Abstract
Objective:
To assess the effects of sevoflurane, the most commonly used inhalation anesthetic, on apoptosis and β-amyloid protein (Aβ) levels in vitro and in vivo.
Subjects:
Naive mice, H4 human neuroglioma cells, and H4 human neuroglioma cells stably transfected to express full-length amyloid precursor protein.
Interventions:
Human H4 neuroglioma cells stably transfected to express full-length amyloid precursor protein were exposed to 4.1% sevoflurane for 6 hours. Mice received 2.5% sevoflurane for 2 hours. Caspase-3 activation, apoptosis, and Aβ levels were assessed.
Results:
Sevoflurane induced apoptosis and elevated levels of β-site amyloid precursor protein-cleaving enzyme and Aβ in vitro and in vivo. The caspase inhibitor Z-VAD decreased the effects of sevoflurane on apoptosis and Aβ. Sevoflurane-induced caspase-3 activation was attenuated by the γ-secretase inhibitor L-685,458 and was potentiated by Aβ. These results suggest that sevoflurane induces caspase activation which, in turn, enhances β-site amyloid precursor protein–cleaving enzyme and Aβ levels. Increased Aβ levels then induce further rounds of apoptosis.
Conclusions:
These results suggest that inhalational anesthetic sevoflurane may promote Alzheimer disease neuropathogenesis. If confirmed in human subjects, it may be prudent to caution against the use of sevoflurane as an anesthetic, especially in those suspected of possessing excessive levels of cerebral Aβ.
Excessive β-amyloid protein (Aβ) accumulation is a major pathological hallmark of Alzheimer disease (AD).1,2 β-amyloid protein is produced via serial proteolysis of the amyloid precursor protein (APP) by aspartyl protease β-site APP-cleaving enzyme (BACE; β-secretase) and γ-secretase. β-site APP-cleaving enzyme cleaves APP to generate a 99-residue membrane-associated C-terminus fragment (APP-C99). This fragment is further cleaved by γ-secretase to release the 4-kDa Aβ and APP intracellular domain.3-5 Increasing evidence suggests a role for caspase activation and apoptosis in AD neuropathogenesis.6-17 Recent studies suggested that caspase activation and apoptosis may enhance BACE levels to facilitate APP processing, leading to increases in Aβ levels.15,18,19
An estimated 200 million patients worldwide have surgery with anesthesia each year. Several studies showed an odds ratio of between 1.2 and 1.6 for the association of previous general anesthesia/surgery and AD. Moreover, the age of onset of AD has been inversely correlated with cumulative exposure to general anesthesia prior to 50 years of age.20,21 A recent study illustrated that patients having coronary artery bypass graft surgery with general anesthesia are at greater risk for the emergence of AD than those having percutaneous transluminal coronary angioplasty with local anesthesia.22 Though there have been no conclusive studies to strongly suggest an association between anesthesia and AD, there have been studies suggesting that anesthetics such as isoflurane may promote AD neuropathogenesis in vitro and in vivo. A recent study showed that an insult from a middle cerebral artery occlusion for 2 hours in rats caused temporary increases in APP and Aβ staining in a brain area near the ischemic region as well as long-term (up to 9 months) APP and Aβ deposits in a brain area distant from the ischemic region.23 These findings suggest that a transient insult, eg, ischemia or anesthesia with isoflurane, could lead to secondary and persistent brain injuries. However, whether inhalation anesthetics other than isoflurane can promote AD neuropathogenesis remains unknown. We therefore set out to determine the effects of sevoflurane, currently the most commonly used inhalational anesthetic, on caspase activation, apoptosis, APP processing, and levels of BACE and Aβ in H4 human neuroglioma cells as well as in naive mice.
METHODS
CELL LINES
We used H4 human neuroglioma cells (naive H4 cells) and H4 human neuroglioma cells stably transfected to express full-length (FL)-APP (H4-APP cells). All cell lines were cultured in Dulbecco Modified Eagle Medium (high glucose) containing 9% heat-inactivated fetal calf serum, 100-Us/mL penicillin, 100-μg/mL streptomycin, and 2mM L-glutamine. Stably transfected H4 cells were additionally supplemented with 200- μg/mL G418.
CELL TREATMENT
The cells were treated with 21% oxygen, 5% carbon dioxide, and 4.1% sevoflurane (2 minimum alveolar concentration) for 6 hours, during which time the cells were incubated in serum-free cell culture media, as described by Xie et al.24 21% O2,5% CO2, and 4.1% sevoflurane were delivered from an anesthesia machine to a sealed plastic box in a 37°C incubator containing 6-well plates seeded with 1 million cells in 1.5-mL cell culture media. A Datex infrared gas analyzer (Puritan-Bennett, Tewks-bury, Massachusetts) was used to continuously monitor the concentrations of delivered CO2, O2, and sevoflurane. In the interaction studies, the cells were treated with Z-VAD (100μM), Aβ40 (7.5μM) plus Aβ42 (7.5μM), and L-685,458 (0.5μM) 1 hour before the treatment with 4.1% sevoflurane. Control conditions included 5% CO2 plus 21% O2, which did not affect caspase-3 activation, cell viability, APP processing, or Aβ generation (data not shown).
CELL LYSIS AND PROTEIN AMOUNT QUANTIFICATION
Cell pellets were detergent-extracted on ice using immunoprecipitation buffer (10mM Tris-hydrochloride [HCl]; pH, 7.4; 150mM sodium chloride [NaCl]; 2mM EDTA; 0.5% Nonidet P-40) plus protease inhibitors (1-μg/mL aprotinin, 1-μg/mL leupeptin, and pepstatin A). The lysates were collected, centrifuged at 12 000 revolutions per minute (rpm) for 10 minutes, and quantified for total proteins by the BCA (bicinchoninic acid) protein assay kit (Pierce, Iselin, New Jersey).
MOUSE ANESTHESIA AND TREATMENT
The animal protocol was approved by the Standing Committee on Animals at Massachusetts General Hospital. Mice (C57/BL6, aged 5-9 months; The Jackson Laboratory, Bar Harbor, Maine) were randomly assigned to an anesthesia or control group. Mice randomized to the anesthesia group received 2.5% sevoflurane in 100% O2 for 2 hours in an anesthetizing chamber, whereas the control group received 100% O2 at an identical flow rate for 2 hours in an identical chamber. The mice breathed spontaneously, and concentrations of anesthetic and O2 were measured continuously (Datex, Tewksbury, Massachusetts). The temperature of the anesthetizing chamber was controlled to maintain a mean (SD) rectal temperature in the animals of 37°C(0.5°C). Mean arterial blood pressure was measured noninvasively using a tail cuff (Kent Scientific Corporation, Torrington, Connecticut) in the anesthetized mice. Anesthesia was terminated by discontinuing sevoflurane and placing animals in a chamber containing 100% O2 until 20 minutes after the return of their righting reflex. They were then returned to individual home cages until they were humanely killed. Mice were killed by decapitation 6, 12, and 24 hours after sevoflurane anesthesia. The brain was removed rapidly and the prefrontal cortex was dissected out and frozen in liquid nitrogen for subsequent processing to determine caspase activation and levels of FL-APP, APP-C99, APP-C83, BACE, and Aβ.
