Abstract
Rising rates of Histoplasma capsulatum infection are an emerging problem among the rapidly growing population of immune-compromised individuals. Although there is a growing understanding of systemic immunity against Histoplasma, little is known about the local granulomatous response, which is an important component in the control of infection. The focus of this article is the characterization of Histoplasma-induced granulomas. Five days after i.p. infection, infected macrophage appear in the liver and lung; however, no granulomas are apparent. Two days later, well-formed sarcoid granulomas are abundant in the lung and liver of infected mice, which contain all visible Histoplasma. Granulomas are dominated by macrophage and lymphocytes. Most of the Histoplasma and most of the apoptotic cells are found in the center of the lesions. We isolated liver granulomas at multiple time points after infection and analyzed the cellular composition, TCR gene usage, and cytokine production of granuloma-infiltrating cells. The lesions contain both CD4+ and CD8+ T cell subsets, and T cells are the primary source of IFN-γ and IL-17. The main source of local TNF-α is macrophage. Chemokines are produced by both infiltrating macrophage and lymphocytes. Dendritic cells are present in granulomas; however, T cell expansion seems to occur systemically because TCR usage is very heterogeneous even at the level of individual lesions. This study is the first direct examination of host cellular responses in the Histoplasma-induced granuloma representing the specific interface between host and pathogen. Our studies will allow further analysis of key elements of host Histoplasma interactions at the site of chronic infection.
The granuloma is a form of delayed-type hypersensitivity. Localized inflammatory lesions composed of infected macrophages and fused giant cells can subsequently form granulomas with the help of CD4+ T lymphocytes. CD4+ T cells are very important for initiating and regulating granuloma function, but macrophage is the dominant cell type (1). A mature granuloma consists of an inflammatory interface surrounded by extracellular matrix that contains both inflammatory cells and the pathogen or inducing agent. The benefit of granuloma formation for the host is that it isolates the inflammation, protects the surrounding healthy tissue, controls the growth of pathogens, and prevents systemic dissemination. At the same time, the microorganism may also benefit from localization to the granuloma. As an isolated microenvironment, granuloma presents a special ecosystem for the pathogen in the host. The chronic granulomatous lesion may be the reservoir from which surviving pathogens emerge to reactivate the infection after a long-term latency is broken by failures of the immune system (2, 3).
Histoplasma capsulatum is a thermally dimorphic fungal pathogen and is an opportunistic pathogen residing in the macrophage phagolysosome (4). Histoplasma infection commonly results in mild or unapparent clinical symptoms in immune-competent individuals and is endemic throughout large areas of the American Midwest (5). However, in immune-compromised individuals deficient in CD4+ T cell function, failure of adequate granuloma function allows the fungus to disseminate systemically and can lead to a serious, life-threatening disease course (6, 7). In endemic areas, histoplasmosis affects a growing population of patients with secondary immune defects, arising from HIV infection, immune suppression after transplantation, anti-TNF-α immunotherapy of rheumatoid arthritis, and inflammatory bowel diseases (6, 8–11).
Histoplasma infection of macrophage induces granuloma formation in different tissues. As in other granuloma-inducing infections, granuloma formation is required to contain fungal growth, prevent systemic dissemination, and protect the organs from widespread inflammatory tissue damage. Both experimental and clinical data indicate that T cells, IFN-γ, and TNF-α are crucial for protection against H. capsulatum infection (12–14).
To better understand the biology of diseases induced by intracellular pathogens, it is important to study the local inflammation site, to dissect and characterize the granuloma, and to expand histopathology data by investigating the granuloma at a cellular level. Despite the growing problems of histoplasmosis in immune-compromised populations, very little is known about the nature of the H. capsulatum-induced granuloma.
One reason is that granulomas are notoriously difficult to isolate and study. Previously, we and others have shown that granulomas induced by Schistosoma mansoni (15), Leishmania chagasi (16), and different species of Mycobacterium (17–19) can be readily isolated from soft tissues such as liver. In the present study, we report that H. capsulatum-induced liver granulomas can also be isolated and describe the cellular composition, cytokine milieu, and TCR gene usage in the lesion during the course of the infection.
These data will allow us to better understand Histoplasma-induced granulomatous inflammation and will serve as a baseline for further studies of how granulomas are regulated by different agents and conditions and how genetic and regulatory processes of the pathogenic yeast and the host contribute to the outcome of the interaction.
Materials and Methods
Mice
C57BL/6 mice were purchased from The Jackson Laboratory and housed in the University of Wisconsin School of Medicine Animal Care Facility. All experiments were approved by the Institutional Animal Care and Use Committee of the School of Medicine and Public Health of the University of Wisconsin.
Fungal strain and infection of mice
H. capsulatum strain G217B (ATCC 26032) was maintained in the yeast form at 37°C in Histoplasma-macrophage medium (HMM)4 broth in 5% CO2. Mice were injected i.p. with 5 × 105 mid-exponential phase yeast cells in 0.5 ml of HMM. For intranasal infections, mice were anesthetized, then 4 × 106 H. capsulatum yeast was introduced into the nose in 20 μ1 of HMM.
Organ load CFU
Liver, lung, and spleen were harvested aseptically from infected mice. Tissue samples were homogenized in RPMI 1640 medium, and serial dilutions were plated on Brain Heart Infusion agar containing gentamicin and cultured at 28°C for 2–3 wk. Data are presented both as total CFU per organ and as total CFU per gram tissue. The limit of sensitivity is >100 CFU.
Histology
Small pieces of liver and lung tissue and liver granulomatous chunks were fixed in 10% buffered formalin and embedded in paraffin. Sectioning and staining were performed by the Histopathology Service of Department of Pathology, University of Wisconsin. Thin tissue sections (8–10 μm) were stained with Gomori's methenamine silver (GMS) to detect H. capsulatum in granulomatous area of the liver by microscopy. For quantitation, GMS-stained yeasts were counted in 30 lesions per group at ×400 total magnification, and the result is presented as the average ± SEM. H&E staining was done for morphometric studies. Liver granuloma burden is the number of lesions per grid at ×100 magnification using an Olympus reticular eyepiece and is presented as the average of nine grids per group ± SEM. Granuloma size was measured at ×400 magnification using the grid and is presented as the average of 30 granulomatous lesions ± SEM.
Isolation of splenocytes and liver granuloma cells
Total splenocytes were isolated from aseptically removed naive and infected spleens by standard methods. Isolation of granuloma-infiltrating cells from infected livers was a modification of previously published methods used to isolate Mycobacterium-induced liver granulomas (17, 20, 21). Briefly, livers were homogenized with a tissue blender, and liver granulomas were allowed to settle by virtue of their higher density. After decanting the supernatant, settled granulomas were washed in RPMI 1640 medium. The granuloma pellet was digested with 5 mg/ml type I collagenase (catalog no. 0130; Sigma-Aldrich) at 37°C for 40 min with shaking. Granulomas were disrupted using a syringe, followed by filtering through a 70-μm nylon cell strainer (BD Falcon) to remove any tissue debris. The live leukocyte count was determined by trypan blue staining. Bulk granuloma cell preps were derived by pooling and processing a minimum of two to three livers from infected animals, allowing us to isolate ∼10–15 million granuloma-infiltrating cells for flow cytometry.
Flow cytometry and Abs
For flow cytometric analysis of cell surface marker expression, splenocytes or granuloma cell suspensions were incubated for 30 min at 4°C with different labeled Abs at saturation, then washed and analyzed. Unlabeled 50 μg/ml anti-FcR Ab (2.4G2) was used to block binding via FcR. Cell surface staining on 20,000–50,000 events was measured using a FACS-Calibur instrument (BD Biosciences) and analyzed using the FlowJo computer program (Macintosh version 6.2.1; Tree Star).
Abs labeled with various fluorochromes and specific for murine LFA-1, CD8, MHC class II (IAb), CD25, γδ TCR (clone GL3), TNF-α, IFN-γ, IL-17, TGF-β1, CD4, B220, and CD8 were purchased from BD Pharmingen. Abs specific for CD5 (clone LyT-1), Vγ1.1 (clone UC2), Mac-1, Cd11c (clone N418), and DEC205 were purified from hybridoma cell lines and labeled with fluorochromes in our laboratory.
