Abstract
Dysregulation of the stress-related corticotropin-releasing factor (CRF) system has been implicated in the development of drug dependence. The present study examined the effects of administering CRF type 1 (CRF1) receptor antagonists on heroin self-administration in animals allowed short (1 hour) or long (8–12 hours) access to intravenous heroin self-administration sessions. The nonpeptide CRF1 antagonists MJL-1-109-2 (1 hour versus 8 hours access) or R121919 (1 hour versus 12 hours access) were systemically injected in both short- and long-access rats. MJL-1-109-2 (10 mg/kg) and R121919 (10 and 20 mg/kg) reduced heroin self-administration in long-access animals without altering heroin intake in short-access animals. Both MJL-1-109-2 and R121919 decreased first-hour intravenous heroin self-administration selectively in long-access rats, with R121919 decreasing cumulative heroin intake across the 12-hour session. The results demonstrate that blockade of the CRF–CRF1 receptor system attenuates the increased heroin intake of rats with extended access to the drug.
Keywords: Addiction, antagonist, CRF, escalation, heroin, self-administration
INTRODUCTION
To understand the underlying neurobiological mechanisms involved in vulnerability to heroin dependence, animal models relevant to components of heroin dependence are being sought. Such models of dependence involve opioid exposure/withdrawal paradigms (e.g. chronic pellet implantation and multiple morphine injections), reinstatement of heroin-seeking and operant self-administration in limited access sessions (Young et al. 1977; Bozarth & Wise 1985; Shaham et al. 1998; Carrera, Schulteis & Koob 1999; Erb & Stewart 1999; Hutcheson et al. 2001; Azar, Jones & Schulteis 2003). Recently, opioid self-administration models have been modified to incorporate the excessive, compulsive drug intake associated with human dependence. Heroin is self-administered in increasing quantities when animals are allowed extended access to the drug, a finding termed ‘escalation’ (Ahmed, Walker & Koob 2000). Access to heroin consumption for 11 hours per day not only increased heroin intake, but also persistently increased the motivation to administer heroin (Ahmed et al. 2000). Rats given 23-hour access to heroin, without an experimenter-initiated increase in the unit dose, also showed a dramatic, spontaneous increase in intravenous heroin self-administration (Chen et al. 2006). Short-access rats, in contrast, limit consumption to lower, stable levels, show lower extinction responding and are less prone to footshock-induced reinstatement than long-access rats (Ahmed et al. 2000). Thus, the short- versus long-access models of intake include controls which, while having experience with opioid self-administration, differ in heroin intake, extinction and reinstatement, helping to differentiate the effects of controlled opioid use versus excessive use. Models of extended access to heroin self-administration have predictive and face validity for modeling the compulsive drug intake associated with heroin dependence in humans (Ahmed & Koob 1998; Ahmed et al. 2000; Koob et al. 2004; Chen et al. 2006; Kenny et al. 2006).
Additionally, rat models of extended access to heroin have been particularly useful for comparing the behavioral profiles associated with different stages in the development of heroin dependence (Chen et al. 2006). Previous research has shown that changes in the amount and pattern of food intake in an animal model may be valuable as a sensitive indicator of the effects of extended access to heroin (Thornhill, Hirst & Gowdey 1976). Meal pattern analysis revealed that smaller and briefer, but more, meals of food were taken as early as 7 days after daily extended (23 hours) access to heroin (Chen et al. 2006).
The challenge now is to identify the neuroadaptive mechanisms that mediate the changes in the motivation for heroin that occur during the transition to dependence. Neurochemical systems that have been implicated include γ-aminobutyric acid, dopamine, norepinephrine, neuropeptide Y, and corticotropin-releasing factor (CRF). Each of these transmitter systems, as well as others (Koob 1992; Koob & Le Moal 2006), appear to mediate some aspects of drug dependence. However, recent findings emphasize the importance of CRF systems in the motivational, affective and somatic signs of drug dependence (Heinrichs et al. 1995; Shaham et al. 1997, 1998; Erb & Stewart 1999; Iredale et al. 2000; Lu et al. 2000; Contarino & Papaleo 2005; Stinus et al. 2005). To address the role of CRF in mediating the neuroadaptations that lead to increased opioid self-administration after extended access to heroin, the present study tested the hypothesis that two CRF type 1 (CRF1) antagonists, MJL-1-109-2 and R121919, would selectively decrease drug self-administration in extended-access rats compared with short-access control rats.
MATERIALS AND METHODS
Animals
Adult male Wistar rats (Charles River, Raleigh, NC, USA) weighing between 200–250 g at the beginning of the experiments were housed in groups of three in a humidity- and temperature-controlled (22°C) vivarium on a 12-hour light/dark cycle with ad libitum access to food and water. The animals were allowed to acclimate to these conditions for at least 7 days. All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Surgery
Rats were anesthetized with an isoflurane/oxygen vapor mixture (2.0–2.5%) and prepared with chronic intravenous catheters (Caine, Lintz & Koob 1993). Catheters consisted of a 14-cm length of silastic tubing fitted to a guide cannula (Plastics One, Roanoke, VA, USA) bent at a right angle. The skull was exposed and cleaned, and four skull screws were implanted, one in each quadrant. The bent guide cannula was secured rostral-caudally to the center of the skull using cranioplastic cement. The catheter tubing was passed subcutaneously from the animal’s skull to the right jugular vein, which was punctured with an 18-gauge needle. Then, 3.7 cm of the silastic tubing was inserted into the vein and tied gently with suture thread. Surgery was conducted under sterile conditions, and all connections involving the catheter were kept as sterile as possible. All animals were allowed to recover for a minimum of 1 week before being given access to heroin self-administration. Catheters were flushed daily with 0.2 ml sterile physiological saline containing heparin (30 USP units/ml) and the antibiotic Timentin (20 mg) (SmithKline Beecham Pharmaceuticals, Philadelphia, PA, USA).
Catheter patency
Catheter integrity was tested whenever an animal not receiving drug pre-treatments displayed behavior outside baseline parameters. In these cases, 0.1 ml of the ultra short-acting barbiturate anesthetic Brevital Sodium (1% methohexital sodium, Eli Lilly, Indianapolis, IN, USA) was administered through the catheter. Animals were assumed to have patent catheters if they exhibited prominent signs of anesthesia (pronounced loss of muscle tone) within 3 seconds of intravenous injection, from which they recovered normal activity within 5 minutes. Animals that lost catheter patency during the course of the experiment were excluded from the experiment and subsequent data analysis.
Self-administration chambers
For each session, the animals were placed into operant cages located inside ventilated, sound-attenuating chambers equipped with a 1.1 W miniature light bulb synchronized to the 6 a.m. (lights on)/6 p.m. (lights off) light/dark cycle. The catheter fittings on the animals’ skulls were connected to polyethylene tubing contained inside a protective metal spring (tether) that was suspended into the operant chamber from a liquid swivel attached to a balance arm. Drug in a syringe was delivered by a syringe pump (Razel Scientific, Stamford, CT; Caine et al. 1993). Modifications from Caine et al. (1993) included using a two-rotation-per-minute syringe pump motor to push a 30-ml syringe for 4.5 seconds to deliver a 0.1 ml infusion; the large 30 ml syringe size was used to ensure that enough drug was available for the full self-administration session without having to replace syringes. Each operant session was performed using one active and one inactive retractable lever that extended approximately 1 inch into the chamber. Following completion of each fixed-ratio 1 (FR1), a 28 V white stimulus light located above the active lever signaled the delivery of a drug and remained on for a 20-second timeout (TO) period, during which responses were recorded but had no scheduled consequences. Once the appropriate time elapsed (1 hour, 8 hours or 12 hours) for drug access, both inactive and active levers retracted.
