Abstract
Background
After 10 years of RA, more than half of patients have focal erosions and the risk of fracture is doubled. However, little information exists about the potential relationship between focal erosions and BMD.
Methods
We enrolled 163 postmenopausal women with RA who did not use osteoporosis medications. Participants underwent a DXA test at the hip and spine, hand x-rays, and answered a questionnaire. The hand x-rays were scored using the Sharp method. We examined the relationship between BMD and erosions using Spearman correlation coefficients and adjusted linear regression models.
Results
The 163 postmenopausal women had an average duration of RA of 13.7 years and almost all patients were currently using a DMARD. 63% were RF positive, the median mHAQ score was 0.7, and the average DAS-28 was 3.8. The erosion score was significantly correlated with the total hip BMD (Spearman R = −0.33, p < 0.0001) but not with the lumbar spine BMD (Spearman R = −0.09, p = 0.27). Hip BMD was significantly lower in RF positive women versus RF negative women (p = 0.02). In multivariable models that included age, BMI, and cumulative oral glucocorticoid dosage, neither total hip nor spine BMD were significantly associated with focal erosions.
Conclusion
These results suggest that hip BMD is associated with focal erosions among postmenopausal women with RA, but that this association disappears after multivariate adjustment. While BMD and erosions may be correlated bone manifestations of RA, their relationship is complex and influenced by other disease-related factors.
INTRODUCTION
Rheumatoid arthritis (RA) is the most common inflammatory arthritis, affecting approximately 1% of the adult population and almost 3% of persons over 65.1 In addition to the pain and functional limitations imposed by arthritis, persons with RA experience two forms of disabling bone disease -- focal erosions and generalized osteoporosis. After five years of disease, 30–50% of patients with RA manifest evidence of focal erosions.2,3 Rheumatoid arthritis doubles the risk of osteoporosis and fractures compared with age and gender-matched controls.4
Focal erosions and osteoporosis cause substantial clinical consequences and may be manifestations of a similar inflammatory cascade. Osteoblasts and osteoclasts play important roles in generalized osteoporosis and both cell types are implicated in focal erosions as well.5, 6 Moreover, inflammatory cytokines, such as TNF-alpha and RANKL, play important roles in both processes.7, 8 Defining the relationship between these two RA related processes may provide insight into the underlying pathophysiology of RA-related bone disease. Moreover, elucidating how osteoporosis is manifest in patients with RA may lead to new therapeutic strategies for both conditions, including novel drug targets and an appreciation for the potential consequences of available osteoporosis treatments in the setting of RA.
In light of the potential importance of the relationship between focal erosions and osteoporosis among patients with RA, we recruited a cohort of postmenopausal women with RA to undergo bone mineral density (BMD) testing and hand radiographs. We hypothesized that lower BMD would be associated with higher erosion scores.
METHODS
Study Population
We recruited postmenopausal women from a single center longitudinal cohort of RA, the Brigham RA Sequential Study (BRASS).9 All patients in BRASS had been diagnosed with RA by a board-certified rheumatologist. From this cohort, eligible women included those reporting postmenopausal status who also were not current users of any prescription medications for osteoporosis, including bisphosphonates, hormone therapy, raloxifene, or calcitonin. 629 eligible women were sent an invitation letter allowing them to opt-out from future contact, and 148 opted out or could not be reached, leaving 481 who we screened through telephone contact. From this group, 163 agreed to participate and completed all parts of the study. Women who agreed to participate signed the IRB-approved consent form. The study protocol was reviewed and approved by the Partners Healthcare System Institutional Review Board (IRB).
Data Collection
Women who agreed to participate underwent a brief interview to confirm that they were postmenopausal and were not currently using any prescription medications for osteoporosis. The interview also determined their personal and family history of fragility fractures, use of calcium and vitamin D supplements, and intake of calcium from dietary sources. All those who remained eligible after the brief interview were invited to undergo a hip and lumbar spine dual energy x-ray absorptiometry (DXA) test on a Hologic Discovery Bone Densitometer (Hologic Inc, Bedford MA). Vertebrae with artifact from sclerosis or compression fracture were removed from the reading. To assure long-term stability of the instrument, anthropomorphic phantoms of the spine of known hydroxyapatite composition are scanned daily. The short-term in vivo measurement standard deviations, estimated from duplicate scans of patients performed in the study scanner on the same day with repositioning, are 0.026 g/cm2 and 0.034 g/cm2, for posterior-anterior spine and lateral spine, respectively. Long-term in vivo reproducibility, determined by calculating the residual variation around a line connecting measurements made at 0, 3, and 6 months for each patient in a prior study, is between 1 and 2% for each of the measurement sites and the reproducibility is independent of the patient’s absolute BMD. A single experienced physician, blinded to the patient’s clinical status and erosion scores, read the DXA scans. In a restricted sensitivity analysis, we dropped women with substantial osteoarthritis or compression fractures on their lumber spine DXA scans.
