Abstract
Toll-like receptors (TLRs) play a pivotal role in innate immunity and inflammation. Here we showed that genetic deficiency of Peli1, an E3 ubiquitin ligase, attenuates induction of proinflammatory cytokines by ligands of TLR3 and TLR4 and renders mice resistant to septic shock. Peli1 was required for TLR3-induced activation of IκB kinase (IKK) and its downstream target transcription factor NF-κB, but was dispensable for IKK–NF-κB activation induced by several other TLRs and the interleukin-1 receptor. Notably, Peli1 bound to and ubiquitinated RIP1, a signaling molecule that mediates IKK activation induced by the TLR3 and TLR4 adaptor TRIF. These findings suggest that Peli1 is a ubiquitin ligase needed for transmission of TRIF-dependent TLR signals.
Innate immunity serves as the first line of host defense against infections. Host cells are equipped with pattern-recognition receptors (PRRs) that recognize diverse pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), peptidoglycan (PGN), single- and double-stranded RNA, and DNA 1. A major family of PRRs is the Toll-like receptor (TLR) family. To date, 10 human and 12 mouse TLRs, each of which recognize distinct PAMPs, have been cloned 2. For example, TLR4 recognizes LPS derived from the outer membrane of gram-negative bacteria, TLR3 recognizes viral double-stranded RNA as well as the synthetic nucleic acid poly(I:C), whereas TLR9 recognizes unmethylated CpG motifs present in bacterial and viral DNAs 2. Recognition of PAMPs by TLRs triggers signaling pathways that lead to the production of proinflammatory cytokines, chemokines, and type I interferons (IFNs). These proteins combat invading pathogens and recruit additional immune cells to the site of infection. TLRs also facilitate induction of costimulatory molecules (e.g. CD80 and CD86) and MHC class II on dendritic cells and B cells, and stimulate B cell proliferation 3, 4.
A key TLR signaling event is activation of the NF-κB family of transcription factors, which mediate induction of various proinflammatory cytokines and many other immune genes 2, 5. In unstimulated cells, NF-κB proteins are sequestered in the cytoplasm by inhibitory proteins, IκBs 5. In response to TLR stimuli, IκB kinases (IKKs) phosphorylate IκBs, causing their degradation and NF-κB nuclear translocation 5. Another TLR signaling pathway, which is stimulated by TLR3 and TLR4, involves activation of the IKK-related kinases, IKKε (also called IKKi) and TBK1 6, 7, which are responsible for activation of IFN-responsive factor 3 (IRF3), a transcription factor that mediates induction of genes encoding type I IFNs and IFN-responsive factors 2.
The signaling function of TLRs depends on intracellular adaptors 2. MyD88 transmits signals emanating from all TLRs, except TLR3, and from the interleukin-1 receptor (IL-1R). Another signaling adaptor, TRIF (also called TICAM1) (http://www.signaling-gateway.org/molecule/query?afcsid=A004068), specifically mediates TLR3 and TLR4 signaling 8-10. TLR4 is unique in that it induces both MyD88- and TRIF-dependent signaling pathways. MyD88-mediated signaling involves recruitment and activation of IL-1R-associated kinases (IRAKs) 11. Upon activation, IRAKs interact with and activate TRAF6, an E3 ubiquitin ligase that specifically catalyzes lysine 63 (K63)-linked ubiquitin chains 12. TRAF6-mediated K63 ubiquitination plays a critical role in transducing TLR-proximal signals to downstream events, particularly the activation of IKK and NF-κB 13.
Recent studies suggest that the function of IRAKs may also involve interaction with the Peli (also called Pellino, hereafter named Peli) family of proteins 12. Peli proteins are mammalian homologues of Drosophila Peli, a molecule that interacts with the Drosophila IRAK-like molecule Pelle 14. Mammalian cells have three Peli members, which share strong sequence homology and contain a RING-like domain 12. In vitro studies suggest that Peli proteins function as E3 ubiquitin ligases that catalyze K63-linked ubiquitin chains 15-17. Like TRAF6, Peli proteins physically interact with IRAKs (IRAK1 and IRAK4) and induce ubiquitination of IRAK1 15-17. Based on cell line transfection studies, Peli proteins have been implicated in the regulation of IL-1R and MyD88-dependent TLR signaling 12. However, to date, the physiological functions of Peli proteins have not been resolved.
Compared to the MyD88-dependent signaling pathway, much less is known regarding how TRIF transduces TLR-proximal signals. Nevertheless, recent studies establish RIP1 (receptor-interacting protein 1) as an essential adaptor mediating IKK activation downstream of TRIF-dependent TLR3 signals 18, 19. RIP1 was originally identified as an adaptor kinase that transduces tumor necrosis factor receptor (TNFR) signals. In the TNFR pathway, the ubiquitin ligase TRAF2 interacts with and ubiquitinates RIP1, which in turn recruits IKK and its upstream kinase, Tak1; these kinases are then activated 13. RIP1 ubiquitination is also induced by the TLR3 ligand, poly(I:C), and is required for poly(I:C)-stimulated IKK activation 18. RIP1 interacts with TRIF via a RIP homotypic interaction motif (RHIM) 19, and the TRIF-RIP1 signaling complex also contains the adaptor molecule TRADD 20-22. Despite these important findings, the ubiquitin ligase responsible for ubiquitinating RIP1 in the TRIF-dependent TLR pathway remains elusive. Although TRAF6 was proposed to ubiquitinate RIP1, TRAF6 knockout studies revealed a dispensable role for this E3 in TRIF-dependent IKK activation 23.
In the present study, we provide genetic evidence that Peli1 has a non-redundant role in regulating RIP1 ubiquitination and IKK activation in the TRIF-dependent TLR pathway. Peli1 deficiency caused a specific defect in pro-inflammatory gene induction by ligands of TLR3 and TLR4 and rendered mice resistant to LPS- and poly(I:C)-induced lethality. Peli1 physically interacted with RIP1 and induced RIP1 ubiquitination. Loss of Peli1 attenuated TLR3-stimulated RIP1 ubiquitination and IKK activation but had no effect on the activation of IKK-related kinases. These findings suggest that Peli1 is an E3 ubiquitin ligase that mediates IKK–NF-κB activation in the TRIF-dependent TLR signaling pathway.
