Abstract
Background
Individuals with advanced chronic kidney disease (CKD) and end stage renal disease (ESRD) have high total plasma homocysteine (tHcy) levels, which may be a risk factor for cognitive impairment. Whether treatment with high dose B-vitamins to reduce high tHcy levels improves cognition in persons with kidney disease is unknown.
Study Design
Randomized controlled trial.
Setting & Participants
A substudy of 659 patients (mean age 67.3 ± 11.7 years) who participated in a randomized, double-blind, clinical trial, 5 years in duration, conducted in 36 US Department of Veterans Affairs medical centers, of the effect on all-cause mortality of vitamin-induced lowering of plasma tHcy. 236 (35.8%) were treated by dialysis (ESRD) and 423 (64.2%) had a Cockcroft-Gault estimated creatinine clearance ≤ 30 ml/min (advanced CKD). All had high tHcy levels (≥15 μmol/L) at baseline. Cognitive assessments began during the follow up period of the main trial, 3 years after treatment began; participants were subsequently retested one year later to assess cognitive change.
Intervention
Daily high dose B-vitamin capsule (40 mg of folic acid, 100 mg of vitamin B6, and 2 mg of vitamin B12) or placebo.
Outcomes
Cognitive function at initial assessment and one year later.
Measurements
The Telephone Interview of Cognitive Status – modified, supplemented with attention, working memory and executive function tests.
Results
Initial cognitive function was impaired in approximately 19% of patients, regardless of treatment assignment (vitamin or placebo) or kidney disease status (advanced CKD or ESRD). Treatment reduced tHcy levels by 26.7%. Unadjusted and adjusted analyses showed that treatment did not improve initial cognitive outcomes or affect subsequent cognitive status one year later.
Limitations
Cognitive assessments began after treatment was initiated; cognitive assessment was limited.
Conclusion
Treatment with high daily doses of B-vitamins, which reduced tHcy levels, did not affect cognitive outcomes in patients with advanced CKD and ESRD.
Index Words: Cognition, Kidney Disease, Homocysteine, Clinical Trial
Introduction
Cognitive impairment and dementia are common in individuals with kidney disease 1-8. They also have high total plasma homocysteine (tHcy) levels9-11. In observational studies of other populations, elevated tHcy levels have been associated with impairment and decline on tests of cognitive function 12-15, and have been implicated as an independent risk factor for dementia in cross-sectional, case-control 16 and prospective17, 18 studies. A recent review19 of clinical trials using B-vitamin therapy (B6, B12, and folic acid either alone or in vitamin combinations) to reduce tHcy levels, however, found little evidence that reducing tHcy improves cognitive outcomes.
Most previous clinical trials were conducted in relatively small samples with moderate elevation of tHcy levels, were of short duration, and employed low doses of vitamins; none was conducted in kidney disease patients who have high tHcy levels. We sought to address these limitations by examining whether high dose B-vitamin treatment for a long duration had a beneficial effect on cognitive outcomes in a large sample of individuals with advanced chronic kidney disease (CKD) or end stage renal disease (ESRD) and high levels of tHcy in the Department of Veterans Affairs (VA) Cooperative Studies Program Homocysteine Study (HOST) 20. The present study examined cognitive function in a subset of these HOST participants.
Methods
Study Design
This was a substudy of the VA HOST Study, which was a 5-year multicenter randomized double blind placebo controlled clinical trial conducted from 2001 to 2006 in 36 VA medical centers. Details of the parent VA HOST study methods and the results have been reported previously 20, 21. In brief, the objective of HOST was to determine if high daily doses of folic acid (40 mg), pyridoxine (vitamin B6, 100 mg) and cyanocobalamin (vitamin B12, 2 mg) to reduce tHcy would reduce all-cause mortality and improve cardiovascular outcomes compared to patients who received placebo. The primary inclusion criteria for the parent study were: age 21 years or older, advanced CKD (estimated creatinine clearance by Cockcroft-Gault22 ≤ 30mL/min, n = 1305) or ESRD (n = 751), and high tHcy levels (≥15 μmol/L). Participants in HOST provided written informed consent.
At study entry, relevant medical history and laboratory data (including baseline fasting tHcy and B-vitamin levels) were collected and patients were given a three month supply of study drug. Patients returned in three months, and fasting tHcy and folate levels were collected, in addition to information about study outcomes. Subsequent patient contact occurred quarterly via telephone from two centers. The median follow-up interval was 3.2 years. Adherence was monitored by having patients return unused study drug when a new three-month supply arrived by mail from the research pharmacy. Among patients assigned to the vitamin treatment group in the HOST study, 90.3% reported taking study medication at 1 year, 87.6% at 2 years, and 85.3% at 3 years21. The results of the HOST Study showed that although the vitamin treatment lowered tHcy by 25.8%, it did not improve survival or reduce the incidence of cardiovascular events 21.
The aim of this cognitive function substudy was to assess whether treatment with high dose B-vitamins leading to tHcy reduction improved cognitive outcomes in a subset of HOST participants. Enrollment into the cognitive function substudy began in June, 2005 during the follow-up period of the parent HOST study (i.e., after enrollment into HOST was completed). Participants in the cognitive function substudy were administered a telephone cognitive battery and a mood questionnaire at enrollment into the substudy and one year later using identical methods. The follow-up calls were made within a four month window; 2 months before to 2 months after each participant's initial test anniversary. Because cognitive function fluctuates between hemodialysis treatments, with better performance seen within 24 hours since dialysis23, we conducted the cognitive assessments in ESRD patients within 24 hours after their last dialysis. Calls were made by trained callers who were periodically assessed to maintain protocol adherence throughout the study. The Human Rights Committee at the VA coordinating center in West Haven and the Institutional Review Boards at the VA Boston Healthcare System and Stanford University approved the study.
Participants/Study Population
Potential participants from the parent HOST study were initially contacted by mail, with telephone follow up. Consent was obtained by telephone from those who agreed to participate and the cognitive battery and mood questionnaire were administered.
