Abstract
Several reviews have highlighted the importance of local tissue production of components of the renin-angiotensin system (RAS). While the concept of tissue RAS is gaining more widespread acceptance, the concept of local angiotensin II (AngII) production, acting in coordinate or independently of the endocrine RAS, continues to be debated. The primary reasons that local AngII production has been studied by many investigators are that components of the RAS are expressed by multiple cell types, and that the endocrine RAS cannot fully explain all effects of AngII. Moreover, through the development and study of genetically altered models for over-expression or knockdown of individual RAS components within specific cell types, it is becoming increasingly more evident that local RAS contribute to effects of AngII in normal physiology and disease. The purpose of this review is to define the presence and physiological significance of a local RAS in adipose tissue in relation to cardiovascular disease.
Keywords: Angiotensin, Adipocyte, Renin-angiotensin system
1. Evidence for an adipocyte RAS
In a search for expression of angiotensinogen (AGT) as the only known precursor to AngII in vascular tissue, our laboratory demonstrated that the majority of AGT mRNA expression was localized to perivascular adipose tissue surrounding the rat aorta (Cassis et al., 1988a,b). Simultaneously, Campbell and co-workers (Campbell and Habener, 1987) demonstrated expression of AGT mRNA in periatrial and periaortic brown adipocytes from rats. To determine if adipose AGT was regulated by stimuli demonstrated to regulate liver AGT expression, we demonstrated that bilateral nephrectomy increased adipose AGT mRNA expression, but that administration of an ACE inhibitor had no effect on adipose expression levels (Cassis et al., 1988a,b). In studies examining developmental expression of AGT in various tissues, results suggested that adipose tissue, rather than the liver, may serve as a primary source of AGT in the fetus (Gomez et al., 1988). To localize AGT expression to adipocytes, we examined AGT mRNA expression during the course of 3T3-L1 adipocyte differentiation, and demonstrated an association of AGT expression with the acquisition of an adipocyte phenotype (Saye et al., 1989). Additional studies using 3T3-F442A adipocytes (Saye et al., 1993), or rat brown adipose tissue (Shenoy and Cassis, 1997), demonstrated renin-like activity in adipose tissue. Processing of angiotensin I to AngII by an angiotensin converting enzyme (ACE)-dependent mechanism was demonstrated in 3T3-F442A adipocytes (Saye et al., 1993). Findings from our laboratory demonstrated AngII receptor binding sites in adipose tissue from lean and obese (Zucker) rats (Cassis et al., 1996). Human adipocytes have also been demonstrated to express AT1 receptors (Crandall et al., 1994; Engeli et al., 1999; Schling et al., 1999). A variety of investigators have demonstrated that human adipocytes express a complete RAS, including ACE, AT1, and AT2 receptors (Cassis, 2000). Recent results support localization of renin receptors to human adipose tissue (Achard et al., 2007), and angiotensin converting enzyme 2 (ACE2) to human and rodent adipose tissue (Galvez-Prieto et al., 2008; Gembardt et al., 2005; Gupte et al., 2008; Li et al., 2007; Zhang et al., 2006). Collectively, these results demonstrate that adipocytes possess components necessary for the synthesis of and responsiveness to AngII.
2. Mechanisms for regulation of the adipocyte RAS
Since AGT was the first RAS component demonstrated in adipocytes, and is abundantly expressed in adipose tissue, several studies have focused on mechanisms of AGT regulation in adipocytes. Early studies demonstrated that a differentiation-specific element is required for sustained transcriptional expression of AGT in differentiating adipocytes (McGehee et al., 1993). Stable transfection of 3T3-L1 cells with an AGT promoter (-501 to +22)-chloramphenicol chimeric construct suggested that the 5′ flanking region of the AGT gene contains the necessary information to trigger adipogenic expression (Tamura et al., 1994). However, the nature of the transcriptional modulator regulating adipogenic expression of AGT was not identified.
A variety of different hormones that are capable of regulating liver AGT mRNA expression have been examined for their ability to regulate AGT mRNA expression in adipocytes. Dexamethasone, a regulator of liver AGT mRNA expression, is frequently employed in the differentiation cocktail of 3T3-L1 adipocytes, and studies by Aubert et al. (1997) demonstrated that adipocyte AGT mRNA expression was positively regulated specifically by glucorticoids, but not by other hormones capable of regulating liver AGT mRNA expression. In isolated rat adipocytes from castrated males, incubation with androgen resulted in an increase in AGT mRNA expression (Serazin-Leroy et al., 2000). Similarly, castration of rats with and without androgen administration was demonstrated to regulate adipose AGT mRNA expression. This finding was suggested to relate andryoid-like obesity to obesity-associated hypertension.
Since adipocytes are a site where insulin-resistance is manifest in type 2 diabetes, insulin has been examined as a potential regulator of adipocyte AGT expression. In human isolated adipocytes, increasing concentrations of insulin (1-1000 nM, 48 h of incubation) were demonstrated to increase AGT protein expression (Harte et al., 2003). In 3T3-L1 adipocytes, insulin increased AGT mRNA expression (at 10 and 1000 nM, 48 h) (Jones et al., 1997). However, in Ob1771 and 3T3-F442A adipocytes, AGT mRNA abundance was decreased by insulin (0-850 nM, 24 h) (Aubert et al., 1998). Since both of these model adipocyte culture systems are derived from mice, it is unclear why insulin would exhibit opposing effects to regulate AGT mRNA expression. However, diverging effects of PGF2α on adipocyte conversion have also been reported in 3T3-L1 compared to Ob17 cells (Russell and Ho, 1976; Nebrel et al., 1981). Surprisingly, in rat adipocytes, insulin had no effect on AGT expression and/or release (30 nM, 2, 4 or 6 h of incubation) (Turban et al., 2001) Species differences (human or rodents), incubation conditions (periods of time, insulin concentrations), expression levels of insulin receptors and/or signaling mediators, or glucose transporter expression in different adipocyte preparations may have contributed to differences in findings for insulin regulation of AGT expression in adipocytes. For example, it is conceivable that AGT regulation by insulin may result from glucose entry into adipocytes, which could vary across adipocyte cell model systems from different species, and/or under different differentiation or cell culture conditions (e.g., glucose concentrations in media).