Results
Normal lymphocyte development in Peli1−/− mice
To understand the physiological function of Peli1 in immune regulation, we performed studies using Peli1−/− mice. These mutant mice were generated using conventional targeting strategy, in which coding exons 1 and 2 were replaced with a lacZ/neomycin cassette (Supplementary Fig. 1). The Peli1 homozygous knockout (Peli1−/−) mice were viable and did not display overt abnormalities in growth and survival. The development of thymocytes appeared to be normal, as Peli1−/− and Peli1+/+ mice produced comparable frequencies of CD4+ and CD8+ single-positive cells and CD4+CD8+ double-positive cells (Supplementary Fig. 2). The number of T cells was also normal in the spleen of Peli1−/− mice (data not shown). Similarly, the Peli1 deficiency did not cause obvious defect in the generation of B cells. Follicular and marginal zone B cells were present in comparable percentages in Peli1−/− and Peli1+/+ mice (Supplementary Fig. 2). Thus, Peli1 appears to be dispensable for the development of immune cells.
Septic shock resistance of Peli1−/− mice
To investigate the in vivo function of Peli1 in regulating TLR signaling, we employed a septic shock model involving intra-peritoneal (i.p.) injection of the TLR4 ligand LPS plus a liver-specific transcription inhibitor, D-galactosamine, which enhances the toxicity of LPS 24. In this acute inflammation model, lethality occurs within hours and is largely dependent on the production of the proinflammatory cytokine TNF 25, 26. As expected, injection of LPS and D-galacosamine caused lethality in the majority of Peli1+/+ mice within 9 hours and killed all of them within 18 hours (Fig. 1a). In sharp contrast, none of the Peli1−/− mice succumbed to the septic shock induction during the entire 24 hour time period (Fig. 1a). We also employed a septic shock model involving injection of high-doses of LPS in the absence of D-galactosamine. As expected, a much higher dose of LPS (45 mg/kg body weight) and a considerably longer time (24 hours) were required to induce a moderate frequency (2 out of 6) of lethality in wild-type mice (Supplementary Fig. 3). At this time, none of the Peli1−/− mice succumbed. After longer times, 1 of the 6 Peli1−/− mice died, compared to 50% of the Peli1+/+ mice (Supplementary Fig. 3). Thus, although less striking than the acute lethality model, the Peli1 deficiency also caused reduced the lethality induced in the high-dose LPS model.
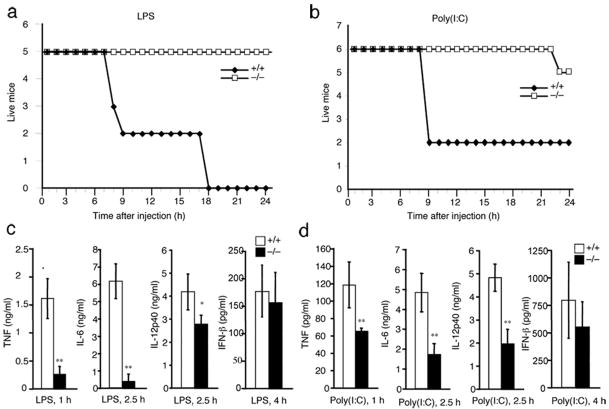
Figure 1. Peli1−/− mice are resistant to LPS- and poly(I:C)-induced lethality.
(a,b) Age- and sex-matched Peli1+/+ and Peli1−/− mice (8 week old; 5 Peli1+/+ and 5 Peli1−/− for a; 6 Peli1+/+ and 5 Peli1−/− for b) were injected (i.p.) with LPS plus D-galactosamine (a) or poly(I:C) plus D-galactosamine (b). Lethality was monitored every hour for 24 hours. (c) Mice shown in a were bled at the indicated times after injection, and the serum concentration of the indicated cytokines was determined by ELISA and presented as mean ± s.d. (d) Mice shown in b were bled at the indicated times after injection, and the serum concentration of the indicated cytokines was determined by ELISA and presented as mean ± sd. * P < 0.05 and ** P < 0.01. Data are representative of 3 independent experiments with similar results.
Like LPS, the TLR3 ligand poly(I:C) also induces lethality in mice through a TNF-dependent mechanism27. We thus examined the effect of Peli1 deficiency on the lethality induced by poly(I:C). Compared to LPS, poly(I:C) was relatively inefficient in inducing lethality. Nevertheless, at a high dose (0.5 μg/g body weight), poly(I:C) induced death of most of the Peli1+/+ mice within 8 hours but none of the Peli1−/− mice at this early time point (Fig. 1b). Even at late times, only 1 out of 6 Peli1−/− mice died.
To understand the molecular mechanism mediating the resistance of Peli1−/− mice to septic shock induction, we bled the LPS- and poly(I:C)-challenged mice during early time periods (1-4 hours) after LPS and poly(I:C) injection and measured the serum concentrations of pro-inflammatory cytokines as well as the anti-viral cytokine interferon (IFN)-β. Injection of Peli1+/+ mice led to the production of high concentrations of TNF, IL-6, IL-12P40, and IFN-β (Fig. 1c). Consistent with the lethality result, the induction of pro-inflammatory cytokines was severely attenuated in the Peli1−/− mice (Fig. 1c). Loss of Peli1 also partially inhibited the proinflammatory cytokine induction by poly(I:C) (Fig. 1d). On the other hand, Peli1 deficiency only slightly reduced the production of IFN-β (Fig. 1c,d). These results suggest a critical role for Peli1 in regulating TLR3 and TLR4-mediated proinflammatory cytokine induction and lethality.
