46, 50]. The ROI masks were created anatomically using the WFU-Pick Atlas tool in SPM2 [35], and applied to the PS > baseline contrast image of each subject. FMRI signal intensity time courses were extracted from the clusters around the peak activation in the GPi and caudate masks for each subject using the Volume of Interest (VOI) tool in SPM2 (adjusted for effects of interest). The time courses were used separately as regressors in a simple correlation analysis at the single subject level. Finally, single subject SPMs were created and entered in a second level analysis using one-sample t-test. Group-averaged SPMs were corrected across the whole brain for multiple voxel-wise comparisons using the family wise error (FWE) correction procedure (p < 0.05) with 5 voxel extent threshold. The differences in the functional connectivity maps between the PD and control groups were examined using a two-sample t-test (FDR-corrected p < 0.05).
2.7.2. Region of Interest (ROI) Analysis
Signal intensity time courses during the PS and CON tasks were extracted from the ROIs of GPi, caudate, and dorsolateral prefrontal cortex (DLPFC). Right and left GPi and caudate volumes were defined anatomically using the WFU-Pick Atlas tool in SPM2, and the time courses were extracted from the whole volumes (not only from the peak clusters) and averaged across four runs. For the DLPFC ROI definition, a combined one-sample t-test group analysis was performed on the PS > baseline and CON > baseline contrasts of all PD and control subjects, and the peak activation was determined in this composite group map (N=26) in the DLPFC bilaterally (x= 52 mm, y= 40 mm, z= 20 mm for right DLPFC and x= −50 mm, y= 30 mm, z= 26 mm for left DLPFC during sequencing; x= 38 mm, y= 44 mm, z= 24 mm for right DLPFC, and x= −50 mm, y= 32 mm, z= 24 mm for left DLPFC during control tasks). This approach has been used previously in fMRI studies investigating the signal intensity changes in the experimental group relative to the control group [6, 47]. The coordinates of the DLPFC activations overlap closely with those from our prior study with young subjects [50]. Spherical ROI masks were created around these peaks with a radius of 5 mm using the Marsbar tool in SPM2 [5]. These masks were applied to each single subject’s PS > baseline and CON > baseline contrasts, and time courses were extracted using the Marsbar tool. Percent signal change was calculated for each subject’s data using the fitted event response option across 48 s blocks in both PS and CON conditions separately, and averaged across four runs. Averaged time courses were analyzed using a repeated measures ANOVA with group as the between-subject factor, and task condition as the within-subject factor. Statistical threshold was set at p < 0.05 (Geisser-Greenhouse corrected).
PD subgroups
We tested approximately equal samples of PD patients with left- (LPD) and right-side (RPD) onset of motor symptoms whose symptoms were mild to moderately bilateral. Even so, due to the asymmetrical disease course, we also evaluated the hypothesis that RPD and LPD patients would show differential hemispheric dysfunction, as has been found in similar samples of PD patients (e.g. [1]). We found no clear evidence for differences between LPD and RPD subgroups in either the fMRI or behavioral results, and therefore we focused all subsequent analyses on the results collapsing across the entire PD group. It remains possible that side of onset effects may be found with a larger subgroup sample.
3. Results
3.1. Behavioral Data
3.1.1. Neuropsychological Results
The repeated measures ANOVA revealed no significant group effect on the neuropsychological test performance (F(1,23) = 2.24, p = 0.15). However, there was a group × test interaction (F(1,7), p = 0.025). Independent-sample t-tests revealed significant slowing in the PD group compared to controls on the Digit Symbol (t = −2.36, p = 0.028) and Trail Making A (t = 2.1, p = 0.048) tests, and significantly higher scores on BDI-II (t= 2.67, p = 0.015) but still clearly within the normal mood range (Table 1). Independent-sample t-tests did not show group differences on span forward (10 PD, 7.8 ± 0.92; 10 control, 7.2 ± 1.14; t = 1.3, p = 0.21) and backward (PD, 5.1 ± 1.52; control, 5.8 ± 1.14, t = −1.17, p = 0.26) and FAS performance (9 PD, 47.8 ± 12.4; 10 control, 53.2 ± 6.7, t = −1.2, p = 0.27).
3.1.2. Task Performance
The average of the median RTs in the PS task was 556 ms (SD=111), in the CON task 566 (SD=88) in the control group, and 579 ms (SD= 154) and 580 ms (SD=126), respectively in the PD group. The repeated measures ANOVA revealed no main effect of group on the RT (F(1,24)= 0.15, p = 0.7). There was also no main effect of task condition on the RT (F(1,1)= 0.29, p = 0.6).
