Abstract
Background
Cell adhesion plays an important role in proliferation, metastasis, and tumor growth and may represent a potential vulnerability in treatment of prostate cancer patients. Bicalutamide (Casodex) has been used as an anti-androgen agent for prostate cancer patients during hormone ablation therapy. This study focuses on the effect of Bicalutamide on cell adhesion to fibronectin in prostate cancer cells.
Methods
LNCaP prostate cancer cells were stimulated with androgen before being irradiated with doses of 0, 5, 10 or 15 Gy. Cell adhesion to fibronectin was then measured to ascertain androgen's role in integrin mediated prostate cancer cell adhesion. Flow cytometry was used to analyze surface expression of integrin subtypes in LNCaP cells upon androgen stimulation and 15 Gy ionizing radiation.
Results
LNCaP cell adhesion to fibronectin (FN) was significantly increased by stimulation with androgen when treated with 10 or 15 Gy ionizing radiations but not at 0 or 5 Gy. This increase was inhibited by treatment with Bicalutamide. LNCaP cells exposed to high dose radiation showed an increased expression of αV and β1 integrins in response to androgen treatment while Bicalutamide abolished this effect.
Conclusions
Our data show that Bicalutamide inhibits the effect of androgen on cell adhesion to FN through changes of integrin subtypes in androgen-dependent prostate cancer cells given high dose radiation. This suggests new molecular targets and possible treatment strategies for prostate cancer patients to improve the outcome during hormone ablation therapy and radiation therapy.
Keywords: LNCaP, Casodex, FN, integrins, extracellular matrix
Introduction
Excluding dermal malignancies, adenocarcinoma (AdCa) of the prostate is now the most common cancer in men and remains the second most lethal malignancy in adult males (1) (2) (3). The transcriptional cascade activated by androgen receptor (AR) plays a key role in prostate function in growth and survival of normal and malignant tissues (4). Methods of early detection have clearly improved tumor identification and stage migration with decreased volume of disease at presentation is now a well-established phenomenon. Although clear progress has been made in the clinical outcomes from patients with low risk clinical features for tumor progression, patients with intermediate and high-risk features remain vulnerable to treatment failure (2)(3)(5)(6). Improved treatment strategies are needed in cases with Gleason scores 7-10 and PSA > 10 patients as these patients appear more vulnerable to treatment failure in spite of optimal treatment planning.
Current treatment strategies include surgery, radiation therapy, and hormone ablation therapy in the treatment of patients at the time of disease presentation. External radiation therapy with and without brachytherapy is the local treatment of choice for patients with intermediate and advanced risk of tumor progression (7). Often these patients are treated with hormone intervention used in a neo-adjuvant fashion or concurrent with radiation treatment. This treatment strategy is commonly used in patient care, however when patients fail treatment, they will ultimately fail with androgen-independent/hormone resistant disease (8) (9). One possible strategy to address this problem will be to identify the mechanism of the benefit of hormone therapy and “mimic” its effect with a targeted therapy, thus keeping hormone therapy as a treatment to use at the time of initial failure (10). This strategy may decrease the rate of failure with androgen-independent disease. Incorporating targeted therapies in a time-sensitive fashion specific to the effects of hormone at presentation may permit hormone to be used at the time of initial radiation treatment instead of primary or neo-adjuvant therapy. This strategy may have the potential of delaying the onset of hormone refractory disease that uniformly predicts poor patient outcome. Strategic use of hormone therapy, either fort course or longer treatment, may also influence this effect. Many investigators are also considering using a shorter course of hormone therapy at presentation in patients with high to intermediate risk factors for recurrence to prevent the development of hormone refractory disease. This idea is under careful evaluation for clinical trials at this time.
Tumor cell adhesion is an important step in the process of tumor cell growth and metastasis (11). Adhesion promotes interactions between cell-cell and cell-extracellular matrix (ECM) (12) (13). Integrin expression is up-regulated at low dose ionizing radiation (14). Inhibiting this process may be important in promoting tumor cell death (15). Our previous study showed this inhibitory effect of high dose radiation therapy on β1 integrin expression in prostate cancer cell lines indicating that radiation therapy plays an important role in decreasing cellular adhesion at high dose ionizing radiation (16). Promoting this effect may be an important aspect of future translational research as treatment directed to this area may be an invaluable co-partner with radiation therapy delivered on a concurrent basis. Integrating targeted therapies or limited course hormone therapy to influence cellular adhesion may become an important translational treatment strategy to improve clinical outcomes (17).
In the present study, we explored the relationship of cell adhesion using fibronectin (FN) based platform and androgen stimulation in prostate cancer upon ionizing radiation. We studied the effect of androgen stimulation on tumor cell adhesion using R1881 (a synthetic androgen that binds strongly to the androgen receptor) or DHT (dihydrotestosterone). Bicalutamide (Casodex) is a non-steroidal anti-androgen chemical that binds to AR in target tissues and competitively inhibits the action of androgen in that tissue by assembly of a transcriptionally inactive receptor (18). The effect of Bicalutamide and its competition for receptor with androgen on tumor cell adhesion is evaluated with or without the effect of ionizing radiation. Identifying the relationships of these interactions will influence strategies for translational research and further development of therapy protocols for patients undergoing treatment for prostate cancer.
