Abstract
We investigated the preclinical characteristics of the neuroprotective effect of the prostaglandin E2 type 1 receptor (EP1) antagonist SC51089 in models of focal cerebral ischemia produced by occlusion of the mouse middle cerebral artery (MCA). We found that systemic administration of SC51089 (5 to 20 μg/kg; i.p.) reduces the brain injury produced by transient (−50% ± 8%; n = 12; P < 0.05) or permanent (−39% ± 7%; n = 12; P < 0.05) MCA occlusion. SC51089 was effective even when administered up to 12 h after ischemia. The protective effect was observed both in male and female mice and was sustained for at least 2 weeks after induction of ischemia. The reduction in injury volume was associated with an improvement in neurological function assessed by the Bederson deficit score, the hanging wire test and the corner test. The data provide proof of principle that EP1 receptor inhibition is a potentially valuable strategy for neuroprotection that deserves further preclinical investigation for therapeutic application in human stroke.
Keywords: SC51089, prostanoids, cyclooxygenase, corner test, middle cerebral artery occlusion
Introduction
Stroke remains a major cause of death and disability for which therapeutic options are extremely limited (Khaja and Grotta, 2007). Although treatment with tissue plasminogen activator has been shown to be beneficial in human ischemic stroke, this agent needs to be administered within 3 h after onset to be maximally effective (Hacke et al, 2004; NINDS 1995). Considering that only a small fraction of stroke patients reaches medical attention so early after the ischemic event, the number of patients that can benefit from tissue plasminogen activator is relatively small (Reeves et al, 2006). Furthermore, risk of hemorrhagic complications and exclusion criteria further reduce the number of patients that can be treated with tissue plasminogen activator (Barber et al, 2001; Heuschmann et al, 2003) Therefore, it would be desirable to develop stroke treatments with a wider therapeutic window that could be used alone or in combination with tissue plasminogen activator and other treatments modalities.
Arachidonic acid metabolites, prostanoids in particular, have long been implicated in the mechanisms of ischemic brain injury (Minghetti, 2007). Prostanoids are produced by cyclooxygenase (COX) enzymes of which three isoforms have been identified: COX-1, COX-2, and COX-3 (Simmons et al, 2004). Recent studies have showed that prostanoids derived from COX-2 are involved in the mechanisms of ischemic and excitotoxic brain damage (Minghetti, 2007). In particular, prostaglandin E2 has emerged as a key downstream effector of COX-2 neurotoxicity (Carlson, 2003; Kawano et al, 2006). Prostaglandin E2 acts on four receptors: EP1, EP2, EP3, and EP4 (Hata and Breyer, 2004). There is accumulating evidence that the EP1 subtype of prostaglandin E2 receptors is involved in the deleterious effects of prostaglandin E2 in the setting of cerebral ischemia (Ahmad et al, 2006; Kawano et al, 2006). Thus, administration of EP1 receptor antagonists have been found to reduce ischemic and excitotoxic brain injury (Ahmad et al, 2006; Kawano et al, 2006; Zhou et al, 2008). Similarly, EP1 receptor null mice are relatively protected from the effects of focal cerebral ischemia and excitotoxicity (Kawano et al, 2006). Therefore, EP1 receptor antagonists could be valuable in the treatment of cerebral ischemia.
However, the characteristics of the neuroprotective effect of EP1 receptor inhibition have not been defined. Many drugs which reduce ischemic injury in animals have been found not to be beneficial in clinical trials (O’Collins et al, 2006). These therapeutic failures have been attributed to a variety of factors, including inadequate preclinical testing of the drugs (Dirnagl, 2006; Drummond et al, 2000; Sena et al, 2007; STAIR, 1999). For example, despite the fact that a substantial number of stroke patients are women, most neuroprotective strategies are not effective in the female sex (Hurn et al, 2005). Some neuroprotective agent are not beneficial when their administration is delayed by several hours, a time interval after which most stroke patients reach medical attention (Gladstone et al, 2002; Reeves et al, 2006). Furthermore, some treatments are effective in transient focal ischemia, but not in the more severe injury produced by permanent ischemia (Beaulieu et al, 1998). In some cases, neuroprotective agents do not prevent brain damage, but only delay its development (Corbett and Nurse, 1998; Dietrich et al, 1993; Drummond et al, 2000; Gladstone et al, 2002; Valtysson et al, 1994). Therefore, to establish the translational value of EP1 receptor inhibition additional information about the protective effect is needed.