At the time of the DXA study, patients were asked to provide a blood specimen to measure serum calcium, parathyroid hormone (PTH), 25-OH vitamin D, and thyroid stimulating hormone (TSH). The time of blood collection was not standardized.
Digitized hand x-ray images were performed every 24 months as part of BRASS. The hand x-ray closest in time to the DXA was selected and read on a Picture Archiving and Communication System display workstation using 2K monitors for image viewing and analysis (Agfa Healthcare Informatics, Greenville, SC). The difference in months between the date of DXA and hand x-ray was controlled for in the adjusted models. Radiographs were analyzed using the method described by Sharp10 by a board-certified radiologist who has been trained in these methods. The Sharp score consists of an erosion score and a joint narrowing score. The current analyses focus only on the erosion score, not the joint space narrowing component. The radiologist was blinded to subject’s identity, BMD scores, and clinical status. A re-reading of 8 films by the same radiologist found a coefficient (r) of 0.97, an intra-rater reliability well within the expected range.11
As part of the BRASS protocol, patients complete questionnaires every six months and their rheumatologists every 12 months. Variables assessed from these questionnaires include oral glucocorticoid use (current and past), disease-modifying antirheumatic drug use (DMARD) (current and past), Disease Activity Score-28 (DAS-28),12 modified Health Assessment Questionnaire (HAQ),13 C-reactive protein (CRP), rheumatoid factor status (RF), anti-cyclic citrullinated peptide antibody (anti-CCP), and duration of RA. For variables whose values may change over time, the questionnaire closest in time to the DXA was selected.
Statistical Analyses
The analyses focused on the relationship between erosion scores and BMD, measured at the hip and lumbar spine. Analyses assessing other relationships should be considered exploratory. We initially looked at Spearman correlation coefficients and then modeled the relationship using linear regression. Unadjusted bivariate models were examined where erosion score was the independent variable and BMD (total hip or lumbar spine) the dependent variable. We then assessed confounding by testing covariates (such as disease duration, age, BMI) in the bivariate linear regression models. Finally, multivariable models were constructed using a stepwise approach, keeping variables that remained significant at the p < 0.2 level. As noted above, a restricted analysis was performed by dropping all women with substantial osteoarthritis or a compression fracture limiting the DXA evaluation of the lumbar spine. In this group, multivariable models were constructed using a stepwise approach as noted above. We also explored effect modification by key variables that were found to be confounders. All analyses were conducted using SAS Statistical Software (Cary NC). Unless otherwise indicated, the data are represented as means ± standard deviations (SD).
RESULTS
The total study cohort consisted of 163 postmenopausal women with RA. The mean age of the women was 62 years (± 9) with an average duration of disease of 13.7 years (see Table 1). Sixty-three percent were rheumatoid factor (RF) positive, the median Modified Health Assessment Questionnaire score was 0.7 (interquartile range 0.3– 1.9), and the average DAS-28 score was 3.8 (± 1.6). Almost all patients reported current use of a synthetic or biologic DMARD and 69% reported current or past use of oral glucocorticoids. Of patients reporting glucocorticoid use, the median cumulative dosage reported was 2,520 milligrams. In addition, 12% of women reported a personal history of fragility fracture (hip, spine, wrist, or upper arm) and 46% of women had taken an osteoporosis medication in the past with 18% having used a bisphosphonate.
Table 1.
Characteristics of 163 postmenopausal women with rheumatoid arthritis participating in study
| N(%) or Mean (± SD) or Median (IQR) | |
|---|---|
| Age | 62.4 (± 9.0) |
| Body mass index | 28.6 (± 6.6) |
| Duration with rheumatoid arthritis | 13.7 (±10.9) |
| Rheumatoid factor status positive | 97 (63%) |
| C-Reactive Protein (mg/dl)* | 2.8 (1.3–6.6) |
| Disease Activity Score (DAS28 – CRP) | 3.8 (± 1.6) |
| History of oral glucocorticoid use | 112 (69%) |
| Cumulative oral glucocorticoid dosage (milligrams)* | 2,520 (960–12,720)† |
| Use of DMARD during follow-up, non-TNF antagonist | 116 (71%) |
| Use of TNF antagonist during follow-up | 72 (44%) |
| Modified Health Assessment Questionnaire* | 0.70 (0.3–1.9) |
| Exercise (days per week) | 1.0 (1 – 4) |
| History of fractures | 19 (12%) |
| Maternal history of fractures | 33 (20%) |
| Total calcium intake (mg) ‡ | 929 (± 332) |
| Use of calcium supplements | 71 (44%) |
| Serum 25-OH vitamin D level (ng/dl) | 29 (± 11) |
| Vitamin D deficient (<20 ng/dl) | 29 (20%) |
| Use of vitamin D supplements | 125 (77%) |
| Use of a bisphosphonate in the past | 30 (18%) |
| Use of a non-bisphosphonate OP medication in the past | 75 (46%) |
OP, osteoporosis.