Intracellular cytokine staining
A total of 106 splenocytes or granuloma cells was cultured for 5 h in a 96-well tissue culture plate, in complete RPMI (cRPMI) 1640 medium, containing 10% FCS and 1% Golgi-Stop (BD Pharmingen) ± 5 μg/ml anti-CD3 Ab. Cells were resuspended in 50 μ1 of FACS staining buffer (1× PBS containing 10% BSA and 0.1% sodium azide) and stained for surface markers CD4, CD8, and Mac-1 for 30 min on ice, with 2 μg of blocking 2.4G2 Ab. Cells were washed three times and fixed by Cytofix/ Cytosperm solution (BD Pharmingen) at room temperature for 20 min. Cells were permeabilized and washed three times in FACS buffer containing 0.1% saponin and incubated for 30 min on ice with anti-TNF-α, anti-IFN-γ, anti-TGF-β, anti-IL-17, or isotype control rat IgG Ab.
Cytokine and chemokine measurements from cell culture supernatant
A total of 106 granuloma cells was cultured for 72 h in a 96-well tissue culture plate, in cRPMI 1640 medium, containing 10% FCS ± 5 μg/ml anti-CD3 Ab. Cell culture supernatants were harvested and stored at − 80°C until analysis. Supernatants were sent to LINCO Research for custom analysis using a 22-plex Mouse Cytokine and Chemokine cytometric bead array. Intra- and interassay variances are <10 and 20%, respectively. For TGF-β measurements, 106 granuloma cells were cultured for 48 h in a 96-well tissue culture plate, in cRPMI 1640 medium without FBS, containing ±5 μg/ml anti-CD3 Ab. Supernatants were stored at − 80°C and assayed by TGF-β1 Emax ELISA kit according to the manufacturer's protocol (Promega).
PCR and primers
Single granulomas were isolated from the preparative suspension before dispersal with collagenase under ×10 magnification using a Pasteur pipette flame-drawn to a finer tip. Individual lesions were stored at − 80°C before processing. mRNA from individual granuloma was isolated using a MicroFast Track mRNA isolation kit (Invitrogen Life Technologies) according to the manufacturer's instructions. cDNA synthesis and RT-PCR was performed as previously described (22, 23) using published primers for CDR3 length analysis (24). Products from these reactions were analyzed on 2% agarose gels and visualized by ethidium bromide staining.
Immunohistochemistry and TUNEL staining
Five-micrometer thick cryosections were cut from OCT-embedded liver tissue samples and fixed for 30 min in 4% paraformaldehyde in PBS, then washed three times with PBS and outlined with a Pap pen. Sections were blocked with 40 μg/ml 2.4G2 Ab in 1% BSA for 30 min and then stained for 30 min using CyChrome-labeled anti-CD4 (RM5-4) and biotin-labeled anti-CD11b (Mac-1) in combination with Alexa568-labeled streptavidin. TUNEL staining was performed following the manufacturer's protocol (Roche Diagnostics). Confocal images were acquired on a Bio-Rad MRC-1024 maintained by the W. M. Keck Laboratory for Biological Imaging (University of Wisconsin).
Results
To investigate granulomatous inflammation induced by H. capsulatum, we established an i.p. infection model of liver granuloma formation. Intraperitoneal infection results in quantitative inoculum delivery, immediate systemic access, and rapid development of disseminated infection. We developed i.p. inoculation as a model to look at systemic, disseminated histoplasmosis at stages beyond the initial respiratory acquisition—specifically dissemination and granulomatous disease in the mononuclear phagocytic system. Liver granulomas are induced naturally after respiratory Histoplasma infection and after intranasal inoculation, but i.p. infection optimizes both yield and synchronization.
H. capsulatum organ load after i.p. infection
To establish our model, we first measured fungal load by quantitative plating (total organ load by CFU) and also by quantitative morphology (counting fungal bodies in GMS-stained liver sections) (Fig. 1). Both methods clearly indicate that the highest fungal load is detected at 7 days after infection, and most of the infection is controlled after 50 days (Fig. 1, B–D). Not surprisingly, the Histoplasma load is higher in spleen and liver (Fig. 1, C and D) but is detectable at low levels in the lung as well (see Fig. 3A, lower panels). While at day 5, H. capsulatum yeasts are observed adjacent to blood vessels in the liver parenchyma without an organized granulomatous response (Fig. 1A, left panel); from day 7, Histoplasma can be seen almost exclusively in the central regions of well-formed granulomas in both liver (Fig. 1A) and lung (Fig. 3A, lower row).
FIGURE 1.

Kinetics of Histoplasma capsulatum growth after i.p. inoculation. A, Photomicrographs at ×400 total magnification of GMS stained thin liver sections at days 5, 7, 10, 14, and 50 after infection of C57BL/6 mice. H. capsulatum cells are indicated by arrows and bar represents 10 μm. B, Quantitation of H. capsulatum in randomly selected lesions was used to calculate the number of yeasts per granulomatous lesion. Error bars represent ±SEM (n = 30 lesions). C, Histoplasma capsulatum organ load cultures from spleen, lung, and liver are presented as LOG CFU ± SEM or (D) LOG CFU per gram tissue ± SEM using four to six mice per time point.
FIGURE 3.

Kinetics of Histoplasma capsulatum induced histopathology in the lung. A, Photomicrographs shown are of thin lung sections at days 5, 7, 10, 14, and 50 after infection of C57BL/6 mice. H&E-stained sections are shown at ×40 (top row) and ×400 (middle row) total magnification, and GMS stain is shown at ×400 total magnification (bottom row). Yeasts are indicated by arrows. B, Lung lesion size was measured as in Fig. 2. Data represent the average ± SEM from three mice per group. Granuloma burden was not quantified because less than one granuloma per field was observed. No more than three granulomas per lobe cross-section were observed.
Histopathology and morphometric analysis
Our next goal was to analyze the kinetics of Histoplasma-induced granuloma formation in the liver. At day 5 after Histoplasma infection, H&E-stained thin liver sections show mild vasculitis and some cellular infiltration (Fig. 2A, top row). Rarely, minor aggregates of inflammatory cells are observed. Higher magnification images (Fig. 2A, bottom row) show macrophage extravasation and focal aggregation of leukocytes. Digital enlargement of the lower left panel of Fig. 2B demonstrates that after extravasation, inflammatory cells migrate in a line (a direct line into the tissue) to H. capsulatum yeasts. A sophisticated well-regulated process of granuloma formation is suggested by the rapid appearance of many well-structured granulomas 2 days later by day 7, which persist through 10 and 14 days (Fig. 2A, top and bottom rows). The appearance of the granulomas is sarcoid, having epithelioid cells in the center. Analysis of the number (Fig. 2C) and size of lesions (Fig. 2D) shows that while the number of granuloma remains high through 14 days, the size of the lesions is down-modulated by day 14 (Fig. 2C). At 50 days postinfection, a few well-formed and small-sized granuloma persist in the liver.
FIGURE 2.

Kinetics of Histoplasma capsulatum induced liver granuloma formation. A, Photomicrographs shown are of H&E-stained thin liver sections at days 5, 7, 10, 14, and 50 after infection of C57BL/6 mice. Total magnification is ×40 (top row) or ×400 (bottom row). Inflammatory lesions are indicated by arrowheads, and track of macrophage extravasation is marked by stars. B, A 3-fold digital enlargement of day 5 ×400 panel is shown. C, Liver granuloma burden is the average of number of granulomatous lesions per field ± SEM counted from randomly selected fields of H&E-stained sections at ×100 total magnification (n = 9). D, Liver granuloma size is the average total area of randomly selected lesions from H&E-stained sections, at ×400 total magnification (n = 30 lesions each from three mice per group). Data represent the average ± SEM.
In the lung (Fig. 3, upper two rows), after the initial appearance of smaller inflammatory cell aggregates, well-formed granulomas dominate from day 10. Interestingly, lung granuloma are much more variable in appearance and size than in the liver but tend to be larger overall. In addition, the very few granulomas present after day 50 (less than or equal to three granulomas per lobe) are still large and are not down-modulated in size as in the liver (Fig. 3, A and B).
Phenotypic analysis and cellular composition of liver granuloma-infiltrating cells during the course of Histoplasma infection
Isolation of granulomatous lesions allows us to study these inflammatory reactions, including cellular composition, cytokine milieu, and gene expression at the local level. This approach has been applied very successfully to granulomas formed in soft tissues like the liver in response to various infectious agents (16, 25–28). Isolation of infiltrating cells after settling and digestion of granulomas from dispersed liver tissue has the advantage over other methods like laser dissection in that it gives a much larger sample size for study. In the present study, we report that H. capsulatum-induced granulomas can be isolated from the liver.
The individual granuloma settles out with a small rim of liver cells. The digested granuloma preparation is enriched for inflammatory cells that can be studied by flow cytometry (Figs. 4A and 5A, lower left insets). For our flow cytometry studies, bulk granuloma cell preps were derived by pooling and processing a minimum of two to three livers from infected animals. The following section will describe the proportion and phenotype of inflammatory cells isolated in this manner.
