1. INTRODUCTION
The RPE performs highly specialized, unique functions essential for homeostasis of the neural retina. These include phagocytosis of photoreceptors shed outer segments, directional transport of nutrients into and removal of waste products from photoreceptor cells and visual pigment transport and regeneration. All of these functions involve the RPE apical microvilli.1–4
The RPE is a low cuboidal epithelium containing very long sheet-like apical microvilli that project into the interphotoreceptor matrix. The microvilli interact with the tips of the rod and cone photoreceptor outer segments extending from the outer retinal surface. The cone-RPE association is much less studied however, as many as 30–40 microvilli can be associated with a single cone. These vary in length with only a few reaching the outer segment. The RPE apical microvilli ensheath the outer segments of photoreceptor cells, extending for as long as half the outer segment.5 A single RPE microvillous may completely surround the outer segment or multiple microvilli can encircle each other while surrounding the photoreceptor outer segments. Intracellular organelles are mostly absent from the cone-ensheathing microvilli while they are very abundant in the microvilli ensheathing the rod outer segments.
The RPE basal surface is highly infolded and interacts with the underlying Bruch’s membrane,1 an acellular layer separating the RPE from the choriocapillaris. The polarized organization of the RPE is essential for the vectorial transport of different molecules between the choriocapillaris and the neural retina and vice-versa. A unique characteristic of the RPE is the “reversed polarity” of select proteins such as the Na,K-ATPase pump, EMMPRIN and the adhesion molecule N-CAM. These proteins are found at the apical surface of the RPE, rather than at the basolateral surface as in other epithelia.6–8
2. RPE MICROVILLI STRUCTURE
The RPE microvillar structure has not been extensively studied. However, available information indicates that RPE microvilli possess an internal core bundle of densely packed actin filaments.9 Myosin VIIa has been detected at the base of apical processes10 while villin, fimbrin and myosin I have not been detected.11,12 The entire length of the RPE microvilli has been shown to contain ezrin and EBP50.11,13–15 The mouse RPE microvilli-enriched fraction, described below, contained several cytoskeletal components, among them various types of actin and tubulin, β-spectrin, ezrin, moesin, EBP50, and profilin.
A more complete definition of the protein composition of the RPE apical microvilli should provide insights into other biochemical processes occurring at this critical interface that are important for the support and maintenance of vision.
3. RPE MICROVILLI PROTEINS AND FUNCTION
Recently, we have improved a method to isolate RPE apical microvilli. The procedure relies on the binding of N-acetylglucosamine and sialic acid-containing glycoconjugates present in abundance on the RPE apical surface16 to the WGA lectin conjugated to agarose beads. Mass interactions of the surface glycoconjugates with the immobilized lectin on the bead allow for the detachment of the RPE microvilli upon physical removal of the WGA beads. The RPE isolated microvilli are resolved by SDS-PAGE, in gel digested with trypsin, and peptides extracted and analyzed by mass spectrometry.3,17 This procedure was done in mice eyecups with the RPE exposed and it has resulted in the identification of over 283 proteins, distributed over functional categories such as retinoid-metabolizing, cytoskeletal, enzymes, extracellular matrix components, membrane proteins and transporters, among others. A summary of selected proteins identified by this method is presented in Table 72.1 and has been recently described.3,17
Table 72.1.
Selected proteins identified on WGA-beads after incubation with apical RPE.
| Proteins | Accession Numbera |
Peptide Matches |
Frequencyb |
|---|---|---|---|
| Annexin A2 | P07356 | 2 | 1 |
| Annexin A5 | P48036 | 3 | 1 |
| Basigin | P18572 | 6 | 3 |
| Carbonic anhydrase XIV | Q9WVT6 | 2 | 1 |
| Chloride intracellular channel 6 | Q96NY7 | 2 | 3 |
| Cellular retinaldehyde-binding protein (CRALBP) | Q9Z275 | 6 | 3 |
| ERM-binding phosphoprotein (EBP50) | Q9JJ19 | 1 | 1 |
| Ezrin | P26040 | 4 | 2 |
| Fibromodulin | P50608 | 4 | 2 |
| Glucose transporter type 1 (Glut-1) | P17809 | 3 | 3 |
| Interphotoreceptor retinoid-binding protein (IRBP) | P49194 | 5 | 3 |
| L-lactate dehydrogenase A chain (LDH) | P06151 | 3 | 1 |
| Lumican | P51885 | 9 | 3 |
| Malate dehydrogenase | P14152 | 2 | 2 |
| Membrane-associated adenylate kinase | Q9R0Y4 | 2 | 1 |
| Monocarboxylate transporter 1 | AAC13720 | 3 | 3 |
| Neuroglycan C | Q9QY32 | 1 | 1 |
| Retinol dehydrogenase, 11-cis (RDH5) | Q27979 | 3 | 3 |
| Sodium/potassium-transporting ATPase alpha-1 | P06685 | 6 | 3 |
| Undulin 1 | A40970 | 7 | 2 |
| Vitronectin receptor a subunit (integrin αv) | P43406 | 3 | 2 |
Swiss Protein database and NCBI (in italics) accession numbers are shown.
