Abstract
Clinical studies link disruption of the neuroendocrine stress system with alcoholism, but remaining unknown is whether functional differences in the hypothalamic-pituitary-adrenal (HPA) axis precede alcohol abuse and dependence or result from chronic exposure to this drug. Using an operant self-administration animal model of alcohol dependence and serial blood sampling, we show that long-term exposure to alcohol causes significant impairment of HPA function in adult male Wistar rats. Acute alcohol (voluntary self-administration or experimenter-administered) stimulated the release of corticosterone and its upstream regulator, adrenocorticotropic hormone, but chronic exposure sufficient to produce dependence led to a dampened neuroendocrine state. HPA responses to alcohol were most robust in ‘low-responding’ non-dependent animals (averaging < 0.2 mg/kg/session), intermediate in non-dependent animals (averaging ~0.4 mg/kg/session), and most blunted in dependent animals (averaging ~1.0 mg/kg/session) following several weeks of daily 30-min self-administration sessions, suggesting that neuroendocrine tolerance can be initiated prior to dependence and relates to the amount of alcohol consumed. Decreased expression of corticotropin-releasing factor (CRF) mRNA expression in the paraventricular nucleus of the hypothalamus and reduced sensitivity of the pituitary to CRF may contribute to, but do not completely explain, neuroendocrine tolerance. The present results, combined with previous studies, suggest that multiple adaptations to stress regulatory systems may be brought about by excessive drinking, including a compromised hormonal response and a sensitized brain stress response that together contribute to dependence.
Keywords: ACTH, alcohol, corticosterone, corticotropin-releasing factor mRNA, self-administration, withdrawal
Introduction
Alcoholism is a chronically relapsing disorder characterized by cycles of repeated high alcohol intake and negative emotional consequences of withdrawal thought to contribute to excessive drinking and susceptibility to relapse (Koob, 2003; Breese et al., 2005; Heilig & Egli, 2006). Similar to human alcoholics, animals made ‘dependent’ using chronic exposure to high alcohol exhibit uncontrolled excessive intake of alcohol during periods of withdrawal and enhanced anxiety-like behaviors (Roberts et al., 1996; Overstreet et al., 2002; Rimondini et al., 2002; Valdez et al., 2002; O’Dell et al., 2004; Koob, 2006). Identifying the effect of alcohol on the nervous system and neuroadaptive changes emerging during the progression of alcohol dependence in rodent models will help elucidate the pathology of alcoholism in humans.
Stress and its related hormones of the hypothalamic-pituitary-adrenal (HPA) axis are implicated in alcohol abuse and dependence (Wand & Dobs, 1991; Froehlich et al., 2003; Koob & Kreek, 2007). Clinical studies link disruption of the HPA axis with alcoholism, including a dampened ability to cope with stress, and negative correlations between cortisol and craving and relapse in alcoholics (Lovallo et al., 2000; O’Malley et al., 2002; Kiefer & Wiedemann, 2004; Adinoff et al., 2005). In rodents, experimenter-administered alcohol stimulates the HPA axis, and the response is significantly blunted by chronic alcohol exposure (Lee & Rivier, 1997). However, investigation of the relationship between voluntary alcohol intake and HPA function is limited. Most animal studies exploring the behavioral components underlying alcohol drinking in self-administering animal models of dependence have not thoroughly and properly explored questions about the HPA axis, largely due to the technical challenges of combining stress-sensitive neuroendocrine and behavioral studies.
To test the hypothesis that the effects of voluntary alcohol administration on the HPA axis vary with alcohol dependence, neuroendocrine measures of HPA activity were determined using serial blood sampling in a well-established operant self-administration animal model of alcohol dependence (O’Dell et al., 2004; Funk et al., 2006; Walker & Koob, 2007; Gilpin et al., 2008b; Richardson et al., 2008; for related models, see Roberts et al., 1996; Overstreet et al., 2002; Rimondini et al., 2002; Valdez et al., 2002). In this model, animals trained to self-administer alcohol and exposed for several weeks to bouts of high alcohol (e.g. vapors) exhibit an alcohol-dependence syndrome characterized by somatic and motivational withdrawal symptoms and engage in excessive drinking when alcohol is made available again (‘dependent animals’). Animals trained to self-administer alcohol but exposed for several weeks to control air do not exhibit the dependence syndrome and continue to respond for alcohol at baseline levels when alcohol is made available again (‘non-dependent’ animals). The present study focused on a particular period of acute withdrawal (6–8 h after removal from chronic alcohol). This is a well-studied period of withdrawal when dependent animals display mild signs of physical withdrawal (Roberts et al., 1996) but robust signs of motivational withdrawal: (i) increased expression of anxiety-like behavior (Baldwin et al., 1991; Rassnick et al., 1993); (ii) elevations in brain reward thresholds (‘reward deficits’; Schulteis et al., 1995); (iii) increased extracellular levels of the stress peptide corticotropin-releasing factor (CRF) in the central nucleus of the amygdala (CeA; Merlo Pich et al., 1995) and lateral bed nucleus of the stria terminalis (BNST; Olive et al., 2002); and (iv) excessive drinking that is sensitive to blockade by CRF1 receptor antagonists (Gilpin et al., 2008a; Richardson et al., 2008). Accordingly, the goal of the present work was to ascertain the neuroendocrine profile of dependent animals at this time to establish the role the HPA axis may play in dependence. We show evidence for acute activation of the HPA axis by alcohol and demonstrate that functional changes in the HPA axis and neuroadaptive changes in CRF gene expression within the hypothalamus can be brought on with chronic exposure to alcohol sufficient to produce dependence.
Materials and methods
Animals
Adult male Wistar rats weighing 150–225 g at the start of the experiment were obtained from Charles River Laboratory (Kingston, NY, USA). Animals weighed ~550–650 g by the end of each experiment (4–6 months later), and body weight between treatment groups was not significantly different (data not shown). Rats were housed two or three per cage with food and water available ad libitum. Lights were on a 12 h light: dark cycle, with lights on at 06.00 h (Experiments 1, 3 and 4) or 20.00 h (Experiment 2). Because of the extensive length and technical challenges associated with these studies, animals were occasionally excluded during the course of the experiment (~25% attrition rate, which did not vary across treatment groups) due to a variety of reasons (e.g. training/baseline criteria, complications during or following surgery or during dependence induction, patency problems, collection and/or processing of plasma samples or brain tissue, etc.). Thus, for simplicity, animal numbers and descriptions below include only those animals that completed the entire experiment. All animals were allowed 4–7 days of acclimation to the laboratory and were frequently handled prior to the start of all experiments. All procedures met the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee at The Scripps Research Institute.
Experimental design and animal model of alcohol dependence
In addition to the animals described below for Experiments 1–4, a group of untrained animals was used to characterize physical signs of withdrawal and blood alcohol levels following several weeks of exposure to alcohol vapors (n = 8) vs. control air (n = 8). Unless stated otherwise, all other animals were processed through the self-administration training, maintenance, dependence induction and operant testing procedures described below in detail and in Fig. 1 before being assigned to separate groups in Experiments 1–4. All behavioral, endocrine and molecular measures in Experiments 1–4 occurred at a time when dependent animals were 6–8 h into withdrawal from alcohol vapors (during the afternoon of the light cycle for Experiments 1, 3 and 4; ~4 h into the dark cycle for Experiment 2).
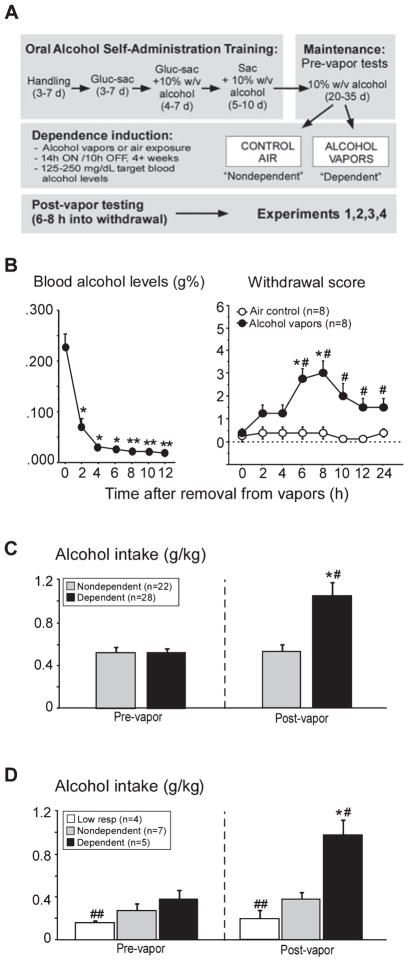
Fig. 1.
Design of the alcohol dependence model (A), blood alcohol levels (left graph), and physical withdrawal signs (right graph) following removal from chronic intermittent exposure to alcohol vapors or air control (B), and operant self-administration data summarizing enhanced responding for alcohol in dependent animals following dependence induction (C). Adult male Wistar rats were trained using a sweetened-solution fading paradigm to orally self-administer alcohol (10% w/v ethanol, alcohol) or water. Following maintenance on 10% w/v alcohol for 20–30 days, stable responders were evenly divided into two groups and exposed to intermittent alcohol vapors, 14 h on/10 h off (‘dependent’) or control air (‘non-dependent’). After 4 weeks of dependence induction, animals were tested at least twice before being assigned to Experiments 1–4. After removal from vapors, blood alcohol levels dropped rapidly, reaching undetectable levels within 4–6 h (*P < 0.001, compared with 0 h; **P < 0.001, blood alcohol levels compared with 2 h; B, left graph). Mild physical withdrawal began to rise 2 h into withdrawal, peaked from 6 to 8 h, and descended thereafter (*P < 0.002, compared with 0 h; #P < 0.01, compared with air control group; B, right graph). Operant data from all trained animals of the present study are shown in (C) (Experiments 1, 3 and 4, light cycle) and (D) (Experiment 2, dark cycle). Post-vapor testing was done when dependent animals were 6–8 h into withdrawal. Bars in (C) represent mean alcohol intake (g/kg) in dependent animals (black bars, n = 22) tested before (‘pre-vapor’) and after (‘post-vapor’) dependence induction compared with non-dependent animals (gray bars, n = 28) tested at these same times (data from Experiments 1, 3 and 4 combined). Bars in (D) represent mean alcohol intake (g/kg) in dependent animals (black bars, n = 5) tested before (‘pre-vapor’) and after (‘post-vapor’) dependence induction compared with non-dependent (gray bars, n = 7) and low-responding non-dependent (white bars, n = 4) controls tested at these same times (Experiment 2). Enhanced responding for alcohol was evident in dependent animals compared with non-dependent controls following chronic intermittent alcohol vapor. Post hoc analysis indicated that post-vapor responding for alcohol was higher in dependent animals (*P < 0.0001, compared with pre-vapor responding; #P < 0.001, compared with non-dependent controls during post-vapor testing; C and D). Low-responding animals had reduced alcohol intake compared with non-dependent and dependent animals pre-vapor (##P < 0.01) and compared with dependent animals post-vapor (##P < 0.01). To convert blood alcohol levels from g% (g/dL) to mM, divide values by 0.0046 (e.g. 0.200 g% is 43.5 mM). Data are expressed as mean ± SEM.