In this study, we sought to better define the preclinical characteristics of the neuroprotection afforded by the EP1 receptor antagonist SC51089, a drug that has been shown to reduce ischemic injury both in vivo and in vitro (Kawano et al, 2006; Zhou et al, 2008). In particular, we examined the dose response and therapeutic window of the protective effect, and we investigated whether the protection is present in permanent ischemia, is long lasting, and it also occurs in female mice. The findings suggest that EP1 receptor inhibition could be valuable in the treatment of cerebral ischemia.
Materials and methods
All procedures were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College. Experiments were performed in 2- to 3-month-old male and female C57BL/6 mice (weight 20 to 22 g; The Jackson Laboratory; Bar Harbor, ME, USA).
Middle Cerebral Artery Occlusion
Procedures for transient middle cerebral artery (MCA) occlusion were identical to those previously described and are only summarized (Cho et al, 2005; Kawano et al, 2006; Kunz et al, 2007). Mice were anesthetized with a mixture of isoflurane (1.5% to 2%), oxygen, and nitrogen. A fiber optic probe was glued to the parietal bone (2 mm posterior and 5 mm lateral to bregma) and connected to a laser Doppler flowmeter (Periflux System 5010) for continuous monitoring of cerebral blood flow (CBF). A heat-blunted black monofilament surgical suture 6-0 was inserted into the exposed external carotid artery, advanced into the internal carotid artery, and wedged into the circle of Willis to obstruct the origin of MCA. The filament was left in place for 25 mins and then withdrawn. Only animals that exhibited 85% reduction in CBF during MCA occlusion and in which CBF recovered by 80% after 10 mins of reperfusion were included in the study (Cho et al, 2005; Kunz et al, 2007). Twenty-six percent of the mice failed to meet these criteria and were not entered in the study. We have previously determined that this protocol for MCA occlusion leads to reliable infarcts of size and distribution comparable to those reported by others (Borsello et al, 2003; Plesnila et al, 2001). For permanent MCA occlusion, the filament was inserted under CBF monitoring and left in place. In all mice, rectal temperature was kept at 37.0°C ± 0.5°C during surgery and in the recovery period until animals regained consciousness. Mice subjected to transient MCA occlusion were allowed to survive for 3 or 14 days. In experiments with permanent MCA occlusion, the survival time was shortened to 1 day because mice invariably died with massive brain swelling 1.5 to 2 days after ischemia. The overall mortality for the entire study was 8.2%. Most of the deaths occurred in vehicle-treated mice (see Supplementary Table).
Measurement of Infarct Volume
As described in detail elsewhere (Cho et al, 2005; Kawano et al, 2006; Kunz et al, 2007), brains were removed, frozen, and sectioned (thickness, 30 mm) in a cryostat. Brain sections were collected serially at 600 mm intervals and stained with cresyl violet. Infarct volume was determined using an image analyzer (MCID; Imaging Research Inc.). The operator was not aware of treatment type (SC51089 or vehicle) received by the mouse whose brain was being analyzed. To eliminate the contribution of postischemic edema to the volume of injury, infarct volumes were corrected for swelling according to the method of Lin et al (1993), as previously described (Zhang and Iadecola, 1994). In mice allowed to survive for 2 weeks, the hemisphere ipsilateral to the occluded MCA was reduced in volume compared with the contralateral one, an effect attributable to shrinkage of the ischemic lesion and atrophy of the nondamaged brain (Bouet et al, 2007; Clark et al, 1993). In these experiments, hemispheric volume was measured, but the correction for swelling was not applied.