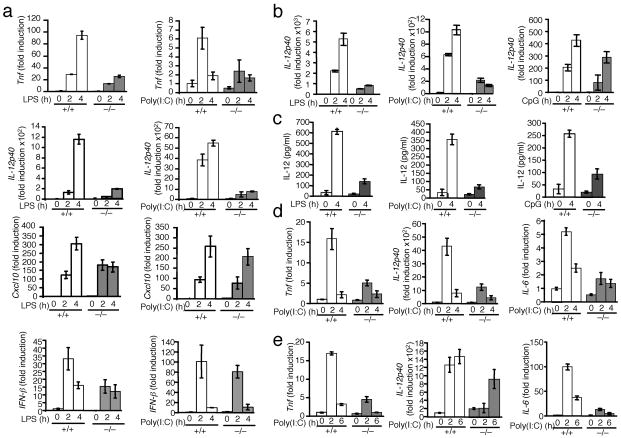
Next we analyzed the effect of Peli1 deficiency on TLR-mediated gene induction in vitro. For these studies, we prepared primary murine embryonic fibroblasts (MEFs) from Peli1+/+ and Peli1−/− littermate embryos (dissected from the same pregnant females). Stimulation of Peli1+/+ MEFs by LPS and poly(I:C) led to the induction of Tnf gene expression (Fig. 2a). Consistent with the in vivo studies, induction of Tnf by both LPS and poly(I:C) was severely inhibited in the Peli1−/− MEFs. Peli1 was also essential for LPS- and poly(I:C)-stimulated expression of another proinflammatory gene, IL-12p40 (Fig. 2a). On the other hand, Peli1 is not a universal signaling component of the TLR pathway, as it was not required for LPS- and poly(I:C)-stimulated expression of IFN-β and an IFN-responsive gene, Cxcl10, which encodes the interferon-inducible protein-10 (IP-10) 28 (Fig. 2a). Real-time PCR and ELISA studies using bone marrow derived macrophages revealed that Peli1 was critical for IL-12p40 induction by LPS and poly(I:C) and was partially required for IL-12p40 induction by CpG (Fig. 2b,c). In addition, in bone marrow-derived dendritic cells and B cells, poly(I:C)-stimulated proinflammatory gene expression was severely attenuated in the absence of Peli (Fig. 2d,e). However, Peli1 deficiency did not appreciably affect gene induction by several other TLR ligands, including Pam3CSK4 (TLR1-TLR2), R837 (TLR7, TLR8), and MALP2 (TLR2-TLR6) (Supplementary Fig. 4). Thus, Peli1 is required for pro-inflammatory cytokine gene induction predominantly by LPS and poly(I:C).
Figure 2. Peli1 is required for TLR3- and TLR4-mediated induction of pro-inflammatory genes.
(a) Peli1+/+ (+/+) and Peli1−/− (−/−) primary MEFs were stimulated with LPS or poly(I:C) and subjected to real-time PCR to detect the relative expression of mRNA transcripts encoding the indicated genes. The data are presented as mean ± s.d. fold induction compared to untreated (0 h) Peli1+/+ samples. (b,c) Peli+/+ (+/+) and Peli1−/− (−/−) bone marrow derived macrophages were stimulated as indicated and subjected to real-time PCR as described in a (b) or ELISA (c). (d,e) Bone marrow derived dendritic cells (d) and splenic B cells (e) were stimulated as indicated and subjected to real-time PCR as described in a. Data are representative of 2 independent experiments.
Peli1 in TLR-mediated B cell activation-survival
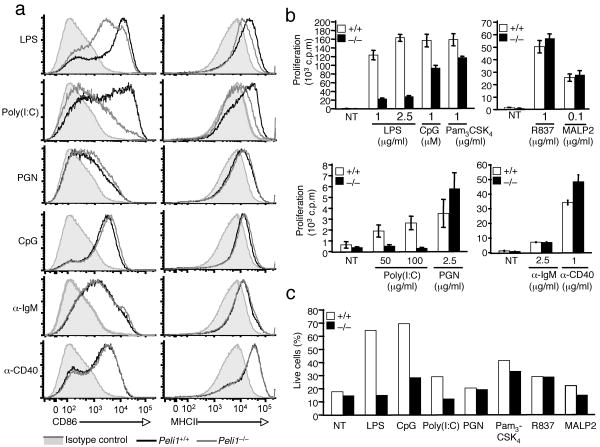
TLRs play an important role in B cell activation 29-31. In response to TLR signals, B cells express costimulatory molecules and increase the expression of MHCII molecules, thus enhancing their ability to activate helper T cells. To examine whether Peli1 is involved in TLR-mediated B cell activation, we analyzed the effect of Peli1 deficiency on the activation of B cells derived from Peli1+/+ and Peli1−/− mice. As expected, stimulation of Peli1+/+ B cells with several TLR ligands (LPS, poly(I:C), PGN, CpG) led to potent induction of the costimulatory molecule CD86 (Fig. 3a, left panels, black line) as well as enhanced expression of MHCII (Fig. 3a, right panels, black line). Induction of these genes was also detected in cells stimulated with antibodies that crosslink IgM and CD40 (Fig. 3a). Induction of both CD86 and MHCII by LPS was attenuated in Peli1−/− B cells (Fig. 3a). The inhibitory effect of Peli1 deficiency on CD86 and MHCII induction was even more profoundly seen in B cells stimulated with the TLR3 ligand, poly(I:C) (Fig. 3a). On the other hand, loss of Peli1 had no effect on the induction of CD86 and MHCII by several other TLR ligands, including PGN and CpG (Fig. 3a) as well as Pam3CSK4, MALP2, and R837 (Supplementary Fig. 5 and data not shown). Furthermore, Peli1 was dispensable for the induction of CD86 and MHCII by anti-IgM and anti-CD40. Thus, Peli1 is required for CD86 and MHCII induction specifically by TLR3 and TLR4 signals.
Figure 3. Peli1 regulates B cell activation and survival.
