Abstract
Background
There is a strong interest in identifying chemopreventive agents that might help decrease the burden of lung cancer. The active metabolite of vitamin D, 1,25-dihydroxycholecalciferol (calcitriol), has been shown to have antiproliferative effects in several tumor types, mediated by the vitamin D receptor (VDR). This is the first comprehensive survey of VDR expression in a series of human lung tissues, including normal and premalignant central airway biopsies and lung tumors.
Methods
Immunohistochemical expression of nuclear and cytoplasmic VDR was examined in 180 premalignant or malignant bronchial biopsies from bronchoscopy of 78 high-risk individuals at the Roswell Park Cancer Institute and also in 63 tumor samples from 35 lung cancer patients from the University of Chicago Hospitals. Associations between clinicopathologic data and VDR expression were examined.
Results
VDR expression was present in many samples. In biopsies, VDR was commonly detected throughout the full epithelial layer. Most histologically normal (60%, 53 of 88) and metaplastic (61%, 39 of 64) samples had moderate to high nuclear intensity; dysplastic samples mostly had low nuclear intensity (10 of 18, 55%). In tumor samples, 62% (38 of 61) were lacking cytoplasmic VDR, with nuclear expression present in 79%(49 of 62). Analysis of all samples revealed a positive linear trend between proportion of samples with greater nuclear than cytoplasmic intensity and increasing histologic grade (P < 0.01).
Conclusions
VDR expression spanned the lung carcinogenesis spectrum. Nuclear expression was similar across various histologies, whereas cytoplasmic expression decreased with increasing histologic grade. These results indicate that there is potential for the use of calcitriol as a chemopreventive agent against the development of lung cancer. (Cancer Epidemiol Bio-markers Prev 2008;17(5):1104–10)
Introduction
Lung cancer is the leading cause of cancer mortality in many countries, accounting for over 1 million deaths annually, worldwide (1). In the United States, lung cancer ranks second in incidence in both males and females (2). It is estimated that lung cancer will be diagnosed in over 213,000 individuals in 2007, and be the leading cause of cancer death, with 160,930 deaths (3). Based on these estimates, lung cancer will be responsible for 31% of the cancer deaths in men and 26% of the cancer deaths in women (3).
Advances in screening and treatment have done little to decrease lung cancer mortality rates (2). This has increased the interest in the identification of effective chemopreventive agents that might help decrease the burden of this disease. One such agent is 1,25-dihydroxycholecalciferol [1,25(OH)2D or calcitriol], the primary active form of vitamin D. It is a steroid hormone that is involved in modulating mineral metabolism, especially that of calcium, by acting on bone, kidneys, and intestinal tract. It is converted to the active 1,25(OH)2D form in many cells, including the lung (4). This active form has been shown to have antiproliferative and anti-invasive properties and to induce apoptosis in a number of tumor cell types (5).
The activity of calcitriol is largely mediated by the vitamin D receptor (VDR), whose expression has been described in some tissues. VDR is a member of the nuclear receptor superfamily present in many types of epithelial and mesenchymal cells, including those found in the lung (6). Studies involving radiolabeled 1,25(OH)2D3 have shown that VDR localization is mostly nuclear (7), although there is evidence of cytoplasmic receptors (8), especially in the absence of ligands and serum in cell culture. In the cell, VDR binds 1,25(OH)2D, and the complex interacts with the retinoid X receptor to form a 1,25(OH)2D*VDR*retinoid X receptor heterodimer complex. This complex can bind vitamin D–responsive elements in the promoter regions of target genes. This interaction causes a variety of downstream events, including the induction of differentiation and the inhibition of proliferation, invasiveness, angiogenesis, and metastatic potential (8, 9).