In addition to hormones as regulators of adipocyte AGT expression, since adipocytes store considerable quantities of lipid in the form of fatty acids, these compounds have been examined as regulators of adipocyte AGT. Exposure of Ob1771 cells to α-bromopalmitate resulted in a 17-fold increase in AGT mRNA expression (Safonova et al., 1997). Interestingly, several different fatty acids, including fatty acids that are ligand activators of PPARγ, also increased AGT mRNA expression in Ob1771 adipocytes. However, using isolated human adipocytes, PPARγ activators failed to stimulate AGT mRNA expression (Rieusset et al., 1999). Mechanisms of fatty acids to induce AGT expression in adipocytes are unclear, and should be an area of future investigation. It would also be of interest to define the effect of fatty acid chain length, and/or saturation, on their ability to regulate adipocyte AGT expression. Interestingly, β-adrenergic-mediated lipolysis at adipocytes elevates cellular levels of cyclic AMP, which was demonstrated to increase AGT mRNA expression in human adipose tissue (Serazin et al., 2004a,b). It is conceivable that β-adrenergic receptor agonists indirectly increase AGT mRNA expression through stimulated lipolysis and fatty acid-induced regulation of AGT. Finally, mediators increased in the obese state, such TNF-α (Wang et al., 2005) or AngII (Lu et al., 2007), have also been reported to regulate AGT expression in adipocytes. (Table 1).
Table 1.
In vitro regulation of AGT expression in adipocytes
| Cell/tissue | Regulator | Reference |
|---|---|---|
| Increase AGT expression | ||
| 3T3-L1 adipocytes | Differentiation-specific element binding protein | (McGehee et al., 1993) |
| 3T3-L1 adipocytes | Dexamethasone | (Aubert et al., 1997) |
| Ob1771 adipocytes | Fatty acids (palmitate, oleate, linoleate, γ-linolenate, ETYA, PPARγ ligand) | (Safonova et al., 1997) |
| Isolated rat adipocytes | Androgen (testosterone, dihydrotestosterone) | (Serazin-Leroy et al., 2000) |
| Isolated human adipocytes | Insulin | (Harte et al., 2003) |
| 3T3-L1 adipocytes | Insulin | (Jones et al., 1997) |
| Human adipose tissue | Cyclic AMP | (Serazin et al., 2004a,b) |
| No effect or decrease AGT expression | ||
| Isolated rat adipocytes | Insulin (no effect) | (Turban et al., 2001) |
| Isolated mouse adipocytes | Insulin (decrease) | (Aubert et al., 1998) |
| Isolated human adipocytes | TNF-α (decrease) | (Wang et al., 2005) |
In addition to in vitro regulation of AGT expression adipocytes, several investigators have examined AGT mRNA expression in a variety of different animal models (Table 2). Studies from our laboratory demonstrated that AGT mRNA expression levels in adipose tissue were decreased in rats made diabetic by injection of streptozotocin (Cassis, 1992). Early studies demonstrated nutritional regulation of adipose AGT mRNA expression, with reductions in fasted mice and elevations in re-fed mice (Frederich et al., 1992). Moreover, release of AGT from explants of adipose tissue from genetically obese mice (ob/ob) was increased, suggesting that obesity promotes adipocyte AGT expression. Further studies in Zucker obese rats with deficiency of leptin receptors initially reported reductions in adipose AGT expression (Jones et al., 1997). However, other investigators reported elevations in adipose AGT mRNA expression and release during the development of obesity in Zucker obese rats (Hainault et al., 2002). Moreover, results from our laboratory demonstrated enhanced effects of AngII to increase sympathetic nerve activity to brown adipose tissue in young Zucker obese rats (Cassis, 1994). It appears that the duration of obesity in models of leptin deficiency may influence AGT mRNA expression in adipose tissue, with early increases during the development of obesity which may not be maintained with chronic obesity. In transgenic mice expressing the human AGT gene under the control of its own promoter, a high fat diet resulted in increased AGT mRNA expression in visceral adipose tissue (Rahmouni et al., 2004). In addition, we demonstrated an increase in AGT mRNA expression in visceral adipose tissue from rats and mice with diet-induced obesity and hypertension (Boustany et al., 2004; Gupte et al., 2008). Collectively, data from rodent models of diet-induced obesity support an elevation of adipose AGT mRNA expression, which may potentially influence local and systemic production of AngII.
Table 2.
In vivo regulation of AGT expression in adipose tissue
| Model | Effect | Reference |
|---|---|---|
| Streptozotocin diabetes in rats | Decrease | (Cassis, 1992) |
| Fasting/re-feeding in mice | Decrease/increase | (Frederich et al., 1992) |
| Ob/ob mice | Increase | (Frederich et al., 1992) |
| Zucker fa/fa rat (adult) | Decrease | (Jones et al., 1997) |
| Zucker fa/fa rat (young) | Increase | (Turban et al., 2002) |
| Human AGT transgene in mice, high fat feeding | Increase | (Rahmouni et al., 2004) |
| Rat, high fat feeding | Increase | (Boustany et al., 2004) |
| Human obesity | Increase | (Van Harmelen et al., 2000a) |
| Human obesity | Decrease | (Gorzelniak et al., 2002) |
| Human obesity | No change | (Faloia et al., 2002) |
| AT2 receptor deficiency | Increase | (Lu et al., 2007) |
| AngII infusion in mice | Increase | (Lu et al., 2007) |
Since the systemic RAS has been reported to be activated with obesity, our laboratory focused on AngII-mediated endocrine feedback regulation of adipose AGT expression. Results demonstrated that AT1aR deficiency in mice decreased AGT mRNA expression in liver, but not in adipose tissue (Lu et al., 2007). Studies using AT2 receptor deficient mice demonstrated that this receptor masked effects of AngII to regulate adipose AGT expression. Accordingly, adipose AGT mRNA expression increased in mice with AT2 receptor deficiency, and this effect was blocked by an AT1 receptor antagonist. Importantly, when mice were infused with exogenous AngII, adipose AT1aR and AGT mRNA expression increased markedly, supporting positive feedback regulation of the adipocyte RAS by AngII. These results suggest that in experimental animal models of obesity, elevations in AngII may maintain continued increases in adipose AGT mRNA expression.