(a) Peli1+/+ and Peli1−/− splenic B cells were incubated in vitro for 24 h in the presence of LPS (1 μg/ml), poly(I:C) (50 μg/ml), PGN (2.5 μg/ml), CpG (1 μM), anti-IgM (2.5 μg/ml) or anti-CD40 (1 μg/ml). Untreated Peli1+/+ cells (shaded area) were included as controls. Surface expression of CD86 and MHCII was detected by flow cytometry. (b) Peli1+/+ and Peli1−/− splenic B cells were incubated in vitro in the absence (NT) or presence of the indicated stimuli. After 48 h, cell proliferation was measured by [3H]thymidine-incorporation and presented as mean ± s.d. (c) Peli1+/+ and Peli1−/− splenic B cells were incubated for 24 h in the absence (NT) or presence of the indicated TLR ligands. Apoptosis was analyzed by annexinV-FITC and PI staining to quantify the annexinV–PI– live cells. Data are representative of 3 independent experiments.
TLR signals also induce the proliferation of B cells 31. Consistently, the proliferation of Peli1+/+ B cells was stimulated by a panel of TLR ligands as well as by IgM and CD40 crosslinking (Fig. 3b). As seen with the induction of CD86 and MHCII expression, the induction of proliferation by LPS and poly(I:C) was severely inhibited in Peli1−/− B cells. This effect was again more profound for poly(I:C). We detected a partial inhibition of CpG- and Pam3CSK4-induced proliferation in Peli1−/− B cells (Fig. 3b). This effect was, however, not generic for all MyD88-binding TLRs, since loss of Peli1 did not inhibit the induction of B cell proliferation by R837, MALP2, or PGN (Fig. 3b). Peli1 deficiency did not affect proliferation induced by IgM or CD40 crosslinking. In fact, we repeatedly detected enhanced proliferation of PGN- and anti-CD40-stimulated Peli1−/− B cells, although it is currently unclear how Peli1 negatively regulates the signaling of these pathways.
TLR ligands, particularly LPS and CpG 32, 33, also stimulate the survival of B cells. To examine the role of Peli1 in TLR-driven survival, we incubated Peli1+/+ and Peli1−/− B cells in vitro in the presence of different TLR ligands. In the absence of TLR ligands, Peli1+/+ B cells underwent massive apoptosis, as determined by staining with annexin-V and PI. After culture for 24 hours, less than 20% of the cells were negative for both PI and annexin-V (Fig. 3c, live cells), but the spontaneous death of Peli1+/+ B cells was greatly inhibited by LPS and CpG and moderately inhibited by several other TLR ligands (Fig. 3c). However, loss of Peli1 severely attenuated the pro-survival effects of LPS, poly(I:C), and CpG (Fig. 3c). Thus, Peli1 mediates TLR3 and TLR4-driven induction of costimulatory gene expression, proliferation and survival in B cells.
Peli1 in IKK-NF-κB activation
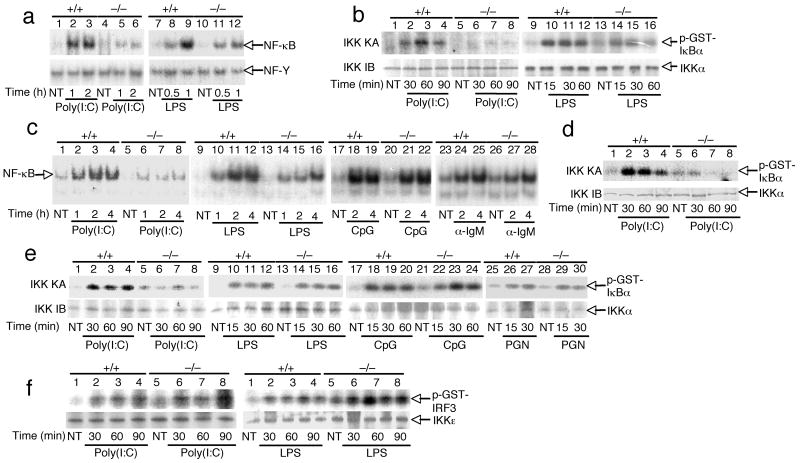
A central signaling event involved in TLR-mediated gene induction is activation of IKK and its downstream transcription factor NF-κB 2. To investigate the function of Peli1 in this process, we examined the effect of Peli1 deficiency on TLR-stimulated activation of IKK and NF-κB using different cell types. Consistent with the gene induction studies (Fig. 2,3), poly(I:C)-stimulated NF-κB activation was severely attenuated in Peli1−/− MEFs (Fig. 4a). The Peli1 deficient MEFs also showed a partial inhibition of NF-κB activation by LPS (Fig. 4a). Parallel kinase assays revealed that Peli1 deficiency largely impaired poly(I:C)-stimulated activation of IKK and also partially inhibited the LPS-stimulated activation of IKK (Fig. 4b). Since TLR4 activates IKK through both MyD88- and TRIF-dependent pathways, whereas TLR3 activates IKK exclusively through TRIF 2, these results suggest that Peli1 plays a non-redundant role in promoting IKK activation in the TRIF-dependent TLR pathway.
Figure 4. Peli1 is essential for IKK-NF-κB activation by TLR3.
(a) Primary Peli1+/+ and Peli1−/− MEFs were unstimulated (NT) or stimulated with poly(I:C) (50 μg/ml) or LPS (1 μg/ml). Nuclear extracts were subjected to EMSA for detection of NF-κB and NF-Y. (b) MEFs were treated as indicated and subjected to kinase assay (KA) by isolating IKK holoenzyme by IP using anti-IKKγ and using GST-IκBα(1-54) as substrate (upper). Kinase assay membrane was subjected to IB using anti-IKKα (lower). (c,d) Peli1+/+ and Peli1−/− B cells were stimulated as indicated and subjected to EMSA (c) and kinase assays (d). (e) Peli1+/+ and Peli1−/− splenocytes were stimulated as indicated and subjected to kinase assays as described in b. (f) Primary Peli1+/+ and Peli1−/− MEFs were stimulated as in A. IKKε was isolated by IP and subjected to kinase assays using GST-IRF3 as substrate. Data are representative of 3 or more independent experiments.
