Abstract
The HSD2 (11-β-hydroxysteroid dehydrogenase type 2-expressing) neurons in the nucleus of the solitary tract (NTS) of the rat are aldosterone-sensitive and have been implicated in sodium appetite (Geerling et al., 2006a; Geerling et al., 2006b). The central nucleus of the amygdala (CeA) has been shown to modulate salt intake in response to aldosterone, so we investigated the connections between these two sites. A prior retrograde tracing study only revealed a minor projection from the HSD2 neurons directly to the CeA, but these experiments suggested that a more substantial projection may be relayed through the parabrachial nucleus. Small injections of cholera toxin beta subunit (CTb) into the external lateral parabrachial subnucleus (PBel) produced both retrograde cell body labeling in the HSD2 neurons and anterograde axonal labeling in the lateral subdivision of the CeA. Also, injections of either CTb or Phaseolus vulgaris leucoagglutinin into the medial subdivision of the CeA labeled a descending projection from the amygdala to the medial NTS. Axons from the medial CeA formed numerous varicosities and terminals enveloping the HSD2 neurons. Complementary CTb injections, centered in the HSD2 subregion of the NTS, retrogradely labeled neurons in the medial CeA. These bidirectional projections could form a functional circuit between the HSD2 neurons and the CeA. The HSD2 neurons may represent one of the functional inputs to the lateral CeA, and their activity may be modulated by a return projection from the medial CeA. This circuit could provide a neuroanatomical basis for the modulation of salt intake by the CeA.
Keywords: 11 beta hydroxysteroid dehydrogenase type 2, HSD2, 11HSD2, aldosterone, mineralocorticoid, nucleus of the solitary tract, nucleus tractus solitarius, NTS, sodium appetite, salt appetite, salt ingestion, ingestive behavior, central nucleus of the amygdala
Introduction
Sodium deficiency activates hard-wired brain circuitry that produces a voracious appetite for salt. Even though this unique drive has been studied for almost seventy years (Richter, 1936), the brain sites and mechanisms responsible for sodium appetite remain poorly understood. Aldosterone enhances the drive to ingest salt (Wolf and Handal, 1966), and a group of neurons in the nucleus of the solitary tract (NTS) may mediate this behavioral effect.
These neurons express the enzyme 11-β-hydroxysteroid dehydrogenase type 2 (HSD2, Geerling et al., 2006b). Consistent with the well-established role of this enzyme in mineralocorticoid-sensitive epithelial cells in the kidney (Funder et al., 1988; Stewart et al., 1988; Naray-Fejes-Toth et al., 1998; Kotelevtsev et al., 1999), the HSD2 neurons are selectively sensitive to the adrenal steroid aldosterone (Geerling et al., 2006a). They are specifically activated by manipulations that elevate aldosterone production and stimulate sodium appetite, including dietary sodium deprivation (Geerling et al., 2006a). These neurons also receive sodium regulatory input signals other than aldosterone because they are still activated by sodium deficiency after removal of the adrenal glands (Geerling et al., 2006a).
The HSD2 neurons innervate brain sites implicated in sodium appetite regulation, including the pontine parabrachial complex and the bed nucleus of the stria terminalis (Geerling and Loewy, 2006). Destruction of either of these sites produces a large reduction in the amount of saline that rats ingest in response to sodium deficiency (Flynn et al., 1991; Reilly et al., 1994; Zardetto-Smith et al., 1994; Scalera et al., 1995).
Another site involved in sodium appetite is the central nucleus of the amygdala (CeA). The CeA modulates salt intake, especially in response to mineralocorticoid administration (Nachman and Ashe, 1974; Schulkin et al., 1989; Galaverna et al., 1992; Johnson et al., 1999), but it only receives a minor direct projection from the HSD2 neurons. Injections of the neural tracer cholera toxin β subunit (CTb) into the CeA only produced retrograde labeling in approximately 1—4 HSD2 neurons per case (Geerling and Loewy, 2006). Although this direct projection is relatively sparse, the HSD2 neurons could exert a more potent influence through another efferent target, the external lateral parabrachial nucleus (PBel, Geerling and Loewy, 2006), which heavily innervates the CeA (Bernard et al., 1993). This was possibility supported in our cases with CTb injections involving PBel, as described below.
This series of CTb experiments was originally designed to identify sites that are innervated by the HSD2 neurons (Geerling and Loewy, 2006), but some tracer injections involving the CeA also produced dense anterograde labeling in the NTS, as described below. In the report, we present evidence consistent with the existence of a relayed projection to the amygdala from the HSD2 neurons, and show that they represent a novel neuronal phenotype targeted by a return projection from the CeA to the NTS. As discussed below, these bidirectional connections with the HSD2 neurons may explain the role of the CeA in sodium appetite modulation.
Materials & Methods
The neural tracing experiments described below were performed in male and female Sprague Dawley albino rats (250–400g; Harlan, Indianapolis, IN). All animal procedures were approved by the Washington University School of Medicine Animal Care Committee and conformed to NIH guidelines. All procedures were performed under sodium pentobarbital (50 mg/kg, i.p.) anesthesia. At the termination of every experiment, rats were killed by perfusion through the aorta with 200 mL saline followed by 500 mL 4% paraformaldehyde in 0.1 sodium phosphate buffer, pH 7.4.
CTb neural tracing experiments
Iontophoretic injections of cholera toxin β-subunit (0.1% solution of salt-free CTb made in distilled water, product #104; List Biological, Campbell, CA) were performed as described previously (Geerling and Loewy, 2006), using stereotaxic coordinates derived from the Paxinos and Watson rat brain atlas (2005). Selected cases with CTb injections involving PBel were chosen from this prior study (Geerling and Loewy, 2006), based upon retrograde labeling in the HSD2 neurons. A 1-in-5 series of sections throughout the entire brain was processed for CTb immunohistochemistry and counterstained with thionin.
CTb injection sites involving the CeA (n=23) and surrounding sites were also analyzed in cases from a prior study (Geerling and Loewy, 2006). Stereotaxic coordinates for these CTb placements ranged from 1.80 to 2.12 mm caudal and 3.00 to 4.00 lateral to bregma, and between 7.25 to 7.4 mm deep to the dural surface. For every case, a 1-in-5 series of 50 μm transverse sections through the caudal medulla (containing the full extent of the distribution of HSD2 neurons in the NTS) had already been stained for HSD2 and CTb in a double-immunofluorescence protocol (Geerling and Loewy, 2006). Descriptions of anterograde and retrograde labeling in the NTS were derived from this material.
CTb injections into the HSD2 subregion of the NTS (n=11) were performed as described before, using the calamus scriptorius as a stereotaxic reference point. The HSD2 neurons were targeted, at a mid-level where they lie parallel to the ventral border of the area postrema (Geerling et al., 2006b), by positioning the pipette tip 0.5 mm rostral to calamus scriptorius, 0.2 mm lateral (right side), and 0.45 mm deep. These coordinates produced CTb injections that, in most cases, were centered within the HSD2 neuron-containing subregion of the NTS (see, for example, Figure 4B). For every case, HSD2 + CTb double-immunohistochemistry was performed on a 1-in-5 series of sections through the NTS at levels containing the HSD2 neurons (not shown), and all injection sites overlapped at least some of the HSD2 neurons. After processing for CTb immunohistochemistry throughout the brain (1-in-5 series of 50 μm transverse sections), we observed a consistent overall pattern of anterograde and retrograde labeling, comparable with prior descriptions (Ricardo and Koh, 1978; van der Kooy et al., 1984; Ter Horst et al., 1989; Herbert et al., 1990). In this study, only the CTb labeling in the CeA is presented.