To determine if AGT expression levels differed depending on the location of adipose tissue, investigators have examined differences in AGT mRNA expression in visceral compared to subcutaneous adipose depots. In biopsies of visceral and abdominal subcutaneous adipose tissue from humans, AGT mRNA expression was higher in visceral fat, with differences between visceral and subcutaneous expression magnified in obese subjects (Dusserre et al., 2000). Similarly, AGT mRNA expression was higher in omental than subcutaneous human adipose tissue, but expression correlated positively to waist-to-hip ratios in both adipose depots (van Harmelen et al., 2000b). In both of these studies, patients with differing body mass indexes were used to examine effects of obesity on adipose AGT expression; however, the patients were generally healthy and did not exhibit cardiovascular diseases such as hypertension. When hypertension was considered in evaluation of adipose AGT expression, levels have been demonstrated to not be different (Faloia et al., 2002) or to be lower in adipose tissue from obese compared to lean subjects (Gorzelniak et al., 2002). In contrast, expression of renin, ACE and AT1 receptors was increased in adipose tissue from hypertensive and non-hypertensive obese compared to lean. Collectively, results demonstrate regional differences in AGT expression favoring higher expression in visceral adipose depots. However, given conflicting findings in obese hypertensives, it is unclear if these differences in regional AGT mRNA expression with human obesity relate to the development of hypertension. Alternatively, if findings from obese rodents are representative of those in humans, it is possible that early elevations in AGT mRNA expression in adipose tissue during the development of obesity initially contribute to hypertension, but are not maintained at a stage of chronic obesity. Moreover, the expanded mass of adipose tissue with obesity suggests that elevated AGT expression in individual adipocytes would not be a necessary requirement to mediate a stimulated systemic RAS in the development of hypertension.
3. Physiologic/pathophysiologic significance of an adipose RAS
As described above, multiple lines of evidence support the existence of an adipocyte RAS, which exhibits somewhat distinct mechanisms for regulation of RAS components. However, while it is intuitive that local production of AngII by cardiovascular-relevant cells and/or tissues might be anticipated since AngII regulates a variety of functions of these same cell types, the significance of local production of AngII by adipocytes has been less clear. Based on the available literature, there are two primary reasons why adipocytes might produce components of the RAS (Fig. 1): (1) to serve as a source of these components for systemic production of AngII, and (2) to produce AngII to exhibit autocrine/paracrine effects on adipocytes. Direct evidence that adipocytes serve as a source of AGT for the systemic RAS comes from studies in transgenic mice with adipocyte over-expression of AGT on a wild type or AGT-deficient background (Massiera et al., 2001). Expression of AGT in adipocytes increased circulating AGT concentrations in AGT-deficient mice, and transgenic over-expression of AGT in adipocytes of wild type mice increased both circulating AGT concentrations and blood pressure. These results were the first to demonstrate that adipocyte-derived AGT could contribute to the circulating RAS. However, no studies have used the alternative approach, namely adipocyte-specific deficiency of AGT, to define the physiologic significance of adipocyte-derived AGT to the circulating RAS. Studies aimed at definition of effects of AngII at adipocytes have focused on AngII regulation of adipocyte growth and differentiation, the adipocyte RAS, inflammation and oxidative stress, lipolysis and local blood flow. The majority of data has come from mice with whole body deficiency of RAS components, including AT1a and AT2 receptors. Below we discuss several potential effects of AngII at adipocytes, and the relationship between these effects and cardiovascular disease.
Fig. 1.
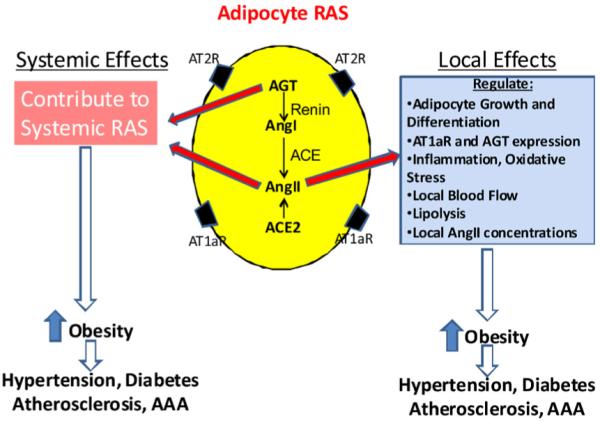
Physiologic/pathophysiologic significance of an adipocyte RAS. Adipocytes could synthesize and secrete components for the systemic RAS, or locally produced angiotensins could exert a variety of effects at adipocytes.
4. AngII as a regulator of adipocyte growth and differentiation
The expression of AngII receptors on adipocytes suggests that locally derived or systemic AngII could be implicated in the regulation of adipose growth and differentiation. AngII may be involved in the control of adiposity through regulation of lipid synthesis and storage in adipocytes. Alternatively, AngII may influence different aspects of the adipocyte differentiation process.
Several approaches have been used to define whether AngII regulates adipocyte differentiation. Initial studies examined expression of RAS components during adipocyte differentiation as an index of effects of the local RAS on adipocyte growth. In differentiating 3T3-L1 cells, mRNA expression of AGT, renin, ACE and AT1 receptors increased during differentiation (Janke et al., 2002; Saye et al., 1989). However, AT2 receptor mRNA expression was reported to decrease during the course of differentiation (Mallow et al., 2000). Similarly, in human preadipocytes, mRNA expression of AT1 and AT2 receptors were inversely regulated during differentiation (Schling and Schafer, 2002).
Several investigators have examined direct effects of angiotensins on adipocyte differentiation, with conflicting results (Ailhaud, 1999; Brucher et al., 2007; Skurk et al., 2004). In primary cultures from human adipose tissue, the stimulation of preadipocytes with AngII (1 μM) resulted in a reduction of cells in the G1 phase of the cell cycle and an increased number of cells in S and G2-M phases (Crandall et al., 1999; Darimont et al., 1994). These effects of AngII were associated with an increased expression of cyclin D1, a regulatory protein involved in cell cycle progression. The selective AT1 receptor antagonist, losartan, significantly attenuated the stimulatory response to AngII, whereas the selective AT2 receptor antagonist, PD 123319, had no effect (Crandall et al., 1999). The authors interpreted this to suggest that AngII influences the early stages of development of preadipocytes in a manner that would promote differentiation to the adipocyte phenotype. Using Ob1771 preadipocytes, AngII (0.1-10 μM) was demonstrated to increase differentiation to adipocytes through a prostacyclin-dependent effect at AT2 receptors (Darimont et al., 1994). Similarly, a recent study demonstrated that low concentrations of AngII (1 pM to 0.01 μM) increased adipocyte differentiation of human preadipocytes isolated from both visceral and perirenal adipose tissue (Sarzani et al., 2007).