We next examined the role of Peli1 in the activation of NF-κB in B cells. As seen in MEFs, the Peli1 deficiency markedly inhibited poly(I:C)-stimulated activation of NF-κB (Fig. 4c) and IKK (Fig. 4d). The loss of Peli1 also partially inhibited LPS-stimulated NF-κB activation. However, Peli1 was largely dispensable for NF-κB activation by CpG and anti-IgM (Fig. 4c). A similar result was obtained in splenocytes, in which Peli1 was required for IKK activation by poly(I:C) but not by other TLR ligands (Fig. 4e). Notably, the effect of Peli1 deficiency on LPS-stimulated IKK activation was not appreciable in splenocytes. These results further suggest a role for Peli1 in mediating IKK–NF-κB activation by the TRIF-dependent TLRs, particularly TLR3.
In addition to activation of IKK, the TRIF-dependent TLR pathway stimulates the activity of IKK-related kinases, IKKε and TBK1. To further investigate the signaling mechanism of Peli1, we examined its role in the activation of IKKε induced by poly(I:C) and LPS. In contrast to the activation of IKK, the activation of IKKε downstream of both poly(I:C) and LPS was similar in Peli1−/− and Peli1+/+ MEFs (Fig. 4f). Thus, Peli1 plays a selective role in regulating IKK–NF-κB activation by TLR3.
Peli1 is dispensable for IL-1R signaling
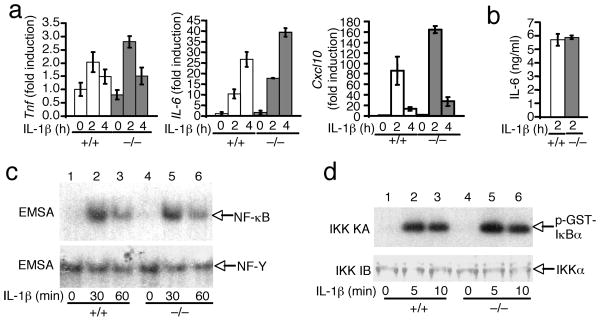
Prior transfection studies performed using a cell line model suggest that Peli1 facilitates IL-1R signaling 34. However, it is unknown whether Peli1 is required for IL-1R signaling under physiological conditions. To address this question, we examined the effect of Peli1 deficiency on IL-1R-mediated pro-inflammatory gene induction in primary MEFs, a cell type that efficiently responds to IL-1β stimulation. In Peli1+/+ MEFs, IL-1β stimulated the expression of Tnf and Il6, although it did not induce appreciable expression of IL-12p40 (Fig. 5a and data not shown). IL-1β also induced expression of Cxcl10, the gene encoding IP-10, in Peli1+/+ MEFs. However, loss of Peli1 did not affect IL-1β-stimulated expression of Tnf, Il6 or Cxcl10 (Fig. 5a). To further examine the role of Peli1 in IL-1R signaling, we employed an in vivo gene induction model. When injected into Peli1+/+ mice, IL-1β stimulated production of high concentrations of IL-6 (Fig. 5b), although it did not induce detectable quantities of TNF (data not shown). Consistent with the in vitro results, Peli1 was completely dispensable for IL-1β-induced IL-6 production in vivo (Fig. 5b).
Figure 5. Dispensable role of Peli1 in IL-1R signaling.
(a) Peli1+/+ and Peli1−/− MEFs were stimulated with IL-1β (10 ng/ml) and subjected to real-time RT-PCR. Data are presented as fold induction (mean ± sd) over the non-stimulated (0 h) Peli1+/+ samples. (b) Peli1+/+ and Peli1−/− mice were injected (i.p.) with IL-1β (20 μg/kg body weight) and bled at 2 h after the injection. The concentration of IL-6 and TNF was determined by ELISA and presented as mean ± sd (n = 5 mice of each genotype). TNF was undetectable at 30 min and 2 h (data not shown). (c,d) MEFs were not treated (NT) or stimulated with IL-1β Nuclear extracts were subjected to EMSA using NF-κB or NF-Y probes (c), and total cell lysates were subjected to in vitro kinase assays using GST-IκBα(1-54) as substrate (d). Data are representative of 2 (a,b) and 3 (c,d) independent experiments.
To directly examine the role of Peli1 in IL-1R signaling, we analyzed the effect of Peli1 deficiency on IL-1β-stimuated activation of NF-κB and IKK. IL-1β stimulated comparable NF-κB DNA-binding activity in Peli1+/+ and Peli1−/− MEFs (Fig. 5c). Consistently, the loss of Peli1 did not affect IL-1β-stimulated activation of IKK (Fig. 5d). Together, these results suggest that Peli1 is either functionally redundant with other Peli proteins or not critical in the IL-1R signaling pathway. As IL-1R transduces signals through MyD88 35, these results are consistent with the dispensable role of Peli1 in IKK–NF-κB activation by the MyD88-dependent TLR2 and TLR9 (Fig. 4c,e).