The control group made an average of 3 errors (SD=2.2) in the PS and 0.85 (SD=1.5) error in the CON task, whereas PD group made an average of 5 (SD= 3.3) and 1.2 (SD= 1.3) errors, respectively. The ANOVA indicated a trend towards an effect of group on accuracy in the PS task (F(1,24)=3.4, p = 0.08) with the PD group performing worse. There was a main effect of task condition with both groups performing more accurately on the CON task compared to the PS task (F(1,1)= 24, p > 0.0001) replicating prior findings with these tasks [50].
3.2. FMRI Results
3.2.1 Within-Group Contrasts
The results of group averaged BOLD data are reported at p < 0.05, corrected for multiple voxel-wise comparisons using the FDR procedure.
3.2.1.1. Task > Baseline (fixation)
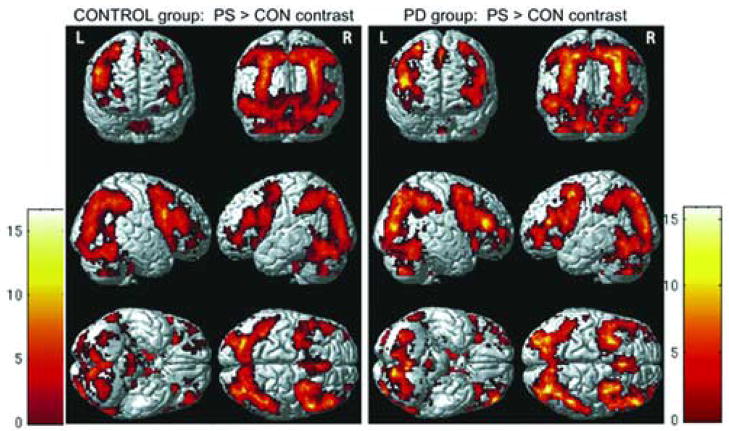
The general activation pattern observed in the task > baseline contrasts was similar in both groups including widespread activation in the occipitotemporal, parietal, and frontal cortices, and basal ganglia. Specifically, in the CON > baseline contrast, the control group showed right dorsolateral prefrontal cortex (DLPFC), left putamen, and left globus pallidus internal part (GPi) activation, whereas the PD group showed bilateral activation in the same areas. The PS > baseline contrast in both groups revealed robust and bilateral activation in the DLPFC and frontopolar areas. Basal ganglia were also bilaterally involved in the PD group, whereas the control group did not show left caudate activation.
3.2.1.2. Picture Sequencing (PS) Task > Object Discrimination Control (CON) Task
Both groups demonstrated robust sequencing-related activation bilaterally in the dorsolateral prefrontal and frontopolar cortices and the basal ganglia, except for the left caudate, which was activated only in the PD group (see Figure 1, and Tables 1–2 for coordinates in supplementary material).
3.2.1.3. Baseline > Task Contrasts
Baseline > CON task revealed no areas of activation in either group. In the baseline > PS task contrast, the control group showed activation in bilateral medial superior frontopolar and anterior cingulate cortices, right medial superior frontal gyrus, left precuneus, left lingual gyrus, right superior and middle temporal gyri, left superior temporal/angular gyrus, and right lateral postcentral gyrus. The same contrast did not reveal any area of activation in the PD group.
3.2.1.4. Object Discrimination Control (CON) Task > Picture Sequencing (PS) Task
This contrast reveals sequencing-related relative decreases in activation. The control group showed activation in this contrast in bilateral dorsal and ventral medial prefrontal cortex, medial posterior parietal/cingulate areas, superior and middle temporal gyri, sensorimotor cortex, and left medial temporal areas. The PD group did not show any activation.
3.2.2. Between-Group Comparisons
The results of the main contrasts of interest, PS > CON and CON > PS (see supplementary material for other contrasts), are reported at p < 0.008 uncorrected, after masking with the respective within-group contrasts.
3.2.2.1. Picture Sequencing (PS) Task > Object Discrimination Control (CON) Task
The control group compared to PDs showed greater activation in the right precentral / inferior frontal gyri (BA 6/44), and middle frontal gyrus (BA 9), and in the left precentral gyrus close to the frontal eye fields, demonstrating regions of hypoactivation in PD. On the other hand, the PD group compared to the control group showed greater activation in the middle frontal gyrus (BA 8), caudate, and lateral orbitofrontal cortex on the left, and in bilateral sensorimotor cortex, demonstrating regions of hyperactivation in PD.
