Summary
The capability of cocaine cues to generate craving in cocaine-dependent humans, even after extended abstinence, is modeled in rats using cue reinstatement of extinguished cocaine-seeking behavior. We investigated neural activity associated with incentive motivational effects of cocaine cues using c-fos mRNA and Fos protein expression as markers. Unlike preceding studies, we used response-contingent presentation of discrete cues to elicit cocaine seeking. Rats were first trained to press a lever, resulting in cocaine reinforcement and light and tone cues. Rats then underwent extinction training, during which lever presses decreased. On the test day, rats either received response-contingent cocaine cues or received no cues. The cues reinstated extinguished cocaine-seeking behavior on the test day. In general, cue-elicited c-fos mRNA and protein expression were similar and both were enhanced in the prefrontal cortex, ventral tegmental area (VTA), dorsal striatum and nucleus accumbens. Cues elicited more widespread Fos protein expression relative to our previous research in which cues were presented non-contingently without prior extinction training, including increases in the VTA, substantia nigra, ventral subiculum, and lateral entorhinal cortex. We also observed a correlation between cocaine-seeking behavior and Fos in the agranular insula (AgI) and basolateral amygdala (BLA). The findings suggest that connections between BLA and AgI play a role in cue-elicited incentive motivation for cocaine and that reinstatement of cocaine seeking by response-contingent cues activates a similar corticolimbic circuit as that observed with other modes of cue presentation; however, activation of midbrain and ventral hippocampal regions may be unique to reinstatement by response-contingent cues.
Keywords: drug craving, addiction, drug conditioning, reinstatement, cocaine, immediate early gene, extinction
Introduction
Recidivism, even after an extended period of abstinence, is a hallmark characteristic of cocaine addiction (Dackis and O’Brien, 2001). The strong desire to take cocaine, known as craving, is associated with relapse and can persist during abstinence (Markou et al., 1993). Craving is induced in cocaine addicts by exposure to paraphernalia, drug-related images, or the environmental context associated with cocaine use (Childress et al., 1988; Grant et al., 1996). Repeated exposure to such cues during the initiation and maintenance of cocaine use is therefore thought to result in these cues acquiring incentive motivational and conditioned reinforcing value (O’Brien et al., 1992). In animal studies, craving is modeled using the extinction/reinstatement procedure (Fuchs et al., 1998; Markou et al., 1993). In this model, rats are first trained to press a lever in an operant conditioning chamber using cocaine reinforcement paired with delivery of cues. Subsequently, animals undergo extinction training for which they are placed in the same chambers, but lever-pressing is no longer reinforced by the delivery of cues or cocaine. This procedure extinguishes drug-seeking behavior that is elicited by the self-administration environment, but leaves the incentive salience of the cocaine-paired response-contingent cues intact (Davis and Smith, 1976; de Wit and Stewart, 1981).
The neural circuitry involved in the incentive motivational effects of cocaine-associated stimuli has been investigated previously using Fos protein expression, which is thought to be a marker for stimulus-elicited brain activity (Chaudhuri, 1997; Harlan and Garcia, 1998). Fos protein expression is transiently increased by cocaine (Cohen et al., 1990; Graybiel et al., 1990; Young et al., 1991), exposure to cocaine-associated environmental cues (Brown et al., 1992; Crawford et al., 1995; Hotsenpiller et al., 2002; Neisewander et al., 2000) or discriminative stimuli that signal drug availability (Ciccocioppo et al., 2001). This study is the first to examine Fos protein expression resulting from cue-elicited reinstatement of extinguished cocaine-seeking behavior. To further examine induction of the c-fos gene, an additional experiment was conducted in order to measure c-fos mRNA using in situ hybridization histochemistry.
Materials and Methods
Animals
Male Sprague-Dawley rats weighing 250–300 g were housed individually in a temperature-controlled colony room with a 12-h reversed light/dark cycle. Animal care and housing conditions were consistent with the specifications of the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). Experimental procedures were approved by the Institutional Care and Use Committee at Arizona State University. Rats were acclimated to handling for 5 days before surgery.
Catheter construction and surgery
Catheters were constructed from Silastic tubing (10 cm length, 0.012 in inner diameter, 0.025 in outer diameter, Dow Corning, Midland, MI) connected to a 22 gauge nonferrous metal cannula encased within a plastic screw connector (Plastics One, Roanoke, VA). A small ball of aquarium sealant was affixed 2.7 cm from the free end of the catheter. Atropine sulfate (10 mg/kg i.p., Sigma, St. Louis, MO) was administered before surgery to reduce bronchial secretions. The rats were anesthetized with sodium pentobarbital (50 mg/kg i.p., Sigma). A burrow was then made subcutaneously from an incision on the neck to an incision across the skull, and the catheter was pulled through the burrow. A small incision was made in the jugular vein, and the catheter was inserted into the vein and secured with sutures on both sides of the ball. The cannula end of the catheter was anchored to the skull using dental acrylic cement and four small anchor screws. The head and neck incisions were then sutured and treated with a topical antibiotic. A flexible obturator made from Tygon tubing was fitted over the cannula to protect the catheter. Patency of the catheters was maintained throughout the experiment by daily flushing with 0.1 ml bacteriostatic saline solution containing heparin (70 U/ml, Elkins-Sinn, Cherry Hill, NJ) and ticarcillin disodium (20 mg/ml GlaxoSmithKline, Philadelphia, PA). Rats also received 0.67 mg/ml urokinase (Astra USA, Westerborough, MA) daily for 1 week after surgery. Catheter patency was tested periodically with 0.8 mg methohexital sodium (Brevital, Sigma), a dose that has produces loss of muscle tone only when administered i.v.
Apparatus
Training and testing were conducted in Plexiglas operant conditioning chambers (20 cm × 28 cm × 20 cm) equipped with two levers mounted on the front wall (Med Associates, St Albans, VT). A cue light was mounted above one lever, a 2.9 kHz tone generator was mounted on the front wall, and a house light was mounted on the rear wall. The lever below the cue light was designated as the active lever. Each conditioning chamber was within its own ventilated, sound-attenuating chamber. An infusion pump contained a 10 mL syringe and was located outside of the sound-attenuating chamber. Tygon tubing connected to the syringe was attached to a liquid swivel (Instech, Plymouth Meeting, PA) suspended above the operant conditioning chamber. The outlet of the swivel was fastened to the catheter via Tygon tubing that ran through a metal spring leash (Plastics One). The leash fastened onto the plastic screw of the catheter that was anchored on the animal’s head.
Self-administration training
Starting five days after surgery, self-administration (SA) training was conducted during daily 2-h sessions over a period of 21 consecutive days. Rats were trained to press the active lever to receive cocaine reinforcement (0.75 mg/kg/0.1 ml, i.v.). Upon completing a schedule of reinforcement, the cue light, house light and tone were activated, followed after one sec by activation of the infusion pump for six sec. Following infusion, the cue light and tone were inactivated. After a 20-s timeout period, the house light was inactivated and active lever presses were accumulated towards the next reinforcement schedule.
During the initial training phase, the rats were placed on a fixed ratio (FR) 1 schedule of reinforcement. After the minimum criterion was met of at least seven schedule completions in an h, the schedule demand was increased from FR 1 to FR 11. Two days prior to the start of training, rats were food-restricted to 18 g of chow per day to facilitate acquisition of SA (Carroll et al., 1981). Food rations were gradually increased as rats acquired operant conditioning. All rats received ad libitum access to food during the last 14 days of SA training and throughout the rest of the experiment.