Results from three independent experiments.
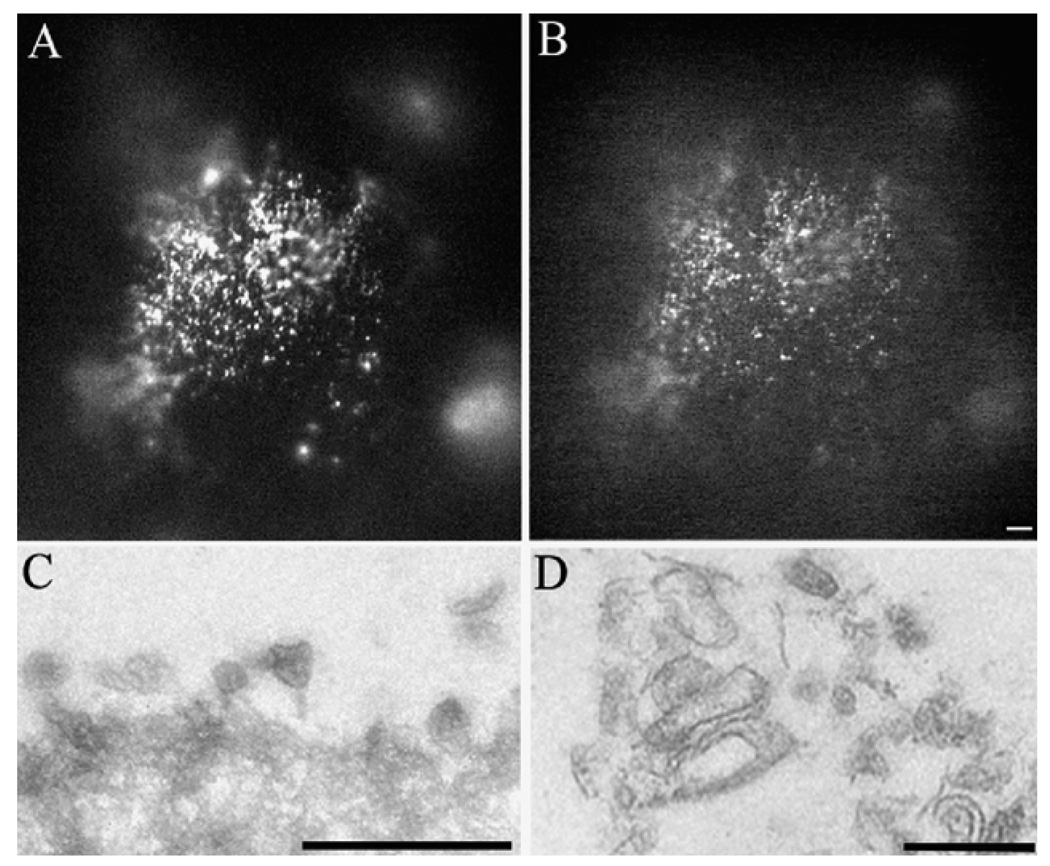
The beads with the isolated RPE microvilli on their surface can be used for immunolabeling experiments, morphological (light and electron microscopy) as well as in biochemical experiments. In Figure 72.1 beads with isolated mouse RPE were fixed in 4% paraformaldehyde, permeabilized in triton X100, reacted with both a rabbit antibody to (A) protein kinase A regulatory subunit II (PKARII) and (B) a mouse antibody to protein kinase A regulatory subunit I (PKARI). Parallel samples were processed for transmission electron microscopy and RPE microvilli are observed on the surface of the agarose beads (C and D).
Figure 72.1. Morphological analysis of isolated WGA-beads with mouse RPE microvilli on their surface.
WGA beads scraped off the mouse eyecups were reacted with antibodies to protein kinase A regulatory subunit alpha II (PKARII) (A) and protein kinase A regulatory subunit I (PKARI) (B). Alternatively, the isolated beads were fixed in 2.5% glutaraldehyde, and processed for transmission electron microscopy (TEM). Low (C) and high (D) magnification of these beads revealed extensive surface areas covered by the RPE microvilli. Bars = 100µm (A, B), 1µm (C) and 0.5µm (D).
