Abstract
Rationale and objectives
Sigma receptors have been implicated in appetitive effects of psychostimulants and in high levels of ethanol intake. This study tested the hypothesis that the sigma-1 receptor subtype (Sig-1R) may modulate ethanol intake.
Material and methods
The effects of acute and repeated treatment with the potent, selective Sig-1R antagonist NE-100 on ethanol intake (10%) were studied in adult, male Sardinian alcohol-preferring (sP) rats, a model of genetic predisposition to high ethanol drinking. To assess the specificity of action, the acute effects of NE-100 on intake of an equally preferred sucrose solution and of a higher concentration of ethanol that sP rats did not prefer over water (28%), were determined. Finally, the ability of NE-100 administration to prevent the increased ethanol intake that occurs after deprivation was evaluated.
Results
Acute treatment with NE-100 dose-dependently (10–30 mg/kg) reduced 1- and 3-h intake of 10% ethanol solution in sP rats, while increasing concurrent water intake and not affecting food intake. NE-100 (17.8–30 mg/kg) comparably reduced intake of the 28% ethanol solution, while not suppressing 1.25% sucrose solution intake, suggesting selectivity of action against ethanol intake. Acute NE-100 (30 mg/kg) also prevented an increase in ethanol intake after a 7-day deprivation period. Repeated, daily NE-100 (30 mg/kg) treatment continued to reduce 24-h ethanol intake across 7 days of administration, with some, but incomplete, tolerance, evident by day 6.
Conclusions
The results implicate the Sig-1R system in alcohol drinking, identifying a potential therapeutic target for the treatment of alcohol use disorders.
Keywords: Ethanol, Addiction, Rat, Alcoholism, Treatment
Introduction
Alcoholism, a global health problem, is a chronic relapsing disorder of compulsive alcohol use (McLellan et al. 2000). Despite progress in understanding the etiology of alcohol dependence, effective, well-tolerated treatments remain elusive. Sigma receptor (SigR) antagonists have been hypothesized to be putative pharmacotherapies for addiction to ethanol and psychostimulants (Brammer et al. 2006; Martin-Fardon et al. 2007; Maurice and Romieu 2004; Menkel et al. 1991; Sabino et al. 2009). SigRs were originally categorized as members of the opiate receptor family (Martin et al. 1976) and a high-affinity phencyclidine (PCP) binding-site (Quirion et al. 1981), but more recent data suggest that SigRs are unique binding sites that differ from other known mammalian proteins (Gundlach et al. 1985; Walker et al. 1990). SigRs are widely expressed in rat brain, throughout the limbic system and brainstem motor structures. The highest levels of immunoreactivity are observed in the olfactory bulb, hypothalamus, and hippocampus; the ventral and dorsal striatum and amygdala also show moderately concentrated, intense labeling (Alonso et al. 2000; Bouchard and Quirion 1997). SigRs are immunohistochemically localized to synaptic contacts, and the anatomical distribution of SigRs (Alonso et al. 2000; Maurice et al. (2002) suggests that SigRs may modulate motivationally relevant synaptic transmission, including that of substances of abuse. Two SigR subtypes are known, Sig-1R and Sig-2R, differing in their binding profile and molecular weight (Hanner et al. 1996; Moebius et al. 1993).
Relevant to ethanol use disorders, nonselective SigR antagonists previously were shown to attenuate ethanol-induced locomotion, block ethanol-induced place and taste conditioning in mice (BD-1047; Maurice et al. 2003), and reduce ethanol intake in rat models of excessive drinking (BD-1063; Sabino et al. 2009). However, the receptor subtype mediating these actions remains unclear. Data implicate the Sig-1R subtype in the actions of cocaine and methamphetamine (Matsumoto et al. 2002; Romieu et al. 2004). Also, Sig-1R gene functional polymorphisms that correlate with altered receptor transcription were over-represented in Japanese alcoholics (Miyatake et al. 2004). In contrast, some pharmacological evidence suggests that some actions which previously have been attributed exclusively to Sig-1Rs may also (or alternatively) involve Sig-2Rs (Matsumoto and Mack 2001; Nuwayhid and Werling 2006). For example, (±)-SM 21, a compound with high and preferential affinity for Sig-2R, relative to the Sig1R subtype has been shown to attenuate some behavioral and toxic effects of cocaine (Matsumoto and Mack 2001; Matsumoto et al. 2007). In addition, while 1,3-di(2-tolyl) guanidine (DTG), a specific, but subtype-nonselective Sig-1R/Sig-2R agonist, markedly worsens cocaine-induced toxicity, (+)pentazocine—a selective Sig-1R agonist—did not significantly alter the responsiveness of the animals to the convulsive effects of cocaine (Matsumoto and Mack 2001; Matsumoto et al. 2007).
The purpose of the present study was to test the hypothesis that the Sig-1R subtype modulates alcohol drinking. We examined the effects of systemic administration of the selective Sig-1R antagonist NE-100 on alcohol drinking in Sardinian alcohol-preferring (sP) rats. The selectively bred sP rat (Colombo 1997) provides a model for identifying potential pharmacotherapies for alcoholism (Colombo et al. 2006; McBride and Li 1998). This line voluntarily drinks ethanol (10% v/v) in high quantities and preference over water, exhibits a heritable component similar to human ethanol dependence (Cloninger et al. 1981; Prescott and Kendler 1999; Sigvardsson et al. 1996), and possesses predictive validity to identify pharmacotherapies for alcohol dependence.
