Abstract
Purpose of review
Drinking and eating are essential skills for survival and benefit from the coordination of several pattern generating networks and their musculoskeletal effectors to achieve safe swallows. Oral-pharyngo-esophageal motility develops during infancy and early childhood, and is influenced by various factors, including neuromuscular maturation, dietary and postural habits, arousal state, ongoing illnesses, congenital anomalies, and the effects of medical or surgical interventions. Gastroesophageal reflux is frequent in neonates and infants, and its role in neonatal morbidity including dysphagia, chronic lung disease, or apparent life-threatening events is not well understood. This review highlights recent studies aimed at understanding the development of oral feeding skills, and cross-system interactions among the brainstem, spinal, and cerebral networks involved in feeding.
Recent Findings
Functional linkages between suck-swallow and swallow-respiration manifest transitional forms during late gestation through the first year of life which can be delayed or modified by sensory experience and/or disease processes. Relevant central pattern generator (CPG) networks and their neuromuscular targets attain functional status at different rates, which ultimately influences cross-system CPG interactions. Entrainment of trigeminal primary afferents accelerates pattern genesis for the suck CPG and transition-to-oral feed in the RDS preterm infant.
Summary
The genesis of within-system CPG control for rate and amplitude scaling matures differentially for suck, mastication, swallow, and respiration. Cross-system interactions among these CPGs represent targets of opportunity for new interventions which optimize experience-dependent mechanisms to promote safe swallows among newborn and pediatric patients.
Keywords: Non-nutritive suck, nutritive suck, swallow, respiration, central pattern generation, preterm, infant, apnea, bolus, brainstem
Introduction
In the mature organism, the processing of ingested food and liquid material occurs in a seemingly stereotyped manner. Upon closer inspection, the dynamics of feeding reveal enormous complexity and coordination among at least 26 pairs of muscles and 5 cranial nerve systems, including trigeminal, facial, glossopharyngeal, vagus, hypoglossal, and the cervical and thoracic spinal cord segments involved in chest wall movements for coordination of respiration with feeding [1,2]. The central patterning of aeroingestive behaviors include volitional and reflexive control mechanisms, and benefit from sensory feedback to modify the spatiotemporal organization of the feed sequence to ensure safe swallow [3–5]. Variation in bolus type (liquid or solid), hardness, homogeneity, volume, viscosity, texture, moisture content, and other sensory characteristics of the bolus (taste) serve to modulate the timing and patterning of motor components which constitute the overall feed sequence.
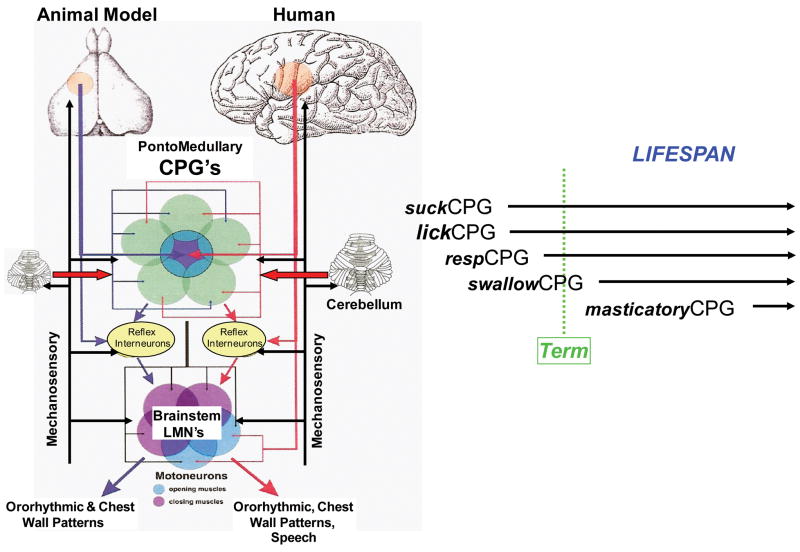
Central pattern generation for suck, lick, mastication, swallow, respiration
Central pattern generators (CPGs) are primarily composed of adaptive networks of interneurons that activate groups of motor neurons to generate task-specific motor patterns [6]. CPGs modulate cycle duration and the duration and intensity of motoneuron bursts in response to CNS and sensory inputs [7]. The development of an in vitro isolated mammalian brainstem preparation [8] was essential for the study of the perinatal rhythmical motor patterns in cranial nerves. In the resting state, rhythmical respiratory activity can be recorded from trigeminal (V), facial (VII) and hypoglossal (XII) motoneurons of neonatal rats [8,9], but a second much faster rhythm appears if the glutamate agonist N-methy-D,L-aspartate (NMDA) is added to the bath, with or without other neurotransmitters or their antagonists [8–13]. Since all neonatal mammals begin to feed on milk, it is presumed that the rhythmic non-respiratory oromotor patterns are related to suckling. Indeed, mastication is not seen in young rats before P12 [14].
The isolated sections of the brainstem of neonates containing the cranial V, VII and XII motor nuclei are each capable of generating the sucking rhythm when isolated from the others [15], as are the left and right halves of the trigeminal section [12]. These observations suggest that there could be at least six separate rhythm generators (two V, two VII, two XII) that are coupled together to coordinate suckling during late gestation and infancy. Exactly how these are coordinated, and whether they all persist into adulthood is not known. However, it is possible that the caudal pair is dominant by birth, because the V pair is tonically inhibited by the caudal brainstem [12]. As shown in Figure 1 [16], CPGs are also modulated by descending inputs from the “sucking area” of the motor cortex [17,18].