Examples of proteins identified in the RPE microvilli by both mass spectrometry and other methods include Na,K-ATPase, Glut-1, monocarboxylate transporter, carbonic anhydrase, basigin, and the chloride intracellular channel 6.17 The cone and rod-associated matrix, present on top of the RPE apical surface, are firmly attached to the RPE apical surface. This is one of the reasons for the mass spectrometric identification of several novel extracellular matrix components such as fibromodulin, lumican, undulin 1, and neuroglycan C in the RPE isolated microvilli. The proteomic method therefore provides an unbiased account of proteins present in the RPE apical microvilli.
RPE apical microvilli play important roles in retinal attachment. Ensheathment of the outer segment tips by apical projections may contribute to adhesion by providing frictional or electrostatic interactions.18 Any disruption of the relationship between cone and rod photoreceptors and the RPE will result in pathological consequences. A retinal detachment, for example, is a separation of the photoreceptor outer segments from its apical RPE microvilli. After clinical reattachment, return of normal vision depends, upon the restoration of a functional relationship between proteins present in the RPE apical surface and the photoreceptors outer segments.
Alterations in the proteins present in the RPE apical microvilli will likely impair vision as a consequence of disrupting the structural and functional nurturing of the photoreceptors by the RPE. Some of the RPE apical proteins identified in the RPE microvilli fraction have already been shown to be involved in retinal degenerations. The list of RPE apical proteins involved in retinal diseases is likely to grow as we learn more about the RPE proteome.
An interaction between cellular retinaldehyde-binding protein (CRALBP) and ERM-binding phosphoprotein 50 (EBP50) in RPE microsomes was recently described.15 Our proteomic analyses was highly enriched in several retinoid processing proteins such as cellular retinaldehyde-binding protein, 11-cis-retinol dehydrogenase, cellular retinol-binding protein 1, interphotoreceptor retinoid-binding protein, EBP50, and ezrin. These results support the existence of a visual cycle protein complex in the RPE apical microvilli.3 Several forms of retinitis pigmentosa are known to be caused by mutations in visual-cycle protein genes such as RPE65, CRALBP, IRBP.19,20
Macular edema resulting from pathologies such as uveitis, postoperative period following cataract extraction,21 retinitis pigmentosa,22 serpiginous choroiditis23 and epiretinal membranes,24 has been widely treated with carbonic anhydrase inhibitors.25 Polarized distribution of carbonic anhydrase activity in the RPE apical surface has been reported. Carbonic anhydrase XIV was one of the proteins we identified by proteomic analysis in isolated RPE microvilli.17
Aging studies have shown a decrease in both the number and the length of epithelial microvilli, and a declined function of plasma membrane enzymes and receptors.26–29 Specifically, a decrease in the activity of some of the enzymes detected in RPE microvilli like Na,K-ATPase, LDH, glutathione S-transferase, phosphoglycerate kinase, adenylate kinase30 and catalase has been established in various epithelia.31–33 Future studies involving these and other proteins may help to improve our understanding of aging diseases such as macular degeneration.
Most recently we have pursued proteomic analysis of rat RPE microvilli. One of the proteins consistently found in rat RPE microvilli is ceruloplasmin. The localization of ceruloplasmin in the RPE has been previously described.34,35 Retinal degeneration has been reported in patients with the autosomal recessive disease called aceruloplasminemia, a deficiency in ceruloplasmin.36
4. CONCLUSIONS
Progress is being made in characterization of the RPE and its apical microvilli. The years to come will bring further definition of key proteins and pathways present in RPE microvilli as well as a better understanding of their function in vision.
ACKNOWLEDGMENTS
Supported by NIH grants EY06603, EY14239, EY014240 and an infrastructure grant EY015638, a Research Center grant from the Foundation Fighting Blindness, a grant from the National Glaucoma Research Program of American Health Assistance Foundation (G2004-047 to SKB), and funds from the Cleveland Clinic Foundation.