To better understand the potential for calcitriol in lung cancer chemoprevention, the expression of VDR was examined in bronchial biopsies obtained from the autofluorescent bronchoscopy examination of high-risk individuals, as well as resected malignant lung tissue from lung cancer patients. Immunohistochemical techniques were used to assess VDR, and associations with clinical and pathologic characteristics of the patients and their specimens were examined. Only one study describes VDR expression in human lung tissue (10), focusing exclusively on lung tumors and cell lines. This study is the first comprehensive description of VDR expression in a series of human lung tissue types, including normal and premalignant central airway biopsies and lung tumors. The presence of VDR in target tissues is considered a prerequisite for a calcitriol-mediated effect and VDR characterization highlights the chemopreventive potential of this agent.
Materials and Methods
Patient and Sample Selection
Tissues analyzed for this study came from two sources: A total of 180 premalignant and malignant biopsies were obtained from the baseline bronchoscopic examination of 78 patients who attended a high-risk surveillance clinic at the Roswell Park Cancer Institute in Buffalo, New York. Additionally, 19 squamous cell carcinoma samples from 19 patients and 44 adenocarcinomas from 16 patients were obtained from the University of Chicago, Department of Pathology, and contained on two tumor micro-arrays.
Patients’ clinical and pathologic information was obtained from lung bronchoscopy database (Roswell Park Cancer Institute) and lung cancer patient database (University of Chicago). Included were such variables as smoking status (never, former, current), histologic diagnosis (biopsies), tumor-node-metastasis staging, and tumor grade.
VDR Immunohistochemistry
Immunohistochemistry was carried out with the monoclonal antibody 9A7 (Affinity BioReagents) for VDR. Four-micrometer-thick tissue sections were deparaffinized by two xylene rinses followed by two rinses with 100% ethanol and two rinses with 95% ethanol. Slides were then pretreated by heating citrate buffer in a steamer and placing the slides in the buffer for 40 min. The slides were cooled for 20 min on Shandon cassettes and snapped onto Shandon racks using DAKO buffer. H2O2 was added and the slides were incubated for 10 min and then rinsed with wash buffer. Two hundred microliters of primary VDR antibody (2.5 μg/mL, 1:500 dilution) were added, and slides were incubated overnight at 4°C. Slides were then incubated for 30 min with biotinylated RAB versus RT (Rat/Rab, 1:300 dilution), rinsed, and incubated for 30 more minutes with a conjugated rabbit polymer (Envision+ horseradish peroxidase). This incubation was followed by two rinses, and slides were visualized with diaminobenzidine chromogen, placed into distilled water, and dipped three times into hematoxylin. This was followed by dipping the slides twice into 95% ethanol for 2 min, dipping twice into 100% ethanol for 2 min, and then dipping in xylene. Finally, a coverslip was added to the slide.
Slides were scored simultaneously by two pathologists (R.C. and A.H.) and the consensus score was recorded. Slides were assessed for intensity of staining relative to external and internal controls (scored from none to high, or 0 to 3, respectively), percentage of cells stained within the scoring region (<10%, 10–50%, >50%), and distribution of staining within the epithelium (basal, suprabasal, full thickness) for the biopsies and squamous cell carcinomas. This was done for nuclear and cytoplasmic expression.
Statistical Analysis
Due to the paucity of studies of VDR expression in human lung tissue, the main goal of this analysis was to describe the VDR expression across the various histologic grades of lung tissue. Summary statistics for the patients involved were presented in frequencies, percentages, means, and SDs. Similar descriptive statistics were provided to describe the various categories of VDR expression (intensity, percentage of cells, epithelial distribution), stratified by tissue histology. For the tumor samples, expression was also examined by differentiation and stage. The χ2 and Mann-Whitney tests were used to examine relationship between the expression variables and tissue characteristics.
In 24 of the patients who received a bronchoscopy, both histologically normal and premalignant (metaplasia or dysplasia) biopsies were obtained. For these samples, intra-individual comparisons of VDR characteristics of the matching normal and abnormal biopsies were made. These comparisons described the differences in staining intensity and percentage of positive cells.