Extinction training
Extinction training began the day after SA training was completed. Rats were exposed to the SA environment for a 2-h session each day across 16–17 consecutive days. During the extinction sessions, lever presses had no scheduled consequences; the rats were connected to the swivel but no infusions or light/tone cues were delivered. Responses on the active and inactive levers were recorded. Since the rats were exposed to the SA environment but not the discrete cocaine-paired cues, the incentive motivational effects of these stimuli remained intact. By the end of extinction training, all rats exhibited active lever response rates of less than 20% of the peak response rates that occurred during extinction training. Responding during the terminal extinction session was used as a baseline for comparison to responding on the test day.
Experimental design
Two experiments were conducted. Experiment 1 examined changes in Fos protein, and Experiment 2 examined changes in c-fos mRNA, associated with cue reinstatement of cocaine-seeking behavior. For both experiments, rats were assigned to either a Cues group or a No Cues group with n = 9 and 7, respectively, for Experiment 1 and n = 5 per group for Experiment 2. The groups were matched to the extent possible for previous cocaine intake and responding during the terminal extinction session.
Test for reinstatement of cocaine-seeking behavior
Cocaine-seeking behavior was operationally defined as responses on the active lever in the absence of cocaine reinforcement. On the day after the final extinction session, rats were placed into their SA chambers for either 90 min (Experiment 1) or 60 min (Experiment 2), during which responses on both the active and the inactive lever were recorded. The test session lengths were chosen for optimal expression of stimulus-induced Fos protein and c-fos mRNA, respectively (Moratalla et al., 1993). The cocaine-paired stimulus complex (i.e. cue light, tone, house light and pump motor) was delivered on a FR1 schedule of reinforcement for the Cues group. Active lever presses had no consequences for rats in the No Cues Group.
Tissue preparation
Experiment 1
Immediately following reinstatement testing, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg i.p.). Their circulatory system was perfused with 150 ml of ice-cold saline followed by 300 ml of ice-cold 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS, pH 7.4). The brains were removed and post-fixed in 4% paraformaldehyde for 24 h, and then cryoprotected by submersion in 30% sucrose for at least 24 h. The brains were then sectioned using a sliding microtome (Microm International, Walldorf, Germany) connected to a freezing stage (Physitemp, Clifton, NJ). Serial coronal 40 μm sections were collected, separated by 120 μm, centered at anatomical locations corresponding to bregma +3.2, +1.6, −2.56 and −5.8 mm (Paxinos and Watson, 1998). The tissue sections were then frozen and stored at 4° C in a cryoprotectant solution comprised of 0.02 M PBS (pH 7.2), 30% sucrose, 10% polyvinyl pyrrolidone and 30% ethylene glycol.
Experiment 2
Rats were decapitated immediately following reinstatement testing. Their brains were removed and immediately frozen in 2-methylbutane (20°C) and stored at −80°C. Sections (20 μm) were collected at four levels as described above and thaw-mounted onto ProbeOn Plus pre-cleaned slides. They were placed in 4% formaldehyde for 60 min at 4°C, rinsed three times in 0.1 M PBS for 5 min and dehydrated in ascending alcohols, ending with 95%. Slides were air-dried completely and again stored at −80°C until processed for in situ hybridization histochemistry.
Fos protein immunocytochemistry
For Experiment 1, free-floating tissue sections were first washed in 0.02 M PBS (pH 7.2, seven times for 10 min each), incubated for 1 h in 0.2% Triton X-100 and 3% normal goat serum (NGS; Vector Laboratories, Burlingame, CA) in 0.02 M PBS. The tissue was then incubated for 48 h at 4°C with rabbit polyclonal anti-Fos serum (sc-52, Santa Cruz Biotechnology, Santa Cruz, CA), diluted 1:10,000 in 0.02 M PBS containing 1% NGS and 0.75% Triton X-100. Following incubation, the sections were washed in 0.02 M PBS (five times for 5 min each) and then incubated for 1 h at room temperature in biotinylated goat anti-rabbit antibody IgG (Vector Laboratories) diluted 1:200 in 0.02 M PBS. The tissue was then washed in 0.02 M PBS (three times for 10 min each) and then incubated for 1 h in avidin-biotinylated horseradish peroxidase complex (ABC Elite Kit; Vector Laboratories) diluted 1:100 in 0.02 M PBS. The sections were then washed with 0.05 M Tris buffer, (pH 7.6, three times for 10 min each) and incubated in 0.05 M Tris buffer containing 0.02% 3,3′-diaminobenzidine (DAB; Sigma), 2.5% nickel ammonium sulfate and 0.005% H2O2. The DAB reaction was terminated after 6.5 min by rinsing the tissue three times for 10 min in 0.02 M PBS. All of the washes and incubations described above were performed on an orbital shaker (Cole-Parmer, Vernon Hills, IL) operating at 130 rpm. The sections were mounted onto gelatin chromium-coated slides, air-dried, dehydrated and protected with a coverslip for light-microscopic inspection.
Fos immunoreactivity analysis
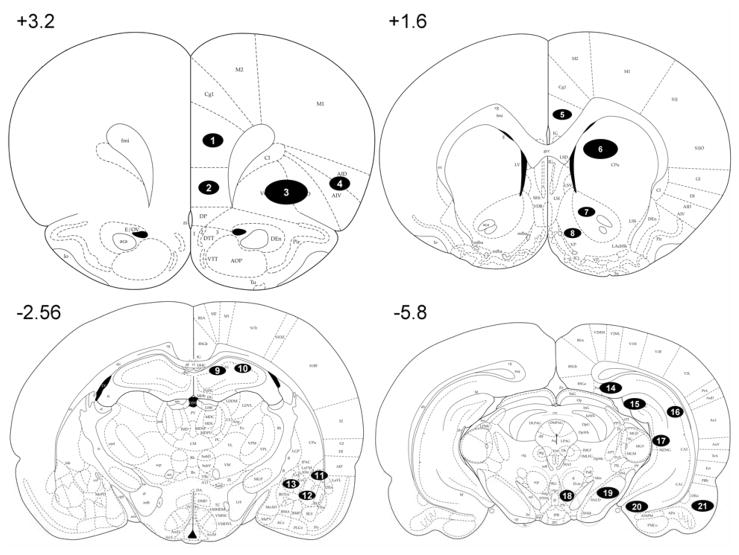
Fos immunoreactivity was examined using a Nikon Eclipse E600 (Nikon Instruments, Melville, NY) microscope set at 20× magnification and counted by an observer blind to treatment conditions using the ImageTool software package (Version 3.0, University of Texas Health Science Center, San Antonio, TX). The anatomical locations and boundaries of each region were determined using a rat brain atlas (Paxinos and Watson, 1998) and are illustrated in Fig 1. Sections taken at +3.2 mm from bregma contained the prelimbic (PrL), infralimbic (IL), orbital (Orb) and agranular insular (AgI) cortices. Sections taken at +1.6 mm from bregma contained the Cg2 region of the anterior cingulate cortex (Cg2), nucleus accumbens shell (NAcS), nucleus accumbens core (NAcC) and dorsal caudate-putamen (dCPu). Sections taken at −2.56 mm from bregma contained the CA1 and CA2 regions of the dorsal hippocampus (dCA1 and dCA2), basolateral amygdala (BlA), central amygdala (CeA) and lateral amygdala (LA). Sections taken at −5.8 mm contained the CA1 and CA3 regions of the ventral hippocampus (vCA1 and vCA3), dentate gyrus (DG), dorsal and ventral subiculum (dS and vS), lateral entorhinal cortex (LEnt), substantia nigra pars reticulata (SNr) and ventral tegmental area (VTA). The sections were taken such that the rostral-caudal extent of each region of interest was sampled (360 μm). Fos immunoreactivity was counted and identified by a blue-black oval-shaped nucleus (Figs. 2A, 2B). Each region of interest was analyzed in both hemispheres from three tissue sections/animal. Two samples from each hemisphere were taken for the Orb and dCPu, and one sample per hemisphere was taken for all of the other regions. The area of each photographed sample was 0.26 mm2. The counts from all the sample areas from a given region were averaged and scaled to provide a mean number of Fos-positive cells per mm2. Analyses were performed blind to group assignment.