Figure 1.
Ororhythmic central pattern generators. An adaptation of a model proposed by Dr. James Lund and colleagues [16], and extended to include modulatory inputs from the peripheral afferents and the cerebellum.
The gross licking motor pattern is controlled by a brainstem CPG distributed within several subdivisions of the medullary reticular formation (RF) [19]. Descending inputs from cerebral cortex and forebrain to the lick CPG project to widely distributed targets of both the medial and lateral reticular formation. Most projections originating from brainstem orosensory nuclei terminate within the lateral reticular formation. Pre-oromotor neurons appear concentrated in the intermediate zone of the reticular formation and receive convergent inputs from the lateral and medial RF sites to control lick [2]. Sensory feedback from intraoral gustatory and somatosensory afferents modulate the motor pattern for lick, suck, mastication and swallow.
The transition from suckling to chewing occurs gradually over a period that can vary enormously between species. In rats, the first masticatory movements appear around post-natal (P) day 12, and the adult patternis established between P18–21 [14]. Human infants begin to chew after several months, and mastication continues to evolve until the secondary dentition is in place. Mastication and suckling both involve jaw opening and closing, but the power stroke for the suction phase is provided by the jaw depressors during suckling and by the elevators during mastication. Nutritive suckling also includes an expression phase that typically lags the suction phase by 100 milliseconds or more. This involves a stripping ‘peristaltic’ motion of the tongue tip along the length of the nipple or teat and requires activation of the intrinsic tongue muscles via the hypoglossal nerve [20,21]. Clearly, many motoneurons are active during all of the oromotor patterns, but it is unclear if the sucking CPG evolves to eventually control mastication, or if the mastication CPG emerges separately during the weaning period. There is evidence that some neurons located in the antero-dorsal region of the trigeminal principal sensory nucleus (NVsnpr) may form the core of the masticatory CPG in rats undergo a rapid maturation during weaning and eventually exhibit pacemaker properties [22].
The essential components of the masticatory CPG are found between the rostral poles of the Vth and VIIth motor nuclei, and each hemisection side can generate a rhythm, although they are normally synchronized by commissural axons [16, 23,24]. Many neurons among several nuclei in this region project to V motoneurons, but also to the VII and XII nuclei, and they are strongly and reciprocally interconnected [25–27]. Furthermore, many of them fire bursts during fictive mastication in phase with either the jaw closing or jaw opening motoneurons [28,29]. The majority of these ‘masticatory’ neurons are activated at very short latency from the masticatory area of sensorimotor cortex, and by sensory inputs.
Mastication patterns vary greatly between foods, and change systematically during a chewing sequence based on sensory feedback. Unlike locomotion, rhythmic orofacial movements including the basic patterns of mastication are represented in the cerebral cortex. Coordinated movements of the jaw, tongue, lips and cheeks can be evoked in anesthetized animals by repetitive electrical stimulation of a large region of the sensorimotor cortex, and other forebrain and midbrain structures. Some of these movements, which strongly resemble natural mastication, including different patterns such as incisal gnawing and left and right molar chewing are represented at specific cortical sites [30]. For example, in rabbit, small rapid vertical movements powered by the digastrics (sucking) are located rostrally; vertical mastication behind these, and patterns with strong lateral swings in the postero-lateral zone of the “masticatory” cortex [30].
Interneurons, which have been identified in the brainstem, are capable of generating a basic swallow pattern in the absence of peripheral or descending cortical inputs [31]. Swallowing is observable in the developing fetus by the 11th week of gestation which is essential for the regulation of amniotic fluid [32]. Swallowing skills develop progressively during fetal and neonatal maturation [33]. At birth, the neonate must transition from swallowing in a liquid environment to integrating swallowing with airway-protective mechanisms. McFarland and Tremblay [31] emphasized that sensory experience is crucial to optimize pattern formation and brain development during the presumed critical period for attainment of swallowing proficiency. Driving intraoral and pharyngeal sensory afferents mediated by the trigeminal and glossopharyngeal system during suck can initiate or modulate a swallow [4,34].
Functional imaging in mature systems has revealed that swallowing is controlled through a network of cortical areas that is considerably more distributed than previously thought, and this network shares loci with other ororhythmic movements including speech. For example, an extensive network of overlapping and distinct BOLD activations for tongue movements and swallowing, including SMA, anterior cingulated area, Brodmann’s areas 3 and 4, cuneus, precuneus, supramarginal gyrus, and cerebellum have been observed [35]. A subsequent meta-analysis, based on 10 published studies and a total of 121 subjects, resulted in the identification of distributed and partly overlapping cortical networks involved in the sensorimotor control of water and saliva swallowing [36]. A between-condition meta-analysis revealed clusters with higher activation likelihood for water than saliva swallowing in the right inferior parietal lobule, right postcentral gyrus, and right anterior insula. Voxel clusters with higher activation likelihood for saliva than for water swallows were found in the bilateral SMA, bilateral ACG, and bilateral precentral gyrus. Therefore, swallowing is not a simple reflex, but rather a complex coordinated sensorimotor process generated by multiple levels of neural control distributed among several physiological systems [31]. The swallowing network is adaptive and subject to modification by experience-dependent plasticity [37].