However, conflicting results have been reported. For example, incubation of human preadipocytes with AngII (0.01-10 μM) decreased expression of PPARγ and fatty acid synthase, markers of adipocyte differentiation (Janke et al., 2002). Since the opposite results were demonstrated in cells treated with an AT1 receptor antagonist, the authors suggested that mature adipocytes release AngII that then acts in an autocrine/paracrine negative feedback manner to inhibit further adipocyte differentiation. Recent studies demonstrated that differentiation of stem cells to adipocytes was associated with an increase in cellular renin and AT2 receptor expression, but reduced expression of AGT and ACE (Matsushita et al., 2006). AngII inhibited differentiation of mesenchymal stem cells to adipocytes, and this effect was primarily mediated through AT2 receptors. The authors suggested that the local RAS might negatively regulate adipose mass. Since effects of AngII to regulate adipocyte differentiation have differed even within one species (e.g., humans), it is unclear what contributed to these opposing findings. Perhaps the source of preadipocytes, differential expression levels of AT2 receptors which could influence differentiation, or cell culture conditions (e.g., modes of differentiation and/or stage of differentiation of preadipocytes) may have contributed to these diverging results. We suggest that future studies using cell specific over-expression or knockdown of individual adipocyte RAS components in whole animal systems may clarify effects of AngII on adipocyte differentiation.
In addition to results from in vitro studies, several investigators have utilized mouse models with RAS manipulation to address the role of AngII to regulate adipose growth. In wild type mice with adipocyte-driven expression of AGT, adipose mass was increased, supporting a positive role of adipocyte-derived AGT in the regulation of adipose growth (Massiera et al., 2001). In male mice with whole body AT1a receptor deficiency, diet-induced obesity was attenuated, again supporting a positive role for AngII to regulate adipose mass (Kouyama et al., 2005). Similarly, in mice lacking AT1a receptors on an apolipoprotein E deficient background, or in obese KK-A(y) mice administered an AT1 receptor antagonist, adipose mass was reduced (Tomono et al., 2008). Surprisingly, similar findings were reported for an effect of AT2 receptor deficiency in mice to protect against the development of diet-induced obesity (Yvan-Charvet et al., 2005). The use of whole animal knockouts of these individual angiotensin receptors makes it difficult to determine the specific role of these receptors on adipocytes in the control of adipose growth and differentiation. Similar to approaches used for over-expression of AGT in adipocytes, studies with adipocyte-specific deficiency of AT1 or AT2 receptors may clarify the direct effects of AngII to control adipose growth.
In addition to angiotensin receptors, deficiency of other components of the RAS has also been demonstrated to influence adiposity. Mice lacking mas receptors, the receptor for Ang(1-7), exhibit a 50% increase in abdominal fat mass, despite normal body weight (Santos et al., 2008). Recent studies demonstrate that mice lacking renin are lean, insulin sensitive, and resistant to the development of diet-induced obesity (Takahashi et al., 2007). Similarly, ACE deficient mice exhibit lower body weight, fat mass, and increased energy expenditure compared to wild type (Jayasooriya et al., 2008). Collectively, mice with deficiencies of the synthetic components of the RAS exhibit a similar phenotype of leanness and resistance to the development of obesity, supporting a role for AngII in regulation of adipose mass. However, these findings in experimental rodents should be taken with caution given that inhibitors of the RAS are widely used, but have not been suggested to have marked effects on body weight. Further studies are warranted to directly address effects of RAS inhibitors on body weight in obese patients, or to define effects of adipocyte-specific over- or under-expression of RAS components on adipose mass and differentiation.
5. Adipocyte RAS and cardiovascular disease
5.1. Hypertension
Pivotal studies examined adipocyte-driven over-expression of AGT in adipose tissue using an aP2-promoter in transgenic mice (Massiera et al., 2001). This model was developed on a wild type background, as well as in AGT-deficient mice. When AGT expression was restricted to adipose tissue (on an AGT-deficient background), circulating AGT was detected and mice were normotensive, suggesting that adipose-derived AGT contributed to the circulating RAS. Moreover, mice over-expressing AGT in adipose tissue (on a wild type background) exhibited elevated circulating AGT, and were hypertensive. Results from this study were the first to demonstrate that adipocyte-derived AGT could contribute to the systemic pool, and to the regulation of blood pressure. Studies in our laboratory demonstrated that AGT mRNA expression was increased in visceral adipose tissue from rats and mice with diet-induced obesity and hypertension (Boustany et al., 2004; Gupte et al., 2008). Moreover, administration of losartan to obese hypertensive rats resulted in a greater reduction in blood pressure than lean controls (Boustany et al., 2005). Vascular contractility was enhanced in obese, hypertensive rats, and coronary artery relaxation was impaired, potentially contributing to elevated blood pressure (Boustany-Kari et al., 2006).
In humans, early studies demonstrated that plasma AGT concentrations and blood pressure correlated positively to body mass index (r = 0.33, P < 0.05), supporting a relationship between the adipose RAS and obesity in blood pressure control (Schorr et al., 1998). In addition, in a study designed to determine the impact of weight loss on the adipose RAS, plasma levels of AGT (-27%), renin (-43%), aldosterone (-31%), and ACE (-12%) decreased with weight loss (-5%) and were associated with a 7 mmHg reduction in systolic blood pressure (Engeli et al., 2005). In contrast to data from rodent models of diet-induced obesity, AGT mRNA expression in adipose tissue from obese hypertensive female patients was not elevated compared to lean subjects. However, weight loss did reduce adipose AGT mRNA expression. In addition, weight loss mediated reductions in adipose AGT mRNA expression correlated to systolic blood pressure and plasma AGT concentrations, suggesting that the effects of weight loss to decrease blood pressure may involve regulation of the adipocyte RAS. Since systemic AGT concentrations correlate to blood pressure and body mass index, the expanded adipose mass with obesity, rather than elevated expression in individual adipocytes, may be sufficient to activate the systemic RAS in human obesity-hypertension. Alternatively, since the majority of data demonstrating an elevation in AGT mRNA expression in adipose tissue comes from rodents with high fat diet-induced obesity, it would be interesting to determine if low fat diets as a mode of inducing weight loss in obese humans results in a more pronounced reduction in adipose AGT mRNA expression.