Figure 4.
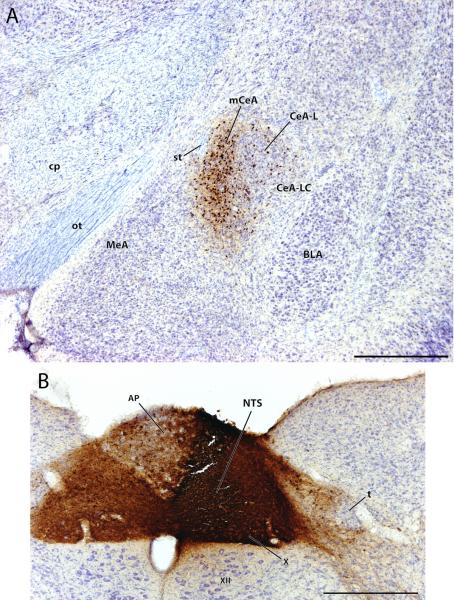
Retrograde mCeA labeling after CTb injection into the NTS (case #8148). (A) Retrogradely labeled neurons in the amygdala were primarily concentrated in mCeA, with a few additional cells scattered in CeA-L (this same pattern continued rostrally and caudally in neighboring sections through the CeA). The overall distribution of NTS-projecting CeA neurons is relatively limited. As shown in panel (B), this case contained a large CTb injection site, centered in the NTS zone that contains the HSD2 neurons (see Geerling et al., 2006b) as well as the axonal projection from the mCeA (Liubashina et al., 2000). Abbreviations: AP = area postrema, t = solitary tract, X = dorsal vagal nucleus, XII = hypoglossal nucleus. Scale bars: (A) 500 μm, (B) 500 μm.
PHAL neural tracing experiments
The anterograde axonal tracer Phaseolus vulgaris leucoagglutinin (PHAL; 2.5% solution made in 0.02 M potassium phosphate buffer, pH 8.0; Vector, Burlingame, CA) was iontophoresed into the CeA (n=10). The full protocol for PHAL iontophoresis has been described previously (Krout et al., 1998). The stereotaxic coordinates used in these PHAL cases were the same as those used for previous CTb injections that produced anterograde labeling in the HSD2 region of the NTS: 2.12 mm caudal to bregma, 1.40 mm lateral, and 7.30 mm deep. Six to seven days after the PHAL injection, rats were perfused as described above.
Histology
Brains were collected and stored in fixative for one or more days, until transverse 50 μm frozen sections were cut. Sections were stored in 0.1 M sodium phosphate buffer containing 0.1% sodium azide until immunohistochemical staining. For HSD2 immunofluorescence staining, we used an affinity-purified sheep polyclonal antibody (product #1296; Chemicon International; Temecula, CA). This antiserum was raised against a recombinant protein generated from nucleotides 385–1204 of the rat HSD2 gene (see Gomez-Sanchez et al., 2001). A dilution of 1:40,000 produces optimal signal-to-noise for staining the restricted group of cytoplasmic-immunoreactive neurons located in the NTS (the subject of the present study), as well as cells in the subcommissural organ and neurons in the ventrolateral division of the ventromedial hypothalamic nucleus (Geerling et al., 2006b), in agreement with the prior localization of HSD2 mRNA by in situ hybridization (Roland et al., 1995). At dilutions under 1:25,000, this antibody labels all neuronal nuclei, but this ubiquitous nuclear labeling was not present at more dilute concentrations. HSD2 is not found within the cell nucleus (Naray-Fejes-Toth and Fejes-Toth, 1996; Naray-Fejes-Toth and Fejes-Toth, 1998); it is tethered to the endoplasmic reticulum by a specialized N-terminal domain (Odermatt et al., 1999).
For these reasons, and because HSD2 mRNA expression is extremely scarce throughout most of the brain, resulting in total lack of detection in original localization attempts (Roland et al., 1995), we have interpreted the ubiquitous nuclear labeling at higher antibody concentrations as nonspecific cross-reactivity. Consistent with this interpretation, Western blot analysis with this antiserum on brain homogenates reveals multiple bands (C. Gomez-Sanchez, unpublished observations). See Geerling et al. (2006b) for further details regarding HSD2 immunoreactivity in the rat brain.
For CTb double-immunofluorescence in combination with HSD2, we used a rabbit polyclonal antiserum raised against purified choleragenoid (1:5000 dilution; product #B65927R; Biodesign, Saco, Maine). For injection site analysis (in all CTb-injected cases) and for whole-brain analysis of labeling (select cases), CTb single-immunohistochemistry was performed using a goat polyclonal antiserum (1:25,000 dilution; product #703; List Biologicals). CTb labeling in these cases was always found in previously described anterograde and retrograde distributions. Neither of these antisera produced any labeling in brain tissue from rats that were not injected with CTb.
The PHAL antiserum used for the double-immunofluorescence staining (in combination with HSD2, see below) was raised in rabbit (1:7,500 dilution; #2300, Vector, Burlingame, California). PHAL injection site single-immunohistochemistry in mCeA-targeted cases was performed using a polyclonal antiserum raised in goat (1:20,000 dilution; product #2224; Vector). Using both these antisera, no immunoreactivity was found in cases without PHAL injections, and PHAL-immunoreactive axons were not found outside the brain sites identified in previous rat CeA tracing studies (van der Kooy et al., 1984; Danielsen et al., 1989; Petrovich and Swanson, 1997; Liubashina et al., 2000).
Double-immunofluorescence staining for PHAL+HSD2 and CTb+HSD2 was performed using protocols described previously (Geerling et al., 2006b). A 1:5 series of nine 50 μm-thick sections through the caudal two-thirds of the NTS were incubated overnight at room temperature in mixture of both primary antibodies (dilutions given above) in a KPBS solution (0.01 M, pH 7.4) containing 5% donkey serum and 0.3% Triton X-100 (Sigma, Saint Louis, Missouri). Then, after two five-minute washes in KPBS, sections were transferred into a mixture of two fluorophore-labeled secondary antibodies: Cy2-donkey anti-sheep (always used for labeling HSD2; 1:500; Jackson ImmunoResearch, West Grove, PA) with Cy3-donkey anti-rabbit or -mouse (used for labeling rabbit anti-PHAL or -CTb; 1:250, Jackson). After three hours in secondary solution, sections were washed in KPBS and mounted onto gelatin-coated glass slides.
Single-immunohistochemical staining (PHAL and CTb injection sites, and whole-brain CTb labeling in NTS-injected cases) was performed via the ABC-DAB method. In some cases, sections were then counterstained with thionin. These histochemical techniques have been described in detail previously (Geerling et al., 2006b).