Whole-cell patch-clamp recording techniques were used to study the isolated respiratory pattern generator located in the pre-Bötzinger complex (pre-BötC) of the ventrolateral medulla in rats [38–41]. Spatially distributed populations of interneurons, premotoneurons (preMNs), and motoneurons (MNs) in the brainstem and spinal cord serve distinct functional roles in mammalian respiration [42]. Interacting populations of interneurons generate temporal features of the motor pattern including network rhythms, preMNs (defined as cells with axonal connections to MNs) function as pattern formation elements and substrates for rhythmic drive transmission, whereas MNs generate motor output. Inspiratory rhythm, generated by interneurons in the pre-BötC of the ventrolateral medulla [39,43,44], propagates through preMN transmission circuits to spinal and cranial MNs.
Drinking, eating, swallowing, and breathing are tightly coupled motor behaviors, with swallowing dominant to respiration in normal individuals [31,45,46]. Swallowing always interrupts the breathing of infants and children [45,47–49]. The cessation or interruption of respiration during swallowing is known as swallowing apnea and is duein part to the closure of the airway and neural suppression of the respiratory pattern generator in the brainstem [45]. Breathing-swallowing coordination is defined by the point in the respiratory phase cycle where swallowing apnea occurs [49]. Swallowing usually begins during the expiratory phase of breathing when drinking. In adults, approximately 75–95% of swallows are initiated during the expiratory phase [46,50] compared to just 39% in newborns [51]. The pause in respiration continues for 0.5 to 1.5 seconds to accommodate the swallow, and respiration resumes with an expiratory cycle to help prevent aspiration of residual food in the pharynx post-swallow [52,53]. Conversely, respiration can resume with an inspiratory cycle when performing sequential swallows while drinking from a cup [54]. The respiratory rhythm is also perturbed during the onset of mastication, with respiratory cycle duration decreased during mastication but increased during swallowing. The ‘exhale-swallow-exhale’ sequence is manifest during eating.
Term infants through the first year of life
Nutritive swallows of healthy term infants occur predominantly at the inspiratory-expiratory cusp, followed by mid-expiration, and occasionally at the expiratory-inspiratory transition [51,55,56]. Infants whose swallow-respiration coordination during feeding deviates from this pattern could be considered ‘disordered.’ For instance, premature infants (33 weeks post-conception), unlike full term infants, swallow predominantly during respiratory pauses [57]. Recently, the maturation of breathing-swallowing coordination was examined during feeding in ten healthy term human infants through the first year of life [48]. More than 15,000 swallows were sampled across ten assessments between 48 hours and 12 months of age. Mid-expiratory swallows represented the dominant pattern of breathing-swallowing coordination within the first 48 h (mean = 45.4%), but the prevalence of this pattern declined rapidly in the first week to 29.1%. Inspiratory-expiratory swallows increased significantly with age, particularly between 9 months (37.0%) and 12 months (50.4%). Nearly 75% of swallows were followed by expiration in the latter 6 months, which is an adult-like characteristic. Post-swallow expiration is a robust feature of breathing-swallowing coordination from birth, with two major shifts in the precise patterns occurring (1)after 1 week of postnatal feeding experience, and (2) between 6 and 12 months, attributed to neural and anatomical maturation [48].
The coordination of nutritive and non-nutritive swallowing with breathing was assessed longitudinally in 10 healthy term infants from birth to 1 year of age [49]. Swallows were classified into five respiratory-phase categories, including mid-inspiration (II), mid-expiration (EE), inspiratory-expiratory cusp (IE), expiratory-inspiratory cusp (EI), and mid-pause (P). Breathing-swallowing coordination differed markedly between the two swallowing conditions, especially between 2 weeks and 2 months. Significant condition effects were found in up to four respiratory-phase categories (II, IE, EI, and P). The condition effect was minimal from 9 months with only IE swallow proportions differing between conditions. A ‘critical period’ is implicated for the genesis and modulation of the neural response to oropharyngeal stimulation during feeding. Interruption of this developmental process may significantly impact infants with neurological and/or respiratory disorders and requires further investigation.
The impact of bolus ingestion and level of consciousness on swallowing apnea duration (SAD) was studied in 10 healthy term infants [58]. SAD during wakefulness, sleep, and feeding (breast or bottle) was measured 10 times from birth to 1 year of age. Based on 19,402 swallows, SAD during feeding was significantly shorter than SAD of non-nutritive swallowing (during wakefulness and sleep) regardless of age. SAD did not change significantly within the first year of life in any of the three conditions and there was no change in the relative durations of nutritive, wake and sleep conditions with age. The absence of an age effect suggests that the neural mechanisms controlling SAD are fundamentally brainstem-mediated and largely determined at birth in healthy term neonates.
Given the known decrease in global cerebral activity during sleep, the sleep-wake paradigm was used to elucidate suprabulbar influences [59]. Non-nutritive breathing-swallow control (BSC)of 10 healthy human infants was monitored longitudinally during wakefulness and sleep from birth to 1 year of age. Digitized recordings of submental muscle activity, nasal airflow, and thyroid acoustics enabled the categorization of swallows as a function of respiratory phase. In contrast to the change in the overall pattern of BSC with age, and despite postnatal cortical proliferation and development over this time, no arousal-related differences were observed during the first year of life. Thus, the non-nutritive BSC in infants appears to be under complete brainstem control.
