Abstract
Polymer-supported α-acylamino ketones were transformed to seven types of structurally unrelated heterocyclic compounds. Syntheses involved variety of chemical routes and comprised diverse chemistries (C-C, C=C, C-N, C=N, C-O bond formations). Different sizes of heterocycles (4-, 5-, 6-, and 7-membered rings) were prepared, including dihydro-pyrrol-2-ones, pyrazin-2-ones, dihydro-triazepin-6-ones, morpholin-3-ones, imidazoles, β-lactams, and isoquinolin-1-ones. Further elaboration to fused ring systems was also documented.
Introduction
A very efficient strategy for diversity-oriented synthesis1 takes advantage of common intermediates that are converted to chemically and structurally unrelated diverse scaffolds. This strategy has been successfully used on several occasions. For example, the Janda group reported syntheses of structurally diverse scaffolds using polymer-bound α-diazo-β-ketoesters.2-4 We prepared polymers-supported o-phenylenediamines with two diversity positions that were subsequently elaborated to various nitrogen containing heterocyclic structures.5 The multiplicity of structural types accessible from a common precursor increased the diversity of compounds: it has been demonstrated that single scaffold libraries are restricted to a limited number of molecular shapes, as opposed to smaller libraries around multiple scaffolds.6
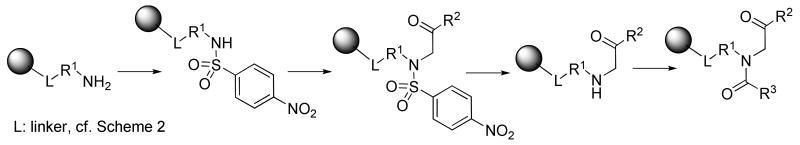
We have recently developed a robust and high-yielding synthesis of α-acylamino ketones on solid phase (Scheme 1) using easily accessible building blocks (polymer-supported amines, bromoketones, and carboxylic acids).7 The synthesis was carried out using polymer-supported primary amines via alkylation of nitrobenzenesulfonyl (Nos) activated/protected amines (a variant of the Fukuyama method8), followed by N-alkylation with bromoketones. We observed a striking difference in the reaction outcome between 2-Nos and 4-Nos derivatives. The attempted cleavage of the 2-Nos protecting group led to an unexpected tandem carbon-carbon followed by nitrogen-nitrogen bonds formations, and ultimately yielded indazole oxides.9 To change the course of this otherwise very useful transformation leading to indazole oxides, we replaced the 2-Nos group by 4-Nos derivative by reacting resin-bound amines with 4-Nos chloride. The alkylation with bromoketones proceeded smoothly under the same condition developed for alkylation of the 2-Nos derivative. Cleavage of 4-Nos group was accomplished by treatment with 2-mercaptoethanol in the presence of a base.8 Subsequent acylation of the secondary amino group yielded resin-bound α-acylamino ketones.
Scheme 1.
Synthesis of resin-bond α-acylamino ketones7
α-Acylamino ketones, in addition to being attractive compounds per se,10-14 represent an intriguing class of compounds that offer an extensive range of chemical transformations and that can serve as a versatile starting point for syntheses of diverse heterocycles. However, their synthetic potential has not been fully explored; so far, α-acylamino ketones have mostly been exploited for the synthesis of imidazoles and oxazoles.15-18 In this contribution we describe the use of resin-bound α-acylamino ketones for the synthesis of structurally unrelated heterocyclic compounds. In addition, we showed that these heterocycles are amenable to subsequent transformations, namely to the formation of an additional fused ring. We have already successfully applied this approach for the synthesis of dihydropyrazino[1,2-b]indazoles.19
Results and Discussion
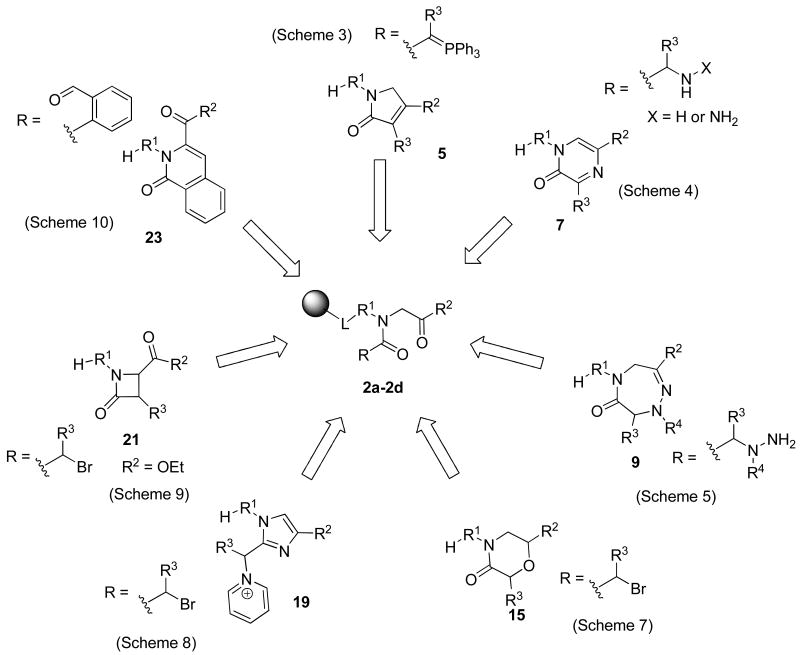
A generic structure of α-acylamino ketones is portrayed in the Figure 1. The R1 side-chain is used for immobilization to the linker-derivatized resin. The R2 substituent is derived from the bromoketones. To increase the multiplicity of target heterocyclic compounds, we carried out the alkylation also with α-bromocarboxylic acid esters. In such cases the α-acylamino esters (R2 = O-alkyl), instead of ketones, were obtained. The R side-chain was introduced by acylation with carboxylic acids.
Figure 1.
α-Acylamino ketones/esters as intermediates for the syntheses of seven different heterocycles
A deliberate selection of acylation agents that introduced the R groups and reaction conditions for the cyclization step enabled the syntheses of diverse and structurally unrelated heterocycles. In this contribution we describe the transformation of α-acylamino ketones/esters to several different heterocyclic motifs: dihydro-pyrrol-2-ones, pyrazin-2-ones, dihydro-triazepin-6-ones, morpholin-3-ones, imidazoles, β-lactams, and isoquinolin-1-ones (Figure 1).