Photomicrographs and data presentation
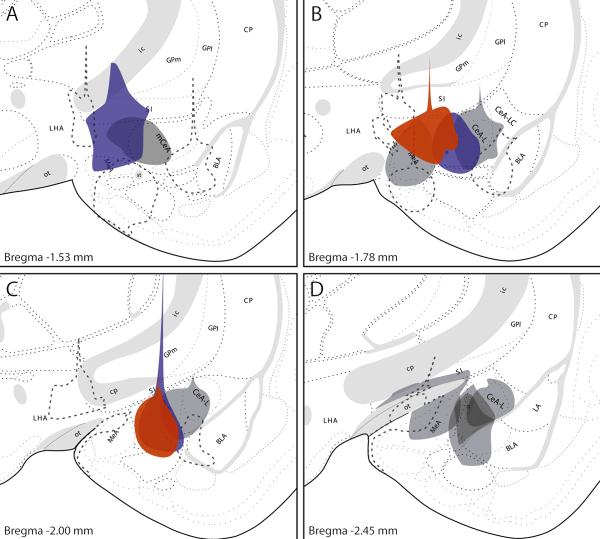
Photomicrographs were captured with a CCD camera using AnalySis software (Soft Imaging Systems, Lakewood, CO). Image cropping, resizing, and adjustments in brightness, contrast, and color balance were made using Adobe Photoshop CS. The injection site drawings shown in Figure 3 (selected examples of CTb injections in and around the amygdala) of were produced by individually aligning photomicrographs of each CTb injection site with standard brain atlas drawings (Swanson, 1998) in Adobe Illustrator.
Figure 3.
Selected examples of CTb injection sites in and around the CeA at multiple levels through the amygdala. Injection sites shown in red represent cases containing anterograde labeling in the NTS that selectively envelops the HSD2 neurons throughout their rostrocaudal distribution. Those in blue produced moderate labeling covering a portion of the HSD2 subregion of the NTS, while gray injection sites represent cases with little or no terminal labeling in the medial NTS; these injections, particularly those located laterally and caudally, labeled axons coursing through the ventrolateral NTS toward the ventrolateral medulla. Most of these three types of injections also produced light retrograde labeling in the NTS, including 1—4 HSD2 neurons. Injection sites that did not involve the CeA (and did not produce anterograde labeling in the medulla) are shown as dashed outlines. Brain atlas drawings were adapted from the Swanson brain atlas (1998). Additional abbreviations: CP = caudate-putamen (striatum), GPl = lateral globus pallidus, GPm = medial globus pallidus, ic = internal capsule, LHA = lateral hypothalamic area, SI = substantia innominata.
Results
Anterograde labeling in lateral CeA after CTb injections into PBel
Cases with small injections of CTb into PBel were examined to determine which part of the CeA is innervated by PB neurons within injection sites that had produced retrograde labeling in HSD2 neurons. In these cases, dense anterograde labeling was found exclusively in the lateral subdivision of CeA (CeA-L). Figure 1 shows the highly restricted anterograde labeling found in case #6075, the case with the largest PBel-restricted injection site and highest percentage of retrogradely-labeled HSD2 neurons (26% in the ipsilateral NTS, see Geerling and Loewy, 2006).
Figure 1.
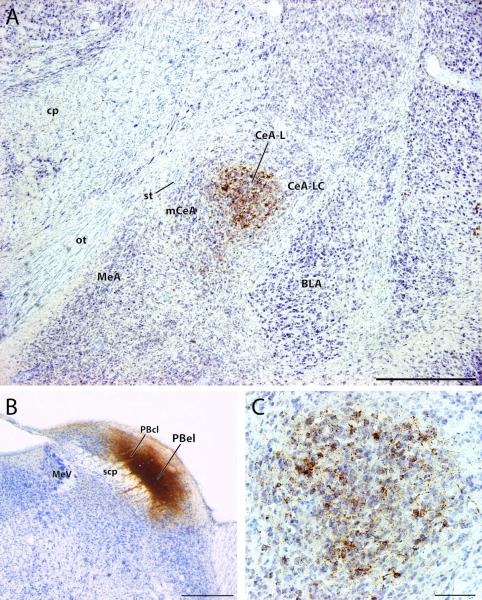
Anterograde labeling in the lateral central nucleus of the amygdala (CeA-L) after a CTb injection involving the external lateral parabrachial subnucleus (PBel). (A) CTb-labeled fibers and terminals are primarily concentrated in CeA-L, with sparse innervation of the medial or lateral capsular CeA subdivisions (mCeA, CeA-LC). Retrogradely labeled cells are present, but much of the labeling represents dense clusters of axon terminals surrounding Nissl-stained cells within CeA-L (see panel C). (B) The CTb injection site in this case (#6075) was centered in the inner subdivision of the external lateral parabrachial nucleus (PBel), but also involved a lateral portion of the central lateral PB (PBcl) and the outer subdivision of PBel. In addition to the CeA-L anterograde labeling shown in panels (A) and (C), this injection produced retrograde labeling in many HSD2 neurons in the NTS (Geerling and Loewy, 2006). (C) High-magnification view of the CeA-L CTb terminal labeling shown in panel (A). Note the numerous examples of boutons forming dense groups of perisomatic contacts, as shown previously (Takeuchi et al., 2004). Other abbreviations: BLA = basolateral nucleus of the amygdala, cp = cerebral peduncle, MeA = medial nucleus of the amygdala, MeV = mesencephalic nucleus of the trigeminal nerve, ot = optic tract, scp = superior cerebellar peduncle, st = stria terminalis. Scale bars: (A) 500 μm, (B) 500 μm, (C) 100 μm.
As shown in Figure 1A, this anterograde labeling was primarily concentrated within CeA-L (including very little territory in the neighboring lateral capsular region), with only minor involvement of mCeA. Retrogradely labeled cells were also observed, complementing previous anterograde tracing data demonstrating the axonal projection from CeA-L to the lateral PB (Petrovich and Swanson, 1997). Axons from PB, shown at higher magnification in Figure 1C, formed numerous terminals within CeA-L, densely surrounding many Nissl-stained cells. Similar to previous descriptions (Takeuchi et al., 2004), these putative perisomatic contacts between PB axons and CeA-L cells were so dense that, at lower magnifications, it was difficult to distinguish them from retrogradely labeled neurons.
Anterograde labeling in the NTS after CTb injections in mCeA
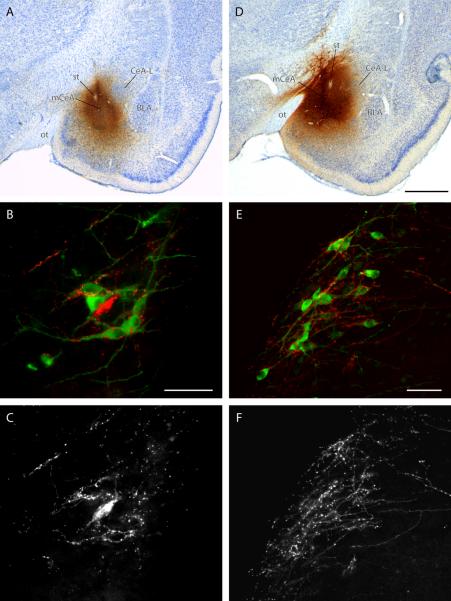
CTb injections involving the CeA (n=23) only produced light retrograde labeling in the NTS; some of these cases (n=11) contained a small number of retrogradely labeled HSD2 neurons (1–4 per case, Geerling and Loewy, 2006). In cases with injection sites overlapping the medial CeA (mCeA, n=14), however, anterograde labeling in the NTS was substantially more prominent. In these cases, many of the HSD2 neurons were surrounded by CTb-filled varicose axons. An example of this prominent anterograde labeling is depicted Figure 2 A–C (case #6474).
Figure 2.