To assess the specificity of action against consumption of ethanol, as opposed to an action on preferred solutions per se, the effects of NE-100 were also evaluated on the intake of an equally consumed, preferred sucrose solution and on the intake of a more concentrated alcohol solution (28% v/v) which sP rats do not prefer over water. The ability of NE-100 to suppress the increase in ethanol intake that occurs after deprivation (the “alcohol deprivation effect”; Koob 2000; Li 2000), a model of relapse drinking in human alcoholics (Boening et al. 2001; Colombo et al. 2006; McBride et al. 2002), was also assessed. Finally, the effects of repeated, daily treatment with NE-100 on ethanol intake were studied to investigate changes in effectiveness that may occur with repeated administration.
Thus, the present study investigated the effects and the selectivity of action of a selective Sig-1R antagonist, NE-100, on alcohol intake in alcohol-preferring sP rats. The overall hypothesis under test was that the Sig-1R system promotes alcohol intake in sP rats such that a subtype-selective antagonist, NE-100, would specifically reduce ethanol intake.
Materials and methods
Animals
Male, genetically selected TSRI Sardinian alcohol-preferring rats (N=60 Scr:sP; 300 g at study onset) were individually housed in a humidity- and temperature-controlled vivarium on a 12-h light–dark cycle (lights off, 10:00A.M.). Rats were generated from the 22nd to the 24th generations of intra-line breeding at The Scripps Research Institute from sP rats obtained after 32 generations of selection from Prof. G.L. Gessa (University of Cagliari, Italy). Different sets of rats were used for each experiment. Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Drugs
Ethanol solutions (10% and 28% v/v) and sucrose solution (1.25% w/v) were prepared using 95% ethyl alcohol or sucrose (Sigma Aldrich) in tap water. NE-100, a gift from Taisho Pharmaceutical, Tokyo, Japan, was dissolved in bacteriostatic saline and injected subcutaneously (s.c., 1 ml/kg), 15 min before drinking sessions (dark cycle onset). NE-100 is a selective Sig-1R antagonist (Ki=1.09 vs. 212 nM at Sig-1R and Sig-2R, respectively; Berardi et al. 2001) that does not show significant affinity for systems other than sigma, including histaminergic, dopaminergic, adrenergic, serotonergic, cholinergic, or glutaminergic receptors (Tanaka et al. 1995).
Procedure for ethanol drinking experiments
Before the experiments, rats received continuous (24 h/day) two-bottle choice access to ethanol (10% v/v) vs. tap water for 8 to 10 consecutive weeks, unless otherwise specified, and were habituated to handling and s.c. injections. Test days in within-subject design experiments were spaced by 2 to 4 intervening treatment-free days. To determine the effect of NE-100 on ethanol drinking, rats (N=10) were pretreated with NE-100 (0, 10, 17.8, and 30 mg/kg, s.c.) in a within-subjects Latin square design. We used the doses of 10 and 30 mg/kg per convention, as approximate half-log intervals. The mid-dose, 17.8 mg/kg, was chosen because it is a quarter-log interval greater than 10 and thus approximately midway between the 10 and 30 doses in log-scale. Ethanol (10% v/v), water, and food intake were determined by weighing bottles and food before session onset using a scale with 0.1 g precision and again 60 and 180 min later.
To determine the effects of NE-100 on intake of a more concentrated ethanol solution that sP rats do not prefer relative to water (28% v/v), sP rats (N=8) received continuous (24 h/day) two-bottle choice access to ethanol (10% v/v for 6 weeks, then 28% v/v for 3 weeks) vs. water and then were pretreated with NE-100 (0 and 30 mg/kg, s.c.) in a counterbalanced design.
In the alcohol deprivation effect experiment, sP rats (N=14) received continuous (24 h/day) two-bottle choice access to ethanol (10% v/v) vs. tap water for 8 weeks. Rats were then pretreated with vehicle. Ethanol, water, and food intake were measured after 60 and 180 min, constituting non-deprived intake. After 2 further days of access to ethanol, rats received a 7-day period of deprivation from ethanol, during which water was the only fluid available. Rats were then pretreated with NE-100 (30 mg/kg) or vehicle in a between-subjects design (n=7/group), and ethanol, water, and food intake were measured after 60 and 180 min, constituting deprived intake.
To determine the effect of repeated treatment with NE-100 on voluntary ethanol drinking, rats (N=9–10/group) received NE-100 (0 or 30 mg/kg, s.c.) daily 15 min before the onset of the dark cycle for 7 consecutive days in a between-subjects design. Ethanol, water, and food were weighed each day, 3 and 24 h after dark cycle onset.
Procedure for sucrose drinking experiment
To determine the effect of acute NE-100 treatment on consumption of a preferred, ethanol-free solution, rats first received continuous (24 h/day) two-bottle choice access to a sucrose solution (1.25% w/v) vs. tap water for 3 consecutive weeks. Rats (N=8) then were pretreated with NE-100 (s.c., 0, 17.8 and 30 mg/kg) in a within-subjects Latin square design. Sucrose solution, water, and food were postweighed 60 and 180 min after the dark cycle onset.