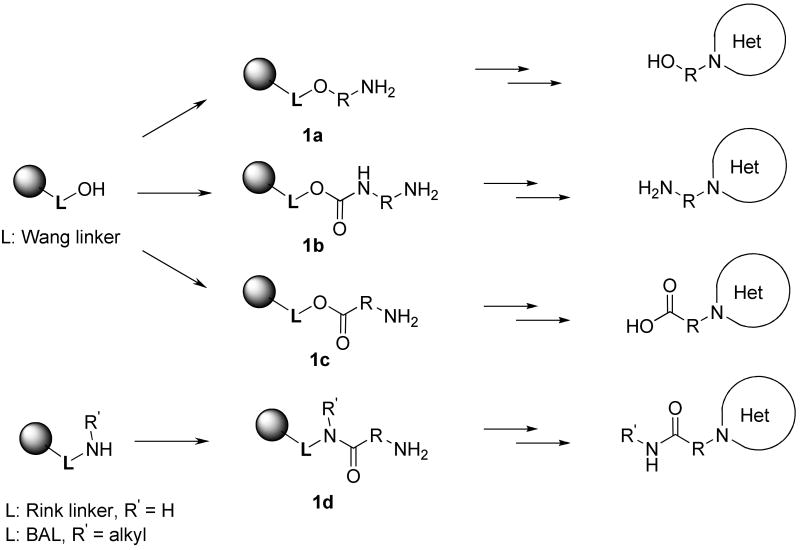
We prepared resin-bound amines used for the synthesis of α-acylamino ketones/esters using three different types of bifunctional building blocks: amino alcohols, diamines, and amino acids (Scheme 2), to obtain target compounds with different functional groups on the R substituent. Immobilization of all amine-containing building blocks was carried out using established protocols and we have already used this approach for the synthesis of indazoles9 and imidazoles.7 Wang resin20 was used to immobilize amino alcohols (1a), diamines (1b), and amino acids (1c) and yielded alcohols, amines, and acids. Rink amide resin21 acylated with amino acids provided amides (1d, R′ = H); the use of backbone amide linker (BAL) resin22 for reductive amination followed by acylation with amino acids (1d, R′ = alkyl) yielded secondary amides.
Scheme 2.
Immobilization of amine-containing building blocks
To address the scope and limitation of resin-bound α-acylamino ketones for the synthesis of diverse heterocycles, we evaluated the transformations leading to seven heterocycles and prepared a set of model compounds for each heterocycle (Table 1). Then we focused our attention on syntheses of 1,5-dihydro-pyrrol-2-ones 5 and 1H-pyrazin-2-ones 7 and prepared small combinatorial arrays of these compounds.
Table 1.
Synthesized heterocycles 5, 7, 9, 13, 15, 19, 21, 23, 24
| Product | R1-H | R2 | R3 | Puritya [%] | Yieldb [%] | MS ESI+ |
|---|---|---|---|---|---|---|
| 5(1,1,1) | –(CH2)2–COOH | 4-Me-Ph | H | 59 | 44 | 246 |
| 5(1,2,1) | –(CH2)2–COOH | 4-OMe-Ph | H | 65 | 75 | 262 |
| 5(1,3,1) | –(CH2)2–COOH | 4-NH2-3,5-diCl-Ph | H | 68 | 9 | 315 |
| 5(2,1,1) | –(CH2)2–OH | 4-Me-Ph | H | 55 | 77 | 218 |
| 5(2,3,1) | –(CH2)2–OH | 4-NH2-3,5-diCl-Ph | H | 58 | 56 | 287 |
| 5(3,1,1) | –(CH2)2–NH2 | 4-Me-Ph | H | 46 | 37 | 217 |
| 5(3,2,1) | –(CH2)2–NH2 | 4-OMe-Ph | H | 53 | 23 | 233 |
| 5(3,3,1) | –(CH2)2–NH2 | 4-NH2-3,5-diCl-Ph | H | 38 | 41 | 286 |
| 5(3,4,1) | –(CH2)2–NH2 | -OEt | H | 71 | 44 | 171 |
| 5(4,1,1) | –(CH2)2–CONH2 | 4-Me-Ph | H | 55 | 28 | 245 |
| 5(4,2,1) | –(CH2)2–CONH2 | 4-OMe-Ph | H | 57 | 38 | 261 |
| 5(4,3,1) | –(CH2)2–CONH2 | 4-NH2-3,5-diCl-Ph | H | 62 | 27 | 314 |
| 5(5,4,1) | –(CH2)2–CONH(4-Me-Bn) | -OEt | H | 80 | 53 | 303 |
| 7(1,1,1) | –(CH2)2–COOH | 4-Me-Ph | H | 81 | 30 | 259 |
| 7(1,2,1) | –(CH2)2–COOH | 4-OMe-Ph | H | 60 | 38 | 275 |
| 7(1,3,1) | –(CH2)2–COOH | 4-NH2-3,5-diCl-Ph | H | 55 | 12 | 328 |
| 7(1,1,3) | –(CH2)2–COOH | 4-Me-Ph | Et | 35 | 24 | 287 |
| 7(2,1,1) | –(CH2)2–OH | 4-Me-Ph | H | 99 | 61 | 231 |
| 7(3,1,1) | –(CH2)2–NH2 | 4-Me-Ph | H | 47 | 40 | 230 |
| 7(3,2,1) | –(CH2)2–NH2 | 4-OMe-Ph | H | 83 | 52 | 246 |
| 7(3,3,1) | –(CH2)2–NH2 | 4-NH2-3,5-diCl-Ph | H | 93 | 62 | 299 |
| 7(3,1,2) | –(CH2)2–NH2 | 4-Me-Ph | Me | 81 | 12 | 244 |
| 7(4,1,1) | –(CH2)2–CONH2 | 4-Me-Ph | H | 57 | 21 | 258 |
| 7(4,2,1) | –(CH2)2–CONH2 | 4-OMe-Ph | H | 36 | 36 | 274 |
| 7(6,1,1) | –(CH2)3–CONH2 | 4-Me-Ph | H | 71 | 39 | 272 |
| 7(6,2,1) | –(CH2)3–CONH2 | 4-OMe-Ph | H | 68 | 42 | 288 |
| 7(7,1,1) | –CONH–(CH2)2CH3 | 4-Me-Ph | H | 66 | 11 | 362 |
| 9(1,1,1)c | –(CH2)2–COOH | 4-Me-Ph | H | 48 | 19 | 366 |
| 9(1,4,1)c | –(CH2)2–COOH | 4-NH2-3,5-diCl-Ph | H | 50 | 25 | 435 |
| 9(3,1,1)c | –(CH2)2–NH2 | 4-Me-Ph | H | 48 | 18 | 337 |
| 13(10,1,1)c | NA | 4-Me-Ph | H | 51 | ND | 319 |
| 15(4,1,1) | –(CH2)2–CONH2 | 4-Me-Ph | H | 31 | 23 | 263 |
| 15(8,1,1) | –CH2Ph– (4–COOH) | 4-Me-Ph | H | 61 | 22 | 326 |
| 19(3,1,4) | –(CH2)2–NH2 | 4-Me-Ph | H | 78 | 16 | 293 |
| 19(5,1,4) | –(CH2)2–CONH(4-Me-Bn) | 4-Me-Ph | H | 68 | 29 | 425 |
| 21(8,4,1) | –CH2Ph– (4–COOH) | –OEt | H | 78 | 23 | 278 |
| 23(1,2,5) | –(CH2)2–COOH | 4-OMe-Ph | NA | 51 | 5 | 352 |
| 23(9,1,5) | –(CH2)2–CONH(CH2)2CH3 | 4-Me-Ph | NA | 58 | 15 | 377 |
| 24(10,1,5) | NA | 4-Me-Ph | NA | 76 | 13 | 289 |
Purity of the crude product before purification;
Yield after purification by HPLC;
R4 = Bn;
NA, not applicable; ND, not determined
1,3,4-Derivatized 1,5-dihydro-pyrrol-2-ones 5
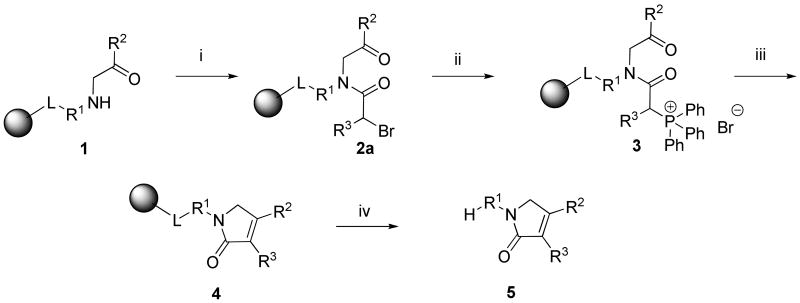
Synthesis of 1,5-dihydro-pyrrol-2-ones 5 involved cyclization between the side chain R3 and the carbonyl group of R2 side-chain by intramolecular Wittig reaction. The resin-bound amino ketones 1 were acylated with bromoacetic acid (Scheme 3) via in situ prepared symmetrical anhydride. L represents any linker described in Scheme 2. A tertiary base (N,N-diisopropylethylamine, DIEA) was added to the reaction mixture to prevent premature cleavage from the resin by the bromoacetic acid.