Results
Patient Characteristics
Characteristics of high-risk patients receiving a bronchoscopy are summarized in Table 1. Most patients were male, with about one fifth having a history of lung cancer. More than one third (37%) were current smokers at the time of their baseline bronchoscopy, which is higher than the current smoking rate in the general population. Due to the types of referrals sent to the clinic, 41 (53%) of the patients had asbestos exposure, and most had chronic obstructive pulmonary disease (74%). Among the cancer patients, a majority were male (57%) and former smokers (57%). All 16 of the adenocarcinoma patients were White.
Table 1.
Descriptive characteristics of 78 high-risk patients who had a premalignant or malignant lung lesion diagnosed on bronchoscopy-aided biopsy at Roswell Park Cancer Institute and 35 patients who underwent lung cancer resection at the University of Chicago Hospitals
| Characteristics | Patients with premalignant lesions* (n = 78), n (%) | Patients with adenocarcinoma (n =16), n (%) | Patients with squamous cell carcinoma (n = 19), n (%) |
|---|---|---|---|
| Age | |||
| Mean (SD) | 61.2 (10.0) | 60.1 (10.7) | 68.5 (7.4) |
| Range | 33–78 | 40–80 | 52–80 |
| Sex | |||
| Female | 19 (24) | 6 (38) | 9 (47) |
| Male | 59 (76) | 10 (62) | 10 (53) |
| Race | |||
| White | 103 (97) | 16 (100) | 11 (58) |
| Black | 3 (3) | — | 8 (42) |
| Smoking status | |||
| Never | 2 (3) | 3 (19) | 1 (5) |
| Former | 47 (60) | 10 (63) | 10 (53) |
| Current | 29 (37) | 2 (13) | 5 (26) |
| Unknown | — | 1 (6) | 3 (16) |
| Previous lung cancer | |||
| Yes | 16 (20) | — | — |
| No | 62 (80) | ||
| COPD | |||
| Yes | 58 (74) | — | — |
| No | 20 (26) | ||
| Asbestos exposure | |||
| Yes | 41 (53) | — | — |
| No | 37 (47) | ||
| Tumor stage | |||
| I | — | 11 (69) | 7 (37) |
| II | — | 6 (31) | |
| III | 5 (31) | 5 (26) | |
| Unknown | — | 1 (5) | |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Includes histologic diagnoses of metaplasia, mild dysplasia, moderate dysplasia, and severe dysplasia.
Bronchial Biopsies
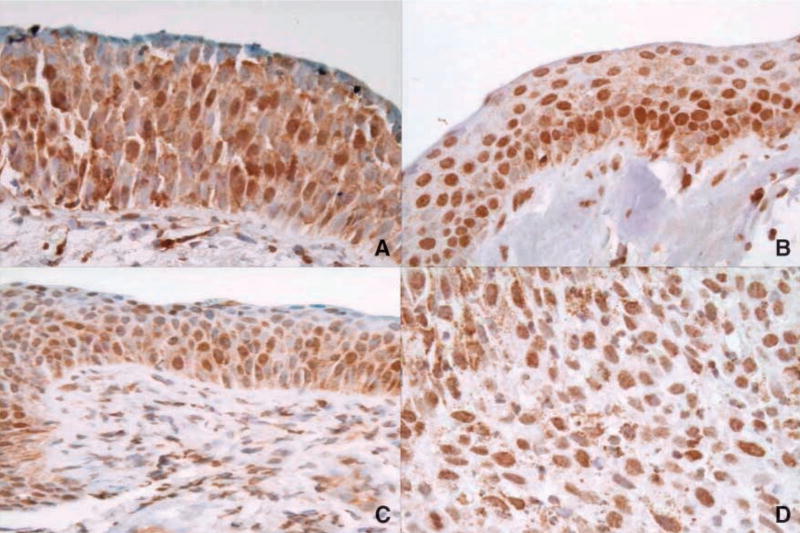
A few of the nonmalignant biopsies had a high staining intensity; most were weak to moderate (Table 2). However, all four of the bronchoscopy-detected cancers were at least of moderate intensity, containing a high percentage of positive-staining cells. VDR expression in the normal and metaplastic biopsies (Fig. 1A and B) had similar nuclear patterns for all four of the categories summarized in Table 2. A majority of the biopsies with positive VDR expression had it throughout the full epithelial layer. A comparison of the nuclear and cytoplasmic intensities in the biopsies revealed that the proportion of biopsies with higher nuclear than cytoplasmic staining intensity increased with histologic grade, although this trend was not statistically significant. The intensity of cytoplasmic VDR staining was significantly greater in normal and metaplastic tissues than dysplastic tissues (P = 0.02 and P = 0.03, respectively). There were no associations between VDR expression and patient characteristics.