Median and interquartile range
Among the 112 with some prior use of oral glucocorticoids.
Dietary sources plus supplements.
During the study period, no patients were receiving abatacept or rituximab.
Total hip BMD results were available for 152 women and lumbar spine results for 144 women. The mean total hip BMD was 0.86 g/cm2 (± 0.12) and lumber spine was 0.98 g/cm2 (± 0.15). Based on total hip T-scores, 7% of women would be considered to have osteoporosis. This figure is 14% based on lumbar spine T-scores. The mean erosion score was 36 (± 37) and 99% of women had at least one erosion.
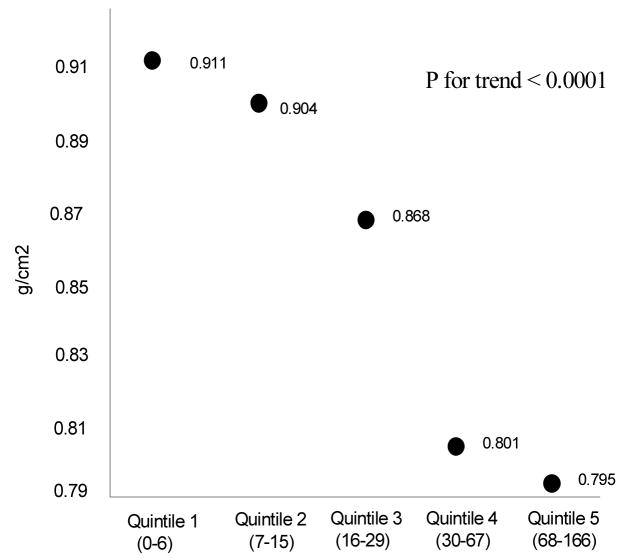
The erosion score was significantly correlated with the total hip BMD (Spearman R = −0.33, p <0.0001) but not with the lumbar spine BMD (Spearman R = −0.09, p = 0.27) (see Table 2). This relationship is illustrated in Figure 1 where hip BMD is compared with erosion scores, demonstrating a strong negative relationship between hip BMD and quintiles of erosion scores. There was no correlation observed between lumbar spine BMD and erosion scores (see Table 2). Body mass index was positively correlated with BMD at the hip and lumbar spine and negatively correlated with the erosion score. Duration of RA did not correlate with lumbar spine BMD but it did correlate with total hip BMD and erosion scores. The DAS-28 did not correlate with either hip or lumbar spine BMD but did correlate with the erosion score. Finally, the cumulative oral glucocorticoid dosage did correlate with both hip and lumbar spine BMD as well as the erosion score.
Table 2.
Spearman correlation coefficients between bone mineral density, focal erosions, and clinical variables among postmenopausal women with rheumatoid arthritis
| Patient variable | Total hip BMD | Lumbar spine BMD | Erosion score |
|---|---|---|---|
| Lumbar spine BMD | 0.60 | --- | −0.09 |
| Total hip BMD | --- | 0.60 | −0.33 |
| Erosion score | −0.33 | −0.09 | --- |
| Duration of rheumatoid arthritis | −0.14 | 0.0006 | 0.59 |
| Exercise, days per week | −0.09 | −0.11 | −0.11 |
| Body mass index | 0.38 | 0.21 | −0.24 |
| Age | −0.27 | −0.08 | 0.32 |
| Age at onset of rheumatoid arthritis | −0.12 | −0.09 | −0.26 |
| C-reactive protein | −0.01 | −0.04 | 0.21 |
| Total calcium intake | −0.11 | −0.09 | 0.11 |
| DAS28-CRP | −0.05 | −0.07 | 0.42 |
| Modified HAQ | −0.04 | −0.02 | 0.11 |
| Cumulative oral GC dose | −0.10 | −0.003 | 0.26 |
| Vitamin D level | −0.17 | −0.12 | 0.16 |
BMD, bone mineral density; BMI, body mass index; DAS, Disease Activity Score; GC, glucocorticoid. HAQ, Health Assessment Questionnaire. Bolded cells have p-values < 0.1. Erosion scores are the erosion component of the Sharp score.