Scheme 3.
Synthesis of 1,5-dihydro-pyrrol-2-ones 5a
aReagents and conditions: (i) bromoacetic acid (2 equiv.), DIC (1 equiv.), DCM, 15 min, then DIEA (1 equiv.), 4 h; (ii) PPh3, anhydrous NMP, overnight; (iii) TEA, anhydrous NMP, 2 h; (iv) 50% TFA in DCM, 1 h
The bromoacylamino ketones 2a (R3 = H) were treated with triphenylphospine (PPh3) in anhydrous 1-methyl-2-pyrrolidinone (NMP) to yield triphenylphosphonium salts 3. Synthesis of triphenylphosphonium salts from bromo derivatives in solution was reported on several occasions23-28 and we observed phosphonium salt formation as a side-product during the Mitsunobu reaction on solid phase.29 Exposure of resins 3 to a base (triethylamine, TEA) afforded polymer-supported 1,5-dihydro-pyrrol-2-ones 4. Cleavage from resin with a 50% solution of TFA in dichloromethane (DCM) yielded 1,5-dihydro-pyrrol-2-ones 5.
This Wittig transformation was used only a few times in solution, e.g., for the synthesis of 3-hydroxypyrroles30,31 and 4-alkoxy-1,5-dihydro-pyrrol-2-one moiety of tetronic acid.32 The Horner-Wadsworth-Emmons reaction was also used to prepare 4-alkyl-1,5-dihydro-pyrrol-2-one derivatives.33,34 The use of Wittig chemistry on solid phase was reviewed.35
To extend the diversity of R2 substituents, we also prepared α-acylamino esters for the synthesis of 1,5-dihydro-pyrrol-2-ones with an alkoxy substituent in the position of R2. On-resin transformation to the triphenylphosphonium derivative 3 was followed by base mediated cyclization. There is one literature precedent for this Wittig reaction in solution with an ester as the carbonyl component in the synthesis of this five-membered ring.32
A small combinatorial array of 1,5-dihydro-pyrrol-2-ones was prepared using Fmoc-β-Ala-OH, ethylenediamine, and ethanolamine in the first combinatorial step and three bromoketones and ethyl bromoacetate in the second step.
3-Pyrrol-2-ones can serve as a starting material for the synthesis of biologicaly relevant compounds such as tetramic acid derivatives.36,37 However, 3-pyrrol-2-one core moiety is also found in natural products, e.g. in pulchellalactam, discovered from the marine fungus Corollospora pulchella, a potent CD45 inhibitor.38
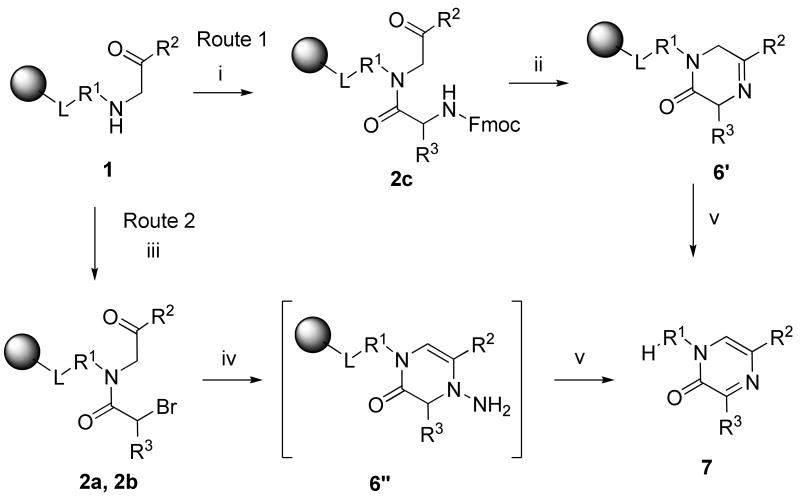
1,3,5-Substituted 1H-pyrazin-2-ones 7
1H-Pyrazin-2-ones 7 were synthesized by two so far unreported routes (Scheme 4). The first route included acylation of polymer-supported amino ketones 1 with Fmoc-protected α-amino acid to yield 2c (R3 = Me). Deprotection of the amino group using 50% piperidine in DMF was followed by a spontaneous ring closure. Cyclization of α-acylamino ketones 2c provided 5,6-dihydro-1H-pyrazin-2-ones 6′, which spontaneously air oxidized, either during TFA cleavage or reaction work up, to yield 1H-pyrazin-2-ones 7.
Scheme 4.
Synthesis of 1H-pyrazin-2-ones 7a
aReagents and conditions: (i) Fmoc-α-amino acid (2 equiv.), DIC (1 equiv.), DCM/DMF (1:1), overnight; (ii) 50% piperidine in DMF, 15 min; (iii) α-bromocarboxylic acid (2 equiv.), DIC (1 equiv.), DCM, 15 min, then DIEA (1 equiv.), 4 h; (iv) hydrazine monohydrate, THF, 2 h; (v) 50% TFA in DCM, 1 h
Although there was a substantial effort dedicated to syntheses of pyrazinones, conversion of α-(2-amino)acylamino ketones has not been reported. The closest analogy to our method is the transformation of dipeptidyl chloromethyl ketones to pyrazinones under rather forcing conditions.39
The second route that afforded 1H-pyrazin-2-ones 7 was rather unexpected. α-Acylamino ketones 2a (R3 = H), 2b (R3 = Et) were prepared by acylation with α-bromocarboxylic acids. Exposure to hydrazine monohydrate afforded, after cleavage from resin with 50% TFA in DCM, 1H-pyrazin-2-ones 7. This plausible mechanism involves the elimination of ammonia by cleavage of the N-N bond. This transformation has a structurally unrelated precedence.40
A small set of 1H-pyrazin-2-ones was synthesized using Fmoc-β-Ala-OH, ethylenediamine, and ethanolamine in the first step, three bromoketones, three α-bromocarboxylic acids and one Fmoc-α-amino acid in the second and third steps, respectively.