Table 2.
VDR expression characteristics of the biopsies, stratified by the histology
| Normal, n (%) |
Metaplasia, n (%) |
Dysplasia, n (%) |
Bronchoscopy-detected cancers, n (%) |
Squamous cell carcinoma TMA, n (%) |
Adenocarcinoma TMA, n (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear | Cytoplasmic | Nuclear | Cytoplasmic | Nuclear | Cytoplasmic | Nuclear | Cytoplasmic | Nuclear | Cytoplasmic | Nuclear | Cytoplasmic | |
| Intensity | ||||||||||||
| 0 | 17 (19) | 15 (17) | 15 (23) | 13 (20) | 3 (17) | 10 (23) | — | — | 3 (17) | 5 (28) | 28 (64) | 10 (59) |
| 1 | 18 (21) | 25 (29) | 10 (16) | 16 (24) | 6 (33) | 14 (32) | — | — | 7 (39) | 9 (50) | 8 (18) | 3 (18) |
| 2 | 50 (57) | 46 (53) | 33 (52) | 37 (56) | 9 (50) | 20 (46) | 3 (75) | 3 (75) | 8 (44) | 4 (22) | 8 (18) | 4 (24) |
| 3 | 3 (3) | 1 (1) | 6 (9) | — | — | — | 1 (25) | 1 (25) | — | — | — | |
| Percentage of cells (%) | ||||||||||||
| 0 | 17 (19) | 15 (17) | 15 (23) | 13 (20) | 4 (21) | 10 (23) | — | — | — | 5 (28) | 28 (64) | 12 (63) |
| <10 | 3 (3) | 7 (8) | 4 (6) | 2 (3) | — | — | — | — | 3 (17) | — | 1 (2) | — |
| 10–50 | 16 (18) | 12 (14) | 12 (19) | 14 (22) | 1 (5) | 4 (9) | — | 1 (25) | 5 (28) | 3 (17) | 5 (11) | 1 (5) |
| >50 | 51 (59) | 53 (61) | 33 (52) | 36 (55) | 14 (74) | 30 (68) | 4 (100) | 3 (75) | 10 (56) | 10 (56) | 10 (23) | 6 (32) |
| Distribution | ||||||||||||
| No staining | 17 (21) | 15 (18) | 15 (25) | 13 (21) | 3 (21) | — | — | — | 3 (18) | 5 (28) | — | 10 (59) |
| Basal | 2 (2) | 5 (6) | 2 (3) | 1 (2) | — | — | — | — | 1 (6) | 0 | — | — |
| Suprabasal | 19 (23) | 17 (20) | 8 (14) | 17 (28) | 2 (14) | — | — | — | 1 (6) | 1 (6) | — | 3 (18) |
| Full | 44 (54) | 46 (55) | 34 (58) | 30 (49) | 9 (64) | — | 2 (100) | 2 (100) | 12 (71) | 12 (67) | — | 4 (24) |
| Cytoplasmic vs nuclear intensity | ||||||||||||
| Nuclear > cytoplasmic | 25 (29) | 22 (34) | 7 (39) | 9 (53) | 30 (68) | |||||||
| Equal | 38 (44) | 26 (41) | 8 (44) | 4 (100) | 4 (24) | 5 (11) | ||||||
| Cytoplasmic > nuclear | 24 (29) | 16 (25) | 3 (17) | 4 (24) | 9 (21) | |||||||
Abbreviation: TMA, tumor microarray.