FIGURE 4.
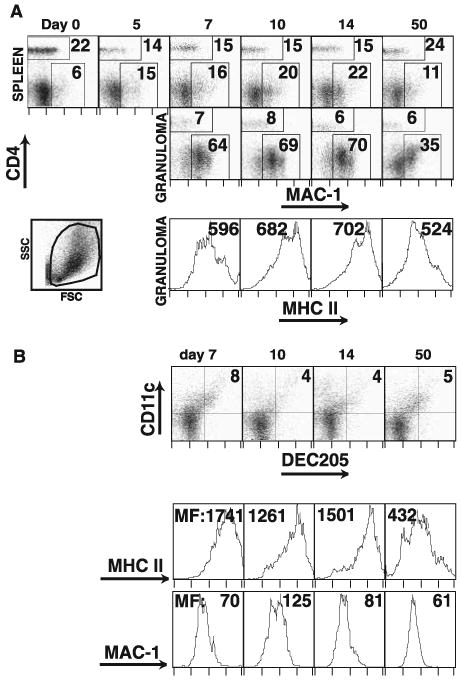
Macrophage and dendritic cells in liver granulomas. Flow cytometric analysis of splenocyte and granuloma-infiltrating cells during the course of Histoplasma infection. A, Dot plots represent expression of Mac-1 and CD4 surface staining on open gated cells from spleen (top row) and granuloma cells (middle row). Lower left dot plot demonstrates forward and orthogonal scatter of the open gate for a granuloma suspension. Values on dot plots represent the percentage of the gated cells in the indicated regions. Histograms represent MHC class II expression on Mac-1 + granuloma cells, and values are mean fluorescent intensity (MFI) (bottom row). B, Dot plots represent expression of DEC205 and CD11c surface staining on open gated cells from granuloma (top row). Indicated values are the quadrant percent of the gated cells. Histograms represent MHC class II expression (middle row) and Mac-1 expression (bottom row) on DEC205+CD11c+ cells. Values are MFI for the gated population. All plots and histograms are representative of three independent experiments.
FIGURE 5.
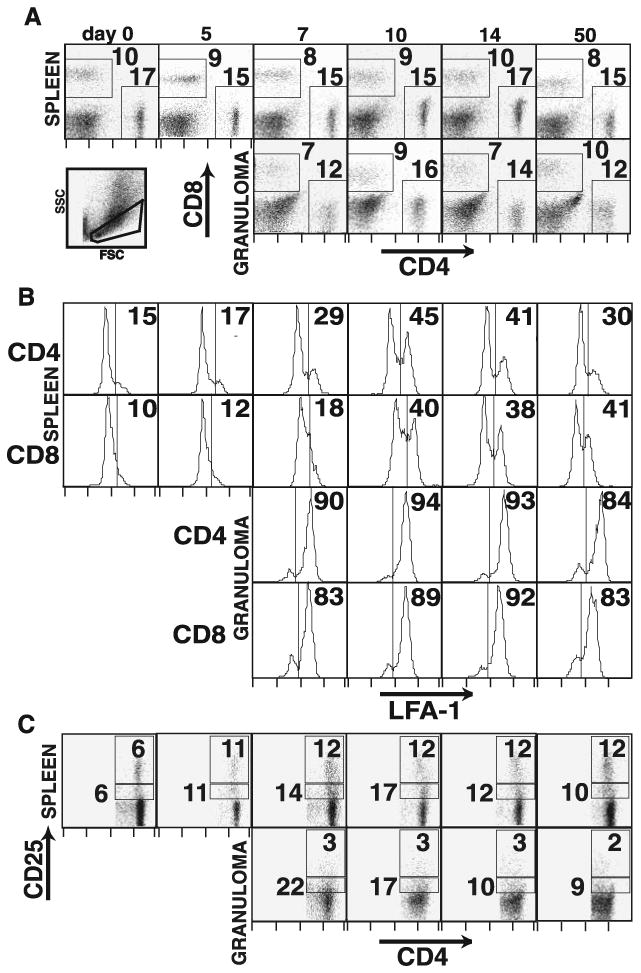
CD4+ and CD8+ T cells in Histoplasma-induced liver granulomas. Flow cytometric analysis of splenocyte and granuloma-infiltrating cells during the course of Histoplasma infection. A, Dot plots represent expression of CD4 and CD8 surface staining on lymphocyte-gated cells from spleen (top row) and granuloma cells (middle row). Lower left dot plot demonstrates forward and orthogonal scatter of the lymphocyte gate for a granuloma suspension. Values on dot plots represent the percentage of the gated cells in the indicated regions. B, Histograms represent expression of LFA-1 on gated CD4+ and CD8+ lymphocyte populations as indicated in total splenocytes (top panels) and granuloma-infiltrating cells (bottom panels). Values represent the percentage of the population having LFA-1 expression higher than the indicated level. C, Dot plots represent expression of CD4 and CD25 surface staining on CD4+ lymphocyte-gated cells from spleen (top row) and granuloma cells (bottom row). Values represent the percentage of the CD4+ gated cells in the indicated (CD25high and CD25int) regions. All plots are representative of three independent experiments.
The proportion of macrophages is somewhat increased in the spleen after the infection; however, macrophages clearly dominate the Histoplasma-induced granulomatous lesion (Fig. 4A, top and middle rows). MHC class II expression on Mac-1+ cells from both spleen and granuloma is elevated (data not shown and Fig. 4A, bottom row). MHC class II expression is the activation marker of macrophages responding to local IFN-γ-mediated signaling. Both total numbers of Mac-1+ cells and MHC class II expression are highest at 10 and 14 days postinfection.
DEC205+CD11c+ dendritic cells represent 1–2% of total spleen cells and that proportion is unaltered during H. capsulatum infection (data not shown). In the early granuloma (day 7; Fig. 4B), a large fraction (8%) of the cells are DEC205+CD11c+ expressing high levels of MHC class II (Fig. 4B, middle row) and intermediate levels of Mac-1 (Fig. 4B, bottom row). The proportion of these cells decreases at later time points but remains consistently higher than in the corresponding spleen samples.
Next, we examined the phenotype of granuloma-infiltrating T cells. Fig. 5A shows that the ratio of CD4+ to CD8+ T cells in splenocytes is essentially unchanged during the course of infection compared with naive splenocytes. In contrast, in granuloma-infiltrating cells, CD4+ T cells are more abundant during the early acute-phase time points, while CD8+ T cells catch up during the chronic stage granuloma at day 50. At the same time, CD4+ T cells with a LFA-1high phenotype arise in the spleen sooner than do CD8+LFA-1high T cells, but the proportion of LFA-1highCD8+ T cells is somewhat higher by day 50. Both CD4+ and CD8+ T cells exhibit a uniformly high expression of LFA-1 in the granuloma (Fig. 5B), indicating an activated phenotype. Both CD4+ effector T cells with intermediate expression of CD25 and T cells with CD25high expression roughly double in the spleen by day 5 of infection and remain elevated through day 50 (Fig. 5C). In the granuloma, CD25int cells are present at a higher frequency than in the spleen, which is consistent with the LFA-1high phenotype of the majority of granuloma-infiltrating T cells. In contrast, CD25highCD4+ T cells thought to be regulatory T cells are present at much lower levels in the granuloma relative to both infected spleen and naive spleen, suggesting an exclusion of those cells from the local inflammatory site that persists through day 50. This is especially interesting since not only does the proportion of these cells double during infection, but the infection increases the total number of splenocytes by 2- to 3-fold.
TCR usage in granuloma-infiltrating T cells
TCR Vβ-chain repertoire studies for Histoplasma infection models have been a subject of extensive investigation (29–32). A consensus exists that the repertoire is heterogeneous overall, but there is some controversy regarding Vβ-chain preferences observed in some models for spleen and lymph node. Our goals were to extend these broad observations to the local inflammatory site.
We looked at the proportional usage of TCR genes in spleen and granuloma during the course of infection using Vβ-chain-specific Abs. Although the Vβ-chain usage is somewhat different between spleen and granuloma, all tested Vβ-chains are present and similarly distributed in spleen and granuloma (Fig. 6A and data not shown). We further studied whether the heterogeneity we observed at the level of pooled cell suspensions (spleens and bulk granuloma) is present in single lesions. Fig. 6B shows a representative single granuloma from a settled preparation before enzymatic digestion. First, the presence of V/β8.2 message was measured in six single granulomas representing three different time points (Fig. 6C). Although the absolute level of message varied, V/β8.2 usage was observed in all single granulomas. Then, the usage of various Jβ genes in Vβ8.2-specific gene transcripts was tested (Fig. 6D), and the data indicated that there are both similarities (Jβ2.4) and many differences in the usage of Jβ genes in different lesions at each examined time point. These data clearly demonstrate TCR heterogeneity even at the single granuloma level and argues that, instead of a few T cells expanding at the inflammatory site, most T cells expand systemically and then home to the granuloma producing a diverse T cell repertoire.