A complementary route for the synthesis of 1H-pyrazin-2-ones from the analogous precursors reported in the literature involved nucleophilic substitution of chloride by azide. Subsequent reaction with PPh3 formed 1H-pyrazin-2-ones via iminophosphoranes.41 An alternative synthesis took advantage of reductive amination of chloroacetyl derivatives with an amine in the presence of borohydride, followed by cyclization to piperazinones.42
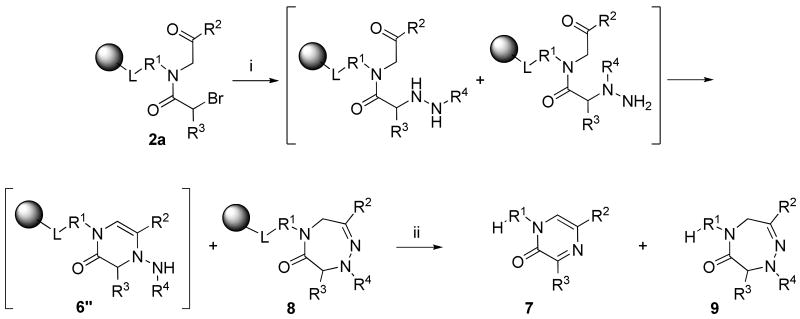
2,3,5,7-Substituted 4,5-dihydro-1,2,5-triazepin-6-ones 9
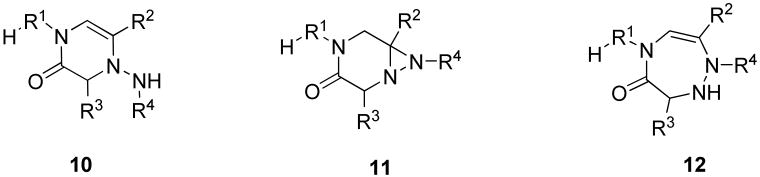
Resin-bound α-acylamino ketones 2a (R3 = H), used for the synthesis of 1H-pyrazin-2-ones, were also reacted with benzylhydrazine monohydrochloride in DMF in the presence of TEA. We observed the formation of two different products (Scheme 5). Besides 1H-pyrazin-2-ones 7, we isolated 4,5-dihydro-1,2,5-triazepin-6-ones 9 (R4 = Bn). The ratio of those two products was 1:2 (at this time, we did not carry out optimization of reaction conditions). The structure of 4,5-dihydro-1,2,5-triazepin-6-one was unequivocally confirmed by 2D NMR spectra (gHMBC) and isomeric structures 10, 11, and 12 (Figure 2) were eliminated.
Scheme 5.
Synthesis of 4,5-dihydro-1,2,5-triazepin-6-ones 9a
aReagents and conditions: (i) benzylhydrazine monohydrochloride, DMF, overnight; (ii) 50% TFA in DCM, 1 h
Figure 2.
Alternative structures with identical molecular mass
The presence of both six- and seven-membered heterocycles can be explained by the formation of two regioisomers when resin-bound bromoacylamino ketones 2a were reacted with alkylhydrazine. Each of the two initially formed regioisomers was transformed to a different product.
To date, 1,2,5-triazepin-6-ones have not been reported in literature. 4H-1,2,5-Triazepine derivatives were prepared from from α-amino hydrazones.43 Two reports described syntheses of 1,2,5-triazepin-3,6-diones44 and their 4-thioxo derivatives.45 Koenig et al.46 attempted to synthesize 3-aryl-tetrahydro-1,2-diazepines by a reaction of aryl-δ-chlorobutyl ketones with unsubstituted and monosubstituted hydrazines. Reaction with unsubstituted hydrazine lead to an unstable product, whereas reaction of aryl-δ-chlorobutyl ketones with monosubstituted hydrazines afforded stable 1-substituted 3-aryl-tetrahydro-1,2-diazepines. Later on the synthesis was improved and the unstable 3-aryl-tetrahydro-1,2-diazepines were converted in-situ to a stable sulfonyl47 and acyl derivatives.48
Fused ring systems
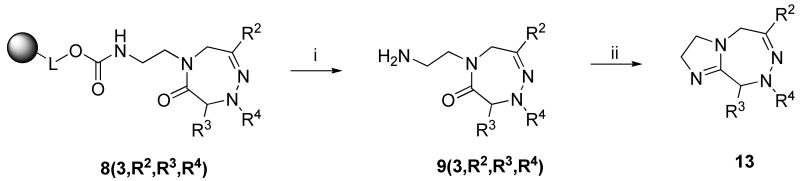
Synthesis of 4,5-dihydro-1,2,5-triazepin-6-ones was carried out with resin 1b prepared using 1,2-diaminoethane. After finishing the synthesis and release from the resin, a fused ring was formed between the side chain R1 and the carbonyl group of amide moiety. Reaction conditions for cyclization of 5-(2-aminoethyl)-4,5-dihydro-1,2,5-triazepin-6-one 9(3,R2,R3,R4) to 3,5,8,9-tetrahydro-2H-imidazo[2,1-d][1,2,5]triazepine 13 (Scheme 6) were optimized. We found it important to neutralize the crude triazepinone 9(3,R2,R3,R4) before subjecting to cyclization. Whereas heating in DMSO and DMF gave low conversion (< 10%), THF resulted in acceptable conversion (albeit with significant contamination) to crude products 13. Gratifyingly, under very mild conditions (DCM, 30 °C, overnight reaction) the fused ring was formed with only 16% of starting material present in the reaction mixture.
Scheme 6.