REFERENCES
- 1.Zinn KM, Benjamin-Henkind JV. Anatomy of the human retinal pigment epithelium. In: Zinn KM, Marmor MF, editors. The Retinal Pigment Epithelium. Cambridge, MA: Harvard University Press; 1979. pp. 3–31. [Google Scholar]
- 2.Bok D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci Suppl. 1993;17:189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- 3.Bonilha VL, Bhattacharya SK, West KA, Crabb JS, Sun J, Rayborn ME, Nawrot M, Saari JC, Crabb JW. Support for a proposed retinoid-processing protein complex in apical retinal pigment epithelium. Exp Eye Res. 2004;79:419–422. doi: 10.1016/j.exer.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Lamb TD, Pugh EN., Jr. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg RH, Wood I. The Relationship of the Retinal Pigment Epithelium to Photoreceptor Outer Segments in Human Retina. In: Zinn KM, Marmor MF, editors. The Retinal Pigment Epithelium. Cambridge, MA: Harvard University Press; 1979. pp. 32–44. [Google Scholar]
- 6.Gundersen D, Orlowski J, Rodriguez-Boulan E. Apical polarity of Na,K-ATPase in retinal pigment epithelium is linked to a reversal of the ankyrin-fodrin submembrane cytoskeleton. J Cell Biol. 1991;112:863–872. doi: 10.1083/jcb.112.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gundersen D, Powell SK, Rodriguez-Boulan E. Apical polarization of N-CAM in retinal pigment epithelium is dependent on contact with the neural retina. J Cell Biol. 1993;121:335–343. doi: 10.1083/jcb.121.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marmorstein AD, Finnemann SC, Bonilha VL, Rodriguez-Boulan E. Morphogenesis of the retinal pigment epithelium: toward understanding retinal degenerative diseases. Ann N Y Acad Sci. 1998;857:1–12. doi: 10.1111/j.1749-6632.1998.tb10102.x. [DOI] [PubMed] [Google Scholar]
- 9.Vaughan DK, Fisher SK. The distribution of F-actin in cells isolated from vertebrate retinas. Exp Eye Res. 1987;44:393–406. doi: 10.1016/s0014-4835(87)80173-2. [DOI] [PubMed] [Google Scholar]
- 10.Hasson T, Heintzelman MB, Santos-Sacchi J, Corey DP, Mooseker MS. Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proc Natl Acad Sci U S A. 1995;92:9815–9819. doi: 10.1073/pnas.92.21.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofer D, Drenckhahn D. Molecular heterogeneity of the actin filament cytoskeleton associated with microvilli of photoreceptors, Muller’s glial cells and pigment epithelial cells of the retina. Histochemistry. 1993;99:29–35. doi: 10.1007/BF00268017. [DOI] [PubMed] [Google Scholar]
- 12.Owaribe K, Eguchi G. Increase in actin contents and elongation of apical projections in retinal pigmented epithelial cells during development of the chicken eye. J Cell Biol. 1985;101:590–596. doi: 10.1083/jcb.101.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonilha VL, Finnemann SC, Rodriguez-Boulan E. Ezrin promotes morphogenesis of apical microvilli and basal infoldings in retinal pigment epithelium. J Cell Biol. 1999;147:1533–1548. doi: 10.1083/jcb.147.7.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonilha VL, Rodriguez-Boulan E. Polarity and developmental regulation of two PDZ proteins in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2001;42:3274–3282. [PubMed] [Google Scholar]
- 15.Nawrot M, West K, Huang J, Possin DE, Bretscher A, Crabb JW, Saari JC. Cellular retinaldehyde-binding protein interacts with ERM-binding phosphoprotein 50 in retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2004;45:393–401. doi: 10.1167/iovs.03-0989. [DOI] [PubMed] [Google Scholar]
- 16.Cooper NG, Tarnowski BI, McLaughlin BJ. Lectin-affinity isolation of microvillous membranes from the pigmented epithelium of rat retina. Curr Eye Res. 1987;6:969–979. doi: 10.3109/02713688709034868. [DOI] [PubMed] [Google Scholar]
- 17.Bonilha VL, Bhattacharya SK, West KA, Sun J, Crabb JW, Rayborn ME, Hollyfield JG. Proteomic characterization of isolated retinal pigment epithelium microvilli. Mol Cell Proteomics. 2004;3:1119–1127. doi: 10.1074/mcp.M400106-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Marmor MF. Mechanisms of Retinal Adhesion. In: Osborne N, Chader G, editors. Progress in Retinal Research. New York, NY: Pergamon Press; 1993. pp. 179–204. [Google Scholar]
