Abstract
Internal ribosome entry site (IRES) RNAs initiate protein synthesis in eukaryotic cells by a noncanonical cap-independent mechanism. IRESes are critical for many pathogenic viruses, but efforts to understand their function are complicated by the diversity of IRES sequences as well as by limited high-resolution structural information. The intergenic region (IGR) IRESes of the Dicistroviridae viruses are powerful model systems to begin to understand IRES function. Here we present the crystal structure of a Dicistroviridae IGR IRES domain that interacts with the ribosome’s decoding groove. We find that this RNA domain precisely mimics the transfer RNA anticodon–messenger RNA codon interaction, and its modeled orientation on the ribosome helps explain translocation without peptide bond formation. When combined with a previous structure, this work completes the first high-resolution description of an IRES RNA and provides insight into how RNAs can manipulate complex biological machines.
Canonical eukaryotic translation initiation is a complex, multistep process in which a modified nucleotide cap on the 5′ end of the mRNA is necessary for protein factor–dependent recruitment of the 40S (small) ribosomal subunit1. The subunit scans the message and recognizes the AUG initiation codon, which leads to GTP hydrolysis, initiation factor protein release, and 60S (large) subunit binding to form the 80S ribosome–mRNA complex. The assembled 80S ribosome, containing an initiator tRNA in the P site, begins translation through eukaryotic elongation factor (eEF) 1A–mediated delivery of an aminoacylated tRNA into the A site. Subsequent peptide bond formation in the ribosome’s peptidyl transferase site is followed by eEF 2-catalyzed translocation, and the ribosome enters the elongation phase of protein synthesis (Fig. 1a). Thus, canonical cap-dependent translation initiation is driven by the action of many protein factors and begins in the P site from an AUG codon in good context; then translocation occurs after the initial peptide bond is made.
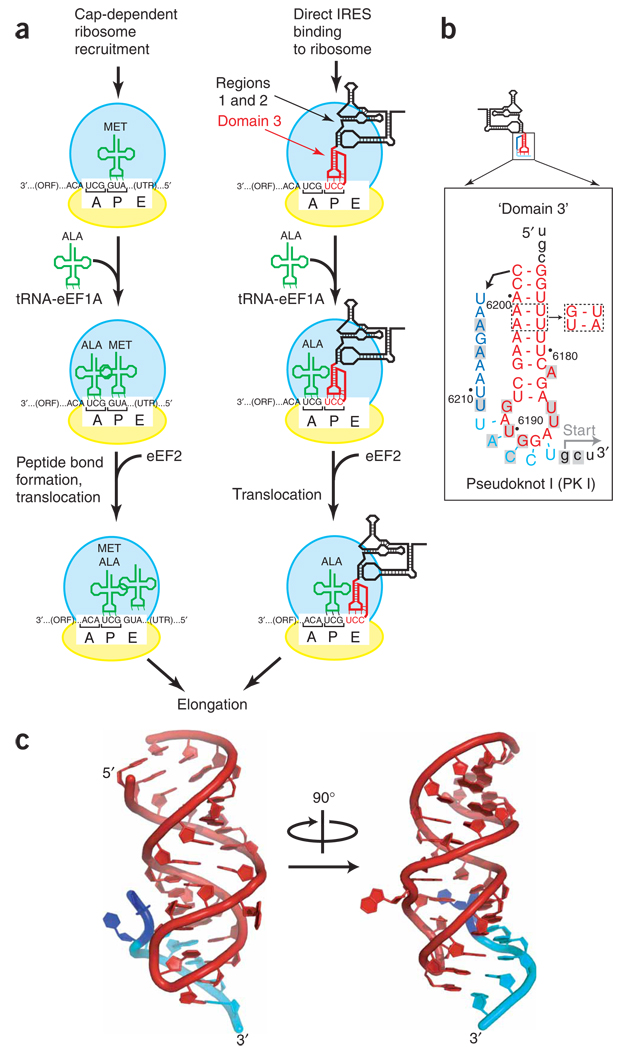
Figure 1. The P site domain of the Dicistroviridae intergenic region IRESes.
(a) Comparison of canonical initiation of protein synthesis compared with that driven by the Dicistroviridae IGR IRES RNA. The steps of initial ribosome recruitment and placement on the message are not shown. 40S subunit, yellow, 60S subunit, blue; tRNA, green; ribosome binding domain (regions 1 + 2) of the IRES, black; domain 3 of the IRES, red. Locations of the A, P and E sites are labeled. (b) Secondary structure of domain 3 from the CrPV IGR IRES. The orientation of this domain has been flipped relative to panel a to reflect how it generally is depicted in the literature. Nucleotides in lowercase are not included in our crystal construct. Gray-shaded boxes, nucleotides that are conserved in six or more of seven members of this IRES class (Supplementary Fig. 1). Dashed boxes, mutations that introduced a heavy atom binding site for obtaining crystallographic phase information (Supplementary Fig. 2). (c) Two views (90° rotation) of the crystal structure of domain 3 of the CrPV IGR IRES, colored to match the secondary structure of panel b. Nts 6203–6209 are not shown because their structure was affected by crystal packing (Supplementary Fig. 3).
In contrast to the canonical cap-dependent pathway, an important alternate mechanism is internal initiation of translation. This mechanism depends on specific cis-acting RNA sequences called IRESes2,3. There is great diversity in IRES sequence, secondary structure and requirements for protein factors, but all IRESes recruit, position and activate the protein-making machinery without recognizing the cap or the 5′ end of the mRNA. IRESes are critical for the infection of many pathogenic viruses and may be important regulatory elements in gene expression2. Insight into the mechanism of many IRESes has been provided by a variety of approaches, but details of their RNA structure-based function remain incomplete. Despite the diversity among IRES RNAs, there may be fundamental structural or mechanistic commonalities that have yet to be discovered. For example, both the IRESes from the IGR of the Dicistroviridae family of viruses and the hepatitis C virus (HCV) interact with ribosomal protein S5 (refs. 4–6), and both contact the ribosome over the E site and through the large subunit’s L1 stalk4,5,7,8. Furthermore, cryo-EM reconstructions of these IRES RNAs bound to the ribosome indicate that both may undergo subtle structural rearrangement during the course of preinitiation complex formation5,7. Answering the question of whether these observations reflect deeper similarities requires more detailed insight into the structure-based mechanisms of model IRES systems.