BRAIN TISSUE LYSIS AND PROTEIN AMOUNT QUANTIFICATION
The harvested brain tissues were homogenized on ice using immunoprecipitation buffer (10mM Tris-HCl; pH, 7.4; 150mM NaCl; 2 mM EDTA; 0.5% Nonidet P-40) plus protease inhibitors (1-μg/mL aprotinin, 1-μg/mL leupeptin, 1-μg/mL pepstatin A). The lysates were collected, centrifuged at 12 000 rpm for 10 minutes, and quantified for total proteins by BCA protein assay kit.
WESTERN BLOT ANALYSIS
The cells and brain tissues were harvested at the end of the experiment and were subjected to Western blot analysis, as described by Xie et al.25 Antibodies A8717 (1:2000; Sigma, St Louis, Missouri), C66 (1:1000; generous gift of Dora Kovacs, PhD, at Massachusetts General Hospital and Harvard Medical School), and anti–β-actin (1:5000; Sigma) were used to visualize FLAPP (110 kDa), APP-C83 (12 kDa), APP-C99 (10 kDa), and β-actin (42 kDa), respectively. A caspase-3 antibody (1:1000; Cell Signaling Technology Inc, Beverly, Massachusetts) was used to recognize the caspase-3 fragment (17-20 kDa) resulting from cleavage at asparate position 175 and caspase-3 FL (35-40 kDa). Rabbit polyclonal anti–BACE1 antibody ab2077 (1:1000; Abcam, Cambridge, Massachusetts) was used to detect the protein levels of BACE (65 kDa). The quantification of Western blots was performed in 2 steps, as described by Xie et al.25 Briefly, the intensity of signals was analyzed by using an image program from the National Institutes of Health (NIH ImageJ; Bethesda, Maryland). First, we used levels of β-actin to normalize (eg, determining ratio of FL-APP amount to β-actin amount) the levels of FL-APP, APP-C83, APP-C99, FLcaspase-3, caspase-3 fragment, BACE, and Aβ to control for the loading differences in total protein amounts. Second, we presented the changes in the levels of FL-APP, APP-C83, APP-C99, FL-caspase-3, caspase-3 fragment, Aβ, and BACE in the cells or animals treated with sevoflurane, Z-VAD, Aβ, and L-685,458 as the percentage of those in the cells or animals treated with controls. We refer to 100% caspase-3 activation, FL-APP, APP-C83, APP-C99, Aβ, and BACE in this article as control levels for the purpose of comparison with experimental conditions.
QUANTIFICATION OF Aβ USING SANDWICH ELISA ASSAY
Secreted Aβ was measured with a sandwich enzyme-linked immunosorbent assay (ELISA) assay using an Aβ measurement kit (Invitrogen, Carlsbad, California) and by the Aβ ELISA Core Facility at the Center for Neurological Diseases, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, as described by Xie et al.26 Specifically, 96-well plates were coated with mouse monoclonal antibodies specific to Aβ40 (2G3) or Aβ42 (21F12). Following blocking with Block Ace, wells were incubated overnight at 4°C with test samples of conditioned cell culture media; anti-Aβ (α-Aβ-HR1) conjugated to horseradish peroxidase was then added. Plates were then developed with tetramethylberizidine reagent and well absorbance was measured at 450 nm. Levels of Aβ in test samples were determined by comparison with the signal from unconditioned media spiked with known quantities of Aβ40 and Aβ42.
IMMUNOBLOT DETECTION OF Aβ
Brain samples were homogenized (150mM NaCl with protease inhibitor cocktail in 50mM Tris; pH, 8.0) and centrifuged (300 000g for 45 minutes), and the supernatant was removed. The pellet was then resuspended by sonication and incubated for 15 minutes in homogenization buffer containing 1% sodium dodecyl sulfate (SDS). Following pelleting of insoluble material (16 000g for 15 minutes), the SDS extract was electrophoresed on SDS–polyacrylimide gel electrophoresis (SDS-PAGE) (4%-12% Bis-Tris polyacrylamide gel; Invitrogen, Carlsbad, California), blotted to a polyvinylidene difluoride membrane and probed with a 1:200 dilution of 6E10 (Signet, Berkeley, California).
CELL APOPTOSIS ASSAY
Cell apoptosis was assessed by a cell death detection ELISA kit (Roche, Palo Alto, California), which assays cytoplasmic histone-associated DNA fragmentation associated with cellular apoptosis.
STATISTICS
Data were expressed as mean (SD). The number of samples varied from 3 to 10, and the samples were normally distributed. We used a 2-tailed t test to compare the difference between the experimental groups. P<.05 and P<.01 were considered statistically significant.