To examine the PD hyperactivation in the left caudate further, we applied Gaussian-field, small-volume correction in 4 mm spherical clusters centered at the peak activations. The results were significant at p < 0.05, corrected for family-wise errors (FWE) (x= −16 mm, y= 26 mm, z= −2 mm (FWE-corrected p = 0.024, z = 2.76) and x= −4, y= 16, z= −2 (FWE-corrected p = 0.03, z = 2.68). We also performed a post-hoc signal intensity time course analysis around the left caudate head activation in both groups during the PS task. A spherical map with 4 mm radius centered around the peak of the left caudate head activation (x= −16 mm, y= 26 mm, z= −2 mm) in the PD group was created. Signal intensity time courses were extracted for each subject by applying this mask to the PS task activation maps using the Marsbar tool in SPM2. An independent-sample t-test in SPSS 11.0.2 revealed significantly higher signal intensity in the PD group compared to controls (t= −2.8, p = 0.01).
3.2.2.2. Object Discrimination Control (CON) Task > Picture Sequencing (PS) Task
The control group compared to the PD group showed activation in bilateral dorsal and ventral medial prefrontal cortices, medial posterior parietal / cingulate areas, superior and middle temporal gyri, sensorimotor cortex bilaterally, and in the caudate, medial temporal areas and orbitofrontal cortex on the left. The PD group compared to the control group did not show any activation in this contrast.
3.3. Functional Connectivity Results
The relative strength of functional connectivity in the sequencing task varied across groups and different basal ganglia structures. Results are reported at FWE-corrected p < 0.05. The correlation maps did not reveal clear lateralization differences between LPD and RPD subgroups. Therefore the reported results reflect the functional connectivity across the entire PD group.
3.3.1. Within-Group Contrasts
3.3.1.1. Left Globus Pallidus, internal part (GPi)
In the control group, the left GPi activation correlated with itself. In the PD group, it correlated with itself and the left precuneus (note, PD results reflect the average of 12 subjects because one PD subject did not show reliable left GPi activation in the PS > fixation contrast).
3.3.1.2. Right Globus Pallidus, internal part (GPi)
In the control group, the right GPi activation correlated with itself and the left GPi. In the PD group, it correlated with itself and bilateral putamen, thalamus, medial superior frontal, inferior and middle frontal (BA 9 and 46) gyri, and supramarginal gyrus, all on the right side.
3.3.1.3. Left Caudate
There was no correlation between the left caudate and any brain area in either group.
3.3.1.4. Right Caudate
In the control group, the right caudate did not correlate with any other brain area. In the PD group, it correlated with itself, and the right putamen/GPi, left caudate, and right lateral orbitofrontal cortex.
3.3.2. Between-Group Comparisons
The functional connectivity maps did not differ significantly between the two groups (two-sample t-test, FDR-corrected, p < 0.05).
3.4. Signal Intensity Time Courses in Regions of Interest (ROIs)
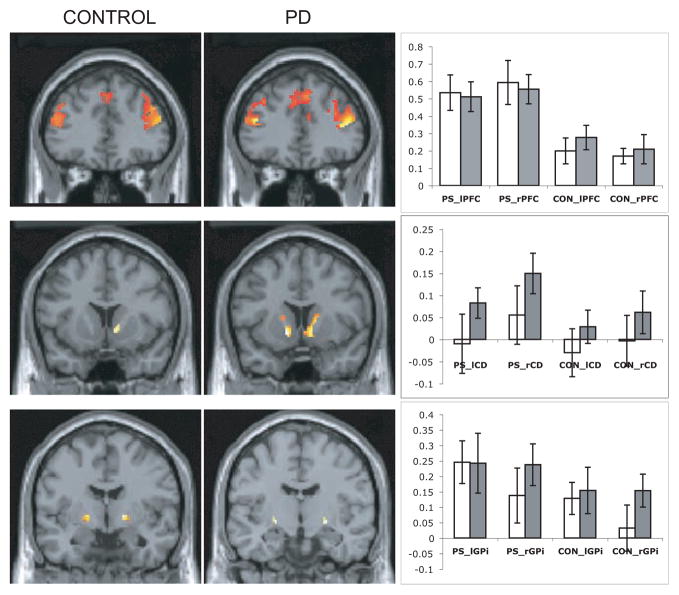
Bar graphs in Figure 2 demonstrate the percent signal change in the caudate, GPi, and DLPFC in both groups during PS and CON tasks. The repeated measures ANOVA revealed no main effect of group for the signal intensity time courses extracted from the caudate, GPi, and DLPFC during the PS and CON tasks (left caudate: F(1,24) = 1.2, p = 0.3; right caudate: F(1,24) = 0.9, p = 0.4; left GPi: F(1,24) = 0.01, p = 0.9; right GPi: F(1,24) = 0.16, p = 0.3; left DLPFC: F(1,24) = 0.06, p = 0.8; right DLPFC: F(1,24) = 0, p = 1). There was a significant main effect of task condition with a higher percent signal change during the PS than the CON task in both groups (left caudate: F(1,1) = 6.3, p = 0.02; right caudate: F(1,1) = 22, p = 0.0001; left GPi: F(1,1) = 15.4, p = 0.001; right GPi: F(1,1) = 16.4, p = 0.0001; left DLPFC: F(1,1) = 40, p = 0.0001; right DLPFC: F(1,1) = 27, p = 0.0001). The focused ROI analysis within the left caudate head activation during the PS task revealed significant differences between the two groups. PD group showed a higher percent signal change compared to controls (PD: 0.09% ± 0.15; Control: −0.1% ± 0.2. Independent-sample t-test, t = −2.8, p = 0.01).