Procedure for blood alcohol level measurements
To determine whether NE-100 altered ethanol pharmacokinetics, rats (n=8/group) were pretreated with NE-100 (30 mg/kg, s.c.) or vehicle in a between-subjects design before receiving ethanol by gavage (1 g/kg, 16% w/v, p.o.) at dark cycle onset. Blood samples were collected from the rats’ tails 15, 30, 60, and 120 min after ethanol administration. Plasma was assayed for alcohol content (Analox Instruments, Lunenburg, MA, USA).
Statistical analysis
Incremental intake in each time bin was normalized for body weight (g/kg, ml/kg, and g/kg, for ethanol, water–sucrose solution, and food, respectively). Intake data from the ethanol and sucrose solution experiments were analyzed by a two-way, repeated-measures analysis of variance (ANOVA), with Antagonist Treatment and Time as within-subject factors. Data on first-hour intake from the alcohol deprivation effect experiment were analyzed by Student’s t-test. Data from the repeated treatment experiment were analyzed by separate two-way, mixed-design ANOVAs (for 3- and 24-h intake), with Antagonist as a between-subjects factor and Day as a within-subjects factor. Significant interactions were interpreted by simple main effects analysis. For pairwise comparisons, Dunnett’s tests were used to determine whether treatment altered performance compared with vehicle conditions. Statistical significance was set at P <0.05.
Results
Effect of acute NE-100 administration on ethanol drinking
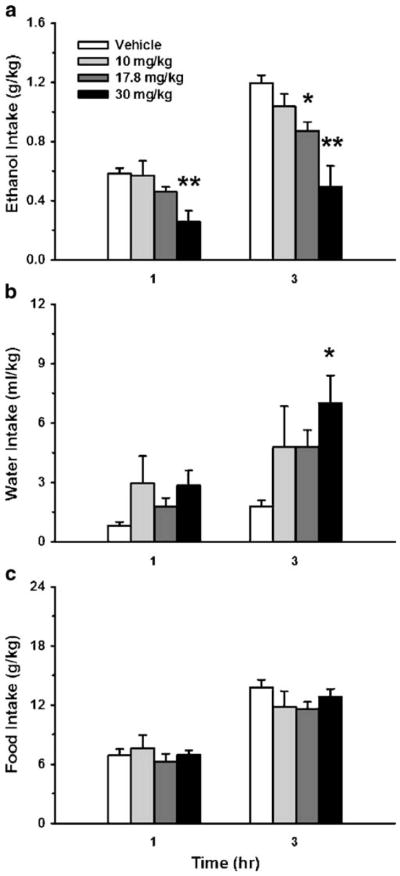
As shown in Fig. 1, treatment with the selective Sig-1R antagonist NE-100 dose-dependently reduced intake of a 10% v/v ethanol solution [F(3,27)=10.84, P<0.0001], while showing a strong trend toward increased concurrent water intake [F(3,27)=2.92, P=0.052]. Pairwise comparisons revealed that the doses of 17.8 and 30 mg/kg both significantly reduced ethanol intake (Fig. 1a, 27% and 58% decrease at the 3-h timepoint, for the 17.8 and 30 mg/kg doses, respectively). Thus, NE-100 decreased 3-h preference for an otherwise preferred ethanol solution (89.8% preference over water in vehicle-treated condition, vs. 72.3% for 17.8 mg/kg and 45.0% for 30 mg/kg). NE-100 treatment did not reliably alter total fluid [F(3,27)=2.78, n.s.] (data not shown) or food intake [F(3,27)=0.85, n.s.] (Fig. 1c).
Fig. 1.
Effect of acute s.c. pretreatment (−15 min) with the selective sigma-1 receptor (Sig-1R) antagonist NE-100 on 1- and 3-h alcohol (a), water (b), and food intake (c), relative to dark cycle onset. Subjects were Sardinian alcohol-preferring (sP) rats (N=10), tested under continuous access (24 h) conditions. Data represent mean+SEM intake, normalized for body weight. *P <0.05, **P<0.01 vs. vehicle-treated group (Dunnett’s test)
NE-100 treatment (30 mg/kg) also reduced 3-h consumption of a more concentrated solution of ethanol (28% v/v) that was not preferred over water (45.8% baseline preference) [F(1,8)= 12.26, P<0.01] (vehicle, 1.68±0.20 vs. NE-100: 0.92± 0.12 g/kg). In this study, NE-100 treatment did not reliably alter concurrent water, food, or total fluid intake (data not shown). Thus, the ability of NE-100 to reduce ethanol intake did not simply reflect reduced intake of a preferred solution.