Among IRES model systems, the Dicistroviridae IGR IRESes possess the most streamlined mechanism of action, because they initiate translation by binding directly to and manipulating the ribosome without any protein initiation factors or initiator tRNA, making them RNA structure-driven translation apparatuses9. The Dicistroviridae IGR IRESes vary in sequence, but all share a three-dimensional architecture comprising two independently folded RNA domains that work together to start protein synthesis10–14. The larger domain interacts with both the 40S and 60S ribosomal subunits to recruit the ribosome directly to the IRES11–13 (Fig. 1a, right). The smaller domain (domain 3) includes the functionally essential pseudoknot I (PK I), which contains a cluster of conserved bases (Fig. 1b and Supplementary Fig. 1 online)14. Domain 3 docks into the small ribosomal subunit’s P site during translation initiation, which is noteworthy because in canonical translation this location must be occupied by initiator tRNAMet (ref. 15) (Fig. 1a). The IGR IRES-driven mechanism now progresses when delivery of a cognate tRNA to the A site leads to eEF 2-catalyzed translocation that is unusual because it occurs without initial peptide bond formation or P site tRNA16. The result of this RNA structure-driven pathway is that unlike typical mRNAs, translation starts immediately downstream of PK I, in the A site, without initiator tRNA, and from a non-AUG codon15,17–21. Because it enters the P site and facilitates the IGR IRES’s streamlined mechanism, domain 3 has been called a ‘tRNA-like element’, although no high-resolution structural data have existed to explain this functional mimicry22. The lack of a structure can be explained by biochemical characterization that shows PK I is dynamic, with transient base-pairing11–13.
To understand how a relatively small structured RNA can manipulate the ribosome to initiate from the A site and translocate without peptide bond formation and to allow the development of detailed structure-based models for IRES function, we have solved the structure of domain 3 of the cricket paralysis virus (CrPV) IGR IRES RNA by X-ray crystallography to a resolution of 2.4 Å. The structure of domain 3, when combined with the previously solved structure of the ribosome binding domain of an IGR IRES23, yields the first complete structural picture of a viral IRES RNA. Combined with mutagenesis and functional and biophysical analysis, this structure suggests a mechanism for translocation without peptide bond formation.
RESULTS
Overview of the structure
The dynamic nature of domain 3′s PK I indicated that it might be difficult to crystallize. Indeed, a NMR spectrum of this RNA indicated dynamic behavior unsuitable for structure determination, and domain 3 RNA from a variety of IGR IRESes failed to crystallize (data not shown). To find diffracting crystals we screened domain 3 RNAs from several members of the Dicistroviridae family of IGR IRES RNAs (Supplementary Figs. 1 and 2 online) and made systematic changes to the 3′ and 5′ ends of the RNA to find a single version that gave crystals that ultimately diffracted to a resolution of 2.4 Å (Fig. 1b). Both wild-type RNA and a version that contained a mutation used for phasing24 crystallized in the same space group and formed the same structure; we present the derivative structure here that diffracted to highest resolution. All 43 nucleotides of domain 3 were visible in the structure, although crystal packing placed nucleotides (nts) 6203–6209 into a conformation that is not biologically relevant, and hence this portion of the structure was not part of our analyses (Supplementary Fig. 3 online).
The structure of domain 3 of the CrPV IGR IRES RNA consists of essentially two elements (Fig. 1c). The first element contains two continuously stacked A-form helices with a single extruded, highly conserved adenosine nucleotide between them (A6182). The second element of the structure is the PK I interaction that forms between the hairpin loop and the last five nts of domain 3 (Fig. 1c), which is the part of the structure that docks into the P site. We observe five base pairs in the pseudoknot, which is the number predicted by phylo-genetic and mutational analyses14,15 and which is two more than those formed in the canonical tRNA-mRNA interaction. Overall, the structure follows classic H-type RNA pseudoknot topology25, with the two stems stacked on each other, loop 1 (containing U6185 and U6186) crossing through the major groove of stem 2 (PK I), loop 2 (containing G6192) stacked between the two stems and loop 3 (nts 6203–6211) poised to interact with the minor groove of stem 1 (Supplementary Fig. 4 online).
The structure reveals a new form of tRNA-mRNA mimicry
Examination of the structure of domain 3 and comparison with the structure of an authentic initiator tRNA-mRNA interaction in the P site of a bacterial ribosome26 reveals that both the topology of the sugar-phosphate backbone and the position of PK I’s bases are virtually indistinguishable from the tRNA’s anticodon loop, and the pathway of the 3′ end of the pseudoknot mimics the pathway taken by an mRNA interacting with an initiator tRNA’s anticodon loop (Fig. 2a). This is the first example of precise mimicry of the initiator tRNA anticodon-mRNA codon intermolecular interaction by a single RNA domain and the first direct demonstration of structural molecular mimicry by an IRES RNA. One difference between the two structures is the presence of A6191 in the CrPV domain 3 loop, which does not correspond to any base in the authentic P site initiator tRNA; this extra base causes G6192 to be tilted and shifted slightly from the position of the corresponding base in the tRNA anticodon loop (Fig. 2a). The three bases that comprise the ‘anticodon’, which are conserved as purines9,14, are stacked in the same manner as in an authentic tRNA. This displays their Watson-Crick faces for base-pairing with the three 3′-most bases of PK I immediately upstream of the translation start site.
Figure 2. Structural details and comparison to authentic tRNA-mRNA interactions.
(a) Left, two views of PK I of domain 3 that mimics the anticodon-codon interaction; right, corresponding views of an authentic initiator tRNA anticodon–mRNA codon interaction in the P site of a ribosome (from PDB 2J00)26. Corresponding bases in the two structures are colored to match each other. Cyan in tRNA–mRNA structure (right), mRNA; cyan in domain 3 structure (left), 3′ end of the IRES RNA that mimics mRNA. (b) Interactions that stabilize the structure, with highly conserved nucleotides (Fig. 1b,c) colored, more variable nucleotides gray, and the U6186 cross-loop hydrogen bond indicated with an asterisk. (c) Location of a constrained iridium(III) hexammine cation (magenta) in the major groove of the anticodon loop of the P site domain structure. Highly conserved bases are colored and variable bases are gray. Partially negatively charged functional groups, including base carbonyl and phosphate oxygens, are in red. Dashed line, location of the U6186 cross-loop hydrogen bond to G6189’s phosphate. The three ‘anticodon’ bases that are conserved as purines are indicated.
Specific interactions and structures are critical for function
The structure of the anticodon loop mimic of the CrPV IGR IRES domain 3 is stabilized by a network of hydrogen bonds between highly conserved nucleotides and well-ordered water molecules (Fig. 2b). The presence of highly conserved bases U6185, U6186 and G6192 in the loop that do not base-pair in the PK I interaction indicates that the sequence conservation may exist in part to maintain a very specific structure in the loop. Specifically, U6186 spans the loop to hydrogen-bond to a phosphate oxygen of G6189, an interaction mirrored in the initiator tRNA anticodon loop, and the bases of G6189 and U6190 form water-mediated hydrogen bonds with U6186 (Fig. 2b). The conservation of the two bases that close the loop (U6185 and G6192) suggests that a base-pairing interaction might occur (Supplementary Fig. 1), but these two bases do not pair and their position matches the analogous bases in initiator tRNA (Fig. 2a). The tight backbone turn in the anticodon loop mimic seems to be stabilized by a bound cation that docks in an electronegative pocket formed by both phosphates and base functional groups, consistent with the observation that cations also interact within tRNA anticodon loops27 (Fig. 2c). In the derivative structure shown here, the cation is a hexammine ion, suggesting the cation provides a general neutralization of the negative charge formed by this classic U-turn motif.