Figure 1.
Schematic representation of coronal sections of the rat brain taken at +3.2, +1.6, −2.56, and −5.8 mm from Bregma (Paxinos and Watson,1998). Encircled numbers in the sections represent the regions analyzed for Fos as follows: (1) prelimbic cortex (PrL); (2) infralimbic cortex (IL); (3) orbital cortex (Orb); (4) agranular insular cortex (AgI); (5) Cg2 region of the anterior cingulate cortex (Cg2); (6) dorsal caudate-putamen (dCPu); (7) nucleus accumbens core (NAcC); (8) nucleus accumbens shell (NAcS); (9) dorsal CA1 region of the hippocampus (dCA1); (10) dorsal CA2 region of the hippocampus (dCA2); (11) lateral amygdala (LA); (12) basolateral amygdala (BlA); (13) central amygdala (CeA); (14) dorsal subiculum (dS); (15) dentate gyrus (DG); (16) ventral CA1 region of the hippocampus (vCA1); (17) ventral CA3 region of the hippocampus (vCA3); (18) ventral tegmental area (VTA); (19) substantia nigra pars reticulata (SNr); (20) ventral subiculum (vS); and (21) lateral entorhinal cortex (LEnt).
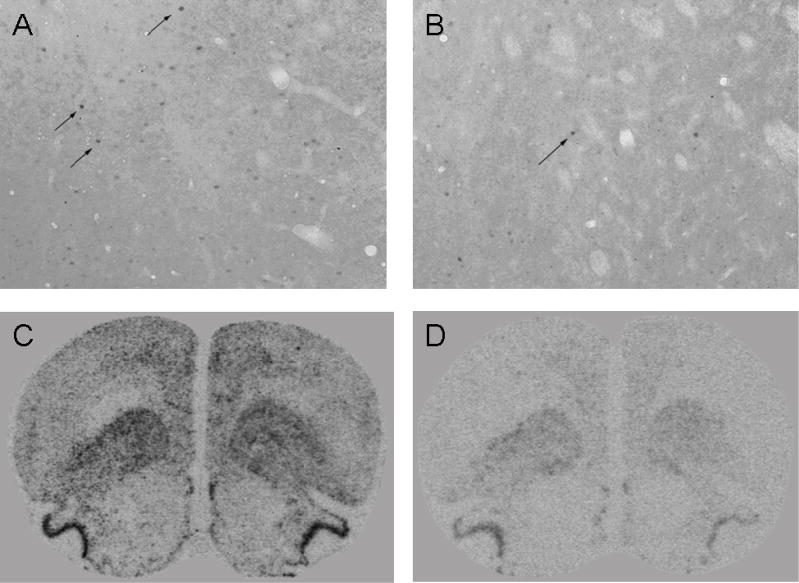
Figure 2.
Representative photomicrographs of Fos protein and c-fos mRNA expression. A magnified sample of Fos-labeled IL taken at 20× magnification is shown from a rat in the Cues group (A) of Experiment 1, demonstrating visible Fos protein expression as dark ovals (highlighted by arrows), and from a rat in the No Cues group (B), demonstrating sparse Fos protein expression. Representative autoradiograph of c-fos mRNA expression in sections taken at +3.2 mm relative to Bregma from a rat in the Cues group (C) and from a rat in the No Cues group (D), with the latter exhibiting far less c-fos mRNA hybridization signal.
c-fos mRNA in situ hybridization histochemistry
For Experiment 2 tissue, slides were placed at −20°C for 30 min, dried on a warming plate and placed in proteinase K solution [100 mM Tris HCl, 50 mM ethylenediaminetetraacetic acid (EDTA)], for 10 min at 37°C. Slides were then rinsed once with diethylpyrocarbonate (DEPC) water and then treated with a solution of 0.1 M triethanolamine and 400:1 triethanolamine:acetic anhydride for 15 min at room temperature. Next, slides were rinsed in 2× sodium chloride/sodium citrate (SSC) at room temperature for 5 min, dehydrated in ascending alcohols, and air-dried. Sections were hybridized with a [33P]UTP –labeled riboprobe 2.1 kb c-fos (GenBank accession number U19866)kindly provided by Dr. T. Curran from NIH. A sense riboprobe was also generated in order to compare labeling to the antisense probe. Probes were transcribed and diluted in hybridization buffer [78.5% formamide, 52 mM Tris (pH 7.8), 3× SSC, 1× Denhardt’s, 26 mM dithiothritol (DTT), 2.6 mM EDTA, 0.2 mg/ml yeast tRNA, 0.2 mg/ml salmon testes DNA and 10% dextran sulfate] and applied to a final concentration of 3.2 × 106 cpm per slide. Following overnight hybridization at 55°C, slides were rinsed in 2× SSC for 5 min at room temperature and then treated with RNase A solution (100 mM Tris, 0.5 M NaCl and 200 μg/ml RNase A) for 1 h at 37°C. Slides were then rinsed in 2 × SSC for 10 min at room temperature, 1× SSC for 10 min at room temperature, 0.5× SSC for 1 h at 55°C and ending with 0.05× SSC for 10 min at room temperature. Slides were again dehydrated in alcohol, air-dried overnight, and apposed to Kodak BioMax film.
c-Fos densitometry
Autoradiographs of brain sections (see Figs. 2C, 2D) were digitally captured using a Nikon camera (model XC-ST70). c-Fos mRNA was measured as the optical density of the regions of interest from four tissue sections using ImageJ imaging software (version 1.34s, National Institute of Health, Bethesda, MD). The boundaries of each region of interest were free-drawn prior to optical density measurement. Optical density values were converted to dpm/cm2 concentration units using a standard curve obtained from optical density measurements of 14C radiolabeled standards calibrated for 33P. The regions and locations analyzed with densitometry matched those analyzed for Fos ICC in Experiment 1 (Fig. 1). Analyses were performed blind to the group assignment.