Drugs
The CRF1 receptor antagonists MJL-1-109-2 (pyrazolo[1, 5-a]-1,3,5-triazin-4-amine,8-[4-(bromo)-2-chlorophenyl]-N,N-bis[2-methoxyethyl]-2,7-dimethyl-[9Cl]; CRF1 Ki = 1.9 nM, ClogP = 3) and the hydrochloride salt of R121919 (3-[6-(dimethylamino)-4-methyl-pyrid-3-yl]-2,5-dimethyl-N,N-dipropyl-pyrazolo[2,3-a]pyrimidin-7-amine, also referred to as NBI-30775; CRF1 Ki = 3.5 nM; CLogP = 4.8) were synthesized as described previously (Jagoda et al. 2003; Chen et al. 2004) by Drs. Kenner Rice and Mei-Jing Lee (National Institute on Drug Abuse, Chemical Biology and Research Branch, Bethesda, MD, USA). The drugs were administered either subcutaneously (R121919 at 2 ml/kg; 0, 5, 10 or 20 mg/kg) or intraperitoneally (MJL-1-109-2 at 4 ml/kg; 0, 1.25, 5 or 10 mg/kg) in 20% w/v hydroxypropyl-β-cyclodextrin (pH 4.5) (Cargill, Cedar Rapids, IA, USA). MJL-1-109-2 and R121919 cross the blood–brain barrier, and CRF1 receptor occupancy data for MJL-1-109-2 (Jagoda et al. 2003) and R121919 (Zobel et al. 2000; Heinrichs et al. 2002) have been reported previously. In animal studies, doses of 2.5–20 mg/kg R121919 have been shown to inhibit stress-induced plasma adrenocorticotropic hormone levels (Chen et al. 2004) and anxiogenic-like behaviors under ‘high-stress’ conditions (Zobel et al. 2000; Keck et al. 2001; Heinrichs et al. 2002; Gutman et al. 2003). Different routes of administration were used because MJL-1-109-2 is less soluble than R121919, and the routes of administration for these drugs were shown previously to be effective (Funk et al. 2007).
Heroin (3,6-diacetylmorphine; International Union of Pure and Applied Chemistry: [5α,6α]-7,8-didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol diacetate ester) was generously provided by the National Institute on Drug Abuse and was diluted in 0.9% sterile saline.
Intravenous heroin self-administration
After 7 days of recovery from surgery, animals were allowed to lever press for intravenous heroin (0.06 mg/kg/0.1 ml infusion/4.5 seconds, FR1 TO 20 seconds, 1 hour/day) for 8 days. The present design used a single unit dose of heroin, similar to previous heroin self-administration studies; the dose is relatively moderate and sustains increases in drug intake when extended access to heroin is provided (Walker et al. 2000; Chen et al. 2006; Kenny et al. 2006). No food restriction was used to establish operant responding. Responding stabilized during the last three consecutive days of the eight baseline days as defined by non-significant differences in the 3 days assessed using repeated-measures analysis of variance (ANOVA). Rats were assigned to receive either daily short (1 hour) or long (8 hours) access to heroin self-administration sessions. Lab chow pellets (40 g; Harlan Teklad LM-485 Diet 7012, Harlan, Indianapolis, IN, USA) and a bottle of water were available at all times in the test chambers. In a representative subset of rats, food was weighed prior to and after test sessions, and the difference was recorded and normalized for body weight. For Experiment 1 (MJL-1-109-2), long- and short-access sessions occurred during the dark cycle.
Experiment 2 (R121919) used methods that were similar to Experiment 1, with the exception that rats were trained for 5 days to nosepoke for food (45 mg pellets, Cat# 5TUM, TestDiet, Richmond, IN, USA) and water (100 μl per nosepoke) prior to the acquisition of heroin responding. After acquisition, rats were separated into short (1 hour) or long (12 hours) access groups, matched for similar heroin responding during the last three baseline sessions. During extended access to heroin (12 hours), lever presses for drug infusion (heroin injections) and nosepokes for food and water were recorded with 10 ms resolution as described previously (Chen et al. 2006; O’Dell et al. 2007).
Experiment 1: MJL-1-109-2 testing
Testing began on Day 40 in one cohort of rats and on Day 21 in a second cohort of rats, after the rats had significantly increased their intake of heroin and vehicle injection did not alter heroin responding, confirmed by repeated-measures ANOVA comparing average baseline responding with vehicle-treated responding. These differences in time of testing in the two groups of animals reflected the time necessary to stabilize responding, defined by no significant difference being found in the repeated-measures ANOVA on mean heroin injections over a 3-day period. MJL-1-109-2 was injected intraperitoneally (4 ml/kg) with one intervening treatment-free day between test days in which heroin responding remained relatively stable compared with levels before testing. Doses of MJL-1-109-2 (vehicle, 1.25, 5 or 10 mg/kg) were given 30–60 minutes prior to the start of test sessions in a Latin square design. All rats received standard lab chow pellets (Harlan Teklad) during self-administration sessions, and the amount of food intake was recorded in a representative subset of rats (n = 7–8 rats/group) by weighing pellets before and after self-administration sessions. Food intake was corrected for differences in body weight per Kleiber’s law [(gram food)/(body weight in kg)0.75] (Sidhu 1992). The attrition rate for the long access rats was 33% (3 out of 10) and 12.5% for the short access rats (1 out of 8). These animals were excluded from the study because of the loss of catheter patency.
Experiment 2: R121919 testing
Testing the effects of R121919 on heroin responding in long (12 hours) and short (1 hour) access rats (n = 7/group) was performed as described for MJL-1-109-2 in Experiment 1, with the following modifications. Rats had received acute intraperitoneal administration of the α1 adrenergic receptor antagonist prazosin 1 month earlier as part of a separate study to be reported elsewhere. Three animals that eventually were used in the R121919 experiments had a history of 23-hour access until Day 21 but then were moved to 12-hour access. Because no difference was observed in their baselines, the animals were pooled with the other four rats that received 12-hour heroin access to form the 12-hour group that was used in the R121919 experiment (Experiment 2). Following prazosin treatment, all subjects were allowed to self-administer heroin for 12 hours/day for 2 weeks and did not begin testing with R121919 until heroin responding after vehicle injection stabilized, as previously operationalized. Testing with R121919 began after 14 days of being drug-free, with the exception of heroin access on Day 76. Doses of R121919 (0, 5, 10 or 20 mg/kg) were administered subcutaneously (2 ml/kg) 60 minutes prior to the onset of heroin availability. Concurrent operant responding for food and water reinforcers was determined in long-access rats in the 12-hour period in the chamber. This methodology allowed for the time course of changes in responding for heroin versus natural reinforcers to be compared and ensured a work requirement for intake of each reinforcer. Rats had ad libitum access to food and water in their home cage during the 12 hours outside the operant chamber. One rat (#93) was removed from testing because of a leaky catheter on two of the test days in the Latin square design. The attrition rate from the beginning of the experiment was 30% for long-access rats and 33% for short-access rats and resulted from loss of catheter patency.
Meal pattern analysis
Meal pattern analysis (Experiment 2) was performed using a drinking-inclusive meal definition validated previously (Zorrilla et al. 2005). A meal was defined as a burst of responses for food or water that contained at least five food-directed responses, or 0.225 g (Demaria-Pesce & Nicolaidis 1998; Zorrilla et al. 2005). The maximum interval between ingestive responses that was considered to continue the ongoing meal was set at 5 minutes between food or water responses, based on previous observations (Zorrilla et al. 2005). The following parameters for nocturnal meal structure were then calculated: (1) total quantity of prandial intake (food intake); (2) total duration of prandial intake; (3) meal frequency (number of meals); (4) average meal size; and (5) average meal duration. Meal duration was calculated as the total time from the first to the last response of a meal, and duration of eating within the meal was calculated as the duration of consecutive responses for food. Thus, transitions between eating and drinking were included in total meal duration but not in duration of eating. Meal sizes for eating were calculated as the average number of food-directed responses during a meal. In the absence of experimental treatments, rats normally exhibit stability in these measures of meal patterning (calculated as an intraclass correlation of absolute agreement; Shrout & Fleiss 1979; average r = 0.77 across 3 weeks of testing; Zorrilla et al. 2005).