Exploring the role of specific bases
The importance of base-pairing in PK I is well established by mutagenesis14. Bases U6185, U6186 and G6192 are not part of the PK I base-pairing, but our structure suggests that they are important for forming the overall codon-anticodon mimic, and mutation of these bases should reduce the ability of the IRES to initiate translation. To test this structure-based prediction, we mutated these bases within a dicistronic–dual luciferase construct (Fig. 3a) and assayed their ability to initiate translation in translationally competent rabbit reticulocyte lysate (RRL) as described13, using the ratio of downstream (firefly, F) to upstream (renilla, R) luciferase as the measure of IRES efficiency (Fig. 3 and Table 1). Wild-type CrPV IGR IRES served as the positive control, and a previously characterized mutant in which both PK I and PK III were altered served as the negative control13. We found that point mutations to any of these bases led to a decrease in IRES efficiency (Fig. 3b–d) but were not as deleterious as mutations that completely abrogate critical pseudoknot interactions19. This supports the conclusion that the specific interactions involving these bases are important for IRES function, although no one point mutation is sufficient to completely block IRES function. For example, in the case of U6186 the base reaches across the loop to form a hydrogen bond with a phosphate oxygen, and mutant U6186A alters the hydrogen-bonding pattern and places a bulky purine in place of the pyrimidine. This probably leads to a suboptimal structure in which the anticodon mimicry is subtly altered, but the IRES still can function at a decreased level. To further examine the importance of these bases we measured the translation initiation efficiency of an IRES in which all three of these bases were mutated (U6185C, U6186A, G6192C). This ‘triple’ mutant IRES showed no measurable IRES activity, demonstrating that these three unpaired bases are critical for function (Fig. 3e).
Figure 3. Translation initiation assays for mutant CrPV IGR IRES RNAs.
(a) Diagram of a dual luciferase construct, with the firefly (F) and renilla (R) luciferase genes shown. (b–e) Bar graphs showing the average F/R ratios of mutant CrPV IGR IRES RNAs compared with wild type and a negative control IRES (ΔPK I/ΔPK III)13. ‘Triple’ refers to the mutant containing U6185C + U6186A + G6192C. Error bars, s.e.m. from at least four experiments. Values are given in Table 1.
Table 1.
Translation initiation efficiency of mutant CrPV IRES RNAs
| Mutant | Translation efficiencya (F/R ratio) |
Translation efficiencya (% wild-type IRES) |
|---|---|---|
| WT | 0.211 ± 0.038 | 100 ± 17.8 |
| ΔPK1/ΔPKIIIb | 0.009 ± 0.001 | 4 ± 1 |
| U6185A | 0.103 ± 0.018 | 49 ± 8.6 |
| U6185C | 0.040 ± 0.017 | 19 ± 8.2 |
| U6185G | 0.082 ± 0.005 | 39 ± 2.6 |
| U6186A | 0.044 ± 0.014 | 21 ± 6.4 |
| U6186C | 0.103 ± 0.019 | 49 ± 9.0 |
| U6186G | 0.068 ± 0.007 | 32 ± 3.4 |
| G6192A | 0.098 ± 0.015 | 47 ± 7.0 |
| G6192C | 0.025 ± 0.006 | 12 ± 2.7 |
| Triple (U6185C + U6186A + G6192C) | 0.0032 ± 0.0004 | 1.5 ± 0.2 |
| Δ6191 | 0.315 ± 0.033 | 149 ± 16 |
| 6190–6191_comp | 0.0032 ± 0.0004 | 1.5 ± 0.2 |
Average values and standard errors from at least three experiments are reported.
Mutant originally characterized in ref.13.
Docking the structure into the ribosome
During translation initiation domain 3 must dock into the P site of the ribosome15. The structure presented here and published biochemical data seem to indicate that it precisely mimics the codon-anticodon interaction. To model this state and thus to explore additional mechanistic implications of this tRNA-mRNA mimicry we docked the structure into its expected location in the P site of a high-resolution crystal structure of a 70S ribosome bound to mRNA and three tRNAs26 (Fig. 4). There is nearly perfect local superposition between PK I and P site tRNA in the anticodon-codon portion (Fig. 4a). However, there is poor overlap between the helical regions. Specifically, in the docked model domain 3 tilts from the P site toward the E site (Fig. 4b). This break in mimicry seems to occur in the vicinity of bases U6190 and A6191, which pair with U6213 and A6212, respectively, to form the ‘extra’ base-pairs within PK I that are not part of the codon-anticodon mimic interaction (Fig. 2a and Fig 4a). This proposed tilt is not obvious in cryo-EM reconstructions of the ribosome-bound IRES, but domain 3′s density in these reconstructions is weak relative to the rest of the IRES, perhaps indicating that domain 3 is not fully docked into complexes that lack an A site tRNA. However, this tilt is consistent with the observation that in the context of the entire IRES, domain 3 connects with the IRES ribosome-binding domain, which binds over the E site4,5.
Figure 4. Model and probing of domain 3 in the ribosome P site.
(a) The part of CrPV IGR IRES domain 3 that mimics the tRNA anticodon loop (red) aligned with the structure of the anticodon loop of an authentic P site–bound tRNAMet (gray, from PDB 2J00)26. Dashed box, portion of the structure that is a precise mimic of the initiator tRNA anticodon loop. (b) View of the structure of CrPV domain 3 docked into its predicted position within the decoding groove, aligned with P site tRNA as in panel a. The CrPV domain 3 is in red and cyan, a portion of A site tRNA is in green, and P and E site tRNAs and mRNA are in gray. During CrPV IGR IRES-driven initiation, only the A site tRNA is present. (c) Native gel of mutants d3_6190–6191_comp (U6190A + A6191U) and d3_Δ6191 next to wild-type domain 3 in the presence of 10 mM MgCl2. (d) A portion of an example gel of PSIV IGR IRES domain 3 modified with NMIA. Lanes containing free IRES RNA, 40S-bound IRES RNA and 80S-bound IRES RNA are shown over a dideoxy sequencing ladder. For clarity, this figure was constructed by splicing lanes from a single gel. Above the gel is a trace quantification of each lane. Some of the nucleotides with strong changes are labeled. (e) Graph of multiple SHAPE probing experiments quantified and analyzed. Gray dashed box, one s.d. between multiple measurements. The difference in modification between 40S-bound and 80S-bound IRES is shown, and changes greater than one s.d. are colored; red bars above the zero line, nucleotides that are modified more in the context of the 80S ribosome; blue bars under the line, those that are modified less. (f) Changes of panel e overlaid on the PSIV IGR IRES domain 3 secondary structure.