Statistical analysis
Separate ANOVAs were used to analyze active lever presses during extinction and active and inactive lever presses on test day, with group (i.e. Cues vs. No Cues on test day) as a between-subjects factor, and day as a repeated measure for the extinction data. Separate ANOVAs were used to analyze Fos protein and c-fos mRNA expression among regions of the cortex (PrL, IL, Orb, Cg2, AgI and LEnt), basal ganglia (NAcC, NAcS, dCPu, VTA and SNr), amygdala (BlA, CeA and LA), dorsal hippocampus (dCA1, dCA2, DG and dS) and ventral hippocampus (vCA1, vCA3 and vS) with group as a between-subjects factor and brain region as a repeated measure. Significant interaction effects were further analyzed using post-hoc Newman-Keuls tests. Main effects of region were not followed up by post-hoc analysis because only the main effects of group and group × region interactions were relevant to the question of neural circuitry activated by cues. Based on effects observed in our previous studies in the NAcC, NAcS, Cg2 and BlA (Neisewander et al., 2000; Zavala et al., 2007), planned t-test comparisons were also made between Cues vs. No Cues groups for Fos expression in these regions, unless they were redundant with the above analyses. Additionally, the correlation between active lever presses on the test day and Fos expression in regions with significant group effects was calculated using the Pearson product-moment correlation (r).
Results
All descriptive statistics are reported as mean ± standard error of the mean.
Cocaine self-administration
The 16 rats in Experiment 1 received a total of 395 ± 6.7 cocaine infusions and the 10 rats in Experiment 2 received 436 ± 12 cocaine infusions during self-administration training. Cocaine intake varied by less than 20% throughout the last 10 days of SA training in both experiments, and there were no group differences in average daily intake or active lever response rates (Table 1).
Table 1.
Mean (± s.e.m.) reinforcement rates and active and inactive lever response rates during SA and extinction.
| SA Reinforcement Rates | SA Response Rates | Extinction Response Rates | Test Day Response Rates | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Infusions | Infusions/day, last 10 days | Active LP/2h, last 10 days | Inactive LP/2h, last 10 days | Peak Active LP/2 h | Last Day Active LP/2 h | Last Day Inactive LP/2 h | Active LP/h | Inactive LP/h | |
| Experiment 1 | |||||||||
| Cues | 385 ± 11 | 22.3 ± 0.5 | 258 ± 6 | 21.0 ± 2.3 | 243 ± 7 | 26.2 ± 1.6 | 18.8 ± 1.7 | 178 ± 7.7* | 35 ± 3.4 |
| No Cues | 393 ± 19 | 22.2 ± 1.1 | 256 ± 12 | 14.1 ± 3.2 | 241 ± 15 | 29 ± 2.8 | 9.3 ± 1.5 | 30 ± 3.4 | 14 ± 1.1 |
| Experiment 2 | |||||||||
| Cues | 431 ± 20 | 21.5 ± 1.3 | 249 ± 17 | 6.0 ± 1.2 | 210 ± 18 | 11.4 ± 1.4 | 6.8 ± 1.3 | 111 ± 12* | 13 ± 0.6 |
| No Cues | 442 ± 26 | 22.3 ± 1.5 | 257 ± 20 | 12.6 ± 3.4 | 195 ± 22 | 14.4 ± 1.0 | 4.4 ± 0.9 | 17 ± 2.9 | 3.6 ± 0.8 |
LP refers to lever presses
signifies significant difference from corresponding activity during the last day of extinction training, p < 0.005.
Extinction and reinstatement of cocaine-seeking behavior
In both experiments, extinction training significantly reduced cocaine-seeking behavior (F16,224 = 46.4, p < 0.0001 for Experiment 1, F16,120 = 20.0, p < 0.0001 for Experiment 2) without group differences in response rates (see Table 1). Furthermore, last-day extinction lever presses were significantly reduced from first-day extinction lever presses for both groups in Experiment 1 (t14 = 11.4, p < 0.0001 for Cues group; t14 = 5.0, p < 0.0005 for No Cues group) and Experiment 2 (t8 = 4.8, p < 0.005 for Cues group; t8 = 3.6, p < 0.01 for No Cues group). No significant changes were observed for inactive lever pressing during extinction.
To illustrate reinstatement of cocaine-seeking behavior, the active lever presses during the last extinction session (i.e., the baseline) were subtracted from the corresponding test day active lever presses. The Cues group exhibited a significantly greater increase from baseline in active lever presses during the test session than the No Cues group in both experiments (t14 = 5.6, p < 0.0001 for Experiment 1; t8 = 2.8, p < 0.05 for Experiment 2; see Fig. 3). Also in both experiments, the Cues group exhibited greater active lever-pressing during the test session than on the final extinction session (t16 = 4.9, p < 0.0005 for Experiment 1; t8 = 2.8, p < 0.05 for Experiment 2). In contrast, the No Cues group demonstrated no significant change from the final extinction session in active or inactive lever presses for either experiment, and the Cues group exhibited no significant change in inactive lever presses for either experiment (see Table 1).
Figure 3.
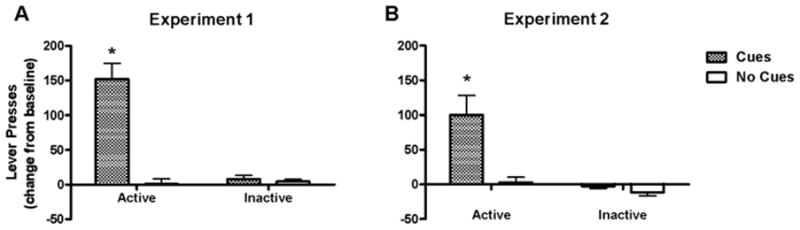
Effect of testing conditions on cocaine-seeking behavior, measured on the test day as the change in active lever presses (± s.e.m.) from baseline that was obtained during the final extinction session. Inactive lever response rates are also shown as a change from baseline. * represents a difference from all other groups (Newman-Keuls test, p < 0.05).
Fos protein immunoreactivity
Rats in the Cues group exhibited significantly more Fos-immunoreactive nuclei than rats in the No Cues group in several limbic and cortical brain regions. Analysis of these data in the cortical regions revealed a main effect for group (F1,80 = 57.2, p < 0.0001) but no group × region interaction (Fig. 4A). Analysis of Fos expression in the basal ganglia regions also revealed a main effect for group (F1,70 = 38.1, p < 0.0001), as well as an interaction (F4,70 = 2.6, p < 0.05; Fig. 5A). Post-hoc tests demonstrated that the Cues group exhibited greater Fos expression in the NAcC, SNr and VTA (Newman-Keuls tests, p < 0.05), but not NAcS or dCPu, relative to the No Cues group. However, the planned comparison yielded a significant difference in Fos expression between groups in the NAcS (t12 = 2.2, p < 0.05). In the amygdala, the ANOVA indicated a significant main effect for group (F1,42 = 7.9, p < 0.01) and a group by region interaction (F2,42 = 7.5, p < 0.002). Post-hoc tests demonstrated that the Cues group exhibited greater Fos expression in the BLA (Newman-Keuls tests, p < 0.05; Fig. 6A), but not the CeA or LA. In the dorsal hippocampal regions (Fig. 7A), there was a main effects of group (F1,56 = 14.9, p < 0.005) and a group × region interaction (F3,56 = 2.8, p < 0.05). Post-hoc analysis showed significantly greater Fos expression in the Cues group compared to the No Cues group in the DG and dS (Newman-Keuls tests, p < 0.05), but not dCA1 or dCA2. In the ventral hippocampal regions (Fig. 8A) there was a marginally significant effect of group (F1,41 = 3.9, p = 0.055) but no interaction. The main effects of group indicate that on average, the Cues group exhibited higher Fos protein expression than the No Cues group.