Direct projection from mCeA to the HSD2 neurons. (A) This CTb injection site (#6474) was centered in mCeA and resulted in light retrograde labeling in the NTS (Geerling and Loewy, 2006), but also produced dense anterograde labeling enveloping the HSD2 neurons. (B) The HSD2 neurons (green) were enveloped in a dense, proximal meshwork of CTb-ir varicose axons (red). One retrogradely labeled NTS neuron is present. This photomicrograph was taken from a caudal level of the NTS, ventral to the area postrema. In panel (C), CTb-immunoreactivity is shown in isolation, highlighting the dense proximal contacts enveloping the HSD2 neurons. (D) This PHAL injection site (case #6605) is similar to the CTb injection site shown in panel (A). Most of the PHAL-filled neurons within this injection site are located in mCeA, with some additional involvement of the medial nucleus of the amygdala (MeA; note that CTb and PHAL injections involving only the MeA did not produce anterograde labeling in the NTS). (E) In this case, anterograde PHAL-ir axons (red) covered the cell bodies and proximal dendrites of many HSD2 neurons (green). This photomicrograph shows HSD2 neurons lying adjacent to the caudal fourth ventricle, along the medial edge of the NTS just rostral to the area postrema. Panel (F) highlights the PHAL-immunoreactive fibers from panel (E). Note the apparent selectivity of labeled axons for HSD2-ir neurons and dendrites. Scale bars: (B) 50 μm (also applies to panel C), (D) 1 mm (also applies to panel A), (E) 50 μm (also applies to panel F).
In all these cases, CTb-labeled varicosities within the medial NTS selectively enveloped the HSD2 neurons. In cases with CTb injections centered largely within mCeA (n=3, see examples in Figure 3), many HSD2-immunoreactive cell bodies and large proximal dendrites were covered with CTb-labeled axons and boutons throughout their rostrocaudal distribution.
At the same brainstem levels, in most cases with this pattern of labeling, an additional group of CTb-labeled axons coursed through the ventrolateral NTS towards the ventral medulla (n=10). In cases with CTb injections centered further laterally or caudally within the CeA (only partially involving mCeA, see examples in Figure 3), anterograde labeling was found primarily within these regions, with little or no labeling near the HSD2 neurons. CTb injections contained entirely within neighboring sites did not produce any anterograde labeling in NTS. These sites included the medial amygdala (MeA, n=3), basolateral amygdala (n=4), and substantia innominata-lateral hypothalamic area (n=7).
These findings did not rule out the possibility that some of the axonal labeling we found in the NTS resulted from uptake of CTb by axon terminals from neurons afferent to the CeA, followed by transport to the NTS via collateral branches. For this reason, these tracing experiments were repeated using the anterograde neural tracer PHAL (Gerfen and Sawchenko, 1984).
Anterograde labeling in the NTS after PHAL injections in mCeA
A series of PHAL injections into the amygdala (n=10) was used to verify that the anterograde axonal labeling observed in the CTb cases described above had originated from neurons within mCeA itself, and not from extra-CeA neurons with branched axons projecting to the CeA and the NTS. Six PHAL injections only filled neurons in the MeA, and, like CTb injections into this site, did not produce anterograde labeling anywhere in the medulla. Injections involving primarily the MeA and a neighboring part of the rostral mCeA (n=1), or primarily the CeA-L (n=1), produced labeling in axons that coursed through the NTS towards the ventrolateral medulla, but did not form any apparent branches, varicosities, or terminations near the HSD2 neurons or anywhere else within the NTS.
Finally, two PHAL injections primarily involved the mCeA and produced anterograde axonal labeling that surrounded many of the HSD2 neurons, in a pattern identical to that found after the mCeA injections of CTb described above. One of these cases contained moderate labeling, which was entirely restricted to the HSD2 subregion of the NTS. The other (#6605) contained denser terminal labeling in the NTS as well as labeled fibers coursing into the ventrolateral medulla. In both cases, the varicose labeled axons in the medial NTS selectively surrounded the ipsilateral HSD2 neurons at multiple rostrocaudal levels throughout their distribution. Figure 2 D–F shows these varicose PHAL-labeled axons (case #6605) enveloping HSD2 neurons lying adjacent to the fourth ventricle, just rostral to the area postrema.
Retrograde labeling in mCeA after CTb injections into the HSD2 zone of the NTS
Injections of CTb into the NTS were used to label the overall distribution of any CeA neurons that may innervate the HSD2 neurons. These injections were centered in the NTS subregion containing HSD2 neurons (n=11, see example in Figure 4B, from case #8148) and produced a consistent pattern of retrograde labeling in the CeA (Figure 4A). The overall pattern of CTb labeling in these brains was similar to that reported previously after retrograde tracer injections into the medial NTS (van der Kooy et al., 1984). As shown in Figure 4A, retrograde labeling within the amygdala was mainly concentrated within the mCeA, with only scattered labeling in a few CeA-L cells.
Discussion
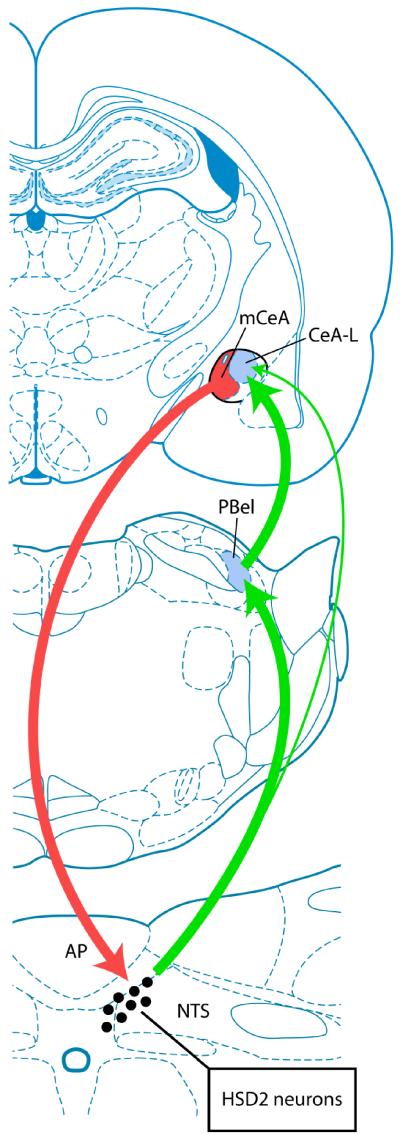
The bidirectional connections between the HSD2 neurons and the CeA may form an important functional circuit involved in the regulation of sodium appetite. The neural tracing data described in this report provide evidence for a parabrachial-relayed pathway from the HSD2 neurons to the lateral CeA, as well as a prominent descending projection from the medial CeA to the HSD2 neurons. In all likelihood, intraamygdalar connections serve to complete this circuit.
PBel as a relay between the HSD2 neurons and the lateral CeA
A major pathway from the NTS (and spinal cord), through the lateral parabrachial nucleus, to the central nucleus of the amygdala, is a key route for the delivery of interoceptive information to the forebrain (Saper and Loewy, 1980; Fulwiler and Saper, 1984; Herbert et al., 1990; Bernard et al., 1993; Saper, 1995). The anterograde neural tracing experiments of Herbert et al. (1990) clearly demonstrated that a specific part of the medial NTS innervates the inner division of PBel, bordering the superior cerebellar peduncle. We showed that the HSD2 neurons project to the inner PBel (Geerling and Loewy, 2006), which provides a major projection to the lateral CeA (Bernard et al., 1993). During the course of this analysis, we observed that PBel injections resulting in the most retrograde labeling in HSD2 neurons (20+% ipsilateral) also produced anterograde labeling within the amygdala. As shown here, this axonal labeling was restricted to the lateral subdivision of the CeA (Figure 1A).