Effect of acute NE-100 administration on sucrose drinking
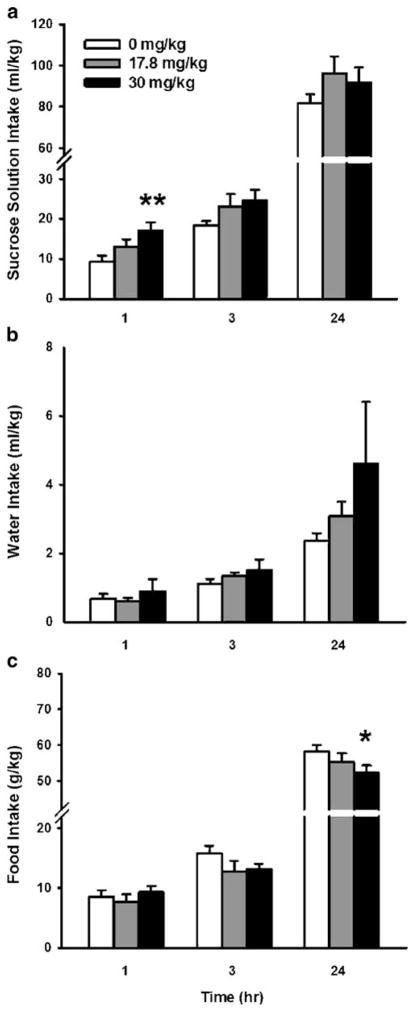
As shown in Fig. 2, treatment with the selective Sig-1R antagonist NE-100 increased sucrose solution intake in a time-dependent manner [Treatment×Time, F(2,16)=6.09, P<0.05]. Post hoc comparisons revealed that the 30 mg/kg dose transiently increased sucrose solution intake by 84% within the first hour compared with vehicle conditions, but not thereafter. NE-100 treatment did not alter intakes of water, food, or total fluid [Treatment, F(2,16)=1.14, 1.92, and 0.84, respectively; Treatment×Time, F(2,16)=0.84, 2.15, and 1.68, respectively, all n.s.].
Fig. 2.
Effect of acute s.c. pretreatment (−15 min) with the selective sigma-1 receptor (Sig-1R) antagonist NE-100 on 1-, 3-, and 24-h intake of 1.25% w/v sucrose solution (a), water (b), and food (c), relative to dark cycle onset in sP rats under continuous access (24 h) conditions (n=8). Data represent mean+SEM intake, normalized for body weight. *P< 0.05, **P<0.01 vs. vehicle-treated group (Dunnett’s test)
Effect of NE-100 on the alcohol deprivation effect
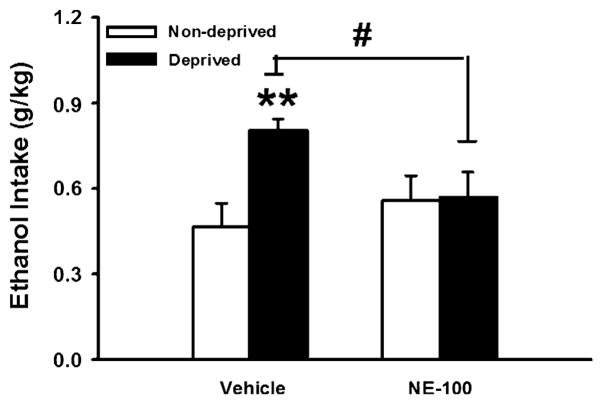
As expected, an imposed 7-day deprivation from ethanol increased ethanol intake during the first hour of renewed access in vehicle-treated subjects [deprived vs. non-deprived intake, t(6)=4.77, P<0.01]. In contrast, as shown in Fig. 3, rats pretreated with NE-100 (30 mg/kg) before renewed access did not show increased ethanol intake during the first hour compared with their nondeprived intake [t(6)=0.07, n.s.]. Consequently, vehicle-treated rats drank more than NE-100-treated rats under deprived conditions [t(12)=2.48, P<0.05].
Fig. 3.
Effect of acute s.c. pretreatment (−15 min) with the selective sigma-1 receptor (Sig-1R) antagonist NE-100 on 1-h alcohol intake in sP rats (n=7/group) after an imposed 7-day deprivation from ethanol. Data represent mean+SEM intake normalized for body weight. **P< 0.01 vs. non-deprived group, #P<0.05 vs. vehicle-treated deprived group (Student’s t tests)
Effect of NE-100 on blood alcohol levels
Blood alcohol levels changed across time following oral ethanol gavage (1 g/kg) [F(3,33)=29.16, p<0.0001]. Table 1 shows that pretreatment with 30 mg/kg NE-100 did not significantly alter blood alcohol levels 15–120 min following ethanol administration [Treatment, F(1,11)=0.46, n.s., Treatment×Time, F(3,33)=0.05, n.s.].
Table 1.
Effect of acute s.c. pretreatment (−15 min) with the selective sigma-1 receptor (Sig-1R) antagonist NE-100 on blood alcohol levels 15, 30, 60, and 120 min following gavage administration of ethanol (1 g/kg, p.o.) to sP rats (n=8/group)
| 15 min | 30 min | 60 min | 120 min | |
|---|---|---|---|---|
| Vehicle | 67.5±14.2 | 104.2±4.4 | 93.2±5.4 | 66.4±7.4 |
| NE-100, 30 mg/kg | 59.4±12.3 | 100.6±8.6 | 85.5±9.2 | 58.5±6.5 |
Data represent the mean±SEM of mg of alcohol per deciliter of plasma.