Cognitive/mood measures
Participants were administered a 20-minute battery of three cognitive tests and a mood questionnaire over the telephone. The Telephone Interview for Cognitive Status – modified (TICSm24) is a brief telephone cognitive assessment similar to the Mini-Mental State Exam (MMSE) that assesses orientation, concentration, memory (immediate and delayed word list recall), responsive naming, comprehension, calculation, reasoning and judgment. The maximum score is 50 and a score of 27 or below is the usual cutoff for possible cognitive impairment 25, 26. The test has been well-validated24, 27, 28. For this study, we added a forced-choice recognition trial after the delayed recall of the 10-item word list to further assess aspects of forgetfulness. Digit span forward and backward was adapted from the Wechsler Adult Intelligence Scale29; we administered one trial, rather than the traditional 2 trials at each span. The forward span measure is considered an index of attention, whereas the backward span measure is an index of working memory30. Verbal fluency was used as an executive function measure, and was assessed by asking the participant to name as many different animals as possible in 60 seconds. The Geriatric Depression Scale – Short Form31 (GDS-SF) was used to assess mood, given the importance of assessing depressed mood when examining cognition in patients with kidney disease32. The GDS-SF is a series of 15 yes/no questions addressing aspects of depressed mood. We selected the GDS-SF because it is easily administered over the telephone, has been well-validated in numerous older adult populations33, and has fewer items focusing on somatic complaints that result in over-estimating depressive symptoms in patients with kidney disease 32.
Outcomes
Cognition was assessed according to three primary outcomes collected at substudy enrollment-- the TICSm total score and separate cognitive and memory composites. The cognitive composite was composed of scores from the TICSm, digit span forward and backward, and verbal fluency. We constructed the cognitive composite by converting the score from each cognitive measure (TICSm total score, digit span forward and backward span scores, and verbal fluency) into z-scores based on each score's respective mean and standard deviation from the entire sample at initial testing. The four z-scores were then averaged to create the cognitive composite z-score. The memory composite was constructed similarly using the word list memory items from the TICSm. We converted the scores for the immediate and delayed recall trials, and the recognition trial to z-scores in a similar fashion as above. We averaged the three memory scores to create the memory composite z-score.
A secondary outcome, cognitive change, was a categorical variable (improve, decline, no change) that was based on the change in TICSm performance over a one-year interval. Estimating true change on cognitive measures is complicated by measurement error and retest effects that occur in repeated administrations of a test. To account for these effects, we calculated a reliable change index (RCI)34 which estimated the degree of change over repeated administrations of the TICSm that exceeded what would be expected due to measurement error and/or retest effects35, 36. In a manner similar to selecting rejection regions, or P values, for statistical tests34, we selected upper (i.e., true improvement) and lower (i.e., true decline) cutoff regions equivalent to a two-tailed test at P < 0.1. Accordingly, an RCI of -1.65 or lower represented reliable decline, an RCI of 1.65 or higher represented reliable improvement, and RCIs between these values represented no change over 1 year (the RCI formula and calculations are provided as Item S1 of the online supplementary material available with this article at www.ajkd.org).
Statistical Analyses
All treatment effects were analyzed according to the randomized treatment groups regardless of adherence. Our analyses of the three cognitive outcomes at initial testing were based on comparing unadjusted mean differences between the placebo and treatment group by t-tests. Subsequent adjusted analyses of treatment group differences at initial testing included relevant baseline covariates and used a priori multiple linear regression models, with follow-up post hoc models based on the initial model results. Prespecified treatment by kidney disease status (advanced CKD, ESRD) interaction tests were performed to examine whether treatment effects on cognition varied as a function of kidney disease status. Chi-square analyses were used to examine treatment group differences in categorical baseline covariates and 1-year change (i.e., RCI) in TICSm performance. Statistical significance was set at P = 0.05 for a two-tailed test for all statistical analyses.
Results
Enrollment in the parent HOST study began in September 2001;enrollment in the cognitive function substudy began in June 2005 and continued until July 2006; annual retests were completed by June 2007. Figure 1 illustrates the timing of the enrollment and follow-up periods for the cognitive function substudy and parent HOST study. At the inception of the cognitive function substudy, 1375 participants were still active in the HOST study. Of those, 659 were recruited into the substudy, 236 with ESRD and 423 with advanced CKD. The differences in baseline characteristics at enrollment into the HOST study between those in the substudy and in the remainder of HOST participants that are statistically significant were, respectively: age (63.7 vs. 66.7 years), past history of myocardial infarction (20.5% vs. 27.0 %), congestive heart failure (17.9% vs. 26.5 %), angina (20.0% vs. 27.6 %), stroke (12.0% vs. 16.7 %), presence of diabetes (50.5% vs. 57.2%), body mass index (28.1 vs. 27.6), and diastolic blood pressure (75.4 mmHg vs. 73.8 mmHg). Otherwise, the characteristics of the cognitive function participants were similar to the characteristics of those not enrolled in the substudy.
Figure 1.
Veterans affairs (VA) Homocysteine Study (HOST) and VA Homocysteine Study Cognitive Function Substudy (HOSTCOG) enrollment and follow-up timelines.