Figure 1.
demonstrates the mean total hip bone mineral density for patients in successive quintiles of erosion score.
The median hip and lumbar spine BMD values were calculated for selected categories of patients (see Table 3). The total hip BMD values were significantly lower in RF positive women (0.83 g/cm2) versus RF negative (0.89 g/cm2, p = 0.02). However, the lumbar spine values were almost identical for the RF positive women (0.96 g/cm2) and the RF negative women (0.97 g/cm2). Patients reporting current use of a TNF antagonist had significantly lower total hip BMD, as well those reporting past bisphosphonate use had lower BMD at the total hip and lumbar spine. However, both of these findings are likely due to confounding by indication. The erosion scores were higher in RF positive versus RF negative women.
Table 3.
Association between discrete patient variables and bone manifestations of rheumatoid arthritis
| Total hip BMD | Lumbar spine BMD | Erosion | |||||
|---|---|---|---|---|---|---|---|
| Patient characteristics | Median (IQR) | P-value | Median (IQR) | P-value | Median (IQR) | P-value | |
| Rheumatoid factor | Positive | 0.83 (0.76–0.91) | 0.96 (0.88–1.03) | 32 (15–71) | |||
| Negative | 0.89 (0.79–0.95) | 0.02 | 0.97 (0.88–1.07) | 0.4 | 13 (7–26) | 0.0001 | |
| C-reactive protein | Elevated | 0.83 (0.77–0.95) | 0.96 (0.86–1.11) | 29 (13–70) | |||
| Normal | 0.85 (0.77–0.92) | 0.9 | 0.97 (0.89–1.05) | 0.4 | 20 (7–46) | 0.04 | |
| Maternal history of fracture | Yes | 0.84 (0.74–0.93) | 0.99 (0.88–1.06) | 24 (7–44) | |||
| No | 0.87 (0.78–0.93) | 0.20 | 0.96 (0.88–1.06) | 0.6 | 22 (8–58) | 0.8 | |
| Prior fragility fracture | Yes | 0.80 (0.71–0.93) | 1.00 (0.89–1.03) | 24 (7–68) | |||
| No | 0.86 (0.77–0.93) | 0.16 | 0.97 (0.88–1.07) | 0.9 | 22 (8–51) | 0.7 | |
| TNF-alpha antagonist use | Yes | 0.83 (0.77–0.90) | 0.97 (0.88–1.03) | 47 (21–83) | |||
| No | 0.88 (0.78–0.96) | 0.04 | 0.97 (0.88–1.07) | 0.8 | 13 (5–27) | <0.001 | |
| DMARD use | Yes | 0.85 (0.76–0.93) | 0.97 (0.89–1.07) | 24 (9–68) | |||
| No | 0.86 (0.79–0.93) | 0.5 | 0.96 (0.87–1.03) | 0.21 | 20 (5–34) | 0.07 | |
| History of oral GC use | Yes | 0.84 (0.76–0.92) | 0.97 (0.88–1.06) | 16 (7–44) | |||
| No | 0.87 (0.80–0.96) | 0.14 | 0.97 (0.88–1.06) | 0.9 | 24 (9–60) | 0.19 | |
| History of BIS use | Yes | 0.78 (0.74–0.88) | 0.90 (0.84–1.02) | 47 (23–70) | |||
| No | 0.87 (0.79–0.94) | 0.009 | 0.97 (0.89–1.07) | 0.08 | 19 (7–38) | 0.0005 | |
| History of non- BIS use | Yes | 0.86 (0.77–0.92) | 0.97 (0.87–1.03) | 22 (9–59) | |||
| No | 0.85 (0.77–0.94) | 0.8 | 0.97 (0.89–1.08) | 0.6 | 20 (7–51) | 0.5 | |
| 25-OH vitamin D level | <20 ng/dl | 0.87 (0.78 – 0.97) | 1.01 (0.89 – 1.07) | 21 (7 – 30) | |||
| 20+ ng/dl | 0.85 (0.77 – 0.92) | 0.2 | 0.96 (0.88 – 1.05) | 0.2 | 23 (9 – 59) | 0.3 | |
GC, glucocorticoid; BIS, bisphosphonate. P values from a Kruskal-Wallis Test.