RESULTS
SEVOFLURANE INDUCES CASPASE-3 ACTIVATION AND APOPTOSIS, ALTERS APP PROCESSING, AND INCREASES LEVELS OF BACE AND Aβ IN H4-APP CELLS
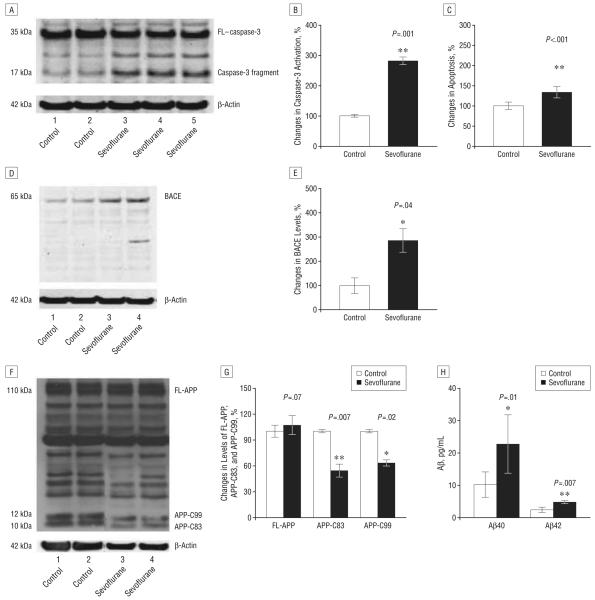
Sevoflurane has previously been reported to induce cytotoxicity in various cell lines.27-32 We have previously reported that isoflurane can induce caspase activation and apoptosis and increase Aβ levels in H4-APP cells.19,24,33 We therefore asked whether the currently most commonly used inhalational anesthetic, sevoflurane, also affects apoptosis and APP processing in H4-APP cells. The H4-APP cells were treated with a clinically relevant concentration (4.1%) of sevoflurane for 6 hours. Because caspase-3 activation is one of the final steps of cellular apoptosis,34 we assessed the effects of sevoflurane on caspase-3 activation by quantitative Western blot analysis. Sevoflurane treatment led to caspase-3 activation (Figure 1A), as evidenced by increased ratios of cleaved (activated) caspase-3 fragment (17-19 kDa) to FL–caspase-3 (35-40 kDa). Quantification of the Western blots, based on the ratio of caspase-3 fragment to FL–caspase-3, revealed that the 4.1% sevoflurane treatment (Figure 1B) led to a 275% increase in caspase-3 activation compared with control cells (Figure 1B) (P=.001). Given that caspase-3 activation alone cannot represent apoptotic cell damage,35 we also assessed the effects of sevoflurane on cellular apoptosis by detecting cytoplasmic histone-associated DNA fragmentation using a cell death detection ELISA kit. We showed that treatment with 4.1% sevoflurane (Figure 1C) led to cellular apoptosis compared with control conditions (Figure 1C) (100% vs 134%; P<.001). We also found that sevoflurane treatment increased terminal deoxynucleotidyl transferase biotin-dUTP nick-end labeling (TUNEL)–positive cells compared with control conditions (data not shown).
Figure 1.
Treatment with 4.1% sevoflurane for 6 hours induces caspase-3 activation and apoptosis, alters amyloid precursor protein (APP) processing, and increases levels of β-site APP-cleaving enzyme (BACE) and β-amyloid protein (Aβ) in H4-APP cells. A, Sevoflurane treatment induces caspase-3 cleavage (activation) compared with control conditions in H4-APP cells in Western blot analysis; there is no significant difference in the amounts of β-actin between the control conditions and the sevoflurane-treated H4-APP cells. B, Caspase-3 activation was assessed by quantifying the ratio of caspase-3 fragment to full-length (FL)–caspase-3 in Western blot analysis. Quantification of the Western blot shows that sevoflurane treatment increases caspase-3 activation compared with control conditions, normalized to β-actin levels. C, Sevoflurane treatment increases apoptosis compared with control conditions in H4-APP cells. D, Sevoflurane treatment enhances levels of BACE compared with control conditions in H4-APP cells in Western blot analysis; there is no significant difference in the amounts of β-actin in the control conditions or the sevoflurane-treated H4-APP cells. E, Quantification of the Western blot shows that sevoflurane treatment enhances BACE levels compared with control conditions in H4-APP cells, normalized to β-actin levels. F, Sevoflurane treatment decreases levels of APP-C83 and APP-C99 compared with control conditions in Western blot analysis; there is no significant difference in the amounts of β-actin in the control conditions or the sevoflurane-treated H4-APP cells. G, Quantification of the Western blot shows that sevoflurane treatment decreases levels of APP-C83 and APP-C99, but not FL-APP, compared with control conditions in H4-APP cells, normalized to β-actin levels. H, Sevoflurane treatment increases levels of both Aβ40 and Aβ42 compared with control conditions. Data are mean (SD); n=3 to 10 for each experimental group; the t test was used to compare the difference between control conditions and sevoflurane treatment conditions; values are significant at *P<.05 and **P<.01.
Recent studies suggest that isoflurane,19 ischemia,15 or the combination of desflurane plus hypoxia18 can induce caspase activation and apoptosis, which, in turn, enhances levels of BACE and Aβ. We thus asked whether sevoflurane can also increase BACE levels and alter APP processing to favor production of Aβ in H4-APP cells. Treatment with 4.1% sevoflurane for 6 hours increased levels of BACE compared with control conditions (Figure 1, D and E) (100% vs 285%; P=.04). Immunoblotting for APP revealed that 4.1% sevoflurane attenuated levels of both APP-C83 and APP-C99 without altering the levels of FL-APP(Figure 1F) in H4-APP cells. Quantification of the results showed that sevoflurane treatment led to 46% (P=.007) and 37% (P=.02) reduction in levels of APP-C83 and APP-C99, respectively, compared with control conditions (Figure 1G). Finally, 4.1% sevoflurane increased Aβ40 (10.2 vs 22.7 pg/mL; P=.01) and Aβ42 (2.4 vs 4.2 pg/mL; P=.007) in the cell culture media of H4-APP cells compared with control conditions (Figure 1H). Collectively, these findings suggest that sevoflurane can induce caspase activation and apoptosis and increase BACE and Aβ levels in H4-APP cells.