FIGURE 6.

TCR Vβ-chain usage in Histoplasma-infected spleen and granuloma cells. A, TCR Vβ3-, Vβ6-, Vβ8.1/8.2-, and Vβ11-chain-specific Abs were used in combination with CD4- and CD8-specific Abs to detect cell surface expression in total splenocytes or granuloma-infiltrating cells at the indicated time points after infection of C57BL/6 mice. Plotted values represent the average percentage of total CD4+ or CD8+ cells expressing the indicated chain from four to six mice per time point. Error bars represent ± SEM. B, The photomicrograph illustrates a H&E-stained individual isolated liver granuloma from day 10 postinfection at ×100 magnification. Individual granulomas are isolated from homogenized liver preparations and further processed for preparation of single-granuloma total mRNA. C, Poly(A)+ RNA was prepared from six single granulomatous lesions isolated after settling of dispersed Histoplasma-infected liver and used as a template for RT-PCR using TCR Vβ8.2 region- and constant region-specific primers. β2-microglobulin-specific primers were used to demonstrate the equivalency of the cDNA template for each reaction. PCR products were separated on 2% agarose gels. D, Vβ8.2-specific RT-PCR product described in B was used as a template for secondary PCR using Vβ8.2 primers and 10 Jβ allele-specific primers. PCR products were separated on 2% agarose gels. The gel images shown are cropped to the estimated size range of the various Jβx PCR products (92–130 bp) and arranged top to bottom to reflect the time points from which the individual granulomas were isolated.
γδ T cells are known to have a role both in controlling infections (33, 34) and in contributing to regulation of granulomas (35). To investigate whether γδ T cells accumulate in Histoplasma-induced liver granulomas, we measured the expression of γδ TCR and the dominant Vγ1.1 chain in both splenocytes and granuloma-infiltrating cells at time points after infection. Fig. 7 shows that γδ T cells as a percentage of the total increase very slightly in splenocytes after Histoplasma infection (0.7% at day 7 vs 0.4% in naive spleen). However, only a few γδ T cells are in Histoplasma-induced granulomas, and their proportion does not change during the course of infection. In addition, TCR Vγ1.1 chain is used by ∼80% of systemic γδ T cells in uninfected mice. This ratio does not change either in the infected spleen or in the granuloma, suggesting an absence of selection for specific γδ TCR.
FIGURE 7.

γδ T cells in Histoplasma-induced liver granulomas. Flow cytometric analysis of splenocytes and granuloma-infiltrating cells at various times after Histoplasma infection of C57BL/6 mice. Dot plots represent γδ TCR and Vγ1.1 chain-specific cell surface staining on CD4−CD8−B220−Mac-1− lymphocyte-gated cells. Values are the percentage of the total sample stained with γδ TCR-specific Ab. γδ TCR+Vγ1.1+ populations are shown by separate regions. The plots are representative of three independent experiments.
While T cells are essential for granuloma function in all instances (36), the presence of B cells varies according to the granuloma-inducing pathogen. Histoplasma-induced liver granulomas also contain a B220+/MHC class II+ B cell component representing ∼10% of lymphocyte-gated cells during the acute infection and increasing to ∼20% of lymphocyte-gated cells at day 50 after infection (Fig. 8A). Alternative B cells expressing the nonconventional marker CD5 represent 5% of splenic B cells. Although CD5 + B cells are the dominant B cell type both in peritoneum and in liver-resident B cells (data not shown), they do not contribute to the B cell pool in the day 10 granuloma (Fig. 8B).
FIGURE 8.

B cells in Histoplasma-induced liver granulomas. Flow cytometric analysis of splenocytes and granuloma-infiltrating cells at various times after Histoplasma infection of C57BL/6 mice. A, Dot plots represent MHC class II- and B220-specific cell surface expression on lymphocytegated cells. Values represent the percentage of MHC class II+B2209+ gated B lymphocytes shown in the upper right quadrant. B, Dot plots represent CD5- and B220-specific cell surface expression on lymphocyte-gated cells at day 10 postinfection. Values represent the percentage of CD5+B220+ cells of lymphocyte-gated cells shown in the oval region. Dot plots are representative of three independent experiments.
Cytokines and chemokines in Histoplasma-induced granulomas
Next, we investigated the local cytokine and chemokine levels in Histoplasma-induced granulomas. Multiplex analysis of cytokines secreted by Histoplasma-induced granuloma-infiltrating cells during 72 h in vitro culture shows that IL-2 production is minimal from day 7 onward and only above the level of detection in response to anti-CD3 stimulation (Table I). Of the Th2 cytokines, IL-5 is the most abundant, and IL-4, IL-9, and IL-13 are at low to undetectable levels. Both TNF-α and IFN-γ have been shown to be important to control of Histoplasma infection (13, 37). Our data show that both cytokines can be produced at very high levels by cells from the granulomatous inflammatory site, demonstrating the strong Th1 bias of infiltrating cells. Comparison of the levels in the supernatants of cultures with and without T cell activating anti-CD3 suggests that IFN-γ is T cell derived and TNF-α is not. Intracellular staining of TNF-α and IFN-γ (Fig. 9) shows that most TNF-α is produced by Mac-1+ cells (macrophages), whereas IFN-γ is produced both by CD4+ and CD8+ T cells. It is clear that the population of cells in the granuloma that produce TNF-α (Fig. 9A) or IFN-γ (data not shown) is much higher than in the spleen. Like IFN-γ, IL-17 is a strong inducer of macrophage effector function and is associated with chronic inflammation. IL-17 is produced by CD4+ granuloma-infiltrating cells (Table I and see Fig. 11). Macrophages are also influenced by high levels of inflammatory cytokines IL-1 and IL-6, in addition to chemokines and macrophage growth factors. These factors favor the recruitment, proliferation, and activation of macrophage that are the dominant cell type in H. capsulatum-induced granulomas and were detected at high levels in the supernatants from granuloma-infiltrating cells. While the level of these molecules are high at all three time points tested, at day 14, the level of proinflammatory mediators decreases. Interestingly, although IL-10 and TGF-β are known to have immunosuppressive functions, their levels in early granuloma culture supernatants are high. The main cellular source of TGF-β is the macrophage (see Fig. 11). These data illustrate that a large number of mediators operate in the local inflammatory lesions and calls for a functional analysis of this local regulatory network.
Table I.
Cytokine and chemokine production of granuloma-infiltrating cellsa
| Day 7 | Day 10 | Day 14 | ||||
|---|---|---|---|---|---|---|
| Media | Anti-CD3 | Media | Anti-CD3 | Media | Anti-CD3 | |
| IL-2 | <3.2 | 4.6 | <3.2 | 3.3 | <3.2 | 9.3 |
| Th1 cytokines | ||||||
| IFN-γ | 545 | >10,000 | 431 | >10,000 | 145 | >10,000 |
| TNF-α | 538 | 565 | 616 | 552 | 542 | 249 |
| IL-17 | <3.2 | 24 | 64 | 231 | 92 | 35 |
| Th2 cytokines | ||||||
| IL-4 | <3.2 | 3.3 | <3.2 | 4.6 | <3.2 | <3.2 |
| IL-5 | 25 | 68 | 13 | 52 | 11 | <3.2 |
| IL-9 | <3.2 | <3.2 | 12 | 18 | 15 | <3.2 |
| IL-13 | <3.2 | 4.7 | <3.2 | 6.3 | 5.4 | <3.2 |
| Inflammatory and anti-inflammatory cytokines | ||||||
| IL-10 | 1,064 | 1,092 | 822 | 842 | 740 | ndb |
| TGF-βactivec | 522 | 521 | 267 | 169 | 224 | 51 |
| TGF-βlatentc | <32 | <32 | <32 | <32 | 236 | 383 |
| IL-1a | 354 | 310 | 759 | 860 | 676 | nd |
| IL-1β | 448 | 343 | 773 | 391 | 517 | nd |
| IL-6 | 16,594 | >18,000 | > 18,000 | >18,000 | 17,962 | nd |
| CC chemokines | ||||||
| MIP-1α | <3.2 | > 10,000 | >10,000 | > 10,000 | 5,782 | 2,682 |
| MCP | 2,102 | 2,121 | 1,390 | 709 | 593 | 740 |
| Rantes | <3.2 | 22 | 95 | 85 | 19 | 237 |
| CXC chemokines | ||||||
| IP-10 | 19 | 15 | 59 | 27 | 17 | 302 |
| KC | 2,053 | 1,730 | 5,821 | 3,599 | 3,625 | 42 |
| Macrophage growth factors | ||||||
| GM-CSF | 575 | 986 | 152 | 425 | 189 | nd |
| G-CSF | 7092 | >10,000 | >10,000 | >10,000 | 8,652 | nd |
All units are pg/ml.
nd, not done.