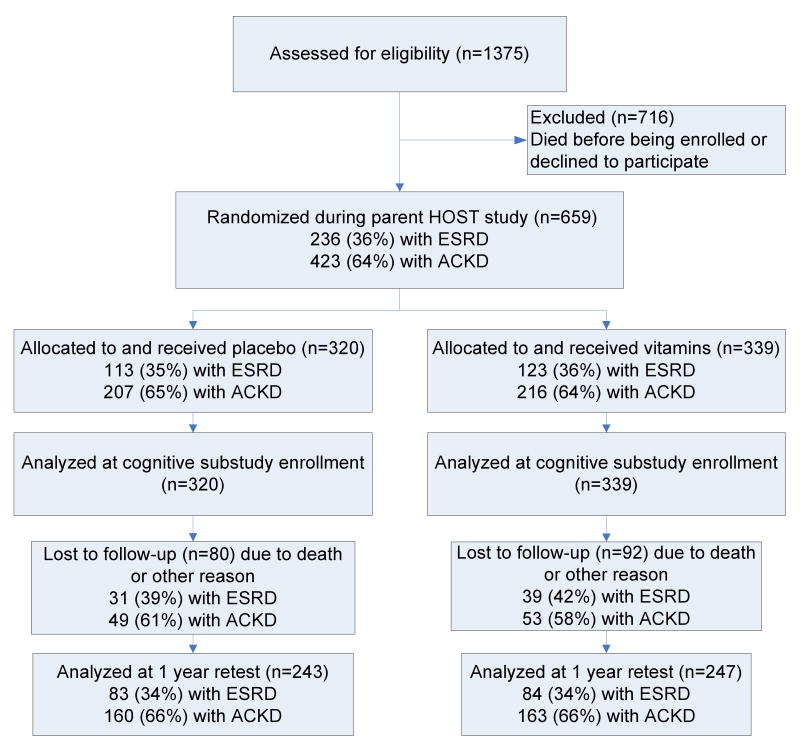
There were 320 participants in the placebo group and 339 in the vitamin group (Figure 2) in the cognitive function study subsample. Baseline data (at HOST randomization) of the participants enrolled in the substudy by treatment group are presented in Table 1. The vitamin group had a lower rate of antiplatelet use, fewer strokes and higher hemoglobin values at baseline; otherwise the two groups were similar.
Figure 2.
Flow of participants into the Veterans Affairs Homocysteine Study (HOST) Cognitive Function Substudy. Abbreviations: ESRD, end stage renal disease; ACKD, advanced chronic kidney disease.
Table 1.
Baseline Characteristics* of HOST Cognitive Function Substudy Population Assigned to Placebo vs. Vitamins
| Characteristic | Placebo (n = 320) |
Vitamins (n = 339) |
P value |
|---|---|---|---|
| Age, mean (SD), y | 64.2 (11.2) | 63.2 (12.2) | 0[nd1].3 |
| Men, No. (%) | 315 (98.4) | 333 (98.2) | 0.8 |
| Racial/ethnic group, No (%) | 0.3 | ||
| White, non-Hispanic | 164 (51.2) | 159 (46.9) | |
| African American, non-Hispanic | 110 (34.4) | 136 (40.1) | |
| Hispanic | 40 (12.5) | 34 (10.0) | |
| Other or missing information | 6 (1.9) | 10 (3.0) | |
| Smoking status, No. (%) | 0.8 | ||
| Never | 88 (27.5) | 87 (25.7) | |
| Former | 177 (55.3) | 189 (55.8) | |
| Current | 55 (17.2) | 63 (18.6) | |
| Body mass index, kg/m2, mean (SD)† | 28.2 (5.0) | 28.0 (4.8) | 0.5 |
| Systolic blood pressure, mean (SD), mm Hg | 140.2 (24.0) | 142.9 (21.7) | 0.1 |
| Diastolic blood pressure, mean (SD), mm Hg | 74.5 (13.2) | 76.3 (12.9) | 0.08 |
| Medical history, No (%) | |||
| Myocardial infarction | 67 (20.9) | 68 (20.1) | 0.8 |
| Congestive heart failure | 57 (17.8) | 61 (18.0) | 0.9 |
| Hypertension | 308 (96.2) | 330 (97.4) | 0.4 |
| Angina | 58 (18.1) | 74 (21.8) | 0.2 |
| Percutaneous coronary angioplasty or stenting | 39 (12.2) | 41 (12.1) | 0.9 |
| Coronary artery bypass graft surgery | 49 (15.3) | 49 (14.4) | 0.8 |
| Stroke | 47 (14.7) | 32 (9.4) | 0.04 |
| Diabetes mellitus | 167 (52.2) | 166 (49.0) | 0.4 |
| Concommitant medication use, No. (%) | |||
| β-Blockers | 188 (58.8) | 192 (56.6) | 0.6 |
| Calcium channel blockers | 199 (62.2) | 207 (61.1) | 0.8 |
| Lipid-lowering agents | 163 (50.9) | 152 (44.8) | 0.1 |
| Aspirin or antiplatelet agents | 138 (43.1) | 121 (35.7) | 0.05 |
| ACE inhibitors | 141 (44.1) | 152 (44.8) | 0.8 |
| Angiotensin II receptor blockers | 39 (12.2) | 35 (10.3) | 0.5 |
| Laboratory values, mean (SD) | |||
| Hemoglobin, g/dL | 11.8 (1.7) | 12.2 (1.6) | <0.001 |
| Albumin, g/dL | 4.0 (0.5) | 4.0 (0.5) | 0.9 |
| Total cholesterol, mg/dL | 168.8 (40.0) | 169.6 (40.6) | 0.8 |
| HDL cholesterol, mg/dL | 42.2 (16.7) | 42.2 (12.3) | 0.9 |
| LDL cholesterol, mg/dL | 91.5 (31.6) | 93.3 (33.2) | 0.5 |
| Triglyerides, mg/dL | 183.1 (128.4) | 180.8 (150.9) | 0.8 |
Note: Conversion factors for units: hemoglobin and albumin in g/dL to g/L, x10; total, HDL, and LDL cholesterol in mg/dL to mmol/L, x0.0259; triglycerides in mg/dL to mmol/L, x0.0113.
Abbreviations: ACE, angiotensin-converting enzyme; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Values at baseline of parent Homocysteine Study.
Body mass index was calculated as weight in kilograms divided by height in meters squared.