Figure 2.
Picture Sequencing (PS) > Object Discrimination Control (CON) contrast. Group-averaged activation patterns in the regions of interest (ROIs) in the control (n=13) and Parkinson’s disease (PD) groups (n=13) are shown on the coronal slices of the canonical brain in SPM2. Anatomically defined masks were used to display the activation in the ROIs using the WFU- Pick Atlas tool in SPM2. Top: Bilateral dorsolateral prefrontal cortex (y = 36 mm for both groups), Middle: Right caudate in the control group (y = 12 mm), bilateral caudate in the PD group (y = 10 mm), Bottom: Bilateral globus pallidus internal part (y = −4 mm for the control group and y = −10 mm for the PD group).
Bar graphs show percent signal change extracted from these ROIs bilaterally for both groups (open bars: Control group, gray bars: PD group) and tasks (PS: Picture Sequencing, CON: Object Discrimination Control). PFC: Dorsolateral prefrontal cortex, CD: Caudate, GPi: globus pallidus internal part, l: Left, r: Right
For the coordinates, and z and p values of these ROIs and of other brain areas see Tables 1 and 2 in the supplementary material.
4. Discussion
The goal of this study was to assess the functional integrity of frontal lobe-basal ganglia systems during a semantic event sequencing task in patients with Parkinson’s disease (PD). Comparisons between PD patients and a matched control group reveal two main findings. First, although the global network pattern of brain systems recruited during the sequencing task is similar between the PD and the normal control groups, the PD patients do not show normal levels of activation. PD patients have semantic event sequencing-related (PS > CON) hypoactivation in frontal areas normally recruited for the PS task, that is, right premotor/inferior frontal areas (BA 6/44) and right dorsolateral prefrontal cortex (BA 9). In addition, PD patients show hyperactivation in one region normally recruited for the PS task (left BA 8) and in several others not specific to the PS task (left caudate and orbitofrontal regions, and bilateral sensorimotor cortices). Additional evidence for abnormal brain activity in PD is our finding that the activation level of a resting or “default” network of brain regions is reduced in the PD group compared with the controls. Second, the functional connectivity findings extend this picture of semantic sequencing-related PD brain dysfunction in two ways. One, we found that the left caudate hyperactivity in PD does not correlate with activity in other brain areas. Two, we found hemispheric differences between the two groups: The PD group demonstrates stronger correlations in frontal - basal ganglia circuits in the right hemisphere even though the semantic sequencing task normally recruits left frontal-basal ganglia loops more strongly [50]. These findings are discussed here in relationship to other studies of executive function in PD.
4.1. Areas of Abnormal Activation
Analysis of signal intensity time courses in the DLPFC, caudate, and GPi did not reveal differences between control and PD groups, and behavioral performance was relatively normal in the PD group. PD patients made slightly more errors than controls on the PS task, although this difference was not significant. We thus consider our PD patients to have performed relatively normally on this task with at most a mild impairment.