Advanced swallow assessment technologies
The use of microphones permit detection of swallow sounds in adults [60] and infants [55,61,62]. Cervical auscultation (CA) utilizing an accelerometer placed over an infant’s throat was developed to include more objective, quantitative parameters that are reproducible among clinical populations to evaluate dysphagia in adults, children, and infants [63,64]. CA was performed recently with an accelerometer and microphone to describe the stability of initial discrete swallow-associated sounds (IDS) of adult and infant feeding [65]. A variance index (VI) was calculated to quantify the stability of IDS. The VI of adults swallowing liquid did not differ from that of preterm infants older than 36 weeks PMA, but was lower than the VI of preterm infants younger than 36 weeks PMA. The authors suggest that stability measurements of swallow-associated sounds may provide a biomarker for neurologic integrity.
Motor evoked potentials (MEPs) recorded from pharyngeal and anterior hyomandibular (submental) muscles at rest have been used to evaluate treatment effects on neural pathways underlying swallowing. A recent study documents a novel method for recording reliable intra-and inter-session MEPs at the submental muscle group during task-related volitional swallowing [66]. MEPs were elicited by single-pulse transcranial magnetic stimulation (TMS), triggered when a pre-set threshold of surface electromyographic activity was attained. Results indicate that surface electromyography-triggered TMS allows reliable measurement of MEP amplitude at the submental muscle group within and across sessions when muscles are pre-activated during volitional swallowing. This methodology will be useful for future investigations on the effects of pathology and modulation of the neural network for swallowing.
A promising approach to the study of pharyngoesophageal motility in infants and children involves the application of pharyngo-UES-esophageal micromanometry [3,5,67–72]. The micromanometric method has been validated and new applications developed to measure the reflexes of interest pertinent to swallowing and primary peristalsis. Recently, Jadcherla and colleagues have defined the maturational changes in enteric nervous system and quantified related motility measures in premature infants. Pharyngeal-upper esophageal sphincter-esophageal body motility characteristics were defined across maturation during infancy. Additionally, this work included a characterization of the sensory motor aspects of the vago-vagal reflex modulation during peristalsis. Pharyngeal reflexive swallowing (PRS) is more frequent than pharyngo-UES-contractile reflex(PUCR). Each reflex type manifests distinctive characteristics in air and water stimuli, and both PRS and PUCR have implications for the evaluation of swallowing in infants [72].
Entrainment of oromotor CPGs to promote the transition to oral feed
Sensorimotor entrainment as a habilitation strategy(i.e., utilizing natural mechanical stimulation, or other modality such as olfactory, vestibular, acoustic), has ecological validity in assisting the infant to produce functional ororhythmic behavior and enhance the transition to oral feeding. This approach is consistent with contemporary ideas on the role of sensory-driven neural activity and critical periods [73,74] during late gestation and early infancy in the formation of functional ororhythmic and deglutition networks. Use of a mechanical entrainment stimulus also has the distinct advantage of being safe, pleasurable, and salient to developing brainstem ororhythmic CPGs [75,76].
Altering the stiffness of the pacifier is also effective in reorganizing the ‘burst-pause’ structure of the NNS CPG in neonates [77]. Infants who were born prematurely were offered the Soothie™ and SuperSoothie™ silicone pacifiers at their3-month follow-up clinical evaluation of feeding skills, health, and development. Even though bulb and cylinder displacement volume and surface geometry of these two widely used pacifiers are virtually identical, their mechanical stiffness profiles differ by a factor of 7x due to the increased wall thickness of the SuperSoothie™ pacifier. The elevated mechanical impedance of the SuperSoothie™ presents a significant challenge to infants and resulted in shorter NNS bursts, reduced numbers of NNS bursts, and modified suck cycle frequency within-burst. These observations provide additional evidence of the sensitivity of the suck CPG to changes in local environment.
Conclusion
Information threads from neuroscience, neonatology, gastroenterology, pediatrics, pulmonary physiology, developmental speech physiology, speech pathology, and medical physics are converging rapidly on the mechanisms underlying the development of aeroingestive functions and the highly adaptive and distributed cortical and brainstem neural networks responsible for central pattern generation. These multidisciplinary information streams will enhance our understanding of cross-system interactions, plasticity, adaptive control, and issues related to neurodevelopmental outcomes, which in turn will facilitate new methods of assessment and novel entrainment including multimodal stimulus interventions for the prohabilitation of the late gestation infant, or rehabilitation of the child with a feeding disorder.