Materials and Methods
Cell line and culture
Androgen-dependent prostate cancer cell lines, LNCaP, were used for this study (19). Cells were cultured in RPMI 1640 culture medium containing 10% heat inactivated fetal bovine serum (FBS), 2 mM L-glutamate, 10 mM HEPES buffer, 1 mM sodium pyruvate, 0.1 mM non-essential amino acids, and 100 unit/ml penicillin, 100 μg/ml streptomycin (all from Invitrogen Carlsbad, California). Cells were maintained at 37°C with 5% CO2.
Reagents and antibodies
FN was purified from human plasma as previously described (20). Synthetic androgen R1881 was purchased from Dupont-NEN Life Technologies (Boston, MA). Poly-L-Lysine and bovine serum albumin (BSA) were from Sigma (St. Louis, MO). FACS analysis was carried out using monoclonal antibodies: TS2/16 to human β1 integrin (ATCC, Manassas, VA); AP-3 to β3 integrin (ATCC); L230 to αv integrin (ATCC); P1B5 to α3 integrin (provided by Elizabeth Wayner, The Fred Hutchinson Cancer Research Center, Seattle, WA) (22); P4C2 to α4 integrin (23) (Biogen-Idec, Cambridge, MA); P1D6 to α5 integrin (Life Technologies, Inc., Gaithersburng, MD); 12CA5 to hemagglutinin (ATCC); 10D5 to β6 integrin (Chemicon, Temecula, CA) and mouse IgG as the negative control (Vector Laboratories, Burlingame, CA).
Radiation exposure
Cells were irradiated at room temperature using a 6 MeV Varian 2300CD linear accelerator (Varian Medical Systems, Palo Alto, CA). Single doses of 5, 10, or 15 Gy were delivered at 3 Gy/minute with a 1 cm surface bolus application to ensure dose uniformity in depth.
Cell starvation and Bicalutamide treatment
LNCaP cells were grown to 70% confluence, washed twice in PBS and starved in RPMI 1640 culture medium supplemented with 2% charcoal stripped FBS (CS-FBS), 2 mM L-glutamate, 10 mM HEPES buffer, 1 mM sodium pyruvate, 0.1 mM non-essential amino acids, 100 unit/ml penicillin and 100 μg/ml streptomycin.
After 24 hrs, cells were washed once and the media was replaced with RPMI 1640 with 2% CS-FBS containing 5 μM Bicalutamide (dissolved in DMSO) or the same volume of DMSO as a control. After 1 hr incubation, cells were stimulated with 1 nM R1881 or ethanol (equal volumes). R1881 was only added once during stimulation. After 24 hr hormone treatment, cells were exposed to x-ray radiation followed by culture for another 24 hrs.
Cell adhesion assay
Plates were coated with 10 μg/ml FN, 100 μg/ml Poly-L-Lysine, or PBS and incubated at 4°C overnight. Plates were washed with PBS (three times) and blocked with 2% BSA for 1 hr at room temperature. Cells were harvested using trypsin/EDTA, and trypsin was neutralized using RPMI 1640 with 2% CS-FBS. After washed with RPMI 1640 with 2% CS-FBS, 100,000 cells were plated on the FN, BSA, or Poly-L-Lysine coated plates in 2% BSA contained RPMI 1640 in the presence of ethanol, DMSO, R1881 or Bicalutamide and maintained in the culture medium as described above.
To measure cell adhesion, cells were incubated at 37°C in 5% CO2 for 3 hrs. After incubation, the medium was removed and the plates were washed three times with PBS. Attached cells were fixed with 3% paraformaldehyde in PBS at 4°C for 30 mins. Cells were washed with PBS and stained with 0.5% crystal violet in PBS for 1 hr at room temperature. Cells were thoroughly washed with water and dried overnight. Attachment of cells was scored by measuring absorbance at 630 nm on a microtiter plate reader (24).
To confirm that cells were equally loaded in each well, the same volumes of suspended cells used in seeding wells were collected and lysed. The protein concentration of the lysate was then determined using BCA Protein Assay Kit (Pierce). Alternatively, cell adhesion to Poly-L-Lysine was measured and compared.
Cell viability
LNCaP cells were harvested with trypsin/EDTA immediately before cell adhesion assays. Cell viabilities were measured using trypan blue exclusion assay.
Fluorescence activated cell sorter (FACS) analysis
LNCaP cells were cultured and starved as previously mentioned. After cells were treated with Bicalutamide, R1881 or ethanol followed by given radiation, cells were collected for analysis of integrin expression using FACS analysis.
One-color FACS analysis was then performed using nonpermeabilized cell suspensions with one of the following monoclonal antibodies to human integrins: TS2/16 to β1, AP-3 to β3; 14E5 to β8; L230 to αv; P1B5 to α3; to α4 integrin (clone P4C2, Biogen); P1D6 to α5; 10D5 to β6 integrin; 12CA5 to hemagglutinin. Mouse IgG was used as a negative control for 10D5. The cells were incubated with goat anti-mouse FITC-conjugated secondary antibody (40 μg/ml; Cappel) at 4°C for 30 mins. FACS analysis was performed after cells were fixed in paraformaldehyde.
Luciferase assay
Luciferase activity was measured as described (25). Plasmids pGL3-(ARE)4-luciferase and CMV-β-galactosidase were from Dr. Michael Lu. Cell lysates were prepared with the reporter lysis buffer (Promega). β-galactosidase activity was assayed using the Galacto-Lite Plus reagent (Tropix). ARE luciferase activity was assayed using Luciferase reagent (Promega).
Statistical analysis
Differences in cell adhesion to FN were measured using paired Student's t-test.