We chose to focus on the DLPFC, caudate, and GPi regions because our previous findings indicated that these regions form the critical “loop” necessary for this task [50]. Here, we demonstrate that early-stage PD patients on medication can perform the high-level cognitive task of semantic event sequencing relatively normally compared to matched controls, and the same sequencing-related DLPFC and basal ganglia regions are active in both groups. However, the relative level of activation of the frontal-basal ganglia circuit was abnormal. PD patients show relative hypoactivation in the critical DLPFC part of the circuit (right BA 9). Hypoactivation of this small portion of the critical DLPFC-basal ganglia circuit may underlie our observation of accuracy for PD patients on the PS task that is slightly reduced but still comparable to normal performance. Hypoactivation was also found in precentral/inferior frontal gyri, areas that are normally active in the PS task, but outside the critical DLPFC-basal ganglia circuit [50].
Abnormal hyperactivation was also noted cortically. In the left middle frontal gyrus (BA 8), the PD group showed relative hyperactivation. This area is involved in maintenance of visuospatial information in working memory [13, 45] and was also active in the PS > CON contrast in our previous study with young subjects [50]. The PS task requires selective updating of neural representations of the pictures held in working memory. BA 8 activity may thus reflect the working memory maintenance demands of the PS task, which may be especially high for PD patients. Relative hyperactivation in BA 8 may reflect compensatory activity to achieve the normal working memory and executive function we observed in our PD patients based on our clinical test assessment.
We also found evidence for abnormal hypoactivation of a “default” resting state network in PD. During the CON relative to the PS task, the control group, but not the PD group, demonstrates recruitment of a task-independent “default” resting state network, including the ventromedial and dorsomedial prefrontal cortices, medial parietal and posterior cingulate cortex, and the lateral occipitotemporal areas [26, 43]. Activation of these “default” areas has been implicated in various aspects of self-referential analysis (i.e., internally-driven, when people are in a state of relative ‘rest’) [21] and has been shown to decrease when subjects perform goal-directed tasks that demand shifting the allocation of attentional resources from these self-referential processes to task-related processing (i.e., externally-driven) [26, 43]. In addition, the “default” areas have been shown to be correlated with each other, while being anticorrelated with areas in the frontoparietal network for attention and working memory [21]. The relationship between these two anticorrelated networks are consistent with our finding that the control group demonstrates decreased recruitment of the “default” resting state network during the sequencing relative to the control task, whereas the PD group did not demonstrate any area of decreased activation during sequencing. This direction of effects and the brain regions involved have also been reported in previous fMRI studies of normal aging and dementia [27, 34]. This may reflect the specific cognitive demands of the PS task for PD patients. In particular, the PS task requires subjects to allocate their attention to the processing of external stimuli, and no external cue is available to guide subjects in solving the sequencing problem. Hence, subjects have to plan a strategy and initiate their plan using internally-generated cues. This process may be harder for PD patients given their well-known deficits in internally-generating and initiating plans [40, 41], requiring the recruitment of additional cortical resources, including the regions in the “default” network.
4.2. Functional Connectivity Changes in Frontal-Basal Ganglia Circuits in PD
Although the signal intensity time courses extracted from the whole caudate volume did not differ between the groups, the head of the left caudate showed relative hyperactivation during sequencing in the PD group and was actually not active in the control group. The ROI analysis of the left caudate head activation also revealed significantly higher signal intensity in the PD group compared to controls. The left caudate head hyperactivation in the PD group might reflect compensatory activity related to working memory demands. Support for this idea comes from a study that found activation in left caudate body, as well as a similar set of prefrontal regions during a semantic working memory task with words [15] and a study that reported bilateral caudate head activation during verbal working memory tasks, though activation is greatest when manipulation processes are required [31]. However, the simple regression analysis did not reveal a correlation between this left caudate head activation and any brain area in the PD group. Thus, alternatively, as the left caudate activity is uncorrelated with other brain structures, most notably the frontal lobe regions that project heavily to the head of the caudate nucleus [38], this activity may be inefficient for task-related processes or might reflect an ongoing disease process, or both.
With young subjects, we have shown that the semantic sequencing task recruits regions in the left hemisphere more [50]. By contrast, in the PD group, the regression analysis in the current study demonstrated that the right caudate and right GPi correlation maps were more significant and widespread compared to those on the left. This pattern of greater right hemisphere recruitment in PD was not observed in the control group, and the different pattern between groups cannot be attributed to differences in participant handedness, as the PD and control groups had comparable handedness, and our bimanual tasks would reduce any handedness effects. These findings suggest that the right hemisphere frontal-basal ganglia circuit shows compensatory hyperactivation in the PD group. This result is consistent with other findings of greater recruitment of the hemisphere opposite the dominant one for the task in other PD studies [7] and evidence for right caudate recruitment for spatial executive tasks in PD patients [8].