Acknowledgments
The author’s work was supported by grants NIHR01 DC003311 (SM Barlow), NIH P30 HD02528, NIH P30 DC005803, and the Sutherland Foundation.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• Of special interest
•• of outstanding interest
- 1••.Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19:691–707. doi: 10.1016/j.pmr.2008.06.001. Excellent review of feeding and swallowing stages in health and disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2•.Travers JB. Motor Control of Feeding and Drinking. In: Squire LR, editor. Encyclopedia of Neuroscience. Vol. 5. Oxford: Academic Press; 2009. pp. 1001–1007. Integrative review of the neural subsystem and sensorimotor control of deglutition with special emphasis on descending control of swallowing, and the extent of shared neural resources and cross-system integration. [Google Scholar]
- 3••.Jadcherla SR, Gupta A, Coley BD, Fernandez S, Shaker R. Esophago-glottal closure reflex in human infants: a novel reflex elicited with concurrent manometry and ultrasonography. Am J Gastroenterol. 2007;102:1–8. doi: 10.1111/j.1572-0241.2007.01401.x. Innovative investigation on the application of micromanometry to characterize the dynamics of esophago-glottal closure reflexes in infants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.Mistry S, Hamdy S. Neural control of feeding and swallowing. Phys Med Rehabil Clin N Am. 2008;19:709–728. doi: 10.1016/j.pmr.2008.05.002. Excellent review of the neuroanatomy, neural control, and central pattern generation of feeding and swallowing. [DOI] [PubMed] [Google Scholar]
- 5••.Jadcherla SR, Gupta A, Fernandez S, Nelin LD, Castile R, Gest AL, Welty S. Spatiotemporal characteristics of acid refluxate and relationship to symptoms of premature and term infants with chronic lung disease. Am J Gastroenterol. 2008;103:720–728. doi: 10.1111/j.1572-0241.2007.01748.x. Insightful study shows the application of micromanometry to localize and characterize the spatiotemporal properties of acid reflux events in the esophagus of preterm and term infants with CLD. [DOI] [PubMed] [Google Scholar]
- 6••.Barlow SM, Lund JP, Estep M, Kolta A. Central pattern generators for speech and orofacial activity. In: Brudzynski SM, editor. Handbook of Mammalian Vocalization. Elsevier; Oxford: 2009. pp. 1–33. Comprehensive review of the distributed neural networks and putative microcircuits known as central pattern generators for suck, lick, mastication, swallow, and respiration in animal and human models. Mechanisms of recombination, and adaptive control by way of peripheral and central neuromodulatory inputs illustrate the range of flexibility offered by CPGs during late gestation, infancy, and across the lifespan. [Google Scholar]
- 7.Grillner S, Markram H, De Schutter E, Silberberg G, LeBeau FEN. Microcircuits in action-from CPGs to neocortex. Trends in Neurosciences. 2005;28(10):525–533. doi: 10.1016/j.tins.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Katakura N, Jia L, Nakamura Y. NMDA-induced rhythmical activity in XII nerve of isolated CNS from newborn rats. Neuroreport. 1995;6:601–604. doi: 10.1097/00001756-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Koizumi H, Ishihama K, Nomura K, Yamanishi T, Kogo M, Matsuya T. Differential discharge patterns of rhythmical activity in trigeminal motorneurons during fictive mastication and respiration in vitro. Brain Res Bulletin. 2002;58:129–133. doi: 10.1016/s0361-9230(02)00767-0. [DOI] [PubMed] [Google Scholar]
- 10.Kogo M, Funk GD, Chandler SH. Rhythmical oral-motor activity recorded in an in vitro brainstem preparation. Somatosensory Motor Res. 1996;13:39–48. doi: 10.3109/08990229609028910. [DOI] [PubMed] [Google Scholar]
- 11.Kogo M, Tanaka S, Chandler SH, Matsuya T. Examination of the relationships between jaw opener and closer rhythmical muscle activity in an in vitro brainstem jaw-attached preparation. Somatosensory Motor Res. 1998;15:200–210. doi: 10.1080/08990229870763. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka S, Kogo M, Chandler SH, Matsuya T. Localization of oral-motor rhythmogenic circuits in the isolated rat brainstem preparation. Brain Research. 1999;821:190–199. doi: 10.1016/s0006-8993(99)01117-8. [DOI] [PubMed] [Google Scholar]
- 13.Enomoto E, Kogo M, Koizumi K, Ishihama K, Yamanishi T. Localization of premotoneurons for an NMDA-induced repetitive rhythmical activity to TMNs. Neuroreport. 2002;13:2303–2307. doi: 10.1097/00001756-200212030-00027. [DOI] [PubMed] [Google Scholar]
- 14.Westneat MW, Hall WG. Ontogeny of feeding motor patterns in infant rats: an electromyographic analysis of suckling and chewing. Behav Neurosci. 1992;106(3):539–554. doi: 10.1037//0735-7044.106.3.539. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura Y, Katakura N, Nakajima M, Liu J. Rhythm generation for food-ingestive movements. Progress BrainRes. 2004;143:97–103. doi: 10.1016/S0079-6123(03)43009-4. [DOI] [PubMed] [Google Scholar]