Administration of SC51089
SC-51089 (8-chlorodibenz11[1,4]oxazepine-10(11H)-carboxylic acid; 2-[1-oxo-3-(3-pyridinyl)propyl]hydrazide; Hallinan et al, 1996) was obtained from Biomol (Plymouth Meeting, PA, USA). Mice were randomly assigned to treatment with vehicle (ddH2O) or the EP1 receptor antagonist SC51089. In dose-response studies, SC51089 was administered i.p. at concentrations ranging from 5 to 20 μg/kg, starting 5 mins after reperfusion. Thereafter SC51089 or vehicle was administered 2 times per day at the specified dose until the time of killing, 3 days after ischemia. When the effect of increasing time intervals between MCA occlusion and treatment was studies (therapeutic window), SC51089 (10 μg/kg; i.p.) was administered starting 6 or 12 and 24 h after reperfusion. The doses of SC51089 used are effective in inhibiting EP1 receptors in brain and do not alter arterial pressure, body temperature, postischemic CBF, or cerebrovascular regulation (Kawano et al, 2006). Treatments were then administered 2 times/day until mice were killed 3 days after ischemia. To our knowledge the blood-brain barrier permeability of SC51089 has not been tested. But, the fact that systemic administration reduced N-methyl-D-aspartic acid lesions in the brain indicates that the drug penetrates the brain (Kawano et al, 2006). The blood-brain barrier opening after stroke would also facilitate drug entry into the brain.
Assessment of Neurological Deficits
The tests described below were administered once a day in animals that were allowed to survive 2 weeks after MCA occlusion. The operator administering the tests was not aware of the treatment of the mice. In the ‘hanging wire’ test, animals were made to grasp with the forepaws a thin wire suspended above ground and the latency to fall was recorded (Cho et al, 2005; Hattori et al, 2000; Li et al, 2004). The test was repeated 3 times for each animal with a 5 mins rest between trials and the falling latencies from 3 trials were averaged. Neurological deficits were also assessed using a modification of the Bederson neurological scale, as previously described (Kawano et al, 2006). Neurological scores were 0 (normal motor function), 1 (flexion of torso and contralateral forelimb when mouse was lifted by the tail), 2 (circling to the contralateral side when mouse was held by the tail on a flat surface, but normal posture at rest), 3 (leaning to the contralateral side at rest), and 4 (no spontaneous motor activity). The corner test was used to assess sensory-motor integration (Li et al, 2004; Zhang et al, 2002). Briefly, the mouse was placed between two cardboard pieces. The two boards were gradually moved closer to form an angle of approximately 30° enclosing the mouse from both sides. The anterior parts of the boards were not allowed to touch, such that a small opening toward the front was always present. When the mouse entered into the deep part of the corner, the facial whiskers were touched by the two boards. Then, the mouse stood up on its hindlimbs, and turned back to face the open end. Ten trials were performed for each mouse and the percentage of right turns was calculated (Zhang et al, 2002). In healthy mice, there was a 50% chance that the mice made a right turn (Li et al, 2004; Zhang et al, 2002).
Statistical Analysis
Data are expressed as mean ± s.e.m. Sample number was determined by power analysis based on the results of a previous study (Kawano et al, 2006). We used a power of 0.8 and a significance level of 0.05. Multiple comparisons were evaluated by the analysis of variance and Neuman–Keuls test. Differences were considered significant at P < 0.05. Neurological deficit data were evaluated by the nonparametric Mann–Whitney test.
Results
Dose-Response Characteristics of the Protective Effect of SC51089
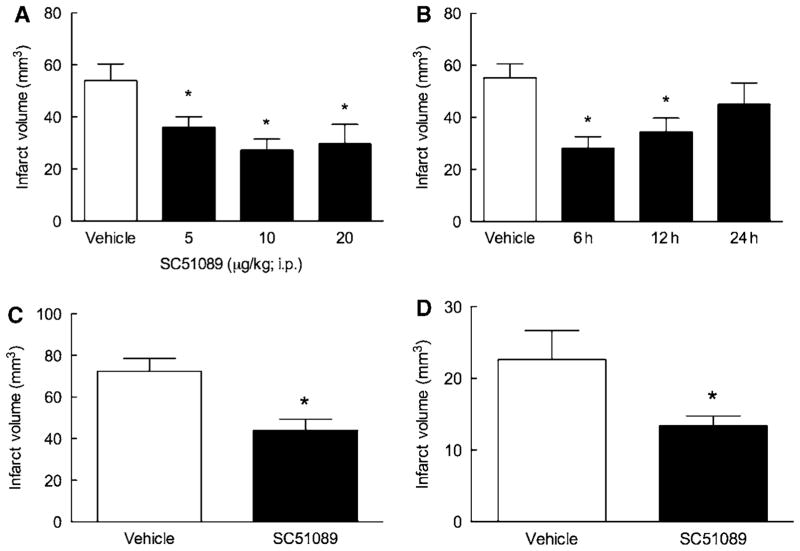
First, we investigated the dose-response characteristics of the reduction in infarct volume induced by SC51089. SC51089 or vehicle was administered i.p. 5 mins after reperfusion and 2 times/day thereafter, until the infarct volume was assessed 3 days later. The reduction in infarct volume was observed at 5 μg/kg (−33% ± 7%; P < 0.05; analysis of variance and Neuman–Keuls test; n = 12) and was greatest at 10 μg/kg (−50% ± 8%; P < 0.05; n = 12; Figure 1A). The infarct volume reduction at 20 μg/kg did not differ from that at 10 μg/kg (P > 0.05; n = 12; Figure 1A).