4.3. Effects of Dopamine
The relatively normal semantic event sequencing performance and normal pattern of task-related brain activity (i.e., PS > CON for PD and control groups) and hyperactivation of left caudate, left orbitofrontal, and bilateral sensorimotor cortex regions outside the normal PS task network, suggests the operation of compensatory mechanisms in our medicated, mild PD patients. The abnormal hyperactivation noted in our study may reflect a combination of compensatory processes outside the PS task network, inefficient processing within the PS task network (in BA 8), and partial dopamine amelioration effects.
Since our PD subjects were all tested while on medication, it is important to consider the extent to which the normalizing effects of dopaminergic medication are responsible for the normal performance and brain activation patterns noted in this study. Executively unimpaired PD patients on dopamine show normal behavioral performance and brain activation in task-related networks [30]. Some neuropsychological findings indicate PD patients perform better on executive function tasks under dopaminergic medication relative to the unmedicated state, for instance in working memory tasks that require manipulating information [32], in spatial working memory tasks [29], task-set switching paradigms [10], in the “perseveration” condition of set-shifting tasks [42], and in planning tasks [29]. However, many other neuropsychological studies find cognitive deficits even in medicated PD patients relative to control groups [14]. On a verbal, semantic event sequencing task, nondemented, mild to moderate PD patients tested on dopaminergic medication made significantly more sequencing and perseverative errors and irrelevant intrusions, and generated scripts more deprived of contextual elements, compared to a control group [23]. However, since this study did not test the PD patients also off dopaminergic medication, it is unclear if dopamine might still have somewhat improved sequencing performance relative to worse performance in the off-state, albeit not to normal levels. The PD group in our study also made more errors in the sequencing task compared to the control group, but this difference did not reach significance, thus we considered our PD subjects mildly impaired at most. We cannot rule out that our PD group performed well on the PS task, and showed a corresponding normal pattern of brain activation, in part due to the normalizing effects of dopamine. This cannot be the whole story, however, because dopamine normalization effects cannot explain our hyperactivation findings.
Overall, neuroimaging findings suggest two alternative explanations for the finding of relatively normal performance associated with hyperactivation in PD patients, whether on or off dopaminergic medication. These two alternatives can be summarized as “compensation” vs. “efficiency”, and are not mutually exclusive. This brain-behavior combination reflects compensatory processing, if found in regions outside of the normal task-related brain network, or instead reflects less efficient neural information processing (leading to hyperactivation), if found in regions within the normal task-related network. Hyperactivation in task-related areas has been found in PD patients off dopaminergic medication and attributed to increased neural activity that compensates for inefficient intrinsic processing that can, however, be made more efficient when dopamine is administered. Imaging studies of PD patients in the off-state while performing planning (e.g., Tower of London task) and spatial working memory tasks have shown cortical hyperactivation compared to controls [11, 17]. In a verbal working memory task, greater cortical activation was found in the off- compared to the on-state in the same PD group [36]. Dopaminergic medication is thought to increase the neurophysiological, information processing efficiency of prefrontal cortex, concomitantly reducing activation of this cortex, relative to the off-state. By contrast, when off medication and in a hypodopaminergic state, the processing is less efficient and so more processing effort must be expended to achieve normal performance, thereby resulting in observed fMRI hyperactivation in brain regions normally involved in the task.
In the present experiment, hyperactivation in left BA 8, which is normally part of the PS task network, could reflect less efficient neural processing that is not fully ameliorated by dopamine in our medicated PD patients.
Most of our hyperactivation was in areas that are outside the PS task network (i.e., left caudate, orbitofrontal, and bilateral sensorimotor cortices). This hyperactivation is consistent with neural information processing that compensates for dysfunction within the critical frontostriatal network for semantic event sequencing (i.e., hypoactivation in right DLPFC (BA9), precentral/inferior frontal gyri, and perhaps hyperactivation in BA 8). The compensatory hyperactivation outside the PS task network may further contribute to achieving the normal semantic event sequencing performance that we observed, perhaps in addition to any benefits from dopamine normalization.
In conclusion, we think that the substantial abnormal brain activation that we observed in PD patients is most compatible with the idea that frontal-basal ganglia circuits are dysfunctional in mild PD, but dopamine has some partial ameliorating effects on the parts of the circuit specifically recruited to accomplish semantic event sequencing.