Effect of repeated NE-100 administration on ethanol drinking
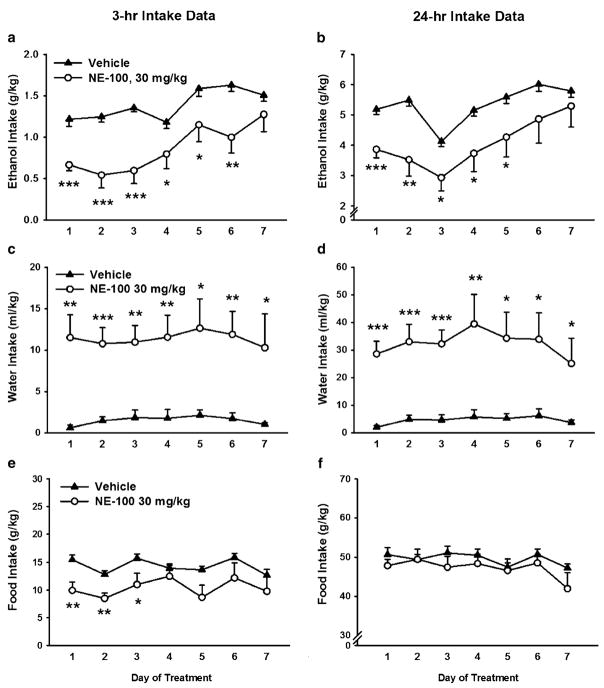
Daily NE-100 (30 mg/kg) administration reduced both 3-and 24-h ethanol intake during the 7 days of treatment [Treatment, 3 h, F(1,17)=11.40, p<0.01; 24 h, F(1,17)= 5.14, p<0.05], with no reliable change in this effect observed across the treatment period [Day×Treatment, 3 h, F(6,102)=2.11, n.s.; 24 h, F(6,102)=1.42, n.s.]. As shown in Fig. 4a, b, pairwise analyses showed that the NE-100-treated group drank significantly less ethanol through the fifth day of treatment.
Fig. 4.
Effect of repeated, 7-day s.c. pretreatment (−15 min) with the selective sigma-1 receptor (Sig-1R) antagonist NE-100 on 3- (a, c, e) and 24-h (b, d, f) intake of alcohol (a and b), water (c and d), and food (e and f), relative to dark cycle onset in sP rats (N=9–10/group), tested under continuous access (24-h) conditions. Data represent mean±SEM intake, normalized for body weight. *P<0.05, **P<0.01, ***P<0.001 vs. vehicle-treated group (Student’s t tests)
As shown in Fig. 4c, d, repeated NE-100 treatment increased water intake [Treatment, 3 h, F(1,17)=16.31, p< 0.001; 24 h, F(1,17)=14.07, p<0.01], leading to a nonsignificant tendency toward increased total fluid intake [Treatment, 3 h, F(1,17)=2.51, n.s.; 24 h, F(1,17)=4.35, n.s.] (data not shown). Figure 4e shows that NE-100 transiently reduced food intake for up to 3 h but only during the first 3 days of treatment [Treatment, F(1,17)=7.95, p<0.05]. However, as Fig. 4f shows, NE-100 treatment did not alter 24 h food intake at any time across the 7-day treatment period [Treatment, F(1,17)=1.12, n.s.].
Discussion
The present study showed that the selective Sig-1R antagonist NE-100 dose-dependently reduced ethanol intake in alcohol-preferring sP rats, a model of genetic predisposition to high ethanol drinking. NE-100 also prevented the increase in ethanol intake that otherwise occurs after a 7-day deprivation period. Suppressive actions of NE-100 were ethanol-selective and not due to changes in ethanol pharmacokinetics, general reductions in preferred fluid intake, or general decreases in fluid intake. NE-100 treatment reduced ethanol intake across 5 days of repeated, daily administration, with little tolerance evident until day 6. The results support the hypothesis that Sig-1R systems modulate alcohol intake.
The highest dose of NE-100 administered (30 mg/kg) decreased 3 h ethanol intake by 58% under continuous access conditions in sP rats, conditions with predictive validity for alcoholism pharmacotherapies (Colombo et al. 2006). The selective reduction of ethanol intake, and not of sucrose solution intake or concurrent food or water intake, suggests that NE-100 did not produce malaise-like, sedative, or other nonspecific behavior-impairing effects. NE-100 treatment also reduced intake of a solution of ethanol which subjects did not prefer over water, discounting the alternative explanation that NE-100 reduced intake of preferred solutions in general. The specific preference shift away from ethanol supports the potential relevance of NE-100, and possibly other Sig-1R antagonists, as therapeutics for ethanol use disorders.
The present results are consistent with findings that subtype nonselective SigR antagonists attenuated behavioral and motivational effects of acute passive ethanol administration in mice (BD-1047; Maurice et al. 2003) and reduced ethanol self-administration in rat models of excessive ethanol intake (BD-1063; Sabino et al. 2009). The findings obtained here with NE-100 more specifically suggest that the Sig-1R subtype may contribute to oral ethanol’s reinforcing effects. This hypothesis is consistent with the overrepresentation of Sig-1R gene functional polymorphisms in alcoholics (Miyatake et al. 2004). Differences in nucleus accumbens Sig-1R transcription also have been observed in relation to genetic preference for ethanol and to ethanol exposure-induced dependence (Sabino et al. 2009). Finally, the present results are also consistent with the apparent role of Sig-1R receptors in the psychomotor stimulant (Menkel et al. 1991; Ujike et al. 1992) and rewarding (Romieu et al. 2000) effects of other substances of abuse.
NE-100 suppressed the alcohol deprivation effect, defined as the transient increase in voluntary alcohol intake seen after alcohol abstinence (Agabio et al. 2000; Romieu et al. 2000). The increase in alcohol intake observed in vehicle-treated rats after a 7-day abstinence period was comparable in magnitude (73% increase) and duration (~1 h) to that reported previously in sP rats (Agabio et al. 2000; Serra et al. 2003). The alcohol deprivation effect models the uncontrolled alcohol consummatory behavior that characterizes alcohol relapse in human alcoholics (Boening et al. 2001) and is attenuated by drugs which reduce relapse frequency in alcoholics (Heilig and Egli 2006), providing some additional predictive validity.