Results
Androgen does not affect LNCaP cell adhesion to FN under low dose ionizing radiation
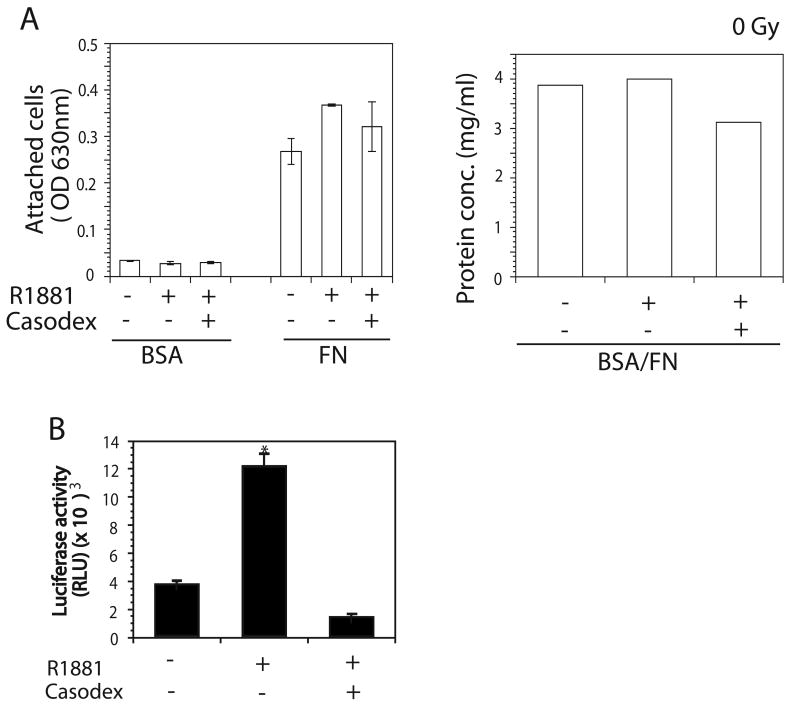
To analyze androgen's role in LNCaP cell adhesion to FN, cell adhesion assays were carried out on FN (10 μg/ml) after cells were stimulated with R1881 (1 nM) or ethanol. R1881 does not affect cell adhesion to FN, as compared to vehicle, of cells exposed to 0 Gy (Fig. 1A) or 5 Gy ionizing radiation (Fig. 2A, left panel). Similar results were obtained when cells were treated with 10 nM DHT (data not shown). We further confirmed our results by treating LNCaP cells with R1881 in the presence of Bicalutamide (5 μM) (18). Bicalutamide showed no effect on cell adhesion to FN. To confirm equal loading, the same volumes of cell suspensions used to seed cells in adhesion assays were collected within an experiment and cell lysates were prepared. The protein concentration of cell lysate was determined by BCA protein assay and observed levels were determined to represent equal loading (right panels in Fig. 1A and Fig. 2). The data show that androgen does not affect prostate cancer cell adhesion to FN at low dose ionizing radiation.
Fig. 1.
Effect of androgen stimulation on LNCaP cell adhesion to FN. LNCaP cells were cultured in 2% CS-FBS containing culture medium for 24 hrs followed by stimulation with 1 nM R1881 for another 24 hrs. Bicalutamide (5 μM) were added into 2% CS-FBS contained culture medium 1 hr before addition of R1881. For adhesion assay, cells were detached and plated onto wells coated with 10 μg/ml FN or BSA (as a control). After 3 hrs of incubation at 37°C, cells were stained with crystal violet. Measuring absorbance at 630 nm scored cell attachment. The same volume of cell suspensions was collected within an experiment and cells were lysed. Protein concentrations of cell lysate were used as a loading control. A: No change in adhesion was observed when LNCaP were stimulated with ethanol or R1881 in the presence or absence of Bicalutamide (left panel). Protein concentrations from the same amount of cells from each treatment for adhesion assay were determined using BSA assay to show the equal cell loading in adhesion assay (right panel). B: LNCaP cells were transiently transfected with plasmid containing ARE luciferase constructs and β-gal. Cells were treated with 1 nM R1881 only or with R1881 in the presence of Bicalutamide (5 μM). AR activity in LNCaP cells was determined by luciferase activity.