- 16.Lund JP, Kolta A. Brainstem circuits that control mastication: do they have anything to say during speech? J Com Disorders. 2006a;39(5):381–390. doi: 10.1016/j.jcomdis.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Nozaki S, Iriki A, Nakamura Y. Localization of central rhythm generator involved in cortically induced rhythmical masticatory jaw-opening movement in the guinea pig. J Neurophysiol. 1986;55(4):806–825. doi: 10.1152/jn.1986.55.4.806. [DOI] [PubMed] [Google Scholar]
- 18.Iriki A, Nozaki S, Nakamura Y. Feeding behavior in mammals: corticobulbar projection is reorganized during conversion from sucking to chewing. Dev Brain Res. 1988;44:189–196. doi: 10.1016/0165-3806(88)90217-9. [DOI] [PubMed] [Google Scholar]
- 19.Travers JB, DiNardo LA, Karimnamazi H. Motor and premotor mechanisms of licking. Neuroscience & Biobehavioral Reviews. 1997;21(5):631–647. doi: 10.1016/s0149-7634(96)00045-0. [DOI] [PubMed] [Google Scholar]
- 20.Lau C. Oral feeding in the preterm infant. NeoReviews. 2006;7(1):e19–e27. [Google Scholar]
- 21.Lau C. Development of oral feeding skills in the preterm infant. Arch Pediatrics. 2007;14(S1):S35–S41. doi: 10.1016/s0929-693x(07)80009-1. [DOI] [PubMed] [Google Scholar]
- 22.Brocard F, Verdier D, Arsenault I, Lund JP, Kolta A. Emergence of intrinsic bursting in trigeminal sensory neurons parallels the acquisition of mastication in weanling rats. J Neurophysiol. 2006;96:2410–2424. doi: 10.1152/jn.00352.2006. [DOI] [PubMed] [Google Scholar]
- 23.Kolta A, Westberg KG, Lund JP. Identification of brainstem interneurons projecting to the trigeminal motor nucleus and adjacent structures in the rabbit. J Chem Neuroanat. 2000;19(3):175–95. doi: 10.1016/s0891-0618(00)00061-2. [DOI] [PubMed] [Google Scholar]
- 24.Lund JP, Kolta A. Generation of the central masticatory pattern and its modification by sensory feedback. Dysphagia. 2006b;21(3):167–174. doi: 10.1007/s00455-006-9027-6. [DOI] [PubMed] [Google Scholar]
- 25.Bourque MJ, Kolta A. Properties and interconnections of trigeminal interneurons of the lateral pontine reticular formation in the rat. J Neurophysiol. 2001;86(5):2583–2596. doi: 10.1152/jn.2001.86.5.2583. [DOI] [PubMed] [Google Scholar]
- 26.Dal Bo G, Lund JP, Verdier D, Kolta A. Inputs to nucleus pontis caudalis from adjacent trigeminal areas. Eur J Neurosci. 2005;22(8):1987–1996. doi: 10.1111/j.1460-9568.2005.04371.x. [DOI] [PubMed] [Google Scholar]
- 27.McDavid S, Lund JP, Auclair F, Kolta A. Morphological and immunohistochemical characterization of interneurons within the rat trigeminal motor nucleus. Neuroscience. 2006;139(3):1049–1059. doi: 10.1016/j.neuroscience.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 28.Westberg KG, Clavelou P, Sandstrom G, Lund JP. Evidence that trigeminal brainstem interneurons form subpopulations to produce different forms of mastication in the rabbit. J Neurosci. 1998;18(16):6466–6479. doi: 10.1523/JNEUROSCI.18-16-06466.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuboi A, Kolta A, Chen CC, Lund JP. Neurons of the trigeminal main sensory nucleus participate in the generation of rhythmic motor patterns. Eur J Neurosci. 2003;17:229–238. doi: 10.1046/j.1460-9568.2003.02450.x. [DOI] [PubMed] [Google Scholar]
- 30.Lund JP, Sasamoto K, Murakami T, Olsson KA. Analysis of rhythmical jaw movements produced by electrical stimulation of motor-sensory cortex of rabbits. J Neurophysiol. 1984;52:1014–1029. doi: 10.1152/jn.1984.52.6.1014. [DOI] [PubMed] [Google Scholar]
- 31.McFarland DH, Tremblay P. Clinical implications of cross-system interactions. Semin Speech Language. 2006;27(4):300–309. doi: 10.1055/s-2006-955119. [DOI] [PubMed] [Google Scholar]
- 32.Bu’Lock F, Woolridge MW, Bairn JD. Development of coordination of sucking, swallowing, and breathing: ultrasound study of term and preterm infants. Dev Med Child Neurol. 1990;32:669–678. doi: 10.1111/j.1469-8749.1990.tb08427.x. [DOI] [PubMed] [Google Scholar]
- 33.Gewolb IH, Vice FL, Schweitzer-Kenney EL, Taciak VL, Bosma JF. Developmental patterns of rhythmic suck and swallow in preterm infants. Dev Med Child Neurol. 2001;43:22–27. doi: 10.1017/s0012162201000044. [DOI] [PubMed] [Google Scholar]
- 34.Jean A. Brainstem control of swallowing: localization and organization of the central pattern generator for swallowing. In: Taylor A, editor. Neurophysiology of the Jaws and Teeth. London: MacMillan Press; 1990. pp. 294–321. [Google Scholar]
- 35.Martin RE, MacIntosh BJ, Smith RC, Barr AM, Stevens TK, Gati GS, Menon RS. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J Neurophysiol. 2004;92(4):2428–2443. doi: 10.1152/jn.01144.2003. [DOI] [PubMed] [Google Scholar]
- 36•.Soros P, Inamotor Y, Martin RE. Functional brain imaging of swallowing: an activation likelihood estimation meta-analysis. Human Brain Mapping. 2008 doi: 10.1002/hbm.20680. Insightful meta-analysis based on 10 fMRI studies to characterize the distributed neural network that is activated during swallowing in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Martin RE. Neuroplasticity and swallowing. Dysphagia. 2009 doi: 10.1007/s00455-008-9193-9. Comprehensive review of the neurophysiology and plasticity of swallowing and hypotheses for developing novel rehabilitation applications. [DOI] [PubMed] [Google Scholar]
- 38.Smith JC, Ballanyi K, Richter DW. Whole-cell patch-clamp recordings from respiratory neurons in neonatal rat brainstem in vitro. Neurosci Letters. 1992;134:153–156. doi: 10.1016/0304-3940(92)90504-z. [DOI] [PubMed] [Google Scholar]