5.2. Atherosclerosis
In 1991, our laboratory demonstrated that perivascular adipose tissue influenced the responsiveness of rat aorta to several contractile agonists (Soltis and Cassis, 1991). Since then, several groups have examined effects of perivascular adipose tissue on blood vessel function (Brandes, 2007; Eringa et al., 2007; Galvez et al., 2006; Gao et al., 2007; Guzik et al., 2007; Iacobellis et al., 2008; Malinowski et al., 2008). Unfortunately, relatively few groups have examined the role of perivascular adipose tissue on atherosclerosis, or focused on a potential role for the adipocyte RAS as a link between obesity and coronary artery disease. However, almost all blood vessels are surrounded by adipose tissue, as is the heart; thus, it is conceivable that atherosclerosis could be influenced by factors derived from perivascular adipose tissue. Moreover, as perivascular adipose tissue increases with obesity (Henrichot et al., 2005), inflammation in adipose tissue may influence developing atherosclerotic lesions. Interestingly, supernatants from human perivascular white adipose tissue induced chemotaxis of peripheral blood leukocytes (Henrichot et al., 2005). This effect was suggested to contribute to obesity-associated atherosclerosis. Recent studies demonstrated that transplantation of adipose tissue from one apolipoprotein E deficient mouse to another promoted local inflammation in transplanted fat, and mice exhibited increased atherosclerosis (Ohman et al., 2008). However, a potential role for AngII or other RAS components from adipocytes in the inflammation of adipose tissue has not been addressed. Administration of an AT1 receptor antagonist to mice with diet-induced obesity ameliorated dysregulation of adipocytokines and reduced oxidative stress in adipose tissue, suggesting a role for the local adipose RAS in atherosclerotic disease associated with the metabolic syndrome (Kurata et al., 2006). In addition, administration of an AT1 receptor antagonist to apolipoprotein E deficient mice reduced expression of proinflammatory chemokines in adipose tissue and decreased atherosclerotic lesion formation, prompting the authors to suggest that atherosclerosis-reducing effects of AT1 receptor antagonists may be partially mediated by effects on the adipocyte RAS (Tomono et al., 2008). Future studies using over- or under-expression of RAS components in adipose tissue would facilitate definition of the role of the adipocyte RAS in adipose tissue inflammation and obesity-associated atherosclerosis.
5.3. Abdominal aortic aneurysms (AAAs)
Similar to blood vessels with atherosclerotic lesions (e.g., aorta and coronary arteries), the abdominal aorta where aneurysms form is surrounded by adipose tissue. Moreover, in contrast to atherosclerosis which is commonly an intimal disease, aneurysms typically involve all layers of the aorta, including the adventitia and extra-adventitial areas. Recent studies demonstrated that measures of obesity (waist circumference and waist-to-hip ratios) are independently associated with AAA formation (Golledge et al., 2007). Mechanisms for enhanced AAAs in obese patients are unknown, but may involve elevated systemic levels of proinflammatory adipokines. Since adipose tissue surrounds the abdominal aorta, it would be of interest to define whether the adipocyte RAS is a source for stimulated inflammation in the development of AAA formation.
6. Conclusions
Components of the RAS are expressed in adipose tissue, exhibit nutritional control by fatty acids, glucose, and other metabolic hormones, with their expression favoring increased AngII in the setting of obesity. Future studies should be aimed at identifying the physiological and pathophysiologic role of adipocyte-derived RAS components as contributors to the systemic system, and to examination of a role for locally derived AngII as a regulator of adipocyte inflammation. Development of models with adipocyte-specific over- or under-expression of individual RAS components would provide definitive evidence for an adipocyte RAS.
References
- Achard V, Boullu-Ciocca S, Desbriere R, Nguyen G, Grino M. Renin receptor expression in human adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R274–R282. doi: 10.1152/ajpregu.00439.2005. [DOI] [PubMed] [Google Scholar]
- Ailhaud G. Cross talk between adipocytes and their precursors: relationships with adipose tissue development and blood pressure. Ann. N Y Acad. Sci. 1999;892:127–133. doi: 10.1111/j.1749-6632.1999.tb07791.x. [DOI] [PubMed] [Google Scholar]
- Aubert J, Darimont C, Safonova I, Ailhaud G, Negrel R. Regulation by glucocorticoids of angiotensinogen gene expression and secretion in adipose cells. Biochem. J. 1997;328(Pt 2):701–716. doi: 10.1042/bj3280701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert J, Safonova I, Negrel R, Ailhaud G. Insulin down-regulates angiotensinogen gene expression and angiotensinogen secretion in cultured adipose cells. Biochem. Biophys. Res. Commun. 1998;250:77–82. doi: 10.1006/bbrc.1998.9185. [DOI] [PubMed] [Google Scholar]
- Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R943–R949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- Boustany CM, Brown DR, Randall DC, Cassis LA. AT1-receptor antagonism reverses the blood pressure elevation associated with diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R181–R186. doi: 10.1152/ajpregu.00507.2004. [DOI] [PubMed] [Google Scholar]
- Boustany-Kari CM, Gong M, Akers WS, Guo Z, Cassis LA. Enhanced vascular contractility and diminished coronary artery flow in rats made hypertensive from diet-induced obesity. Int. J. Obes. (Lond.) 2006;31:1652–1659. doi: 10.1038/sj.ijo.0803426. [DOI] [PubMed] [Google Scholar]