Synthesis of 3,5,8,9-tetrahydro-2H-imidazo[2,1-d][1,2,5]triazepine fused ring system 13a
aReagents and conditions: (i) 50% TFA in DCM, 1 h; (ii) filtration through C18 cartridge, then DCM, 30 °C, overnight
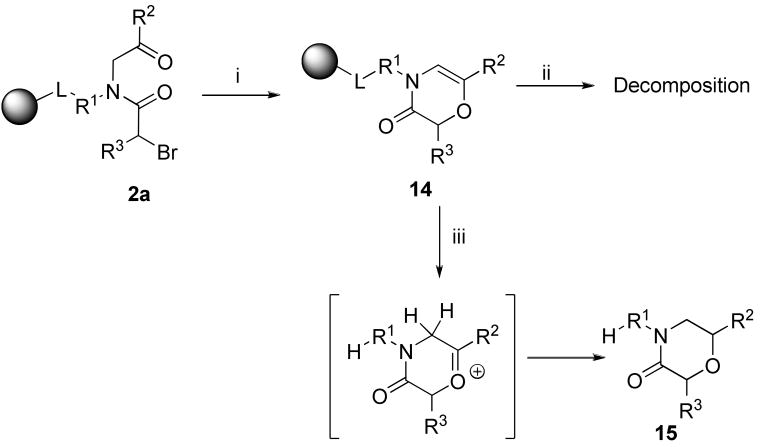
2,4,6-Substituted morpholin-3-ones 15
The next heterocyclic compounds assembled from α-acylamino ketones were morpholin-3-ones 15. The polymer-supported amino ketones 1 were acylated with bromoacetic acid to yield α-acylamino ketones 2a (R3 = H). 2-Tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphorine (BEMP)-mediated cyclization afforded polymer-supported 1,4-oxazine-3-ones 14. Because the 2H-1,4-oxazin-3(4H)-ones were found not to be stable in the TFA used for cleavage from the resin, the resin-bound oxazines were cleaved from resin in solution of TFA, triethylsilane (TES), and DCM in a ratio of 5:1:4 and yielded morpholin-3-ones 15. TES was added to the cleavage cocktail to prevent decomposition of 1,4-oxazine-3-ones 14 via oxonium ions, which could be formed during the cleavage.
Formation of 4H-[1,4]oxazin-3-one has been described as a side-reaction during exposure of (2-chloroacetyl)amino acetic acid methyl ester to a base (4-aminometylpiperidine).49 Synthesis of morpholin-3-ones on solid phase was reported.50
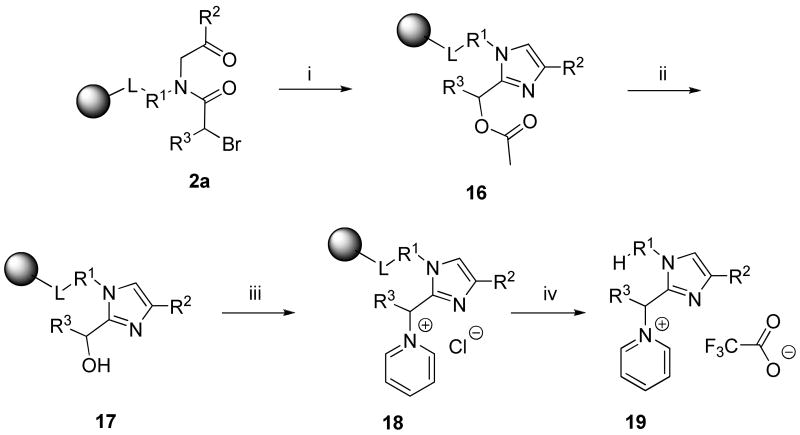
1,2,4-Substituted 1H-imidazoles 19
Synthesis of imidazoles by forming the five-membered ring from resin-bound α-acylamino ketones in the presence of ammonium acetate at elevated temperature has been reported.15-18,51 We have also used this route for synthesis of imidazoles and imidazolyl-quinoxalinones.7 Here we extend the previously reported imidazole set to include 2-hydroxymethyl imidazoles. Cyclization of resin-bound acylamino ketone 2a in acetic acid solution of ammonium acetate yielded O-acetylderivatives 16. After saponification and attempted reaction with mesyl chloride (for further derivatization) and cleavage from the resin, we isolated the pyridinium imidazoles 19 as the only product.
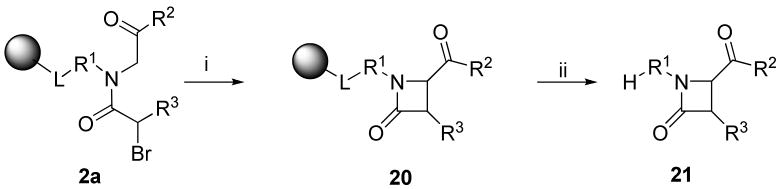
1,3-Substituted 4-oxo-azetidine-2-carboxylic acid esters (β-lactams) 21
The role of β-lactams in drugs and drug-like compounds has been well established. α-Amino acetates 1 (R2 = OEt) were acylated with bromoacetic acid. To convert α-acylamino esters 2a (R3 = H) to β-lactams 21, we tested several different bases for the cyclization; Schwesinger BEMP-mediated cyclization afforded (upon resin cleavage) β-lactams 21 in high crude purity (78%). There are several reports describing this type of cyclization52-57 including examination of the enantioselectivity of the cyclization.53,54 The synthetic route has also been applied to solid-phase58 via immobilization of Fmoc-amino acids to Wang and BAL resins, followed by N-alkylation and acylation with chloroacetic acid.
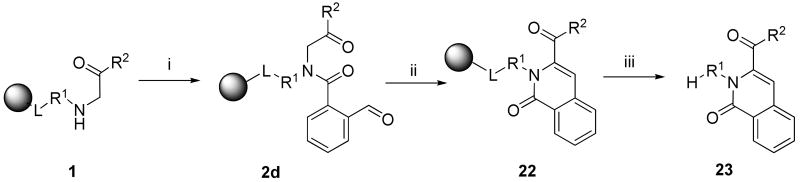
2,3,6-Substituted 2H-isoquinolin-1-ones 23
Resin-bound α-acylamino ketones 2d for the synthesis of 2H-Isoquinolin-1-ones 23 were prepared by acylation with 2-carboxybenzaldehyde. Ring closure was accomplished by intramolecular aldol condensation between the methylene carbon alpha to the ketone and the carbonyl of the carboxybenzaldehyde. DBU-mediated ring closure afforded the isoquinolinone ring 23. An intramolecular aldol condensation that yielded the isoquinolinone moiety has already been reported and used to prepare the framework of camptothecin59 and tetrahydroisoquinolinone derivatives.60 Solid-phase synthesis of highly substituted 2H-isoquinolin-1-ones was reported by Goff and Zuckermann.61,62
Fused ring systems
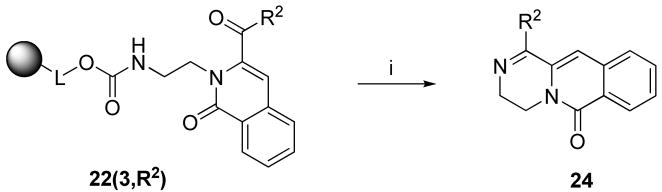
The second example of fused ring closure involved condensation between the amino group residing on the side chain R1 and the carbonyl of R2 side-chain of the 2H-isoquinolin-1-one. 1,2-Diaminoethane was attached to Wang resin via carbamate linkage using carbonyldiimidazole (CDI) activation (Scheme 2). The synthesis was carried out according to the same protocol as described for the synthesis of 2H-isoquinolin-1-ones 23. After cleavage from the resin, the intermediate spontaneously closed the six-membered fused ring and provided access to 3,4-dihydro-pyrazino[1,2-b]isoquinolin-6-ones 24.