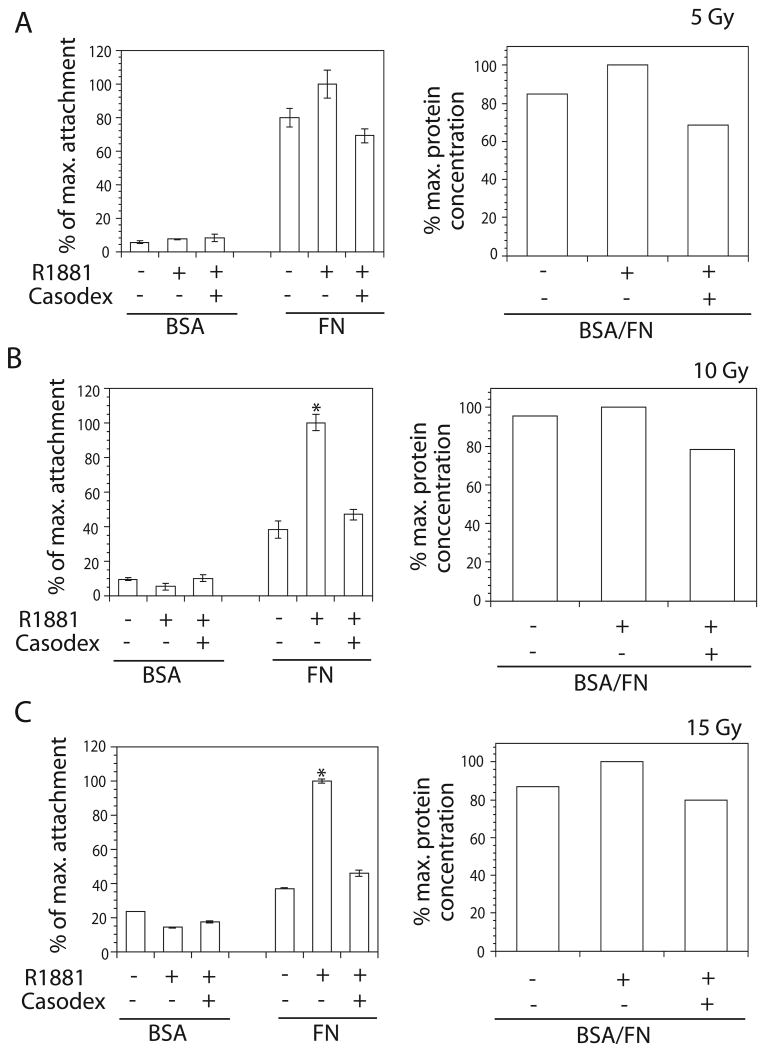
Fig. 2.
Effect of androgen stimulation and ionizing radiation on LNCaP cell adhesion to FN. After starvation in 2% CS-FBS containing cultured medium followed by stimulation with R1881 in the presence or absence of Bicalutamide, LNCaP cells were irradiated with ionizing radiation at doses of 5, 10 or 15 Gy at room temperature. At 24 hr post-irradiation, cells were detached for adhesion assay the same way as mentioned in Figure 1. Adhesion of cells with different treatments was scored as the percentage when compared to maximum attachment. A: No change in adhesion was observed among cells treated with vehicle or R1881 in the presence or absence of Bicalutamide (left panel) when cells were exposed to 5 Gy (left panel). B: A statistically significant (*P ≤ 0.05) increase in adhesion was observed when cells were treated with R1881 and irradiated with 10 Gy (left panel). C: A statistically significant (*P ≤ 0.05) increase in adhesion was observed when cells were treated with R1881 and irradiated with 15 Gy. Protein concentrations from the same amount of cells from each treatment for adhesion assay were determined using BSA assay to show the equal cell loading in adhesion assay (right panels in A, B, C).
To confirm inhibition of AR activity by Bicalutamide, we performed luciferase assays by transiently transfecting cells with a pGL3-(ARE)4-luciferase construct. LNCaP cells were treated with Bicalutamide 1 hr before stimulation with R1881. Our data show that Bicalutamide completely inhibits AR activity of cells in the presence of androgen (Fig. 1B) indicating the specificity of ligand function.
Androgen stimulates adhesion to FN of LNCaP cells irradiated with high dose ionizing radiation
To analyze the effect of high dose ionizing radiation, LNCaP cells were irradiated with doses of 10 Gy (Fig. 2B) or 15 Gy (Fig. 2C). Cell adhesion to FN (10 μg/ml) was greater when cells were stimulated with R1881 (1 nM) compared to ethanol (10 Gy p=0.005; 15 Gy p=0.0009). Moreover, Bicalutamide (5 μM) abrogates androgen-stimulated increase in adhesion of LNCaP cells exposed to high dose ionizing radiation (10 Gy p=0.008; 15 Gy p=0.001866). Similar results were observed when stimulating with DHT (10 nM, data not shown). Loading control was carried out to compare the number of cells seeded for adhesion assays by collecting the same volume of cell suspension, lysing the cells, and measuring the protein concentration by BCA assay. Equal loading was confirmed by similar protein concentrations in cell lysates (Fig. 2B and 2C, right panels). Our data indicate that androgen increased cell adhesion to FN upon exposure to high-dose ionizing radiation. However, inhibition of AR activity by Bicalutamide reduced this effect.
LNCaP surface expression of integrin subunits is affected by androgen stimulation at high dose radiation
To ascertain whether a change in surface expression of any subunits of integrin known to bind FN resulted in the observed difference in cell adhesion, FACS analysis was carried out on LNCaP cells stimulated with R1881 (1 nM) and exposed to 15 Gy ionizing radiation. Cells were probed with a variety of antibodies specific to integrin subtypes. The surface expression of these integrins (β1, β3, β6, αv, α3, α4, or α5) was determined when comparing cells in the presence or absence of androgen (Fig. 3 and 4). Among these integrins, αv and β1 integrins show an increase in response to R1881 treatment (Fig. 3). Specifically, 10% of cells that do not express αv integrin did express the subunit upon R1881 stimulation; 9.8% of cells that do not express β1 integrin express the subunit in response to R1881 treatment. However, in cells treated with Bicalutamide before addition of R1881, αv or β1 increase was abolished and was reduced to a basal level similar to ethanol-treated cells (Fig. 3). Meanwhile, the expression of other subtypes of integrins, such as α3, α4, α5, β3 and β6 did not show changes in cells treated with androgen or Bicalutamide (Fig. 3 and Fig. 4). Our data show that surface expression of αv and β1 integrin is increased in androgen-treated LNCaP cells in response to androgen upon exposure to high-dose radiation.