In multivariable models that included age, body mass index, and cumulative oral glucocorticoid dosage, neither hip nor spine BMD were significantly associated with focal erosions (see Table 4). In the full multivariable model, the only variable that remained an independent predictor of total hip and lumbar spine BMD was body mass index, where a higher body mass index was associated with higher BMD. Several variables modified the effect of the relationship between the erosions and total hip BMD, including age, duration of RA, body mass index, and cumulative oral glucocorticoid dosage. These effects are explored in Table 5 where the parameter estimate for erosion scores in the fully adjusted model on all patients is compared with the fully adjusted parameter estimate in models stratified on one variable at a time. The relationship between erosion scores and hip BMD was strengthened among younger patients (< 62 years old versus older, where 62 was the median), patients with fewer years of disease (<5 years versus longer), higher body mass index (≥ 28 kg/m2 versus less, where 28 kg/m2 was the median), and less cumulative oral glucocorticoid use (< 960 mg versus greater, where 960 mg was the median). The sensitivity analysis removing patients with less than four vertebral bodies yielded slightly different results for the relationship between lumbar spine and erosions (see Table 4, Full Model B). Of note, the relationship between lumbar spine BMD and erosions appeared to be stronger in this restricted analysis.
Table 4.
Adjusted analysis for hip and lumbar spine bone mineral density
| Total hip BMD as outcome | Lumbar spine BMD as outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Partial model | Full model | Partial Model | Full Model A | Full Model B | ||||||
| Variables | Parameter estimate |
P value | Parameter estimate |
P-value | Parameter estimate |
P-value | Parameter estimate |
P- value |
Parameter estimate |
P- value |
| Erosion score* | −0.0047 | 0.070 | −0.0044 | 0.10 | 0.00007 | 0.9 | −0.0017 | 0.6 | −0.0068 | 0.13 |
| Age, per decade* | −0.0038 | 0.0001 | −0.0040 | 0.0001 | −0.0012 | 0.4 | −0.0023 | 0.1 | −0.0020 | 0.9 |
| Body mass index* | 0.0073 | <0.0001 | 0.0069 | < 0.001 | 0.0052 | 0.006 | 0.0040 | 0.05 | 0.0044 | 0.10 |
| Cumulative oral GC dose* | −0.0000011 | 0.17 | −0.0000008 | 0.34 | 0.0000002 | 0.9 | −0.0000003 | 0.6 | −0.00058 | 0.6 |
| Prior fragility fracture | --- | --- | −0.046 | 0.17 | --- | --- | --- | --- | --- | --- |
| DMARD use, ever | --- | --- | --- | --- | --- | --- | 0.055 | 0.06 | 0.075 | 0.04 |
| Prior BIS use | --- | --- | −0.05 | 0.03 | --- | --- | --- | --- | --- | --- |
| Prior non-BIS use | --- | --- | 0.03 | 0.02 | --- | --- | --- | --- | --- | --- |
| CRP, per one unit increase | --- | --- | --- | --- | --- | --- | --- | --- | 0.0032 | 0.03 |
N = 144 for lumbar spine model and 152 for total hip model. GC, glucocorticoids; DMARD, disease-modifying antirheumatic drugs. BIS, bisphosphonate.
Variables forced into all models. In the full model, all variables listed in Table 1 were tested for entry and remained if the p-value < 0.2. The difference in months between the date of DXA and hand x-ray was controlled for in the partially and fully adjusted models. Full Model A includes all patients with lumbar spine BMD measurements. Full Model B excludes any woman whose DXA scan was limited by osteoarthritis or a fracture.
Table 5.
Modification of the effect of erosions on total hip bone mineral density by selected patient characteristics
| Total hip BMD as outcome | ||
|---|---|---|
| Parameter estimate | P value | |
| Fully adjusted model* | −0.0044 | 0.10 |
| Stratified models on: | ||
| Patient age | ||
| < 62 years | −0.006 | 0.08 |
| 63 and older | −0.004 | 0.4 |
| Disease duration | ||
| < 5 years | −0.016 | 0.3 |
| ≥5 years | −0.005 | 0.1 |
| Body mass index | ||
| < 28 kg/m2 | −0.001 | 0.8 |
| 28+ kg/m2− | −0.008 | 0.04 |
| Cumulative glucocorticoid dose | ||
| < 960mg | −0.010 | 0.02 |
| 960+ mg | 0.0004 | 0.9 |
Same as model shown in Table 5.
DISCUSSION
The skeletal system is a major target organ for RA, but relatively little is known about how focal erosions relate to generalized osteoporosis. We studied this relationship among a cohort of postmenopausal women with a median duration of RA of 14 years. Total hip BMD correlated with erosion scores in unadjusted analyses, however this relationship was not significant after controlling for other variables. This relationship appeared stronger among certain subgroups of patients, including those with a shorter duration of disease, a higher body mass index, a lower cumulative oral glucocorticoid dosage, and lower 25-OH vitamin D levels.