In May 2006 the Data and Safety Monitoring Board recommended the parent HOST study be halted early because the required number of primary endpoints (deaths) had occurred. All participants in HOST stopped taking the study drug and the parent study ended August 31, 2006. Although all participants underwent initial cognitive testing while still taking the study drug, 454 of the 490 participants who were retested had discontinued the drug before being retested. There were no differences between the groups for the mean length of treatment (placebo, 1144 days; vitamin, 1128 days, P = 0.4). The double blind was not broken, and the results of the parent HOST study were not given to participants until after the final cognitive retest had occurred.
Treatment effects on tHcy and B-vitamin levels
During the parent HOST study, baseline and 3-month median tHcy levels for the treatment and placebo groups in the cognitive substudy were as follows: vitamins, 22.5 μmol/L and 16.5 μmol/L (P < 0.001); placebo, 22.2 μmol/L and 21.1 μmol/L (P = 0.09). Over the same interval, median folate levels for the treatment and placebo groups were as follows: vitamins, 15.9 ng/mL and 2050.0 ng/mL (P < 0.001); placebo, 14.8 ng/mL and 17.1 ng/mL (P = 0.004). These treatment results were similar to those in the parent study21. The mean interval between enrollment in HOST and enrollment in the substudy was 3.1 years for both the vitamin and placebo groups; thus, those in the vitamin group had been treated for 3.1 years before being tested.
Treatment effects on cognitive outcomes at initial testing
There were no differences between placebo and vitamin groups on any cognitive measure or outcome, or on the GDS-SF (Table 2). We also examined whether there were differences in the prevalence of cognitive impairment between the groups, using a TICSm score of 27 or below as the cutoff for impairment 25, 26, and found that the rates of impairment were similar (placebo, 17.5%; vitamin, 20.7; P = 0.3).
Table 2.
Cognitive Function Measures at Initial Assessment Among Participants Assigned to Placebo vs. Vitamins
| Cognitive Measure | Placebo | Vitamins | P value |
|---|---|---|---|
| TICSm | 32.1 (5.2) | 31.8 (5.1) | 0.5 |
| Digit span forward – span | 5.6 (1.6) | 5.6 (1.4) | 0.8 |
| Digit span backward – span | 3.8 (1.4) | 3.7 (1.3) | 0.3 |
| Verbal Fluency | 15.7 (5.3) | 16.2 (4.8) | 0.2 |
| Global cognition z-score composite | 0.003 (0.8) | -0.009 (0.7) | 0.8 |
| Memory z-score composite | 0.003 (0.8) | -0.005 (0.8) | 0.9 |
| Geriatric Depression Scale | 3.4 (3.3) | 3.5 (3.3) | 0.6 |
Note. Values expressed as mean± SD
Abbreviation: TICSm, Telephone Interview for Cognitive Status – modified
Vitamin treatment had no effect on any cognitive outcome in models adjusted for the following variables collected at HOST baseline: demographic information, kidney disease status (advanced CKD, ESRD), cardiovascular disease, diabetes, blood pressure, hypertension treatment, and tHcy and GDS-SF score at the time of cognitive testing (Table 3). Baseline tHcy was negatively associated only with TICSm performance; follow-up analyses determined that this association was not related to baseline levels of folate and B-vitamins (P values ranged from .09 to 0.9). Cognitive outcomes were not significantly different for advanced CKD compared to ESRD participants at baseline or during follow-up (i.e., adjusted for the dialysis status at the time of initial testing in this substudy; P values ranged from 0.07 to 0.9) and there were no significant treatment by kidney disease status interactions on any cognitive outcome (P values ranged from 0.6 to 0.9). Although there were significant baseline differences between the treatment groups in stroke rate and hemoglobin level (Table 1), neither was significantly related to the cognitive outcomes in our models (P values ranged from 0.1 to 0.9).
Table 3.
Association Between Baseline Homocysteine, Vitamin Treatment, and Cognitive Outcomes in Adjusted Models Using Multiple Regression*
| Baseline Homocysteine (μmol/L)† | Vitamin Treatment‡ | |||
|---|---|---|---|---|
| Cognitive Outcome | Regression coefficient (SE) | P value | Regression coefficient (SE) | P value |
| TICSm | -.05 (.02) | .04 | -.28 (.34) | 0.4 |
| Cognitive composite | -.00 (.00) | .4 | -.01 (.05) | 0.9 |
| Memory composite | -.00 (.00) | .6 | -.02 (.06) | 0.7 |
Note: Homocysteine reported in umol/L, unit for conversion to mg/L, x0.135. Cognitive composite = Cognitive z-score composite composed of TICSm totals score, digit span forward and backward span scores, and verbal fluency total correct. Memory composite = Memory composite composed of TICSm word list immediate and delayed recall, and the recognition trials.
Abbreviations: TICSm = Telephone Interview of Cognitive Status – modified, SE = standard error
Models adjusted for the following variables collected at HOST baseline: age, education, race, kidney disease status (advanced chronic kidney disease, end-stage renal disease), cardiovascular disease, diabetes, systolic and diastolic blood pressure, hypertension treatment, and plasma total homocysteine. Geriatric Depression Scale score at the time of cognitive testing was also included in the model.
Values at baseline of parent VA Homocysteine Study.
Effect of vitamin treatment compared with placebo.
Prevalence of cognitive impairment at initial testing
We further examined the prevalence of cognitive impairment at initial testing using a TICSm score of 27 or below as the cutoff for impairment. Prevalence rates were similar across kidney disease groups (advanced CKD, 18.7%; ESRD, 19.9%; P = 0.7); these rates did not change when we performed subsequent analyses adjusted for the dialysis status at the time of initial testing in this substudy to account for any effects of change in kidney disease status from baseline. The prevalence of cognitive impairment increased by age decade (collapsed across kidney disease status) as follows: 40s, 3.4%; 50s, 10.9%; 60s, 14.0%; 70s, 28.2%; 80s, 28.7% (P < 0.001).