Experiment 1: effect of voluntary alcohol self-administration on the HPA axis and blood alcohol levels in non-dependent and dependent animals
The goals of this experiment were to determine whether: (i) voluntary operant self-administration of alcohol stimulated the HPA axis at the level of corticosterone and its upstream regulator, adrenocorticotropic hormone (ACTH); (ii) whether HPA hormone levels were blunted under basal conditions in dependent animals; (iii) whether self-administration of alcohol elevated ACTH and corticosterone to levels observed in non-dependent animals. Fifteen trained animals (n = 7 non-dependent, n = 8 dependent) were fitted with jugular catheters, and blood samples were measured before, during and after a 30-min operant self-administration session in which animals were allowed to respond for alcohol and water. Blood alcohol levels, ACTH and corticosterone levels were assessed at 0, 15, 30 and 45 min time-points. Details of all procedures are described below.
Experiment 2: effect of 1 g/kg alcohol on the HPA axis and blood alcohol levels in low-responding non-dependent, non-dependent and dependent animals
This experiment sought to characterize HPA responses to a controlled dose of alcohol (1 g/kg) in animals self-administrating alcohol chronically but consuming different levels. Alcohol self-administration elicited a mild increase in ACTH and corticosterone (Experiment 1) compared with the robust HPA responses observed in alcohol-naive animals following an experimenter-administered alcohol challenge (e.g. Lee & Rivier, 1997; Lee et al., 2000), suggesting that some degree of HPA downregulation may occur following chronic daily use of alcohol, even under non-dependent conditions. Thus, the primary goal of Experiment 2 was to measure ACTH and corticosterone responses to alcohol in three groups of trained animals: (i) dependent animals (n = 5); (ii) non-dependent animals (n = 7); and (iii) ‘low-responding’ non-dependent animals (n = 4). The low-responding group consisted of non-dependent animals consuming low levels of alcohol (averaging <10 responses for alcohol or 0.2 g/kg per session throughout training). Low-responding animals are normally eliminated from our studies during baseline training because of limited space and resources but nonetheless show the same relative susceptibility to dependence induction (doubling alcohol intake) as ‘normal’ non-dependent animals (N.W. Gilpin, A. Smith, M. Cole, F. Weiss, G.F. Koob and H.N. Richardson, unpublished results). An alcohol dose of 1 g/kg (i.v.) was chosen for the alcohol challenge because it reflected the amount voluntarily consumed by dependent animals during self-administration sessions. Animals were fitted with jugular catheters, and ACTH and corticosterone were serially measured before (0 min) and after (15, 45 and 75 min) the alcohol injection. The timing of withdrawal (6–8 h) was shifted to occur during the active (dark) phase of the animals’ light: dark cycle, allowing us to determine whether dampened HPA function in dependent animals persisted beyond the afternoon of the light cycle. Details of all procedures are described below.
Experiment 3: effect of CRF administration on the HPA axis in alcohol-naive, non-dependent and dependent animals
We next established whether reduced HPA axis activity associated with dependence was of peripheral or central origin by testing dependent animals for decreased responsiveness of the pituitary gland to CRF. ACTH responses were measured following administration of rat/human CRF (r/hCRF). A dose–response curve was first conducted in alcohol-naive animals to determine the dose of r/hCRF that elicits a mid-range ACTH response; this same dose was then tested in non-dependent and dependent animals. Thus, 24 alcohol-naive (n = 6, for each of four r/hCRF doses: 3.0, 1.0, 0.3 or 0.1 μg/kg) and nine trained animals (non-dependent, n = 5; dependent, n = 4) were fitted with jugular catheters, blood samples were obtained before (0 min) and after (15, 30 and 45 min) an injection of r/hCRF, and ACTH levels were measured. Details of all procedures are described below.
Experiment 4: CRF mRNA in the paraventricular nucleus of the hypothalamus (PVN) in alcohol-naive, non-dependent and dependent animals
The final experiment determined whether decreased function of the HPA axis associated with long-term alcohol exposure and dependence was associated with alterations in CRF expression in the hypothalamic CRF system. CRF mRNA levels were measured using in situ hybridization histochemistry in the PVN in alcohol-naive (n = 6), non-dependent (n = 10) and dependent (n = 13) animals. Details of all procedures are described below.
Operant self-administration apparatus
The self-administration system consisted of operant boxes (Coulbourn Instruments, Allentown, PA, USA) contained within wooden sound-attenuated ventilated cubicles. The operant boxes were equipped with two retractable levers located 4 cm above the grid floor and 4.5 cm to either side of a small stainless steel receptacle containing two drinking cups. Two infusion pumps (Razel Scientific Instruments, Stamford, CT, USA) were connected to the system so that a lever press resulted in delivery of 0.1 mL of solution. Tap water was delivered to one dish, and the experimental solution (e.g. sweetened solution or alcohol) was delivered to the other dish. Fluid delivery and recording of operant self-administration were controlled by a microcomputer. Lever presses were not recorded during the 0.5 s inter-response time-out interval when solution was being delivered.
Operant self-administration procedure
Animals were trained to orally self-administer alcohol or water in a concurrent, two-lever, free-choice contingency. A continuous reinforcement (fixed ratio-1, FR1) schedule was used in which each lever press was reinforced. Animals acquired alcohol self-administration using a variation of the previously described saccharin fading free-choice operant conditioning protocol (Samson, 1986). The present procedure culminates in pharmacologically relevant levels of alcohol self-administration, defined by blood alcohol levels, in non-dependent animals with limited access to alcohol over a 6-week period (Roberts et al., 1999). The modified procedure in the present study utilized a sweetened solution containing 3% glucose and 0.125% saccharin (Sigma, St Louis, MO, USA) instead of water restriction, and 0.2% saccharin to initiate and maintain operant responding (Funk et al., 2006). Animals respond for this solution within just one–two training sessions, thereby circumventing the need for water restriction to initiate lever pressing. Operant sessions during training and post-vapor testing were conducted 5 days per week between 09.00 h and 15.00 h (lights on at 06.00 h for Experiments 1, 3 and 4, and lights off at 08.00 h for Experiment 2). Operant sessions were 30 min in duration, except during the initial days of training in which sessions lasted up to 2 h to permit acquisition of responding for the sweetened solution. Alcohol (10% w/v) then was added to the sweetened solution and, once mean responding stabilized (about 1 week), the glucose was removed from the solution, leaving only 0.125% saccharin and 10% w/v alcohol. Animals were kept at this stage until mean responding again stabilized (about 1 week), and saccharin concentrations were gradually reduced in ~50% successive steps over 2–10 days, ultimately leaving an unsweetened 10% w/v alcohol solution. Animals then were maintained on 10% w/v alcohol for at least 3 weeks, and stable responders (± 25% across three consecutive sessions and averaging > 10 presses for alcohol) were evenly divided into two groups matched for baseline responding and exposed to intermittent alcohol vapors (dependent) or air (non-dependent) as described below. As stated above, Experiment 2 included an additional group of trained animals normally excluded before groups are matched for baseline responding and split into non-dependent vs. dependent groups. Low-responding non-dependent animals that average ~10 or fewer alcohol responses (< 0.20 g/kg/30 min session) were included in this experiment for comparison with the non-dependent group that averages ~20–25 alcohol responses (~4.0 g/kg/30 min session) and the dependent group that averages ~55–70 alcohol responses (~1.0 g/kg/30 min session) 6–8 h into withdrawal from alcohol vapors (e.g. O’Dell et al., 2004; Walker & Koob, 2007; Richardson et al., 2008; see Fig. 1).
Solutions for oral self-administration
Alcohol (10% w/v) was prepared with 95% ethyl alcohol and tap water. Glucose (3%) and/or saccharin (0–0.125%; Sigma Chemical, St Louis, MO, USA) were added to water or the alcohol solutions to achieve the appropriate concentration.
Dependence induction by alcohol vapor chambers
A recent modification of the alcohol dependence model was made to reflect the natural progression of alcohol dependence in which alcohol exposure occurs in a series of extended exposures followed by periods of withdrawal (O’Dell et al., 2004). Chronic exposure to intermittent vapor exposure elicits even higher alcohol self-administration than continuous vapor (O’Dell et al., 2004); therefore, the procedure was used to induce dependence in trained animals in the present study. Vapors were delivered on an intermittent schedule (on at 18.00 h, off at 08.00 h) for a period of 4 weeks before post-vapor testing began. This schedule of exposure has been shown to induce dependence (O’Dell et al., 2004). In the chambers, 95% alcohol flows from a large reservoir to a peristaltic pump (model QG-6, FMI Laboratory; Fluid Metering, Syosett, NY, USA). Alcohol is delivered from the pump to a sidearm flask at a flow rate that can be regulated. The flask is placed on a heater so that the drops of alcohol hitting the bottom of the flask are vaporized. Airflow controlled by a pressure gage is delivered to the flask and carries the alcohol vapors to the vapor chamber that contains the animal cages. The flow rate was set between 22 and 27 mg/L to deliver vapors that result in blood alcohol levels between 0.125 and 0.250 g% (g/dL) or 27.2–54.4 mM, which was confirmed by weekly blood samples. Blood samples were collected by the tail-snip method (0.1–0.2 mL) from all animals (both alcohol vapor-exposed dependent and control air-exposed non-dependent groups) just after the vapors turned off (08.00 h), and 5 μL of plasma was used for measurement of blood alcohol levels, as described below. When dependent animals had blood alcohol levels outside the target range, the evaporated alcohol values were adjusted to reestablish the correct range. As expected, blood alcohol levels were always undetectable (< 0.02 g% or 4.4 mM) in non-dependent animals, but tail bleeding was performed to control for any stress experienced during this procedure.