Done by ELISA.
FIGURE 9.

IFN-γ and TNF-α production by cells from Histoplasma-induced granuloma. Intracellular cytokine analysis of splenocytes and granuloma-infiltrating cells at various times after Histoplasma infection of C57BL/6 mice. A, Dot plots represent Mac-1 and TNF-α staining on splenocytes and granuloma-infiltrating cells after 5 h of in vitro culture. Values represent the percentage of cells from the indicated quadrant in an open gate. B, Dot plots represent CD4 and IFN-γ staining on granuloma-infiltrating cells after 5 h of culture ± anti-CD3. Values represent the percentage of IFN-γ+CD4+ lymphocyte-gated cells. C, Dot plots represent CD8 and IFN-γ staining on granuloma-infiltrating cells after 5 h of culture ± anti-CD3. Values represent the percentage of IFN-γ+CD8+ lymphocyte-gated cells. Dot plots are representative of three independent experiments.
FIGURE 11.
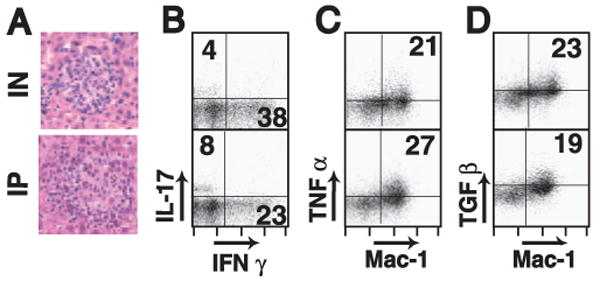
Granulomas induced by i.p. (IP) or intranasal (IN) Histoplasma infection. Liver granulomas were induced via intranasal or i.p. infection routes. A, Images represent ×400 total magnification of H&E-stained thin liver sections from mice infected intranasally (upper image) or i.p. (lower image). Intracellular cytokine staining was performed on granuloma-infiltrating cells after 5 h of incubation with 5 μg/ml anti-CD3 and analyzed by flow cytometry. B, Dot plots represent IFN-γ and IL-17 intracellular expression in granuloma-infiltrating CD4+ T cells. Numbers in quadrants show the percentage of IFN-γ+ or IL-17+ cells in the CD4+ gate. Dot plots represent TNF-α (C) and TGF-β (D) production by granuloma-infiltrating Mac-1+ cells. Numbers on plots are the percentage of (C) TNF-α+Mac-1+ or (D) TGF-β+Mac-1+ double-positive cells in an open gate.
Apoptosis of granuloma-infiltrating leukocytes in Histoplasma infection
Others have suggested that Histoplasma-induced T cell apoptosis is a requirement for developing effective antifungal protection (38). Microscopic examination of Histoplasma-infected liver granulomas by H&E staining did not show frequent central necrosis in C57BL/6 mice. To characterize the presence of apoptotic cells in Histoplasma granulomas, we used immunofluorescent Ab staining and TUNEL staining. Mac-1+ and CD4+ cells (Fig. 10, A and B) were enriched in Histoplasma liver granulomas in proportions consistent with our FACS data (Fig. 4A). In addition, immunofluorescent end-labeling of DNA fragments was enriched within granulomas, suggesting a relatively high occurrence of apoptotic cells (Fig. 10C). Costained sections demonstrate that both macrophage and T cells contribute to the apoptotic fraction of granuloma-infiltrating cells (Fig. 10D). The granuloma centers are where the large majority of the apoptotic cells are found.
FIGURE 10.

Apoptosis in Histoplasma-induced liver granuloma. TUNEL staining of liver sections from day 10 Histoplasma infection was done to detect apoptosis of liver granuloma-infiltrating macrophages and CD4+ T cells. Confocal microscopy was performed on cryosections stained with (A) biotin-conjugated anti-CD11b (Mac-1) in combination with Alexa 568-labeled streptavidin (red), (B) CyChrome-labeled anti-CD4 (RM5-4, blue), and (C) after end-labeling DNA with fluorescein-dUTP (TUNEL, green). D, Merged confocal image of liver granuloma costained with anti-CD11b, anti-CD4, and TUNEL.
Comparison of Histoplasma liver granulomas induced via i.p. and intranasal routes
In this article, we used an i.p. route of infection as a model for disseminated histoplasmosis. It is possible that the natural infection route through the airways might induce a different type of disseminated liver granuloma due to the modifying effect of a complex lung infection and altered local APC. We compared the i.p. model granulomas to liver granulomas induced after intranasal infection. Fig. 11A shows that the morphology of the liver granulomas induced is very similar. Analysis of cellular composition, T cell activation, TCR usage, DC, and macrophage subsets were also very similar in intranasal and i.p. infection models (data not shown).
Importantly, measurement of intracellular cytokine production by granuloma cells suggests that lesions induced after either infection route contain CD4+ T cells, which produce similar levels of IFN-γ and IL-17 (Fig. 11B). Both cytokines are important for activating macrophage. Lastly, Mac-1+ cells from both lesion types are able to produce TNF-α (Fig. 11C) and TGF-β (Fig. 11D).
Discussion
The success of host immunity in controlling H. capsulatum infection is underscored by the wide prevalence of asymptomatic or clinically mild infections despite skin test reactivity rates over 90% in endemic areas along the Ohio and the Mississippi River valleys (5, 39). Granulomas formed in response to macrophages infected by Histoplasma are most likely a dominant component of the highly effective antifungal immune response. Conversely, the progressive, disseminated histoplasmosis observed in immune-compromised persons arises in large part from failures of established granulomas and failure to form new inflammatory lesions in response to recently infected macrophages. While there is growing knowledge about the nature of systemic immunity during the course of histoplasmosis (32, 40–42), there is little known about local immune responses within granulomas despite these lesions representing the main interface between the fungi and the host. Previously, one of the limiting factors was lack of an isolation procedure for Histoplasma-induced granulomas. Effective techniques for isolating granulomas from the liver have been published for S. mansoni (21)-, Mycobacterium (17)-, and Leishmania (16)-infected mice among others. Subsequent studies of granuloma-in-filtrating cells significantly enriched our understanding of similarities and differences between local and systemic immunity in response to these infectious agents (43). The goal of the present studies was to isolate H. capsulatum-induced granulomas and describe the cellular composition and cytokine milieu of granuloma-infiltrating cells during the course of infection. From this foundation of basic knowledge, mechanistic studies can be initiated to understand local immune regulatory networks in H. capsulatum infection and their relevance in the control of this infectious agent.
As soon as 5 days after i.p. infection, yeast-infected macrophages are detectable in the liver by histopathology. At that early time, no granulomas are visible, although there is an evident vasculitis and evidence for extravasation of leukocytes toward infected macrophages (Fig. 2). Just 2 days later, the liver is marked by an abundance of organized sarcoid-type granulomas. The ability to form granulomas in just 2 days provides a well-timed model to study granuloma formation. The complexity and similarity of these synchronized lesions suggest a well-orchestrated and regulated process.
Morphometric analysis of liver granuloma size showed a dynamic change throughout the time course of the infection, which is mirrored in the kinetics of Histoplasma clearance. The average granuloma size reaches a maximum at day 10 of the infection and subsequently declines. This observation recalls the down-regulation of acute lesion size during chronic disease reported for Schistosoma and Leishmania infections (44, 45). In the latter models, the down-modulation of granuloma size is driven by IL-10 and TGF-β (46–48). Multiplex data show that IL-10 levels are elevated in ex vivo cultures of H. capsulatum granuloma-infiltrating cells with and without anti-CD3 stimulation, suggesting that IL-10 is elevated in the Histoplasma granuloma. Interestingly, both IL-10 and TGF-β are highest in the early granuloma. The main source of TGF-β is macrophage, according to our intracellular FACS data. In addition to IL-10, fungal clearance leading to decreased antigenic stimuli, inflammatory agents and chemoattractants, and attenuated cellular recruitment may contribute to the time-dependent size decrease of liver granulomas. Interestingly, the size of lung granulomas remains at a maximum for a longer time, perhaps indicating that the host organ affects the phenotype of granulomatous lesions. Another interesting observation is that new granuloma formation is clearly present after organ load cultures indicate clearance of the yeast. It is possible that fragments of the yeast persist and serve as granuloma-inducing irritants. However, it is also possible that granulomatous protection is not completely sterilizing, and very low numbers of yeast survive and serve as a source for reactivation disease during immune deficiency.