Figure 4.
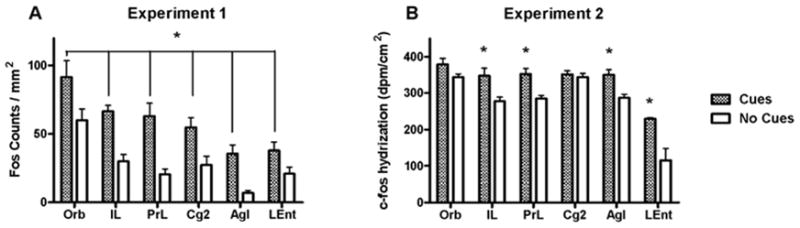
Number of Fos-positive nuclei/mm (A) and c-fos mRNA hybridization (dpm/cm2) (B) in cortical regions of rats receiving response-contingent cue presentations (Cues group) and rats whose lever presses produced no consequences (No Cues group). In A, * represents significant main effect of group (p < 0.05). In B, * represents a significant difference from the No Cues group (Newman-Keuls test, p < 0.05).
Figure 5.
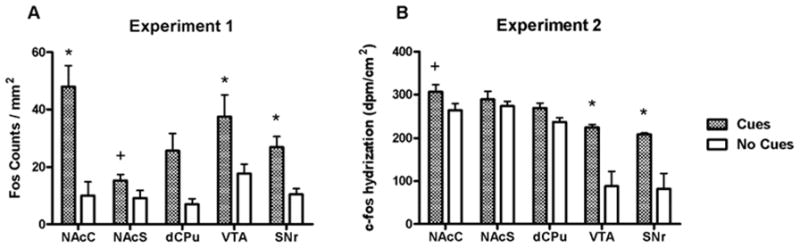
Fos protein (A) and c-fos mRNA (B) expression in basal ganglia regions of rats receiving response-contingent cue presentations (Cues group) and rats whose lever presses produced no consequences (No Cues group). A * above the column pair for a region represents a post hoc significant difference (Newman-Keuls test, p < 0.05) in expression between Cues and No Cues groups for that region. A + above the column pair denotes a significant difference (t-test, p < 0.05) found by a planned comparison.
Figure 6.
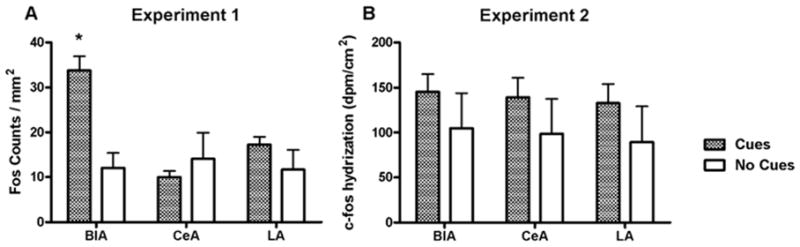
Fos protein (A) and c-fos mRNA (B) expression in amygdala of rats receiving response-contingent cue presentations (Cues group) and rats whose lever presses produced no consequences (No Cues group). In A, a * above the column pair for a region represents a post hoc significant difference (Newman-Keuls test, p < 0.05) in expression between Cues and No Cues groups for that region.
Figure 7.
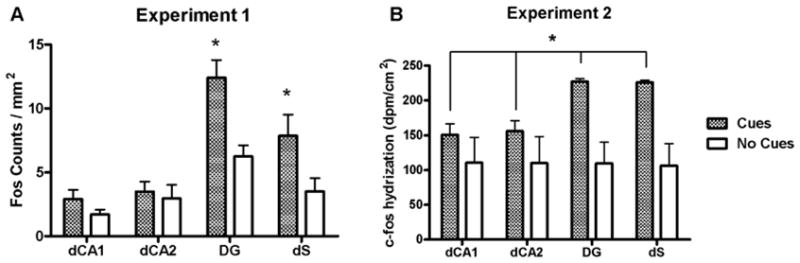
Fos protein (A) and c-fos mRNA (B) expression in dorsal hippocampal regions of rats receiving response-contingent cue presentations (Cues group) and rats whose lever presses produced no consequences (No Cues group). In A, a * above the column pair for a region represents a post hoc significant difference (Newman-Keuls test, p < 0.05) in expression between Cues and No Cues groups for that region. In B, the * represents significant main effect of group (p < 0.05).
Figure 8.
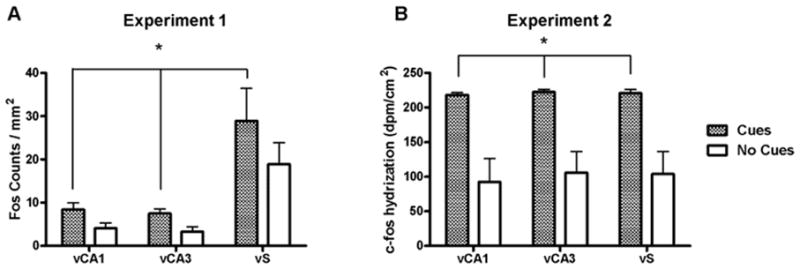
Fos protein (A) and c-fos mRNA (B) expression in ventral hippocampal regions of rats receiving response-contingent cue presentations (Cues group) and rats whose lever presses produced no consequences (No Cues group). * represents significant main effect of group (p < 0.05).
c-fos mRNA hybridization
Analysis of c-fos mRNA expression in the cortical regions (Fig. 4B) revealed main effects for group (F1,48 = 45.9, p < 0.0001), as well as a group × region interaction (F5,48 = 2.8, p < 0.05). Post-hoc tests found that the Cues group exhibited greater c-fos mRNA expression than the No Cues group in IL, PrL, AgI and LEnt (Newman-Keuls tests, p < 0.05), but not Orb or Cg2. The planned comparison for Cues vs. No Cues groups c-fos expression in Cg2 was not significant. Analysis of c-fos mRNA expression in the basal ganglia (Fig. 5B) revealed main effects for group (F1,40 = 33.5, p < 0.0001), as well as a group × region interaction (F4,40 = 4.2, p < 0.01). Post-hoc tests demonstrated that the Cues group exhibited greater c-fos mRNA expression in the SNr and VTA (Newman-Keuls tests, p < 0.05), but not in the NAcC, NAcS or dCPu, relative to the No Cues group. However, planned comparisons did reveal that the Cues group exhibited greater c-fos mRNA expression than the No Cues group in NAcC (t8 = 1.9, p < 0.05) but not NAcS. In the amygdala regions (Fig. 6B) there were no significant effects, and the planned comparison of BlA c-fos mRNA expression was not significant. In the dorsal hippocampal regions (Fig. 7B), there was a main effect of group (F1,32 = 19.9, p < 0.0001) but no interaction. Similarly, in the ventral hippocampal regions (Fig. 8B), there was a main effect of group (F1,24 = 40.4, p < 0.0001) but no interaction. These main effects indicate that on average, the Cues group exhibited higher c-fos mRNA expression than the No Cues group.