Treatment effects on 1-year change in TICSm performance
One-year retests were completed in 490 participants. Retests were not conducted on 169 participants because of: loss to follow-up (73), death (72), refusal (20), and illness or confusion (4). The proportions of those who were not retested were similar in the placebo and vitamin groups, 25% (80/320) and 27% (92/339), respectively. There were no effects of treatment on 1-year change on the TICSm at the RCI cutoffs of +/- 1.65 (Table 4). Follow-up sensitivity analyses using RCI cutoffs that were either more liberal (i.e., +/- 1.29) or conservative (i.e., +/- 1.96) also were nonsignificant (P values = 0.9 in both cases).
Table 4.
1-year Change on TICSm Among Participants Assigned to Placebo vs. Vitamins
| RCI | Placebo | Vitamins |
|---|---|---|
| Improve | 19 | 23 |
| Decline | 20 | 22 |
| No change | 204 | 202 |
Note: 490 participants completed Time 2 assessments. Improve = 1-year TICSm improvement of 6 or more points. Decline = 1-year TICSm decline of 5 or more points. No change = TICSm 1-year differences between these values.
There were no differences between placebo and vitamin groups in the number of participants who improved, declined or showed no change on the TICSm over a 1-year interval via Chi-Square analyses, P = 0.8.
Abbreviations: TICSm = Telephone Interview for Cognitive Status – modified, RCI = Reliable Change Index.
Discussion
Treatment with high daily doses of folic acid and vitamins B6 and B12 reduced tHcy levels by 26.7%, and the reduction was maintained for over three years21. However, in this subset of participants, treatment did not influence cognitive outcomes at initial testing or change in cognition over a 1-year interval. These findings do not support the administration of B-vitamin therapy to improve cognitive outcomes in persons with advanced CKD and ESRD.
Our findings confirm the null findings of other clinical trials of the effects of B-vitamin therapy or tHcy reduction on cognitive outcomes in other populations 19, 37-41, and extend these findings to advanced CKD and ESRD patients with higher baseline tHcy values receiving a higher dose B-vitamin intervention than those in previous studies. In contrast, the Folic Acid and Carotid Intima-Media Thickness (FACIT) trial cognitive substudy 42, found that tHcy reduction via daily administration of 800 μg of folic acid improved cognitive outcomes in a large sample of community-dwelling older adults. It is unclear why the FACIT study found a beneficial effect of tHcy reduction on cognition whereas other similarly designed studies 38, 39 did not.
Two other notable findings were that the advanced CKD and ESRD groups had similar rates (approximately 19%) of cognitive impairment on the TICSm, and that cognitive impairment was present in nearly a third of patients aged 70 and older. In comparison, previous studies found that the prevalence of cognitive impairment was between 15-20% in advanced CKD 1-3, 43. The prevalence of cognitive impairment in our ESRD sample was lower than the 27-87% prevalence seen in similar studies 1, 5, 44-46, and may be due to differences in sample composition (e.g., age, comorbid conditions) or the cognitive measures used. The higher cognitive impairment prevalence in older members of our sample confirms recent findings about cognitive impairment in an aging CKD population 8, 47.
There are several explanations for the failure to find a treatment effect on cognitive outcomes in our study. First, our participants had a high comorbidity burden of hypertension, diabetes and vascular disease. These comorbidities affect cognition 48-51, and may have obscured any treatment effect on cognition. Second, although tHcy levels were reduced by 26.7%, only a third of the participants had their tHcy levels reduced to normal levels of 8 - 10 μmol/L21. The results of the FACIT trial42 suggested that tHcy may need to be lowered into the normal range to produce beneficial effects on cognition; however, McMahon et al.39 reduced tHcy to normal levels in their trial and found no cognitive effect of this reduction.
Several limitations of this study may have contributed to the failure to demonstrate a treatment effect on cognition. The lack of baseline measurement of cognitive function at initial randomization in the parent study is an important limitation. However, two other trials examining cognitive function decline also enrolled a cohort during the follow up period of the main study; one found treatment effects on cognition52 whereas another did not53. Because of this design limitation, we assumed that baseline cognitive function was similar in the two treatment groups at randomization, and any differences at the initial cognitive function assessment at 3 years after randomization would be due to treatment effects. It is likely that cognitive function was similar across the treatment groups at baseline because the groups did not differ in risk factors for cognitive dysfunction, such as age or hypertension, but it cannot be known for certain.
Moreover, we could not determine whether cognitive decline occurred in the 3-year interval between enrollment into HOST and the cognitive substudy. Thus, we may not have detected a treatment effect because our sample may have been cognitively stable during this interval. There are two reasons why this latter possibility is unlikely. First, although longitudinal data about cognitive function in CKD are limited, a significant decline has been demonstrated over 2-4 year intervals in both CKD54 and ESRD55 patients. For example, Kurella-Tamura54 showed that those with moderate to advanced CKD (i.e., estimated glomerular filtration rate [eGFR] < 45 mL/min/1.73 m2) were nearly 2.5 times more likely to decline over 4 years on the Modified Mini Mental State Exam (3MS; decline defined as either a decline of > 5 points, or 1 standard deviation, or a score that decreased to < 80) compared with those without CKD (i.e., eGFR ≥ 60 mL/min/1.73 m2). Second, cognitive decline over a similar interval is related to increasing age56-58 and vascular disease risk factors such as those present in our sample59 in relatively healthier community-dwelling samples. Therefore, it is likely that cognitive decline would have occurred in the participants after initial randomization and before enrollment into the substudy. The participants enrolled in the cognitive function substudy represent a survivor cohort, since sicker patients would have died prior to initiation of the substudy. This possibility is reflected in the younger age and lower rates of vascular disease in the substudy participants. Nevertheless, risk factors for cognitive decline were balanced by treatment group in the substudy participants and the burden of vascular comorbidities was still high.