Statistical analysis
For 8-hour and 12-hour access, a two-way ANOVA was performed on the first hour of heroin intake from Day 1 to Day 53 (the first vehicle day) to analyze the effect of extended heroin access on separation between long- and short-access. A one-way ANOVA was performed on total heroin intake during days of heroin access to determine when long-access rats (8 hours and 12 hours) significantly increased heroin intake. Because of high variability in the 8-hour group, the data were log-transformed before the one-way ANOVA was performed for total intake of heroin over 28 days. A t-test was also performed to determine possible differences in heroin intake between the 8-hour and 12-hour long-access groups. Separate mixed two-way ANOVAs (dose × access) were performed to analyze the effect of MJL-1-109-2 and R121919 on the first hour of heroin responding in long- and short-access groups. Note that the error bars in the figures reflect between-subject variability, whereas the statistical test included each animal as its own control.
With dose-response functions, a powerful way of using ANOVA is to perform linear trend analysis (Rosner 1995; Bewick, Cheek & Ball 2004; Bretz, Pinheiro & Branson 2004; Sheskin 2004). With a significant dose × access linear trend contrast, a simple main effect of dose on heroin intake within each group was performed using the error term MSB×subjw·groups [mean squares of the within-subjects dose factor (B) × subjects within-groups], and the F ratio = [(MSbata1)/(MSB×subjw·groups)], where b = a given dose, and a = a given access condition (Winer 1962) with Dunnett’s post hoc test for individual means comparisons. Fischer’s protected least significant differences (LSD) were used for post hoc tests when a significant omnibus F-ratio was obtained in the ANOVA, with the exception of Bonferroni-corrected t-tests (t < 0.0125, or 0.05/4 doses) being used to determine whether effects of drug access on heroin responding during the first hour persisted following CRF1 antagonist treatment. The possibility of rate-sensitive effects was examined by comparing the effects of R121919 between high-responding/short-access and low-responding/long-access subjects, which exhibited similar response rates. ‘High’ responding and ‘low’ responding were defined by median split analyses of each access group (Walker & Koob 2007).
The effects of MJL-1-109-2 on food intake were assessed using a one-way ANOVA. For R121919 testing, paired sample t-tests were performed to determine the difference in meal pattern measures between Day 0 and Day 73. Separate one-way repeated-measures ANOVAs (dose) were performed on measures of food and water intake in long-access rats to determine whether the CRF1 antagonists had any non-specific effects on meal patterns or the total quantity of food or water responding. When assumption of sphericity was not met, the Greenhouse–Geisser correction was used. Statistical analyses were conducted using SPSS v.12.0 (SPSS Inc., Chicago, IL, USA) and Microsoft Excel 2003 (Redmond, WA, USA). The level of significance was set at P < 0.05.
RESULTS
Extended access to heroin increases heroin intake
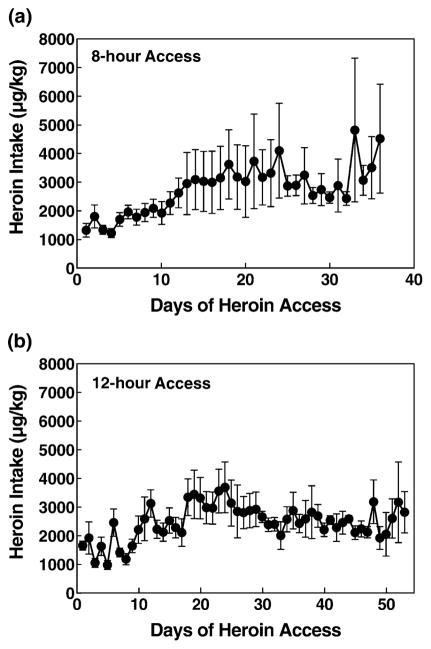
The 8-hour and 12-hour long-access rats doubled their intake to 4 mg/kg from Day 1 to Day 28 (Fig. 1). A two-way repeated-measures ANOVA revealed a linear contrast interaction of time × access [F(1,12) = 13.263, P < 0.01], indicating that the long-access rats (12-hour access group) significantly increased their first-hour intake to a greater degree than the short-access rats (Fig. 2). A one-way ANOVA on long-access (12 hours) total heroin intake from Day 1 to Day 53 revealed a significant increase of heroin responding over days of access [overall ANOVA: F(1,52) = 4.835, P < 0.001; linear contrast: F(1,6) = 24.592, P < 0.01], whereas a one-way ANOVA on 1-hour heroin intake in short-access rats yielded no significant increase in heroin intake over time (P > 0.05). For the 8-hour group of rats, a one-way repeated measures ANOVA on the 8-hour total heroin intake from Day 1 to Day 28 revealed a significant increase in heroin responding over days of access [F(1,27) = 2.762, P < 0.001]. A t-test for total heroin intake on Day 28 revealed no difference in heroin intake between the 8-hour and the 12-hour long-access groups (P > 0.05).
Figure 1.
Increased heroin intake in long-access (a: 8 hours; b: 12 hours) rats. Mean ±SEM heroin intake in μg/kg. (a) 8-hour rats (n = 7) increased their intake from Day 1 to Day 24 (to 4000 μg/kg). (b) 12-hour rats (n = 4) also increased intake to approximately 3800 μg/kg of heroin/day
Figure 2.
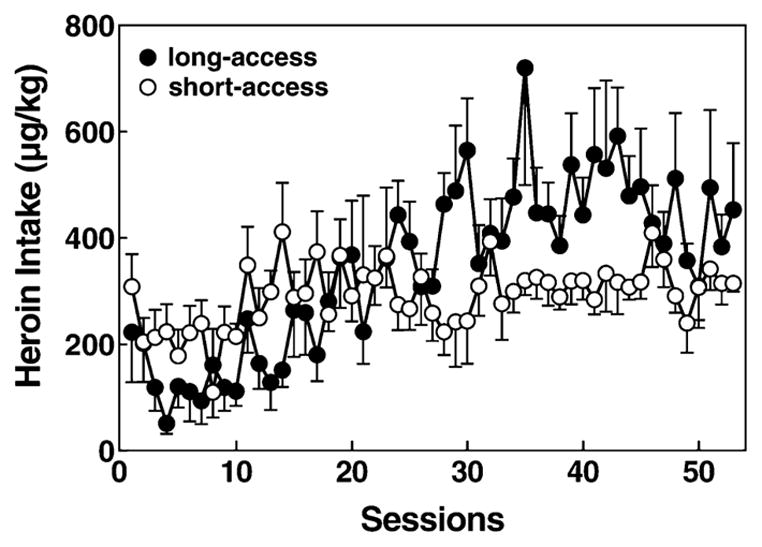
Comparison of 1-hour heroin intake in long-access (12 hours) and short-access (1 hour). Mean ±SEM heroin intake in μg/kg in long-access (n = 7, closed circles) and short-access (n = 7, open circles) rats. Although the short-access rats started slightly higher in heroin responding, by Day 24 long-access rats surpassed short-access rats in heroin intake. Post hoc analysis of a significant time × access interaction indicated that escalated drug intake was only observed in long-access rats. A significant time × access interaction revealed that the long-access rats were different than the short-access rats (P < 0.001)
Effects of MJL-1-109-2 on heroin self-administration
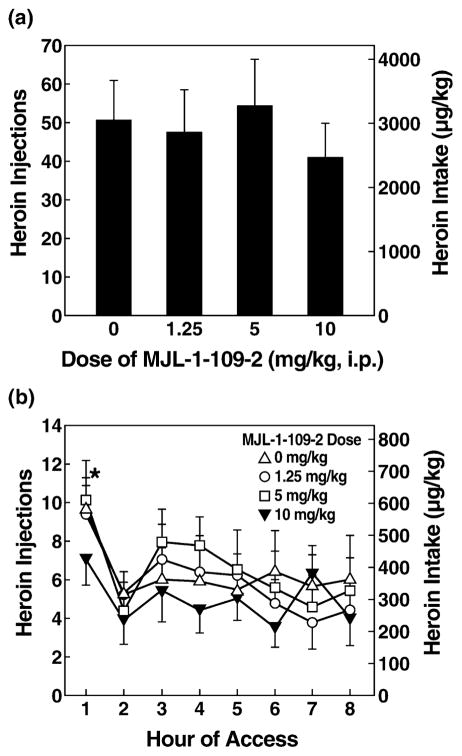
A two-way ANOVA indicated a significant effect of MJL-1-109-2 dose on the first hour of self-administration of long- (8-hour group) and short-access rats [F(3,66) = 5.300, P < 0.01], and a significant effect of access [F(1,22) = 4.804, P < 0.05] but no dose × access interaction. Post hoc tests comparing self-administration of long-and short-access groups at each dose indicated greater heroin responding in long-access (8-hour group) rats (t = 0.002) at the vehicle dose (Fig. 3), showing a behavioral difference between the two groups. One-way ANOVAs showed that MJL-1-109-2 administration significantly reduced 1-hour heroin intake [F(3,30) = 3.033, P < 0.05] and revealed a trend in the linear dose contrast in the long-access (8-hour heroin access) group [F(1,10) = 4.418, P = 0.062] but no significant effects of dose in the short-access (1-hour access) group (Fig. 3). In the long-access (8 hours) group, post hoc LSD tests showed that the highest dose of MJL-1-109-2 (10 mg/kg) significantly reduced heroin self-administration compared with vehicle (P = 0.012) and the 5 mg/kg dose (P = 0.019). MJL-1-109-2 treatment did not alter body weight-normalized food intake in either the short- or long-access (8 hours) groups (Table 1). Mean total heroin intake across 8 hours of observation was not altered significantly by MJL-1-109-2 (Fig. 4, Table 2).