Correlation between Fos expression and cocaine-seeking behavior
Of all the brain regions that demonstrated significant increases in Fos expression in the Cues group relative to the No Cues group, only BlA and AgI also demonstrated a significant correlation in the Cues group between Fos counts and active lever presses on the test day (Fig. 9). In the BlA, r = 0.70, which was significant by the Pearson test for correlation (p < 0.02). In the AgI, r = 0.75, which was also significant (p < 0.02). The regions Orb (r = 0.25), PrL (r = 0.45), dS (r = 0.46) and LEnt (r = 0.28) demonstrated positive but non-significant correlations, whereas dCPu (r = −0.14), DG (r = −0.38), NAcC (r = 0.03) and NAcS (r = −0.19) exhibited small r values that were mostly negative. c-Fos mRNA expression in the BlA, but not the AgI, also exhibited a significant correlation to active lever pressing (r = 0.92, p < 0.05).
Figure 9.
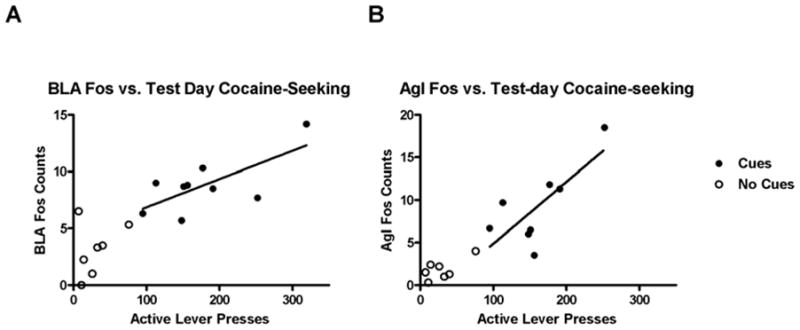
Scatter plot of BlA (A) and AgI (B) Fos expression versus test session cocaine-seeking behavior exhibited by Experiment 1 rats. The closed circles represent rats in the Cues group and open circles represent the No Cues group. The line indicates significant linear correlation (p < 0.05) among the active-lever presses and number of Fos-labeled cells for the Cues group.
Discussion
To our knowledge, the present results are the first to demonstrate c-fos induction using the extinction/reinstatement model, where the rats were exposed to the self-administration environment but not discrete cues during extinction training. c-Fos was induced in several limbic and cortical brain regions during the reinstatement test, during which cues were presented response-contingently. Both an increase in Fos protein expression after a 90-min test and an increase in c-fos mRNA after a 60-min test were observed in the IL, PrL, AgI, Orb, NAcC, dCPu, VTA and subregions of the hippocampus. Measuring mRNA and protein allows for verification of significant gene expression by independent measurements (Rhodes et al., 1996), which is important given the inherent uncertainties in antibody specificity (Rhodes and Trimmer, 2006) and the regulation of c-fos gene expression by multiple receptor systems and signal transduction pathways (Chaudhuri, 1997). Expression of Fos protein was more widespread than that of c-fos mRNA, with only the former sensitive to cue effects in the NAcS, BlA and Cg2. The regions exhibiting conditioned expression of c-fos mRNA and Fos are, for the most part, consistent with our previous findings of Fos protein expression following passive re-exposure to discrete and contextual cocaine-paired cues (Neisewander et al., 2000; Zavala et al., 2007; 2008). Exceptions include the present increases in Fos in the VTA, SNr, vCA3 and LEnt, which had not previously exhibited increased Fos expression (Neisewander et al., 2000), and the negative results in dCA1, CeA and LA, which had exhibited increased Fos expression in one of our previous studies (Zavala et al., 2007). Additionally, we had not examined the AgI previously.
Although increases in c-fos induction were observed only in the rats engaged in cocaine seeking reinforced by cues, it is not likely that the increases were due to either a sensory or locomotor response. First, all rats in our previous studies (Neisewander et al., 2000; Zavala et al., 2007; 2008), including saline-yoked control rats, were exposed to the same sensory stimuli during the testing period. Further, rats tested without levers available demonstrated similar Fos induction patterns as rats tested with available levers, who actively engaged in cocaine-seeking behavior (Neisewander et al., 2000). Also in the present study, the two activated brain regions that exhibited a correlation between the degree of Fos expression and active lever presses during the test period were the BlA and AgI, which are not primarily involved in motor function.
A likely reason for the more widespread Fos protein expression than c-fos mRNA expression could be the difference in testing periods (90 min for Experiment 1 versus 60 min for Experiment 2). The 90-min test session for Fos protein expression was chosen based on the pioneering study of cocaine-conditioned locomotion (Brown et al., 1992) as well as our study of re-exposure to a cocaine-paired environment (Neisewander et al., 2000). Expression of c-fos mRNA was expected to peak between 30 to 60 min after presentation of motivationally salient stimuli (Ennulat et al., 1994; Graybiel et al., 1990; Hearing et al., 2008), but a preliminary study that we conducted using the exact same procedure except for a 30-min, rather than 60-min, testing period yielded no significant group effects in c-fos mRNA in any brain region (unpublished observation). Nevertheless, c-fos mRNA expression is more transient than Fos protein expression, which may account for differences between these two measures.
Surprisingly, some regions exhibiting Fos expression in response to cue reinstatement in this study (i.e. VTA, SNr, vS and LEnt) had failed to exhibit increased Fos in our previous study using exposure to contextual cues plus passive presentation of discrete cues (Neisewander et al., 2000). It is possible that extinction of the contextual cues in this study may have served to increase the salience of the discrete cues on the test day, resulting in more widespread activation. Alternatively, the use of a different primary anti-fos antibody in the present study and more recent papers (Zavala et al., 2007; 2008) versus Neisewander et al., 2000 could have increased the sensitivity to detect changes in Fos expression. Another possibility is that the additional regions exhibiting c-fos induction in this study may be involved in conditioned reinforcement, which occurs with response-contingent cue presentations.
The presence of increased c-fos mRNA but not, unequivocally, Fos protein expression in the dCA1 and dCA2 subregions of hippocampus is unexpected, given past observations of elevated Fos levels in DG, dCA1 and dCA3 following exposure to a cocaine SA environment (Zavala et al., 2007). The dorsal hippocampus has been shown to be crucial for context-induced, but not cue-induced, reinstatement of cocaine-seeking behavior (Fuchs et al., 2005; Rogers and See, 2007). Furthermore, lesions of the dorsal hippocampus, but not the ventral hippocampus, disrupt cocaine-conditioned place preference (Meyers et al., 2003). Hence, we expected the dorsal hippocampus would fail to exhibit c-fos induction as a result of reinstatement by discrete cues. It is possible that the presentation of discrete cues may have reinstated the incentive motivational effects of the context to some degree.
Fos expression associated with response-contingent discrete cues in the NAcC, NAcS, PrL, IL and BlA (Figs. 4–7) overlaps with Fos expression patterns previously observed with cocaine-paired contextual cues presented alone (Brown et al., 1992; Crawford et al., 1995; Hotsenpiller et al., 2002) or in conjunction with discrete cues (Neisewander et al., 2000; Zavala et al., 2007; 2008). The NAcC is a principal target of the mesolimbic dopaminergic system and a critical substrate of cue reinstatement (Fuchs et al., 2004a). NAcC Fos expression has been found after re-exposure to a cocaine-paired environment (Franklin and Druhan, 2000) as well as reinstatement of cocaine-conditioned place preference (Miller and Marshall, 2004; 2005). However, a study using reinstatement of cocaine seeking by discriminative cues found no Fos increases in the nucleus accumbens (Ciccocioppo et al., 2001), possibly reflecting activation of different neural circuits depending on mode of cue presentation. Additionally, NAcS Fos expression by cocaine-associated cues appears to depend on the method of conditioning, where increases occurred in rats that previously underwent SA training (Neisewander et al., 2000; Zavala et al., 2007), but not in rats conditioned to passive cocaine injections (Brown et al., 1992; Hotsenpiller et al., 2002; Miller and Marshall, 2005).