Figure 1.
Characteristics of the neuroprotective effect of SC51089. (A) Dose-response characteristics in transient MCA occlusion; (B) therapeutic window in transient MCA occlusion (3 days survival); (C) effect in permanent MCA occlusion (1 day survival). (D) The neuroprotective effect of SC51089 is present in female mice. *P < 0.05 from vehicle (analysis of variance and Neuman–Keuls Multiple Comparison test); n = 12/group.
Therapeutic Window
We then sought to define the time interval after ischemia at which SC51089 was still able to protect the brain (therapeutic window). A single dose of SC51089 (10 μg/kg; i.p.) or vehicle was administered 6, 12, or 24 h after reperfusion, and then 2 times/day until mice were killed 3 days after ischemia. Significant stroke volume reductions were observed when SC51089 was administered 6 h (−49% ± 8%; n = 12) or 12 h (−38% ± 10%; n = 12) after reperfusion, but not when the administration was delayed by 24 h (P > 0.05; n = 12; Figure 1B).
SC51089 Reduces Infarct Volume also in Permanent Ischemia
In this study, SC51089 (10 μg/kg; i.p.) or vehicle was administered 6 h after permanent MCA occlusion and 2 times/day thereafter. Mice were killed 1 day after ischemia because they did not survive for 3 days due to massive cerebral edema and cerebral herniation. SC51089 reduced infarct volume also in permanent cerebral ischemia (−39% ± 7%; P < 0.05; n = 12; Figure 1C).
SC51089 Reduces Infarct Volume also in Female Mice
Many neuroprotective strategies are not effective in females (Hurn et al, 2005). Therefore, we next sought to determine whether SC51089 is also effective in reducing infarct volume in female mice. Middle cerebral artery was transiently occluded in female mice and SC51089 (10 μg/kg; i.p.) or vehicle was administered 5 mins after reperfusion and 2 times/day thereafter. Infarct volume was determined 3 days after ischemia. As in previous studies (Park et al, 2006), female mice had smaller infarcts than male mice (Figure 1D; P < 0.05; n = 12). However, SC51089 reduced infarct volume also in female mice (−41% ± 6%; P < 0.05; n = 12; Figure 1D).