Repeated, daily s.c. NE-100 treatment (30 mg/kg) significantly reduced alcohol intake by 44% on the first day of treatment, with a peak reduction of 61% by the third treatment day. Beginning from the sixth treatment day, some tolerance to NE-100’s actions was evident. This time course is similar to that of clinically utilized opioid receptor antagonists (e.g., naloxone and naltrexone), for which tolerance develops after 5–14 days of treatment (Cowen et al. 1999; Overstreet et al. 1999; Parkes and Sinclair 2000).
Ethanol is not thought to interact directly with SigRs, but SigR ligands may alter reinforcing actions of ethanol by indirectly modulating activity of other ethanol-sensitive transmitter systems. For example, SigR ligands affect the synthesis, release, and uptake of dopamine (Bastianetto et al. 1995; Iyengar et al. 1990; Weatherspoon and Werling 1999; Weiser et al. 1995), and they also modulate the firing activity of both the A9 and A10 dopaminergic pathways, with an activation of SigRs generally increasing firing rate (Minabe et al. 1999; Sanchez-Arroyos and Guitart 1999; Steinfels and Tam 1989). Therefore, Sig-1R antagonists might attenuate ethanol reinforcement by reducing mesolimbic dopaminergic transmission. The hypothesis that NE-100 might alter the reinforcing properties of ethanol by interfering with the mesolimbic dopaminergic pathway is, however, challenged by the fact that the effect of the drug does not correspond with preference/palatability, either on ethanol solutions or on sucrose solution. Rather than altering mesolimbic responsiveness in general, NE-100 might perhaps alter the pharmacological interaction of ethanol with mesolimbic neurons. Alternatively, NE-100’s action may be mediated via a different neural substrate.
For example, ethanol actions are thought to be mediated by neurosteroids, which are endogenous SigR ligands (for a review, see Maurice 2004). Therefore, an alternative (or complementary) hypothesis is that Sig-1R antagonists might prevent the ethanol-induced release, synthesis, or action of neurosteroids (Maurice 2004; Ueda et al. 2001). While the brain regions subserving Sig-1R antagonist-induced decreases in ethanol intake are unknown, the distribution of Sig-1Rs (Alonso et al. 2000) suggests that the nucleus accumbens, which integrates reward-relevant information with the generation of goal-directed behaviors (Carelli and Wightman 2004), may be involved.
In summary, acute or repeated administration of NE-100, a selective Sig-1R antagonist, selectively reduced ethanol intake in a model of genetic predisposition to high ethanol drinking. The effects were specific to ethanol and did not involve general reductions in total fluid intake or preferred solution intake. The results implicate the Sig-1R subtype in ethanol drinking and suggest the therapeutic potential of NE-100, or other selective Sig-1R antagonists, for the detoxification and abstinence phases of alcohol use disorders.
Acknowledgments
Research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (5K99AA016731-02, 2P60AA006420-25, and 5R01AA012602-09) and by the Pearson Center for Alcoholism and Addiction Research. This is manuscript number 19878 from The Scripps Research Institute. The authors thank Taisho Pharmaceuticals for the gift of NE-100, the excellent technical assistance of Maury Cole, Molly Brennan, Jeanette Helfers and Robert Lintz, and the editorial assistance of Mike Arends.
Footnotes
Disclosure/conflicts of interest The authors declare no conflicts of interest.
References
- Agabio R, Carai MA, Lobina C, Pani M, Reali R, Vacca G, Gessa GL, Colombo G. Development of short-lasting alcohol deprivation effect in Sardinian alcohol-preferring rats. Alcohol. 2000;21:59–62. doi: 10.1016/s0741-8329(00)00072-0. [DOI] [PubMed] [Google Scholar]
- Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T. Immunocytochemical localization of the sigma (1) receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Rouquier L, Perrault G, Sanger DJ. DTG-induced circling behaviour in rats may involve the interaction between sigma sites and nigrostriatal dopaminergic pathways. Neuropharmacology. 1995;34:281–287. doi: 10.1016/0028-3908(94)00156-m. [DOI] [PubMed] [Google Scholar]
- Berardi F, Ferorelli S, Colabufo NA, Leopoldo M, Perrone R, Tortorella V. A multireceptorial binding reinvestigation on an extended class of sigma ligands: N-[omega-(indan-1-yl and tetralin-1-yl) alkyl] derivatives of 3,3-dimethylpiperidine reveal high affinities towards sigma1 and EBP sites. Bioorg Med Chem. 2001;9:1325–1335. doi: 10.1016/s0968-0896(01)00011-6. [DOI] [PubMed] [Google Scholar]
- Boening JA, Lesch OM, Spanagel R, Wolffgramm J, Narita M, Sinclair D, Mason BJ, Wiesbeck GA. Pharmacological relapse prevention in alcohol dependence: from animal models to clinical trials. Alcohol Clin Exp Res. 2001;25:127S–131S. doi: 10.1097/00000374-200105051-00022. [DOI] [PubMed] [Google Scholar]