Figure 3.
MJL-1-109-2 decreased first-hour heroin responses in long-access, but not short-access, rats. The CRF1 antagonist MJL-1-109-2 at a dose of 10 mg/kg significantly (*P < 0.05) reduced heroin intake (mean ±SEM) in the first hour of 8-hour (long-access, n = 7, left panel) rats. No effect on heroin intake was observed in short-access rats (n = 8, right panel) at any of the doses tested. Symbols indicate an overall linear effect of dose ($P < 0.05), significant differences from vehicle treatment (*P < 0.05) and significant differences between groups at the vehicle dose (#P = 0.002)
Table 1.
Effects of MJL-1-109-2 on food intake.
| MJL-1-109-2 dose (i.p.) |
||||
|---|---|---|---|---|
| Vehicle | 1.25 mg/kg | 5 mg/kg | 10 mg/kg | |
| Long access total food intake (g/kg) | 33.48 ± 5.11 | 37.00 ± 2.80 | 41.05 ± 4.46 | 35.82 ± 5.97 |
| Short access total food intake (g/kg) | 4.84 ± 0.85 | 4.57 ± 1.9 | 5.70 ± 0.82 | 4.57 ± 0.91 |
Food intake in both 8-hour and 1-hour access groups. Mean ± SEM of food intake measured in g/kg body weight to the 0.75 power using Kleiber’s correction (Sidhu 1992). No significant effect on food intake was observed in either group.
Figure 4.
Total heroin responses in rats after MJL-1-109-2 administration. (a) MJL-1-109-2 had no effect on total responses (8 hours) for heroin (mean ±SEM). (b) Responses (mean ±SEM) by hour in 8-hour access rats. In the first hour, MJL-1-109-2 had a significant effect (*P < 0.05, overall Dose effect compared with vehicle) on heroin intake, but at the other time points, no effect of MJL-1-109-2 on heroin intake was observed
Table 2.
Effects of MJL-1-109-2 on total heroin intake (8 hours).
| MJL-1-109-2 dose (i.p.) |
||||
|---|---|---|---|---|
| Vehicle | 1.25 mg/kg | 5 mg/kg | 10 mg/kg | |
| Long access total heroin intake (μg/kg) | 50.45 ± 10.82 | 47.45 ± 11.29 | 54.45 ± 12.16 | 41.00 ± 9.13 |
Total heroin intake in rats treated with MJL-1-109-2. Mean ± SEM of 8 hours heroin responses in long-access rats (n = 7) treated with MJL-1-109-2. No effect on total heroin responding was observed, similar to the results depicted in Fig. 4.
Effects of R121919 on heroin self-administration
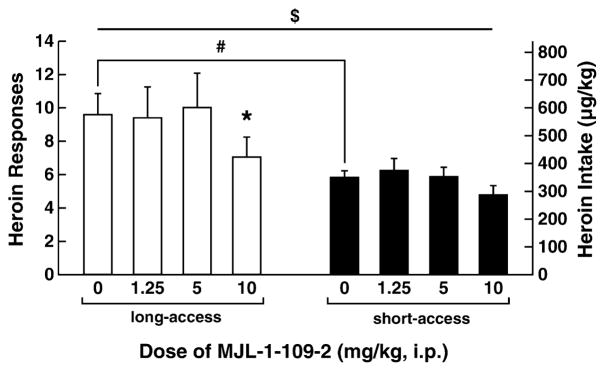
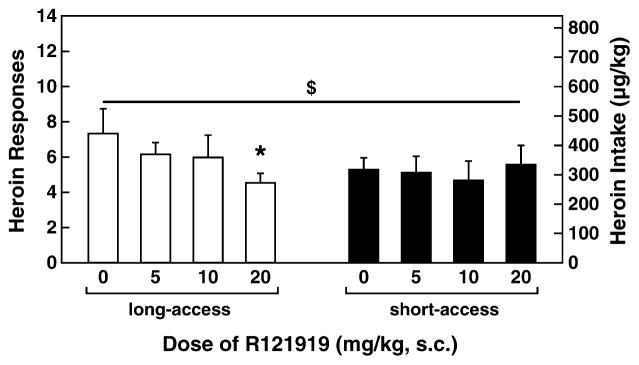
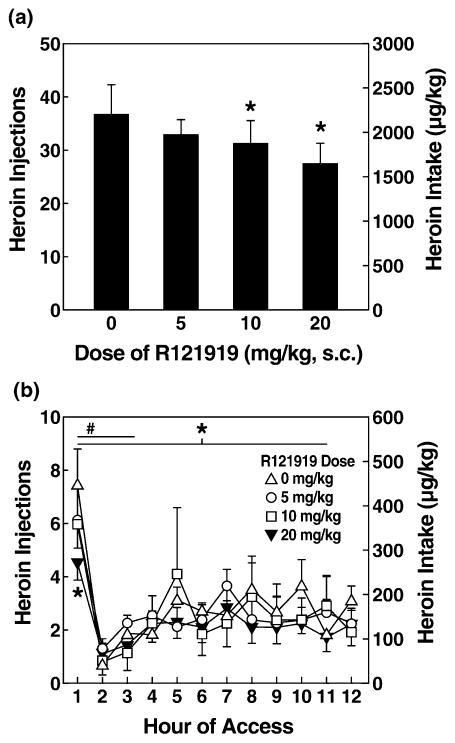
A two-way ANOVA revealed a significant dose × access interaction in 1-hour heroin self-administration in long-(12-hour access) and short-access rats [F(3,36) = 2.781, P < 0.05]. Although no main effect of dose was observed on self-administration during the first hour in long-(12-hour access) and short-access rats, a significant linear dose contrast [F(1,12) = 6.782, P < 0.05] showed a dose-dependent effect of R121919 on heroin intake, a relationship that differed according to access condition [linear contrast effect of dose × access: F(1,12) = 8.258, P < 0.05]. Figure 5 shows the first-hour data for long-(12-hour access) and short-access rats. Similar to the results for MJL-1-109-2, R1291919 pre-treatment significantly reduced first-hour heroin self-administration in long-access (12 hours) rats [overall ANOVA: F(3,18) = 3.153, P < 0.05; linear contrast: F(1,6) = 15.678, P < 0.01] but not in short-access (1-hour access) rats. Pairwise comparisons revealed that the highest dose of R121919 (20 mg/kg) significantly reduced heroin self-administration in long-access (12 hours) rats during the first hour compared with vehicle (P = 0.016) and low doses of antagonist (P = 0.010). R121919 also dose-dependently reduced total heroin self-administration (12-hour access) across the entire 12-hour session in long-access rats [overall ANOVA: F(3,18) = 4.150, P = 0.021; linear contrast: F(1,6) = 20.348, P < 0.01]. Post hoc tests revealed that both the 20 mg/kg (P = 0.017) and 10 mg/kg (P = 0.032) doses significantly reduced heroin responding compared with the vehicle condition (Fig. 6). The 20 mg/kg dose also significantly reduced heroin responding compared with the 5 mg/kg dose (P = 0.025).