SEVOFLURANE INDUCES CASPASE-3 ACTIVATION AND INCREASES LEVELS OF BACE AND Aβ IN NAIVE MICE
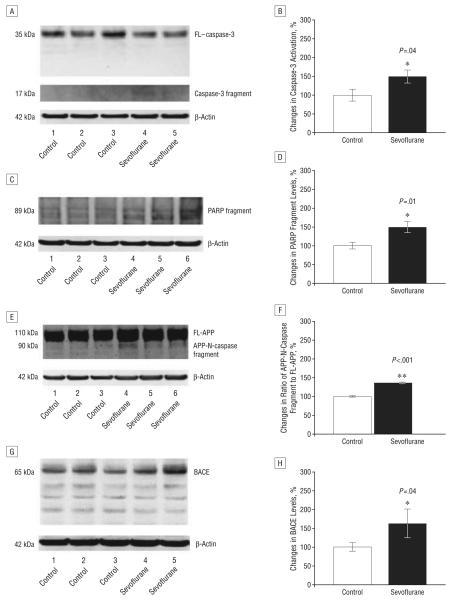
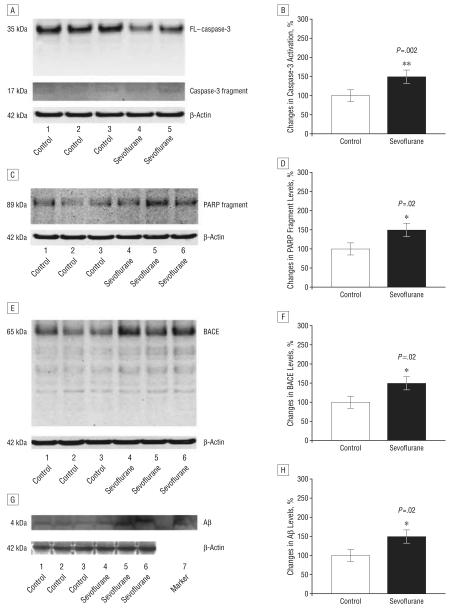
We then assessed the in vivo relevance of these effects of sevoflurane. Naive mice were given anesthesia with 2.5% sevoflurane for 2 hours. The mice exhibited no significant effects on blood pressure or blood gas (Table). Anesthesia with 2.5% sevoflurane for 2 hours led to caspase-3 activation for 6 (Figure 2A) and 12 hours (Figure 3A) after anesthesia. Quantification of these results revealed that sevoflurane anesthesia led to a 150% (Figure 2B) (P=.04) and 159% (Figure 3B) (P=.002) increase in the ratio of caspase-3 fragment to FL–caspase-3 levels 6 and 12 hours, respectively, but not 24 hours (data now shown) after anesthesia compared with control conditions. Anesthesia with 2.5% sevoflurane for 2 hours also led to poly (adenosine diphosphate–ribose) polymerase (PARP) cleavage, as evidenced by a 149% (Figure 2D) (P=.01) and 140% (Figure 3D) (P=.02) increase in levels of PARP fragment at 6 (Figure 2C) and 12 hours (Figure 3C), respectively, following anesthesia. Finally, sevoflurane anesthesia increased levels of caspase-cleaved APP–N (N-terminal)–fragment (Figure 2, E and F, a 135% increase) (P<.001) 6 hours after anesthesia compared with control conditions. Collectively, these results suggest that sevoflurane can induce caspase activation in the brain tissues of naive mice for up to 12 hours after anesthesia.
Table.
Blood Pressure and Blood Gas in Mice Under Control Condition and Sevoflurane Anesthesiaa
| Mean (SD) Blood or Gas Pressure, mm Hg |
||
|---|---|---|
| Controlb | 2.5% Sevofluranec | |
| MAP | 107 (2.5) | 95.4 (9.3) |
| pH | 7.31 (0.04) | 7.33 (0.04) |
| Pv̄O2 | 226 (55) | 234 (75) |
| Pv̄CO2 | 42.2 (4.1) | 43.8 (3.1) |
Abbreviations: MAP, mean arterial pressure; Pv̄CO2, mixed venous carbon dioxide tension; Pv̄O2, mixed venous oxygen tension.
Treatment with 2.5% sevoflurane for 2 hours does not significantly alter blood pressure and blood gas in C57 mice. Sevoflurane treatment does not significantly alter values of MAP, pH, Pv̄O2, or Pv̄CO2 in naive C57 mice compared with control conditions.
Baseline blood pressure in C57 mice with 21% O236 and baseline blood gas in C57 mice with 100% oxygen.
Blood pressure and blood gas in C57 mice during the sevoflurane anesthesia in 100% O2.
Figure 2.
Anesthesia with 2.5% sevoflurane for 2 hours induces caspase-3 activation and increases β-site amyloid precursor protein (APP)–cleaving enzyme (BACE) levels 6 hours after anesthesia in naive mice. A, Sevoflurane anesthesia induces caspase-3 cleavage (activation) by decreasing full-length (FL)–caspase-3 levels and increasing caspase-3 fragment compared with control conditions in Western blot analysis. B, Quantification of the Western blots shows that sevoflurane anesthesia increases the ratio of caspase-3 fragment to FL–caspase-3 levels compared with control conditions. C, Sevoflurane anesthesia induces poly–(adenosine diphosphate–ribose) polymerase (PARP) cleavage by increasing PARP fragment compared with control conditions in Western blot analysis. D, Quantification of the Western blots shows that sevoflurane anesthesia increases levels of PARP fragment compared with control conditions. E, Sevoflurane anesthesia increases levels of caspase-cleaved APP-N-fragment (APP-N-caspase fragment) compared with control conditions in Western blot analysis. F, Quantification of the Western blots shows that sevoflurane anesthesia increases levels of APP-N-caspase fragment compared with control conditions. G, Sevoflurane anesthesia increases BACE levels compared with control conditions in Western blot analysis. H, Quantification of the Western blots shows that sevoflurane anesthesia increases BACE levels compared with control conditions. There is no significant difference in amounts of β-actin in control conditions or sevoflurane-treated mouse brain tissue. Data are means (SD); n=3 to 6 for each experimental group; the t test was used to compare the difference between control conditions and sevoflurane anesthesia; values are significant at * P<.05 and ** P<.01.
Figure 3.
Anesthesia with 2.5% sevoflurane for 2 hours induces caspase activation and increases levels of β-site amyloid precursor protein (APP)–cleaving enzyme (BACE) and β-amyloid protein (Aβ) 12 hours after anesthesia. A, Sevoflurane anesthesia induces caspase-3 cleavage (activation) by decreasing full-length (FL)–caspase-3 levels and increasing caspase-3 fragment compared with control conditions in Western blot analysis. B, Quantification of the Western blots shows that sevoflurane anesthesia increases the ratio of caspase-3 fragment to FL–caspase-3 levels compared with control conditions. C, Sevoflurane anesthesia induces poly–(adenosine diphosphate–ribose) polymerase (PARP) cleavage by increasing PARP fragment compared with control conditions in Western blot analysis. D, Quantification of the Western blots shows that sevoflurane anesthesia increases levels of PARP fragment compared with control conditions. E, Sevoflurane anesthesia increases BACE levels compared with control conditions in Western blot analysis. F, Quantification of the Western blot shows that sevoflurane anesthesia increases BACE levels compared with control conditions. G, Sevoflurane anesthesia increases Aβ levels compared with control conditions in Western blot analysis. Synthetic Aβ was used as a marker to identify position of Aβ in Western blot analysis. H, Quantification of the Western blot shows that sevoflurane anesthesia increases Aβ levels compared with control conditions. There is no significant difference in amounts of β-actin in control conditions or sevoflurane-treated mouse brain tissue. Data are mean (SD); n=3 to 6 for each experimental group; the t test was used to compare the difference between control conditions and sevoflurane anesthesia; values are significant at *P<.05 and **P<.01.