- 39.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger Complex: A brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butera RJ, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger Complex. I. Bursting pacemaker neurons. J Neurophysiol. 1999;82:382–397. doi: 10.1152/jn.1999.82.1.382. [DOI] [PubMed] [Google Scholar]
- 41.Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature. 1999;400:360–363. doi: 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- 42••.Rybak IA, Abdala APL, Markin SN, Paton JFR, Smith JC. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog Brain Res. 2007;165:201–220. doi: 10.1016/S0079-6123(06)65013-9. Detailed neurophysiological study characterizing the mechanisms of the respiratory central pattern generator, including new information on premotor, CPG, and motor neuron properties contributing to bursting and modulation of the respiratory rhythm in a mammalian animal model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feldman JL, Smith JC. Neural control of respiratory pattern in mammals: an overview. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. Dekker; New York: 1995. pp. 39–69. [Google Scholar]
- 44.Rekling JC, Feldman JL. PreBotzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annual Review Physiology. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- 45.Nishino T, Hiraga K. Coordination of swallowing and respiration in unconscious subjects. J Appl Physiol. 1991;70(3):988–993. doi: 10.1152/jappl.1991.70.3.988. [DOI] [PubMed] [Google Scholar]
- 46.McFarland DH, Lund JP. Modification of mastication and respiration during swallowing in the adult human. J Neurophysiol. 1995;74(4):1509–1517. doi: 10.1152/jn.1995.74.4.1509. [DOI] [PubMed] [Google Scholar]
- 47.Stevenson RD, Allaire JH. The development of normal feeding and swallowing. Pediatr Clin North Am. 1991;38(6):1439–1453. doi: 10.1016/s0031-3955(16)38229-3. [DOI] [PubMed] [Google Scholar]
- 48.Kelly BN, Huckabee ML, Jones RD, Frampton CM. The early impact of feeding on infant breathing-swallowing coordination. Respir Physiol Neurobiol. 2007a;156(2):147–153. doi: 10.1016/j.resp.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 49••.Kelly BN, Huckabee ML, Jones RD, Frampton CM. The first year of human life: coordinating respiration and nutritive swallowing. Dysphagia. 2007b;22(1):37–43. doi: 10.1007/s00455-006-9038-3. Insight report on the spatiotemporal patterns and coordination for nutritive swallow-respiration in infants. [DOI] [PubMed] [Google Scholar]
- 50.Hiss SG, Treole K, Stuart A. Effects of age, gender, bolus volume, and trial on swallowing apnea duration and swallow/respiratory phase relationships of normal adults. Dysphagia. 2001;16(2):128–135. doi: 10.1007/s004550011001. [DOI] [PubMed] [Google Scholar]
- 51.Bamford O, Taciak V, Gewolb IH. The relationship between rhythmic swallowing and breathing during suckle feeding in term neonates. Pediatr Res. 1992;31(6):619–624. doi: 10.1203/00006450-199206000-00016. [DOI] [PubMed] [Google Scholar]
- 52.Martin-Harris B, Brodsky MB, Michel Y. Breathing and swallowing dynamics across the adult lifespan. Arch Otolaryngol Head Neck Surg. 2005;131(9):762–770. doi: 10.1001/archotol.131.9.762. [DOI] [PubMed] [Google Scholar]
- 53••.Martin-Harris B. Clinical implications of respiratory-swallowing interactions. Curr Opin Otolaryngol Head Neck Surg. 2008;16:194–199. doi: 10.1097/MOO.0b013e3282febd4b. This review emphasizes the significance of cross-system neural control on swallow-respiration, and the need to explore the effects of therapeutic strategies that modulate respiratory-swallow phase patterning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dozier TS, Brodsky MB, Michel Y, Walters BC, Martin-Harris B. Coordination of swallowing and respiration in normal sequential cup swallows. The Laryngoscope. 2006;116:1489–1493. doi: 10.1097/01.mlg.0000227724.61801.b4. [DOI] [PubMed] [Google Scholar]
- 55.Selley WG, Ellis RE, Flack FC, Brooks WA. Coordination of sucking, swallowing and breathing in the newborn: its relationship to infant feeding and normal development. Br J Disord Commun. 1990;25(3):311–327. doi: 10.3109/13682829009011980. [DOI] [PubMed] [Google Scholar]
- 56.Selley WG, Ellis RE, Flack FC, Curtis H, Callon M. Ultrasonographic study of sucking and swallowing by newborn infants. Dev Med Child Neurol. 1986;28(6):82–1823. doi: 10.1111/j.1469-8749.1986.tb03945.x. [DOI] [PubMed] [Google Scholar]
- 57.Mizuno K, Ueda A. The maturation and coordination of sucking, swallowing, and respiration in preterm infants. J Pediatrics. 2003;142(1):36–40. doi: 10.1067/mpd.2003.mpd0312. [DOI] [PubMed] [Google Scholar]
- 58.Kelly BN, Huckabee ML, Jones RD, Frampton CM. Nutritive and non-nutritive swallowing apnea duration in term infants: implications for neural control mechanisms. Respir Physiol Neurobiol. 2006;154(3):372–378. doi: 10.1016/j.resp.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 59••.Kelly BN, Huckabee ML, Frampton CM, Jones RD. Arousal has no effect on non-nutritive breathing-swallowing coordination during the first year of human life. Int J Dev Neurosci. 2008;26(5):385–390. doi: 10.1016/j.ijdevneu.2008.03.006. This study demonstrates the role of brainstem pattern generation on the coordination of non-nutritive swallow-respiration in infancy. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi K, Groher ME, Michi K. Methodology for detecting swallowing sounds. Dysphagia. 1994;9(1):54–62. doi: 10.1007/BF00262760. [DOI] [PubMed] [Google Scholar]