Peli1 induces RIP1 ubiquitination
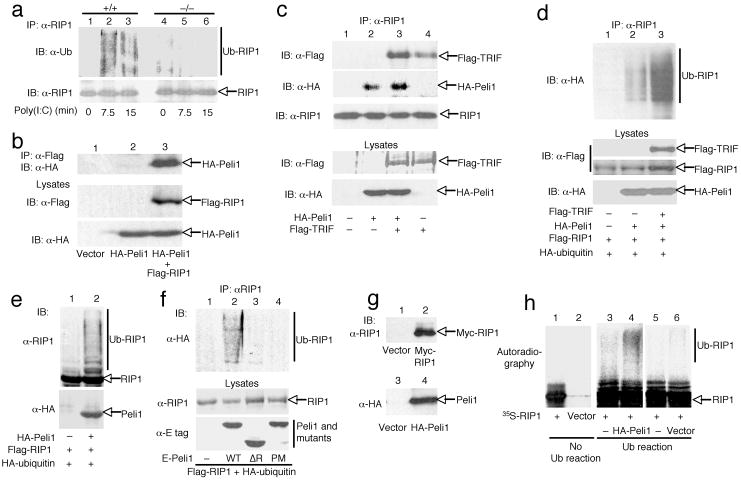
The signaling studies presented above suggest a role for Peli1 in regulating IKK activation in the TRIF-dependent TLR pathway. A hallmark of this pathway of IKK activation is the ubiquitination of a TRIF-interacting adaptor kinase, RIP1 18. In response to poly(I:C) stimulation, RIP1 undergoes K63 ubiquitination, which is critical for recruitment and activation of IKK. To date, the ubiquitin ligase of RIP1 in the TRIF-dependent TLR pathway remains unidentified. Since Peli1 is an E3 ubiquitin ligase 15-17 that is critical for IKK activation by TLR3, we examined the potential role of Peli1 in ubiquitinating RIP1. We first analyzed whether Peli1 is essential for poly(I:C)-stimulated ubiquitination of RIP1. Consistent with previous studies 18, 21, stimulation of Peli1+/+ MEFs with poly(I:C) led to accumulation of ubiquitinated RIP1 (Fig. 6a). In contrast, the poly(I:C)-stimulated RIP1 ubiquitination was largely blocked in the Peli1−/− cells (Fig. 6a and Supplementary Fig. 6).
Figure 6. Peli1 interacts with and ubiquitinates RIP1.
(a) Peli1+/+ and Peli1−/− MEFs were stimulated with poly(I:C) (100 μg/ml) and subjected to RIP1 ubiquitination (top) and RIP1 IB (bottom) assays. (b,c) 293 cells were transfected with the indicated expression vectors. Flag-RIP1 (b) or endogenous RIP1 (c) was isolated by IP followed by IB to detect the indicated proteins. Cell lysates were also subjected to IBs. (d-f) 293 cells were transfected with (+) or without (–) the indicated expression vectors. RIP1 was isolated by IP, and the ubiquitinated RIP1 was detected using anti-HA (d,f) or anti-RIP1 (e). (g,f) Myc-RIP1, HA-Peli1, or vector control were translated in vitro as 35S-labeled (Myc-RIP1) or cold (HA-Peli1) proteins and detected by IB (g). The indicated translation products were incubated in the absence (no ub reaction) or presence (ub reaction) of ubiquitination components. Ubiquitinated RIP1 and unmodified RIP1 were separated by SDS-PAGE and visualized by autoradiography.
We next examined whether Peli1 physically associates with RIP1. When coexpressed in 293 cells, Peli1 and RIP1 indeed formed a stable complex that was readily detected by coimmunoprecipitation assays (Fig. 6b). Peli1 also interacted with endogenous RIP1 (Fig. 6c). Peli1-RIP1 binding seemed to be further enhanced by expression of TRIF, and Peli1 seemed to enhance the association between TRIF and endogenous RIP1 (Fig. 6c). These enhanced physical interaction activities were not due to increases in the expression of RIP1, TRIF, or Peli1 (Fig. 6c). As Peli1 was essential for poly(I:C)-stimulated RIP1 ubiquitination, we examined whether Peli1 was able to induce RIP1 ubiquitination. Indeed, when coexpressed with Peli1, RIP1 became potently ubiquitinated (Fig. 6d,e). Moreover, the Peli1-mediated RIP1 ubiquitination was further enhanced by TRIF (Fig. 6d). Peli1-mediated RIP1 ubiquitination required the RING domain of Peli1, since the ubiquitination of RIP1 was not induced by Peli1 mutants harboring RING deletion (ΔR) or point mutations (PM) (Fig. 6f). Peli1-mediated RIP1 ubiquitination appeared to be direct, as Peli1 also induced RIP1 ubiquitination in a cell-free system (Fig. 6h). These results suggest that Peli1 is an E3 ubiquitin ligase that mediates RIP1 ubiquitination in the TRIF-dependent TLR pathway.
Discussion
Peli proteins possess K63 ubiquitin ligase function 15-17, although their physiological role has remained unknown. The results presented in this paper demonstrate a critical role for Peli1 in facilitating TLR3-stimulated activation of IKK and its downstream transcription factor NF-κB. Peli1 interacted with the TRIF-signaling adaptor kinase RIP1, and mediated RIP1 ubiquitination, a key step in the TRIF-dependent IKK-signaling pathway. Consequently, Peli1 was required for TLR3-stimulated expression of pro-inflammatory cytokines and B cell activation. Peli1 was also important for TLR4-mediated gene induction, although it was only partially required for LPS-stimulated activation of IKK and NF-κB. This result is reminiscent of the phenotype of TRIF- and RIP1-deficient cells, and can be attributed to the involvement of both TRIF- and MyD88-dependent pathways in TLR4-mediated IKK–NF-κB activation 10, 18, 33. Our study further revealed that, like RIP1, Peli1 was dispensable for IKK–NF-κB activation induced by IL-1R and the MyD88-dependent TLR2 and TLR9.
Peli1 joins TRAF6 as the second E3 ubiquitin ligase known to mediate IKK–NF-κB activation in the TLR-signaling pathways. However, these two E3 molecules appear to have distinct roles. Extensive studies have established TRAF6 as an essential E3 in the MyD88-dependent TLR and IL-1R signaling pathways 2. On the other hand, TRAF6 appears to be less important for TRIF-dependent TLR signaling. Although TRAF6 binds to TRIF and modulates TRIF-specific signaling function under transfection conditions 36, TRAF6 deficiency does not impair poly(I:C)-stimulated NF-κB activation 23. In contrast to TRAF6, Peli1 is critical for the TRIF-dependent TLR signaling but is dispensable for the signaling function of MyD88-dependent TLRs and IL-1R. The finding that Peli1 is not required for IL-1R signaling was unexpected, as a previous transfection study suggests a role for Peli1 in regulating IL-1R signaling in 293 cells 34. It is currently unclear whether Peli1 is functionally redundant with TRAF6 or other Peli members in mediating the signaling of IL-1R and MyD88-dependent TLRs. As the different Peli family members share strong sequence homology 12, it is possible that they possess functional redundancy in certain signaling pathways and cell types. Nevertheless, our findings suggest a crucial function of Peli1 in the TRIF-dependent NF-κB signaling pathway. It is important to note, however, that the defect of Peli1−/− mice and cells in cytokine induction is less severe than that of the TRIF–/– mice and cells. This finding implies that Peli1 homologues or additional E3 proteins may also participate in TRIF signaling. An alternative explanation is that the ubiquitination of RIP1 is important, but not absolutely essential, for mediating TRIF-dependent NF-κB activation. The signaling function of Peli1 appears to be cell type-specific, with the most obvious phenotype observed in B cells and MEFs. Future studies will examine whether the cell type-specific function of Peli1 is due to its expression amount or redundancy with other Peli members.