Fig. 3.
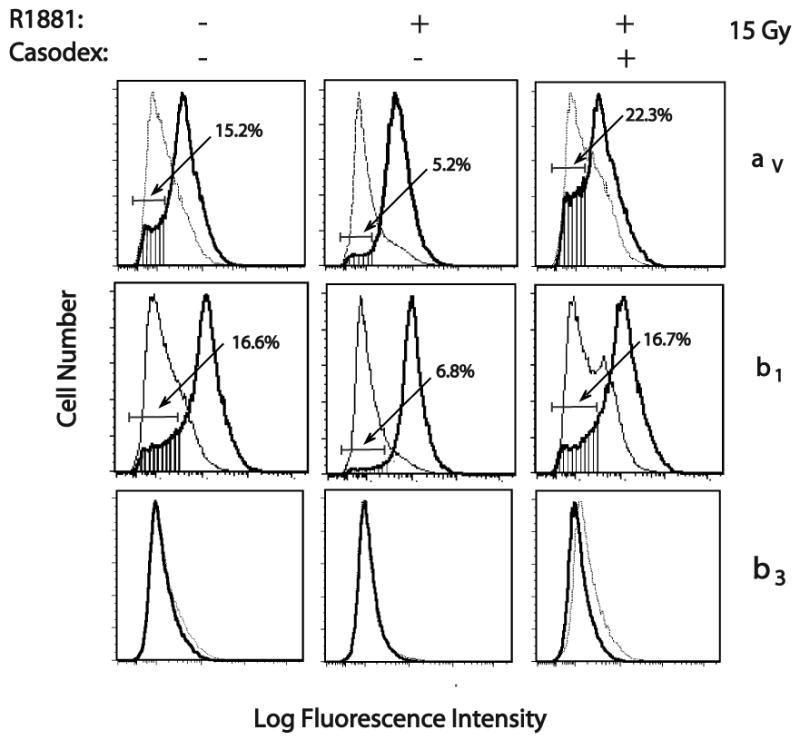
Effect of androgen stimulation on surface expression of integrin subtypes by LNCaP irradiated with 15 Gy. LNCaP cells were irradiated with 6 MeV X-rays 24 hrs after stimulation. At 24 hr post-irradiation, integrin expression was measured by FACS analysis using monoclonal antibodies (Thick line): TS2/16 to human β1 integrin; AP-3 to β3 integrin; L230 to αv integrin. The mouse IgG and 12CA5 (specific to hemagglutinin) were used as negative controls (Thin line).
Fig. 4.
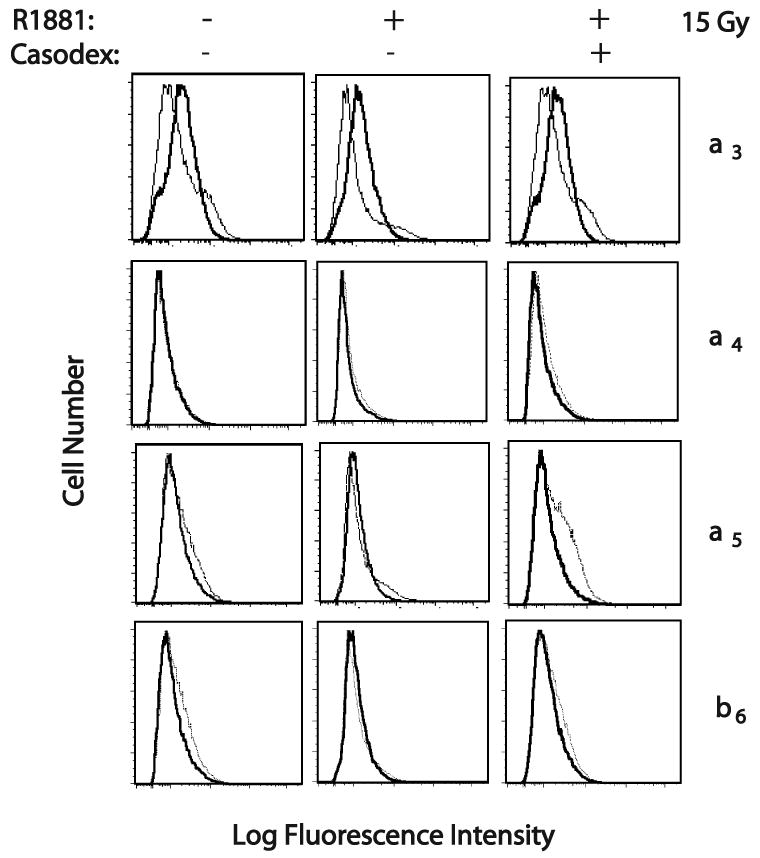
Effect of androgen stimulation on surface expression of integrin subtypes by LNCaP irradiated with 15 Gy. LNCaP were irradiated with 6 MeV X-rays 24 hrs after stimulation. At 24 hr post-irradiation, integrin expression was measured by FACS analysis using monoclonal antibodies (Thick line): P1B5 to α3 integrin; P4C2 to α4 integrin; P1D6 to α5 integrin; 10D5 to β6 integrin. The mouse IgG and 12CA5 (specific to hemagglutinin) were used as negative controls (Thin line).