- Brandes RP. The fatter the better? Perivascular adipose tissue attenuates vascular contraction through different mechanisms. Br. J. Pharmacol. 2007;151:303–304. doi: 10.1038/sj.bjp.0707229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucher R, Cifuentes M, Acuna MJ, Albala C, Rojas CV. Larger antiadipogenic effect of angiotensin II on omental preadipose cells of obese humans. Obesity (Silver Spring) 2007;15:1643–1646. doi: 10.1038/oby.2007.196. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Habener JF. Cellular localization of angiotensinogen gene expression in brown adipose tissue and mesentery: quantification of messenger ribonucleic acid abundance using hybridization in situ. Endocrinology. 1987;121:1616–1626. doi: 10.1210/endo-121-5-1616. [DOI] [PubMed] [Google Scholar]
- Cassis LA. Downregulation of the renin-angiotensin system in streptozotocin-diabetic rats. Am. J. Physiol. 1992;262:E105–E109. doi: 10.1152/ajpendo.1992.262.1.E105. [DOI] [PubMed] [Google Scholar]
- Cassis LA. Angiotensin II in brown adipose tissue from young and adult Zucker obese and lean rats. Am. J. Physiol. 1994;266:E453–E458. doi: 10.1152/ajpendo.1994.266.3.E453. [DOI] [PubMed] [Google Scholar]
- Cassis LA. Fat cell metabolism: insulin, fatty acids, and renin. Curr. Hypertens. Rep. 2000;2:132–138. doi: 10.1007/s11906-000-0072-5. [DOI] [PubMed] [Google Scholar]
- Cassis LA, Fettinger MJ, Roe AL, Shenoy UR, Howard G. Characterization and regulation of angiotensin II receptors in rat adipose tissue. Angiotensin receptors in adipose tissue. Adv. Exp. Med. Biol. 1996;396:39–47. doi: 10.1007/978-1-4899-1376-0_5. [DOI] [PubMed] [Google Scholar]
- Cassis LA, Lynch KR, Peach MJ. Localization of angiotensinogen messenger RNA in rat aorta. Circ. Res. 1988a;62:1259–1262. doi: 10.1161/01.res.62.6.1259. [DOI] [PubMed] [Google Scholar]
- Cassis LA, Saye J, Peach MJ. Location and regulation of rat angiotensinogen messenger RNA. Hypertension. 1988b;11:591–596. doi: 10.1161/01.hyp.11.6.591. [DOI] [PubMed] [Google Scholar]
- Crandall DL, Armellino DC, Busler DE, McHendry-Rinde B, Kral JG. Angiotensin II receptors in human preadipocytes: role in cell cycle regulation. Endocrinology. 1999;140:154–158. doi: 10.1210/endo.140.1.6430. [DOI] [PubMed] [Google Scholar]
- Crandall DL, Herzlinger HE, Saunders BD, Armellino DC, Kral JG. Distribution of angiotensin II receptors in rat and human adipocytes. J. Lipid Res. 1994;35:1378–1385. [PubMed] [Google Scholar]
- Darimont C, Vassaux G, Gaillard D, Ailhaud G, Negrel R. In situ microdialysis of prostaglandins in adipose tissue: stimulation of prostacyclin release by angiotensin II. Int. J. Obes. Relat. Metab. Disord. 1994;18:783–788. [PubMed] [Google Scholar]
- Dusserre E, Moulin P, Vidal H. Differences in mRNA expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose tissues. Biochim. Biophys. Acta. 2000;1500:88–96. doi: 10.1016/s0925-4439(99)00091-5. [DOI] [PubMed] [Google Scholar]
- Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- Engeli S, Gorzelniak K, Kreutz R, Runkel N, Distler A, Sharma AM. Co-expression of renin-angiotensin system genes in human adipose tissue. J. Hypertens. 1999;17:555–560. doi: 10.1097/00004872-199917040-00014. [DOI] [PubMed] [Google Scholar]
- Eringa EC, Bakker W, Smulders YM, Serne EH, Yudkin JS, Stehouwer CD. Regulation of vascular function and insulin sensitivity by adipose tissue: focus on perivascular adipose tissue. Microcirculation. 2007;14:389–402. doi: 10.1080/10739680701303584. [DOI] [PubMed] [Google Scholar]
- Faloia E, Gatti C, Camilloni MA, Mariniello B, Sardu C, Garrapa GG, Mantero F, Giacchetti G. Comparison of circulating and local adipose tissue renin-angiotensin system in normotensive and hypertensive obese subjects. J. Endocrinol. Invest. 2002;25:309–314. doi: 10.1007/BF03344010. [DOI] [PubMed] [Google Scholar]
- Frederich RC, Jr., Kahn BB, Peach MJ, Flier JS. Tissue-specific nutritional regulation of angiotensinogen in adipose tissue. Hypertension. 1992;19:339–344. doi: 10.1161/01.hyp.19.4.339. [DOI] [PubMed] [Google Scholar]
- Galvez B, de Castro J, Herold D, Dubrovska G, Arribas S, Gonzalez MC, Aranguez I, Luft FC, Ramos MP, Gollasch M, Fernandez Alfonso MS. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler. Thromb. Vasc. Biol. 2006;26:1297–1302. doi: 10.1161/01.ATV.0000220381.40739.dd. [DOI] [PubMed] [Google Scholar]
- Galvez-Prieto B, Bolbrinker J, Stucchi P, de Las Heras AI, Merino B, Arribas S, Ruiz-Gayo M, Huber M, Wehland M, Kreutz R, Fernandez-Alfonso MS. Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. J. Endocrinol. 2008;197:55–64. doi: 10.1677/JOE-07-0284. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br. J. Pharmacol. 2007;151:323–331. doi: 10.1038/sj.bjp.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gembardt F, Sterner-Kock A, Imboden H, Spalteholz M, Reibitz F, Schultheiss HP, Siems WE, Walther T. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides. 2005;26:1270–1277. doi: 10.1016/j.peptides.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golledge J, Clancy P, Jamrozik K, Norman PE. Obesity, adipokines, and abdominal aortic aneurysm: health in men study. Circulation. 2007;116:2275–2279. doi: 10.1161/CIRCULATIONAHA.107.717926. [DOI] [PubMed] [Google Scholar]
- Gomez RA, Cassis L, Lynch KR, Chevalier RL, Wilfong N, Carey RM, Peach MJ. Fetal expression of the angiotensinogen gene. Endocrinology. 1988;123:2298–2302. doi: 10.1210/endo-123-5-2298. [DOI] [PubMed] [Google Scholar]
- Gorzelniak K, Engeli S, Janke J, Luft FC, Sharma AM. Hormonal regulation of the human adipose-tissue renin-angiotensin system: relationship to obesity and hypertension. J. Hypertens. 2002;20:965–973. doi: 10.1097/00004872-200205000-00032. [DOI] [PubMed] [Google Scholar]
- Gupte MN, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, English VL, Cassis LA. ACE2 is expressed in mouse adipocytes and regulated by a high fat diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R781–R788. doi: 10.1152/ajpregu.00183.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik TJ, Marvar PJ, Czesnikiewicz-Guzik M, Korbut R. Perivascular adipose tissue as a messenger of the brain-vessel axis: role in vascular inflammation and dysfunction. J. Physiol. Pharmacol. 2007;58:591–610. [PubMed] [Google Scholar]