Figure 1.
VDR expression across various lung histologies. A. Normal. B. Metaplasia. C. Dysplasia. D. Squamous cell carcinoma. In many samples, VDR was expressed in a large percentage of the cells, in multiple layers of the bronchial epithelium. Cytoplasmic expression decreased with increasing histologic grade.
Matched Normal and PremalignantBiopsies
Both histologically normal and premalignant (metaplasia or dysplasia) biopsies were obtained from 24 of the patients receiving bronchoscopy. Table 3 summarizes the intra-individual comparison of VDR intensity and percent of positive cells for these patients. Whereas the cytoplasmic variables were similar in the matched samples, nuclear intensity and percentage of positive cells was at least twice as likely to be higher in the premalignant lesions than in the matching normal biopsies. However, the differences were not statistically significant, likely due to lack of statistical power and the small sample size.
Table 3.
Intra-individual comparison of VDR expression in matched histologically normal and premalignant biopsies, obtained from 24 individuals during bronchoscopy
| Normal > premalignant* | Normal = premalignant* | Normal < premalignant* | P† | |
|---|---|---|---|---|
| Nuclear intensity, n (%) | 5 (21) | 9 (38) | 10 (42) | 0.42 |
| Cytoplasmic intensity, n (%) | 7 (29) | 10 (42) | 7 (29) | 0.69 |
| Percentage of cells positive for nuclear expression, n (%) | 4 (17) | 10 (42) | 10 (42) | 0.28 |
| Percentage of cells positive for cytoplasmic, n (%) | 6 (26) | 9 (39) | 8 (35) | 0.74 |
Includes histologic diagnoses of metaplasia, mild dysplasia, moderate dysplasia, and severe dysplasia,
P for deviation from expected distribution.
Tumor Expression of VDR
VDR expression in the squamous cell carcinomas was similar to the adenocarcinomas (Table 2). In general, there was more nuclear than cytoplasmic expression for all of the variables (Fig. 1D), with distributions similar to those reported in the biopsies. A key difference, however, was the decrease in cytoplasmic VDR expression, best illustrated by the intensity and the higher proportion of samples with greater nuclear than cytoplasmic expression.
In the adenocarcinomas, there was a higher proportion of moderate and well-differentiated samples lacking VDR expression than was found in the poorly differentiated samples (Table 4). In the advanced-stage samples, expression was almost exclusively nuclear, which was not the case in the early-stage tumors.
Table 4.
VDR expression in adenocarcinoma samples, stratified by tumor stage
| Stage, n (%) |
||||
|---|---|---|---|---|
| I |
III |
|||
| Nuclear | Cytoplasmic | Nuclear | Cytoplasmic | |
| Intensity | ||||
| 0 | 9 (31) | 15 (52) | 1 (7) | 13 (87) |
| 1 | 8 (28) | 7 (24) | 6 (40) | 1 (7) |
| 2 | 12 (41) | 7 (24) | 8 (53) | 1 (7) |
| 3 | — | — | — | — |
| Percentage* of cells (%) | ||||
| 0 | 9 (31) | 15 (52) | 1 (7) | 13 (87) |
| <10 | — | — | — | 1 (7) |
| 10–50 | 3 (10) | 5 (17) | 1 (7) | — |
| >50 | 17 (59) | 9 (31) | 13 (87) | 1 (7) |
VDR Expression across All Samples
Comparisons of VDR expression in the nonmalignant biopsies and tumor revealed differences for cytoplasmic intensity, which was significantly higher in the biopsies than in the tumors (P < 0.01). This held true when restricting the tumors to squamous cell carcinomas, which arise from the central part of the airways, the same area from where the bronchoscopy-aided biopsies were obtained.