- 61.Hanlon MB, Tripp JH, Ellis RE, Flack FC, Selley WG, Shoesmith HJ. Deglutition apnea as indicator of maturation of suckle feeding in bottle-fed preterm infants. Dev Med Child Neurol. 1997;39(8):534–542. doi: 10.1111/j.1469-8749.1997.tb07482.x. [DOI] [PubMed] [Google Scholar]
- 62.Pinnington LL, Smith CM, Ellis RE, Morton RE. Feeding efficiency and respiratory integration in infants with acute viral bronchiolitis. J Pediatrics. 2000;137(4):523–526. doi: 10.1067/mpd.2000.108396. [DOI] [PubMed] [Google Scholar]
- 63.Reynolds EW, Vice FL, Bosma JF, Gewolb IH. Cervical accelerometry in preterm infants. Dev Med Child Neurol. 2002;44:587–592. doi: 10.1017/s0012162201002626. [DOI] [PubMed] [Google Scholar]
- 64.Reynolds EW, Vice FL, Gewolb IH. Cervical accelerometry in preterm infants with and without bronchopulmonary dsyplasia. Dev Med Child Neurol. 2003;45:442–446. doi: 10.1017/s0012162203000835. [DOI] [PubMed] [Google Scholar]
- 65••.Reynolds EW, Vice FL, Gewolb IH. Variability of swallow-associated sounds in adults and infants. Dysphagia. 2009 doi: 10.1007/s00455-008-9160-5. Cervical auscultation using accelerometry is compared to microphone to objectively identify swallow sounds in adults and infants. [DOI] [PubMed] [Google Scholar]
- 66•.Doeltgen SH, Ridding MC, O’Beirne GA, Dalrymple-Alford J, Huckabee ML. Test-retest reliability of motor evoked potentials (MEPs) at the submental muscle group during volitional swallowing. J Neurosci Methods. 2009;178(1):134–137. doi: 10.1016/j.jneumeth.2008.12.005. This study shows the effects of central modulation on volitional swallowing using a novel technique combining spike-triggered TMS and submental electromyographic monitoring. [DOI] [PubMed] [Google Scholar]
- 67.Jadcherla SR. Manometric evaluation of esophageal protective reflexes in infants and children. Am J Med. 2003;115(3A):157–160S. doi: 10.1016/s0002-9343(03)00215-8. [DOI] [PubMed] [Google Scholar]
- 68.Jadcherla SR. Esophageal motility in the human neonate. Neo Reviews. 2006;7(1):e7–e12. [Google Scholar]
- 69.Jadcherla SR, Duong HQ, Hoffman R, Shaker R. Esophageal body and UES motor responses due to abrupt esophageal provocation in premature infants. J Pediatr. 2003;143:31–38. doi: 10.1016/S0022-3476(03)00242-7. [DOI] [PubMed] [Google Scholar]
- 70.Jadcherla SR, Duong HQ, Hofmann C, Hoffmann RG, Shaker R. Characteristics of upper esophageal sphincter and esophageal body during maturation in healthy human neonates compared with adults. Neurogastroenterol Motil. 2005;17:663–670. doi: 10.1111/j.1365-2982.2005.00706.x. [DOI] [PubMed] [Google Scholar]
- 71.Jadcherla SR, Hoffmann RG, Shaker R. Effect of maturation on the magnitude of mechanosensitive and chemosensitive reflexes in the premature human esophagus. J Pediatrics. 2006;141(1):77–82. doi: 10.1016/j.jpeds.2006.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jadcherla SR, Gupta A, Stoner E, Fernandex S, Shaker R. Pharyngeal swallowing: defining pharyngeal and upper esophageal sphincter relationships in human neonates. J Pediatrics. 2007;151:597–603. doi: 10.1016/j.jpeds.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Penn AA, Shatz CJ. Brain waves and brain wiring: the role of endogenous and sensory-driven neural activity in development. Pediatric Research. 1999;45(4):447–458. doi: 10.1203/00006450-199904010-00001. [DOI] [PubMed] [Google Scholar]
- 74.Hensch T. Critical period regulation. Ann Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 75.Finan DS, Barlow SM. Mechanosensory modulation of non-nutritive sucking in human infants. Early Hum Dev. 1998;52:181–97. doi: 10.1016/s0378-3782(98)00029-2. [DOI] [PubMed] [Google Scholar]
- 76••.Barlow SM, Finan DS, Chu S, Lee J. Patterns for the premature brain: Synthetic orocutaneous stimulation entrains preterm infants with feeding difficulties to suck. J Perinatology. 2008;28:541–548. doi: 10.1038/jp.2008.57. This intervention study in 31 preterm RDS infants demonstrates the effects of patterned orocutaneous stimulation delivered through a pneumatic silicone pacifier in accelerating the development of the suck central pattern generator. A secondary outcome illustrates the relation between entrainment stimulation and the transition to oral feeds in these infants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77•.Zimmerman E, Barlow SM. Pacifier stiffness alters the dynamics of the suck central pattern generator. J Neonatal Nursing. 2008;14(3):79–86. doi: 10.1016/j.jnn.2007.12.013. The suck central pattern generator was modulated by controlled changes in nipple stiffness, providing new direct evidence on the influence of peripheral mechanical properties on ororhythmic motor output in infants during the first year of life. [DOI] [PMC free article] [PubMed] [Google Scholar]