Figure 5.
R121919 decreased first-hour responding in long-access rats, but not in short-access rats. Mean ± SEM of heroin responses in 1 hour. (Left) Gray bars show long-access rats (n = 7). (Right) Black bars show short-access rats (n = 7). R121919 (20 mg/kg) significantly decreased heroin responding in long-access rats (*P < 0.05). Symbols indicate an overall linear effect of dose ($P < 0.05) and significant differences from vehicle treatment (*P < 0.05)
Figure 6.
R121919 reduced total heroin responses in long-access rats. (a) Mean ± SEM of heroin self-administration responses during R121919 treatment in long-access rats (n = 7). The 10 and 20 mg/kg doses were effective at reducing heroin self-administration in the long-access rats (P < 0.05). (b) Effect (mean ± SEM) of each dose of R121919 on heroin responding by hour of access. Heroin responses were the highest in the first hour for all doses. Heroin responding at the 20 mg/kg R121919 dose stayed relatively low across time, with some recovery after the third hour for the 10 mg/kg dose. #P < 0.05 for the first 3 hours of intake. *P < 0.05 for total intake and first-hour intake at the 20 mg/kg dose
The three rats in the long-access group that showed the greatest increases in heroin responding from baseline had heroin responses equaling more than 10 responses under vehicle conditions in which the highest dose of R12919 decreased their heroin responding by at least 50% (e.g. from 10 active heroin responses to five active heroin responses). In contrast, only one of four rats in the long-access group with fewer than 10 responses under vehicle conditions showed 50% decreases in heroin responding.
R121919 affected self-administration of the ‘low responding/long-access’ subgroup (n = 4) [overall ANOVA: F(3,9) = 4.167, P < 0.05; linear contrast: F(1,3) = 29.824, P = 0.012; mean responses ± SEM: vehicle, 5.25 ± 0.25; 5 mg/kg, 5.50 ± 0.65; 10 mg/kg, 3.5 ± 0.87; 20 mg/kg, 3.75 ± 0.25] but not that of the ‘high responding/short-access’ subgroup (n = 4) (mean responses ± SEM: vehicle, 6.5 ± 0.96; 5 mg/kg, 6.25 ± 1.49; 10 mg/kg, 5.75 ± 1.80; 20 mg/kg, 6.50 ± 1.66). Because of the similar baseline intakes of these groups, the results argue against a rate-sensitive interpretation of R121919’s differential effects per access condition.
Total food and water responding
R121919 did not reliably alter concurrent 1-, 3- or 12-hour responding for food (Table 3) or water (not shown) in long-access (12 hours) rats, determined by overall ANOVA or linear trend analysis.
Table 3.
R121919 has no effect on food intake in long-access rats.
| R121919 dose (s.c.) |
||||
|---|---|---|---|---|
| Vehicle | 5 mg/kg | 10 mg/kg | 20 mg/kg | |
| First-hour food intake (g/kg) | 3.80 ± 1.27 | 3.19 ± 0.87 | 3.55 ± 0.68 | 3.43 ± 1.31 |
| 3-hour food intake (g/kg) | 9.18 ± 1.25 | 8.55 ± 1.48 | 7.27 ± 0.87 | 7.43 ± 1.23 |
| Total food intake (g/kg) | 32.22 ± 1.65 | 31.55 ± 1.80 | 31.00 ± 2.18 | 31.16 ± 2.61 |
Food responding over 1, 3 and 12 hours in rats treated with R121919. Mean ± SEM of food responses expressed as g/kg after Kleiber’s correction (Sidhu 1992) for long-access rats (n = 7) treated with R121919. No significant effect of R121919 was observed on food intake.
Meal pattern analysis
Similar to previous findings (Chen et al. 2006; Greenwell et al. 2008), meal patterns changed from Day 0 (pre-heroin) through Day 73 of heroin access for the long-access (12 hours) rats. Meals became significantly more frequent (number of meals; P = 0.04) but also briefer (meal duration; P = 0.05) and smaller (meal size; P = 0.05) by Day 73 (Table 4). No change was observed in total prandial food intake (body weight-corrected) (Table 4).
Table 4.
Effects of long-access heroin exposure on meal microstructure.
| Day 0 | Day 73 | |
|---|---|---|
| 12-hour intake | ||
| Total food intake (g/kg0.75) | 32.77 ± 2.50 | 35.75 ± 1.08 |
| Total duration of prandial intake (food only) (min) | 46.02 ± 3.56 | 61.92 ± 2.32* |
| Meal frequency | 10.00 ± 1.41 | 14.57 ± 0.50* |
| Average meal size (g/kg0.75) | 3.50 ± 0.31 | 2.52 ± 0.08* |
| Average meal duration (food only) (min) | 4.99 ± 0.53 | 4.39 ± 0.20 |
P < 0.05, between Day 0 and Day 73 of heroin self-administration. No other measures were significant.
Effect of extended heroin access on meal pattern analysis measures for long-access rats. Mean ± SEM of meal pattern measures for long-access rats (n = 7).
R121919 treatment did not significantly alter 12-hour meal microstructure in long-access rats, measured by the total quantity or duration of prandial intake, number of meals, average meal duration or meal size (P > 0.05 for all measures; Table 5).
Table 5.
Effects of R121919 on meal microstructure.
| R121919 dose (s.c.) |
||||
|---|---|---|---|---|
| Vehicle | 5 mg/kg | 10 mg/kg | 20 mg/kg | |
| 12-hour intake | ||||
| Total food intake (g/kg0.75) | 35.19 ± 1.66 | 31.13 ± 2.11 | 30.90 ± 2.33 | 29.36 ± 2.51 |
| Total duration of prandial intake (food only) (min) | 63.40 ± 4.65 | 55.84 ± 4.16 | 55.24 ± 6.25 | 57.33 ± 7.62 |
| Meal frequency | 14.86 ± 1.50 | 13.14 ± 1.12 | 12.86 ± 1.60 | 13.57 ± 0.78 |
| Average meal size (g/kg0.75) | 2.49 ± 0.24 | 2.46 ± 0.27 | 2.56 ± 0.30 | 2.24 ± 0.31 |
| Average meal duration (food only) (min) | 4.40 ± 0.36 | 4.42 ± 0.49 | 4.49 ± 0.60 | 4.33 ± 0.64 |
Meal pattern analysis measures for long-access rats treated with R121919. Mean ± SEM of meal pattern measures for long-access rats (n = 7). No significant effect of R121919 was observed on meal pattern measures.
DISCUSSION
This study demonstrated that CRF1 antagonists decreased heroin self-administration in long-access rats that were allowed daily extended access to heroin but not in short-access rats with daily limited access to heroin. The CRF1 receptor antagonist MJL-1-109-2 reduced first-hour heroin intake in long-access rats (8 hours/day) but not in short-access rats, without altering total food or water consumption in the long-access rats. A second CRF1 antagonist, R121919, reduced not only first-hour intake, but also total heroin intake in rats that were allowed daily 12-hour access to heroin, without affecting their concurrent meal microstructure or quantity of food or water consumption, and also without modifying heroin responding of short-access rats.
Further analysis revealed that access length to heroin is necessary for CRF antagonists to produce selective effects. R121919 decreased responding of ‘low-responding’ long-access, but not ‘high-responding’ short-access rats, subgroups that showed similar baseline responding. Thus, we suggest that R121919 only reduced heroin self-administration relative to access history (i.e. only in the long-access subgroup of rats), rather than as a rate-dependent effect in either access group.
The ability of R121919 to reduce heroin responding in rats that were given extended access (12 hours) to heroin was related to the degree to which rats had escalated their heroin self-administration from short-access conditions. Three long-access rats had greater increases in heroin intake over days of testing compared with the other long-access rats, and these three rats were found to be more sensitive to R121919. The evidence presented here showed that the action of R121919 in decreasing heroin intake was greatest in long-access, high-responding rats.