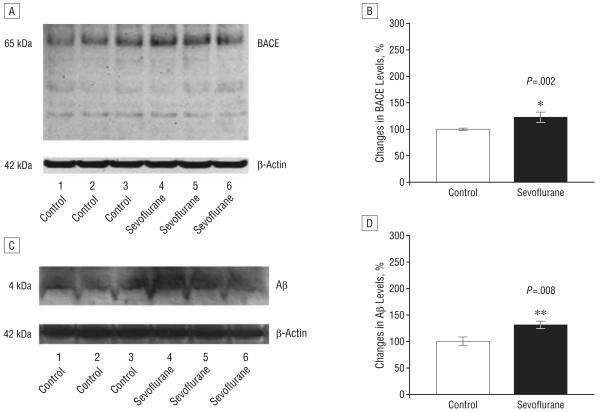
We next assessed whether sevoflurane can elevate levels of BACE and Aβ in the mouse brain. Western blot analyses revealed that exposure to 2.5% sevoflurane anesthesia increased levels of BACE for 6 (Figure 2G), 12 (Figure 3E), and 24 hours (Figure 4A) after the anesthesia compared with control conditions. Quantification of the Western blots, normalized to β-atin, showed that sevoflurane anesthesia led to a 164% (Figure 2H) (P = .04), 143% (Figure 3F) (P = .02), and 123% (Figure 4B) (P=.002) increases in BACE levels 6, 12, and 24 hours, respectively, following anesthesia, compared with control conditions. The 2.5% sevoflurane anesthesia also led to a 141% increase at 12 hours (Figure 3, G and H) (P=.02) and a 132% increase in Aβ levels at 24 hours (Figure 4, C and D) (P=.008). No increase in Aβ levels was observed at 6 hours (data not shown). Collectively, these findings suggest that anesthesia with 2.5% isoflurane for 2 hours, a clinically relevant regimen, can induce a time-dependent cascade of caspase activation, elevation in BACE levels, and increase in Aβ levels.
Figure 4.
Anesthesia with 2.5% sevoflurane for 2 hours increases levels of β-site amyloid precursor protein–cleaving enzyme (BACE) and β-amyloid protein (Aβ) 24 hours following anesthesia. A, Sevoflurane anesthesia increases BACE levels compared with control conditions in Western blot analysis. B, Quantification of the Western blot shows that sevoflurane anesthesia increases BACE levels compared with control conditions. C, Sevoflurane anesthesia increases Aβ levels compared with control conditions in Western blot analysis. D, Quantification of the Western blot shows that sevoflurane anesthesia increases Aβ levels compared with control conditions. There is no significant difference in amounts of β-actin in control conditions or sevoflurane-treated mouse brain tissue. Data are mean (SD); n=3 to 6 for each experimental group; the t test was used to compare the difference between control conditions and sevoflurane anesthesia; values are significant at *P<.05 and **P<.01.
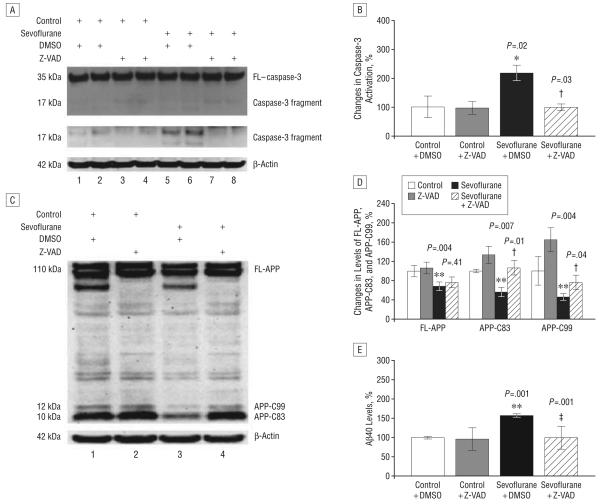
ATTENUATION OF SEVOFLURANE-INDUCED ALTERATIONS IN APP PROCESSING AND Aβ GENERATION BY THE CASPASE INHIBITOR Z-VAD IN H4-APP CELLS
Given that sevoflurane can alter APP processing and increase Aβ levels in H4-APP cells, we next asked whether these effects are dependent on caspase activation. For this purpose, we incubated H4-APP cells with Z-VAD (100μM), a caspase inhibitor, for 1 hour, followed by treatment with 4.1% sevoflurane for 6 hours. Sevoflurane induced caspase-3 activation, which was attenuated by treatment with Z-VAD (Figure 5A). Quantification of the Western blots revealed that treatment with sevoflurane and Z-VAD reduced caspase-3 activation from 210% to 107% (Figure 5B) (P=.03). Treatment with Z-VAD also attenuated sevoflurane-induced alterations in APP processing and Aβ generation. As can be seen in Figure 5, APP immunoblotting revealed that sevoflurane treatment decreased protein levels of FL-APP, APP-C83, and APP-C99 compared with control conditions. While ZVAD treatment alone had no effect, the Z-VAD treatment attenuated the sevoflurane-induced changes in FLAPP, APP-C83, and APP-C99 (Figure 5C). Quantification of the Western blots showed that treatment with sevoflurane led to 34% (P=.004), 44% (P=.007), and 54% (P=.004) reductions in levels of FL-APP, APP-C83, and APP-C99, respectively, compared with control conditions (Figure 5D). Treatment with Z-VAD attenuated the effects of sevoflurane on levels of APP-C83 (44% reduction vs no reduction; P=.01) and APP-C99 (54% reduction vs 24% reduction; P=.04) compared with dimethyl sulfoxide treatment (Figure 5D).
Figure 5.