Measurements of physical withdrawal
Previous work using a related model (chronic continuous vapor exposure) indicated that dependent animals show mild, but not severe (e.g. spontaneous tonic–clonic convulsions; Majchrowicz, 1975) signs of physical withdrawal following removal from alcohol vapors (Roberts et al., 1996). To characterize withdrawal severity in the present model, withdrawal signs were quantified in 2-h bouts over 24 h after removal from chronic intermittent alcohol vapors or control air using methodology similar to earlier reports (Macey et al., 1996; Roberts et al., 1996). Specifically, distal limb flexion response, tail stiffness and abnormal body posture were measured using a subjective 0–2 point scale to score withdrawal symptoms (0 = undetectable, 1 = moderate, 3 = severe). Ventromedial distal limb flexion responses were determined by lifting the rat up by the scruff of the neck and observing retraction of the lower legs toward the body. Tails were examined for rigidity and/or curvature upward toward the back. The presence of a broad-based stance and/or abnormal gait indicated abnormal body posture. Statistical analyses were conducted on overall withdrawal severity scores, that were determined for each animal by summing the scores for the three signs, yielding a range from 0 to 6.
Intravenous catheterization surgery
Animals used in blood sampling experiments were anesthetized by isoflurane inhalation, and intravenous catheters were aseptically inserted in the right jugular vein using a modified version of a procedure previously described (Caine et al., 1993). The catheter assembly consisted of a 14-cm length of silastic tubing (0.025 inch inner diameter, 0.047 inch outer diameter; Dow Corning, Midland, MI, USA) that was attached to a guide cannula (Plastics One, Roanoke, VA, USA) bent at a near right angle and embedded in dental acrylic anchored with a 2 × 2 cm square of mesh. The catheter was fed subcutaneously from where it was secured in the jugular vein to the animal’s back. It exited through a small incision on the back, and the base was sealed with a small plastic cap and metal cover cap. This design helps keep the catheter base sterile and protected from cage mates (animals were housed in groups of two–three). Catheters were flushed with heparinized saline (10 U/mL of heparin sodium; American Pharmaceutical Partners, Schaumburg, IL, USA; in 0.9% bacteriostatic sodium chloride; Hospira, Lake Forest, IL, USA) containing antibiotic (20 mg/0.2 mL; Timetin, GlaxoSmithKline; once daily for 1 week, then once every 7–14 days) and heparinized saline afterward until the end of the experiment.
Blood sampling: alcohol challenge, self-administration and CRF challenge experiments
Blood sampling experiments were conducted in the following manner. In Experiments 1, 3 and 4, hormone levels were measured during the afternoon of the light phase of the light: dark cycle (lights on at 06.00 h and off at 18.00 h) when dependent animals were 6–8 h into withdrawal (~14.00–16.00 h) and blood alcohol levels were back at baseline (preliminary work; see also Fig. 1B). In Experiment 2, the animals were housed in a reverse light cycle (lights on at 20.00 h and off at 08.00 h) that allowed for analysis of HPA hormones during the dark phase while maintaining the same withdrawal period as Experiments 1–3 (6–8 h after removal from alcohol vapors for dependent animals). To minimize the effects of extraneous stress on stress-sensitive hormones, animals were allowed at least 1 week of recovery following surgery and were sampled under basal conditions (see below). In the self-administration blood sampling experiment (Experiment 1) following surgical implantation of jugular catheters and post-surgery recovery, animals were tested every 2–4 days in the operant chambers while connected to tethers and sampling tubing until baseline responding stabilized. This ensured that on the day of the experiment, animals were fully habituated to the sampling apparatus, and the experimental procedures would not interfere with operant responding, that is highly sensitive to environmental changes.
On the day of sampling, catheters were connected to tubing attached to a syringe containing heparinized saline. Rats were placed in individual buckets (alcohol and CRF challenge experiments) or operant boxes (self-administration experiment) where they remained awake and freely moving, allowing for collection of blood without disturbing the animals. Animals rested for 3 h after connection to allow stress-responsive hormones to return to basal levels (C. Rivier, unpublished results) before serial sampling began. Blood samples were taken via the catheter (0.3 mL each sample), and fluid was replaced with heparinized saline. In the self-administration behavior/sampling experiment (Experiment 1), the first sample (0 min, basal) was followed by presentation of the operant levers, two samples were taken during self-administration (15 and 30 min), and a final sample was taken 15 min after retraction of the levers (45 min). In the alcohol challenge experiment (Experiment 2), the first sample (basal) was followed by i.v. administration of alcohol (1 g/kg of 20% w/v alcohol diluted in heparinized saline), and three samples were taken thereafter (15, 45 and 75 min). During the injection, alcohol was delivered in small pulses evenly distributed over the course of 5 min to approximate the blood alcohol levels observed during self-administration of alcohol in the previous experiment. In the CRF challenge experiment (Experiment 3), the first sample (basal) was followed by i.v. administration of r/hCRF, and three subsequent blood samples were taken (15, 30 and 45 min). Blood was collected into 1.5-mL Eppendorf tubes on ice containing 10 μL EDTA and capped immediately to avoid evaporation of alcohol in the blood. Samples were spun at 3000 rpm for 15 min, and plasma was collected and stored at −80°C until ACTH, corticosterone and blood alcohol level assays were performed (described below).
Reagent
r/hCRF was synthesized by solid phase methodology and generously provided by Dr Jean Rivier (The Salk Institute, La Jolla, CA, USA; Kornreich et al., 1992). It was dissolved in 0.04 M phosphate-buffered saline, pH 7.4, containing 0.1% bovine serum albumin and 0.01% ascorbic acid.
Measurement of ACTH levels
Plasma ACTH levels were determined using a commercially available two-site immunoradiometric assay (IRMA) kit (Allegro kit; Nichols Institute, San Juan Capistrano, CA, USA). IRMA was selected for measurement of plasma ACTH levels because it uses two monoclonal antibodies to measure ACTH1–39 in plasma, resulting in cross-reactivity < 0.5% with ACTH fragments (Gibson et al., 1989). Data are expressed in pg/mL plasma, and the lowest limit of detectability was 5 pg/mL or 1.1 pM. This assay has been validated for rats (Rivier & Shen, 1994) and has intra- and interassay coefficients of variation of 3.2% and 6.8%, respectively.
Measurement of corticosterone levels
Total plasma corticosterone levels were determined using a commercially available radioimmunoassay kit (MP Biomedicals, Orangeburg, NY, USA). Data are expressed in ng/mL plasma, and the lowest limit of detectability was 5 ng/mL or 14.4 nM. Intra- and interassay coefficients of variation are 7.3% and 13.2%, respectively.
Measurement of blood alcohol levels
Plasma (5 μL) was used for measurement of blood alcohol levels using an Analox AM 1 analyzer (Analox Instruments, Lunenburg, MA, USA). The reaction is based on the oxidation of alcohol by alcohol oxidase in the presence of molecular oxygen (alcohol + O2 → acetaldehyde + H2O2). The rate of oxygen consumption is directly proportional to the alcohol concentration. Single-point calibrations were done for each set of samples with reagents provided by Analox Instruments (0.025–0.400 g% or 5.4–87.0 mM).
Intracardial perfusion and brain tissue preparation for in situ hybridization histochemistry
Brain tissue collection was timed to correspond to the period when animals would normally be tested for self-administration to best represent the neural state under which animals were motivated to lever press for alcohol. Thus, 2–4 days after the last 30-min self-administration session and at a time when dependent animals were 6–8 h into withdrawal from vapors, dependent, non-dependent and alcohol-naive control rats (matched for age, body weight and handling) were deeply anesthetized with 35% chloral hydrate, a drug that does not increase immediate-early gene RNA levels or ACTH concentrations (C. L. Rivier, unpublished results). Tissue collection and processing procedures were similar to those described previously (Potter et al., 1994). Briefly, animals were perfused intracardially with saline followed by cold 4% paraformaldehyde/0.1 M borate buffer, pH 9.5. The brains were removed, post-fixed in 4% paraformaldehyde for 4–5 days, and placed overnight in 10% sucrose/4% paraformaldehyde/0.1 M borate buffer. Brains were sectioned into 30-μm coronal slices obtained at 120-μm intervals and stored at −20°C in a cryoprotectant solution (50% 0.1 M phosphate-buffered saline, 30% ethylene glycol and 20% glycerol) until in situ hybridization histochemistry.
Synthesis and preparation of probe for in situ hybridization histochemistry
All solutions were treated with diethylpyrocarbonate (DepC) and autoclaved to prevent degradation of RNA. The pGEM4 plasmid containing a 1.2-kb EcoRI fragment of rat CRF cDNA (kindly provided by Dr K. Mayo) was linearized with HindIII. The antisense probe was transcribed in a reaction mixture containing 250 ng of linearized plasmid in 6 mM MgCl2, 36 mM Tris (pH 7.5), 2 mM spermidine, 8 mM dithiothreitol, 25 mM ATP/GTP/CTP, 5 μM of γ35S-UTP, 1 U of RNasin (Promega, Madison, WI, USA) and 10 U of SP6 for 60 min at 37°C. Unincorporated nucleotides were removed using Quick-Spin columns (Boehringer Mannheim, Indianapolis, IN, USA). A sense probe was used as a control for non-specific signals in some adjacent sections for in situ hybridization.