- Hainault I, Nebout G, Turban S, Ardouin B, Ferre P, Quignard-Boulange A. Adipose tissue-specific increase in angiotensinogen expression and secretion in the obese (fa/fa) Zucker rat. Am. J. Physiol. Endocrinol. Metab. 2002;282:E59–E66. doi: 10.1152/ajpendo.2002.282.1.E59. [DOI] [PubMed] [Google Scholar]
- Harte AL, McTernan PG, McTernan CL, Crocker J, Starcynski J, Barnett AH, Matyka K, Kumar S. Insulin increases angiotensinogen expression in human abdominal subcutaneous adipocytes. Diab. Obes. Metab. 2003;5:462–467. doi: 10.1046/j.1463-1326.2003.00274.x. [DOI] [PubMed] [Google Scholar]
- Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler. Thromb. Vasc. Biol. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- Iacobellis G, Gao YJ, Sharma AM. Do cardiac and perivascular adipose tissue play a role in atherosclerosis? Curr. Diab. Rep. 2008;8:20–24. doi: 10.1007/s11892-008-0005-2. [DOI] [PubMed] [Google Scholar]
- Janke J, Engeli S, Gorzelniak K, Luft FC, Sharma AM. Mature adipocytes inhibit in vitro differentiation of human preadipocytes via angiotensin type 1 receptors. Diabetes. 2002;51:1699–1707. doi: 10.2337/diabetes.51.6.1699. [DOI] [PubMed] [Google Scholar]
- Jayasooriya AP, Mathai ML, Walker LL, Begg DP, Denton DA, Cameron-Smith D, Egan GF, McKinley MJ, Rodger PD, Sinclair AJ, Wark JD, Weisinger HS, Jois M, Weisinger RS. Mice lacking angiotensin-converting enzyme have increased energy expenditure, with reduced fat mass and improved glucose clearance. Proc. Natl. Acad. Sci. USA. 2008;105:6531–6536. doi: 10.1073/pnas.0802690105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BH, Standridge MK, Taylor JW, Moustaid N. Angiotensinogen gene expression in adipose tissue: analysis of obese models and hormonal and nutritional control. Am. J. Physiol. 1997;273:R236–R242. doi: 10.1152/ajpregu.1997.273.1.R236. [DOI] [PubMed] [Google Scholar]
- Kouyama R, Suganami T, Nishida J, Tanaka M, Toyoda T, Kiso M, Chiwata T, Miyamoto Y, Yoshimasa Y, Fukamizu A, Horiuchi M, Hirata Y, Ogawa Y. Attenuation of diet-induced weight gain and adiposity through increased energy expenditure in mice lacking angiotensin II type 1a receptor. Endocrinology. 2005;146:3481–3489. doi: 10.1210/en.2005-0003. [DOI] [PubMed] [Google Scholar]
- Kurata A, Nishizawa H, Kihara S, Maeda N, Sonoda M, Okada T, Ohashi K, Hibuse T, Fujita K, Yasui A, Hiuge A, Kumada M, Kuriyama H, Shimomura I, Funahashi T. Blockade of Angiotensin II type-1 receptor reduces oxidative stress in adipose tissue and ameliorates adipocytokine dysregulation. Kidney Int. 2006;70:1717–1724. doi: 10.1038/sj.ki.5001810. [DOI] [PubMed] [Google Scholar]
- Li J, Gao J, Xu YP, Zhou TL, Jin YY, Lou JN. Expression of severe acute respiratory syndrome coronavirus receptors, ACE2 and CD209L in different organ derived microvascular endothelial cells. Zhonghua Yi Xue Za Zhi. 2007;87:833–837. [PubMed] [Google Scholar]
- Lu H, Boustany-Kari CM, Daugherty A, Cassis LA. Angiotensin II increases adipose angiotensinogen expression. Am. J. Physiol. Endocrinol. Metab. 2007;292:E1280–E1287. doi: 10.1152/ajpendo.00277.2006. [DOI] [PubMed] [Google Scholar]
- Malinowski M, Deja MA, Golba KS, Roleder T, Biernat J, Wos S. Perivascular tissue of internal thoracic artery releases potent nitric oxide and prostacyclin-independent anticontractile factor. Eur. J. Cardiothorac. Surg. 2008;33:225–231. doi: 10.1016/j.ejcts.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Mallow H, Trindl A, Loffler G. Production of angiotensin II receptors type one (AT1) and type two (AT2) during the differentiation of 3T3-L1 preadipocytes. Horm. Metab. Res. 2000;32:500–503. doi: 10.1055/s-2007-978676. [DOI] [PubMed] [Google Scholar]
- Massiera F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, Quignard-Boulange A, Negrel R, Ailhaud G, Seydoux J, Meneton P, Teboul M. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15:2727–2729. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Wu Y, Okamoto Y, Pratt RE, Dzau VJ. Local renin-angiotensin expression regulates human mesenchymal stem cell differentiation to adipocytes. Hypertension. 2006;48:1020–1022. doi: 10.1161/01.HYP.0000248211.82232.a7. [DOI] [PubMed] [Google Scholar]
- McGehee RE, Jr., Ron D, Brasier AR, Habener JF. Differentiation-specific element: a cis-acting developmental switch required for the sustained transcriptional expression of the angiotensinogen gene during hormonal-induced differentiation of 3T3-L1 fibroblasts to adipocytes. Mol. Endocrinol. 1993;7:551–560. doi: 10.1210/mend.7.4.7684818. [DOI] [PubMed] [Google Scholar]
- Nebrel R, Grimaldi P, Ailhaud G. Differentiation of ob 17 preadipocytes to adipocytes. Effects of prostaglandin F2alpha and relationship to prostaglandin synthesis. Biochim. Biophys. Acta. 1981;666:15–24. doi: 10.1016/0005-2760(81)90086-2. [DOI] [PubMed] [Google Scholar]
- Ohman MK, Shen Y, Obimba CI, Wright AP, Warnock M, Lawrence DA, Eitzman DT. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2008;117:798–805. doi: 10.1161/CIRCULATIONAHA.107.717595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni K, Mark AL, Haynes WG, Sigmund CD. Adipose depot-specific modulation of angiotensinogen gene expression in diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2004;286:E891–E895. doi: 10.1152/ajpendo.00551.2003. [DOI] [PubMed] [Google Scholar]
- Rieusset J, Auwerx J, Vidal H. Regulation of gene expression by activation of the peroxisome proliferator-activated receptor gamma with rosiglitazone (BRL 49653) in human adipocytes. Biochem. Biophys. Res. Commun. 1999;265:265–271. doi: 10.1006/bbrc.1999.1657. [DOI] [PubMed] [Google Scholar]
- Russell TR, Ho R. Conversion of 3T3 fibroblasts into adipose cells: triggering of differentiation by prostaglandin F2alpha and 1-methyl-3-isobutyl xanthine. Proc. Natl. Acad. Sci. USA. 1976;73:4516–4520. doi: 10.1073/pnas.73.12.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safonova I, Aubert J, Negrel R, Ailhaud G. Regulation by fatty acids of angiotensinogen gene expression in preadipose cells. Biochem. J. 1997;322(Pt 1):235–239. doi: 10.1042/bj3220235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SH, Fernandes LR, Mario EG, Ferreira AV, Porto LC, Alvarez-Leite JI, Botion LM, Bader M, Alenina N, Santos RA. Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes. 2008;57:340–347. doi: 10.2337/db07-0953. [DOI] [PubMed] [Google Scholar]