The TRIF-dependent TLR3 signaling pathway activates IKKε and TBK1, which mediate activation of IRF3 and IFN-responsive genes. Like the activation of IKK, the activation of IKKε and TBK1also involves K63 ubiquitination. We showed that Peli1 deficiency only had moderate effects on LPS- and poly(I:C)-induced expression of type I IFNs both in vivo and in vitro. Furthermore, Peli1 was not required for TLR3-mediated activation of IKKε. Consistent with our finding, TRAF3 has been implicated as the E3 ubiquitin ligase that mediates IKKε and TBK1 activation 37. Our finding that Peli1 regulates RIP1 further suggests a specific function of Peli1 in the IKK–NF-κB signaling axis, as RIP1 mediates activation of NF-κB but not that of IRF3 18. When injected in vivo, poly(I:C) also induces a cytoplasmic PRR signaling pathway involving the adaptors MDA5 and IPS-1 (also known as MAVS and Cardif) 38, 39. Future studies will examine whether Peli1 has a role in regulating the MDA5-IPS-1 signaling pathway.
Our data suggest that in addition to its role in regulating the signaling function of TRIF-dependent TLRs (TLR3 and TLR4), Peli1 is partially required for CpG (TLR9)- and Pam3CSK4 (TLR1-TLR2)-stimulated B cell proliferation and survival. However, this function is not generic for all MyD88-dependent TLRs, because Peli1 is not required for B cell activation induced by the ligands of TLR2 (PGN), TLR2-TLR6 (MALP2), or TLR7-TLR8 (R837). It is currently unclear how Peli1 regulates the function of specific MyD88-dependent TLRs, but circumstantial evidence suggests the involvement of RIP1 in some MyD88-dependent TLRs, particularly TLR9. Like Peli1−/− B cells, RIP1-deficient B cells are partially defective in CpG-induced B cell proliferation 33. As Peli1 is not required for CpG-stimulated NF-κB activation, we propose that Peli1 might regulate CpG-mediated activation of other signaling factors or specific members of NF-κB.
Additional signaling functions of Peli1 are also implicated by the interaction of Peli proteins with IRAK family members, particularly IRAK1 and IRAK4 12. Whether IRAKs are involved in the signaling function of Peli1 in the TLR3 signaling pathway remains to be investigated. However, this possibility is low, as IRAKs do not seem to have a critical role in TRIF-dependent TLR signaling, despite their critical participation in the MyD88-dependent TLR and IL-1R signaling pathways 40-42. It is more likely that IRAKs modulate the function of Peli1 in MyD88-dependent TLR and IL-1R pathways. As discussed above, this function of Peli1 might be masked by its functional redundancy with other Peli members or TRAF6. Generation of mouse models deficient in different Peli proteins is important for addressing this question. Notwithstanding, our current work establishes Peli1 is an E3 ubiquitin ligase that is specifically required for TRIF-induced RIP1 ubiquitination and IKK activation.
Methods
The Methods and their associated references appear only online.
Supplementary Material
Acknowledgments
We thank Texas Institute for Genomic Medicine for providing the Peli1 knockout mice. We also thank R Beyaert (Ghent University), J Hiscott (McGill University), V Chau (Pennsylvania State University College of Medicine), MK Offermann (Emory University), and WJ. Kaiser (Emory University) for reagents. This study was supported by grants from National Institutes of Health (AI057555, AI064639, GM84459).
Footnotes
Author Contributions: M.C. and W.J. designed and performed the research and prepared the figures. S.C.S. designed the research and wrote the manuscript.