Telephone assessment of cognition may not be as sensitive as an in-person interview to detect effects of treatment, but in-person assessments have also failed to disclose cognitive benefits of tHcy reduction39. The early discontinuation of the parent HOST trial may have contributed to our not finding treatment effects over a one-year duration; however, since there were no differences in initial cognitive assessment after 3 years of treatment, it is unclear why discontinuing treatment would effect a change over one year. We were not able to conduct 1-year retests on 169 participants. Although the loss of follow-up data from these participants could have introduced bias into our 1-year retest results, it is unlikely that this affected the treatment effect results because the proportion of those who were not retested was similar in the placebo (25%) and vitamin (27%) groups. Finally, this trial was conducted in the United States after folic acid fortification of the food supply was completed in 1998, which may have reduced the power to detect the effect of folic acid intervention on cognition60, 61.
In summary, we found a high prevalence of cognitive impairment in a large cohort of patients with advanced CKD and ESRD. The prevalence of cognitive dysfunction increased with age, with 28% of those over the age of 70 years having cognitive impairment. Treatment with high daily doses of B-vitamins, which reduced tHcy levels, did not improve cognitive outcomes compared to placebo and should not be recommended for treatment or prevention of cognitive dysfunction in patients with advanced CKD or ESRD.
Supplementary Material
Acknowledgments
We thank those who coordinated the study and conducted the telephone assessments, data scoring, entry, and accuracy checks at the HOST sites in Boston (Nicholas Best, Zilia Castrillon, Dana Jones, Nancy Pastore, Allison Rayne) and Palo Alto (Emily Ach, Marsha Anderson, Gerald Georgette, Michele Helmuth). We also thank Elizabeth Jobes, Shirley Joyner, Elizabeth Petrokaitis, and Scott Zellner at the VA Cooperative Studies Program (CSP) Coordinating Center at West Haven, CT for assistance in study and data coordination. Additionally, we thank Rebecca Brown, Zilia Castrillon, (VA Boston Healthcare System), and Carlos Rosado (VA Caribbean Healthcare System, San Juan, PR) for assistance in translating the cognitive assessment battery into Spanish. Finally, we thank the following groups from the parent HOST study (CSP #453), whose members are listed individually in Jamison et al21: Executive Committee, Data Safety Monitoring board, Human Rights Committee, and the Principal Investigators, Co-investigators, and Coordinators at each VA Medical Center who recruited participants into the parent study. The views expressed in this paper are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Support: The present work was supported by funds from the Cooperative Studies and Merit Review Programs of the United States Department of Veterans Affairs Office of Research and Development (Clinical Science Research and Development Program), the National Institute of Diabetes and Digestive and Kidney Diseases (grant R21 DK071292) and PamLab Pharmaceuticals, L.L.C. Dr Gaziano received research support in the form of vitamin pills and packaging from BASF, DMS Pharmaceuticals, and Wyeth Pharmaceuticals. The CSP approved the manuscript for submission but was not involved in the design of the study or the collection, management, analysis, or interpretation of the data. None of the other funding sources were involved in the design or conduct of the study or the collection, management, analysis, and interpretation of the data or review or approval of the manuscript.
Footnotes
For N Section: Trial registration: www.clinicaltrials.gov; study number: NCT00032435
Because an author of this manuscript is an editor for AJKD, the peer-review and decision-making processes were handled entirely by an Associate Editor (Michael Shlipak, MD, MPH, University of California, San Francisco) who served as Acting Editor-in-Chief. Details of the journal's procedures for potential editor conflicts are given in the Editorial Policies section of the AJKD website.
Financial Disclosure: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52(11):1863–1869. doi: 10.1111/j.1532-5415.2004.52508.x. [DOI] [PubMed] [Google Scholar]
- 2.Kurella M, Yaffe K, Shlipak MG, Wenger NK, Chertow GM. Chronic kidney disease and cognitive impairment in menopausal women. Am J Kidney Dis. 2005;45(1):66–76. doi: 10.1053/j.ajkd.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 3.Kurella Tamura M, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis. 2008;52(2):227–234. doi: 10.1053/j.ajkd.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madero M, Gul A, Sarnak MJ. Cognitive function in chronic kidney disease. Semin Dial. 2008;21(1):29–37. doi: 10.1111/j.1525-139X.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 5.Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67(2):216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 6.Pereira AA, Weiner DE, Scott T, Sarnak MJ. Cognitive function in dialysis patients. Am J Kidney Dis. 2005;45(3):448–462. doi: 10.1053/j.ajkd.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate Renal Impairment and Risk of Dementia among Older Adults: The Cardiovascular Health Cognition Study. Journal of the American Society of Nephrology. 2004;15(7):1904–1911. doi: 10.1097/01.asn.0000131529.60019.fa. [DOI] [PubMed] [Google Scholar]
- 8.Weiner DE. The cognition-kidney disease connection: lessons from population-based studies in the United States. Am J Kidney Dis. 2008;52(2):201–204. doi: 10.1053/j.ajkd.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Bostom AG, Shemin D, Verhoef P, et al. Elevated fasting total plasma homocysteine levels and cardiovascular disease outcomes in maintenance dialysis patients. A prospective study. Arteriosclerosis, Thrombosis & Vascular Biology. 1997;17(11):2554–2558. doi: 10.1161/01.atv.17.11.2554. [DOI] [PubMed] [Google Scholar]