Our findings suggest that the relationship between focal erosions and generalized osteoporosis is complicated and modified by many aspects of RA as well as other factors. The fact that the relationship was stronger among patients with shorter duration of RA suggests that with longer disease duration other variables dilute the relationship between focal erosions and total hip BMD. These other variable might include the use of specific DMARDs (nearly universal in our study cohort), disease activity and inflammatory markers. While we have good information on these factors for adjustment in regression model, our information is not perfect on these longitudinal variables. Similar to disease duration’s effects, the cumulative oral glucocorticoid dosage appears to blunt the relationship between focal erosions and BMD over time. Prior studies have reported that higher body mass index is associated with a lower erosion score.14 This is supported by our findings (see Table 2). Moreover, the relationship between total hip BMD and erosion score was most evident at the higher body mass index.
We found that there was a stronger relationship between erosions and total hip BMD than with lumbar spine BMD. Several potential explanations exist for this apparent discrepancy across anatomic sites. It is possible that the inflammatory process underlying RA affects BMD at the hip more so than the lumbar spine. On the one hand, because the vertebral bodies have a greater trabecular bone content than the total hip and the trabecular bone is more metabolically active, one might anticipate that cytokine perturbations related to RA would affect lumbar spine more dramatically than total hip BMD. On the other hand, since total hip BMD may more closely relate to joint mobility and overall functional status than lumbar spine BMD, it is possible that the effects of RA would be more apparent at the hip than lumbar spine. Furthermore, it is possible that DXA artifacts, known to affect the lumbar spine BMD more than the proximal femur, dilute the relationship between BMD and erosions differentially by anatomic site. This concept is supported by the sensitivity analysis removing patients with fewer than four lumbar vertebrae that showed a strengthened relationship.
Several prior studies have examined the bone manifestations of RA. The COBRA randomized controlled trial tested a treatment regimen of high dose glucocorticoids, methotrexate, and sulfasalazine versus sulfasalazine alone.15 Bone resorption markers, such as urinary pyridinoline and doxypyridinoline, were correlated with erosion scores, but no analyses were performed testing the correlation between erosions and BMD. While several studies have found a significant positive correlation between hand BMD and erosions, the relevance of this finding to the current study is not clear because of the difference in anatomic sites.7,16,17 A cohort study similar to the one we report was conducted in Canada among 204 patients with early onset RA. The BMD at the hip and spine were significantly correlated with Larsen scores at baseline and after two years of follow-up for the women but not the men.18 A very recent post-hoc analysis of the BeST study of patients with < 2 years of RA found that progression of erosion scores was associated with reduction in BMD.
There are some important limitations to our study. First, while we had adequate statistical power to look at the relationships between BMD and erosions in the total cohort, the subgroups of patients were quite small. This precludes meaningful statements about important groups, such as those with early RA. Second, the DXAs and hand x-rays were not performed simultaneously. The mean lag between images was 3.4 months months and this lag was included as a covariate in our models. However, this lag may have diluted the relationship between BMD and erosions. Third, almost all of the patients in this cohort received DMARDs for their RA. TNF antagonists and DMARD use were included as separate covariates, but such treatments may blunt the relationship between erosions and BMD. Some agents, such as the TNF antagonists, may lead to erosion healing in some patients and reduce the loss (or actually improve) BMD.19 The effect of glucocorticoids on this relationship may be very complex and our cumulative dosage variable may misclassify some patients. Fourth, some women had received medications for osteoporosis in the past. Restricted analyses removing women who had used bisphosphonates (n = 30) showed very similar results. Fifth, our study was not extremely large and it is possible that a true effect could have been missed. However, post-hoc power calculations suggest that we had > 80% power to detect a clinically important correlation between BMD and erosions. Sixth, it is possible that the relationship between BMD and erosions was blunted in our population because of intensive treatment and supplemental vitamin D use. While it is true that our subjects received aggressive care, our cohort does not differ from several other cohorts in vitamin D status and DAS scores.20–23 Seventh, our analysis included many clinical and some biologic variables, but some potential confounders such as tobacco use were not accurately collected in our study database. Finally, only one radiologist, trained in musculoskeletal radiology, read all the radiographs. This precludes consensus readings for difficult to interpret radiographs. However, the radiologist’s intra-rater reliability was excellent (r = 0.97), films were obtained at a single center on very similar imaging processors without any films needing to be excluded, and standard methods for interpretation (e.g., erosion atlas) were used.