While most Histoplasma granulomas appear free of necrosis, TUNEL staining (Fig. 10) shows that at day 10 when the granulomas are the largest, the lesion centers are enriched in apoptotic cells. Clearing of apoptotic cells by local macrophages may contribute to the decrease in granuloma size, perhaps as part of a continuous turnover of the cellular composition of the lesion. It may also be important in the control of the yeast by clearing infected cells. An intriguing report (38) shows that apoptosis is a prerequisite for a successful immune response. Mycobacteria are also known to evade immune clearance by inhibiting apoptosis in the infected macrophage (49, 50).
Both liver histology and flow cytometry of the granuloma-infiltrating cells indicate that macrophage are the dominant cell type in the lesion reaching up to 70% of the granuloma. Most of them are activated because the levels of IFN-γ and other macrophage-activating factors are high in the lesions. Many of the Mac-1+ macrophage have elevated MHC class II expression and produce TNF-α (Figs. 4A and 9A and Table I). There are very few other myeloid cells in granulomas. H&E-stained sections suggest that most lesions have few, if any, neutrophils, while flow cytometry indicates that only a low percentage of granuloma-infiltrating cells express a dendritic cell phenotype (CD11c+DEC205+) (Fig. 4B). The presence of dendritic cells may provide a local reactivation for the recruited effector T cells and raises the possibility that they sample granuloma Ags and may carry them to draining lymph nodes. The idea that granuloma-contained Ags might prime systemic T cells needs further investigation.
Both CD4+ and CD8+ T cells are recruited to the lesions. At early stages, there are more CD4+ T cells present, but later the ratio is close to 1:1. This temporal change in the CD4/CD8 T cell ratio likely reflects the somewhat earlier systemic activation of CD4+ T cells relative to activation of CD8+ T cells. Both T cells contribute to the cytokine milieu of the granulomas. A subpopulation of cells has IL-2R expression levels above the level on activated effector T cells and consistent with regulatory T cells. Interestingly, the proportion of CD4+IL-2Rhigh cells is higher systematically than in the granuloma, suggesting that this population of cells fails to accumulate in the lesions. The role of regulatory T cells in Histoplasma-induced granuloma formation warrants more investigation since these cells are reported to regulate Leishmania- and Schistosoma-induced lesions (51–54). Furthermore, given the limitation of regulatory T cell classification based on CD25 expression alone, additional phenotypic and functional characterization will be needed in further studies of regulatory T cells in Histoplasma infection.
The level of γδ T cell is also lower in granulomas compared with systemic sites. γδ T cells have been reported in granulomas (55) affecting granuloma size during the chronic stage of infection (56–58). A low level of B cells is also present having a conventional B cell phenotype (CD5−,Mac-1−) characteristic of peripheral blood. The potential for local Ab production in the granuloma is potentially important since there are reports that Abs can protect against intracellular yeasts (58).
The TCR repertoire of systemic T cells during Histoplasma infection has been studied before. While some studies reported relative enrichment of T cells using certain TCR Vβ-chains (29–31, 59, 60) and others did not (29, 32, 60), there is a consensus that H. capsulatum activates a diverse repertoire of T cells. In the present study, we extend this finding to granuloma-infiltrating T cells. Our data clearly indicate a heterogeneous repertoire in the granuloma with no major superantigen-type shifts in TCR usage. Moreover, using J allele-specific primer pairs, we demonstrated that there is a large level of T cell heterogeneity even at the single granuloma level (Fig. 6D). Additionally, we looked at TCR usage at days 7, 10, and 14 and saw no shifts consistent with clonal dominance or with founder effects through the time course.
This finding argues that instead of a model in which a few T cell founders home to the early granuloma and expand there, the repertoire of the Histoplasma-induced granuloma derives from the entire systemically expanded T cell population. This is similar to our previously reported observations for both Mycobacterium bovis bacillus Calmette-Guérin- and S. mansoni-induced granuloma formation (22, 23), indicating that similar principles drive T cell accumulation in various granulomas. Likewise, TCR repertoire analysis of the low numbers of γδ T cells infiltrating the granuloma indicates that they express the Vγ1.1 receptor allele in a similar proportion those found in the blood. Collectively, B cells, αβ T cells, and γδ T cells in the Histoplasma-induced liver granuloma all appear to reflect the peripheral blood populations.
IFN-γ plays a central role in the immunity against H. capsulatum. In fact, IFN-γ-deficient animals are killed by H. capsulatum infection (12, 37). We extended this finding by showing that IFN-γ is produced at high levels by granuloma-infiltrating cells (Table I and Fig. 9, B and C). The main cellular source of IFN-γ in the granulomas is CD4+ and CD8+ T cells (Fig. 9, B and C). Local IFN-γ activates macrophage to produce reactive radicals crucial to control of the yeast. The granuloma-infiltrating cells also produce high levels of TNF-α. Intracellular staining (Fig. 9A) and the absence of anti-CD3-induced TNF-α production (Table I) show that the primary cellular source of TNF-α is the macrophage, and T cells contribute little if any to local TNF-α levels. Therapeutic anti-TNF-α treatments induced reactivation of latent H. capsulatum infection in some patients, emphasizing the role of this mediator in control of the yeast and preventing dissemination (8, 10, 61). The granuloma has strong Th1-type and very little Th2-type cytokine activity (Table I). Interestingly, the newly described Th-17 cells (61) may also be present, as IL-17 levels increase in response to anti-CD3. Intracellular staining also shows the presence of CD4+ IL-17-producing cells in the lesions (Fig. 11B). The Th-17 lineage is proposed to be a major effector population responsible for tissue damage in chronic autoimmune diseases. One of the reported functions of Th-17 cells is in the induction of chemokines. We observed that granuloma-infiltrating cells produce high levels of multiple chemokines capable of mediating the recruitment of macrophages and lymphocytes to the lesion (Table I). The in situ production of myeloid growth factors may help macrophage recruitment and survival in the granulomas.
All of the previous investigations of immune responses to Histoplasma infection characterized either systemic responses or acute peripheral responses. To our knowledge, ours is the first study looking at the local inflammatory site at a stage corresponding to Histoplasma-induced delayed-type hypersensitivity responses. We compared our granuloma responses to systemic responses represented by the spleen and found a very good correspondence in the systemic parameters we measured with previous characterizations by other investigators. Specifically, other investigators have seen that systemic responses against Histoplasma infection are dominated by CD4+ T cell responses and a requirement for IFN-γ and TNF-α production as basic factors of fungal control. Likewise, Histoplasma granulomas are dominated by activated macrophage producing TNF-α and infiltrated by IFN-γ-producing CD4+ T cells. Overall, the Histoplasma-induced delayed-type hypersensitivity response in the liver is characterized by diffuse sarcoid-type granuloma formation, which contains fungal growth and is associated with a relatively rapid fungal clearance.
There are three nonexclusive paradigms about granuloma formation. Traditionally, granuloma formation is considered a pathology in which host responses to infectious pathogens induce organ damage. Simultaneously, it is widely accepted that granulomas are a protective form of delayed-type hypersensitivity controlling the expansion of infectious agents. The formation of the granulomatous lesion walls off pathogens from further access to the host, thus preventing dissemination and organ damage arising from the host inflammatory response. Finally, in the field of Mycobacterium infection, Ramakrishnan and coworkers (62) describe granulomas as ecosystems of mutual benefit to host and infectious organism. They posit granuloma formation as an evolutionary compromise (62) in which the host restricts both dissemination and damaging acute inflammation, while allowing a small number of pathogens to survive for extended intervals. The granuloma:pathogen balance results in relatively little harm to the healthy host, yet dormant bacteria are able to reactivate and disseminate when host immunity wanes and granuloma formation fails. Mutant Mycobacteria eliciting an altered host pathology after infection, pat mutants, provide support for the concept that the infectious agent participates in forming the granuloma (reviewed in Ref. 63). Studies indicate that during secondary infection, Mycobacterium preferentially “home” to pre-existing granulomas where immunity is strong but not sterilizing (62). The occurrence of reactivation disease in immune-deficient hosts for both tuberculosis and histoplasmosis makes it possible that a similar host:pathogen accommodation may be operating during Histoplasma infection. All of these paradigms stress the importance of further knowledge regarding H. capsulatum-induced granulomas to understand the biology of infection and provide a sound basis for more efficacious therapeutic interventions.