Conclusion
We have shown that resin-bound α-acylamino ketones represent versatile intermediates for transformation to diverse and structurally unrelated heterocyclic compounds. Synthetic routes included a variety of chemistries (C-C, C=C, C-N, C=N, C-O bond formations) and size of heterocycles (4-, 5-, 6-, and 7-membered rings). On two examples, we documented that the primary ring closure can be accompanied by the formation of a fused ring. Synthesized compounds were submitted for evaluation of biological activities to High Throughput Screening in the Molecular Libraries Probe Production Centers Network. The results are available in PubChem (http://pubchem.ncbi.nlm.nih.gov/).
Experimental Section
Solid-phase syntheses were carried out on manually operated Domino Block synthesizer63 (www.torviq.com) in disposable polypropylene reaction vessels. Commercially available solvents, resins, and reagents were used. The Rink resin (100-200 mesh, 1% DVB, 0.75 mmol/g), aminomethyl resin (100-200 mesh, 1% DVB, 0.9 mmol/g) and Wang resin (100-200 mesh, 1% DVB, 1.0 mmol/g) were obtained from Advanced ChemTech (Louisville, KY, www.peptide.com). Swelling of resins in DCM was measured before syntheses and resins with swollen volume greater than 7 mL/g of dry resin were used.64 All reactions were carried out at ambient temperature (21 °C) unless stated otherwise. The yield was calculated based on resin loading of the first building block.
Acylation with α-bromocarboxylic acid (resins 2a, 2b)
Resin 1, ∼1 g, was washed 3 × with DCM. A solution of α-bromocarboxylic acid (5 mmol) in 10 mL DCM was made in polypropylene reaction vessels and DIC (2.5 mmol, 387 μL) was added. After 5 min N,N′-di-i-propylurea (DIU) was filtered, DIEA (2.5 mmol, 436 μL) added and solution added to resin 1 and reacted 4 h. The resin was washed 5 × with DCM.
Acylation with Fmoc-α-amino acid (resins 2c)
Resin 1, ∼250 mg, was washed 3 × with DCM. A solution of Fmoc-α-amino acid (1 mmol) and DIC (0.5 mmol, 77 μL) in 2.5 mL DCM/DMF (1:1) was added to the resin and reaction slurry was shaken overnight. The resin was washed 3 × with DMF and 3 × with DCM.
Acylation with 2-carboxybenzaldehyde (resins 2d)
Resin 1, ∼1 g, was washed 3 × with DCM and solution of 2-carboxybenzaldehyde (10 mmol, 1.5 g) and DIC (5 mmol, 770 μL) in 10 mL DMF/DCM (1:1) was added. After overnight reaction the resin was washed 3 × with DMF and 3 × with DCM.
1,3,4-Derivatized 1,5-dihydro-pyrrol-2-ones 5
Preparation of triphenylphosphonium salt (resins 3)
Resin 2a, ∼250 mg, was washed 3 × with anhydrous DCM and 3 × with anhydrous NMP. A solution of triphenylphosphine (1 mmol, 262 mg) in 2.5 mL anhydrous NMP was added to the resin and slurry was shaken overnight. The resin was washed 3 × with DCM.
Cyclization (resins 4)
Resin 3, ∼250 mg, was washed 3 × with anhydrous DCM and 3 × with anhydrous NMP. A solution of TEA (0.25 mmol, 35 μL) in 2.5 mL anhydrous NMP was added to the resin and slurry was shaken two hours. The resin was washed 3 × with DCM.
1,3,5-Substituted 1H-pyrazin-2-ones 7
Route 1
Cyclization (resin 6′)
Resin 2c, ∼250 mg, was washed 3 × with DMF and treated with 2.5 mL 50% piperidine in DMF for 15 minutes. The resin was washed 3 × with DMF and 3 × with DCM.
Route 2
Cyclization (resins 6″)
Resins 2a, 2b, ∼250 mg, was washed 3 × with THF and a solution of hydrazine monohydrate (6 mmol, 0.3 mL) in 2.5 mL THF was added and reacted two hours. The resin was washed 3 × with THF and 3 × with DCM.
2,3,5,7-Substituted 4,5-dihydro-1,2,5-triazepin-6-ones 9
Cyclization (resins 8)
Resin 2a, ∼250 mg, was washed 3 × with DMF and a solution of benzylhydrazine monohydrochloride (1 mmol, 170 mg) and TEA (1 mmol, 0.15 mL) in 3 mL DMF was added and reacted overnight. The resin was washed 3 × with DMF and 3 × with DCM.
2,4,6-Substituted morpholin-3-ones 15
Cyclization to 1,4-oxazine-3-ones (resins 14)
Resin 2a (R2 = aryl), ∼250 mg, was washed 3 × with anhydrous NMP and a solution of BEMP (0.09 mmol, 25 μL) in 2.5 mL anhydrous NMP and resin slurry was shaken 5 min. The resin was neutralized with 3% AcOH in DMF, washed 3 × with DMF, 3 × with DCM, 3 × with MeOH and 3 × with DCM.
Cleavage from resin to obtain morpholin-3-ones (15)
Resin 14, ∼250 mg, was treated with a solution of TFA, TES and DCM (5:1:4) for one hour. TFA solution was collected, resin was washed 3 × with 50% TFA in DCM, combined extracts were evaporated by a stream of nitrogen and crude products were purified by reversed-phase HPLC.
1,2,4-Substituted 1H-imidazoles 19
Cyclization by ammonium acetate in AcOH (resins 16)
Resin 2a, ∼250 mg, was heated in a solution of 2.5 M ammonium acetate (25 mmol, 1.93 g) in 10 mL AcOH at 100 °C overnight. The reaction was repeated when the cyclization was not complete. The resin was washed 5 × with DCM.
Ester cleavage (resins 17)
Resin 16, ∼250 mg, was treated washed 3 × with THF and reacted with solution of 0.5 mL 10 M NaOH in 10 mL THF/MeOH (1:1) for 1 h. Resin was washed 3 × with THF, 3% AcOH in THF, 3 × with DCM.
Reaction with mesyl chloride (resins 18)
Resin 17, ∼250 mg, was washed 3 × with pyridine. A solution of mesyl chloride (2.5 mmol, 192 μL) in 5 mL pyridine was added and left for 1 h. Resin was washed 3 × with pyridine and 3 × with DCM.
1,3-Substituted β-lactams 21
Cyclization (resins 20)
Resin 2a (R2 = OEt), ∼400 mg, was washed with DCM and anhydrous NMP and treated with BEMP (40 μL) in 4 mL NMP for 30 min. Resin was washed 3 × with DMF, 3 × with DCM, 3 × with MeOH, and 3 × with DCM.