- Bouchard P, Quirion R. [3H]1, 3-di(2-tolyl) guanidine and [3H] (+) pentazocine binding sites in the rat brain: autoradiographic visualization of the putative sigma1 and sigma2 receptor subtypes. Neuroscience. 1997;76:467–477. doi: 10.1016/s0306-4522(96)00221-7. [DOI] [PubMed] [Google Scholar]
- Brammer MK, Gilmore DL, Matsumoto RR. Interactions between 3, 4-methylenedioxymethamphetamine and sigma1 receptors. Eur J Pharmacol. 2006;553:141–145. doi: 10.1016/j.ejphar.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Wightman RM. Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Curr Opin Neurobiol. 2004;14:763–768. doi: 10.1016/j.conb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Colombo G. ESBRA-Nordmann 1996 Award Lecture: ethanol drinking behaviour in Sardinian alcohol-preferring rats. Alcohol Alcohol. 1997;32:443–453. doi: 10.1093/oxfordjournals.alcalc.a008279. [DOI] [PubMed] [Google Scholar]
- Colombo G, Lobina C, Carai MA, Gessa GL. Phenotypic characterization of genetically selected Sardinian alcohol-preferring (sP) and -non-preferring (sNP) rats. Addict Biol. 2006;11:324–338. doi: 10.1111/j.1369-1600.2006.00031.x. [DOI] [PubMed] [Google Scholar]
- Cowen MS, Rezvani AH, Jarrott B, Lawrence AJ. Ethanol consumption by Fawn-Hooded rats following abstinence: effect of naltrexone and changes in mu-opioid receptor density. Alcohol Clin Exp Res. 1999;23:1008–1014. [PubMed] [Google Scholar]
- Gundlach AL, Largent BL, Snyder SH. Phencyclidine and sigma opiate receptors in brain: biochemical and autoradiographical differentiation. Eur J Pharmacol. 1985;113:465–466. doi: 10.1016/0014-2999(85)90100-1. [DOI] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sic USA. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Dilworth VM, Mick SJ, Contreras PC, Monahan JB, Rao TS, Wood PL. Sigma receptors modulate both A9 and A10 dopaminergic neurons in the rat brain: functional interaction with NMDA receptors. Brain Res. 1990;524:322–326. doi: 10.1016/0006-8993(90)90709-k. [DOI] [PubMed] [Google Scholar]
- Koob GF. Animal models of craving for ethanol. Addiction. 2000;95 (Suppl 2):S73–S81. doi: 10.1080/09652140050111663. [DOI] [PubMed] [Google Scholar]
- Li TK. Clinical perspectives for the study of craving and relapse in animal models. Addiction. 2000;95(Suppl 2):S55–S60. doi: 10.1080/09652140050111645. [DOI] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology. 2007;32:1967–1973. doi: 10.1038/sj.npp.1301323. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Mack AL. (+/−)-SM 21 attenuates the convulsive and locomotor stimulatory effects of cocaine in mice. Eur J Pharmacol. 2001;417:R1–R2. doi: 10.1016/s0014-2999(01)00891-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, McCracken KA, Pouw B, Zhang Y, Bowen WD. Involvement of sigma receptors in the behavioral effects of cocaine: evidence from novel ligands and antisense oligo-deoxynucleotides. Neuropharmacology. 2002;42:1043–1055. doi: 10.1016/s0028-3908(02)00056-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Pouw B, Mack AL, Daniels A, Coop A. Effects of UMB24 and (+/−)-SM 21, putative sigma2-preferring antagonists, on behavioral toxic and stimulant effects of cocaine in mice. Pharmacol Biochem Behav. 2007;86:86–91. doi: 10.1016/j.pbb.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T. Neurosteroids and sigma1 receptors, biochemical and behavioral relevance. Pharmacopsychiatry. 2004;37(Suppl 3):S171–S182. doi: 10.1055/s-2004-832675. [DOI] [PubMed] [Google Scholar]
- Maurice T, Romieu P. Involvement of the sigma1 receptor in the appetitive effects of cocaine. Pharmacopsychiatry. 2004;37(Suppl 3):S198–S207. doi: 10.1055/s-2004-832678. [DOI] [PubMed] [Google Scholar]
- Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR. Sigma (1) (sigma(1)) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci Biobehav Rev. 2002;26:499–527. doi: 10.1016/s0149-7634(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Maurice T, Casalino M, Lacroix M, Romieu P. Involvement of the sigma 1 receptor in the motivational effects of ethanol in mice. Pharmacol Biochem Behav. 2003;74:869–876. doi: 10.1016/s0091-3057(03)00002-9. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Le AD, Noronha A. Central nervous system mechanisms in alcohol relapse. Alcohol Clin Exp Res. 2002;26:280–286. [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Menkel M, Terry P, Pontecorvo M, Katz JL, Witkin JM. Selective sigma ligands block stimulant effects of cocaine. Eur J Pharmacol. 1991;201:251–252. doi: 10.1016/0014-2999(91)90355-t. [DOI] [PubMed] [Google Scholar]