Acknowledgments
We acknowledge Dr. Bruce S. Klein for valuable comments on the study. We thank Khen Macvilay and Toshi Kinoshita for excellent technical support.
Footnotes
This study was funded by National Institutes of Health Grants R01 AI48087 (to M.S.), R01 AI52303 (to J.P.W), R01 HL55949 (to J.P.W.), and R37 AI42747 (J.P.W. subcontract on award to George S. Deepe, Jr.).
Abbreviations used in this paper: HMM, Histoplasma-macrophage medium; GMS, Gomori's methenamine silver; MFI: mean fluorescence intensity; cRPMI, complete RPMI.
Disclosures: The authors have no financial conflict of interest.
References
- 1.Kobayashi K, Kaneda K, Kasama T. Immunopathogenesis of delayed-type hypersensitivity. Microsc Res Tech. 2001;53:241–245. doi: 10.1002/jemt.1090. [DOI] [PubMed] [Google Scholar]
- 2.Flynn JL, Chan J. Tuberculosis: latency and reactivation. Infect Immun. 2001;69:4195–4201. doi: 10.1128/IAI.69.7.4195-4201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan J, Flynn J. The immunological aspects of latency in tuberculosis. Clin Immunol. 2004;110:2–12. doi: 10.1016/s1521-6616(03)00210-9. [DOI] [PubMed] [Google Scholar]
- 4.Woods JP. Knocking on the right door and making a comfortable home: Histoplasma capsulatum intracellular pathogenesis. Curr Opin Microbiol. 2003;6:327–331. doi: 10.1016/s1369-5274(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 5.Woods JP. Histoplasma capsulatum molecular genetics, pathogenesis, and responsiveness to its environment. Fungal Genet Biol. 2002;35:81–97. doi: 10.1006/fgbi.2001.1311. [DOI] [PubMed] [Google Scholar]
- 6.Zerbe CS, Holland SM. Disseminated histoplasmosis in persons with interferon γ receptor 1 deficiency. Clin Infect Dis. 2005;41:e38–e41. doi: 10.1086/432120. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg JD, Scheinfeld NS. Cutaneous histoplasmosis in patients with acquired immunodeficiency syndrome. Cutis. 2003;72:439–445. [PubMed] [Google Scholar]
- 8.Giles JT, Bathon JM. Serious infections associated with anticy-tokine therapies in the rheumatic diseases. J Intensive Care Med. 2004;19:320–334. doi: 10.1177/0885066604267854. [DOI] [PubMed] [Google Scholar]
- 9.Conant MA. Fungal infections in immunocompromised individuals. Dermatol Clin. 1996;14:155–162. doi: 10.1016/s0733-8635(05)70336-3. [DOI] [PubMed] [Google Scholar]
- 10.Wood KL, Hage CA, Knox KS, Kleiman MB, Sannuti A, Day RB, Wheat LJ, Twigg HL., III Histoplasmosis after treatment with anti-tumor necrosis factor α therapy. Am J Respir Crit Care Med. 2003;167:1279–1282. doi: 10.1164/rccm.200206-563OC. [DOI] [PubMed] [Google Scholar]
- 11.Nath DS, Kandaswamy R, Gruessner R, Sutherland DE, Dunn DL, Humar A. Fungal infections in transplant recipients receiving alemtuzumab. Transplant Proc. 2005;37:934–936. doi: 10.1016/j.transproceed.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 12.Clemons KV, Darbonne WC, Curnutte JT, Sobel RA, Stevens DA. Experimental histoplasmosis in mice treated with anti-murine interferon γ antibody and in interferon γ gene knockout mice. Microbes Infect. 2000;2:997–1001. doi: 10.1016/s1286-4579(00)01253-3. [DOI] [PubMed] [Google Scholar]
- 13.Allendoerfer R, Deepe GS., Jr Blockade of endogenous TNF-α exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J Immunol. 1998;160:6072–6082. [PubMed] [Google Scholar]
- 14.Williams DM, Graybill JR, Drutz DJ. Histoplasma capsulatum infection in nude mice. Infect Immun. 1978;21:973–977. doi: 10.1128/iai.21.3.973-977.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstock JV, Elliott D, Metwali A, Blum A, Li J, Qadir K, Sandor M. Immunoregulation within the granulomas of murine schistosomiasis mansoni. Microbes Infect. 1999;1:491–498. doi: 10.1016/s1286-4579(99)80087-2. [DOI] [PubMed] [Google Scholar]
- 16.Wilson ME, Weinstock JV. Hepatic granulomas in murine visceral Leishmaniasis chagasi. Methods. 1996;9:248–254. doi: 10.1006/meth.1996.0031. [DOI] [PubMed] [Google Scholar]
- 17.Sacco RE, Jensen RJ, Thoen CO, Sandor M, Weinstock J, Lynch RG, Dailey MO. Cytokine secretion and adhesion molecule expression by granuloma T lymphocytes in Mycobacterium avium infection. Am J Pathol. 1996;148:1935–1948. [PMC free article] [PubMed] [Google Scholar]
- 18.Co DO, Hogan LH, Kim SI, Sandor M. Mycobacterial granulomas: keys to a long-lasting host-pathogen relationship. Clin Immunol. 2004;113:130–136. doi: 10.1016/j.clim.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Adams LB, Krahenbuhl JL. Granulomas induced by Mycobacterium leprae. Methods. 1996;9:220–232. doi: 10.1006/meth.1996.0029. [DOI] [PubMed] [Google Scholar]
- 20.Sandor M, Sperling AI, Cook GA, Weinstock JV, Lynch RG, Bluestone JA. Two waves of γδ T cells expressing different Vδ genes are recruited into schistosome-induced liver granulomas. J Immunol. 1995;155:275–284. [PubMed] [Google Scholar]
- 21.Metwali A, Elliott D, Blum AM, Li J, Sandor M, Lynch R, Noben-Trauth N, Weinstock JV. The granulomatous response in murine Schistosomiasis mansoni does not switch to Th1 in IL-4-deficient C57BL/6 mice. J Immunol. 1996;157:4546–4553. [PubMed] [Google Scholar]
- 22.Hogan LH, Macvilay K, Barger B, Co D, Malkovska I, Fennelly G, Sandor M. Mycobacterium bovis strain bacillus Calmette Guerin-induced liver granulomas contain a diverse TCR repertoire, but a monoclonal T cell population is sufficient for protective granuloma formation. J Immunol. 2001;166:6367–6375. doi: 10.4049/jimmunol.166.10.6367. [DOI] [PubMed] [Google Scholar]
- 23.Hogan LH, Wang M, Suresh M, Co DO, Weinstock JV, Sandor M. CD4+ TCR repertoire heterogeneity in Schistosoma mansoni-induced granulomas. J Immunol. 2002;169:6386–6393. doi: 10.4049/jimmunol.169.11.6386. [DOI] [PubMed] [Google Scholar]
- 24.Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T cell receptor β-chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci USA. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandor M, Houlden B, Bluestone J, Hedrick SM, Weinstock J, Lynch RG. In vitro and in vivo activation of murine γ/δ T cells induces the expression of IgA, IgM, and IgG Fc receptors. J Immunol. 1992;148:2363–2369. [PubMed] [Google Scholar]
- 26.Hogan LH, Markofski W, Bock A, Barger B, Morrissey JD, Sandor M. Mycobacterium bovis BCG-induced granuloma formation depends on γ interferon and CD40 ligand but does not require CD28. Infect Immun. 2001;69:2596–2603. doi: 10.1128/IAI.69.4.2596-2603.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McHardy P, Riley J, Huntley JF. The recruitment of mast cells, exclusively of the mucosal phenotype, into granulomatous lesions caused by the pentastomid parasite Porocephalus crotali: recruitment is irrespective of site. Parasitology. 1993;106(Pt 1):47–54. doi: 10.1017/s0031182000074801. [DOI] [PubMed] [Google Scholar]
- 28.Nibbering PH, van der Heide GA, van Furth R. Immunocytochemical analysis of cellular responses to BCG. Clin Exp Immunol. 1989;75:147–154. [PMC free article] [PubMed] [Google Scholar]
- 29.Deepe GS, Jr, Gibbons RS, Ward SR. Discordance between T cell receptor expression and effector function in mice infected with Histoplasma capsulatum. Infect Immun. 2002;70:1648–1652. doi: 10.1128/IAI.70.3.1648-1652.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheckelhoff M, Deepe GS., Jr The protective immune response to heat shock protein 60 of Histoplasma capsulatum is mediated by a subset of Vβ8.1/8.2+ T cells. J Immunol. 2002;169:5818–5826. doi: 10.4049/jimmunol.169.10.5818. [DOI] [PubMed] [Google Scholar]