This finding complements the patterns of PHAL anterograde axonal labeling of projections from various PB subnuclei to the amygdala reported by Bernard and colleagues (1993). The injection site from our case #6075, shown in Figure 1B, lies between the PB injection sites shown for their PHAL case #s 27 and 60 (see Figures 9 & 10 of Bernard et al., 1993), involving the central lateral and external lateral PB subnuclei, respectively. The intermediate territory covered by the CTb injection shown from our case #6075 includes a large medial part of inner PBel (and possibly a small part of outer PBel) and a lateral portion of the central lateral subnucleus. The anterograde labeling in Figure 1A shows that the projection from these PB neurons is targeted almost exclusively to CeA-L, similar to the labeling shown in case #27 of Bernard et al. (1993). Our finding that CTb injections within PBel can simultaneously produce retrograde labeling in the HSD2 neurons and anterograde labeling within CeA-L suggests that an important part of the NTS→PB→CeA pathway includes information from the HSD2 neurons, which may selectively influence the lateral CeA subdivision (Figure 5).
Figure 5.
Bidirectional connections between the HSD2 neurons and the CeA. These connections, based upon the neural tracing experiments described in this report and in a previous study (Geerling and Loewy, 2006), include: (1) An input from the HSD2 neurons, relayed through the parabrachial nucleus, selectively targeting the lateral subdivision of the CeA (CeA-L). Our neural tracing data also revealed a minor direct projection from the HSD2 neurons to the CeA (indicated by the long thin ascending arrow at right, see Geerling and Loewy, 2006) and (2) A descending projection from the medial CeA (mCeA) that directly targets the HSD2 neurons. The CeA-L contains striatal-like medium spiny neurons (McDonald, 1982), which provide direct input to the projection neurons in the adjacent mCeA (Jolkkonen and Pitkanen, 1998). Thus, the connections summarized here may form a modulatory loop from the HSD2 neurons, through the CeA, and back to the HSD2 neurons (see Figure 6). Brain drawings were adapted from the atlas of Paxinos and Watson (2005). Abbreviations: AP = area postrema.
We have also found that neurons in PBel project to the ventrolateral bed nucleus of the stria terminalis (BSTvl), a part of the extended amygdala complex that receives a major direct projection from the HSD2 neurons (see Figure 15A of Geerling and Loewy, 2006). As we suggested in this previous report, output from the HSD2 neurons may be relayed (via PBel) to the CeA-L and BSTvl in parallel. Also noted previously, CTb injections into the BSTvl and the pre-locus coeruleus (pre-LC, another major efferent target for the HSD2 neurons) both produced labeling in the CeA (Geerling and Loewy, 2006). The network formed by these interconnected sites may play a special role in processing the information delivered by the HSD2 neurons.
Direct projection from the mCeA to the HSD2 neurons
In addition to peripheral viscerosensory inputs, the NTS receives projections from a limited number of sites within the brain (van der Kooy et al., 1984). One major input arises from the CeA (Hopkins and Holstege, 1978; Price and Amaral, 1981; Schwaber et al., 1982; van der Kooy et al., 1984; Danielsen et al., 1989; Liubashina et al., 2000). Our CTb injections in some other forebrain sites (substantia innominata, BST, paraventricular and lateral hypothalamic nuclei, in CTb cases from Geerling and Loewy, 2006) produced varying degrees of light anterograde labeling in the medial NTS, but never in the dense and selective pattern that was consistently produced by injections involving the mCeA.
The localization of brainstem-projecting neurons in the mCeA (as shown in Figure 4A), and their general pattern of termination within the NTS have been described in a number of previous reports (Schwaber et al., 1982; van der Kooy et al., 1984; Danielsen et al., 1989; Liubashina et al., 2000). The distribution of anterograde labeling within the NTS in our CTb and PHAL cases was consistent with these prior descriptions. In particular, axonal labeling was primarily found within the medial NTS subregion that contains HSD2 neurons, similar to the NTS terminal distribution described by Liubashina et al. (2000). Also, projections were restricted to the NTS or the ventral medulla in a few cases, consistent with previous retrograde tracing evidence for separate projections arising from largely separate and partially non-overlapping populations of neurons within the CeA (Thompson and Cassell, 1989).
The data presented here reveal that the HSD2 neurons are a major neuronal phenotype targeted by the mCeA projection to the medial NTS. Evidence from two different anterograde neural tracing techniques showed that the HSD2 neurons are selectively targeted by neurons in the mCeA.
Varicose axons from the mCeA were prominently apposed to the cell bodies and large, primary dendrites of the HSD2 neurons, as shown in Figure 2B. The proximal location of these contacts, many of which formed basket-like perisomatic meshes, suggests that they may form inhibitory-type synapses. Their morphology is consistent with existing evidence that the projection from the CeA to the NTS is predominantly inhibitory (>90%, Jia et al., 1997; Saha et al., 2000). Neurons in the mCeA express the GABA-synthetic enzyme glutamic acid decarboxylase (Pitkanen and Amaral, 1994). Their axon terminals in the NTS form predominantly symmetric, inhibitory-type synapses (Pickel et al., 1996), and contain both GABA and its vesicular transporter (Saha et al., 2000).
This GABAergic projection to the NTS could allow mCeA neurons to potently modulate the activity of the HSD2 neurons. This connection may play a functionally significant role in the circuit between the CeA and the NTS (Figure 5), and may explain the important modulatory role of the CeA in sodium appetite (see below).
Bidirectional circuitry between HSD2 neurons and the CeA: parallels withstriatal-pallidal looped architecture through the basal ganglia
As described above, the HSD2 neurons, in parallel with other ascending projections from the brainstem and spinal cord, may exert a prominent influence over the activity of neurons within the lateral CeA. The lateral CeA contains GABAergic medium spiny neurons (similar to medium spiny neurons in the caudate-putamen, McDonald, 1982), which innervate the GABAergic projection neurons of the mCeA (Jolkkonen and Pitkanen, 1998; Cassell et al., 1999; Alheid, 2003). These GABAergic projection neurons in the mCeA innervate a variety of targets (Hopkins and Holstege, 1978; Price and Amaral, 1981), including the HSD2 neurons in the NTS.
The pathway through the CeA-L and the mCeA bears many similarities to the well-known striatal-pallidal pathways at the heart of the well-known cortical “loops” through the basal ganglia (Cassell et al., 1999; Alheid, 2003). Striatal-pallidal modulatory loops have been demonstrated for other noncortical sites such as the superior colliculus (McHaffie et al., 2005). It is possible that the lateral and medial subdivisions of the CeA perform a striatal-pallidal-like function, through which they modulate the activity of subcortical functional groups like the HSD2 neurons in the NTS (see Figure 6). Similar models for feedback modulation by the CeA may help explain other physiological and behavioral effects involving parallel transfer of information from the NTS/AP to the CeA, such as cardiorespiratory modulation and learned taste aversions.
Figure 6.