In situ hybridization histochemistry and microscopic analysis
Hybridization labeling of CRF-expressing cells was performed using 35S-labeled CRF cRNA probe. Protocols for riboprobe synthesis, hybridization and autoradiographic localization of mRNA signals were adapted from Simmons et al. (1989) and Potter et al. (1994). Brain paste standards containing serial dilutions of 35S-UTP, used for quantification of mRNA signal, were prepared concurrently to ensure that optical density was found within the linear range of the standard curve. Brain tissue was processed in two hybridization runs, and tissue from dependent and non-dependent rats was included in both runs for CRF mRNA levels. Transcript expression did not differ across runs, and animals were combined for data analyses. All solutions were treated with DepC and sterilized to prevent RNA degradation. Sections mounted onto gelatin- and poly-L-lysine-coated slides were desiccated under vacuum overnight, fixed in 4% paraformaldehyde for 30 min and digested by proteinase K (10 μg/mL in 50 mM Tris–HCl, pH 7.5, and 5 mM EDTA at 37°C for 25 min). Slides were then rinsed in sterile DepC water followed by a solution of 0.1 M triethanolamine (TEA), pH 8.0, acetylated in 0.25% acetic anhydride in 0.1 M TEA and dehydrated via graded concentrations of alcohol (50, 70, 95 and 100%). After vacuum drying for a minimum of 2 h, 90 μL of hybridization mixture (1 × 107 cpm/mL) was spotted on each slide, sealed under a coverslip and incubated at 60°C overnight in a slide warmer. Coverslips were then removed, and the slides were rinsed in 4× standard sodium citrate (SSC) (1× SSC: 0.15 M NaCl, 15 mM trisodium citrate buffer, pH 7.0) at room temperature. Sections were digested by RNase A (20 μg/mL, 37°C, 30 min), rinsed in descending concentrations of SSC (2×, 1×, 0.5× SSC), washed in 0.1× SSC for 30 min at 65°C, and dehydrated via graded concentrations of alcohol. Slides were then vacuum-dried and exposed at 4°C to X-ray film (Eastman Kodak, Rochester, NY, USA) for 24–48 h, defatted in xylene and dipped in NTB2 nuclear emulsion (Kodak; diluted 1: 1 with distilled water). Slides were exposed for 7–10 days, developed in D19 developer (Kodak) for 3.5 min at 15°C, and fixed in rapid fixer (Kodak) for 6 min. Thereafter, slides were rinsed in running distilled water, counterstained with thionin (0.25%), dehydrated via graded concentrations of alcohol, cleared in xylenes and coverslipped with DPX.
Semi-quantitative densitometric microscopic analyses of CRF mRNA were conducted on silver grain-developed emulsion-dipped slides using darkfield microscopy. All microscopic analyses were obtained using a Zeiss Axiophot photomicroscope fitted with a Zeiss ZVS video camera in which images were captured under darkfield illumination at 100× magnification. Captured sections were analyzed for optical density (OD, arbitrary units) by measuring silver grain intensity over the area of interest and subtracting background silver grain intensity using NIH Scion Image 4.03 software. CRF mRNA signal was measured from both sides of the PVN (parvocellular and magnocellular divisions, −1.4 to −2.12 mm from bregma). Three–four matched sections were used to determine mean values for each animal.
Data presentation and statistical analyses
Operant behavior averages presented in Fig. 1 and Table 1 were obtained from three–six operant tests prior to and two–eight operant tests following dependence induction by chronic exposure to intermittent alcohol vapors (dependent animals) or air control (non-dependent animals) from Experiments 1–4. Data collected from operant sessions during the light phase (Experiments 1, 3 and 4) were analyzed separately from data collected from operant sessions during the dark phase (Experiment 2) of the light: dark cycle. All behavioral (operant responding and withdrawal signs), plasma blood alcohol, ACTH and corticosterone levels, and brain transcript data were analyzed using analyses of variance ANOVAs). Because animals were allowed 3 h of rest after hook-up before the sample was obtained, separate analyses of ACTH and corticosterone levels at the 0 min time-point were performed as an index of basal HPA activity. Simple regression analyses were used to establish the relationship between total alcohol consumption, blood alcohol levels and HPA responses. Unless stated otherwise, all analyses were followed by Bonferroni–Dunn post hoc tests, and P < 0.05 was considered statistically significant. To allow comparisons with in vitro studies (Little, 2003), conversion constants are provided for blood alcohol levels and ACTH and corticosterone levels in the figure legends.
Table 1.
Mean operant responding for water (± SEM) during the light or dark phase prior to (‘pre-vapor’) and following (‘post-vapor’) dependence induction by alcohol vapors in dependent animals compared with non-dependent control animals
| Operant testing | Group | Pre-vapor responses for water | Post-vapor responses for water |
|---|---|---|---|
| Experiments 1, 3 and 4 (light cycle) | Non-dependent (n = 22) | 14.85 ± 3.52 | 11.59 ± 3.00 |
| Dependent (n = 28) | 10.61 ± 2.02 | 10.73 ± 2.56 | |
| Experiment 2 (dark cycle) | Non-dependent (n = 7) | 26.81 ± 8.18 | 28.43 ± 8.33 |
| Dependent (n = 5) | 18.13 ± 4.76 | 22.40 ± 6.96 | |
| Low-responding non-dependent (n = 4) | 32.67 ± 11.48 | 27.50 ± 12.43 |
In all experiments, operant responses for water were not different between treatment groups before or after dependence induction by alcohol vapors in dependent animals (all P > 0.05). Animals were tested when dependent animals were 6–8 h into withdrawal from alcohol vapors.
Results
Animal model of alcohol dependence
Figure 1 illustrates the design of the alcohol dependence model (Fig. 1A), blood alcohol levels and physical withdrawal signs following removal from chronic intermittent exposure to alcohol vapors or air control in untrained animals (Fig. 1B), and operant self-administration data from trained animals of Experiments 1–4, summarizing enhanced responding for alcohol in dependent animals following dependence induction by chronic intermittent exposure to alcohol vapors (Fig. 1C and D). Figure 1B (left graph) shows the drop in blood alcohol levels following removal from vapors, reaching undetectable levels by 4–6 h into withdrawal (main effect of Withdrawal Time, F6,42 = 80.57, P < 0.0001, Power = 1.00). Blood alcohol levels were significantly lower at all withdrawal times compared with 0 h, and blood alcohol levels at 8, 10 and 12 h were significantly lower compared with blood alcohol levels at 2 h (all P < 0.001; data are from alcohol vapor-exposed animals only). Figure 1B (right graph) shows that mild physical withdrawal signs were evident as early as 2 h into withdrawal, peaked from 6 to 8 h, and descended thereafter (main effect of Group, F1,98 = 25.89, P = 0.0002, Power = 1.00; and Withdrawal Time, F1,98 = 4.75, P = 0.0001, Power = 1.00; interaction between both factors, F1,98 = 4.26, P = 0.0004, Power = 0.99). Post hoc analyses indicated significant differences between air control and alcohol vapor-exposed animals at 6, 8, 10, 12 and 24 h time-points (all P < 0.01) and significant increases in withdrawal signs at 6 and 8 h compared with 0 h in dependent animals only (P < 0.00). Operant response data in Fig. 1C and D are self-administration averages (g/kg/session) occurring pre- or post-vapor (i.e. compared with dependence induction using chronic exposure to intermittent alcohol vapors in dependent animals or control air in non-dependent animals; model shown in Fig. 1A). All post-vapor testing was conducted when dependent animals were 6–8 h into withdrawal from vapors, which occurred in the light (Experiments 1, 3 and 4; Fig. 1C) or dark (Experiment 2; Fig. 1D) phase of the light: dark cycle. Main effects of Group (F1,48 = 9.45, P = 0.003, Power = 0.87) and Testing Period (F1,48 = 22.60, P < 0.0001, Power = 1.00) were observed compared with dependence induction by alcohol vapors, with an interaction between the two variables (F1,48 = 22.73, P < 0.0001, Power = 1.00) on alcohol self-administration (g/kg) when animals were tested during the light cycle (Fig. 1C). Post hoc analysis indicated that pre-vapor alcohol self-administration was not different between the groups, but post-vapor responding for alcohol was higher in dependent compared with non-dependent animals and compared with pre-vapor responding (P < 0.0001; Fig. 1C). Figure 1D shows the alcohol intake during the dark phase in Experiment 4. Main effects of Group (F2,13 = 11.95, P = 0.001, Power = 0.98) and Testing Period (F2,13 = 28.56, P < 0.0001, Power = 1.00) were observed compared with dependence induction by alcohol vapors, with an interaction between the two variables (F2,13 = 13.45, P < 0.0002, Power = 0.99) on alcohol self-administration (g/kg; Fig. 1D). Post hoc analysis indicated that low-responding non-dependent animals consumed less alcohol pre-vapor compared with both non-dependent and dependent animals (P < 0.01; Fig. 1D). Post-vapor alcohol self-administration was higher in dependent animals compared with pre-vapor responding in this group and compared with post-vapor responding in both low-responding and non-dependent animals (P < 0.01; Fig. 1D).
Self-administration of water (number of responses/session) is shown in Table 1. Water responding did not differ between non-dependent and dependent groups or with dependence induction [pre-vs. post-vapor testing in animals tested during the light (Experiments 1, 3 and 4) or dark (Experiment 2) cycle (all P > 0.05; Table 1)]. As expected, the data confirm that chronic intermittent vapor-induced increases in alcohol drinking in dependent animals were not attributable to non-specific increases in self-administration behavior.
Experiment 1: effect of voluntary alcohol self-administration on the HPA axis and blood alcohol levels in non-dependent and dependent animals
Alcohol consumption and blood alcohol levels during self-administration
Alcohol consumption (cumulative responses and total g/kg intake) and blood alcohol levels during a 30-min operant session in non-dependent and dependent animals 6–8 h into withdrawal are shown in Fig. 2A (alcohol consumption), Fig. 2B (regression plot for alcohol consumption and 30 min blood alcohol levels) and Fig. 2C (group average blood alcohol levels at 0, 15, 30 and 45 min). As expected, one-way ANOVAs indicated that dependent animals self-administered significantly more alcohol than non-dependent animals over the course of the operant session (total alcohol responses: F1,16 = 4.29, P = 0.05, Power = 0.49, Fig. 3A; total alcohol g/kg intake: F1,16 = 6.09, P = 0.02, Power = 0.64, Fig. 3A inset), while responding for water was not different between groups (non-dependent: 2.71 ± 0.18; dependent: 2.63 ± 0.31 responses; P > 0.05).