- 10.Friedman AN, Bostom AG, Selhub J, Levey AS, Rosenberg IH. The kidney and homocysteine metabolism. J Am Soc Nephrol. 2001;12(10):2181–2189. doi: 10.1681/ASN.V12102181. [DOI] [PubMed] [Google Scholar]
- 11.Jungers P, Massy ZA, Nguyen Khoa T, et al. Incidence and risk factors of atherosclerotic cardiovascular accidents in predialysis chronic renal failure patients: a prospective study. Nephrol Dial Transplant. 1997;12(12):2597–2602. doi: 10.1093/ndt/12.12.2597. [DOI] [PubMed] [Google Scholar]
- 12.Dufouil C, Alperovitch A, Ducros V, Tzourio C. Homocysteine, white matter hyperintensities, and cognition in healthy elderly people. Annals of Neurology. 2003;53(2):214–221. doi: 10.1002/ana.10440. [DOI] [PubMed] [Google Scholar]
- 13.Elias MF, Sullivan LM, D'Agostino RB, et al. Homocysteine and cognitive performance in the Framingham offspring study: age is important. Am J Epidemiol. 2005;162(7):644–653. doi: 10.1093/aje/kwi259. [DOI] [PubMed] [Google Scholar]
- 14.McCaddon A, Hudson P, Davies G, Hughes A, Williams JHH, Wilkinson C. Homocysteine and cognitive decline in healthy elderly. Dementia and Geriatric Cognitive Disorders. 2001;12(5):309–313. doi: 10.1159/000051275. [DOI] [PubMed] [Google Scholar]
- 15.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Hyperhomocysteinemia associated with poor recall in the third National Health and Nutrition Examination Survey. American Journal of Clinical Nutrition. 2001;73(5):927–933. doi: 10.1093/ajcn/73.5.927. [DOI] [PubMed] [Google Scholar]
- 16.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B-12, and serum total homocysteine levels in confirmed Alzheimer disease. Archives of Neurology. 1998;55(11):1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 17.Haan MN, Miller JW, Aiello AE, et al. Homocysteine, B vitamins, and the incidence of dementia and cognitive impairment: results from the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2007;85(2):511–517. doi: 10.1093/ajcn/85.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. New England Journal of Medicine. 2002;346(7):476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 19.Balk EM, Raman G, Tatsioni A, Chung M, Lau J, Rosenberg IH. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167(1):21–30. doi: 10.1001/archinte.167.1.21. [DOI] [PubMed] [Google Scholar]
- 20.Jamison RL, Hartigan P, Gaziano JM, et al. Design and statistical issues in the homocysteinemia in kidney and end stage renal disease (HOST) study. Clin Trials. 2004;1(5):451–460. doi: 10.1191/1740774504cn038oa. [DOI] [PubMed] [Google Scholar]
- 21.Jamison RL, Hartigan P, Kaufman JS, et al. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. Jama. 2007;298(10):1163–1170. doi: 10.1001/jama.298.10.1163. [DOI] [PubMed] [Google Scholar]
- 22.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 23.Griva K, Newman SP, Harrison MJ, et al. Acute Neuropsychological Changes in Hemodialysis and Peritoneal Dialysis Patients. Health Psychology. 2003;22(6):570–578. doi: 10.1037/0278-6133.22.6.570. [DOI] [PubMed] [Google Scholar]
- 24.Welsh KA, Breitner JC, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 1993;6(2):103–110. [Google Scholar]
- 25.Crooks VC, Clark L, Petitti DB, Chui H, Chiu V. Validation of multi-stage telephone-based identification of cognitive impairment and dementia. BMC Neurol. 2005;5(1):8. doi: 10.1186/1471-2377-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallo JJ, Breitner JCS. Alzheimer's disease in the NAS-NRC Registry of ageing twin veterans: IV. Performance characteristics of a two-stage telephone screening procedure for Alzheimer's dementia. Psychological Medicine. 1995;25(6):1211–1219. doi: 10.1017/s0033291700033183. [DOI] [PubMed] [Google Scholar]
- 27.Jarvenpaa T, Rinne JO, Raiha I, et al. Characteristics of two telephone screens for cognitive impairment. Dementia & Geriatric Cognitive Disorders. 2002;13(3):149–155. doi: 10.1159/000048646. [DOI] [PubMed] [Google Scholar]
- 28.Plassman BL, Newman TT, Welsh KA, Helms M, et al. Properties of the telephone interview for cognitive status: Application in epidemiological and longitudinal studies. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 1994;7(3):235–241. [Google Scholar]
- 29.Wechsler D. Wechsler Adult Intelligence Scale. Third. San Antonio, CA: Psychological Corporation; 1997. [Google Scholar]
- 30.Lezak MD. Neuropsychological Assessment. New York: Oxford University Press; 1995. [Google Scholar]
- 31.Yesavage JA, Brink TL. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 32.Pliskin NH, Kiolbasa TA, Hart RP, Umans JG. Neuropsychological function in renal disease and its treatment. In: Tarter RE, Butters M, editors. Medical neuropsychology. 2nd. 2001. pp. 107–126. [Google Scholar]
- 33.Montorio I, Izal M. The Geriatric Depression Scale: a review of its development and utility. International Psychogeriatrics. 1996;8(1):103–112. doi: 10.1017/s1041610296002505. [DOI] [PubMed] [Google Scholar]
- 34.Chelune GJ, Naugle RI, Lueders H, Sedlak J, et al. Individual change after epilepsy surgery: Practice effects and base-rate information. Neuropsychology. 1993;7(1):41–52. [Google Scholar]
- 35.Collie A, Maruff P, Makdissi M, McStephen M, Darby DG, McCrory P. Statistical procedures for determining the extent of cognitive change following concussion. Br J Sports Med. 2004;38(3):273–278. doi: 10.1136/bjsm.2003.000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini-Mental State Examination. J Neurol Neurosurg Psychiatry. 2007;78(12):1298–1303. doi: 10.1136/jnnp.2006.109074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aisen PS, Schneider LS, Sano M, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008;300(15):1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang JH, Cook N, Manson J, Buring JE, Albert CM, Grodstein F. A trial of B vitamins and cognitive function among women at high risk of cardiovascular disease. Am J Clin Nutr. 2008;88(6):1602–1610. doi: 10.3945/ajcn.2008.26404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMahon JA, Green TJ, Skeaff CM, Knight RG, Mann JI, Williams SM. A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med. 2006;354(26):2764–2772. doi: 10.1056/NEJMoa054025. [DOI] [PubMed] [Google Scholar]