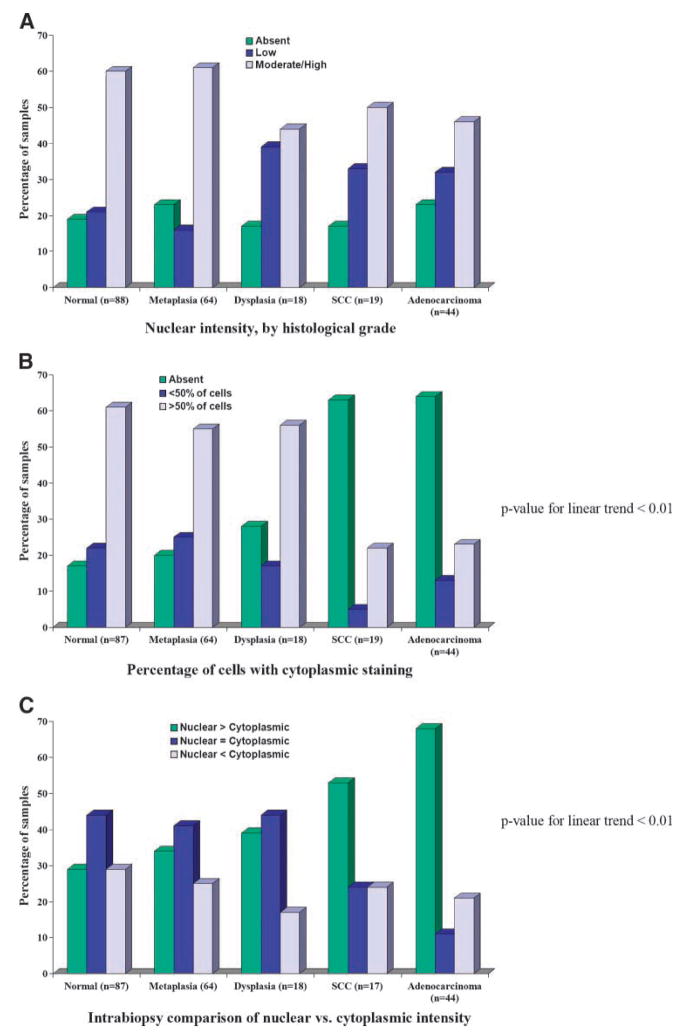
Figure 2 illustrates information described in Tables 2 to 4. As noted above, the key difference between the tumor and nontumor samples is that cytoplasmic VDR expression is generally lacking in tumors. This is reflected in the intensity, the percentage of cells expressing VDR, and the distribution of VDR staining within the epithelial layer; it also drives the intrabiospy comparison of nuclear versus cytoplasmic intensity. In Fig. 2A, the proportions of samples with no nuclear VDR expression are similar across the various histologies, with the higher-grade samples having a higher proportion with low intensity than those of a lower grade. As with intensity, the percentage of cells expressing cytoplasmic VDR decreased with increasing grade (Fig. 2B), with a significant linear trend when the squamous cell carcinomas and adenocarcinomas were combined together (P < 0.01). There was also a linear trend in the proportion of samples with a higher nuclear than cytoplasmic intensity, across histologic grade (P < 0.01).
Figure 2.
VDR expression, by histologic grade.
Discussion
Vitamin D has been implicated in the inhibition of growth in murine and human breast and colon cancer models, and the induction of differentiation, cell cycle arrest, and apoptosis in leukemic and tumor cells (6). More specifically, 1,25(OH)2D has been reported to cause G0–G1 arrest in breast cancer cell lines (11), differentiation in the U937 myelomonocytic cell line (12), induction of apoptosis in MCF-7 breast cancer cells, and the down-regulation of Bcl-2 in HL-60 leukemic cells (13). In humans, much of the evidence for a protective role of vitamin D against cancer was recently reviewed in Garland et al. (14) and Giovannucci (9), with associations being most convincing for colorectal, prostate, and breast cancers.