The Protection Afforded by SC51089 is Sustained in Time and is Associated with Improved Neurological Outcome
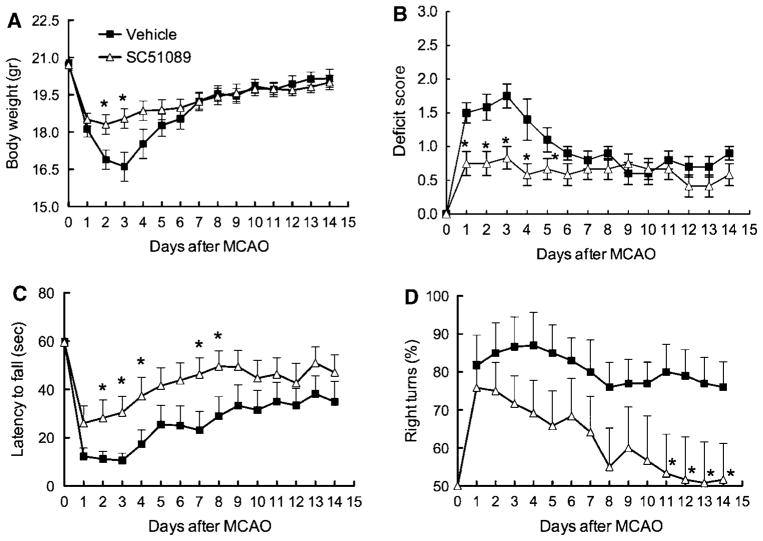
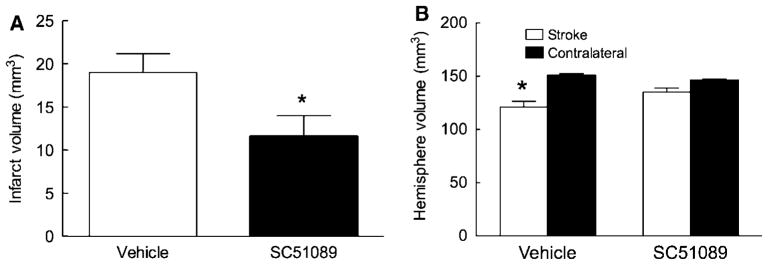
Next, we investigated whether the protective effect of SC51089 is sustained in time. The middle cerebral artery was transiently occluded and SC51089 (10 μg/kg; i.p.) or vehicle was administered 6 h after reperfusion and 2 times/day until day 3. Mice were weighed and evaluated neurologically daily by the deficit score, the hanging wire test, and the corner test. Infarct volume and the volume of the hemispheres were measured 14 days after ischemia. Mice treated with SC51089 showed a less pronounced weight loss after induction of ischemia (P < 0.05; n = 12; Figure 2A). In vehicle-treated mice, ischemia led to an increase in the deficit score (Figure 2B) and reduction in the latency to fall at the hanging wire test (Figure 2C), reflecting the neurological deficits induced by the infarct. Spontaneous improvements were noted starting on day 3 or 4 (Figures 2B and 2C). In mice treated with SC51089 the deficits were less severe (Figures 2B and 2C). The effect of the treatment was observed at day 1 for the deficit score and at day 2 for the hanging wire test (P < 0.05; n = 12; Mann–Whitney test; Figures 2B and 2C). Because of the spontaneous recovery of the mice, no differences were observed between treated and untreated mice starting from day 6 for the deficit score and day 9 for the hanging wire test (P > 0.05; n = 12; Figures 2B and 2C). Vehicle treated mice also exhibited deficits at the corner test, but they failed to show spontaneous improvement after 2 weeks (Figure 2D). In contrast, SC51089 treatment resulted in improved performance in the corner test that reached statistical significance starting on day 11 (P < 0.05; n = 12). By day 14, mice reached performance levels not different from intact mice (no side preference in turning; Figure 2D). These neurological improvements in SC51089-treated mice were associated with a reduction in the volume of the infarct and less atrophy of the ipsilateral hemisphere (P < 0.05; n = 12; Figures 3A and 3B).
Figure 2.
Effect of SC51089 on body weight and neurological function up to 2 weeks after transient MCA occlusion. (A) Body weight; (B) deficit score (modified Bederson scale); (C) latency to fall at the hanging wire test; (D) corner test. *P < 0.05 from vehicle (Mann–Whitney test); n = 12/group.
Figure 3.
Effect of SC51089 on injury volume 14 days after transient MCA occlusion. (A) Infarct volume; (B) volume of the hemisphere ipsilateral and contralateral to the stroke; *P < 0.05; n = 12/group.
Discussion
We showed that the EP1 receptor antagonist SC51089 reduces infarct volume in models of focal cerebral ischemia. The protective effect is related to the dose of SC51089, is sustained for at least 2 weeks after MCA occlusion, and is associated with an improvement of the neurological deficits resulting from the arterial occlusion. Importantly, SC51089 is effective even if the treatment was delayed up to 12 h after ischemia. Furthermore, the reduction in infarct volume is also observed in a model of permanent cerebral ischemia and is present in female mice. These observations suggest that EP1 receptor inhibition produces a sustained reduction of ischemic brain damage that is not sexually dimorphic, occurs with a wide therapeutic window and is associated with neurological improvements.