Fig. 2.
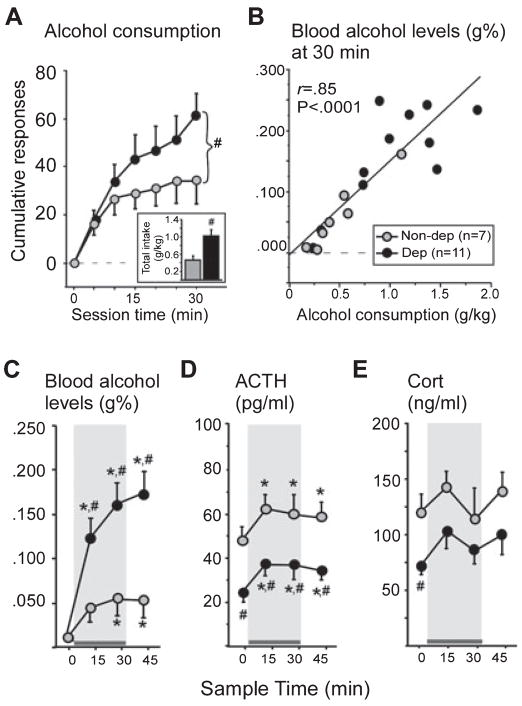
Alcohol consumption (cumulative responses, A, and total g/kg intake, inset), blood alcohol levels (blood alcohol levels, g%; simple regression scatter plot of alcohol consumption and 30 min blood alcohol levels, B; blood alcohol levels over time, C), plasma adrenocorticotropic hormone (ACTH; pg/mL, D), and plasma corticosterone (cort; ng/mL, E) responses to self-administered alcohol (10% w/v) in non-dependent (gray bar/circles, n = 7) and dependent (black bar/circles, n = 11) animals 6–8 h into withdrawal from vapors. Gray shading indicates when the levers were available in the operant chambers. Dependent animals consumed twice as much alcohol as non-dependent animals (#P = 0.01, A, inset), reflected in blood alcohol levels that were > 0.150 g% (32.6 mM) in this group (C). The amount of alcohol consumed was strongly correlated with blood alcohol levels immediately following the session (B). (C) Self-administration of alcohol significantly increased blood alcohol levels in both groups (*P < 0.05, compared with 0 min, C), but blood alcohol levels were significantly higher in dependent animals at all time-points except at 0 min, when blood alcohol levels were at baseline (#P < 0.05, compared with non-dependent animals). Self-administration of alcohol elevated ACTH in both groups of animals (*P = 0.02, compared with 0 min, D), and the degree to which ACTH was increased from basal levels was positively correlated with blood alcohol levels (15 min blood alcohol levels and percent increase in ACTH from basal levels, r = 0.52, P = 0.02, no indicator shown). Despite the increase, ACTH remained lower at all time-points in dependent animals (#P = 0.01, compared with non-dependent animals, D). Corticosterone was also lower in dependent animals at 0 min (#P < 0.02, compared with non-dependent animals, E). Self-administration of alcohol elicited a slight increase in corticosterone at 15 min, and the degree to which corticosterone increased was positively correlated with blood alcohol levels (r = 0.58, P = 0.04, no indicator shown). To convert blood alcohol levels from g% (g/dL) to mM, divide values by 0.0046 (e.g. 0.150 g% is 32.6 mM); to convert ACTH1–39 (pg/mL) to pM, divide values by 4.5410 (e.g. 50 pg/mL is 11.01 pM); to convert corticosterone (ng/mL) to nM, divide values by 0.3464 (e.g. 100 ng/mL is 288.7 nM). Data are expressed as mean ± SEM.
Fig. 3.
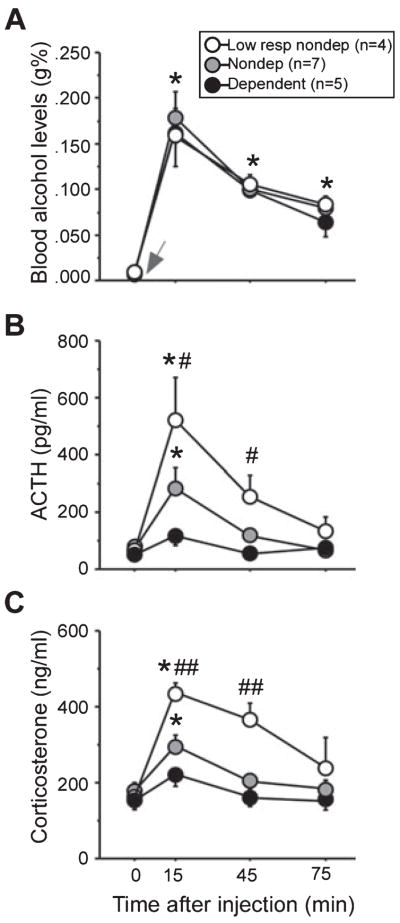
Blood alcohol levels (g%, A), adrenocorticotropic hormone (ACTH; pg/mL, B) and corticosterone levels (ng/mL, C) before and after an alcohol challenge (1 g/kg, i.v.) in low-responding (white circles, n = 4), non-dependent (n = 7) and dependent (black circles, n = 5) animals 6–8 h into withdrawal from vapors (dark cycle). Alcohol was administered (gray arrow) immediately after the first sample was collected (basal, 0 min). Baseline (0 min) blood alcohol levels were undetectable, and alcohol elicited equivalent increases in blood alcohol levels in all groups (*P < 0.0001; compared with 0 min). No differences in HPA axis activity were evident under basal conditions, but alcohol elicited vastly different ACTH and corticosterone responses in the three groups. The most robust HPA responses were observed in low-responding animals, intermediate responses in non-dependent animals and nearly flattened responses in dependent animals. Post hoc analyses indicated significant peaks in ACTH and corticosterone at 15 min in low-responding and non-dependent animals, and corticosterone levels remained elevated at 45 min only in low-responding animals (*P < 0.008, compared with 0 min). Low-responding animals had significantly higher ACTH and corticosterone levels 15 and 30 min after the alcohol challenge (#P < 0.01, compared with dependent animals; ##P < 0.01, compared with dependent and non-dependent animals). To convert blood alcohol levels from g% (g/dL) to mM, divide values by 0.0046 (e.g. 0.200 g% is 43.5 mM); to convert ACTH1–39 (pg/mL) to pM, divide values by 4.5410 (e.g. 500 pg/mL is 110.1 pM); to convert corticosterone (ng/mL) to nM, divide values by 0.3464 (e.g. 400 ng/mL is 1154.7 nM). Data are expressed as mean ± SEM.
Self-administration of an average of 1 g/kg of alcohol in 30 min produced average blood alcohol levels above 0.150 g% (32.6 mM) in dependent animals vs. self-administration of an average of 0.5 g/kg (~0.050 g% or 10.9 mM blood alcohol levels) in non-dependent animals (Fig. 3B and C). Simple regression analysis of total alcohol consumption and blood alcohol levels at the end of the session (30 min) confirmed a strong positive correlation between the two variables (r = 0.85, F1,16 = 44.89, P < 0.0001, Power = 1.00; Fig. 3B). Significant main effects of Group (F1,48 = 7.60, P = 0.01, Power = 0.72) and Sample Time (F3,48 = 25.20, P < 0.0001, Power = 1.00), and a significant Group × Sample Time interaction (F3,48 = 6.50, P = 0.0009, Power = 0.97) were observed on blood alcohol levels during the self-administration session. Specifically, blood alcohol levels were significantly higher at 15, 30 and 45 min in dependent animals (all P < 0.001), and at 30 and 45 min in non-dependent animals (all P < 0.05) compared with basal levels (0 min, Fig. 3C). Blood alcohol levels were significantly higher in dependent animals compared with non-dependent animals at all sampling time-points after the start of the self-administration session (15, 30 and 45 min, all P < 0.05; Fig. 3C), reflecting the higher amount of alcohol consumed in dependent animals during the test session.
Attenuated ACTH and corticosterone in dependent animals: stimulation of the HPA axis by alcohol self-administration
ACTH levels obtained before (basal, 0 min), during (15 and 30 min) and after (45 min) self-administration of alcohol are shown in Fig. 3D. Under basal conditions, ACTH levels were significantly blunted in dependent animals (F1,16 = 11.60, P = 0.003, Power = 0.91; Fig. 3D). Self-administration of alcohol elevated ACTH in both groups of animals (F3,48 = 3.56, P = 0.02, Power = 0.76; Fig. 3D) at 15 min, and the degree to which ACTH was increased from basal levels was positively correlated with blood levels of alcohol [15 min blood alcohol levels and the percent change in ACTH at 15 min compared with basal levels (~60% increase), r = 0.52, F1,17 = 5.98, P = 0.02]. Blood alcohol levels were not correlated with percent increase in ACTH at any other time-point. Two-way ANOVA indicated that, despite alcohol-elicited increases, ACTH remained lower at all time-points in dependent compared with non-dependent animals (main effect of Group, F1,48 = 7.78, P = 0.01, Power = 0.75).
Corticosterone levels obtained before (basal, 0 min), during (15 and 30 min) and after (45 min) self-administration of alcohol are shown in Fig. 3E. Basal corticosterone was lower in dependent compared with non-dependent animals (0 min, F1,16 = 4.77, P = 0.04, Power = 0.53; Fig. 3E). Self-administration of alcohol elicited a slight increase in corticosterone at 15 min, demonstrated by a significant positive correlation between 15 min blood alcohol levels and the percent change in corticosterone at 15 min (~17% increase) compared with basal levels (r = 0.48, F1,16 = 4.79, P = 0.04). Blood alcohol levels were not correlated with percent increase in corticosterone at any other time-point.