- 19.Maw MA, Kennedy B, Knight A, Bridges R, Roth KE, Mani EJ, Mukkadan JK, Nancarrow D, Crabb JW, Denton MJ. Mutation of the gene encoding cellular retinaldehyde-binding protein in autosomal recessive retinitis pigmentosa. Nat Genet. 1997;17:198–200. doi: 10.1038/ng1097-198. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Chen Q, Zhao K, Wang L, Traboulsi EI. Update on the molecular genetics of retinitis pigmentosa. Ophthalmic Genet. 2001;22:133–154. doi: 10.1076/opge.22.3.133.2224. [DOI] [PubMed] [Google Scholar]
- 21.Farber MD, Lam S, Tessler HH, Jennings TJ, Cross A, Rusin MM. Reduction of macular oedema by acetazolamide in patients with chronic iridocyclitis: a randomised prospective crossover study. Br J Ophthalmol. 1994;78:4–7. doi: 10.1136/bjo.78.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fishman GA, Gilbert LD, Fiscella RG, Kimura AE, Jampol LM. Acetazolamide for treatment of chronic macular edema in retinitis pigmentosa. Arch Ophthalmol. 1989;107:1445–1452. doi: 10.1001/archopht.1989.01070020519031. [DOI] [PubMed] [Google Scholar]
- 23.Chen JC, Fitzke FW, Bird AC. Long-term effect of acetazolamide in a patient with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1990;31:1914–1918. [PubMed] [Google Scholar]
- 24.Marmor MF. Hypothesis concerning carbonic anhydrase treatment of cystoid macular edema: example with epiretinal membrane. Arch Ophthalmol. 1990;108:1524–1525. doi: 10.1001/archopht.1990.01070130026013. [DOI] [PubMed] [Google Scholar]
- 25.Wolfensberger TJ. The role of carbonic anhydrase inhibitors in the management of macular edema. Doc Ophthalmol. 1999;97:387–397. doi: 10.1023/a:1002143802926. [DOI] [PubMed] [Google Scholar]
- 26.Weisse I. Changes in the aging rat retina. Ophthalmic Res. 1995;27:154–163. doi: 10.1159/000267862. [DOI] [PubMed] [Google Scholar]
- 27.Hirai T, Kojima S, Shimada A, Umemura T, Sakai M, Itakura C. Age-related changes in the olfactory system of dogs. Neuropathol Appl Neurobiol. 1996;22:531–539. doi: 10.1111/j.1365-2990.1996.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 28.Jang I, Jung K, Cho J. Influence of age on duodenal brush border membrane and specific activities of brush border membrane enzymes in Wistar rats. Exp Anim. 2000;49:281–287. doi: 10.1538/expanim.49.281. [DOI] [PubMed] [Google Scholar]
- 29.Serot JM, Bene MC, Faure GC. Choroid plexus, aging of the brain, and Alzheimer’s disease. Front Biosci. 2003;8:s515–s521. doi: 10.2741/1085. [DOI] [PubMed] [Google Scholar]
- 30.Kadlubowsk M, Agutter PS. Changes in the activities of some membrane-associated enzymes during in vivo ageing of the normal human erythrocyte. Br J Haematol. 1977;37:111–125. [PubMed] [Google Scholar]
- 31.Napoleone P, Bronzett E, Amenta F. Enzyme histochemistry of aging rat kidney. Mech Ageing Dev. 1991;61:187–195. doi: 10.1016/0047-6374(91)90016-s. [DOI] [PubMed] [Google Scholar]
- 32.Teillet L, Preisser L, Verbavatz JM, Corman B. Kidney aging: cellular mechanisms of problems of hydration equilibrium. Therapie. 1999;4:147–154. [PubMed] [Google Scholar]
- 33.Schmucker DL, Thoreux K, Owen RL. Aging impairs intestinal immunity. Mech Ageing Dev. 2001;122:1397–1411. doi: 10.1016/s0047-6374(01)00276-7. [DOI] [PubMed] [Google Scholar]
- 34.Hahn P, Dentchev T, Qian Y, Rouault T, Harris ZL, Dunaief JL JL. Immunolocalization and regulation of iron handling proteins ferritin and ferroportin in the retina. Mol Vis. 2004;10:598–607. [PubMed] [Google Scholar]
- 35.Hahn P, Qian Y, Dentchev T, Chen L, Beard J J, Harris ZL, Dunaief JL. Disruption of ceruloplasmin and hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration. Proc Natl Acad Sci U S A. 2004;101:13850–13855. doi: 10.1073/pnas.0405146101. Epub 12004 Sep 13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi K, Takahash S, Kawanami T, Kato T, Sasaki H. Retinal degeneration in hereditary ceruloplasmin deficiency. Ophthalmologica. 1998;212:11–14. doi: 10.1159/000027251. [DOI] [PubMed] [Google Scholar]