Hypothetical model for the modulation of HSD2 neurons by a striatal-pallidal-like loop through the CeA, based upon the findings presented in this study. (A) Traditional striatal-pallidal “loop” model for interactions between the cerebral cortex and the basal ganglia. In this model, parallel cortical regions send massive projections to the striatum (caudate, putamen, nucleus accumbens). Striatal medium spiny neurons (MSNs) are GABAergic and primarily innervate neurons in the globus pallidus or ventral pallidum. Pallidal GABAergic neurons project to various target sites, including thalamic neurons that innervate the same cortical regions providing the original projections to the striatum (Middleton and Strick, 2000; McHaffie et al., 2005). (B) A similar model may help explain the functional role of reciprocal connections between the HSD2 neurons and the CeA. As suggested above, the HSD2 neurons may influence the activity of the CeA-L via a relay in PBel (in addition to their minor direct projection). The GABAergic MSNs in the CeA-L are developmentally derived from the neighboring caudate-putamen and are morphologically identical to striatal MSNs (Cassell et al., 1999; Alheid, 2003; McDonald, 2003). These CeA-L MSNs directly innervate neurons in the mCeA (Jolkkonen and Pitkanen, 1998), which are also GABAergic and project to a variety of extra-amygdalar subcortical targets (Pitkanen and Amaral, 1994), including the HSD2 neurons in the NTS. These connections may form a modulatory loop through which the CeA can influence the activity of the HSD2 neurons. Such modulation would be subject to changes in a variety of other inputs to the amygdala, such as PB-relayed gustatory or interoceptive information (Bernard et al., 1993) and emotional/fear-related stimuli (LeDoux, 2000).
In this arrangement, inhibitory input from the mCeA could exert an important modulatory influence on the activity of the HSD2 neurons, and possibly sodium appetite. Such modulation would be shaped by changes in the activity of various direct inputs to the mCeA, and those arriving through CeA-L (including a relayed projection from the HSD2 neurons). In this respect, it is interesting to note that gustatory information arrives via a projection from medial PB directly to the mCeA, not CeA-L (Bernard et al., 1993). This information, positioned to provide the amygdala with information about taste detection of environmental salt, could play a key role in modulating the motivated ingestive behavior that occurs once salt is tasted by an animal with an increased need for sodium (Geerling and Loewy, 2006). Also, given the demonstrated role of the CeA in fear conditioning (LeDoux, 2000), the HSD2 neurons (and salt appetite) may be modulated in response to emotional/fear-related stimuli.
CeA modulates sodium appetite
The amygdala has been implicated in the modulation of salt ingestive behavior. Early studies showed that electrical stimulation and electrolytic lesions in different subregions of the amygdala could increase or decrease saline ingestion (Gentil et al., 1968; Gentil et al., 1971). These findings led subsequent investigators to test whether a rat without an amygdala would still increase its salt intake in response to sodium appetite stimuli such as hypovolemia or the natriorexic hormone aldosterone.
Total destruction of the amygdala (massive bilateral electrolytic lesions extending rostrocaudally from the level of the optic chiasm to the hippocampus, and laterally from the optic tract out to the external capsule) depressed baseline fluid intakes of both water and saline and reduced the increase in saline ingestion that occurs in response to sodium depletion and mineralocorticoid administration (Cox et al., 1978). A reduction in sodium appetite was also produced by large injections of colchicine into the amygdala, and the effect reversed after recovery from this pharmacological inactivation (Zolovick et al., 1980).
Importantly, the amygdala is not necessary for sodium appetite. In the experiments described above, and in later studies with more restricted lesions, rats continued to drink a significantly increased volume of saline (albeit less than unlesioned controls) in response to sodium depletion (Cox et al., 1978; Zolovick et al., 1980). Thus, the basic input and output sites responsible for sodium appetite lie outside the amygdala.
Later studies reproduced these basic findings with more restricted electrolytic and excitotoxin lesions in and around the CeA (Nitabach et al., 1989; Schulkin et al., 1989; Galaverna et al., 1992; Zhang et al., 1993; Zardetto-Smith et al., 1994). One interesting observation of these studies was that more restricted lesions could reduce or eliminate mineralocorticoid-stimulated sodium appetite without necessarily affecting salt intake in response to an actual sodium deficiency (Nitabach et al., 1989; Schulkin et al., 1989; Galaverna et al., 1992; Zhang et al., 1993). Lesions located medially, involving the MeA and mCeA, eliminated sodium appetite in response to mineralocorticoid administration (Nitabach et al., 1989; Schulkin et al., 1989; Galaverna et al., 1992). Lesions involving more of the lateral CeA still eliminated mineralocorticoid-induced sodium appetite, but also reduced the amount of sodium ingested in response to actual sodium depletion (Galaverna et al., 1992; Zardetto-Smith et al., 1994).
It is impossible to determine the boundaries of the critical amygdalar subnuclei (i.e. MeA vs. CeA) from these studies, which involved a variety of electrolytic lesions without any systematic neuroanatomical cell counts to demonstrate the extent of damage to individual subnuclei. The low-magnification histological examples presented for selected cases show gross damage that usually spanned multiple cytoarchitectonically-defined amygdalar nuclei, such as both MeA and mCeA (see, for example, Figure 1 of Schulkin et al., 1989).
The data presented here demonstrate a bidirectional linkage between the CeA and the aldosterone-sensitive HSD2 neurons. Combined with prior neuroanatomical data showing that the CeA receives relayed gustatory and gastrointestinal inputs (Bernard et al., 1993), our findings suggest that the critical neurons affected in behavioral studies involving lesions of the “medial” amygdala are located within the CeA. Also, the CeA, and not the MeA, projects to the ventrolateral bed nucleus of the stria terminalis (BSTvl), another part of the extended amygdala innervated by the HSD2 neurons (Geerling and Loewy, 2006) and implicated in the regulation of sodium appetite (Reilly et al., 1994; Zardetto-Smith et al., 1994).
Any physiological role of the CeA in mineralocorticoid-stimulated sodium appetite may depend upon its connections with the intrinsically aldosterone-sensitive HSD2 neurons. The distribution of these neurons overlaps a region of the NTS, just ventral to the area postrema, that lacks a complete blood-brain barrier (BBB, Gross et al., 1990). This location may allow the HSD2 neurons increased exposure to blood-borne aldosterone, which only weakly penetrates the BBB, particularly in comparison to the glucocorticoid corticosterone (Pardridge and Mietus, 1979; Funder and Myles, 1996), with which it must compete for mineralocorticoid receptors (MRs) in cells without HSD2.
The amygdala is protected by a functional BBB and lacks detectable levels of HSD2 immunoreactivity (Geerling et al., 2006b), so it is unlikely to be a direct site of action for aldosterone. Weak mRNA signal for HSD2 in the amygdala was reported in one in situ hybridization study (Robson et al., 1998), but could not be detected in another (Roland et al., 1995). A lack (or extremely low level) of HSD2 expression by neurons in the amygdala is consistent with the ability of glucocorticoids to out-compete aldosterone for MR binding sites here (McEwen et al., 1986), in contrast to the aldosterone-selective cells in the kidney (Funder et al., 1988) and the NTS (Geerling et al., 2006a).