- Minabe Y, Matsuno K, Ashby CR., Jr Acute and chronic administration of the selective sigma1 receptor agonist SA4503 significantly alters the activity of midbrain dopamine neurons in rats: an in vivo electrophysiological study. Synapse. 1999;33:129–140. doi: 10.1002/(SICI)1098-2396(199908)33:2<129::AID-SYN3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Miyatake R, Furukawa A, Matsushita S, Higuchi S, Suwaki H. Functional polymorphisms in the sigma1 receptor gene associated with alcoholism. Biol Psychiatry. 2004;55:85–90. doi: 10.1016/j.biopsych.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Moebius FF, Burrows GG, Hanner M, Schmid E, Striessnig J, Glossmann H. Identification of a 27-kDa high affinity phenylalkylamine-binding polypeptide as the sigma 1 binding site by photoaffinity labeling and ligand-directed antibodies. Mol Pharmacol. 1993;44:966–971. [PubMed] [Google Scholar]
- Nuwayhid SJ, Werling LL. Sigma(2) (sigma(2)) receptors as a target for cocaine action in the rat striatum. Eur J Pharmacol. 2006 doi: 10.1016/j.ejphar.2005.12.077. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Kampov-Polevoy AB, Rezvani AH, Braun C, Bartus RT, Crews FT. Suppression of alcohol intake by chronic naloxone treatment in P rats: tolerance development and elevation of opiate receptor binding. Alcohol Clin Exp Res. 1999;23:1761–1771. [PubMed] [Google Scholar]
- Parkes H, Sinclair JD. Reduction of alcohol drinking and upregulation of opioid receptors by oral naltrexone in AA rats. Alcohol. 2000;21:215–221. doi: 10.1016/s0741-8329(00)00091-4. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Quirion R, Hammer RP, Jr, Herkenham M, Pert CB. Phencyclidine (angel dust)/sigma “opiate” receptor: visualization by tritium-sensitive film. Proc Natl Acad Sic USA. 1981;78:5881–5885. doi: 10.1073/pnas.78.9.5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu P, Martin-Fardon R, Maurice T. Involvement of the sigma1 receptor in the cocaine-induced conditioned place preference. Neuroreport. 2000;11:2885–2888. doi: 10.1097/00001756-200009110-00011. [DOI] [PubMed] [Google Scholar]
- Romieu P, Meunier J, Garcia D, Zozime N, Martin-Fardon R, Bowen WD, Maurice T. The sigma1 (sigma1) receptor activation is a key step for the reactivation of cocaine conditioned place preference by drug priming. Psychopharmacology (Berl) 2004;175:154–162. doi: 10.1007/s00213-004-1814-x. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Zhao Y, Iyer MR, Steardo LJ, Steardo L, Rice KC, Conti B, Koob GF, Zorrilla EP. The sigma-receptor antagonist BD-1063 decreases ethanol intake and reinforcement in animal models of excessive drinking. Neuropsychopharmacology. 2009;34(6):1482–1493. doi: 10.1038/npp.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Arroyos R, Guitart X. Electrophysiological effects of E-5842, a sigma1 receptor ligand and potential atypical antipsychotic, on A9 and A10 dopamine neurons. Eur J Pharmacol. 1999;378:31–37. doi: 10.1016/s0014-2999(99)00440-9. [DOI] [PubMed] [Google Scholar]
- Serra S, Brunetti G, Vacca G, Lobina C, Carai MA, Gessa GL, Colombo G. Stable preference for high ethanol concentrations after ethanol deprivation in Sardinian alcohol-preferring (sP) rats. Alcohol. 2003;29:101–108. doi: 10.1016/s0741-8329(03)00003-x. [DOI] [PubMed] [Google Scholar]
- Sigvardsson S, Bohman M, Cloninger CR. Replication of the Stockholm Adoption Study of alcoholism. Confirmatory cross-fostering analysis. Arch Gen Psychiatry. 1996;53:681–687. doi: 10.1001/archpsyc.1996.01830080033007. [DOI] [PubMed] [Google Scholar]
- Steinfels GF, Tam SW. Selective sigma receptor agonist and antagonist affect dopamine neuronal activity. Eur J Pharmacol. 1989;163:167–170. doi: 10.1016/0014-2999(89)90413-5. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Shirasaki T, Kaku S, Muramatsu M, Otomo S. Characteristics of binding of [3H]NE-100, a novel sigma-receptor ligand, to guinea-pig brain membranes. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:244–251. doi: 10.1007/BF00233243. [DOI] [PubMed] [Google Scholar]
- Ueda H, Yoshida A, Tokuyama S, Mizuno K, Maruo J, Matsuno K, Mita S. Neurosteroids stimulate G protein-coupled sigma receptors in mouse brain synaptic membrane. Neurosci Res. 2001;41:33–40. doi: 10.1016/s0168-0102(01)00258-9. [DOI] [PubMed] [Google Scholar]
- Ujike H, Kanzaki A, Okumura K, Akiyama K, Otsuki S. Sigma (sigma) antagonist BMY 14802 prevents methamphetamine-induced sensitization. Life Sic. 1992;50:PL129–PL134. doi: 10.1016/0024-3205(92)90466-3. [DOI] [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Walker FO, Matsumoto RR, De CB, Rice KC. Sigma receptors: biology and function. Pharmacol Rev. 1990;42:355–402. [PubMed] [Google Scholar]
- Weatherspoon JK, Werling LL. Modulation of amphetamine-stimulated [3H]dopamine release from rat pheochromocytoma (PC12) cells by sigma type 2 receptors. J Pharmacol Exp Ther. 1999;289:278–284. [PubMed] [Google Scholar]
- Weiser SD, Patrick SL, Mascarella SW, Downing-Park J, Bai X, Carroll FI, Walker JM, Patrick RL. Stimulation of rat striatal tyrosine hydroxylase activity following intranigral administration of sigma receptor ligands. Eur J Pharmacol. 1995;275:1–7. doi: 10.1016/0014-2999(94)00718-m. [DOI] [PubMed] [Google Scholar]