We hypothesized that the ‘extra’ base pairs in PK I that involve U6190 and A6191 help to form the overall structure of domain 3 and are important for function. To test this we used native gel electrophoresis, in which RNAs with altered three-dimensional shapes show aberrant migration relative to wild-type, and translation initiation assays. The mutations we tested were deletion of A6191 (Δ6191) and simultaneously changing both U6190 and A6191 to their Watson-Crick complements resulting in abrogation of the ‘extra’ base pairs of PK I (6190–6191_comp) (Fig. 4c). When subjected to native gel analysis, both 6190–6191_comp and Δ6191 display a retarded migration rate, indicating that as the structure predicts, mutation of these conserved features of domain 3 affects its conformation. However, whereas the 6190–6191_comp mutation was highly deleterious to IRES function, the Δ6191 mutation actually increased the efficiency of the IRES in cell-free extract relative to wild type (Fig. 3e and Table 1). Thus, the Δ6191 deletion both alters the equilibrium structure of CrPV domain 3 relative to wild type and increases the IRES’s functional efficiency. There is precedent for this type of effect; in the HCV IRES, mutations that destabilize a stem-loop structure containing the start codon lead to an increase in translation efficiency, indicating that disruption or alteration of structure in the decoding groove may be part of the HCV IRES mechanism28. The observation that Δ6191 increases translation efficiency of the CrPV IGR IRES indicates that docking of the domain into the P site may be accompanied by structural changes important for function.
Structural changes of domain 3 within the 80S ribosome
The domain 3 crystal structure suggests that it docks precisely into the P site but does not show whether this docking is accompanied by structural changes to the RNA. Cryo-EM reconstructions of IGR IRES–40S subunit and IGR IRES–80S ribosome complexes show IRES conformational differences between these two states, including movement of domain 3. However, these structures do not reveal local changes within domain 3. Therefore, we probed the structure of an IGR IRES RNA related to CrPV (Plautia stali intestine virus, PSIV) in the free, 40S subunit-and 80S ribosome-bound forms using selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE), which modifies available 2′-OH groups that are not constrained by base-pairing29,30. We found that the modification pattern of specific nucleotides in domain 3 changes when the IRES moves from a 40S subunit-bound state to the 80S ribosome-bound state, indicating that there may be structural changes at these locations (Fig. 4d–f). Specifically, nts 6156 and 6183–6185 are modified more in the 80S-bound complex, whereas nts 6165, 6170–6172, 6176, 6181 and 6188–6189 are modified less. Increases in modification are interpreted as increases in the dynamic character of the RNA, indicating that the structures associated with these nucleotides are being somewhat destabilized upon 80S binding. These data suggest not only that domain 3 docks precisely into the decoding groove but that subtle structural changes within domain 3 upon 80S binding are part of the IGR IRES mechanism. Interestingly, structural changes and destabilization of the P site codon-anticodon interaction also are associated with canonical translocation and tRNA dynamics, specifically the movement of the P site tRNA into the P/E hybrid state31.
Domain 3′s model position resembles a hybrid-state tRNA
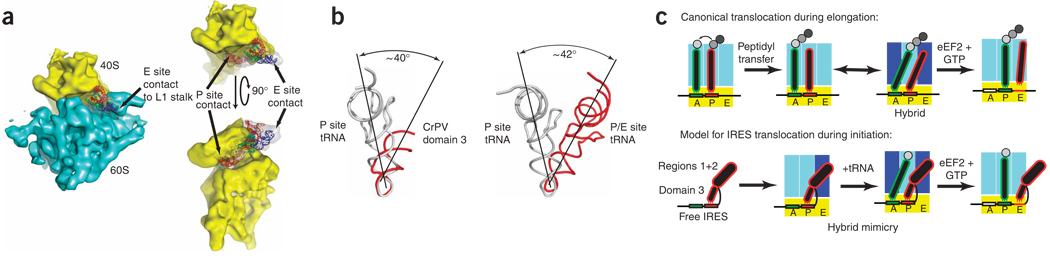
The A/P and P/E hybrid tRNA states, adopted by tRNAs after peptide bond formation, are orientations in which the acceptor stem of the tRNA has shifted on the large subunit to its next position, but the anticodon loop has not yet shifted in the decoding groove on the small subunit32,33. As pointed out by Yamamoto et al.34, the IGR IRES RNA simultaneously contacts ribosome features that interact with E site tRNA on the large subunit and the P site on the small subunit5, a defining characteristic of a P/E hybrid tRNA32,33,35,36 (Fig. 5a). When combined with the structure presented here, this observation indicates that the IGR IRES may globally position itself to mimic a hybrid-state tRNA while locally mimicking the precise structures and interactions within the P site of the decoding groove. In further support of this idea, we note that the angle between the docked model CrPV IGR IRES domain 3 and a P site tRNA is ~40–42°, similar to that between a P/E hybrid tRNA and a P site tRNA observed by cryo-EM37 (Fig. 5b). Because the P/E hybrid state is an authentic intermediate of translocation38, this putative P/E hybrid-state mimicry could explain translocation without peptide bond formation (Fig. 5c).
Figure 5. Docking of both IRES domain structures into a cryo-EM reconstruction and hypothesized mimicry mechanism.
(a) Three views of the crystal structure of the ribosome binding domain of the PSIV IRES23 and the domain 3 structure docked into the cryo-EM structure of the CrPV IRES on a human 80S ribosome5. At left is a view from the ‘top’ of the 80S; at top right the 60S subunit has been removed computationally, and at bottom right is this same view rotated 90° with the 60S subunit included. IGR IRES contacts to P and E site interaction sites are indicated. (b) Comparison of the angle between docked domain 3 and P site tRNA (from PDB 2J00)26, and P/E hybrid state and P site tRNAs (from PDB 1Z03)37. (c) Proposed mechanistic model of tRNA hybrid-state mimicry by the Dicistroviridae IRES. Top, canonical method of translocation in which the tRNAs adopt a hybrid state after peptide bond formation but before translocation. Amino acids in the peptide chain, gray circles, P and A site tRNAs, red and green outlines, respectively. 40S subunit, yellow; 60S, blue. Darker shades of blue, conformational changes in the subunit that are associated with the tRNA hybrid states. Bottom, proposed corresponding steps in the IGR IRES mechanism that mimic the hybrid state. IRES, red; the two IRES domains are indicated. Figure is based on figure from ref. 36.
DISCUSSION
IRES RNAs bypass the need for the 5′ cap structure, in some cases dispensing with the need for any of the eukaryotic translation initiation factors2,3. IRESes are diverse, but it is possible that some aspects of IRES-driven translation are conserved, and finding an explanation for these features requires more in-depth understanding of model IRES systems. The Dicistroviridae IGR IRESes represent a good model system to begin to gain this understanding. We have solved the structure of domain 3 of a Dicistroviridae IGR IRES that docks into the P site of the ribosome during factorless translation initiation. This structure, when combined with the structure of the ribosome binding domain of a Dicistroviridae IGR IRES23, allows us to model the entire IRES into position on the ribosome (Supplementary Fig. 5 online) and to construct testable structure-based models for IGR IRES function.