Discussion
Prostate carcinoma remains a significant clinical challenge for surgeons and oncologists involved in the care of these patients. It remains the most common non-dermal malignancy in men and is responsible for a significant number of patient deaths (1). Although progress has been made in the area of disease identification and early detection, a significant number of patients remain vulnerable to treatment failure and progress with disease in spite of optimal current therapies. A more comprehensive understanding of the science of treatment and its downstream effectors may improve protocol development, treatment strategy, and patient outcome.
Radiation therapy remains the primary modality of care for many patients with AdCa of the prostate. Patients with Gleason 7-10 pathology, large irregularly shaped glands, elevated PSA, and other intermediate and high risk features are often treated with radiation therapy as their primary treatment in combination with sequential or concurrent hormone therapy (8). This standard has been the pattern of care for an extended period. Often investigators use hormone ablation therapy before undergoing brachytherapy to decrease the size of the prostate gland prior to implantation. The use of hormone therapy in these clinical settings has been considered reasonable treatment strategy for many years, however extended duration of hormone therapy may promote and perhaps accelerate the presence of hormone refractory disease as well as impose unwanted sequels in normal tissue function. The presence of hormone refractory disease bodes for a uniformly poor clinical outcome (7) and the quality of life for patients having hormone treatment can be compromised. It would be interesting to identify the effects of hormone on cell signaling and cell cycle pathways and determine if these effects could be mimicked by other therapies, thus possibly reserving extended hormone therapy and its unwanted side effects for a later time point if needed for progressive disease. Another strategy that has been proposed by many investigators is to consider short-targeted course hormone therapy during radiation therapy as a strategy to inhibit tumor progression and potentially decrease the likelihood of developing hormone refractory disease at a later time point. This would also serve to limit sequels of hormone therapy.
Identifying the manner upon which hormone and radiation therapy affects tumor cells gives us insight into the development of protocol strategies for patients undergoing treatment for prostate cancer. For example, our laboratory has demonstrated the effect of radiation therapy on the expression of β1 integrin in prostate cancer cell lines. The effect is primarily noted at high dose radiation therapy (16). In this study, we further showed that cell surface expression of αv and β1 integrins was increased in LNCaP cells in response to androgen at high dose radiation. Moreover, the adhesion of cells exposed to high dose of ionizing radiation (10 or 15 Gy) to FN is significantly enhanced in the presence of androgen in cells suggesting that integrins play an important role in mediating cell adhesion during radiation therapy. Several papers report that integrin mediates adhesion of cells to extracellular matrix components and subsequently modulates the cellular response to ionizing radiation in vitro (26)(27)(28). This mechanism might be in part causative for radiation resistance phenotypes in prostate cancer cells. Facilitating this effect with a medical co-partner/targeted therapy may be a very good approach to sensitize tumor cells to the effects of radiation therapy. Identifying medical partners to enhance the effect of radiation treatment may prove to be a valuable strategy for translational research. For years, patients with prostate cancer have been treated with androgen deprivation as part of primary treatment strategy. There is evidence that this strategy may improve outcome for patients with intermediate to high risk factors for tumor recurrence treated with radiation therapy (29) (30). However, some investigators are more cautious of the use of hormone therapy as part of primary management secondary to 1) the promotion of androgen-independent disease process and 2) possibly placing cells into non-radiation sensitive phases of the cell cycle (31) (32). A more comprehensive understanding of the science of the interactions may help promote strategic treatment approach to these patients.
The results of these experiments are interesting to evaluate as our data show that Bicalutamide abolished androgen-stimulated increase of both αv and β1 integrin expression while the expression of other subtypes of integrins, such as α3, α4, α5, β3 and β6 did not show changes. Prostate cancer often develops from an androgen-dependent into an androgen-independent phenotype, making the complex mechanism of Bicalutamide inhibition of AR activity more challenging. Although it is well known to act as an AR antagonist by suppressing the receptor (18), Bicalutamide can also switch from antagonist to agonist of the AR (33). While some patients obtain hormonal ablation therapy using Bicalutamide, many patients are also treated for prostate cancer with surgery and definitive radiation therapy without androgen deprivation. The presence of androgen during radiation treatment has never been fully evaluated from the perspective of tumor biology. Our results demonstrate that androgen promotes tumor cell adhesion in this LNCaP/FN model in irradiated tissue. This would imply that androgen interferes with one of the demonstrated effects of radiation therapy by promoting tumor cell adhesion that is normally inhibited by high dose radiation therapy. Therefore, promoting tumor adhesion may be counterproductive during radiation therapy and may lead to more limited patient outcome. Finding an agent that inhibits this effect may prove to be a valuable adjunct to radiation therapy. Finding an agent that functions through an integrin ligand may also be of value as the primary modulator of adhesion affected by radiation therapy is through an integrin-mediated mechanism. Thus, attacking adhesion through another mechanism such as FN may be valuable in patient care. The length of hormone therapy can be limited to the duration of teletherapy or decay of brachytherapy in patients with favorable intermediate risk factors for tumor control.