Increased Fos expression in the cingulate cortex and subregions of medial PFC in the Cues group (Fig. 4A) is in agreement with previous Fos studies of incentive motivational effects of cues in animals following cocaine SA training (Ciccocioppo et al., 2001; Neisewander et al., 2000; Zavala et al., 2007). Increased c-fos mRNA expression in the medial PFC in the Cues group (Fig. 4B) is also in agreement with published observations of enhanced c-fos mRNA after exposure to the SA environment (Hearing et al., 2008). These regions have been cortical targets of pharmacological and surgical manipulations in the reinstatement model (Shaham et al., 2003). Inactivation of the medial PFC by tetrodotoxin infusions attenuates cue-induced reinstatement of cocaine-seeking (Fuchs et al., 2005; See, 2002). Inactivation of the PrL subregion by lidocaine also attenuates cue-induced reinstatement, but this effect was present for light/sound and not light/odor cues (Di Pietro et al., 2006). Attenuation of cue reinstatement by inactivating PrL also was found to require prior extinction training, as opposed to abstinence in a different environment (Fuchs et al., 2006). Therefore, the roles of these regions in discrete cue-elicited cocaine-seeking behavior may involve interactions with the neural processing of contextual information. Neuroimaging studies of cocaine-dependent humans have found that the anterior cingulate and medial PFC respond to visual cues associated with cocaine use, such as images of paraphernalia or people using cocaine (Childress et al., 1999; Garavan et al., 2000; Grant et al., 1996; Kilts et al., 2004; Wexler et al., 2001). Discrepancies in the observed brain activation patterns among these studies have been associated with differences in subject drug history and/or expectation of drug reinforcement, indicating the potential complexity of interpreting clinical information about the cingulate and PFC (Bonson et al., 2002; Kosten et al., 2006; Wexler et al., 2001). The present results confirm that the cingulate and medial PFC are part of the brain circuit engaged by cocaine-paired cues, but their apparent sensitivity to environmental factors must be accounted for when relating studies of the rat model to clinical experiments of cocaine craving.
The BlA activation by discrete, response-contingent cue reinstatement is in agreement with prior Fos research using a variety of methods to reinstate cocaine-seeking behavior, including a combination of discrete and contextual cues (Neisewander et al., 2000; Zavala et al., 2007), a cocaine-paired discriminative cue (Ciccocioppo et al., 2001) and re-exposure to a cocaine-paired environment (Franklin and Druhan, 2000). Studies of BlA lesions or transient inactivation have demonstrated that this region is essential for extinction and cue-elicited reinstatement of cocaine-seeking behavior (Fuchs et al., 2005; 2002; McLaughlin and See, 2003; Yun and Fields, 2003). However, Fos expression in BlA by cocaine-conditioned cues has been inconsistent in experiments that involved no or very limited (3–4 days) abstinence after passive cocaine administration (Brown et al., 1992; Hotsenpiller et al., 2002). The accumulated results of BlA Fos expression suggest an important link between cocaine-paired cues and cocaine seeking, which may depend on a history of both cocaine exposure and either forced abstinence or extinction.
A novel finding in this study is the correlation of AgI and BlA Fos expression with active lever pressing exhibited by rats that demonstrated cue-induced reinstatement on the test day (Fig. 9). The AgI is considered to be part of the rat analogue of the primate orbitofrontal cortex (OFC), a region implicated in clinical studies of visual cue-induced cocaine craving (Bonson et al., 2002; Kilts et al., 2004; Schoenbaum et al., 2006). The rat AgI shares reciprocal connections with BlA (Kita and Kitai, 1990; Krettek and Price, 1977), forming a limbic circuit comparable to the primate lateral OFC (Schoenbaum et al., 2006). Structural and functional abnormalities within the OFC have been reported in several studies of human cocaine abusers (Dom et al., 2005; Volkow and Fowler, 2000), and the OFC of these individuals demonstrates activation by systemic cocaine (Kufahl et al., 2005) as well as cocaine cues (Bonson et al., 2002; Kilts et al., 2004). The amygdala and lateral OFC are also selectively engaged by the presentation of i.v. cocaine when unanticipated by the subject, but not when anticipated (Kufahl et al., 2008). This sensitivity suggests an important role for this circuit in processing the interaction between craving and cues, but not necessarily the craving itself.
The present findings, as well as published reports in cocaine-craving humans, appear to fit with current thinking that the BlA communicates with the lateral OFC in the rat during reinforcement and reversal learning. The firing patterns of OFC neural ensembles in conditioned animals have been shown to adapt to changes in the reinforcement value of predictive stimuli (Schoenbaum et al., 2000). Selective damage to the OFC as well as repeated cocaine exposure inhibits this adaptation (Calu et al., 2007; Schoenbaum and Setlow, 2005). A suggested interpretation of these findings is that the lateral OFC holds expectancies regarding the outcome of light/tone cue presentation (Fuchs et al., 2004b), and the BlA resolves these expectancies with the actual outcome, with BlA neurons becoming cue-selective during training (Pickens et al., 2003; Schoenbaum et al., 2006). Therefore, the correlation of lever pressing with Fos in the AgI could reflect the representation of the animal’s expectancy of the drug reward, whereas the correlation in the BlA could reflect an ongoing modification of the incentive value of the current cue stimulus (Fuchs et al., 2002), which is then used to adapt behavior.
The present findings are also in line with the view that learning and memory of contextual cues and discretely presented cues involve different mechanisms (Holland and Bouton, 1999; Phillips and LeDoux, 1992). Reinstatement of cocaine-seeking behavior has been accomplished by both passive and response-contingent discrete cues, but the latter method is more reliable, unless the cues serve as discriminative stimuli (Alleweireldt et al., 2001; Shalev et al., 2002; Weiss et al., 2000). Reinstatement by the presentation of response-contingent cues activated a range of cortical, limbic and hippocampal brain regions, many of which have been previously associated with cocaine-seeking behavior. The presence of both NAcC and NAcS increased Fos expression in this study, as opposed to previous studies, is possibly tied to the pairing of cocaine delivery with active lever pressing (Brown et al., 1992; Hotsenpiller et al., 2002; Miller and Marshall, 2005). The mode of cue presentation in the present study could also account for the presence of VTA and SNr Fos effects, which was absent in our previous investigation (Neisewander et al., 2000).
In conclusion, the results suggest that the neural circuitry activated by cocaine-associated cues may vary depending on the mode of cue presentation and/or whether animals have undergone extinction training prior to measuring cocaine-seeking behavior. Furthermore, we suggest that connections between the BlA and AgI in the rat may be critical for processing incentive value of cocaine cues and modifying cocaine-seeking behavior as the value changes. This hypothesis warrants further study.