CrPV IGR IRES domain 3 is a precise mimicry of the anticodon loop-codon structure of an initiator tRNA. The mimicry suggests that docked CrPV domain 3 can form all the specific intermolecular contacts that occur between the ribosome and an authentic anticodon loop–mRNA complex within the decoding center. This is an interesting observation given that the tRNA affinity for the ribosome is highest in the P site39, and in bacteria the P site performs a diverse set of functions through very specific contacts with the codon-anticodon structure40. Cryo-EM reconstructions of yeast ribosome–tRNA complexes show that most interactions between the 40S subunit and P site tRNA are with the anticodon loop part of the tRNA41, and these interactions and changes in ribosome structure maintain the reading frame and lock the ribosome into a pretranslocation conformation26,42. The precise tRNA–mRNA mimicry displayed by CrPV domain 3 indicates that this domain may have related roles during IGR IRES translation initiation. Specifically, the mimicry probably docks PK I precisely to establish the reading frame, positions the next codon in the A site, and also contributes to moving the ribosome into a pretranslocation state. In addition, our probing data suggest that there are structural changes within domain 3 as the IRES progresses from the 40S-bound to 80S-bound state, consistent with the idea that the mechanism of the IGR IRES RNAs involves dynamic changes in both the RNA and the ribosome and not a ‘lock-and-key’ docking mechanism. The proposal that domain 3 undergoes some sort of subtle structural changes during ribosome assembly and possibly translocation is further supported by the observation that a mutant (Δ6191) that alters the structure of domain 3 also leads to an increase in IRES function; one might speculate that this mutation promotes these structural changes. However, this leads to the question of why the IRES would have evolved a sequence that inhibits IRES function. This question also has been asked of the HCV IRES, where a conserved stem-loop structure that contains the start codon has been shown to decrease IRES efficiency28. One hypothesis is that these IRESes have evolved to work at a very specific level of efficiency, not necessarily maximal efficiency, because the correct amount of viral proteins must be regulated precisely for successful viral infection28. The mutational data are consistent with the idea that both the structural mimicry and perhaps the position of domain 3 in the P site are due to the contribution of many nucleotides. Additional studies to understand the specifics of these conformational changes, the efficiency of IRES activity and the involvement of various nucleotides in domain 3 in these changes will reveal additional details of this dynamic mechanism.
Current models for IGR IRES function describe the IRES initially folding and then binding directly to the ribosome using regions 1 + 2, followed by binding-induced allosteric changes that alter the position of domain 3 in relation to the A and P sites4,5,23. The structure and observations presented here combined with our previous structure of an IGR IRES ribosome binding domain23 (regions 1 + 2) and a wealth of other published data now suggest a detailed mechanistic model that extends to IRES-driven translocation after ribosome binding (Fig. 5c and Supplementary Fig. 6 online). Specifically, after ribosome binding, domain 3 is placed in the P site, where it forms contacts with the ribosome that precisely mimic an initiator tRNA–mRNA complex. This docking also displays the next codon in the A site, where it is recognized by aminoacylated tRNA. Entry of A site tRNA stabilizes the docked position of domain 3, and in this state the entire IRES may mimic the P/E tRNA hybrid state as suggested elsewhere34, allowing the A site tRNA to likewise enter the A/P hybrid state. This elongation-like/ pretranslocation configuration is recognized by eEF 2, inducing translocation before a peptide bond is made. Hence, in this model the IGR IRES initiates translation by directly entering and co-opting the ribosome’s elongation cycle through molecular mimicry of tRNA.
This model is speculative but is consistent with several other observations. First, IGR IRES binding changes the conformation of the 60S subunit’s L1 stalk5, and similar conformational changes are associated with eEF 2 binding, P/E hybrid-state tRNA and the translocation cycle43. Second, the tRNA hybrid-state configuration is an authentic translocation intermediate38 that stimulates the action of an elongation factor44, thus hybrid-state mimicry by the IRES would facilitate the recruitment and action of eEF 2 and translocation. Third, this model provides an explanation of how the IGR IRES can be removed from the ribosome despite the high affinity (Kd = 2–20 nM) of this interaction; it is treated as a deaminoacylated tRNA during the translocation process. In fact, adoption of the P/E hybrid state is known to destabilize interaction in the P site31. Fourth, this model is consistent with the overall global position of the IRES on the ribosome, contacting the P site on the small subunit and the E site on the large subunit4,5. Importantly, the hybrid-state model is consistent with, but does not depend on, the tilted modeled position of domain 3; the ability of the IGR IRES to contact both the P site and E sites simultaneously and to mimic the P site codon-anticodon interaction precisely is not dependent on this modeled tilt. Finally, binding of tRNA to the A site of the ribosome is enhanced by eEF 2, and rRNA helix 18 is destabilized upon IGR IRES binding34. eEF 2 is known to recognize hybrid-state tRNAs and facilitate translocation, thus this result suggests that the A site tRNA binds and assumes a hybrid state immediately and is consistent with the structure-based model proposed here.
tRNA mimicry in translation is common, but the mode of mimicking both tRNA and mRNA as well as the putative hybrid-state mimicry used here has not been previously described. Elongation and release factor proteins have been shown to mimic the overall shape of tRNAs45, but none also mimic the mRNA codon, and they do not occupy the P and E sites. Certain viral RNAs can be aminoacylated by cellular synthetases, but direct evidence that they interact with the ribosome is scarce and they also do not mimic mRNA46. tRNA and mRNA mimicry is found in bacterial tmRNAs, which act to rescue stalled ribosomes through trans-translation, but they do not have anticodon-codon mimicry and they require SmpB proteins to function47. One of these proteins contacts the ribosome in the vicinity of the E and P site in the decoding groove, and a recent structure suggests that it replaces the codon-anticodon interaction48, raising the possibility that similar manipulation of the ribosome occurs with tmRNA and some IRESes. Fundamentally similar strategies might be used by a variety of IRESes and other structured noncoding RNAs to manipulate complex biological machines and to respond to specific cellular conditions.
METHODS
Cloning and plasmid production
Plasmid pCrPV1-1 (encoding the CrPV IRES) was used as template for PCR reactions to generate inserts that contain the T7 polymerase promoter and nts 6174–6216 of the CrPV RNA flanked by cis-acting ribozymes. Inserts were ligated into the EcoRI/BamHI site of pUC19, amplified in DH5α cells and sequenced to yield plasmid pCrPV11. Mutants were generated using the QuickChange mutagenesis kit (Stratagene).
RNA preparation
The transcription template was prepared by BamHI linearization of pCrPV11 plasmid or by PCR from pCrPV11 using M13 forward and reverse primers. RNA was transcribed in vitro, ethanol-precipitated, purified by denaturing gel electrophoresis, concentrated and stored as described11.
Preparation of cobalt(III) and iridium(III) hexammine acetate
Cobalt(III) hexammine acetate and iridium(III) hexammine acetate were prepared from the chloride salts as described24. Iridium hexammine was synthesized as described24.