The total intake for the 8-hour and 12-hour rats was the same and was consistent with the intake of rats with 23-hour access if one considers only 12-hour intake in the dark cycle (Chen et al. 2006). These results suggest that animals may reach a level of opioid titration sufficient to alleviate acute withdrawal, but because of the 12- and 16-hour off periods, these durations of exposure are not sufficient to drive escalation further, similar to observations in 23-hour access rats (Chen et al. 2006).
Based on the present findings, CRF, antagonists may have a unique effect of selectively reducing heroin intake in dependent versus non-dependent animals, whereas opioid drugs (agonists, antagonists and partial agonists) have different profiles. Unlike what was observed for the CRF1 antagonists, buprenorphine and naloxone are known to decrease heroin self-administration in both dependent and non-dependent animals. Naloxone and buprenorphine caused a dose-dependent rightward shift in the heroin dose-effect curve in non-dependent monkeys (Negus 2006). Furthermore, buprenorphine decreased withdrawal-associated increases in heroin intake but not as effectively as methadone (Negus 2006). In contrast, methadone had no effect on heroin responding in non-dependent animals but prevented the emergence of withdrawal signs and leftward shifts in dose-effect curves in dependent animals (Negus 2006).
R121919 exhibited a longer duration of action and greater efficacy of reducing heroin self-administration in long-access rats than did MJL-1-109-2. One possible explanation for the different efficacies of the CRF1 antagonists may relate to the very slow ‘off’ kinetics of R121919, which reportedly dissociates from the CRF1 receptor with a t1/2 of approximately 11–12 hours (D. Grigoriadis, pers. comm.). Thus, although MJL-1-109-2 has greater acute in vitro CRF1 affinity than R121919, the pharmacokinetics and pharmacodynamics of R121919 might ultimately culminate in greater or more prolonged receptor occupancy. Heinrichs et al. (2002) reported 50% and 100% occupancy 1 hour following oral administration of 2.5 and 20 mg/kg doses of R121919, respectively. In the present study, the different routes of administration also may be relevant because MJL-1-109-2 (i.p.) and R121919 (s.c.) were subject to differential metabolism. Different routes of administration were used because MJL-1-109-2 is less soluble than R121919, and the routes of administration for these drugs were shown previously to be effective (Funk et al. 2007). The longer daily (12 hours versus 8 hours) and cumulative [74 days versus 21 (cohort 1) and 40 (cohort 2) days] heroin access of rats that received R121919 compared with those that received MJL-1-109-2 also may lead to a greater recruitment of CRF1 systems in the maintenance of drug intake, resulting in increased sensitivity to CRF1 antagonism (Koob et al. 2004).
The CRF1 antagonist-mediated decrease of heroin responding in long-access rats may have resulted from a number of different mechanisms, including weakened positive reinforcement (Dworkin et al. 1988), attenuation of incentive learning (Hutcheson et al. 2001), blunting of the incentive salience of the drug reinforcer (Pecina, Schulkin & Berridge 2006), positive reinforcement from CRF antagonists (Broadbear et al. 2002) or alleviation of negative reinforcement processes that drive long-access heroin intake (Koob et al. 2004; Chen et al. 2006; Kenny et al. 2006). Several reports provide support for the latter possibility (Heinrichs et al. 1995; Lu et al. 2000; Funada, Hara & Wada 2001; Contarino & Papaleo 2005; Stinus et al. 2005). Two prevalent characteristics of extended access to drugs are elevated, drug intake and the emergence of a negative emotional state, among other withdrawal symptoms, during drug abstinence (Koob & Le Moal 2001). A transition has been hypothesized to occur in the development of drug dependence by which positive reinforcement mechanisms (e.g. euphoria) may drive initial drug intake, but with increasing drug-taking experience, negative reinforcement mechanisms, such as the alleviation of the negative emotional components of withdrawal, gain prominence in motivating drug intake (Koob & Le Moal 2001). Evidence for such a transition from positive to negative reinforcement mechanisms has recently been seen in rats with extended access to heroin using conditioned withdrawal and intracranial self-stimulation procedures (Ahmed et al. 2002; Kenny et al. 2006).
One explanation for such changes in negative emotional state is interactions of CRF in the extended amygdala. CRF secretion is increased in the central nucleus of the amygdala (CeA) during opiate withdrawal (Weiss et al. 2001), and activation of CRF1 receptors has aversive and anxiogenic-like effects in animals (Heinrichs, Britton & Koob 1991; Smith et al. 1998; Zorrilla & Koob 2004). Administration of a CRF antagonist into the CeA reverses withdrawal-induced place aversion resulting from methylnaloxonium microinfusions into the CeA (Heinrichs et al. 1995). The selective CRF1 antagonist antalarmin blocked place aversion produced by naloxone in morphine-dependent rats (Stinus et al. 2005). CRF1 receptor knockout and heterozygous mice showed a lack of conditioned place aversion to opioid withdrawal compared with wild type mice (Contarino & Papaleo 2005). These results show a key role for CRF receptors in mediating the aversive stimulus properties of opiate withdrawal. Paradoxically, CRF1-deficient mice showed more somatic signs of withdrawal than their wild type counterparts, demonstrating a dissociation between the affective and somatic signs of opioid withdrawal (Contarino & Papaleo 2005). However, in the rat, CRF1 receptors also appear to be important for somatic withdrawal signs as well as relapse to morphine dependence (Iredale et al. 2000; Lu et al. 2000). Perhaps opioid administration gives rise to an opponent process involving activated CRF systems in the amygdala that are counterbalanced by opioids in the steady state and unmasked by an opioid antagonist during withdrawal (Koob et al. 1993). The reduction in heroin administration resulting from CRF1 receptor blockade may be attributable to alleviation of a negative emotional state that is hypothesized to drive the maintenance and resumption of heroin self-administration (Koob et al. 1989; Ahmed & Koob 2005). The negative emotional state of opioid withdrawal has been observed in three animal models in our laboratory: the elevated plus maze (Schulteis et al. 1998), conditioned place aversion (Heinrichs et al. 1995; Stinus et al. 2005) and brain stimulation reward (Kenny et al. 2006). Conditioned place aversion was reversed by systemic administration of a CRF1 antagonist, and the elevation in reward thresholds was observed during escalation in heroin intake (Kenny et al. 2006). These studies provide the conceptual rationale for the current CRF1 antagonist tests on heroin self-administration in rats with extended access. Other neurotransmitter systems also may be involved in mediating heroin intake, including dopamine (Di Chiara & Imperato 1988; Maldonado et al. 1997; Rowlett, Platt & Spealman 2004) and norepinephrine (Maldonado 1997; Georges & Aston-Jones 2003; Greenwell et al. 2008).
CRF1-selective antagonists also might increase dopamine levels in long- and short-access rats given heroin, similar to rats given intraperitoneal cocaine (Lu et al. 2003). However, the effects of the CRF1 antagonist on dopamine release were observed after acute injection of cocaine. Thus, because we did not observe a reduction in heroin intake in the short-access rats, the reduction of heroin consumption that is specific to long-access rats appears to be attributable to negative emotional states rather than a generalized increase in dopamine release. Previous studies have shown that opioid withdrawal increases CRF in the amygdala, and the aversive effects of opioid withdrawal are reversed by systemic administration of CRF1 antagonists (Stinus et al. 2005) and administration of CRF1/2 antagonists injected directly into the CeA (Heinrichs et al. 1995). Thus, based on our data and previous findings, CRF mechanisms in the amygdala may be important for driving heroin self-administration in long-access rats.
One potential caveat of the hypothesis of an action of CRF1 antagonists on negative reinforcement mechanisms is that CRF antagonists themselves might be reinforcing under certain conditions (e.g. drug withdrawal). Transient reinforcing properties of a CRF1 antagonist, antalarmin, have been observed in rhesus macaques. Eight monkeys had an extensive history of barbiturate self-administration, and only one with no history of drug self-administration had self-administered antalarmin (Broadbear et al. 2002). Thus, antalarmin self-administration might partly have reflected an acquired reinforcing action of antalarmin as a function of extensive drug experience, perhaps to ‘self-medicate’ the negative emotional symptoms of drug abstinence (Zorrilla & Koob 2004).