While there are important limitations to this study, it is also important to note that this is one of the only studies to date that has focused on the relationship between two skeletal manifestations of RA. The patients included were very well characterized from a clinical standpoint. The vast majority of DXA measurements were conducted on a single bone densitometer that has very careful quality controls in place. In conclusion, we found that hip BMD correlated with erosion scores among postmenopausal women with RA. However, this relationship was no longer statistically significant after adjustment for clinical factors. It appears that the relationship between BMD and erosions is stronger in patients with early RA. In conjunction with the findings from the BeST trial showing a longitudinal relationship between erosions and BMD in patients with < 2 years of disease, this suggests that early RA would be an important population for future studies.24 It may be that the presumed association between erosions and BMD is most relevant for patients with severe or early untreated RA. This information is likely to become increasingly important as more bone-directed treatments find their way into RA management paradigms. Thus, it may become possible to treat multiple skeletal manifestations of RA with a single agent. Data from denosumab (a monoclonal antibody directed against RANKL) trials suggest that it may be effective at improving BMD and reducing erosion progression.25, 26 In addition to the clinical implications of unraveling this relationship, further studies that include more biologic information may clarify important inflammatory underpinnings of both focal erosions and osteoporosis in RA.
Acknowledgments
Support: This project was supported by the NIH (AG 027066). In addition, Dr. Solomon receives salary support from the NIH (AR047782 and AR055989). Dr. Shadick receives research support from the Amgen Med Foundation, Bristol Myers Squibb Foundation, the CDC, and the NIH. Dr. Weinblatt receives support from Abbott, Amgen, Biogen/Idec, Bristol Myers Squibb Foundation, Genentech, Millenium Pharmaceuticas, and Riley Genomics and serves as consultant to those companies. Dr. Gravallese receives support from the Worcester Foundation and the NIH (AG 027066 and AR047665). Dr. Finkelstein receives support from NIH (K24 DK02759). Dr. Winalski receives research support from Abbott and is a shareholder in Pfizer. The BRASS registry was supported by funds from Millenium Pharmaceuticals, Riley Genomics, and Biogen-Idec.
References
- 1.Rasch EK, Hirsch R, Paulose-Ram R, Hochberg MC. Prevalence of rheumatoid arthritis in persons 60 years of age and older in the United States: effect of different methods of case classification. Arthritis & Rheumatism. 2003;48(4):917–926. doi: 10.1002/art.10897. [DOI] [PubMed] [Google Scholar]
- 2.Gabriel SE. The epidemiology of rheumatoid arthritis. Rheumatic Diseases Clinics of North America. 2001;27(2):269–281. doi: 10.1016/s0889-857x(05)70201-5. [DOI] [PubMed] [Google Scholar]
- 3.Lee DM, Weinblatt ME. Rheumatoid arthritis.[see comment] Lancet. 2001;358(9285):903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 4.van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis & Rheumatism. 2006;54(10):3104–3112. doi: 10.1002/art.22117. [DOI] [PubMed] [Google Scholar]
- 5.Walsh NC, Gravallese EM. Bone loss in inflammatory arthritis: mechanisms and treatment strategies. Curr Opin Rheumatol Jul. 2004;16(4):419–427. doi: 10.1097/01.bor.0000127824.42507.68. [DOI] [PubMed] [Google Scholar]
- 6.Gravallese EM, Harada Y, Wang JT, Gorn AH, Thornhill TS, Goldring SR. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. American Journal of Pathology. 1998;152(4):943–951. [PMC free article] [PubMed] [Google Scholar]
- 7.Haugeberg G, Lodder MC, Lems WF, et al. Hand cortical bone mass and its associations with radiographic joint damage and fractures in 50–70 year old female patients with rheumatoid arthritis: cross sectional Oslo-Truro-Amsterdam (OSTRA) collaborative study. Annals of the Rheumatic Diseases. 2004;63(10):1331–1334. doi: 10.1136/ard.2003.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redlich K, Gortz B, Hayer S, et al. Repair of local bone erosions and reversal of systemic bone loss upon therapy with anti-tumor necrosis factor in combination with osteoprotegerin or parathyroid hormone in tumor necrosis factor-mediated arthritis. American Journal of Pathology. 2004;164(2):543–555. doi: 10.1016/S0002-9440(10)63144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon DH, Stedman M, Licari A, Weinblatt ME, Maher N, Shadick N. Agreement between patient report and medical record review for medications used for rheumatoid arthritis: the accuracy of self-reported medication information in patient registries. Arthritis & Rheumatism. 2007;57(2):234–239. doi: 10.1002/art.22549. [DOI] [PubMed] [Google Scholar]