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Hemmi H, Akira S. TLR signalling and the function of dendritic cells. Chem Immunol Allergy. 2005;86:120–135. doi: 10.1159/000086657. [DOI] [PubMed] [Google Scholar]
- 4.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol. 2005;560:11–18. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- 5.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald KA, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 7.Sharma S, et al. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto M, et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 9.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 11.Janssens S, Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell. 2003;11:293–302. doi: 10.1016/s1097-2765(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 12.Schauvliege R, Janssens S, Beyaert R. Pellino proteins: novel players in TLR and IL-1R signalling. J Cell Mol Med. 2007;11:453–461. doi: 10.1111/j.1582-4934.2007.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- 14.Grosshans J, Schnorrer F, Nüsslein-Volhard C. Oligomerisation of Tube and Pelle leads to nuclear localisation of dorsal. Mech Dev. 1999;81:127–138. doi: 10.1016/s0925-4773(98)00236-6. [DOI] [PubMed] [Google Scholar]
- 15.Schauvliege R, Janssens S, Beyaert R. Pellino proteins are more than scaffold proteins in TLR/IL-1R signalling: a role as novel RING E3-ubiquitin-ligases. FEBS Lett. 2006;580:4697–4702. doi: 10.1016/j.febslet.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 16.Butler MP, Hanly JA, Moynagh PN. Kinase-active interleukin-1 receptor-associated kinases promote polyubiquitination and degradation of the Pellino family: direct evidence for PELLINO proteins being ubiquitin-protein isopeptide ligases. J Biol Chem. 2007;282:29729–29737. doi: 10.1074/jbc.M704558200. [DOI] [PubMed] [Google Scholar]
- 17.Ordureau A, et al. The IRAK-catalysed activation of the E3 ligase function of Pellino isoforms induces the Lys63-linked polyubiquitination of IRAK1. Biochem J. 2008;409:43–52. doi: 10.1042/BJ20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cusson-Hermance N, Khurana S, Lee TH, Fitzgerald KA, Kelliher MA. Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-{kappa}B activation but does not contribute to interferon regulatory factor 3 activation. J Biol Chem. 2005;280:36560–36566. doi: 10.1074/jbc.M506831200. [DOI] [PubMed] [Google Scholar]
- 19.Meylan E, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 20.Chen NJ, et al. Beyond tumor necrosis factor receptor: TRADD signaling in toll-like receptors. Proc Natl Acad Sci USA. 2008;105:12429–12434. doi: 10.1073/pnas.0806585105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ermolaeva MA, et al. Function of TRADD in tumor necrosis factor receptor 1 signaling and in TRIF-dependent inflammatory responses. Nat Immunol. 2008;9:1037–1046. doi: 10.1038/ni.1638. [DOI] [PubMed] [Google Scholar]
- 22.Pobezinskaya YL, et al. The function of TRADD in signaling through tumor necrosis factor receptor 1 and TRIF-dependent Toll-like receptors. Nat Immunol. 2008;9:1047–1054. doi: 10.1038/ni.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gohda J, Matsumura T, Inoue J. Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not toll/IL-1 receptor domain-containing adaptor-inducing IFN-beta (TRIF)-dependent pathway in TLR signaling. J Immunol. 2004;173:2913–2917. doi: 10.4049/jimmunol.173.5.2913. [DOI] [PubMed] [Google Scholar]
- 24.Galanos C, F M, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfeffer K, et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 26.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dejager L, Libert C. Tumor necrosis factor alpha mediates the lethal hepatotoxic effects of poly(I:C) in D-galactosamine-sensitized mice. Cytokine. 2008;42:55–61. doi: 10.1016/j.cyto.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Vanguri P, Farber JM. Identification of CRG-2. An interferon-inducible mRNA predicted to encode a murine monokine. J Biol Chem. 1990;265:15049–15057. [PubMed] [Google Scholar]
- 29.Pasare C, Medzhitov R. Control of B cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 30.Ruprecht CR, Lanzavecchia A. TLR stimulation as a third signal required for activation of human naive B cells. Eur J Immunol. 2006;36:810–816. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- 31.Gerondakis S, Grumont RJ, Banerjee A. Regulating B cell activation and survival in response to TLR signals. Immunol Cell Biol. 2007;85:471–475. doi: 10.1038/sj.icb.7100097. [DOI] [PubMed] [Google Scholar]
- 32.Yi AK, Chang M, Peckham DW, Krieg AM, Ashman RF. CpG oligodeoxyribonucleotides rescue mature spleen B cells from spontaneous apoptosis and promote cell cycle entry. J Immunol. 1998;160:5898–5906. [PubMed] [Google Scholar]
- 33.Vivarelli MS, et al. RIP links TLR4 to Akt and is essential for cell survival in response to LPS stimulation. J Exp Med. 2004;200:399–404. doi: 10.1084/jem.20040446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang Z, et al. Pellino 1 is required for interleukin-1 (IL-1)-mediated signaling through its interaction with the IL-1 receptor-associated kinase 4 (IRAK4)-IRAK-tumor necrosis factor receptor-associated factor 6 (TRAF6) complex. J Biol Chem. 2003;278:10952–10956. doi: 10.1074/jbc.M212112200. [DOI] [PubMed] [Google Scholar]
- 35.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 36.Sato S, et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol. 2003;171:4304–4310. doi: 10.4049/jimmunol.171.8.4304. [DOI] [PubMed] [Google Scholar]
- 37.Kayagaki N, et al. DUBA: a deubiquitinase that regulates type I interferon production. Science. 2007;318:1628–1632. doi: 10.1126/science.1145918. [DOI] [PubMed] [Google Scholar]
- 38.Kato H, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 39.Sun Q, et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Z, et al. Poly(I-C)-induced Toll-like receptor 3 (TLR3)-mediated activation of NFkappa B and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR. J Biol Chem. 2003;278:16713–16719. doi: 10.1074/jbc.M300562200. [DOI] [PubMed] [Google Scholar]
- 41.Kawagoe T, et al. Essential role of IRAK-4 protein and its kinase activity in Toll-like receptor-mediated immune responses but not in TCR signaling. J Exp Med. 2007;204:1013–1024. doi: 10.1084/jem.20061523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawagoe T, et al. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol. 2008;9:684–691. doi: 10.1038/ni.1606. [DOI] [PubMed] [Google Scholar]
- 43.Kaiser WJ, Offermann MK. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol. 2005;174:4942–4952. doi: 10.4049/jimmunol.174.8.4942. [DOI] [PubMed] [Google Scholar]
- 44.Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 45.Reiley W, Zhang M, Sun SC. Tumor suppressor negatively regulates JNK signaling pathway downstream of TNFR members. J Biol Chem. 2004;279:55161–55167. doi: 10.1074/jbc.M411049200. [DOI] [PubMed] [Google Scholar]
- 46.Racoosin EL, Swanson JA. Macrophage colony-stimulating factor (rM-CSF) stimulates pinocytosis in bone marrow-derived macrophages. J Exp Med. 1989;170:1635–1648. doi: 10.1084/jem.170.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang M, et al. Regulation of IkappaB kinase-related kinases and antiviral responses by tumor suppressor CYLD. J Biol Chem. 2008;283:18621–18626. doi: 10.1074/jbc.M801451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reiley WW, et al. Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat Immunol. 2006;7:411–417. doi: 10.1038/ni1315. [DOI] [PubMed] [Google Scholar]
- 49.Rivera-Walsh I, Cvijic ME, Xiao G, Sun SC. The NF-kappa B signaling pathway is not required for Fas ligand gene induction but mediates protection from activation-induced cell death. J Biol Chem. 2000;275:25222–25230. doi: 10.1074/jbc.M000444200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.