MJL-1-109-2 is less studied than other CRF1 antagonists, but is related to DMP696 and has specificity for CRF1 receptors (Jagoda et al. 2003). Blockade of the CRF1 receptor reduces anxiety-like behavior under ‘high-stress’ conditions (Zorrilla & Koob 2004). R121919 is highly selective for the CRF1 receptor (Heinrichs et al. 2002) and has no other reported pharmacological interactions (Zorrilla & Koob 2004). Additionally, in animal studies, doses of 2.5–20 mg/kg of R121919 have been shown to inhibit stress-induced plasma adrenocorticotropic hormone levels (Chen et al. 2004) and anxiogenic-like behaviors under high-stress test conditions (Zobel et al. 2000; Keck et al. 2001; Heinrichs et al. 2002; Gutman et al. 2003). MJL-1-109-2 and R121919 also reduced 30-minute ethanol self-administration in ethanol-dependent rats that were tested during acute withdrawal, without affecting self-administration in non-dependent rats (Funk et al. 2007). Thus, motivational components of opioid withdrawal may be blocked by 10 mg/kg MJL-1-109-2, a dose that selectively decreased heroin self-administration in the present long-access rats. Consistent with the present results, MJL-1-109-2 did not significantly reduce food intake at doses as high as 10 mg/kg (Sabino et al. 2006).
Similar to MJL-1-109-2, R121919 did not affect total food or water intake, indicating a specific effect of the CRF1 antagonist on heroin-maintained operant responding. Although R121919 was hypothesized to possibly reverse or attenuate the heroin-access-related changes in meal patterning, this was not the case. No measures of meal microstructure changed significantly as a result of R121919 treatment. Whether the changes in meal patterning are dissociable from long-access heroin responding or whether chronic administration of R121919 may be necessary for reversing changes in feeding microstructure remain to be determined.
The injections of CRF1 antagonists are hypothesized to act in the brain to decrease long-access heroin responding. Although some interactions with the hypothalamic-pituitary-adrenal axis may occur (Jutkiewicz et al. 2005). Many of the anti-stress-like behavioral effects of CRF1 antagonists appear to depend more on extrahypothalamic than neuroendocrine CRF systems (Swiergiel et al. 1992; Nijsen et al. 2001; Muller et al. 2003; Swiergiel 2003; Zorrilla & Koob 2004).
One limitation of the present study is that one group of rats was treated with a noradrenergic α1 receptor antagonist (prazosin) before being treated with R121919, thus leaving the possibility that cross-tolerance or cross-sensitization may have occurred. Although this is possible, this group of rats received a 2-week washout period of heroin self-administration without drug treatment. The other CRF1 antagonist (MJL-1-109-2) also reduced heroin responding, with no history of other drug treatments in this group of subjects. Another limitation of the present study may be that long access to heroin could have impaired the metabolism, detoxification and excretion that could increase the plasma levels and duration of action of the compounds in dependent animals. In an open label clinical study, R121919 increased liver enzymes in some patients, usually in those who received higher doses of R121919 (Zobel et al. 2000). In vitro studies in human liver cells showed that cytochrome P450 3A4 (CYP3A4) and 3A5 (CYP3A5) isozymes are involved in R121919 metabolism, yielding the active metabolite R142900 (Zobel et al. 2000). How these effects of R121919 or any potential effects from MJL-1-109-2 on metabolic liver function may interact with heroin metabolism is unclear. Acute heroin administration increases the hepatic content of cytochrome P450 (126%), also with induction of aryl hydrocarbon hydroxylase and NADPH-cytochrome c reductase activities (Sheweita 2003). Narcotic drugs changed the expression of CYP2E1 and CYP2C6 and other activities of carcinogenic-metabolizing enzymes in the liver of male mice (Sheweita 2003). Although metabolic explanations remain possible, we did see a difference in the rate of responding between high-rate heroin responders and low-rate heroin responders in the long-access groups that received high levels of heroin each day. Therefore, the effect of the CRF1 antagonists in long-access, but not short-access, animals is not believed to be attributable to pharmacokinetic differences induced by heroin access.
In summary, the present data suggest a possible role for CRF–CRF1 systems in maintaining heroin self-administration in rats with a history of extended (either 8 hours or 12 hours) drug access. CRF1 antagonists selectively attenuated heroin self-administration in long-access animals that showed a history of increased drug intake but not in short-access animals. The data presented here provide potential new insights into the neurotransmitter systems that may drive increased intake of opioids when increased drug access is provided.
Acknowledgments
This study was supported by National Institutes of Health grants DA04043 (G.F.K.) and DA019295 (T.N.G.) from the National Institute on Drug Abuse, DK64871 (E.P.Z.) from the National Institute of Diabetes and Digestive and Kidney Diseases, a merit fellowship from the University of Rome La Sapienza (P.C.) and the Pearson Center for Alcoholism and Addiction Research. The authors thank Mr. Robert Lintz, Mrs. Yanabel Grant and Mr. Daniel Bowling for their excellent technical assistance. We also thank Mr. Michael Arends for his editorial assistance and Dr. Olivier George for his comments on the manuscript. This is publication number 18771 from The Scripps Research Institute.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology (Berl) 2005;180:473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Azar MR, Jones BC, Schulteis G. Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology (Berl) 2003;170:42–50. doi: 10.1007/s00213-003-1514-y. [DOI] [PubMed] [Google Scholar]
- Bewick V, Cheek L, Ball J. Statistics review 9: one-way analysis of variance. Crit Care. 2004;8:130–136. doi: 10.1186/cc2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Toxicity associated with long-term intravenous heroin and cocaine self-administration in the rat. JAMA. 1985;254:81–83. [PubMed] [Google Scholar]
- Bretz F, Pinheiro JC, Branson M. On a hybrid method in dose finding studies. Methods Inf Med. 2004;43:457–460. [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Rice KC, Woods JH. Antalarmin, a putative CRH-RI antagonist, has transient reinforcing effects in rhesus monkeys. Psychopharmacology (Berl) 2002;164:268–276. doi: 10.1007/s00213-002-1187-y. [DOI] [PubMed] [Google Scholar]
- Caine SB, Lintz R, Koob GF. Intravenous drug self-administration techniques in animals. In: Sahgal A, editor. Behavioral Neuroscience: A Practical Approach. New York: Oxford University Press; 1993. pp. 117–143. [Google Scholar]
- Carrera MR, Schulteis G, Koob GF. Heroin self-administration in dependent Wistar rats: increased sensitivity to naloxone. Psychopharmacology (Berl) 1999;144:111–120. doi: 10.1007/s002130050983. [DOI] [PubMed] [Google Scholar]
- Chen C, Wilcoxen KM, Huang CQ, Xie YF, McCarthy JR, Webb TR, Zhu YF, Saunders J, Liu XJ, Chen TK, Bozigian H, Grigoriadis DE. Design of 2,5-dimethyl-3-(6-dimethyl-4-methylpyridin-3-yl)-7-dipropylaminopyrazolo[1,5-a]pyrimidine (NBI 30775/R121919) and structure—activity relationships of a series of potent and orally active corticotropin-releasing factor receptor antagonists. J Med Chem. 2004;47:4787–4798. doi: 10.1021/jm040058e. [DOI] [PubMed] [Google Scholar]
- Chen SA, O’Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology. 2006;31:2692–2707. doi: 10.1038/sj.npp.1301008. [DOI] [PubMed] [Google Scholar]
- Contarino A, Papaleo F. The corticotropin-releasing factor receptor-1 pathway mediates the negative affective states of opiate withdrawal. Proc Natl Acad Sci USA. 2005;102:18649–18654. doi: 10.1073/pnas.0506999102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria-Pesce VH, Nicolaidis S. Mathematical determination of feeding patterns and its consequence on correlational studies. Physiol Behav. 1998;65:157–170. doi: 10.1016/s0031-9384(98)00159-0. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin SI, Guerin GF, Goeders NE, Smith JE. Kainic acid lesions of the nucleus accumbens selectively attenuate morphine self-administration. Pharmacol Biochem Behav. 1988;29:175–181. doi: 10.1016/0091-3057(88)90292-4. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funada M, Hara C, Wada K. Involvement of corticotropin-releasing factor receptor subtype 1 in morphine withdrawal regulation of the brain noradrenergic system. Eur J Pharmacol. 2001;430:277–281. doi: 10.1016/s0014-2999(01)01402-9. [DOI] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Prolonged activation of mesolimbic dopaminergic neurons by morphine withdrawal following clonidine: participation of imidazoline and norepinephrine receptors. Neuropsychopharmacology. 2003;28:1140–1149. doi: 10.1038/sj.npp.1300161. [DOI] [PubMed] [Google Scholar]