Supplementary Material
Acknowledgments
We thank Alice Cronin-Golomb, Ph.D., Courtney Horwitz, Anne Nisenzon, Stephen M. Maher, Kim Celone, and Sigurros Davidsdottir for their assistance with the study. Research was supported by NIMH award R21 MH066213. We acknowledge imaging support from the Athinoula A. Martinos Center for Biomedical Imaging and NCRR P41RR14075. H.E.S. was supported by Tufts University faculty research funds and FRAC semester fellowship, NIA award F32 AG05914 and NINDS award R01 NS052914.
Footnotes
Disclosure Statement
The authors do not state conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amick MM, Schendan HE, Ganis G, Cronin-Golomb A. Frontostriatal circuits are necessary for visuomotor transformation: Mental rotation in Parkinson’s disease. Neuropsychologia. 2006;44(3):339–49. doi: 10.1016/j.neuropsychologia.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Andersson JL, Hutton C, Ashburner J, Turner R, Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–19. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- 3.Beatty WW, Monson N. Picture and motor sequencing in Parkinson’s disease. J Geriatr Psychiatry Neurol. 1990;3:192–7. doi: 10.1177/089198879000300403. [DOI] [PubMed] [Google Scholar]
- 4.Beck AT. Beck Depression Inventory-II. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 5.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract]. Presented at the 8th International Conferance on Functional Mapping of the Human Brain; June 2–6; Sendai, Japan. 2002. Available on CD-ROM in NeuroImage, Vol 16, No 2. [Google Scholar]
- 6.Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC. Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci. 2000;12 (Suppl 2):24–34. doi: 10.1162/089892900564046. [DOI] [PubMed] [Google Scholar]
- 7.Carbon M, Marie RM. Functional imaging of cognition in Parkinson’s disease. Curr Opin Neurol. 2003;16(4):475–80. doi: 10.1097/01.wco.0000084225.82329.3c. Review. [DOI] [PubMed] [Google Scholar]
- 8.Cheesman AL, Barker RA, Lewis SJ, Robbins TW, Owen AM, Brooks DJ. Lateralisation of striatal function: evidence from 18F-dopa PET in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2005;76(9):1204–10. doi: 10.1136/jnnp.2004.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: a new graphic interactive environment for designing psychology experiments. Behav Res Methods Instrum Comput. 1993;25(2):257–71. [Google Scholar]
- 10.Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11(12):1136–43. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- 11.Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM. Dopaminergic modulation of high-level cognition in Parkinson’s disease: the role of the prefrontal cortex revealed by PET. Brain. 2002;125:584–94. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- 12.Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV. Cognitive impairment in early, untreated Parkinson’s disease and its relationship to motor disability. Brain. 1991;114 (Pt 5):2095–122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- 13.Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279(5355):1347–51. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- 14.Cronin-Golomb A, Amick MM. Spatial abilities in aging, Alzheimer’s disease, and Parkinson’s disease. In: Boller F, Cappa S, editors. Aging and dementia. 2. Vol. 6. Amsterdam: Elsevier; 2001. pp. 119–44. [Google Scholar]
- 15.Crosson B, Rao SM, Woodley SJ, Rosen AC, Bobholz JA, Mayer A, et al. Mapping of semantic, phonological, and orthographic verbal working memory in normal adults with functional magnetic resonance imaging. Neuropsychology. 1999;13(2):171–87. doi: 10.1037//0894-4105.13.2.171. [DOI] [PubMed] [Google Scholar]
- 16.Dagher A, Owen AM, Boecker H, Brooks DJ. Mapping the network for planning: a correlational PET activation study with the Tower of London task. Brain. 1999;122 (Pt10):1973–87. doi: 10.1093/brain/122.10.1973. [DOI] [PubMed] [Google Scholar]
- 17.Dagher A, Owen AM, Boecker H, Brooks DJ. The role of the striatum and hippocampus in planning: a PET activation study in Parkinson’s disease. Brain. 2001;124:1020–32. doi: 10.1093/brain/124.5.1020. [DOI] [PubMed] [Google Scholar]
- 18.Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J Neurol. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- 19.Fahn S, Elton R. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne D, Goldstein M, editors. Recent Developments in Parkinson’s Disease. New Jersey: MacMillan Health Care Information; 1987. pp. 153–63. [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102(27):9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–78. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 23.Godbout L, Doyon J. Defective representation of knowledge in Parkinson’s disease: evidence from a script-production task. Brain Cogn. 2000;44(3):490–510. doi: 10.1006/brcg.2000.1213. [DOI] [PubMed] [Google Scholar]