- Sarzani R, Marcucci P, Salvi F, Bordicchia M, Espinosa E, Mucci L, Lorenzetti B, Minardi D, Muzzonigro G, Dessi-Fulgheri P, Rappelli A. Angiotensin II stimulates and atrial natriuretic peptide inhibits human visceral adipocyte growth. Int. J. Obes. (Lond.) 2007;32:259–267. doi: 10.1038/sj.ijo.0803724. [DOI] [PubMed] [Google Scholar]
- Saye JA, Cassis LA, Sturgill TW, Lynch KR, Peach MJ. Angiotensinogen gene expression in 3T3-L1 cells. Am. J. Physiol. 1989;256:C448–C451. doi: 10.1152/ajpcell.1989.256.2.C448. [DOI] [PubMed] [Google Scholar]
- Saye JA, Ragsdale NV, Carey RM, Peach MJ. Localization of angiotensin peptide-forming enzymes of 3T3-F442A adipocytes. Am. J. Physiol. 1993;264:C1570–C1576. doi: 10.1152/ajpcell.1993.264.6.C1570. [DOI] [PubMed] [Google Scholar]
- Schling P, Mallow H, Trindl A, Loffler G. Evidence for a local renin angiotensin system in primary cultured human preadipocytes. Int. J. Obes. Relat. Metab. Disord. 1999;23:336–341. doi: 10.1038/sj.ijo.0800821. [DOI] [PubMed] [Google Scholar]
- Schling P, Schafer T. Human adipose tissue cells keep tight control on the angiotensin II levels in their vicinity. J. Biol. Chem. 2002;277:48066–48075. doi: 10.1074/jbc.M204058200. [DOI] [PubMed] [Google Scholar]
- Schorr U, Blaschke K, Turan S, Distler A, Sharma AM. Relationship between angiotensinogen, leptin and blood pressure levels in young normotensive men. J. Hypertens. 1998;16:1475–1480. doi: 10.1097/00004872-199816100-00011. [DOI] [PubMed] [Google Scholar]
- Serazin-Leroy V, Morot M, de Mazancourt P, Giudicelli Y. Androgen regulation and site specificity of angiotensinogen gene expression and secretion in rat adipocytes. Am. J. Physiol. Endocrinol. Metab. 2000;279:E1398–E1405. doi: 10.1152/ajpendo.2000.279.6.E1398. [DOI] [PubMed] [Google Scholar]
- Serazin V, Dieudonne MN, Morot M, de Mazancourt P, Giudicelli Y. cAMP-positive regulation of angiotensinogen gene expression and protein secretion in rat adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2004a;286:E434–E438. doi: 10.1152/ajpendo.00188.2003. [DOI] [PubMed] [Google Scholar]
- Serazin V, Dos Santos E, Morot M, Giudicelli Y. Human adipose angiotensinogen gene expression and secretion are stimulated by cyclic AMP via increased DNA cyclic AMP responsive element binding activity. Endocrine. 2004b;25:97–104. doi: 10.1385/endo:25:2:097. [DOI] [PubMed] [Google Scholar]
- Shenoy U, Cassis L. Characterization of renin activity in brown adipose tissue. Am. J. Physiol. 1997;272:C989–C999. doi: 10.1152/ajpcell.1997.272.3.C989. [DOI] [PubMed] [Google Scholar]
- Skurk T, van Harmelen V, Hauner H. Angiotensin II stimulates the release of interleukin-6 and interleukin-8 from cultured human adipocytes by activation of NF-kappaB. Arterioscler. Thromb. Vasc. Biol. 2004;24:1199–1203. doi: 10.1161/01.ATV.0000131266.38312.2e. [DOI] [PubMed] [Google Scholar]
- Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin. Exp. Hypertens. A. 1991;13:277–296. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Li F, Hua K, Deng J, Wang CH, Bowers RR, Bartness TJ, Kim HS, Harp JB. Increased energy expenditure, dietary fat wasting, and resistance to diet-induced obesity in mice lacking renin. Cell. Metab. 2007;6:506–512. doi: 10.1016/j.cmet.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Umemura S, Ishii M, Tanimoto K, Murakami K, Fukamizu A. Molecular mechanism of transcriptional activation of angiotensinogen gene by proximal promoter. J. Clin. Invest. 1994;93:1370–1379. doi: 10.1172/JCI117113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomono Y, Iwai M, Inaba S, Mogi M, Horiuchi M. Blockade of AT1 receptor improves adipocyte differentiation in atherosclerotic and diabetic models. Am. J. Hypertens. 2008;21:206–212. doi: 10.1038/ajh.2007.50. [DOI] [PubMed] [Google Scholar]
- Turban S, Hainault I, Andre J, Ferre P, Quignard-Boulange A, Guerre-Millo M. Molecular and cellular mechanisms of adipose secretion: comparison of leptin and angiotensinogen. J. Cell. Biochem. 2001;82:666–673. doi: 10.1002/jcb.1187. [DOI] [PubMed] [Google Scholar]
- Turban S, Hainault I, Truccolo J, Andre J, Ferre P, Quignard-Boulange A, Guerre-Millo M. Specific increase in leptin production in obese (falfa) rat adipose cells. Biochem. J. 2002;362:113–118. doi: 10.1042/0264-6021:3620113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harmelen V, Ariapart P, Hoffstedt J, Lundkvist I, Bringman S, Arner P. Increased adipose angiotensinogen gene expression in human obesity. Obes. Res. 2000a;8:337–341. doi: 10.1038/oby.2000.40. [DOI] [PubMed] [Google Scholar]
- van Harmelen V, Elizalde M, Ariapart P, Bergstedt-Lindqvist S, Reynisdottir S, Hoffstedt J, Lundkvist I, Bringman S, Arner P. The association of human adipose angiotensinogen gene expression with abdominal fat distribution in obesity. Int. J. Obes. Relat. Metab. Disord. 2000b;24:673–678. doi: 10.1038/sj.ijo.0801217. [DOI] [PubMed] [Google Scholar]
- Wang B, Jenkins JR, Trayhurn P. Expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture: integrated response to TNF-alpha. Am. J. Physiol. Endocrinol. Metab. 2005;288:E731–E740. doi: 10.1152/ajpendo.00475.2004. [DOI] [PubMed] [Google Scholar]
- Yvan-Charvet L, Even P, Bloch-Faure M, Guerre-Millo M, Moustaid-Moussa N, Ferre P, Quignard-Boulange A. Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes. 2005;54:991–999. doi: 10.2337/diabetes.54.4.991. [DOI] [PubMed] [Google Scholar]
- Zhang XH, Zeng ZP, Li HZ, Zhou YR, Zhang J, Tong AL, Yan ZL. Expression of renin-angiotensin-aldosterone system in human adipose tissues. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006;28:766–769. [PubMed] [Google Scholar]