The caspase inhibitor Z-VAD inhibits caspase-3 activation and attenuates sevoflurane-induced increases in β-amyloid protein (Aβ) in H4 amyloid precursor protein (H4-APP) cells. A, Western blot analysis shows that 4.1% sevoflurane induces caspase-3 cleavage (activation) compared with control conditions or Z-VAD (100μM) treatment. The Z-VAD treatment inhibits the caspase-3 cleavage (activation) induced by 4.1% sevoflurane treatment. The blot showing the band of caspase-3 fragment only is the same blot with more exposure time in developing the film. There is no significant difference in the amounts of β-actin in H4-APP cells with the above treatments. B, Quantification of the Western blot shows that sevoflurane treatment increases caspase-3 activation compared with control conditions or the Z-VAD (100μM) treatment, normalized to β-actin levels. Sevoflurane-induced caspase-3 activation is inhibited by Z-VAD treatment. C, Z-VAD inhibits the sevoflurane-induced changes in APP processing in H4-APP cells. Treatment with 4.1% sevoflurane decreases the protein levels of full-length (FL)–APP and APP–C-terminal fragments (APP-C83 and APP-C99) compared with control conditions or Z-VAD (100μM) treatment in Western blot analysis. Treatment with Z-VAD inhibits the sevoflurane-induced decreases in levels of APP-C83 and APP-C99. There is no significant difference in the amounts of β-actin in the H4-APP cells with all of the above treatments. D, Quantification of the Western blot shows that 4.1% sevoflurane treatment decreases the protein levels of FL-APP, APP-C83, and APP-C99 compared with control conditions or Z-VAD treatment, normalized to β-actin levels. The sevoflurane-induced decrease in the protein levels of APP-C83 and APP-C99 is inhibited by the Z-VAD treatment. E, Z-VAD inhibits the sevoflurane-induced increases in Aβ levels. Treatment with 4.1% sevoflurane increases levels of Aβ40 compared with control conditions. Treatment with Z-VAD alone does not change the levels of Aβ40; however, Z-VAD treatment inhibits the sevoflurane-induced increases in Aβ40 levels. Data are mean (SD); n=3 to 10 for each experimental group; the t test was used to compare the difference between control conditions and 4.1% sevoflurane treatment. Values are significant at *P<.05, **P<.01, and the difference between dimethyl sulfoxide (DMSO) treatment and Z-VAD treatment at †P<.05 and ‡P<.01.
Sevoflurane treatment, but not Z-VAD treatment, alone, significantly increased Aβ levels in the conditioned media compared with control conditions (Figure 5E). Treatment with Z-VAD attenuated the sevoflurane-induced increase in Aβ levels (152% vs 98%; P=.001) (Figure 5E). We have also found that treatment with 4.1% sevoflurane for 6 hours can induce caspase-3 activation without detectable changes in APP processing and Aβ levels in H4 naive cells (data not shown). Collectively, these results suggest that sevoflurane-induced alterations in APP processing and Aβ levels are largely dependent on the ability of sevoflurane to induce caspase-3 activation and apoptosis.
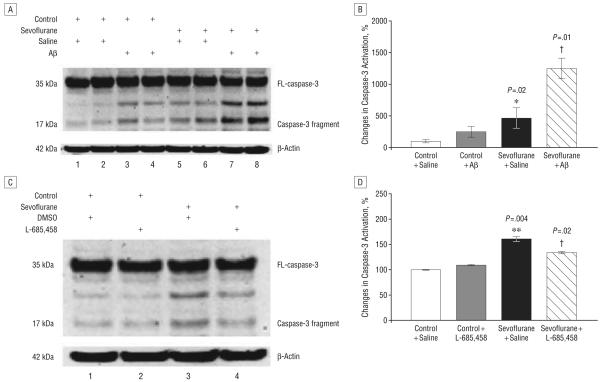
POTENTIATION OF SEVOFLURANE-INDUCED CASPASE-3 ACTIVATION BY EXOGENOUSLY ADDED Aβ AND ATTENUATION BY THE γ-SECRETASE INHIBITOR L-685,458
To assess the possibility that sevoflurane-induced increases in Aβ levels can lead to further caspase activation beyond that induced by sevoflurane, we next asked whether the γ-secretase inhibitor L-685,458 could attenuate, but Aβ could potentiate, sevoflurane-induced caspase-3 activation in H4-APP and naive H4 cells. Sevoflurane treatment led to a 460% increase in caspase-3 activation over control conditions (Figure 6, A and B) (P=.02) in H4 naive cells. Addition of 7.5μM Aβ40 plus 7.5μM Aβ42 further potentiated sevoflurane-induced caspase-3 activation in H4 naive cells (460% vs 1249%; P=.01) (Figure 6, A and B). Caspase-3 activation induced by sevoflurane in H4-APP cells was reduced by L-685,458 (134% vs 160%; P=.02) (Figure 6, C and D). However, L-685,458 alone did not significantly induce caspase-3 activation compared with control conditions (Figure 6, C and D). These results suggest that Aβ can further potentiate the effects of sevoflurane on caspase activation.
Figure 6.
Sevoflurane-induced caspase-3 activation was potentiated by β-amyloid protein (Aβ) and attenuated by the γ-secretase inhibitor L-685,458 in H4 cells. A, Sevoflurane treatment induces caspase-3 cleavage (activation) compared with control conditions. Treatment with Aβ40 (7.5μM) plus Aβ42 (7.5μM) potentiates caspase-3 activation induced by sevoflurane. There is no significant difference in amounts of β-actin in H4 naive cells with above treatments. B, Quantification of the Western blot shows that sevoflurane increases caspase-3 activation compared with control conditions, normalized to β-actin levels. Also, Aβ potentiates the sevoflurane-induced caspase-3 activation. C, Treatment with 4.1% sevoflurane induces caspase-3 cleavage (activation) compared with control conditions or L-685,458 (0.5μM) treatment. Treatment with L-685,458 (0.5μM) attenuates caspase-3 cleavage (activation) induced by sevoflurane in Western blot analysis. There is no significant difference in amounts of β-actin in H4-APP cells with above treatments. D, Quantification of the Western blot shows that sevoflurane increases caspase-3 activation compared with control conditions, normalized to β-actin levels. The sevoflurane-induced caspase-3 activation is attenuated by L-685,458 (0.5μM). Data are mean (SD); n=3 to 10 for each group; the t test was used to compare the difference between control conditions and 4.1% sevoflurane treatment; values are significant at *P<.05 and **P<.01, and for the difference between treatment with 4.1% sevoflurane plus dimethyl sulfoxide (DMSO) and 4.1% sevoflurane plus L-685,458 or Aβ, †P<.05; FL indicates full-length.
COMMENT
We have previously shown that the commonly used inhalational anesthetic isoflurane can induce caspase activation and apoptosis and increase Aβ generation in H4-APP cells.19,24,33,37 However, it is unknown whether other inhalational anesthetics can also promote AD neuro-pathogenesis. Here we assessed the effects of sevoflurane, currently the most commonly used inhalational anesthetic, on caspase activation, apoptosis, APP processing, and Aβ levels in H4 cells and naive mice.
First, we found that sevoflurane can induce caspase-3 activation and apoptosis in H4-APP cells. Given that isoflurane, desflurane plus hypoxia, and ischemia have all been shown to enhance BACE and Aβ levels subsequent to caspase activation,15,18,19 we next asked whether sevoflurane has similar effects. We were able to show that a clinically relevant regimen of sevoflurane anesthesia enhanced BACE levels, altered APP processing, and increased Aβ levels in H4-APP cells. These results, along with previous findings, indicate that AD neuropatho-genesis can be promoted by multiple inhalational anesthetics, suggesting that it may be prudent to carry out systematic and comprehensive assessment of the effects of all currently used inhalational anesthetics on AD-related neuropathogenic events.