The protective effect of SC51089 cannot be attributed to alterations in body temperature, blood gases, and arterial pressure, or to effects on the regulation of the CBF resulting in a better preservation of postischemic flow, because we have previously shown that SC51089 does not affect these parameters (Kawano et al, 2006). Thus, inhibition of EP1 receptors does not affect resting CBF, intra ischemic CBF, postischemic CBF, functional hyperemia, endothelium-dependent relaxation, and endothelium-independent relaxation (Kawano et al, 2006). On these bases we doubt that the protective effect can be attributed to cerebrovascular factors. Anesthesia is also unlikely to play a role because SC51089 is also neuroprotective in neuronal cultures and hippocampal slices (Kawano et al, 2006; Zhou et al, 2008). Furthermore, it is unlikely that effects of SC51089 are unrelated to EP1 receptor inhibition because we have previously shown that this antagonist is not effective in EP1-null mice (Kawano et al, 2006). Therefore, the findings of this study cannot be attributed to alterations in physiological parameters or to nonspecific effects of SC51089.
Treatment with SC51089 was associated with reduced weight loss and an improvement in neurological function. Thus, testing by the neurological score based on the Bederson scale and the hanging wire test, which are more sensitive to acute neurological deficits (Li et al, 2004; Zhang et al, 2002), showed an improved outcome in mice treated with SC51089. Improvements in treated mice were also observed with the corner test, a test that is better suited to detect chronic improvements in neurological function, especially sensory-motor integration (Bouet et al, 2007; Li et al, 2004; Zhang et al, 2002). Therefore, the reduction in brain lesion volume is coupled with improved neurological function both in the acute and chronic stages of ischemic brain injury.
The mechanisms of the protective effects of EP1 receptor inhibition with SC51089 have recently been investigated. In models of excitoxicity, SC51089 attenuates the Ca2+ overload produced by exposure of neuronal cultures to N-methyl-D-aspartic acid (Kawano et al, 2006). The effect is related by the ability of SC51089 to enhance the function of the Na+/Ca2+ exchanger, resulting in a reduction in N-methyl-D-aspartic acid-induced intracellular Ca2+ accumulation (Kawano et al, 2006). The neuroprotective effect afforded by SC51089 is comparable to that observed in EP1-null mice (Kawano et al, 2006). In a model of ischemic-hypoxic damage produced by oxygen-glucose deprivation in hippocampal slice cultures, SC51089 reduced the injury in the CA1 sector of the hippocampus (Zhou et al, 2008). The effect was mediated by activation of protein kinase B/Akt, which phosphorylates the proapoptotic protein BAD leading to a reduction in its mitochondrial translocation and in the ensuing apoptotic cell death (Zhou et al, 2008). Therefore, SC51089 is neuroprotective in models of cerebral ischemia both in vivo and in vitro. However, the molecular links between the effects of EP1 receptor inhibition on the Na+/Ca2+ exchanger and on the Akt pathway remain to be defined.
The protective effect of SC51089 provides proof of principle that EP1 receptor inhibition has preclinical characteristics compatible with therapeutic use in human stroke. However, more work is needed to fully characterize the protective effect of SC51089. This study constitutes a first step in this process and many questions remain to be answered. For example, the ability of EP1 receptor inhibition to reduce ischemic injury in aged animals and in animals with stroke risk factors, such as hypertension and diabetes, remain to be established. The full therapeutic window in females needs to be determined. Furthermore, it remains to be established whether EP1 receptor inhibition is effective in species other than rodents. Although we have tested SC51089, more potent antagonists of EP1 receptors are available (Watanabe et al, 2000) and a full evaluation of their neuroprotective effect, as well as their pharmacokinetic characteristics and suitability for human use, remain to be defined. Irrespective of these open questions, our data suggest that EP1 receptor inhibition is a valuable strategy for ischemic brain injury, which is worthy of further investigation for the treatment of ischemic stroke.