Experiment 2: effect of 1 g/kg of alcohol on the HPA axis and blood alcohol levels in low-responding, non-dependent and dependent animals
Administration of alcohol (1 g/kg, i.v.) elicited drastically different HPA responses among the three self-administration groups, with the most robust responses occurring in the low-responding non-dependent group, intermediate responses occurring in the standard non-dependent group and significantly blunted responses occurring in the dependent group (Fig. 3). Alcohol stimulated similar elevations in blood alcohol levels in all groups equally, with peaks occurring at 15 min (main effect of Sampling Time, F3,39 = 66.71, P < 0.0001, Power = 1.00), with no significant main effect of Group and no interaction (P > 0.05; Fig. 3A). Despite similar blood alcohol level responses, main effects of Group (F2,36 = 4.36, P = 0.03, Power = 0.64) and Sample Time (F3,36 = 23.51, P < 0.0001, Power = 1.00), and a significant Group × Sample Time interaction (F6,36 = 5.13, P = 0.0007, Power = 0.99) were observed on ACTH levels (Fig. 2B). Post hoc analyses indicated significant elevations in ACTH at 15 min in low-responding and non-dependent groups (all P < 0.008; Fig. 3B). Likewise, main effects of Group (F2,39 = 15.98, P = 0.0003, Power = 1.00) and Sample Time (F3,39 = 12.41, P < 0.0001, Power = 1.00), and a significant interaction between these two variables (F6,39 = 2.39, P = 0.04, Power = 0.75) were observed on corticosterone levels (Fig. 2C). Post hoc analyses indicated significant elevations in corticosterone at 15 and 45 min in low-responding animals, and at 15 min in non-dependent animals compared with 0 min (all P < 0.008; Fig. 2B). Low-responding animals had higher ACTH and corticosterone levels compared with dependent animals at 15 and 45 min (P < 0.01; Fig. 2B and C). Accordingly, trained animals that chronically self-administered low levels of alcohol (averaging less than 0.2 g/kg/session) elicited higher or ‘normal’ (comparable with the alcohol-naive animals in Lee & Rivier, 1997; Lee et al., 2000) HPA responses to an alcohol challenge compared with animals chronically self-administering moderate (non-dependent animals, averaging ~0.3–0.4 g/kg/session) or high (dependent animals, averaging 1.0 g/kg/session) levels of alcohol. Interestingly, prior to the alcohol injection, basal levels of ACTH and corticosterone were not significantly lower in dependent animals compared with non-dependent animals or low-responding non-dependent animals (all P > 0.05), suggesting that dependence-induced reductions in basal HPA activity that were observed in the afternoon of the light cycle (Experiments 1, 3, 4) may reflect potential perturbation of diurnal regulation of the HPA axis.
Experiment 3: effect of a CRF challenge on the HPA axis in alcohol-naive, non-dependent and dependent animals
The next experiment explored whether ACTH and corticosterone responses to exogenously administered CRF differed in dependent and non-dependent animals because pituitary responsiveness could mediate neuroendocrine tolerance. A dose–response curve was first conducted in alcohol-naive animals (Fig. 4A). As expected, CRF induced dose-related (F3,60 = 55.94, P < 0.0001, Power = 1.00) and time-related (F3,60 = 507.40, P < 0.0001, Power = 1.00) increases in plasma ACTH levels, with peak responses measured at the 15 min time-point (Fig. 4A). The 0.3 μg/kg dose of CRF elicited a mid-range ACTH response in alcohol-naive control animals (elevated ACTH to levels ~300 pg/mL or 66.1 pM) and was selected for administration in dependent and non-dependent rats. Similar to Experiment 1, basal ACTH was lower in dependent animals (F1,7 = 32.66, P = 0.0007, Power = 0.99; Fig. 4B). However, CRF elicited an increase in ACTH in dependent and non-dependent animals, eliminating the difference between the two groups (F3,21 = 71.13, P < 0.0001, Power = 1.00; Fig. 4B). Notably, CRF elicited ACTH responses that were about half the magnitude observed in alcohol-naive controls (0.3 μg/kg dose in Fig. 4A and B).
Fig. 4.
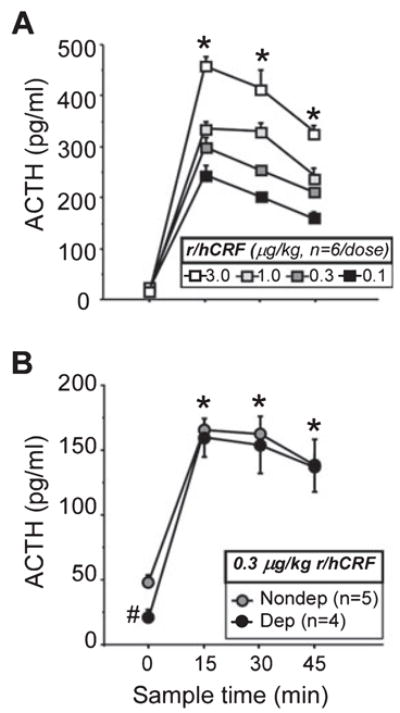
Adrenocorticotropic hormone (ACTH) responses following exogenously administered rat/human corticotropin-releasing factor (r/hCRF). A dose–response curve for r/hCRF was first conducted in alcohol-naive animals (n = 6 per dose: 0.1, 0.3, 1.0 and 3.0 μg/kg, i.v.), indicating that all doses increased ACTH (*P < 0.0001, A), with a peak at 15 min, and that the 0.3 μg/kg dose of CRF elicited a mid-range ACTH response. The 0.3 μg/kg dose of r/hCRF was then administered to non-dependent (gray circles, n = 5) and dependent (black circles, n = 4) rats 6–8 h into withdrawal from vapors (14.00–16.00 h), and blood samples were obtained 0 (basal, before injection), 15, 30 and 45 min later for measurement of plasma ACTH. Although basal ACTH was significantly lower in dependent animals (#P = 0.0007, compared with non-dependent animals at 0 min, B), CRF elicited a rapid rise in ACTH and eliminated the hormonal difference between groups (*P < 0.0001, compared with 0 min, B). To convert ACTH1–39 (pg/mL) to pM, divide values by 4.5410 (e.g. 200 pg/mL is 44.0 pM). Data are expressed as mean ± SEM.
Experiment 4: CRF mRNA in the PVN in alcohol-naive, non-dependent and dependent animals
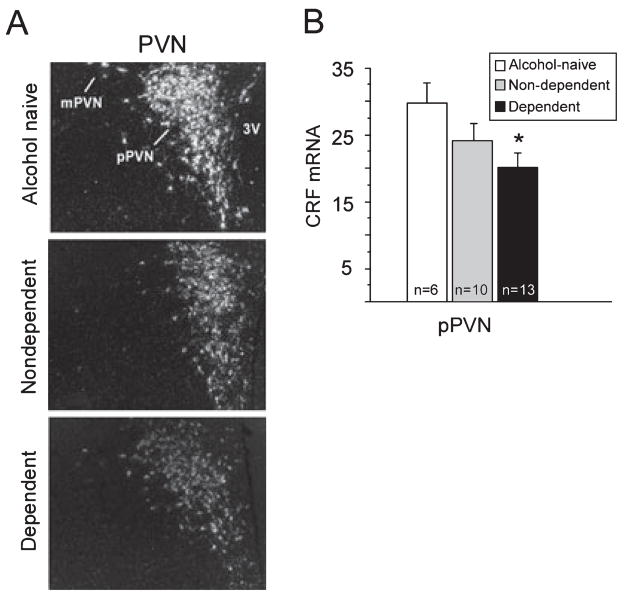
Photomicrographs illustrating CRF mRNA signal in the PVN from alcohol-naive, non-dependent and dependent animals are shown in Fig. 5A. Group means of OD (arbitrary units) are shown in Fig. 5B for the parvocellular division of the PVN (pPVN). CRF mRNA levels in the pPVN were reduced in alcohol-exposed animals compared with alcohol-naive controls, but this reduction reached significance only in dependent animals (main effect of Group: F2,26 = 3.20, P = 0.05, Power = 0.55; naive > dependent, P = 0.01; Fig. 5B). Non-dependent and dependent animals did not differ in CRF expression levels in the pPVN, and no groups differed in CRF mRNA levels in the magnocellular division of the PVN, a region that contains a small number of CRF cells (data not shown; Swanson et al., 1983).
Fig. 5.
Photomicrographs showing corticotropin-releasing factor (CRF) mRNA signal in the paraventricular nucleus of the hypothalamus (PVN, A) and group means of transcript optical density (OD, arbitrary units of signal intensity corrected for background) in the parvocellular portion of the PVN (pPVN) in alcohol-naive (white bar, n = 6), non-dependent (gray bar, n = 10) and dependent (black bar, n = 13) animals 6–8 h into withdrawal from vapors (14.00–16.00 h, B). CRF mRNA was significantly decreased in the pPVN in dependent animals compared with alcohol-naive controls (*P = 0.01, B), but not compared with non-dependent animals. Groups did not differ in CRF mRNA levels in the magnocellular division of the PVN (mPVN, data not shown). Data are expressed as mean ± SEM.
Discussion
The HPA axis has long been hypothesized to play a role in alcoholism and dependence (Wand & Dobs, 1991; Froehlich et al., 2003). Combined behavioral, neuroendocrine and molecular approaches were used to investigate the relationship between alcohol drinking, chronic alcohol exposure and the HPA axis using an animal model of alcohol dependence. Operant self-administration of alcohol acutely elevated HPA hormone levels, but chronic exposure resulted in significant impairment of HPA function. Dependence was associated with reduced basal levels of corticosterone and its upstream regulator, ACTH, in the afternoon of the light cycle and robust deficits in HPA responsivity to alcohol (voluntary and involuntary) that persisted into the dark phase. An injection of CRF ‘normalized’ ACTH levels in dependent animals compared with non-dependent controls, implicating central rather than peripheral mechanisms in this deficit. While neuroendocrine tolerance may be accompanied by decreased CRF mRNA in the PVN in dependent animals compared with alcohol-naive controls, alterations in hypothalamic CRF expression do not fully explain the severe impairment of HPA function observed in dependent animals compared with non-dependent animals. Overall, the findings support clinical reports showing impaired stress responsivity in alcoholics (Lovallo et al., 2000; O’Malley et al., 2002; Kiefer & Wiedemann, 2004; Adinoff et al., 2005) and suggest a pathway by which high chronic alcohol use could lead to a dampened neuroen-docrine state. The neuroendocrine changes may underlie some of the negative characteristics experienced in the acute withdrawal state that lead to excessive alcohol intake, but this remains to be determined. Nevertheless, severe alterations in HPA function associated with chronic alcohol exposure are particularly important because they have the potential to negatively impact overall health and the ability to respond appropriately to internal and environmental challenges.