Crystallization
RNA was combined with buffer and salt to yield a solution that was 5 mg ml−1 RNA, 10 mM potassium-HEPES buffer, pH 7.5, 2.5 mM MgCl2 and 0.5 mM spermidine HCl, then heated to 65 °C for 3 min and allowed to cool on the bench. This RNA solution was combined in a 1:1 ratio with well solution (1.4 M Li2SO4, 40 mM magnesium acetate, 50 mM HEPES-NaOH, pH 7.5) in a hanging drop at 30 °C. To obtain a heavy atom derivative, RNA mutant CrPV-11_GU3 (Supplementary Fig. 1) was prepared and crystallized under conditions identical to wild type. The crystals were stabilized by replacing the well solution with 2.0 M Li2SO4, 40 mM magnesium acetate, 50 mM HEPES-NaOH, pH 7.5, 0.5 mM spermidine HCl and by subjecting them to overnight equilibration, then transferred to a soaking tray containing 50 µl of this well solution. The soaking solution was replaced in increments to the derivative solution: 3.0 M lithium acetate, 40 mM magnesium acetate, 50 mM HEPES-NaOH, pH 7.5, 0.5 mM spermidine HCl, plus 100 mM iridium(III) hexammine acetate or 100 mM cobalt(III) hexammine acetate. The crystals were soaked for 1 additional hour then cryo-cooled directly in liquid nitrogen. Further details are in Supplementary Methods online and ref. 24.
Data collection, phasing, building and refinement
The cobalt(III) hexammine–soaked crystals were used in a single-wavelength SAD experiment with Cu-Kα radiation (1.5418 Å) under cryo-conditions. Data were collected and processed in HKL2000 (ref. 49), and initial phases were found using PHENIX50,51. The initial electron density map showed typical features of an RNA such as stacked bases and phosphate backbone. The iridium(III) hexammine–soaked CrPV domain 3 RNA crystals were used in a two-wavelength (1.1055Å, 1.1053 Å) MAD experiment at BL 12.3.1 of the Advanced Light Source (Berkeley, California, USA) under cryo-conditions. The MAD data set was processed with ELVES52, MOSFLM53, the CCP4 Suite54 and SOLVE55. Three iridium(III) hexammine sites were used to obtain initial phases with a figure of merit of 0.62, which improved to 0.78 after solvent flattening. The correct space group and handedness were determined by visually inspecting all possible experimental electron density maps. The model was built in O (ref. 56) and refined in CNS57 using iterative rounds of manual building, simulated annealing, energy minimization, B-factor refinement and automated water-picking. A summary of data collection and statistics is contained in Table 2, and details of the structure determination and refinement process are contained in Supplementary Methods and Supplementary Table 1 online.
Table 2.
Data collection, phasing and refinement statisticsa
| Cobalt crystal | Iridium crystal | ||
|---|---|---|---|
| Data collection | |||
| Space group | P41212 | P41212 | |
| Cell dimensions | |||
| a, b, c (Å) | 58.58, 58.58, | 58.69, 58.69, | |
| 98.90 | 99.00 | ||
| Inflection | Peak | ||
| Wavelength | 1.5418 (Cu-Kα) | 1.1055 | 1.1053 |
| Resolution (Å) | 50.00–3.00 | 23.2–2.40 | 23.2–2.40 |
| Rmerge | 11.8 (53.8) | 9.8 | 9.0 |
| I / σI | 21.8 (4.5) | 27.9 (2.3) | 27.2 (2.0) |
| Completeness (%) | 99.8 (100) | 99.7 (99.8) | 99.7 (99.7) |
| Redundancy | 14.8 (14.9) | 31.7 (18.1) | 31.9 (18.3) |
| Refinement | |||
| Resolution (Å) | 50–2.4 | ||
| No. reflections (unique) | 7,225 | ||
| Rwork / Rfree | 22.9% / 26.0% | ||
| No. atoms | 1,106 | ||
| Nucleic acid | 932 | ||
| Ligand/ion | 28 | ||
| Water | 146 | ||
| B-factors | |||
| Nucleic acid | 44.17 | ||
| Ligand/ion | 62.0 | ||
| Water | 43.7 | ||
| R.m.s deviations | |||
| Bond lengths (Å ) | 0.005316 | ||
| Bond angles (°) | 1.35 | ||
Data were collected from two crystals; no native crystals were used. Values in parentheses are for highest resolution shell.
Translation assays
Translation assays were performed essentially as described13. Briefly, mutants of pCrPV1-1 were generated and the plasmids linearized with XbaI. RNA was in vitro–transcribed (100-µl reactions) and the reactions were extracted with phenol-chloroform-isoamyl alcohol and precipitated with ethanol. Pelleted RNA was resuspended in 50 ml ddH2O and passed through Micro Bio-Spin P-30 columns (Bio-Rad). To 35 µl RRL (Promega) supplemented with amino acids and 154 mM potassium acetate was added 1 µg RNA in a final volume of 50 µl. Reactions were incubated in a 30 °C water bath for 90 min and assayed using the Dual-Luciferase Reporter Assay System (Promega) on a Sirius FB12 luminometer (Zylux Corporation).
Native gels
Purified RNA was used in native gel electrophoresis experiments as described11.
SHAPE probing of RNA–ribosome complexes
Salt-washed ribosomes (containing both subunits) and purified 40S subunit were prepared from RRL58 and SHAPE probing was done essentially as described29. Details are contained in Supplementary Methods. Briefly, 310 pmol RNA in 150 µl water were heated to 85 °C for 60 s, then 81 µl of folding solution (222 mM HEPES, pH 7.5, 20 mM MgCl2, 333 mM NaCl) were added and the solutions cooled to room temperature (~22 °C). After 54 µl of salt-washed ribosome mix were added, the solutions were incubated for 10 min at 35 °C; this was followed by addition of 100 µg of emitene and another 5 min incubation. To modify the RNA, 33 µl of 65 mM N-methyl-isatoic anhydride (NMIA) dissolved in DMSO were added and incubated for 1 h at 35 °C, followed by addition of 300 µl of dilution buffer (50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM DTT, 50 mM NaCl, 100 µg ml−1 emitine); then the reaction was placed on ice. Several control reactions containing only DMSO (no NMIA) also were performed but are not shown in Figure 4.
Preinitiation complexes were separated by ultracentrifugation through 10–35% (w/v) sucrose gradients containing 50 mM Tris-HCl, pH 7.5, 50 mM NaCl, 5 mM MgCl2, 1 mM DTT, 100 µg emitine as described10. After fractionation, the IRES–ribosome complexes were ethanol-precipitated and resuspended in 15 µl water. Control reactions without ribosomal subunits, with purified 40S subunit, in the presence of the ribosome mix, and both with and without NMIA were performed as well but were not processed with a sucrose gradient (data not shown). Reverse transcription was conducted and sequencing ladders generated essentially as described, with the exception that annealing and extension temperatures were 37 °C and 2.3 mM ddNTP was used29. Reactions were resolved on 10% (w/v) denaturing polyacrylamide sequencing gels, run at 65 W for various time periods, dried and analyzed as described11. Quantitative analysis was performed using SAFA59 and normalized as described in Supplementary Methods.
Supplementary Material
Note: Supplementary information is available on the Nature Structural & Molecular Biology website.