Nonetheless, the reduction of MR expression by injection of antisense oligodeoxynucleotides directly into the amygdala has been reported to reduce the amount of saline that rats ingest in response to treatment with the mineralocorticoid deoxycorticosterone (Sakai et al., 1996; Sakai et al., 2000). Unlike aldosterone, deoxycorticosterone readily crosses the BBB (Kraulis et al., 1975), so it is possible that it activates MR directly within forebrain sites like the amygdala, in addition to aldosterone-like MR activation in the HSD2 neurons in the NTS. Whether or not MR activation directly within the amygdala plays a role, the HSD2 neurons in the NTS represent the only identified cells in the brain with high levels of both HSD2 and MR expression and demonstrated aldosterone-sensitivity and -selectivity (Geerling et al., 2006a; Geerling et al., 2006b).
Conclusion
A circuit between the HSD2 neurons and the CeA provides a neuroanatomical basis for the modulation of sodium appetite by the amygdala. The importance of the CeA for increasing salt intake in response to mineralocorticoid administration may be due, at least in part, to an indirect input from the HSD2 neurons, which may provide information about the need for sodium. The descending projection from the mCeA to the HSD2 neurons may play a major role in modulating their activity. This direct modulation of the HSD2 neurons may help explain CeA modulation of sodium appetite.
Acknowledgements
Special thanks to Xay Van Nguyen for his expert surgical and histological assistance.
Grant Support: National Institute of Heart, Lung, and Blood of the NIH, Grant number HL-25449 American Heart Association, Predoctoral Fellowship award (Joel Geerling) # 0510050Z
LITERATURE CITED
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Cox JR, Cruz CE, Ruger J. Effect of total amygdalectomy upon regulation of salt intake in rats. Brain Res Bull. 1978;3:431–435. doi: 10.1016/0361-9230(78)90071-0. [DOI] [PubMed] [Google Scholar]
- Danielsen EH, Magnuson DJ, Gray TS. The central amygdaloid nucleus innervation of the dorsal vagal complex in rat: a Phaseolus vulgaris leucoagglutinin lectin anterograde tracing study. Brain Res Bull. 1989;22:705–715. doi: 10.1016/0361-9230(89)90090-7. [DOI] [PubMed] [Google Scholar]
- Flynn FW, Grill HJ, Schulkin J, Norgren R. Central gustatory lesions: II. Effects on sodium appetite, taste aversion learning, and feeding behaviors. Behav Neurosci. 1991;105:944–954. doi: 10.1037//0735-7044.105.6.944. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. 1984;319:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Funder J, Myles K. Exclusion of corticosterone from epithelial mineralocorticoid receptors is insufficient for selectivity of aldosterone action: in vivo binding studies. Endocrinology. 1996;137:5264–5268. doi: 10.1210/endo.137.12.8940344. [DOI] [PubMed] [Google Scholar]
- Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242:583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- Galaverna O, De Luca LA, Jr., Schulkin J, Yao SZ, Epstein AN. Deficits in NaCl ingestion after damage to the central nucleus of the amygdala in the rat. Brain Res Bull. 1992;28:89–98. doi: 10.1016/0361-9230(92)90234-o. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Engeland WC, Kawata M, Loewy AD. Aldosterone target neurons in the nucleus tractus solitarius drive sodium appetite. J Neurosci. 2006a;26:411–417. doi: 10.1523/JNEUROSCI.3115-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Kawata M, Loewy AD. Aldosterone-sensitive neurons in the rat central nervous system. J Comp Neurol. 2006b;494:515–527. doi: 10.1002/cne.20808. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Aldosterone-sensitive neurons in the nucleus of the solitary tract: efferent projections. J Comp Neurol. 2006 doi: 10.1002/cne.20993. in press. [DOI] [PubMed] [Google Scholar]
- Gentil CG, Antunew-Rodrigues J, Negro-Vilar A, Covian MR. Role of amygdaloid complex in sodium chloride and water intake in the rat. Physiology and Behavior. 1968;3:981–985. [Google Scholar]
- Gentil CG, Mogenson GJ, Stevenson JA. Electrical stimulation of septum, hypothalamus, and amygdala and saline preference. Am J Physiol. 1971;220:1172–1177. doi: 10.1152/ajplegacy.1971.220.5.1172. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Sawchenko PE. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L) Brain Res. 1984;290:219–238. doi: 10.1016/0006-8993(84)90940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Ganjam V, Chen YJ, Liu Y, Clark SA, Gomez-Sanchez CE. The 11beta hydroxysteroid dehydrogenase 2 exists as an inactive dimer. Steroids. 2001;66:845–848. doi: 10.1016/s0039-128x(01)00119-2. [DOI] [PubMed] [Google Scholar]
- Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol. 1990;259:R1131–1138. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- Jia HG, Rao ZR, Shi JW. Evidence of gamma-aminobutyric acidergic control over the catecholaminergic projection from the medulla oblongata to the central nucleus of the amygdala. J Comp Neurol. 1997;381:262–281. doi: 10.1002/(sici)1096-9861(19970512)381:3<262::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Johnson AK, de Olmos J, Pastuskovas CV, Zardetto-Smith AM, Vivas L. The extended amygdala and salt appetite. Ann N Y Acad Sci. 1999;877:258–280. doi: 10.1111/j.1749-6632.1999.tb09272.x. [DOI] [PubMed] [Google Scholar]
- Jolkkonen E, Pitkanen A. Intrinsic connections of the rat amygdaloid complex: projections originating in the central nucleus. J Comp Neurol. 1998;395:53–72. doi: 10.1002/(sici)1096-9861(19980525)395:1<53::aid-cne5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kotelevtsev Y, Brown RW, Fleming S, Kenyon C, Edwards CR, Seckl JR, Mullins JJ. Hypertension in mice lacking 11beta-hydroxysteroid dehydrogenase type 2. J Clin Invest. 1999;103:683–689. doi: 10.1172/JCI4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraulis I, Foldes G, Traikov H, Dubrovsky B, Birmingham Distribution, metabolism and biological activity of deoxycorticosterone in the central nervous system. Brain Res. 1975;88:1–14. doi: 10.1016/0006-8993(75)90942-7. [DOI] [PubMed] [Google Scholar]
- Krout KE, Jansen AS, Loewy AD. Periaqueductal gray matter projection to the parabrachial nucleus in rat. J Comp Neurol. 1998;401:437–454. [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Liubashina O, Jolkkonen E, Pitkanen A. Projections from the central nucleus of the amygdala to the gastric related area of the dorsal vagal complex: a Phaseolus vulgaris-leucoagglutinin study in rat. Neurosci Lett. 2000;291:85–88. doi: 10.1016/s0304-3940(00)01392-6. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol. 1982;208:401–418. doi: 10.1002/cne.902080409. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Is there an amygdala and how far does it extend? An anatomical perspective. Ann N Y Acad Sci. 2003;985:1–21. doi: 10.1111/j.1749-6632.2003.tb07067.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Lambdin LT, Rainbow TC, De Nicola AF. Aldosterone effects on salt appetite in adrenalectomized rats. Neuroendocrinology. 1986;43:38–43. doi: 10.1159/000124506. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stanford TR, Stein BE, Coizet V, Redgrave P. Subcortical loops through the basal ganglia. Trends Neurosci. 2005;28:401–407. doi: 10.1016/j.tins.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Nachman M, Ashe JH. Effects of basolateral amygdala lesions on neophobia, learned taste aversions, and sodium appetite in rats. J Comp Physiol Psychol. 1974;87:622–643. doi: 10.1037/h0036973. [DOI] [PubMed] [Google Scholar]