Chronic exposure to high alcohol induces physical and motivational dependence, as measured by increased anxiety-like responses and elevated brain reward thresholds during acute withdrawal (Rassnick et al., 1993; Schulteis et al., 1995; Roberts et al., 1996; Valdez et al., 2002; Overstreet et al., 2004; Gehlert et al., 2007). Increased responding for alcohol in dependent animals was two–threefold higher than pre-vapor responding, which is consistent with previous reports that tested during acute withdrawal (2 h; Funk et al., 2006; 6–8 h, O’Dell et al., 2004; Walker & Koob, 2007; Richardson et al., 2008) and protracted abstinence (Roberts et al., 1996; Valdez et al., 2002). Animals made dependent by exposure to intermittent alcohol vapors had mild physical signs of withdrawal that began to rise about 2 h following removal from alcohol vapors, peaked at 8 h and gradually descended thereafter. The physical signs of withdrawal observed here were milder and dissipated earlier compared with animals made dependent using continuous alcohol vapor exposure (Roberts et al., 1996). In contrast, responding for alcohol is higher when animals are exposed to alcohol vapors on an intermittent vs. continuous schedule (O’Dell et al., 2004), further confirming the dissociation between physical withdrawal and high alcohol consumption. Together these results provide support for the hypothesis that the ‘emotional’ aspects of withdrawal (indexed by changes in anxiety-like behavior and reward thresholds in animal studies) are motivators at least as powerful as, if not more than, physical aspects of withdrawal for driving excessive drinking in dependent animals (Rassnick et al., 1993; Schulteis et al., 1995; Valdez et al., 2002; Overstreet et al., 2004; Sabino et al., 2006; Chu et al., 2007; Funk et al., 2007; Gehlert et al., 2007; Richardson et al., 2008). Consistent with this hypothesis, physical symptoms of withdrawal are not always observed in alcoholics, and the motivational signs of withdrawal, such as anxiety, dysphoria and malaise, are considered important in continued use and relapse. Alcoholics are hypothesized to drink alcohol not simply for its euphorigenic effects, but also to avoid or reduce the negative affective states associated with withdrawal or to self-medicate existing negative affective states (Cappell & LeBlanc, 1979; Lowman et al., 1996; Koob, 2003).
The present study showed that basal ACTH and corticosterone levels were attenuated in dependent compared with non-dependent animals self-administering alcohol, extending earlier studies showing blunted HPA hormones several weeks after withdrawal from chronic alcohol liquid diet compared with alcohol-naive controls (Rasmussen et al., 2000; Zorrilla et al., 2001). Additionally, consistent with these findings, clinical studies link disruption of the HPA axis with alcoholism, including a dampened ability to cope with stress and negative correlations between cortisol and craving and relapse in alcoholics (Lovallo et al., 2000; O’Malley et al., 2002; Kiefer & Wiedemann, 2004; Adinoff et al., 2005). Conversely, some clinical and rodent studies have demonstrated elevated, rather than reduced, basal glucocorticoid levels during acute withdrawal or early abstinence. Many factors may contribute to the seemingly opposite findings, including species differences in rodent models (Tabakoff et al., 1978; Roberts et al., 1992), co-presence of a depression syndrome (Heinz et al., 1995; Sher, 2007), developmental age (Sher, 2007) and measurements of free vs. total glucocorticoid levels (Keedwell et al., 2001). Future studies in which free corticosterone levels are also measured (a better index of bioavailability) in non-dependent and dependent self-administering animals could also elucidate whether the acute and chronic effects of alcohol on total corticosterone levels (measured in the present study) parallel changes in free circulating hormone levels more readily available for actions within the brain and periphery.
Another important factor that may explain some differences among studies is glucocorticoid rhythmicity (Adinoff et al., 1991). In the afternoon of the light cycle, when glucocorticoid levels are normally rising to peak levels, dependent animals had lower ACTH and corticosterone compared with non-dependent animals (Experiments 1, 3 and 4). However, a few hours into the dark phase (Experiment 2), basal HPA hormone levels were elevated in dependent animals and no longer differed from non-dependent animals. Thus, detection of attenuated basal HPA activity in dependent animals depends on circadian time, suggesting possible perturbation in the diurnal regulation of ACTH and corticosterone with dependence. Nevertheless, blunted release of ACTH and corticosterone following voluntary and involuntary alcohol in dependent animals persisted across light and dark cycles, indicating dissociation in the underlying mechanisms driving the dependence-induced changes in basal HPA hormone levels vs. HPA responsivity.
The current work indicates that a dampened neuroendocrine state did not occur prior to, but rather was an ‘outcome’ of, chronic exposure to alcohol vapors in dependent animals (but see Gianoulakis et al., 2005). A dampened neuroendocrine state may still underlie some of the negative emotions experienced during withdrawal and motivate drinking behavior to establish normal endocrine function. Glucocorticoids are hypothesized to initially play a stimulatory role on alcohol consumption during training in non-dependent animals through positive reinforcement mechanisms (Piazza et al., 1993, 1996; Fahlke et al., 1995; Koenig & Olive, 2004). Subsequently in the dependent state (present report), attenuated HPA could function as a motivating factor for excessive drinking because considerably higher alcohol consumption would be needed to achieve the same level of glucocorticoids. Notably, baseline ACTH and corticosterone prior to initiation of drinking are not tightly linked to the amount of alcohol dependent animals consume (regression analyses not shown), indicating that low basal hormone levels by themselves are not enough to ‘initiate’ high drinking. Instead, the failure to significantly elevate ACTH and corticosterone during self-administration would be one means by which a dampened neuroendocrine state could affect drinking behavior possibly through altered reward or satiety thresholds. In support of this hypothesis, anti-craving drugs used to prevent relapse, such as opioid antagonists, activate the HPA axis, and sensitivity to this activation is most predominant in subjects with a strong family history for alcohol dependence (Wand et al., 1999; O’Malley et al., 2002; Kiefer et al., 2006).
Several important inferences can be made by inclusion of alcohol-naive animals and low alcohol-consuming animals (low-responding, non-dependent group) for comparison to moderate (non-dependent groups) and high (dependent groups) alcohol-consuming animals. First, neuroendocrine tolerance appears to be first initiated prior to dependence. HPA responses to alcohol (Experiment 2) and CRF (Experiment 3) were substantially more robust (‘normal’) in groups that had little (low-responding, non-dependent animals) or no exposure (alcohol-naïve) to alcohol compared with non-dependent animals that consumed moderate levels of alcohol and dependent animals that consumed high levels of alcohol. CRF mRNA normally shows high constitutive expression in the PVN (Sawchenko et al., 1993), and transcript levels were high in alcohol-naive controls, slightly (albeit not significantly) reduced in non-dependent animals and significantly reduced in dependent animals. The findings altogether suggest that downregulation of the HPA axis may be initiated before dependence. The early stages of neuroendocrine tolerance could potentially be mediated at the level of the pituitary (decreased sensitivity to CRF; Rivier et al., 1990) and eventually through adaptations in the hypothalamus. The data also argue against these mechanisms completely explaining the robust blunting of basal ACTH and corticosterone levels and HPA responsivity in dependent compared with non-dependent animals. The two groups did not differ in either pituitary responsivity to CRF or constitutive expression of CRF mRNA in the PVN, precluding peripheral and implicating (but not identifying) other central mediators of neuroendocrine tolerance in the later stages of addiction.
Although all of the exact mechanisms driving reduced HPA function in dependent animals remain unknown, the current report, in conjunction with previous findings, suggests a possible pathway by which high alcohol consumption may eventually lead to dependence. CRF neurons in limbic structures, such as the CeA and BNST are involved in mediating the negative affective state experienced during withdrawal (Alheid & Heimer, 1988; Herman & Cullinan, 1997; Wand, 2005; Koob, 2006). The present study demonstrates that oral self-administration of alcohol stimulates the HPA axis to release ACTH and corticosterone, one means by which the HPA axis can drive neuroadaptive changes in the limbic CRF system. High corticosterone increases CRF mRNA in the CeA and lateral BNST and decreases CRF mRNA in the PVN (Makino et al., 1994; Albeck et al., 1997; Schulkin et al., 1998; Shepard et al., 2000). Furthermore, the CeA has stimulatory influence and the lateral BNST has both stimulatory and inhibitory influence on the CRF system in the PVN, and this complex interplay is vulnerable to allostatic overload under chronic high stress conditions (Cullinan et al., 1993; Gray et al., 1993; Herman et al., 1994, 1996; Herman & Cullinan, 1997; Schulkin et al., 1998; Schulkin, 1999). Given that moderate to high drinking (present study) stimulates the HPA axis, the initial exposure to high corticosterone may stimulate CRF expression in the CeA and lateral BNST, eventually leading to neuroadaptive changes, including further sensitization of CRF activation in the extended amygdala and decreased HPA function. Thus, multiple mechanisms of the brain stress axis may be engaged with excessive drinking, including a compromised hormonal response (present study) and a sensitized brain stress response (previous studies), both of which contribute to dependence.
In conclusion, the present study shows that alcohol dependence is associated with dysregulation of the HPA axis. Alterations of the HPA axis brought on by heavy binge drinking may begin with a history of high drinking, whether or not the subject is physically dependent, and may ultimately lead to the dysregulation associated with the dependent state. HPA adaptations occur at various stages of the addictive cycle and appear to be mediated by multiple mechanisms. Decreased HPA function provides support for the hypothesized shift in stress regulation (decreased activation of the hypothalamic/neuroendocrine CRF system and enhanced activation and sensitivity of the extrahypothalamic/behavioral CRF system; Koob, 2008). Changes in HPA function could directly or indirectly contribute to the negative affective state experienced during acute withdrawal to motivate excessive alcohol intake. Moreover, impaired HPA function associated with dependence could ultimately negatively impact overall health because of the decreased ability to respond appropriately to internal and environmental challenges.