The finding that androgen stimulates cell adhesion upon ionizing radiation and Bicalutamide inhibits this effect at high-dose radiation (10 and 15 Gy) is an exciting result. This may explain in part how these treatments interact and affect tumor control for patients with recurrence after hormonal ablation treatment and/or radiation therapy. Inhibiting this effect during radiation treatment may make radiation therapy more effective in patient care. Further promoting this strategy is far more pronounced in irradiated cells, lending further support to the use of adhesion inhibition with radiation therapy. Bicalutamide appears effective in this role. Radiation therapy to patients undergoing androgen ablation treatment has been shown to improve the patient outcome. Alternative methods of achieving the beneficial effects of androgen ablation in counteracting cellular adhesion would possibly spare patients the adverse side effects of androgen suppression. Thus, treatment strategies incorporating adhesion interruption into patient care may prove to be an extremely valuable adjunct to radiation therapy in the care of patients with prostate cancer. Further studies may identify other therapies, including small molecules and specific adhesion target strategies that may further promote this effect beyond what is currently observed with Bicalutamide. Nevertheless, the effect of Bicalutamide on cellular adhesion is exciting and suggests that treatment of prostate cancer patients with Bicalutamide during radiation therapy, both with external therapy and brachytherapy, may enhance the effectiveness of radiation treatment in the care of these patients.
Acknowledgments
This work was supported by the following grants: NIH R01 CA-89720 and CA109874, DOD PCRP DAMD PC040221 (L. R. Languino) and Our Danny Cancer Funds P00010003300000 to (T. Wang).
The authors would like to thank Dr. ML Lu of Department of Surgery of Harvard Medical School for ARE construct, E. Wayner, The Fred Hutchinson Cancer Research Center for antibody P1B5 and Dr. N Alam from Department of Cancer Biology for his critical reading during paper preparation. We would also like to thank Thomas Lozeau, Diane Safer, Ginette Bailey, Linda Ding and Julie Trifone at Department of Radiation Oncology of the University of Massachusetts Medical School for their effort and patience that made this study possible.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. CA: a cancer journal for clinicians. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. 2008. [DOI] [PubMed] [Google Scholar]
- 2.Aaltomaa S, Lipponen P, Ala-Opas M, Eskelinen M, Kosma VM. α-catenin expression has prognostic value in local and locally advanced prostate cancer. Br J Cancer. 1999;80(3-4):477–482. doi: 10.1038/sj.bjc.6690381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdollahi A, Lipson KE, Han X, Krempien R, Trinh T, Weber KJ, Hahnfeldt P, Hlatky L, Debus J, Howlett AR, Huber PE. SU5416 and SU6668 attenuate the angiogenic effects of radiation-induced tumor cell growth factor production and amplify the direct anti-endothelial action of radiation in vitro. Cancer Res. 2003;63(13):3755–3763. [PubMed] [Google Scholar]
- 4.Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. J Cell Biochem. 2006;99(2):333–344. doi: 10.1002/jcb.20794. [DOI] [PubMed] [Google Scholar]
- 5.Aus G, Abrahamsson PA, Ahlgren G, Hugosson J, Lundberg S, Schain M, Schelin S, Pedersen K. Hormonal treatment before radical prostatectomy: a 3-year followup. The Journal of urology. 1998;159(6):2013–2017. doi: 10.1016/S0022-5347(01)63230-0. [DOI] [PubMed] [Google Scholar]
- 6.Brachman DG, Thomas T, Hilbe J, Beyer DC. Failure-free survival following brachytherapy alone or external beam irradiation alone for T1-2 prostate tumors in 2222 patients: results from a single practice. International journal of radiation oncology, biology, physics. 2000;48(1):111–117. doi: 10.1016/s0360-3016(00)00598-8. [DOI] [PubMed] [Google Scholar]
- 7.FitzGerald TJ, Simon E, Meyer J. Prostate carcinoma: opportunities for translational research. J Cell Biochem. 2004;91(3):433–442. doi: 10.1002/jcb.10693. [DOI] [PubMed] [Google Scholar]
- 8.Dorff TB, Quek ML, Daneshmand S, Pinski J. Evolving treatment paradigms for locally advanced and metastatic prostate cancer. Expert Rev Anticancer Ther. 2006;6(11):1639–1651. doi: 10.1586/14737140.6.11.1639. [DOI] [PubMed] [Google Scholar]
- 9.Kirschenbaum A, Liu X, Yao S, Levine AC. The role of cyclooxygenase-2 in prostate cancer. Urology. 2001;58(2 Suppl 1):127–131. doi: 10.1016/s0090-4295(01)01255-9. [DOI] [PubMed] [Google Scholar]
- 10.De Marzo AM, Nelson WG, Isaacs WB, Epstein JI. Pathological and molecular aspects of prostate cancer. Lancet. 2003;361(9361):955–964. doi: 10.1016/S0140-6736(03)12779-1. [DOI] [PubMed] [Google Scholar]
- 11.Fornaro M, Manes T, Languino LR. Integrins and prostate cancer metastases. Cancer Metastasis Rev. 2001;20(3-4):321–331. doi: 10.1023/a:1015547830323. [DOI] [PubMed] [Google Scholar]
- 12.Alam N, Goel HL, Zarif MJ, Butterfield JE, Perkins HM, Sansoucy BG, Sawyer TK, Languino LR. The integrin-growth factor receptor duet. Journal of cellular physiology. 2007;213(3):649–653. doi: 10.1002/jcp.21278. [DOI] [PubMed] [Google Scholar]