ACKNOWLEDGMENTS
We thank K. Bjornsen for obtaining initial crystals and for technical assistance, Q. Vicens, T. Evans, R. Batey, B. Hodges, T. Blumenthal, E. Eisenmesser, R. Zhao, M. Filbin and A. Keel for critically reading this manuscript, R. Batey for useful discussions and for providing the iridium(III) hexammine, P. Sarnow (Stanford University), E. Jan (University of British Columbia) and N. Nakashima (Japanese National Institute of Agrobiological Sciences) for plasmids, and the staffs of beam lines 4.2.2 and 12.3.1 at the Advanced Light Source. This work was supported by grants AI072187 and GM072560 from the US National Institutes of Health (J.S.K.).
Footnotes
Accession codes. Protein Data Bank: Coordinates of the CrPV IGR IRES RNA domain 3 have been deposited with accession code 3B31.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
References
- 1.Hershey JWB, Merrick WC. Pathway and mechanism of initiation of protein synthesis. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor, New York, USA: Cold Spring Harbor Laboratory Press; 2000. pp. 33–88. [Google Scholar]
- 2.Stoneley M, Willis AE. Cellular internal ribosome entry segments: structures, trans-acting factors and regulation of gene expression. Oncogene. 2004;23:3200–3207. doi: 10.1038/sj.onc.1207551. [DOI] [PubMed] [Google Scholar]
- 3.Jackson RJ. Alternative mechanisms of initiating translation of mammalian mRNAs. Biochem. Soc. Trans. 2005;33:1231–1241. doi: 10.1042/BST0331231. [DOI] [PubMed] [Google Scholar]
- 4.Schuler M, et al. Structure of the ribosome-bound cricket paralysis virus IRES RNA. Nat. Struct. Mol. Biol. 2006;13:1092–1096. doi: 10.1038/nsmb1177. [DOI] [PubMed] [Google Scholar]
- 5.Spahn CM, et al. Cryo-EM visualization of a viral internal ribosome entry site bound to human ribosomes; the IRES functions as an RNA-based translation factor. Cell. 2004;118:465–475. doi: 10.1016/j.cell.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Fukushi S, et al. Ribosomal protein S5 interacts with the internal ribosomal entry site of hepatitis C virus. J. Biol. Chem. 2001;276:20824–20826. doi: 10.1074/jbc.C100206200. [DOI] [PubMed] [Google Scholar]
- 7.Boehringer D, Thermann R, Ostareck-Lederer A, Lewis JD, Stark H. Structure of the hepatitis C virus IRES bound to the human 80S ribosome: remodeling of the HCV IRES. Structure. 2005;13:1695–1706. doi: 10.1016/j.str.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Spahn CM, et al. Hepatitis C virus IRES RNA-induced changes in the conformation of the 40S ribosomal subunit. Science. 2001;291:1959–1962. doi: 10.1126/science.1058409. [DOI] [PubMed] [Google Scholar]
- 9.Jan E. Divergent IRES elements in invertebrates. Virus Res. 2006;119:16–28. doi: 10.1016/j.virusres.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Pfingsten JS, Costantino DA, Kieft JS. Conservation and diversity among the three-dimensional folds of the Dicistroviridae intergenic region IRESes. J. Mol. Biol. 2007;370:856–869. doi: 10.1016/j.jmb.2007.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costantino D, Kieft JS. A preformed compact ribosome-binding domain in the cricket paralysis-like virus IRES RNAs. RNA. 2005;11:332–343. doi: 10.1261/rna.7184705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishiyama T, et al. Structural elements in the internal ribosome entry site of Plautia stali intestine virus responsible for binding with ribosomes. Nucleic Acids Res. 2003;31:2434–2442. doi: 10.1093/nar/gkg336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jan E, Sarnow P. Factorless ribosome assembly on the internal ribosome entry site of cricket paralysis virus. J. Mol. Biol. 2002;324:889–902. doi: 10.1016/s0022-2836(02)01099-9. [DOI] [PubMed] [Google Scholar]
- 14.Kanamori Y, Nakashima N. A tertiary structure model of the internal ribosome entry site (IRES) for methionine-independent initiation of translation. RNA. 2001;7:266–274. doi: 10.1017/s1355838201001741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson JE, Pestova TV, Hellen CU, Sarnow P. Initiation of protein synthesis from the A site of the ribosome. Cell. 2000;102:511–520. doi: 10.1016/s0092-8674(00)00055-6. [DOI] [PubMed] [Google Scholar]
- 16.Pestova TV, Hellen CU. Translation elongation after assembly of ribosomes on the cricket paralysis virus internal ribosomal entry site without initiation factors or initiator tRNA. Genes Dev. 2003;17:181–186. doi: 10.1101/gad.1040803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki J, Nakashima N. Methionine-independent initiation of translation in the capsid protein of an insect RNA virus. Proc. Natl. Acad. Sci. USA. 2000;97:1512–1515. doi: 10.1073/pnas.010426997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki J, Nakashima N. Translation initiation at the CUU codon is mediated by the internal ribosome entry site of an insect picorna-like virus in vitro. J. Virol. 1999;73:1219–1226. doi: 10.1128/jvi.73.2.1219-1226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jan E, et al. Initiator Met-tRNA-independent translation mediated by an internal ribosome entry site element in cricket paralysis virus-like insect viruses. Cold Spring Harb. Symp. Quant. Biol. 2001;66:285–292. doi: 10.1101/sqb.2001.66.285. [DOI] [PubMed] [Google Scholar]
- 20.Thompson SR, Gulyas KD, Sarnow P. Internal initiation in Saccharomyces cerevisiae mediated by an initiator tRNA/eIF2-independent internal ribosome entry site element. Proc. Natl. Acad. Sci. USA. 2001;98:12972–12977. doi: 10.1073/pnas.241286698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson JE, Powell MJ, Hoover SE, Sarnow P. Naturally occurring dicistronic cricket paralysis virus RNA is regulated by two internal ribosome entry sites. Mol. Cell. Biol. 2000;20:4990–4999. doi: 10.1128/mcb.20.14.4990-4999.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jan E, Kinzy TG, Sarnow P. Divergent tRNA-like element supports initiation, elongation, and termination of protein biosynthesis. Proc. Natl. Acad. Sci. USA. 2003;100:15410–15415. doi: 10.1073/pnas.2535183100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfingsten JS, Costantino DA, Kieft JS. Structural basis for ribosome recruitment and manipulation by a viral IRES RNA. Science. 2006;314:1450–1454. doi: 10.1126/science.1133281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keel AY, Rambo RP, Batey RT, Kieft JS. A general strategy to solve the phase problem in RNA crystallography. Structure. 2007;15:761–772. doi: 10.1016/j.str.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilbers CW, Michiels PJ, Heus HA. New developments in structure determination of pseudoknots. Biopolymers. 1998;48:137–153. doi: 10.1002/(SICI)1097-0282(1998)48:2<137::AID-BIP4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 26.Selmer M, et al. Structure of the 70S ribosome complexed with mRNA and tRNA. Science. 2006;313:1935–1942. doi: 10.1126/science.1131127. [DOI] [PubMed] [Google Scholar]