Because the above findings were in vitro–based, we next sought in vivo confirmation of sevoflurane effects in naive mice. We found that a clinically relevant concentration of sevoflurane induces caspase activation and PARP cleavage, and elevates levels of caspase-cleaved APP–N-fragment, BACE, and Aβ for up to 24 hours after the anesthesia. However, sevoflurane anesthesia did not significantly alter blood pressure and blood gas in naive mice (Table). These findings suggest that a clinically relevant regimen of sevoflurane anesthesia induces a time-dependent cascade of caspase activation and elevated BACE and Aβ levels in vivo.
We next showed that the broad caspase activation inhibitor Z-VAD could attenuate sevoflurane-induced caspase-3 activation, indicating that sevoflurane-induced alterations in APP processing and Aβ levels are at least partially dependent on caspase activation. We also showed that the γ-secretase inhibitor L-685,458 reduced Aβ levels (data not shown) and attenuated sevoflurane-induced caspase activation and apoptosis in H4-APP cells. In contrast, exogenously added Aβ potentiated sevoflurane-induced caspase-3 activation in naive H4 cells. These data suggest that enhanced Aβ generation, subsequent to sevoflurane-induced caspase-3 activation, can lead to further caspase-3 activation, resulting in additional rounds of apoptosis and Aβ generation.
Wei et al38 showed that treatment with 4.1% sevoflurane for 24 hours did not induce cell death in rat PC12 pheochromocytoma cells and primary cortical neurons. In addition, many studies have suggested that sevoflurane can protect cells from cytotoxicity.39-47 However, many other studies have suggested that sevoflurane may induce cytotoxic effects.27-32 This discrepancy could be owing to the use of different cell lines, eg, rat kidney cells vs human neural-derived cells, and the duration and concentration of sevoflurane exposure in these studies. Future studies need to assess the effects of sevoflurane on apoptosis, APP processing, and Aβ levels with different concentrations and durations. A recent study by Wei et al48 showed that isoflurane inhibited the cytotoxicity induced by isoflurane itself. These findings suggest that isoflurane could have neuroprotective effects through induction of endogenous neuroprotective mechanisms, eg, preconditioning, while different concentrations of isoflurane with different exposure times could cause inherent neurotoxic effects. We have postulated that sevoflurane may also have dual effects on cytotoxicity. Future studies are necessary to further test this hypothesis.
The exact molecular mechanisms by which sevoflurane induces caspase activation and apoptosis, alters APP processing, and increases Aβ levels are unknown. A recent study showed that caspase activation can reduce levels of the golgi-associated, γ-adaptin ear containing adenosine diphosphate–ribosylation factor binding protein 3 (GGA-3), a protein involved in BACE degradation.15 We therefore hypothesized that sevoflurane induces caspase activation, which then reduces GGA-3 levels. The reduced GGA-3 levels will result in accumulation of BACE; the increased BACE will finally increase Aβ levels by facilitating amyloidogenic processing of APP. Our in vivo findings that sevoflurane induces caspase-3 activation at 6 or 12 hours after anesthesia but enhances Aβ levels at a later times (eg, 12 and 24 hours after anesthesia) further support this hypothesis.
Sevoflurane might also affect APP processing and Aβ generation through energy inhibition. Velliquette et al49 reported that insulin, 2-deoxyglucose, 3-nitropropionic acid, and kainic acid can induce acute energy inhibition to enhance levels of BACE and Aβ in wild-type and AD transgenic (Tg2576) mice. A recent neuroimaging study showed that sevoflurane blocked emotional memory in humans and suppressed cerebral metabolism, as evidenced by the fact that sevoflurane induced a 17% reduction of the cerebral metabolic rate of glucose use in human brains.50 Future studies will be necessary to determine whether a sevoflurane-induced increase in levels of BACE and Aβ is dependent on sevoflurane-induced changes in glucose use or GGA-3 levels.
A recent study by Groen et al23 showed that an insult from a 2-hour occlusion of the middle cerebral artery increased levels of APP and Aβ in axons at the corpus callosum and in neurons at the border of the ischemic region. Moreover, this transient insult caused persistent APP and Aβ deposits in the thalamic nuclei (ventroposterior lateral and ventroposterior medial nuclei) that eventually developed into dense plaque-like deposits 9 months after the initial insult.23 This secondary and persistent brain harm could be due to axonal damage of the thalamic neurons, leading to retrograde degeneration,51 damage from vasogenic edema and some noxious substance,52 or hypometabolism.53,54 Both anesthetics19 and brain ischemia15 have been shown to induce caspase activation and apoptosis, which then enhance levels and activities of BACE to facilitate APP processing and to increase Aβ generation. Based on the study by Groen et al,23 it is possible that exposure to sevoflurane for 2 hours not only induces transient injuries (eg, caspase activation and apoptosis, increases in levels of BACE and Aβ), but could also lead to longer-term neurodegeneration and Aβ accumulation in other brain regions. Future studies will be necessary to address the potential longer-term effects of sevoflurane on AD neuropathogenesis in the mouse brain to test this hypothesis.
The limitation of the current study is that there is currently no satisfactory way to extrapolate the findings of caspase activation and Aβ metabolism in cultured cells and in the mouse brain to the human brain. Furthermore, some of these changes are moderate, and the measured effects on the mouse brain tissue are not long-lasting. Collectively, these findings do not present any direct evidence that inhalation of anesthetic sevoflurane can cause irreversible harm to the human brain. Determination of the in vivo relevance of sevoflurane on AD neuropathogenesis in the human brain will be necessary before we can conclude that anesthetic sevoflurane facilitates or exacerbates AD neuropathogenesis in humans.