- Naray-Fejes-Toth A, Colombowala IK, Fejes-Toth G. The role of 11beta- hydroxysteroid dehydrogenase in steroid hormone specificity. J Steroid Biochem Mol Biol. 1998;65:311–316. doi: 10.1016/s0960-0760(98)00009-0. [DOI] [PubMed] [Google Scholar]
- Naray-Fejes-Toth A, Fejes-Toth G. Subcellular localization of the type 2 11beta- hydroxysteroid dehydrogenase. A green fluorescent protein study. J Biol Chem. 1996;271:15436–15442. doi: 10.1074/jbc.271.26.15436. [DOI] [PubMed] [Google Scholar]
- Naray-Fejes-Toth A, Fejes-Toth G. Extranuclear localization of endogenous 11beta-hydroxysteroid dehydrogenase-2 in aldosterone target cells. Endocrinology. 1998;139:2955–2959. doi: 10.1210/endo.139.6.6036. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Schulkin J, Epstein AN. The medial amygdala is part of a mineralocorticoid-sensitive circuit controlling NaCl intake in the rat. Behav Brain Res. 1989;35:127–134. doi: 10.1016/s0166-4328(89)80113-5. [DOI] [PubMed] [Google Scholar]
- Odermatt A, Arnold P, Stauffer A, Frey BM, Frey FJ. The N-terminal anchor sequences of 11beta-hydroxysteroid dehydrogenases determine their orientation in the endoplasmic reticulum membrane. J Biol Chem. 1999;274:28762–28770. doi: 10.1074/jbc.274.40.28762. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Mietus LJ. Transport of steroid hormones through the rat blood- brain barrier. Primary role of albumin-bound hormone. J Clin Invest. 1979;64:145–154. doi: 10.1172/JCI109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier; Burlington: 2005. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res. 1997;763:247–254. doi: 10.1016/s0006-8993(96)01361-3. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Van Bockstaele EJ, Chan J, Cestari DM. GABAergic neurons in rat nuclei of solitary tracts receive inhibitory-type synapses from amygdaloid efferents lacking detectable GABA-immunoreactivity. J Neurosci Res. 1996;44:446–458. doi: 10.1002/(SICI)1097-4547(19960601)44:5<446::AID-JNR5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Amaral DG. The distribution of GABAergic cells, fibers, and terminals in the monkey amygdaloid complex: an immunohistochemical and in situ hybridization study. J Neurosci. 1994;14:2200–2224. doi: 10.1523/JNEUROSCI.14-04-02200.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JJ, Maki R, Nardozzi J, Schulkin J. The effects of lesions of the bed nucleus of the stria terminalis on sodium appetite. Acta Neurobiol Exp (Wars) 1994;54:253–257. [PubMed] [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Richter CP. Increased salt appetite in adrenalectomized rats. Am J Physiol. 1936;115:155–161. [Google Scholar]
- Robson AC, Leckie CM, Seckl JR, Holmes MC. 11 Beta-hydroxysteroid dehydrogenase type 2 in the postnatal and adult rat brain. Brain Res Mol Brain Res. 1998;61:1–10. doi: 10.1016/s0169-328x(98)00161-2. [DOI] [PubMed] [Google Scholar]
- Roland BL, Li KX, Funder JW. Hybridization histochemical localization of 11 beta-hydroxysteroid dehydrogenase type 2 in rat brain. Endocrinology. 1995;136:4697–4700. doi: 10.1210/endo.136.10.7664691. [DOI] [PubMed] [Google Scholar]
- Saha S, Batten TF, Henderson Z. A GABAergic projection from the central nucleus of the amygdala to the nucleus of the solitary tract: a combined anterograde tracing and electron microscopic immunohistochemical study. Neuroscience. 2000;99:613–626. doi: 10.1016/s0306-4522(00)00240-2. [DOI] [PubMed] [Google Scholar]
- Sakai RR, Ma LY, Zhang DM, McEwen BS, Fluharty SJ. Intracerebral administration of mineralocorticoid receptor antisense oligonucleotides attenuate adrenal steroid-induced salt appetite in rats. Neuroendocrinology. 1996;64:425–429. doi: 10.1159/000127148. [DOI] [PubMed] [Google Scholar]
- Sakai RR, McEwen BS, Fluharty SJ, Ma LY. The amygdala: site of genomic and nongenomic arousal of aldosterone-induced sodium intake. Kidney Int. 2000;57:1337–1345. doi: 10.1046/j.1523-1755.2000.00972.x. [DOI] [PubMed] [Google Scholar]
- Saper CB. The spinoparabrachial pathway: shedding new light on an old path. J Comp Neurol. 1995;353:477–479. doi: 10.1002/cne.903530402. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Scalera G, Spector AC, Norgren R. Excitotoxic lesions of the parabrachial nuclei prevent conditioned taste aversions and sodium appetite in rats. Behav Neurosci. 1995;109:997–1008. [PubMed] [Google Scholar]
- Schulkin J, Marini J, Epstein AN. A role for the medial region of the amygdala in mineralocorticoid-induced salt hunger. Behav Neurosci. 1989;103:179–185. doi: 10.1037/0735-7044.103.1.178. [DOI] [PubMed] [Google Scholar]
- Schwaber JS, Kapp BS, Higgins GA, Rapp PR. Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. J Neurosci. 1982;2:1424–1438. doi: 10.1523/JNEUROSCI.02-10-01424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PM, Corrie JE, Shackleton CH, Edwards CR. Syndrome of apparent mineralocorticoid excess. A defect in the cortisol-cortisone shuttle. J Clin Invest. 1988;82:340–349. doi: 10.1172/JCI113592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Elsevier; Amsterdam: 1998. [Google Scholar]
- Takeuchi Y, Xie Q, Miki T, Matsumoto Y, Satriotomo I, Li HP, Gu H. Parabrachial inputs to Fos-immunoreactive neurons in the lateral central nucleus of amygdala activated by hypotension: a light and electron microscopic study in the rat. Brain Res Bull. 2004;64:171–180. doi: 10.1016/j.brainresbull.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ, de Boer P, Luiten PG, van Willigen JD. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience. 1989;31:785–797. doi: 10.1016/0306-4522(89)90441-7. [DOI] [PubMed] [Google Scholar]
- Thompson RL, Cassell MD. Differential distribution and non-collateralization of central amygdaloid neurons projecting to different medullary regions. Neurosci Lett. 1989;97:245–251. doi: 10.1016/0304-3940(89)90605-8. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- Wolf G, Handal PJ. Aldosterone-induced sodium appetite: dose-response and specificity. Endocrinology. 1966;78:1120–1124. doi: 10.1210/endo-78-6-1120. [DOI] [PubMed] [Google Scholar]
- Zardetto-Smith AM, Beltz TG, Johnson AK. Role of the central nucleus of the amygdala and bed nucleus of the stria terminalis in experimentally-induced salt appetite. Brain Res. 1994;645:123–134. doi: 10.1016/0006-8993(94)91645-4. [DOI] [PubMed] [Google Scholar]
- Zhang DM, Epstein AN, Schulkin J. Medial region of the amygdala: involvement in adrenal-steroid-induced salt appetite. Brain Res. 1993;600:20–26. doi: 10.1016/0006-8993(93)90396-5. [DOI] [PubMed] [Google Scholar]
- Zolovick AJ, Avrith D, Jalowiec JE. Reversible colchicine-induced disruption of amygdaloid function in sodium appetite. Brain Res Bull. 1980;5:35–39. doi: 10.1016/0361-9230(80)90280-4. [DOI] [PubMed] [Google Scholar]