- Greenwell TN, Walker BM, Cottone P, Zorilla EP, Koob GF. The α1 adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacol Biochem Behav. 2008 July 23; doi: 10.1016/j.pbb.2008.07.012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman DA, Owens MJ, Skelton KH, Thrivikraman KV, Nemeroff CB. The corticotropin-releasing factor1 receptor antagonist R121919 attenuates the behavioral and endocrine responses to stress. J Pharmacol Exp Ther. 2003;304:874–880. doi: 10.1124/jpet.102.042788. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Britton KT, Koob GF. Both conditioned taste preference and aversion induced by corticotropin-releasing factor. Pharmacol Biochem Behav. 1991;40:717–721. doi: 10.1016/0091-3057(91)90075-d. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, De Souza EB, Schulteis G, Lapsansky JL, Grigoriadis DE. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type 1 receptor selective antagonist. Neuropsychopharmacology. 2002;27:194–202. doi: 10.1016/S0893-133X(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Schulteis G, Koob GF, Stinus L. Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol. 1995;6:74–80. [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A. The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci. 2001;4:943–947. doi: 10.1038/nn0901-943. [DOI] [PubMed] [Google Scholar]
- Iredale PA, Alvaro JD, Lee Y, Terwilliger R, Chen YL, Duman RS. Role of corticotropin-releasing factor receptor-1 in opiate withdrawal. J Neurochem. 2000;74:199–208. doi: 10.1046/j.1471-4159.2000.0740199.x. [DOI] [PubMed] [Google Scholar]
- Jagoda E, Contoreggi C, Lee MJ, Kao CH, Szajek LP, Listwak S, Gold P, Chrousos G, Greiner E, Kim BM, Jacobson AE, Rice KC, Eckelman W. Autoradiographic visualization of corticotropin releasing hormone type 1 receptors with a nonpeptide ligand: synthesis of [76Br]MJL-1-109-2. J Med Chem. 2003;46:3559–3562. doi: 10.1021/jm034077k. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Wood SK, Houshyar H, Hsin LW, Rice KC, Woods JH. The effects of CRF antagonists, antalarmin, CP154,526, LWH234, and R121919, in the forced swim test and on swim-induced increases in adrenocorticotropin in rats. Psychopharmacology (Berl) 2005;180:215–223. doi: 10.1007/s00213-005-2164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ME, Welt T, Wigger A, Renner U, Engelmann M, Holsboer F, Landgraf R. The anxiolytic effect of the CRH1 receptor antagonist R121919 depends on innate emotionality in rats. Eur J Neurosci. 2001;13:373–380. doi: 10.1046/j.0953-816x.2000.01383.x. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiology of Addiction. San Diego, CA: Academic Press; 2006. [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, Britton KT. The role of corticotropin-releasing factor in behavioural responses to stress. Ciba Found Symp. 1993;172:277–289. doi: 10.1002/9780470514368.ch14. [DOI] [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le MM, Bloom FE. Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neurosci Biobehav Rev. 1989;13:135–140. doi: 10.1016/s0149-7634(89)80022-3. [DOI] [PubMed] [Google Scholar]
- Lu L, Liu D, Ceng X, Ma L. Differential roles of corticotropin-releasing factor receptor subtypes 1 and 2 in opiate withdrawal and in relapse to opiate dependence. Eur J Neurosci. 2000;12:4398–4404. [PubMed] [Google Scholar]
- Lu L, Liu Z, Huang M, Zhang Z. Dopamine-dependent responses to cocaine depend on corticotropin-releasing factor receptor subtypes. J Neurochem. 2003;84:1378–1386. doi: 10.1046/j.1471-4159.2003.01635.x. [DOI] [PubMed] [Google Scholar]
- Maldonado R. Participation of noradrenergic pathways in the expression of opiate withdrawal: biochemical and pharmacological evidence. Neurosci Biobehav Rev. 1997;21:91–104. doi: 10.1016/0149-7634(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Saiardi A, Valverde O, Samad TA, Roques BP, Borrelli E. Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature. 1997;388:586–589. doi: 10.1038/41567. [DOI] [PubMed] [Google Scholar]
- Muller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, Kormann MS, Droste SK, Kuhn R, Reul JM, Holsboer F, Wurst W. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Negus SS. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther. 2006;317:711–723. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- Nijsen MJ, Croiset G, Diamant M, De Wied D, Wiegant VM. CRH signalling in the bed nucleus of the stria terminalis is involved in stress-induced cardiac vagal activation in conscious rats. Neuropsychopharmacology. 2001;24:1–10. doi: 10.1016/S0893-133X(00)00167-6. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Markou A, Zorrilla EP, Koob GF. Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007;320:180–193. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- Pecina S, Schulkin J, Berridge KC. Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of Biostatistics. Boston, MA: Duxbury; 1995. [Google Scholar]
- Rowlett JK, Platt DM, Spealman RD. Cocaine-like discriminative stimulus effects of heroin: modulation by selective monoamine transport inhibitors. J Pharmacol Exp Ther. 2004;310:342–348. doi: 10.1124/jpet.104.065631. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Yackey M, Risbrough V, Koob GF. Anxiogenic-like effects of spontaneous and naloxone-precipitated opiate withdrawal in the elevated plus-maze. Pharmacol Biochem Behav. 1998;60:727–731. doi: 10.1016/s0091-3057(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheskin DJ. Handbook of Parametric and Nonparametric Statistical Procedures. Boca Raton, FL: Chapman and Hall; 2004. [Google Scholar]
- Sheweita SA. Narcotic drugs change the expression of cytochrome P450 2E1 and 2C6 and other activities of carcinogen-metabolizing enzymes in the liver of male mice. Toxicology. 2003;191:133–142. doi: 10.1016/s0300-483x(03)00252-x. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Sidhu KS. Basis for body weight exponent (0.75) as a scaling factor in energy metabolism and risk assessment. J Appl Toxicol. 1992;12:309–310. doi: 10.1002/jat.2550120503. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Stinus L, Cador M, Zorrilla EP, Koob GF. Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology. 2005;30:90–98. doi: 10.1038/sj.npp.1300487. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH. Effects of infusion of corticotropin-releasing factor antagonist into the locus coeruleus on freezing behavior and brain catecholamines in rats. Acta Neurobiol Exp (Wars) 2003;63:9–16. doi: 10.55782/ane-2003-1449. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Takahashi LK, Rubin WW, Kalin NH. Antagonism of corticotropin-releasing factor receptors in the locus coeruleus attenuates shock-induced freezing in rats. Brain Res. 1992;587:263–268. doi: 10.1016/0006-8993(92)91006-z. [DOI] [PubMed] [Google Scholar]
- Thornhill JA, Hirst M, Gowdey CW. Disruption of diurnal feeding patterns of rats by heroin. Pharmacol Biochem Behav. 1976;4:129–135. doi: 10.1016/0091-3057(76)90004-6. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The γ-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JR, Ahmed SH, Gracy KN, Koob GF. Microinjections of an opiate receptor antagonist into the bed nucleus of the stria terminalis suppress heroin self-administration in dependent rats. Brain Res. 2000;854:85–92. doi: 10.1016/s0006-8993(99)02288-x. [DOI] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. New York: McGraw-Hill; 1962. [Google Scholar]
- Young GA, Moreton JE, Meltzer LT, Khazan N. 1-alpha-acetylmethadol (LAAM), methadone and morphine abstinence in dependent rats: EEG and behavioral correlates. Drug Alcohol Depend. 1977;2:141–148. doi: 10.1016/0376-8716(77)90014-x. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, Holsboer F. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34:171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1450–R1467. doi: 10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]