The evidence for a possible role of the vitamin D pathway against lung cancer comes largely from in vitro or in vivo studies. Higashimoto et al. (15) reported that calcitriol inhibited the growth of lung cancer cell lines, mediated by VDR, and the metabolite has also been shown to affect cell cycle regulation in squamous cell carcinoma models (16, 17). Jones et al. (4) reported expression of the vitamin D–metabolizing enzymes CYP1α and CYP24 in human non–small cell lung carcinoma cell lines, whereas Anderson et al. (18) reported a significant up-regulation of CYP24 mRNA in lung tumors relative to normal tissue. Calcitriol has been shown to inhibit lung tumor growth and lung metastases in mouse models (19). The only study to examine VDR expression in human lung tissue focused on lung tumors and reported positivity, as determined by immunohistochemistry, in 64% (9 of 14) of adenocarcinomas and 67% (10 of 15) of squamous cell carcinomas (10). VDR expression was mostly absent in large-cell carcinoma (25%).
Lung cancer is thought to arise in a stepwise fashion, characterized by pathologic changes. These changes have been better defined for central squamous cell carcinomas than the peripheral adenocarcinomas and small-cell lung cancers (20). Studies of the premalignant lesions have identified molecular abnormalities that have taken place, and many lesions are characterized by a dysregulated cell cycle, decreased apoptosis, and increased proliferation (21), areas that are positively affected by the vitamin D pathway.
We decided to record nuclear and cytoplasmic VDR expression separately. Because the published evidence describing the significance of bronchial nuclear versus cytoplasmic expression is sparse, it is unclear what the differences mean. It is possible that increased nuclear expression relative to cytoplasmic expression may be indicative of the cell preparing itself for pathways that control aberrant growth. Similarly, the nuclear to cytoplasmic ratio may be a marker of actual or potential vitamin D pathway activity, because the receptor must be translocated to the nucleus for the pathway to function. Another possibility is that a decrease in nuclear expression, accompanied by an increase in cytoplasmic expression, is a positive adaptive mechanism for cells that are tending toward aberrant growth. In this study, the proportion of samples with higher nuclear than cytoplasmic intensity increased with histologic grade, with a statistically significant trend. Considering the previously mentioned report of cytoplasmic receptors in the absence of ligands and serum in cell culture, the results of this study favor the first or second explanation and might further indicate that calcitriol and related compounds can have a positive role in preventive and therapeutic interventions among high-risk patients and cancer patients. However, it must be noted that the intensity levels were most often low or moderate and rarely high, although VDR was usually expressed beyond the basal epithelial layer in a high percentage of cells.
The present study reports that a key prerequisite for the vitamin D pathway to have its effects, VDR expression, was found to be present both early and late in the lung carcinogenesis spectrum. This study is the first to describe VDR expression in a series of human lung tissue types, including normal and premalignant central airway biopsies and lung tumors. Additionally, the nonmalignant samples were obtained from high-risk individuals who would be suitable candidates for a chemoprevention intervention. Further studies of VDR expression and the effects of calcitriol on bronchial tissue are warranted, with the eventual objective of developing calcitriol as a chemopreventive agent in high-risk individuals or therapeutic agent in lung cancer patients.