- 27.Cabello-Villegas J, Tworowska I, Nikonowicz EP. Metal ion stabilization of the U-turn of the A37 N6-dimethylallyl-modified anticodon stem-loop of Escherichia coli tRNAPhe. Biochemistry. 2004;43:55–66. doi: 10.1021/bi0353676. [DOI] [PubMed] [Google Scholar]
- 28.Honda M, Brown EA, Lemon SM. Stability of a stem-loop involving the initiator AUG controls the efficiency of internal initiation of translation on hepatitis C virus RNA. RNA. 1996;2:955–968. [PMC free article] [PubMed] [Google Scholar]
- 29.Merino EJ, Wilkinson KA, Coughlan JL, Weeks KM. RNA structure analysis at single nucleotide resolution by selective 2′-hydroxyl acylation and primer extension (SHAPE) J. Am. Chem. Soc. 2005;127:4223–4231. doi: 10.1021/ja043822v. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson KA, Merino EJ, Weeks KM. Selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE): quantitative RNA structure analysis at single nucleotide resolution. Nat. Protoc. 2006;1:1610–1616. doi: 10.1038/nprot.2006.249. [DOI] [PubMed] [Google Scholar]
- 31.McGarry KG, Walker SE, Wang H, Fredrick K. Destabilization of the P site codon-anticodon helix results from movement of tRNA into the P/E hybrid state within the ribosome. Mol. Cell. 2005;20:613–622. doi: 10.1016/j.molcel.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moazed D, Noller HF. Intermediate states in the movement of transfer RNA in the ribosome. Nature. 1989;342:142–148. doi: 10.1038/342142a0. [DOI] [PubMed] [Google Scholar]
- 33.Bretscher MS. Translocation in protein synthesis: a hybrid structure model. Nature. 1968;218:675–677. doi: 10.1038/218675a0. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto H, Nakashima N, Ikeda Y, Uchiumi T. Binding mode of the first aminoacyl-tRNA in translation initiation mediated by Plautia stali intestine virus internal ribosome entry site. J. Biol. Chem. 2007;282:7770–7776. doi: 10.1074/jbc.M610887200. [DOI] [PubMed] [Google Scholar]
- 35.Blanchard SC, Kim HD, Gonzalez RL, Jr., Puglisi JD, Chu S. tRNA dynamics on the ribosome during translation. Proc. Natl. Acad. Sci. USA. 2004;101:12893–12898. doi: 10.1073/pnas.0403884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munro JB, Altman RB, O’Connor N, Blanchard SC. Identification of two distinct hybrid state intermediates on the ribosome. Mol. Cell. 2007;25:505–517. doi: 10.1016/j.molcel.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen GS, Zavialov A, Gursky R, Ehrenberg M, Frank J. The cryo-EM structure of a translation initiation complex from Escherichia coli. Cell. 2005;121:703–712. doi: 10.1016/j.cell.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 38.Dorner S, Brunelle JL, Sharma D, Green R. The hybrid state of tRNA binding is an authentic translation elongation intermediate. Nat. Struct. Mol. Biol. 2006;13:234–241. doi: 10.1038/nsmb1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olejniczak M, Dale T, Fahlman RP, Uhlenbeck OC. Idiosyncratic tuning of tRNAs to achieve uniform ribosome binding. Nat. Struct. Mol. Biol. 2005;12:788–793. doi: 10.1038/nsmb978. [DOI] [PubMed] [Google Scholar]
- 40.Noller HF, Hoang L, Fredrick K. The 30S ribosomal P site: a function of 16S rRNA. FEBS Lett. 2005;579:855–858. doi: 10.1016/j.febslet.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 41.Spahn CM, et al. Structure of the 80S ribosome from Saccharomyces cerevisiae-tRNA-ribosome and subunit-subunit interactions. Cell. 2001;107:373–386. doi: 10.1016/s0092-8674(01)00539-6. [DOI] [PubMed] [Google Scholar]
- 42.Berk V, Zhang W, Pai RD, Cate JH. Structural basis for mRNA and tRNA positioning on the ribosome. Proc. Natl. Acad. Sci. USA. 2006;103:15830–15834. doi: 10.1073/pnas.0607541103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valle M, et al. Locking and unlocking of ribosomal motions. Cell. 2003;114:123–134. doi: 10.1016/s0092-8674(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 44.Sharma D, Southworth DR, Green R. EF-G-independent reactivity of a pre-translocation-state ribosome complex with the aminoacyl tRNA substrate puromycin supports an intermediate (hybrid) state of tRNA binding. RNA. 2004;10:102–113. doi: 10.1261/rna.5148704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kristensen O, Laurberg M, Liljas A, Selmer M. Is tRNA binding or tRNA mimicry mandatory for translation factors? Curr. Protein Pept. Sci. 2002;3:133–141. doi: 10.2174/1389203023380837. [DOI] [PubMed] [Google Scholar]
- 46.Fechter P, Rudinger-Thirion J, Florentz C, Giege R. Novel features in the tRNA-like world of plant viral RNAs. Cell. Mol. Life Sci. 2001;58:1547–1561. doi: 10.1007/PL00000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore SD, Sauer RT. The tmRNA system for translational surveillance and ribosome rescue. Annu. Rev. Biochem. 2007;76:101–124. doi: 10.1146/annurev.biochem.75.103004.142733. [DOI] [PubMed] [Google Scholar]
- 48.Bessho Y, et al. Structural basis for functional mimicry of long-variable-arm tRNA by transfer-messenger RNA. Proc. Natl. Acad. Sci. USA. 2007;104:8293–8298. doi: 10.1073/pnas.0700402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 50.Adams PD, et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 2002;58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 51.Adams PD, et al. Recent developments in the PHENIX software for automated crystallographic structure determination. J. Synchrotron Radiat. 2004;11:53–55. doi: 10.1107/s0909049503024130. [DOI] [PubMed] [Google Scholar]
- 52.Holton J, Alber T. Automated protein crystal structure determination using ELVES. Proc. Natl. Acad. Sci. USA. 2004;101:1537–1542. doi: 10.1073/pnas.0306241101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leslie AGW. Recent changes to the MOSFLM package for processing film and image plate data. Joint CCP4 + ESF-EAMCB Newslett. Protein Crystallogr No. 26. 1992 [Google Scholar]
- 54.Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 55.Terwilliger T. SOLVE and RESOLVE: automated structure solution, density modification and model building. J. Synchrotron Radiat. 2004;11:49–52. doi: 10.1107/s0909049503023938. [DOI] [PubMed] [Google Scholar]
- 56.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A Foundations Crystallogr. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 57.Brunger AT, et al. Crystallography & NMR system: a new software suite for macro-molecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 58.Kieft JS, Zhou K, Jubin R, Doudna JA. Mechanism of ribosome recruitment by hepatitis C IRES RNA. RNA. 2001;7:194–206. doi: 10.1017/s1355838201001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Das R, Laederach A, Pearlman SM, Herschlag D, Altman RB. SAFA: semi-automated footprinting analysis software for high-throughput quantification of nucleic acid footprinting experiments. RNA. 2005;11:344–354. doi: 10.1261/rna.7214405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: Supplementary information is available on the Nature Structural & Molecular Biology website.