Acknowledgments
The authors thank Jenny Browning, Ryan Meyers and Tracy Osredkar for technical contributions and Dr. T. Curran at NIH for generously providing the c-fos riboprobe. This work was supported by NIDA grants DA11064, DA13649 (to JLN), and DA021485 (to PRK). The authors declare no financial conflicts of interest.
References
- Alleweireldt AT, Weber SM, Neisewander JL. Passive exposure to a contextual discriminative stimulus reinstates cocaine-seeking behavior in rats. Pharmacology, biochemistry, and behavior. 2001;69(3–4):555–560. doi: 10.1016/s0091-3057(01)00573-1. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26(3):376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci. 1992;12(10):4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learning & memory (Cold Spring Harbor, NY) 2007;14(5):325–328. doi: 10.1101/lm.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Intravenous self-administration of etonitazene, cocaine and phencyclidine in rats during food deprivation and satiation. The Journal of pharmacology and experimental therapeutics. 1981;217(2):241–247. [PubMed] [Google Scholar]
- Chaudhuri A. Neural activity mapping with inducible transcription factors. Neuroreport. 1997;8(16):v–ix. [PubMed] [Google Scholar]
- Childress A, Ehrman R, McLellan AT, O’Brien C. Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res Monogr. 1988;81:74–80. [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. The American journal of psychiatry. 1999;156(1):11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A. 2001;98(4):1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP. The effects of the kappa agonist U-50,488 on cocaine-induced conditioned and unconditioned behaviors and Fos immunoreactivity. Psychopharmacology. 1995;120(4):392–399. doi: 10.1007/BF02245810. [DOI] [PubMed] [Google Scholar]
- Dackis CA, O’Brien CP. Cocaine dependence: a disease of the brain’s reward centers. Journal of substance abuse treatment. 2001;21(3):111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Davis WM, Smith SG. Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. The Pavlovian journal of biological science. 1976;11(4):222–236. doi: 10.1007/BF03000316. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75(2):134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. The European journal of neuroscience. 2006;24(11):3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Dom G, Sabbe B, Hulstijn W, van den Brink W. Substance use disorders and the orbitofrontal cortex: systematic review of behavioural decision-making and neuroimaging studies. Br J Psychiatry. 2005;187:209–220. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- Ennulat DJ, Babb S, Cohen BM. Persistent reduction of immediate early gene mRNA in rat forebrain following single or multiple doses of cocaine. Brain Res Mol Brain Res. 1994;26(1–2):106–112. doi: 10.1016/0169-328x(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur J Neurosci. 2000;12(6):2097–2106. doi: 10.1046/j.1460-9568.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26(13):3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30(2):296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2004a;176(3–4):459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004b;24(29):6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Tran-Nguyen LT, Specio SE, Groff RS, Neisewander JL. Predictive validity of the extinction/reinstatement model of drug craving. Psychopharmacology. 1998;135(2):151–160. doi: 10.1007/s002130050496. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Weber SM, Rice HJ, Neisewander JL. Effects of excitotoxic lesions of the basolateral amygdala on cocaine-seeking behavior and cocaine conditioned place preference in rats. Brain research. 2002;929(1):15–25. doi: 10.1016/s0006-8993(01)03366-2. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. The American journal of psychiatry. 2000;157(11):1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93(21):12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A. 1990;87(17):6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan RE, Garcia MM. Drugs of abuse and immediate-early genes in the forebrain. Molecular neurobiology. 1998;16(3):221–267. doi: 10.1007/BF02741385. [DOI] [PubMed] [Google Scholar]
- Hearing MC, Miller SW, See RE, McGinty JF. Relapse to cocaine seeking increases activity-regulated gene expression differentially in the prefrontal cortex of abstinent rats. Psychopharmacology. 2008;198(1):77–91. doi: 10.1007/s00213-008-1090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Bouton ME. Hippocampus and context in classical conditioning. Current opinion in neurobiology. 1999;9(2):195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Horak BT, Wolf ME. Dissociation of conditioned locomotion and Fos induction in response to stimuli formerly paired with cocaine. Behav Neurosci. 2002;116(4):634–645. [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. The American journal of psychiatry. 2004;161(2):233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Amygdaloid projections to the frontal cortex and the striatum in the rat. The Journal of comparative neurology. 1990;298(1):40–49. doi: 10.1002/cne.902980104. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31(3):644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. The Journal of comparative neurology. 1977;172(4):687–722. doi: 10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- Kufahl P, Li Z, Risinger R, Rainey C, Piacentine L, Wu G, Bloom A, Yang Z, Li SJ. Expectation modulates human brain responses to acute cocaine: a functional magnetic resonance imaging study. Biol Psychiatry. 2008;63(2):222–230. doi: 10.1016/j.biopsych.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, Li SJ. Neural responses to acute cocaine administration in the human brain detected by fMRI. NeuroImage. 2005;28(4):904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology. 1993;112(2–3):163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168(1–2):57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR, Neisewander JL. Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. Neuroreport. 2003;14(16):2127–2131. doi: 10.1097/00001756-200311140-00023. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered Prelimbic Cortex Output during Cue-Elicited Drug Seeking. J Neurosci. 2004;24(31):6889–6897. doi: 10.1523/JNEUROSCI.1685-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered Fos expression in neural pathways underlying cue-elicited drug seeking in the rat. European Journal of Neuroscience. 2005;21(5):1385–1393. doi: 10.1111/j.1460-9568.2005.03974.x. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Vickers EA, Robertson HA, Cochran BH, Graybiel AM. Coordinate expression of c-fos and jun B is induced in the rat striatum by cocaine. J Neurosci. 1993;13(2):423–433. doi: 10.1523/JNEUROSCI.13-02-00423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20(2):798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Annals of the New York Academy of Sciences. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J Neurosci. 2003;23(35):11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Monaghan MM, Barrezueta NX, Nawoschik S, Bekele-Arcuri Z, Matos MF, Nakahira K, Schechter LE, Trimmer JS. Voltage-gated K+ channel beta subunits: expression and distribution of Kv beta 1 and Kv beta 2 in adult rat brain. J Neurosci. 1996;16(16):4846–4860. doi: 10.1523/JNEUROSCI.16-16-04846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Trimmer JS. Antibodies as valuable neuroscience research tools versus reagents of mass distraction. J Neurosci. 2006;26(31):8017–8020. doi: 10.1523/JNEUROSCI.2728-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, See RE. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiology of learning and memory. 2007;87(4):688–692. doi: 10.1016/j.nlm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. J Neurosci. 2000;20(13):5179–5189. doi: 10.1523/JNEUROSCI.20-13-05179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends in neurosciences. 2006;29(2):116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal-amygdalar function. Cereb Cortex. 2005;15(8):1162–1169. doi: 10.1093/cercor/bhh216. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacology, biochemistry, and behavior. 2002;71(3):517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168(1–2):3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacological reviews. 2002;54(1):1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97(8):4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. The American journal of psychiatry. 2001;158(1):86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Yun IA, Fields HL. Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience. 2003;121(3):747–757. doi: 10.1016/s0306-4522(03)00531-1. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, Neisewander JL. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145(2):438–452. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Browning JR, Dickey ED, Biswas S, Neisewander JL. Region-specific involvement of AMPA/Kainate receptors in Fos protein expression induced by cocaine-conditioned cues. Eur Neuropsychopharmacol. 2008;18(8):600–611. doi: 10.1016/j.euroneuro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]