- 10.Sharp JT, Young DY, Bluhm GB, et al. How many joints in the hands and wrists should be included in a score of radiologic abnormalities used to assess rheumatoid arthritis? Arthritis & Rheumatism. 1985;28(12):1326–1335. doi: 10.1002/art.1780281203. [DOI] [PubMed] [Google Scholar]
- 11.Boini S, Guillemin F. Radiographic scoring methods as outcome measures in rheumatoid arthritis: properties and advantages. Ann Rheum Dis Sep. 2001;60(9):817–827. [PMC free article] [PubMed] [Google Scholar]
- 12.Smolen JS, Breedveld FC, Schiff MH, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology. 2003;42(2):244–257. doi: 10.1093/rheumatology/keg072. [DOI] [PubMed] [Google Scholar]
- 13.Katz JN, Larson MG, Phillips CB, Fossel AH, Liang MH. Comparative measurement sensitivity of short and longer health status instruments. Medical Care. 1992;30(10):917–925. doi: 10.1097/00005650-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann J, Kielstein V, Kilian S, Stein G, Hein G. Relation between body mass index and radiological progression in patients with rheumatoid arthritis. Journal of Rheumatology. 2003;30(11):2350–2355. [PubMed] [Google Scholar]
- 15.Garnero P, Landewe R, Boers M, et al. Association of baseline levels of markers of bone and cartilage degradation with long-term progression of joint damage in patients with early rheumatoid arthritis: the COBRA study. Arthritis & Rheumatism. 2002;46(11):2847–2856. doi: 10.1002/art.10616. [DOI] [PubMed] [Google Scholar]
- 16.Jensen T, Hansen M, Jensen KE, Podenphant J, Hansen TM, Hyldstrup L. Comparison of dual X-ray absorptiometry (DXA), digital X-ray radiogrammetry (DXR), and conventional radiographs in the evaluation of osteoporosis and bone erosions in patients with rheumatoid arthritis. Scandinavian Journal of Rheumatology. 2005;34(1):27–33. doi: 10.1080/03009740510017986. [DOI] [PubMed] [Google Scholar]
- 17.Stewart A, Mackenzie LM, Black AJ, Reid DM. Predicting erosive disease in rheumatoid arthritis. A longitudinal study of changes in bone density using digital X-ray radiogrammetry: a pilot study. Rheumatology. 2004;43(12):1561–1564. doi: 10.1093/rheumatology/keh385. [DOI] [PubMed] [Google Scholar]
- 18.Forslind K, Keller C, Svensson B, Hafstrom I, Group BS. Reduced bone mineral density in early rheumatoid arthritis is associated with radiological joint damage at baseline and after 2 years in women. Journal of Rheumatology. 2003;30(12):2590–2596. [PubMed] [Google Scholar]
- 19.Lange U, Teichmann J, Muller-Ladner U, Strunk J. Increase in bone mineral density of patients with rheumatoid arthritis treated with anti-TNF-alpha antibody: a prospective open-label pilot study. Rheumatology. 2005;44(12):1546–1548. doi: 10.1093/rheumatology/kei082. [DOI] [PubMed] [Google Scholar]
- 20.Matsui T, Kuga Y, Kaneko A, et al. Disease Activity Score 28 (DAS28) using C-reactive protein underestimates disease activity and overestimates EULAR response criteria compared with DAS28 using erythrocyte sedimentation rate in a large observational cohort of rheumatoid arthritis patients in Japan. Ann Rheum Dis Sep. 2007;66(9):1221–1226. doi: 10.1136/ard.2006.063834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aletaha D, Nell VP, Stamm T, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7(4):R796–806. doi: 10.1186/ar1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel S, Farragher T, Symmons D. Reply to letter by Lippi et al commenting on the relationship between serum vitamin D levels and levels of inflammation markers. Arthritis Rheum Jun. 2008;58(6):1882. doi: 10.1002/art.23784. [DOI] [PubMed] [Google Scholar]
- 23.Cutolo M, Otsa K, Uprus M, Paolino S, Seriolo B. Vitamin D in rheumatoid arthritis. Autoimmun Rev Nov. 2007;7(1):59–64. doi: 10.1016/j.autrev.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Guler-Yuskel MBJ, Goekoop-Ruiterman YPM, et al. Changes in bone mineral density in patients with recent onset, active rheumatoid arthritis. Annals of the Rheumatic Diseases. 2008;67:823–828. doi: 10.1136/ard.2007.073817. [DOI] [PubMed] [Google Scholar]
- 25.McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density.[see comment] New England Journal of Medicine. 2006;354(8):821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 26.Cohen SBDRK, Lane NE, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: A twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis & Rheumatism. 2008;58(5):1299–1309. doi: 10.1002/art.23417. [DOI] [PubMed] [Google Scholar]