- 40.Stott DJ, MacIntosh G, Lowe GD, et al. Randomized controlled trial of homocysteine-lowering vitamin treatment in elderly patients with vascular disease. Am J Clin Nutr. 2005;82(6):1320–1326. doi: 10.1093/ajcn/82.6.1320. [DOI] [PubMed] [Google Scholar]
- 41.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. Jama. 2004;291(5):565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 42.Durga J, van Boxtel MP, Schouten EG, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369(9557):208–216. doi: 10.1016/S0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- 43.Nulsen RS, Yaqoob MM, Mahon A, Stoby-Fields M, Kelly M, Varagunam M. Prevalence of cognitive impairment in patients attending pre-dialysis clinic. J Ren Care. 2008;34(3):121–126. doi: 10.1111/j.1755-6686.2008.00028.x. [DOI] [PubMed] [Google Scholar]
- 44.Kutlay S, Nergizoglu Gk, Duman N, et al. Recognition of neurocognitive dysfunction in chronic hemodialysis patients. Renal Failure. 2001;23(6):781–787. doi: 10.1081/jdi-100108189. [DOI] [PubMed] [Google Scholar]
- 45.Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. American Journal of Kidney Diseases. 1997;30(1):41–49. doi: 10.1016/s0272-6386(97)90563-1. [DOI] [PubMed] [Google Scholar]
- 46.Tyrrell J, Paturel L, Cadec B, Capezzali E, Poussin G. Older patients undergoing dialysis treatment: cognitive functioning, depressive mood and health-related quality of life. Aging Ment Health. 2005;9(4):374–379. doi: 10.1080/13607860500089518. [DOI] [PubMed] [Google Scholar]
- 47.Monk RD, Bennett DA. Reno-cerebrovascular disease? The incognito kidney in cognition and stroke. Neurology. 2006;67(2):196–198. doi: 10.1212/01.wnl.0000231530.04240.f8. [DOI] [PubMed] [Google Scholar]
- 48.Elias MF, D'Agostino RB, Elias PK, Wolf PA. Neuropsychological test performance, cognitive functioning, blood pressure and age: the Framingham Heart Study. Experimental Aging Research. 1995;21:369–391. doi: 10.1080/03610739508253991. [DOI] [PubMed] [Google Scholar]
- 49.Elias MF, Elias PK, Robbins MA, Wolf PA, D'Agostino RB. Cardiovascular risk factors and cognitive functioning: An epidemiological perspective. In: Waldstein SR, Elias MF, editors. Neuropsychology of cardiovascular disease. Mahwah, NJ: Lawrence Erlbaum Associates; 2001. pp. 83–104. [Google Scholar]
- 50.Ryan CM. Diabetes-associated cognitive dysfunction. In: Waldstein SR, Elias MF, editors. Neuropsychology of cardiovascular disease. Mahwah, NJ: Lawrence Erlbaum Associates; 2001. pp. 61–82. [Google Scholar]
- 51.Waldstein SR, Katzel LI. Hypertension and cognitive function. In: Waldstein SR, Elias MF, editors. Neuropsychology of cardiovascular disease. Mahwah, NJ: Lawrence Erlbaum Associates; 2001. pp. 15–36. [Google Scholar]
- 52.Grodstein F, Kang JH, Glynn RJ, Cook NR, Gaziano JM. A randomized trial of beta carotene supplementation and cognitive function in men: the Physicians' Health Study II. Arch Intern Med. 2007;167(20):2184–2190. doi: 10.1001/archinte.167.20.2184. [DOI] [PubMed] [Google Scholar]
- 53.Grady D, Yaffe K, Kristof M, Lin F, Richards C, Barrett-Connor E. Effect of postmenopausal hormone therapy on cognitive function: the Heart and Estrogen/progestin Replacement Study. The American Journal of Medicine. 2002;113(7):543–548. doi: 10.1016/s0002-9343(02)01270-6. [DOI] [PubMed] [Google Scholar]
- 54.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005;16(7):2127–2133. doi: 10.1681/ASN.2005010005. [DOI] [PubMed] [Google Scholar]
- 55.Altmann P, Barnett ME, Finn WF. Cognitive function in Stage 5 chronic kidney disease patients on hemodialysis: no adverse effects of lanthanum carbonate compared with standard phosphate-binder therapy. Kidney Int. 2007;71(3):252–259. doi: 10.1038/sj.ki.5001932. [DOI] [PubMed] [Google Scholar]
- 56.Colsher PL, Wallace RB. Longitudinal application of cognitive function measures in a defined population of community-dwelling elders. Annals of Epidemiology. 1991;1:215–230. doi: 10.1016/1047-2797(91)90001-s. [DOI] [PubMed] [Google Scholar]
- 57.Sliwinski M, Buschke H. Cross-sectional and longitudinal relationships among age, cognition, and processing speed. Psychology and Aging. 1999;14(1):18–33. doi: 10.1037//0882-7974.14.1.18. [DOI] [PubMed] [Google Scholar]
- 58.Small BJ, Basun H, Backman L. Three-year changes in cognitive performance as a function of apolipoprotein E genotype: Evidence from very old adults without dementia. Psychology and Aging. 1998;13(1):80–87. doi: 10.1037//0882-7974.13.1.80. [DOI] [PubMed] [Google Scholar]
- 59.Brady CB, Spiro A, McGlinchey-Berroth R, Milberg W, Gaziano JM. Stroke risk predicts verbal fluency decline in healthy older men: Evidence from the Normative Aging Study. Journal of Gerontology: Psychological Sciences. 2001;56B(6):P340–P346. doi: 10.1093/geronb/56.6.p340. [DOI] [PubMed] [Google Scholar]
- 60.B-Vitamin Treatment Trialists' Collaboration. Homocysteine-lowering trials for prevention of cardiovascular events: a review of the design and power of the large randomized trials. Am Heart J. 2006;151(2):282–287. doi: 10.1016/j.ahj.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 61.National Kidney Foundation. K/DOQI clinical practice guidelines on hypertension and anti hypertensive agents in chronic kidney disease. American Journal of Kidney Diseases. 2004;43(5):S14–S290. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.