2,3,6-Substituted 2H-isoquinolin-1-ones 23
Cyclization (resins 22)
Resin 2d, ∼250 mg, was washed 3 × with DMF and solution of 0.2 M DBU (150 μL) in 5 mL DMF was added and tumbled for 30 min. The cyclization was monitored by LCMS analysis of a sample cleaved from the resn. The resin was washed with 3 × with DMF, 5 × with DCM, 3% n-propylamine in DCM, DCM, 3% AcOH in DCM, and 3 × with DCM.
Cleavage from resin (compounds 5, 7, 9, 13, 19, 21, 23, 24)
Target compounds were obtained after cleavage from resin with 50% TFA in DCM for 1 h. TFA solution was collected, resin was washed 3 × with 50% TFA in DCM and combined extracts were evaporated by a stream of nitrogen. All crude products were purified by semi-preparative HPLC.
The crude compound 9(3,R2,R3,R4) was dissolved in 2 mL acetonitrile, diluted with 10 mL 10 mM ammonium acetate buffer and filtered through C18 cartridge. The compound was eluted by acetonitrile and the solvent was evaporated, oily residue dissolved in 10 mL DCM and heated at 30 °C overnight to yield fused ring system 13.
Supplementary Material
Scheme 7.
Synthesis of morpholin-3-ones 15a
aReagents and conditions: (i) BEMP, anhydrous NMP, 15 min; (ii) 50% TFA, DCM, 1 h; (iii) TFA/TES/DCM (5:1:4), 1 h
Scheme 8.
Synthesis of 1H-imidazoles 19a
aReagents and conditions: (i) ammonium acetate, acetic acid, 100 °C, overnight; (ii) KOTMS, THF, 1 h; (iii) mesyl chloride, pyridine, 1 h; (iv) 50% TFA in DCM, 1 h
Scheme 9.
Synthesis of β-lactams 21a
aReagents and conditions: (i) BEMP, NMP, 30 min; (ii) 50% TFA, DCM, 1 h
Scheme 10.
Synthesis of 2H-isoquinolin-1-ones 23a
aReagents and conditions: (i) 2-carboxybenzaldehydes (2 equiv.), DIC (1 equiv.), DMF, overnight; (ii) DBU, DMF, 30 min; (iii) 50% TFA, DCM, 1 h
Scheme 11.
Synthesis of pyrazino[1,2-b]isoquinolinone fused ring system 24a
aReagents and conditions: (i) 50% TFA in DCM, 1 h
Acknowledgments
The work was supported by the Department of Chemistry and Biochemistry at the University of Notre Dame and the NIH (GM079576). We gratefully appreciate the use of the NMR facility at the University of Notre Dame.
Footnotes
Supporting Information Available. Details of experimental procedures, spectroscopic data and NMR spectra for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Burke MD, Schreiber SL. Angew Chem, Int Ed. 2004;43:46–58. doi: 10.1002/anie.200300626. [DOI] [PubMed] [Google Scholar]
- 2.Lee SH, Clapham B, Koch G, Zimmermann J, Janda KD. Org Lett. 2003;5:511–514. doi: 10.1021/ol020244j. [DOI] [PubMed] [Google Scholar]
- 3.Lee SH, Clapham B, Koch G, Zimmermann J, Janda KD. J Comb Chem. 2003;5:188–196. doi: 10.1021/cc020079z. [DOI] [PubMed] [Google Scholar]
- 4.Matsushita H, Lee SH, Yoshida K, Clapham B, Koch G, Zimmermann J, Janda KD. Org Lett. 2004;6:4627–4629. doi: 10.1021/ol047933a. [DOI] [PubMed] [Google Scholar]
- 5.Krchňák V, Holladay MW. Chem Rev. 2002;102:61–91. doi: 10.1021/cr010123h. [DOI] [PubMed] [Google Scholar]
- 6.Sauer WHB, Schwarz MKJ. Che Inf Comput Sci. 2003;43:987–1003. doi: 10.1021/ci025599w. [DOI] [PubMed] [Google Scholar]
- 7.Kočí J, Pudelová N, Krchňák V. J Comb Chem. 2009;11:397–402. doi: 10.1021/cc800210q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuyama T, Jow CK, Cheung M. Tetrahedron Lett. 1995;36:6377–6374. [Google Scholar]
- 9.Bouillon I, Zajíček J, Pudelová N, Krchňák V. J Org Chem. 2008;73:9027–9032. doi: 10.1021/jo8018895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tice CM, Michelotti EL, Mata EG, Nicolŕs E, Garcia J, Albericio F. Tetrahedron Lett. 2002;43:7491–7494. [Google Scholar]
- 11.Garcia J, Nicolàs E, Albericio F, Michelotti EL, Tice CM. Tetrahedron Lett. 2002;43:7495–7498. [Google Scholar]
- 12.Tice CM, Hormann RE, Thompson CS, Friz JL, Cavanaugh CK, Saggers JA. Bioorg Med Chem Lett. 2003;13:1883–1886. doi: 10.1016/s0960-894x(03)00315-9. [DOI] [PubMed] [Google Scholar]
- 13.Morwick T, Hrapchak M, DeTuri M, Campbell S. Org Lett. 2002;4:2665–2668. doi: 10.1021/ol020092s. [DOI] [PubMed] [Google Scholar]
- 14.Garcia J, Mata EG, Tice CM, Hormann RE, Nicolas E, Albericio F, Michelotti EL. J Comb Chem. 2005;7:843–863. doi: 10.1021/cc0500396. [DOI] [PubMed] [Google Scholar]
- 15.Sarshar S, Siev D, Mjalli AMM. Tetrahedron Lett. 1996;37:835–838. [Google Scholar]
- 16.Lee HB, Balasubramanian S. Org Lett. 2000;2:323–326. doi: 10.1021/ol991271l. [DOI] [PubMed] [Google Scholar]
- 17.Cobb JM, Grimster N, Khan N, Lai JYQ, Payne HJ, Payne LJ, Raynham T, Taylor J. Tetrahedron Lett. 2002;43:7557–7560. [Google Scholar]
- 18.Li W, Lam Y. J Comb Chem. 2005;7:644–647. doi: 10.1021/cc049818x. [DOI] [PubMed] [Google Scholar]
- 19.Pudelová N, Krchňák V. J Comb Chem. 2009;11:370–374. doi: 10.1021/cc800181y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang SS. J Am Chem Soc. 1973;95:1328–1333. doi: 10.1021/ja00785a602. [DOI] [PubMed] [Google Scholar]
- 21.Rink H. Tetrahedron Lett. 1987;28:3787–3790. [Google Scholar]