In conclusion, we have showed that the EP1 receptor antagonist SC51089 is a relatively potent neuroprotective agent, effective both in transient and permanent focal cerebral ischemia. Reduction in injury volume is obtained even if the drug is administered 12 h after induction of ischemia. The protective effect is sustained for at least 2 weeks and is associated with marked improvement of neurological function in the acute and chronic phase of cerebral ischemic injury. In addition, SC51089 is equally effective in male and female mice, indicating that unlike many other neuroprotective strategies, the reduction in injury is not sexually dimorphic. The data provide preclinical evidence that EP1 receptor inhibition is a valuable strategy for the treatment of cerebral ischemia that deserve further exploration for its applicability to human stroke.
Supplementary Material
Acknowledgments
This study was supported by NIH Grant NS35806.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow and Metabolism website (http://www.nature.com/jcbfm)
References
- Ahmad AS, Saleem S, Ahmad M, Dore S. Prostaglandin EP1 receptor contributes to excitotoxicity and focal ischemic brain damage. Toxicol Sci. 2006;89:265–70. doi: 10.1093/toxsci/kfj022. [DOI] [PubMed] [Google Scholar]
- Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology. 2001;56:1015–20. doi: 10.1212/wnl.56.8.1015. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Busch E, Rother J, de Crespigny A, Hsia CJ, Moseley ME. Polynitroxyl albumin reduces infarct size in transient focal cerebral ischemia in the rat: potential mechanisms studied by magnetic resonance imaging. J Cereb Blood Flow Metab. 1998;18:1022–1031. doi: 10.1097/00004647-199809000-00012. [DOI] [PubMed] [Google Scholar]
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9:1180–6. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- Bouet V, Freret T, Toutain J, Divoux D, Boulouard M, Schumann-Bard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp Neurol. 2007;203:555–67. doi: 10.1016/j.expneurol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Carlson NG. Neuroprotection of cultured cortical neurons mediated by the cyclooxygenase-2 inhibitor APHS can be reversed by a prostanoid. J Neurosci Res. 2003;71:79–88. doi: 10.1002/jnr.10465. [DOI] [PubMed] [Google Scholar]
- Cho S, Park EM, Febbraio M, Anrather J, Park L, Racchumi G, Silverstein RL, Iadecola C. The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J Neurosci. 2005;25:2504–12. doi: 10.1523/JNEUROSCI.0035-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RK, Lee EV, Fish CJ, White RF, Price WJ, Jonak ZL, Feuerstein GZ. Development of tissue damage, inflammation and resolution following stroke: an immunohistochemical and quantitative planimetric study. Brain Res Bull. 1993;31:565–72. doi: 10.1016/0361-9230(93)90124-t. [DOI] [PubMed] [Google Scholar]
- Corbett D, Nurse S. The problem of assessing effective neuroprotection in experimental cerebral ischemia. Prog Neurobiol. 1998;54:531–48. doi: 10.1016/s0301-0082(97)00078-6. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Busto R, Alonso O, Globus MY, Ginsberg MD. Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1993;13:541–9. doi: 10.1038/jcbfm.1993.71. [DOI] [PubMed] [Google Scholar]
- Dirnagl U. Bench to bedside: the quest for quality in experimental stroke research. J Cereb Blood Flow Metab. 2006;26:1465–78. doi: 10.1038/sj.jcbfm.9600298. [DOI] [PubMed] [Google Scholar]
- Drummond JC, Piyash PM, Kimbro JR. Neuroprotection failure in stroke. Lancet. 2000;356:1032–3. doi: 10.1016/S0140-6736(05)72654-4. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–36. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, Brott T, Frankel M, Grotta JC, Haley EC, Jr, Kwiatkowski T, Levine SR, Lewandowski C, Lu M, Lyden P, Marler JR, Patel S, Tilley BC, Albers G, Bluhmki E, Wilhelm M, Hamilton S. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–74. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- Hallinan EA, Hagen TJ, Tsymbalov S, Husa RK, Lee AC, Stapelfeld A, Savage MA. Aminoacetyl moiety as a potential surrogate for diacylhydrazine group of SC-51089, a potent PGE2 antagonist, and its analogs. J Med Chem. 1996;39:609–13. doi: 10.1021/jm950454k. [DOI] [PubMed] [Google Scholar]
- Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–66. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Hattori K, Lee H, Hurn PD, Crain BJ, Traystman RJ, DeVries AC. Cognitive deficits after focal cerebral ischemia in mice. Stroke. 2000;31:1939–44. doi: 10.1161/01.str.31.8.1939. [DOI] [PubMed] [Google Scholar]
- Heuschmann PU, Berger K, Misselwitz B, Hermanek P, Leffmann C, Adelmann M, Buecker-Nott HJ, Rother J, Neundoerfer B, Kolominsky-Rabas PL. Frequency of thrombolytic therapy in patients with acute ischemic stroke and the risk of in-hospital mortality: the German Stroke Registers Study Group. Stroke. 2003;34:1106–13. doi: 10.1161/01.STR.0000065198.80347.C5. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Vannucci SJ, Hagberg H. Adult or perinatal brain injury: does sex matter? Stroke. 2005;36:193–5. doi: 10.1161/01.STR.0000153064.41332.f6. [DOI] [PubMed] [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–9. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- Khaja AM, Grotta JC. Established treatments for acute ischaemic stroke. Lancet. 2007;369:319–30. doi: 10.1016/S0140-6736(07)60154-8. [DOI] [PubMed] [Google Scholar]
- Kunz A, Park L, Abe T, Gallo EF, Anrather J, Zhou P, Iadecola C. Neurovascular protection by ischemic tolerance: role of nitric oxide and reactive oxygen species. J Neurosci. 2007;27:7083–93. doi: 10.1523/JNEUROSCI.1645-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–21. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- Minghetti L. Role of COX-2 in inflammatory and degenerative brain diseases. Subcell Biochem. 2007;42:127–141. doi: 10.1007/1-4020-5688-5_5. [DOI] [PubMed] [Google Scholar]
- NINDS. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–77. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- Park EM, Cho S, Frys KA, Glickstein SB, Zhou P, Anrather J, Ross ME, Iadecola C. Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. J Cereb Blood Flow Metab. 2006;26:392–401. doi: 10.1038/sj.jcbfm.9600194. [DOI] [PubMed] [Google Scholar]
- Plesnila N, Zinkel S, Le DA, Amin-Hanjani S, Wu Y, Qiu J, Chiarugi A, Thomas SS, Kohane DS, Korsmeyer SJ, Moskowitz MA. BID mediates neuronal cell death after oxygen/glucose deprivation and focal cerebral ischemia. Proc Natl Acad Sci USA. 2001;98:15318–15323. doi: 10.1073/pnas.261323298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MJ, Broderick JP, Frankel M, LaBresh KA, Schwamm L, Moomaw CJ, Weiss P, Katzan I, Arora S, Heinrich JP, Hickenbottom S, Karp H, Malarcher A, Mensah G. The Paul Coverdell National Acute Stroke Registry: initial results from four prototypes. Am J Prev Med. 2006;31:S202–9. doi: 10.1016/j.amepre.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Sena E, van der Worp HB, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. 2007;30:433–9. doi: 10.1016/j.tins.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- STAIR. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–8. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Valtysson J, Hillered L, Andine P, Hagberg H, Persson L. Neuropathological endpoints in experimental stroke pharmacotherapy: the importance of both early and late evaluation. Acta Neurochir (Wien) 1994;129:58–63. doi: 10.1007/BF01400874. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kawamori T, Nakatsugi S, Ohta T, Ohuchida S, Yamamoto H, Maruyama T, Kondo K, Narumiya S, Sugimura T, Wakabayashi K. Inhibitory effect of a prostaglandin E receptor subtype EP(1) selective antagonist, ONO-8713, on development of azoxy-methane-induced aberrant crypt foci in mice. Cancer Lett. 2000;156:57–61. doi: 10.1016/s0304-3835(00)00440-7. [DOI] [PubMed] [Google Scholar]
- Zhang F, Iadecola C. Reduction of focal cerebral ischemic damage by delayed treatment with nitric oxide donors. J Cereb Blood Flow Metab. 1994;14:574–80. doi: 10.1038/jcbfm.1994.71. [DOI] [PubMed] [Google Scholar]
- Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, Lu M, Chopp M. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002;117:207–14. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]
- Zhou P, Qian L, Chou T, Iadecola C. Neuroprotection by PGE2 receptor EP1 inhibition involves the PTEN/AKT pathway. Neurobiol Dis. 2008;29:543–51. doi: 10.1016/j.nbd.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.