- 13.Rennebeck G, Martelli M, Kyprianou N. Anoikis and survival connections in the tumor microenvironment: is there a role in prostate cancer metastasis? Cancer Res. 2005;65(24):11230–11235. doi: 10.1158/0008-5472.CAN-05-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onoda JM, Piechocki MP, Honn KV. Radiation-induced increase in expression of the alpha IIb beta 3 integrin in melanoma cells: effects on metastatic potential. Radiation research. 1992;130(3):281–288. [PubMed] [Google Scholar]
- 15.Cordes N, van Beuningen D. Cell adhesion to the extracellular matrix protein fibronectin modulates radiation-dependent G2 phase arrest involving integrin-linked kinase (ILK) and glycogen synthase kinase-3β (GSK-3β) in vitro. Br J Cancer. 2003;88(9):1470–1479. doi: 10.1038/sj.bjc.6600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon EL, Goel HL, Teider N, Wang T, Languino LR, Fitzgerald TJ. High dose fractionated ionizing radiation inhibits prostate cancer cell adhesion and β1 integrin expression. Prostate. 2005;64(1):83–91. doi: 10.1002/pros.20227. [DOI] [PubMed] [Google Scholar]
- 17.Goel HL, Languino LR. Integrin signaling in cancer. Cancer Treat Res. 2004;119:15–31. doi: 10.1007/1-4020-7847-1_2. [DOI] [PubMed] [Google Scholar]
- 18.Masiello D, Cheng S, Bubley GJ, Lu ML, Balk SP. Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor. J Biol Chem. 2002;277(29):26321–26326. doi: 10.1074/jbc.M203310200. [DOI] [PubMed] [Google Scholar]
- 19.Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via αvβ3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59(7):1655–1664. [PubMed] [Google Scholar]
- 20.Engvall E, Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- 21.Jackson T, Clark S, Berryman S, Burman A, Cambier S, Mu D, Nishimura S, King AM. Integrin αvβ8 functions as a receptor for foot-and-mouth disease virus: role of the β-chain cytodomain in integrin-mediated infection. J Virol. 2004;78(9):4533–4540. doi: 10.1128/JVI.78.9.4533-4540.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skubitz AP, Grossman MD, McCarthy JB, Wayner EA, Cameron JD. The decreased adhesion of Y79 retinoblastoma cells to extracellular matrix proteins is due to a deficit of integrin receptors. Invest Ophthalmol Vis Sci. 1994;35(6):2820–2833. [PubMed] [Google Scholar]
- 23.Iida J, Skubitz AP, Furcht LT, Wayner EA, McCarthy JB. Coordinate role for cell surface chondroitin sulfate proteoglycan and α4β1 integrin in mediating melanoma cell adhesion to fibronectin. J Cell Biol. 1992;118(2):431–444. doi: 10.1083/jcb.118.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng DQ, Woodard AS, Tallini G, Languino LR. Substrate specificity of αvβ3 integrin-mediated cell migration and phosphatidylinositol 3-kinase/AKT pathway activation. J Biol Chem. 2000;275(32):24565–24574. doi: 10.1074/jbc.M002646200. [DOI] [PubMed] [Google Scholar]
- 25.Lu ML, Schneider MC, Zheng Y, Zhang X, Richie JP. Caveolin-1 interacts with androgen receptor. A positive modulator of androgen receptor mediated transactivation. J Biol Chem. 2001;276(16):13442–13451. doi: 10.1074/jbc.M006598200. [DOI] [PubMed] [Google Scholar]
- 26.Cordes N, Seidler J, Durzok R, Geinitz H, Brakebusch C. β1-integrin-mediated signaling essentially contributes to cell survival after radiation-induced genotoxic injury. Oncogene. 2006;25(9):1378–1390. doi: 10.1038/sj.onc.1209164. [DOI] [PubMed] [Google Scholar]
- 27.Sandfort V, Koch U, Cordes N. Cell adhesion-mediated radioresistance revisited. Int J Radiat Biol. 2007;83(11-12):727–732. doi: 10.1080/09553000701694335. [DOI] [PubMed] [Google Scholar]
- 28.Pawar SC, Dougherty S, Pennington ME, Demetriou MC, Stea BD, Dorr RT, Cress AE. ༟6 Integrin Cleavage: Sensitizing human prostate cancer to ionizing radiation. Int J Radiat Biol. 2007;83(11):761–767. doi: 10.1080/09553000701633135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilepich MV, Caplan R, Byhardt RW, Lawton CA, Gallagher MJ, Mesic JB, Hanks GE, Coughlin CT, Porter A, Shipley WU, Grignon D. Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: report of Radiation Therapy Oncology Group Protocol 85-31. J Clin Oncol. 1997;15(3):1013–1021. doi: 10.1200/JCO.1997.15.3.1013. [DOI] [PubMed] [Google Scholar]
- 30.Lawton CA, DeSilvio M, Roach M, 3rd, Uhl V, Kirsch R, Seider M, Rotman M, Jones C, Asbell S, Valicenti R, Hahn S, Thomas CR., Jr An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. International journal of radiation oncology, biology, physics. 2007;69(3):646–655. doi: 10.1016/j.ijrobp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell RE, Chang SS. Current controversies in the treatment of high-risk prostate cancer. Current opinion in urology. 2008;18(3):263–268. doi: 10.1097/MOU.0b013e3282f9b37f. [DOI] [PubMed] [Google Scholar]
- 32.Kremer CL, Schmelz M, Cress AE. Integrin-dependent amplification of the G2 arrest induced by ionizing radiation. Prostate. 2006;66(1):88–96. doi: 10.1002/pros.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Culig Z, Hoffmann J, Erdel M, Eder IE, Hobisch A, Hittmair A, Bartsch G, Utermann G, Schneider MR, Parczyk K, Klocker H. Switch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model system. Br J Cancer. 1999;81(2):242–251. doi: 10.1038/sj.bjc.6690684. [DOI] [PMC free article] [PubMed] [Google Scholar]