- 22.Jensen KJ, Alsina J, Songster MF, Vagner J, Albericio F, Barany G. J Am Chem Soc. 1998;120:5441–5452. [Google Scholar]
- 23.Dauben WG, Gerdes JM, Bunce RA. J Org Chem. 1984;49:4293–4295. [Google Scholar]
- 24.Nader FW, Brecht A, Kreisz S. Chem Ber. 1986;119:1208–1216. [Google Scholar]
- 25.Ikuta H, Shirota H, Kobayashi S, Yamagishi Y, Yamada K, Yamatsu I, Katayama K. J Med Chem. 1987;30:1995–1998. doi: 10.1021/jm00394a011. [DOI] [PubMed] [Google Scholar]
- 26.IbrahimOuali M, Sinibaldi ME, Troin Y, Gardette D, Gramain JC. Synth Commun. 1997;27:1827–1848. [Google Scholar]
- 27.Boisse T, Rigo B, Millet R, Henichart JP. Tetrahedron. 2007;63:10511–10520. [Google Scholar]
- 28.Creech GS, Kwon O. Org Lett. 2008;10:429–432. doi: 10.1021/ol702462w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krchňák V. J Comb Chem. 2005;7:507–509. doi: 10.1021/cc0498276. [DOI] [PubMed] [Google Scholar]
- 30.Flitsch W, Hampel K, Hohenhorst M. Tetrahedron Lett. 1987;28:4395–4396. [Google Scholar]
- 31.Flitsch W, Hohenhorst M. Liebigs Ann Chem. 1990:397–399. [Google Scholar]
- 32.Brennan J, Murphy PJ. Tetrahedron Lett. 1988;29:2063–2065. [Google Scholar]
- 33.Inagaki H, Takeda T, Miyauchi RN, Kawakami K, Takahashi H, Takemura M. Heterocycles. 2004;63:699–706. [Google Scholar]
- 34.Irie H, Shibata K, Matsuno K, Zhang Y. Heterocycles. 1989;29:1033–1035. [Google Scholar]
- 35.Lorsbach BA, Kurth M. J Chem Rev. 1999;99:1549–1581. doi: 10.1021/cr970109y. [DOI] [PubMed] [Google Scholar]
- 36.Huang PQ, Wu TJ, Ruan YP. Org Lett. 2003;5:4341–4344. doi: 10.1021/ol035617a. [DOI] [PubMed] [Google Scholar]
- 37.Paintner FF, Metz M, Bauschke G. Synlett. 2003:627–630. [Google Scholar]
- 38.Alvi KA, Nair B, Gallo C, Baker D. J Antibiotics. 1997;50:264–266. [PubMed] [Google Scholar]
- 39.Taguchi H, Yokoi T, Tsukatani M, Okada Y. Tetrahedron. 1995;51:7361–7372. [Google Scholar]
- 40.Gilchrist TL, Healy MAM. Tetrahedron. 1993;49:2543–2556. [Google Scholar]
- 41.Ackrell J, Galeazzi E, Muchowski JM, Tokes L. Can J Chem. 1979;57:2696–2702. [Google Scholar]
- 42.Dinsmore CJ, Zartman CB. Tetrahedron Lett. 2000;41:6309–6312. [Google Scholar]
- 43.Gnichtel H, Salem WI. Liebigs Ann Chem. 1982:729–733. [Google Scholar]
- 44.Mahran MA, El-Sayed OA, Fahmy HTY, Ashour FA. Alexandria J Pharm Sci. 1996;10:133–135. [Google Scholar]
- 45.Zaleska B, Trzewik B, Grochowski J, Serda P. Synthesis. 2003:2559–2563. [Google Scholar]
- 46.Koenig JJ, Wermuth CG. Tetrahedron Lett. 1973;14:603–606. [Google Scholar]
- 47.Wiethe RW, Stewart EL, Drewry DH, Gray DW, Mehbob A, Hoekstra WJ. Bioorg Med Chem Lett. 2006;16:3777–3779. doi: 10.1016/j.bmcl.2006.04.055. [DOI] [PubMed] [Google Scholar]
- 48.Kalyanam D, Dave KG, Likhate MA. Synth Commun. 1988;18:2183–2191. [Google Scholar]
- 49.Feng DZ, Song YL, Jiang XH, Chen L, Long YQ. Org Biomol Chem. 2007;5:2690–2697. doi: 10.1039/b707175b. [DOI] [PubMed] [Google Scholar]
- 50.Dolle RE, Macleod C, Martinez-Teipel B, Barker W, Seida PR, Herbertz T. Angew Chem, Int Ed. 2005;44:5830–5833. doi: 10.1002/anie.200501665. [DOI] [PubMed] [Google Scholar]
- 51.Zhang CZ, Moran EJ, Woiwode TF, Short KM, Mjalli AMM. Tetrahedron Lett. 1996;37:751–754. [Google Scholar]
- 52.Sheehan JC, Bose AK. J Am Chem Soc. 1951;73:1761–1765. [Google Scholar]
- 53.Perez-Faginas P, Garcia-Lopez IAMT, Gonzalez-Muniz R. Tetrahedron Lett. 2008;49:215–218. [Google Scholar]
- 54.Perez-Faginas P, O'Reilly F, O'Byrne A, Garcia-Aparicio C, Martin-Martinez M, de Vega MJP, Garcia-Lopez MT, Gonzalez-Muniz R. Org Lett. 2007;9:1593–1596. doi: 10.1021/ol070533d. [DOI] [PubMed] [Google Scholar]
- 55.Santa Z, Nagy J, Nyitrai J. Tetrahedron-Asymmetry. 2006;17:3111–3127. [Google Scholar]
- 56.Bonache MA, Cativiela C, Garcia-Lopez MT, Gonzalez-Muniz R. Tetrahedron Lett. 2006;47:5883–5887. [Google Scholar]
- 57.Bonache MA, Gerona-Navarro G, Garcia-Aparicio C, Alias M, Martin-Martinez M, Garcia-Lopez MT, Lopez P, Cativiela C, Gonzalez-Muniz R. Tetrahedron-Asymmetry. 2003;14:2161–2169. [Google Scholar]
- 58.Gerona-Navarro G, Royo M, Garcia-Lopez MT, Albericio F, Gonzalez-Muniz R. Molecular Diversity. 2003;6:75–84. doi: 10.1023/b:modi.0000006837.36303.eb. [DOI] [PubMed] [Google Scholar]
- 59.Shamma M, Novak L. Tetrahedron. 1969;25:2275–2279. [Google Scholar]
- 60.Alonso R, Castedo L, Dominguez D. Tetrahedron Lett. 1986;27:3539–3542. [Google Scholar]
- 61.Goff DA, Zuckermann RN. J Org Chem. 1995;60:5748–5749. [Google Scholar]
- 62.Trotter BW, Nanda KK, Kett NR, Regan CP, Lynch JJ, Stump GL, Kiss L, Wang J, Spencer RH, Kane SA, White RB, Zhang RN, Anderson KD, Liverton NJ, McIntyre CJ, Beshore DC, Hartman GD, Dinsmore CJ. J Med Chem. 2006;49:6954–6957. doi: 10.1021/jm060927v. [DOI] [PubMed] [Google Scholar]
- 63.Krchňák V, Paděra V. Bioorg Med Chem Lett. 1998;22:3261–3264. doi: 10.1016/s0960-894x(98)00594-0. [DOI] [PubMed] [Google Scholar]
- 64.Bouillon I, Soural M, Miller MJ, Krchňák V. J Comb Chem. 2009;11:213–215. doi: 10.1021/cc800143e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.