Acknowledgments
This is publication number 18460 from The Scripps Research Institute. The authors thank Maury Cole, Bryant Silbaugh, Yanabel Grant, Yaira Haas and Dr Han-E Zhao for excellent technical assistance, Drs John Polich and Eric Zorrilla for statistical expertise and scientific discussion, and Mike Arends and Mellany Santos for editorial assistance. This study was supported by the Pearson Center for Alcoholism and Addiction Research and National Institutes of Health grants AA06420, AA08459 and AA12602 from the National Institute on Alcohol Abuse and Alcoholism.
Abbreviations
- ACTH
adrenocorticotropic hormone
- BNST
bed nucleus of the stria terminalis
- CeA
central nucleus of the amygdala
- CRF
corticotropin-releasing factor
- CRF1
CRF type I receptor
- DepC
diethylpyrocarbonate
- HPA
hypothalamic-pituitary-adrenal
- IRMA
immunoradiometric assay
- OD
optical density
- pPVN
parvocellular division of the paraventricular nucleus of the hypothalamus
- PVN
paraventricular nucleus of the hypothalamus
- r/hCRF
rat/human CRF
- SSC
standard sodium citrate
- TEA
triethanolamine
References
- Adinoff B, Risher-Flowers D, De Jong J, Ravitz B, Bone GH, Nutt DJ, Roehrich L, Martin PR, Linnoila M. Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. Am J Psychiatry. 1991;148:1023–1025. doi: 10.1176/ajp.148.8.1023. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nikulina J, McEwen BS, Sakai RR. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J Neurosci. 1997;17:4895–4903. doi: 10.1523/JNEUROSCI.17-12-04895.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: a “kindling”/stress hypothesis. Psychopharmacology (Berl) 2005;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Lintz R, Koob GF. Intravenous drug self-administration techniques in animals. In: Sahgal A, editor. Behavioural Neuroscience: A Practical Approach. Vol. 2. IRL Press; Oxford: 1993. pp. 117–143. [Google Scholar]
- Cappell H, LeBlanc AE. Tolerance to, and physical dependence on, ethanol: why do we study them? Drug Alcohol Depend. 1979;4:15–31. doi: 10.1016/0376-8716(79)90038-3. [DOI] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hard E, Eriksson CJ, Engel JA, Hansen S. Consequence of long-term exposure to corticosterone or dexamethasone on ethanol consumption in the adrenalectomized rat, and the effect of type I and type II corticosteroid receptor antagonists. Psychopharmacology (Berl) 1995;117:216–224. doi: 10.1007/BF02245190. [DOI] [PubMed] [Google Scholar]
- Froehlich J, O’Malley S, Hyytia P, Davidson D, Farren C. Preclinical and clinical studies on naltrexone: what have they taught each other? Alcohol Clin Exp Res. 2003;27:533–539. doi: 10.1097/01.ALC.0000057943.57330.AB. [DOI] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee M-J, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C, Dai X, Thavundayil J, Brown T. Levels and circadian rhythmicity of plasma ACTH, cortisol, and beta-endorphin as a function of family history of alcoholism. Psychopharmacology (Berl) 2005;18:437–444. doi: 10.1007/s00213-005-0129-x. [DOI] [PubMed] [Google Scholar]
- Gibson S, Pollock A, Littley M, Shalet S, White A. Advantages of IRMA over RIA in the measurement of ACTH. Ann Clin Biochem. 1989;26:500–507. doi: 10.1177/000456328902600608. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Koob GF. Effects of CRF-1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008a;32:1535–1542. doi: 10.1111/j.1530-0277.2008.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Lumeng L, Koob GF. Dependence-induced alcohol drinking by alcohol-preferring (P) rats and outbred Wistar rats. Alcohol Clin Exp Res. 2008b;32:1688–1696. doi: 10.1111/j.1530-0277.2008.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TS, Piechowski RA, Yracheta JM, Rittenhouse PA, Bethea CL, Van de Kar LD. Ibotenic acid lesions in the bed nucleus of the stria terminalis attenuate conditioned stress-induced increases in prolactin, ACTH and corticosterone. Neuroendocrinology. 1993;57:517–524. doi: 10.1159/000126400. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Heinz A, Rommelspacher H, Gräf KJ, Kürten I, Otto M, Baumgartner A. Hypothalamic-pituitary-gonadal axis, prolactin, and cortisol in alcoholics during withdrawal and after three weeks of abstinence: comparison with healthy control subjects. Psychiatry Res. 1995;56:81–95. doi: 10.1016/0165-1781(94)02580-c. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Watson SJ. Involvement of the bed nucleus of the stria terminalis in tonic regulation of paraventricular hypothalamic CRH and AVP mRNA expression. J Neuroendocrinol. 1994;6:433–442. doi: 10.1111/j.1365-2826.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996;10:371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Poon L, Papadopoulos AS, Marshall EJ, Checkley SA. Salivary cortisol measurements during a medically assisted alcohol withdrawal. Addict Biol. 2001;6:247–256. doi: 10.1080/13556210120056580. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Wiedemann K. Neuroendocrine pathways of addictive behaviour. Addict Biol. 2004;9:205–212. doi: 10.1080/13556210412331292532. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Otte C, Naber D, Wiedemann K. Hypothalamic-pituitary-adrenocortical axis activity: a target of pharmacological anticraving treatment? Biol Psychiatry. 2006;60:74–76. doi: 10.1016/j.biopsych.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Koenig HN, Olive MF. The glucocorticoid receptor antagonist mifepristone reduces ethanol intake in rats under limited access conditions. Psychoneuroendocrinology. 2004;29:999–1003. doi: 10.1016/j.psyneuen.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcohol dependence: the importance of neurobiology to treatment. Medscape Psychiatry Ment Health. 2006;11:1–7. [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornreich WD, Galyean R, Hernandez JF, Craig AG, Donaldson CJ, Yamamoto G, Rivier C, Vale W, Rivier J. Alanine series of ovine corticotropin releasing factor (oCRF): a structure-activity relationship study. J Med Chem. 1992;35:1870–1876. doi: 10.1021/jm00088a024. [DOI] [PubMed] [Google Scholar]
- Lee S, Rivier C. An initial, three-day-long treatment with alcohol induces a long-lasting phenomenon of selective tolerance in the activity of the rat hypothalamic-pituitary-adrenal axis. J Neurosci. 1997;17:8856–8866. doi: 10.1523/JNEUROSCI.17-22-08856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Cole M, Smith A, Rivier C. Prolonged exposure to intermittent alcohol vapors blunts hypothalamic responsiveness to immune and non-immune signals. Alcohol Clin Exp Res. 2000;24:110–122. [PubMed] [Google Scholar]
- Little HJ. Alcohol is a drug; a cautionary note on its use as a drug solvent. Psychopharmacology (Berl) 2003;171:234–235. doi: 10.1007/s00213-003-1580-1. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res. 2000;24:651–658. [PubMed] [Google Scholar]
- Lowman C, Allen J, Stout RL. Replication and extension of Marlatt’s taxonomy of relapse precipitants: overview of procedures and results. The Relapse Research Group. Addiction. 1996;91(Suppl):S51–S71. [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13:163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Res. 1994;657:141–149. doi: 10.1016/0006-8993(94)90961-x. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci USA. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci USA. 1996;93:8716–8720. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci USA. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol Clin Exp Res. 2000;24:1836–1849. [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, Zorrilla EP, Koob GF. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol Biochem Behav. 2008;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Rivier C, Shen GH. In the rat, endogenous nitric oxide modulates the response of the hypothalamic-pituitary-adrenal axis to interleukin-1 beta, vasopressin, and oxytocin. J Neurosci. 1994;14:1985–1993. doi: 10.1523/JNEUROSCI.14-04-01985.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Imaki T, Vale W. Prolonged exposure to alcohol: effect on CRF mRNA levels, and CRF- and stress-induced ACTH secretion in the rat. Brain Res. 1990;520:1–5. doi: 10.1016/0006-8993(90)91685-a. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Crabbe JC, Keith LD. Genetic differences in hypothalamic-pituitary-adrenal axis responsiveness to acute ethanol and acute ethanol withdrawal. Brain Res. 1992;579:296–302. doi: 10.1016/0006-8993(92)90064-g. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Koob GF. Operant self-administration of sweetened versus unsweetened ethanol: effects on blood alcohol levels. Alcohol Clin Exp Res. 1999;23:1151–1157. [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology. 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Imaki T, Potter E, Kovács K, Imaki J, Vale W. The functional neuroanatomy of corticotropin-releasing factor. Ciba Found Symp. 1993;172:5–21. doi: 10.1002/9780470514368.ch2. [DOI] [PubMed] [Google Scholar]
- Schulkin J. Corticotropin-releasing hormone signals adversity in both the placenta and the brain: regulation by glucocorticoids and allostatic overload. J Endocrinol. 1999;161:349–356. doi: 10.1677/joe.0.1610349. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci USA. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. \. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861:288–295. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- Sher L. The role of the hypothalamic-pituitary-adrenal axis dysfunction in the pathophysiology of alcohol misuse and suicidal behavior in adolescents. Int J Adolesc Med Health. 2007;19:3–9. doi: 10.1515/ijamh.2007.19.1.3. [DOI] [PubMed] [Google Scholar]
- Simmons D, Arriza JL, Swanson LW. A complete protocol for in situ hybridization of messenger RNA in brain and other tissues with radiolabeled single-stranded RNA probes. J Histotechnol. 1989;12:169–181. [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Jafee RC, Ritzmann RF. Corticosterone concentrations in mice during ethanol drinking and withdrawal. J Pharm Pharmacol. 1978;30:371–374. doi: 10.1111/j.2042-7158.1978.tb13259.x. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The γ-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand G. The anxious amygdala: CREB signaling and predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2697–2699. doi: 10.1172/JCI26436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand GS, Dobs AS. Alterations in the hypothalamic-pituitary-adrenal axis in actively drinking alcoholics. J Clin Endocrinol Metab. 1991;72:1290–1295. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Wand GS, Mangold D, Ali M. Adrenocorticotropin responses to naloxone in sons of alcohol-dependent men. J Clin Endocrinol Metab. 1999;84:64–68. doi: 10.1210/jcem.84.1.5373. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]