- 24.Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13(6):933–49. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- 25.Groth-Marnat G. Handbook of Psychological Assessment. 3. New York: John Wiley & Sons, Inc; 1999. The Wechsler subtests; pp. 132–204. [Google Scholar]
- 26.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. [Review] Nat Rev Neurosci. 2001;2(10):685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 27.Herholz K, Salmon E, Perani D, Baron JC, Holthoff V, Frolich L, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17(1):302–16. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- 28.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 29.Lange KW, Robbins TW, Marsden CD, James M, Owen AM, Paul GM. L-dopa withdrawal in Parkinson’s disease selectively impairs cognitive performance in tests sensitive to frontal lobe dysfunction. Psychopharmacology (Berl) 1992;107(2–3):394–404. doi: 10.1007/BF02245167. [DOI] [PubMed] [Google Scholar]
- 30.Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci. 2003;23(15):6351–6. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. Eur J Neurosci. 2004;9(3):755–60. doi: 10.1111/j.1460-9568.2004.03108.x. [DOI] [PubMed] [Google Scholar]
- 32.Lewis SJ, Slabosz A, Robbins TW, Barker RA, Owen AM. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson’s disease. Neuropsychologia. 2005;43(6):823–32. doi: 10.1016/j.neuropsychologia.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Lezak MD. Neuropsychological Assessment. 3. New York: Oxford University Press; 1995. Neuropathology for neuropsychologists; pp. 170–276. [Google Scholar]
- 34.Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA. 2003;100(24):14504–9. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 36.Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg TE, Chase TN, et al. Dopaminergic modulation of cortical function in patients with Parkinson’s disease. Ann Neurol. 2002;51(2):156–64. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- 37.Mattis S. Dementia Rating Scale. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 38.Middleton FA, Strick PL. A Revised Neuroanatomy of Frontal-Subcortical Circuits. In: Lichter DG, Cummings JL, editors. Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders. New York: The Guilford Press; 2001. pp. 44–58. [Google Scholar]
- 39.Oldfield R. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 40.Owen AM, Doyon J, Dagher A, Sadikot A, Evans AC. Abnormal basal ganglia outflow in Parkinson’s disease identified with PET. Implications for higher cortical functions. Brain. 1998;121(Pt 5):949–65. doi: 10.1093/brain/121.5.949. [DOI] [PubMed] [Google Scholar]
- 41.Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, et al. Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain. 1992;115(Pt 6):1727–51. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- 42.Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, Robbins TW. Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson’s disease. Brain. 1993;116(Pt 5):1159–75. doi: 10.1093/brain/116.5.1159. [DOI] [PubMed] [Google Scholar]
- 43.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- 45.Rowe JB, Passingham RE. Working memory for location and time: activity in prefrontal area 46 relates to selection rather than maintenance in memory. Neuroimage. 2001;14(1Pt1):77–86. doi: 10.1006/nimg.2001.0784. [DOI] [PubMed] [Google Scholar]
- 46.Schendan HE, Searl MM, Melrose RJ, Stern CE. An FMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37(6):1013–25. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- 47.Schon K, Atri A, Hasselmo ME, Tricarico MD, LoPresti ML, Stern CE. Scopolamine reduces persistent activity related to long-term encoding in the parahippocampal gyrus during delayed matching in humans. J Neurosci. 2005;25(40):9112–23. doi: 10.1523/JNEUROSCI.1982-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spielberger CD, Gorusch RL, Lushene R. Spielberger State Trait Anxiety Inventory I and II. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 49.Sullivan EV, Sagar HJ, Gabrieli JD, Corkin S, Growdon JH. Different cognitive profiles on standard behavioral tests in Parkinson’s disease and Alzheimer’s disease. J Clin Exp Neuropsychol. 1989;11:799–820. doi: 10.1080/01688638908400937. [DOI] [PubMed] [Google Scholar]
- 50.Tinaz S, Schendan HE, Schon K, Stern CE. Evidence for the importance of basal ganglia output nuclei in semantic event sequencing: An fMRI study. Brain Res. 2006;1067(1):239–49. doi: 10.1016/j.brainres.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 51.Wechsler D. Wechsler Adult Intelligence Scale-III. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 52.Zalla T, Sirigu A, Pillon B, Dubois B, Grafman J, Agid Y. Deficit in evaluating predetermined sequences of script events in patients with Parkinson’s disease. Cortex. 1998;34:621–27. doi: 10.1016/s0010-9452(08)70519-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.