- 31.Gomez FJ, Woodward EO, Pilcher-Roberts R, Gibbons RS, Deepe GS., Jr Vβ6+ and Vβ4+ T cells exert cooperative activity in clearance of secondary infection with Histoplasma capsulatum. J Immunol. 2001;166:2855–2862. doi: 10.4049/jimmunol.166.4.2855. [DOI] [PubMed] [Google Scholar]
- 32.Lin JS, Wu-Hsieh BA. Functional T cells in primary immune response to histoplasmosis. Int Immunol. 2004;16:1663–1673. doi: 10.1093/intimm/dxh168. [DOI] [PubMed] [Google Scholar]
- 33.Ladel CH, Blum C, Dreher A, Reifenberg K, Kaufmann SH. Protective role of γ/δ T cells and α/β T cells in tuberculosis. Eur J Immunol. 1995;25:2877–2881. doi: 10.1002/eji.1830251025. Published erratum appears in. [DOI] [PubMed] [Google Scholar]; Eur J Immunol. 1995;25:3525. [Google Scholar]
- 34.Ladel CH, Hess J, Daugelat S, Mombaerts P, Tonegawa S, Kaufmann SH. Contribution of α/β and γ/δ T lymphocytes to immunity against Mycobacterium bovis bacillus Calmette Guerin: studies with T cell receptor-deficient mutant mice. Eur J Immunol. 1995;25:838–846. doi: 10.1002/eji.1830250331. [DOI] [PubMed] [Google Scholar]
- 35.D'Souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, Orme IM. An anti-inflammatory role for γδ T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–1221. [PubMed] [Google Scholar]
- 36.Co D, Hogan LH, Kim SI, Sandor M. T cell contributions to the different phases of granuloma formation. Immunol Lett. 2004;92:135–147. doi: 10.1016/j.imlet.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 37.Allendoerfer R, Deepe GS., Jr Intrapulmonary response to Histoplasma capsulatum in γ interferon knockout mice. Infect Immun. 1997;65:2564–2569. doi: 10.1128/iai.65.7.2564-2569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen HL, Deepe GS. Apoptosis modulates protective immunity to the pathogenic fungus Histoplasma capsulatum. J Clin Invest. 2005;115:2875–2885. doi: 10.1172/JCI25365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cano MV, Hajjeh RA. The epidemiology of histoplasmosis: a review. Semin Respir Infect. 2001;16:109–118. doi: 10.1053/srin.2001.24241. [DOI] [PubMed] [Google Scholar]
- 40.Newman SL. Cell-mediated immunity to Histoplasma capsulatum. Semin Respir Infect. 2001;16:102–108. doi: 10.1053/srin.2001.24240. [DOI] [PubMed] [Google Scholar]
- 41.Deepe GS, Jr, Gibbons RS. Protective and memory immunity to Histoplasma capsulatum in the absence of IL-10. J Immunol. 2003;171:5353–5362. doi: 10.4049/jimmunol.171.10.5353. [DOI] [PubMed] [Google Scholar]
- 42.Deepe GS., Jr Modulation of infection with Histoplasma capsulatum by inhibition of tumor necrosis factor α activity. Clin Infect Dis. 2005;41(Suppl 3):S204–S207. doi: 10.1086/429999. [DOI] [PubMed] [Google Scholar]
- 43.Sandor M, Weinstock JV, Wynn TA. Granulomas in schistosome and mycobacterial infections: a model of local immune responses. Trends Immunol. 2003;24:44–52. doi: 10.1016/s1471-4906(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 44.Boros DL, Pelley RP, Warren KS. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975;114:1437–1441. [PubMed] [Google Scholar]
- 45.Gutierrez Y, Maksem JA, Reiner NE. Pathologic changes in murine leishmaniasis (Leishmania donovani) with special reference to the dynamics of granuloma formation in the liver. Am J Pathol. 1984;114:222–230. [PMC free article] [PubMed] [Google Scholar]
- 46.Mola PW, Farah IO, Kariuki TM, Nyindo M, Blanton RE, King CL. Cytokine control of the granulomatous response in Schistosoma mansoni-infected baboons: role of exposure and treatment. Infect Immun. 1999;67:6565–6571. doi: 10.1128/iai.67.12.6565-6571.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadler CH, Rutitzky LI, Stadecker MJ, Wilson RA. IL-10 is crucial for the transition from acute to chronic disease state during infection of mice with Schistosoma mansoni. Eur J Immunol. 2003;33:880–888. doi: 10.1002/eji.200323501. [DOI] [PubMed] [Google Scholar]
- 48.Stadecker MJ. The shrinking schistosomal egg granuloma: how accessory cells control T cell-mediated pathology. Exp Parasitol. 1994;79:198–201. doi: 10.1006/expr.1994.1080. [DOI] [PubMed] [Google Scholar]
- 49.Winau F, Hegasy G, Kaufmann SH, Schaible UE. No life without death-apoptosis as prerequisite for T cell activation. Apoptosis. 2005;10:707–715. doi: 10.1007/s10495-005-2940-6. [DOI] [PubMed] [Google Scholar]
- 50.Schaible UE, Winau F, Sieling PA, Fischer K, Collins HL, Hagens K, Modlin RL, Brinkmann V, Kaufmann SH. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat Med. 2003;9:1039–1046. doi: 10.1038/nm906. [DOI] [PubMed] [Google Scholar]
- 51.McKee AS, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol. 2004;173:1224–1231. doi: 10.4049/jimmunol.173.2.1224. [DOI] [PubMed] [Google Scholar]
- 52.Freeman CM, Chiu BC, Stolberg VR, Hu J, Zeibecoglou K, Lukacs NW, Lira SA, Kunkel SL, Chensue SW. CCR8 is expressed by antigen-elicited, IL-10-producing CD4+CD25+ T cells, which regulate Th2-mediated granuloma formation in mice. J Immunol. 2005;174:1962–1970. doi: 10.4049/jimmunol.174.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh KP, Gerard HC, Hudson AP, Reddy TR, Boros DL. Retroviral Foxp3 gene transfer ameliorates liver granuloma pathology in Schistosoma mansoni infected mice. Immunology. 2005;114:410–417. doi: 10.1111/j.1365-2567.2004.02083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 55.Modlin RL, Pirmez C, Hofman FM, Torigian V, Uyemura K, Rea TH, Bloom BR, Brenner MB. Lymphocytes bearing antigen-specific γδ T cell receptors accumulate in human infectious disease lesions. Nature. 1989;339:544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka S, Itohara S, Sato M, Taniguchi T, Yokomizo Y. Reduced formation of granulomata in γδT cell knockout BALB/c mice inoculated with Mycobacterium avium subsp. paratuberculosis. Vet Pathol. 2000;37:415–421. doi: 10.1354/vp.37-5-415. [DOI] [PubMed] [Google Scholar]
- 57.Saunders BM, Frank AA, Cooper AM, Orme IM. Role of γδT cells in immunopathology of pulmonary Mycobacterium avium infection in mice. Infect Immun. 1998;66:5508–5514. doi: 10.1128/iai.66.11.5508-5514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nosanchuk JD, Steenbergen JN, Shi L, Deepe GS, Jr, Casadevall A. Antibodies to a cell surface histone-like protein protect against Histoplasma capsulatum. J Clin Invest. 2003;112:1164–1175. doi: 10.1172/JCI19361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gomez FJ, Cain JA, Gibbons R, Allendoerfer R, Deepe GS., Jr Vβ4+ T cells promote clearance of infection in murine pulmonary histoplasmosis. J Clin Invest. 1998;102:984–995. doi: 10.1172/JCI2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deepe GS, Jr, Gibbons RS. Functional properties of the T cell receptor repertoire in responding to the protective domain of heat-shock protein 60 from Histoplasma capsulatum. J Infect Dis. 2002;186:815–822. doi: 10.1086/342602. [DOI] [PubMed] [Google Scholar]
- 61.Wynn TA. T(H)-17: a giant step from T(H)1 and T(H)2. Nat Immunol. 2005;6:1069–1070. doi: 10.1038/ni1105-1069. [DOI] [PubMed] [Google Scholar]
- 62.Cosma CL, Humbert O, Ramakrishnan L. Superinfecting mycobacteria home to established tuberculous granulomas. Nat Immunol. 2004;5:828–835. doi: 10.1038/ni1091. [DOI] [PubMed] [Google Scholar]
- 63.Hogan LH, Co D, Sandor M. Mycobacterial granulomas: a genomic approach. In: Falus A, editor. Immunogenomics and Human Disease. John Wiley & Sons; London: 2005. pp. 497–514. [Google Scholar]