Footnotes
Disclosure of Potential Conflicts of Interest
M. Tretiakova: University of Chicago employee. The other employees disclosed no potential conflicts of interest.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Thun MJ, et al. Cancer statistics. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Jones G, Ramshaw H, Zhang A, et al. Expression and activity of vitamin D-metabolizing cytochrome P450s (CYP1α and CYP24) in human nonsmall cell lung carcinomas. Endocrinology. 1999;140:3303–10. doi: 10.1210/endo.140.7.6799. [DOI] [PubMed] [Google Scholar]
- 5.Jiang F, Bao J, Li P, Nicosia SV, Bai W. Induction of ovarian cancer cell apoptosis by 1,25-dihydroxyvitamin D3 through the down-regulation of telomerase. J Biol Chem. 2004;279:53213–21. doi: 10.1074/jbc.M410395200. [DOI] [PubMed] [Google Scholar]
- 6.Trump DL, Hershberger PA, Bernardi RJ, et al. Anti-tumor activity of calcitriol: pre-clinical and clinical studies. J Steroid Biochem Mol Biol. 2004:89–90. 519–26. doi: 10.1016/j.jsbmb.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 7.Stumpf WE, Sar M, Reid FA, Tanaka Y, DeLuca HF. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science. 1979;206:1188–90. doi: 10.1126/science.505004. [DOI] [PubMed] [Google Scholar]
- 8.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 9.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States) Cancer Causes Control. 2005;16:83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser U, Schilli M, Wegmann B, et al. Expression of vitamin D receptor in lung cancer. J Cancer Res Clin Oncol. 1996;122:356–9. doi: 10.1007/BF01220803. [DOI] [PubMed] [Google Scholar]
- 11.Sheikh MS, Rochefort H, Garcia M. Overexpression of p21WAF1/CIP1 induces growth arrest, giant cell formation and apoptosis in human breast carcinoma cell lines. Oncogene. 1995;11:1899–905. [PubMed] [Google Scholar]
- 12.Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10:142–53. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- 13.Elstner E, Linker-Israeli M, Umiel T, et al. Combination of a potent 20-epi-vitamin D3 analogue (KH 1060) with 9-cis-retinoic acid irreversibly inhibits clonal growth, decreases bcl-2 expression, and induces apoptosis in HL-60 leukemic cells. Cancer Res. 1996;56:3570–6. [PubMed] [Google Scholar]
- 14.Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–61. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higashimoto Y, Ohata M, Nishio K, et al. 1α,25-Dihydroxyvitamin D3 and all-trans -retinoic acid inhibit the growth of a lung cancer cell line. Anticancer Res. 1996;16:2653–9. [PubMed] [Google Scholar]
- 16.Light BW, Yu WD, McElwain MC, Russell DM, Trump DL, Johnson CS. Potentiation of cisplatin antitumor activity using a vitamin D analogue in a murine squamous cell carcinoma model system. Cancer Res. 1997;57:3759–64. [PubMed] [Google Scholar]
- 17.Hershberger PA, Modzelewski RA, Shurin ZR, Rueger RM, Trump DL, Johnson CS. 1,25-Dihydroxycholecalciferol (1,25-D3) inhibits the growth of squamous cell carcinoma and down-modulates p21(Waf1/Cip1) in vitro and in vivo. Cancer Res. 1999;59:2644–9. [PubMed] [Google Scholar]
- 18.Anderson MG, Nakane M, Ruan X, Kroeger PE, Wu-Wong JR. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother Pharmacol. 2006;57:234–40. doi: 10.1007/s00280-005-0059-7. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa K, Kawaura A, Kato S, Takeda E, Okano T. 1 α,25-Dihydroxyvitamin D(3) is a preventive factor in the metastasis of lung cancer. Carcinogenesis. 2005;26:429–40. doi: 10.1093/carcin/bgh332. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch FR, Franklin WA, Gazdar AF, Bunn PA., Jr Early detection of lung cancer: clinical perspectives of recent advances in biology and radiology. Clin Cancer Res. 2001;7:5–22. [PubMed] [Google Scholar]
- 21.Hirsch FR, Merrick DT, Franklin WA. Role of biomarkers for early detection of lung cancer and chemoprevention. Eur Respir J. 2002;19:1151–8. doi: 10.1183/09031936.02.00294102. [DOI] [PubMed] [Google Scholar]