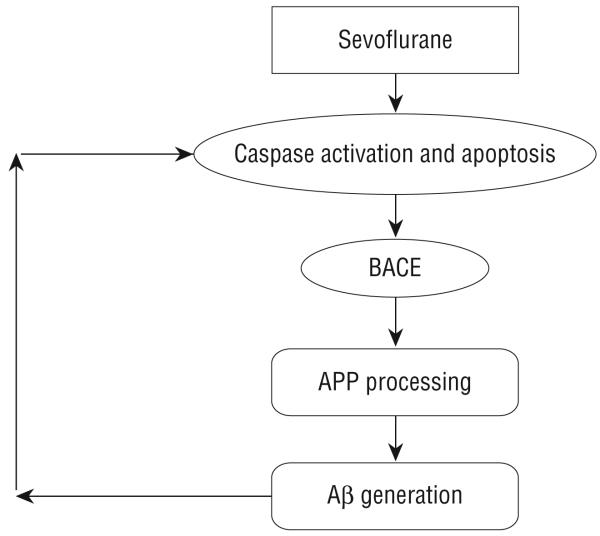
Collectively, our studies have illustrated that sevoflurane can induce caspase activation and apoptosis, alter APP processing, and increase Aβ levels, key changes associated with AD neuropathogenesis, in vitro and in vivo. Moreover, these studies have defined the underlying molecular pathways. As can be seen in Figure 7, sevoflurane can induce caspase activation and apoptosis, which then increase the levels and activities of BACE, leading to elevated Aβ levels. Finally, increased Aβ levels can further potentiate caspase activation and apoptosis, resulting in subsequent rounds of apoptosis and Aβ generation following sevoflurane treatment. We would like to emphasize that though our current findings suggest that sevoflurane may induce key aspects of AD neuropathogenesis in vitro and in vivo, the in vivo relevance of these effects in humans remains unclear. Nonetheless, our current findings should ultimately help to facilitate the design of safer anesthetics and improved anesthesia care for patients, especially elderly individuals and patients with AD.
Figure 7.
Hypothetical pathway by which sevoflurane induces apoptosis and β-amyloid protein (Aβ) generation. Sevoflurane induces caspase-3 activation and apoptosis. Caspase activation and apoptosis, in turn, increase β-site APP-cleaving enzyme (BACE) levels, which serves to increase Aβ generation. Elevated Aβ generation can then further induce caspase-3 activation and apoptosis.
Acknowledgments
Funding/Support: Dr Xie had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This study was supported by grants NS048140, AG029856, and GM088801 from the National Institutes of Health; the American Geriatrics Society Jahnigen Award; the William F. Milton Fund of Harvard University; an Investigator-Initiated Research Grant from Alzheimer's Association (Dr Xie); grants AG2025 (Dr Crosby) and GM077507 (Dr Culley) from the National Institutes of Health and the Cure Alzheimer's Fund (Dr Tanzi). The cost of anesthetic sevoflurane and partial salary support for Dr Dong were generously provided by the Department of Anesthesia and Critical Care, Massachusetts General Hospital and Harvard Medical School, Boston.
Footnotes
Financial Disclosure: None reported.
REFERENCES
- 1.Glenner GG, Wong CW. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 2.Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120(4):545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Gu Y, Misonou H, Sato T, Dohmae N, Takio K, Ihara Y. Distinct intramembrane cleavage of the beta-amyloid precursor protein family resembling gamma-secretase-like cleavage of Notch. J Biol Chem. 2001;276(38):35235–35238. doi: 10.1074/jbc.C100357200. [DOI] [PubMed] [Google Scholar]
- 4.Sastre M, Steiner H, Fuchs K, et al. Presenilin-dependent gamma-secretase processing of beta-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO Rep. 2001;2(9):835–841. doi: 10.1093/embo-reports/kve180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu C, Kim SH, Ikeuchi T, et al. Characterization of a presenilin-mediated amyloid precursor protein carboxyl-terminal fragment gamma. Evidence for distinct mechanisms involved in gamma-secretase processing of the APP and Notch1 trans-membrane domains. J Biol Chem. 2001;276(47):43756–43760. doi: 10.1074/jbc.C100410200. [DOI] [PubMed] [Google Scholar]
- 6.Holtzman DM, Deshmukh M. Caspases: a treatment target for neurodegenerative disease? Nat Med. 1997;3(9):954–955. doi: 10.1038/nm0997-954. [DOI] [PubMed] [Google Scholar]
- 7.Lunkes A, Trottier Y, Mandel JL. Pathological mechanisms in Huntington's disease and other polyglutamine expansion diseases. Essays Biochem. 1998;(33):149–163. doi: 10.1042/bse0330149. [DOI] [PubMed] [Google Scholar]
- 8.Namura S, Zhu J, Fink K, et al. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18(10):3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim TW, Pettingell WH, Jung YK, Kovacs DM, Tanzi RE. Alternative cleavage of Alzheimer-associated presenilins during apoptosis by a caspase-3 family protease. Science. 1997;277(5324):373–376. doi: 10.1126/science.277.5324.373. [DOI] [PubMed] [Google Scholar]
- 10.Loetscher H, Deuschle U, Brockhaus M, et al. Presenilins are processed by caspase-type proteases. J Biol Chem. 1997;272(33):20655–20659. doi: 10.1074/jbc.272.33.20655. [DOI] [PubMed] [Google Scholar]
- 11.Barnes NY, Li L, Yoshikawa K, Schwartz LM, Oppenheim RW, Milligan CE. Increased production of amyloid precursor protein provides a substrate for caspase-3 in dying motoneurons. J Neurosci. 1998;18(15):5869–5880. doi: 10.1523/JNEUROSCI.18-15-05869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacs DM, Mancini R, Henderson J, et al. Staurosporine-induced activation of caspase-3 is potentiated by presenilin 1 familial Alzheimer's disease mutations in human neuroglioma cells. J Neurochem. 1999;73(6):2278–2285. doi: 10.1046/j.1471-4159.1999.0732278.x. [DOI] [PubMed] [Google Scholar]
- 13.Su JH, Anderson AJ, Cummings BJ, Cotman CW. Immunohistochemical evidence for apoptosis in Alzheimer's disease. Neuroreport. 1994;5(18):2529–2533. doi: 10.1097/00001756-199412000-00031. [DOI] [PubMed] [Google Scholar]
- 14.Su JH, Deng G, Cotman CW. Bax protein expression is increased in Alzheimer's brain: correlations with DNA damage, Bcl-2 expression, and brain pathology. J Neuropathol Exp Neurol. 1997;56(1):86–93. doi: 10.1097/00005072-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Tesco G, Koh YH, Kang EL, et al. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54(5):721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattson MP. Contributions of mitochondrial alterations, resulting from bad genes and a hostile environment, to the pathogenesis of Alzheimer's disease. Int Rev Neurobiol. 2002;53:387–409. doi: 10.1016/s0074-7742(02)53014-2. [DOI] [PubMed] [Google Scholar]
- 17.Raina AK, Hochman A, Ickes H, et al. Apoptotic promoters and inhibitors in Alzheimer's disease: who wins out? Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):251–254. doi: 10.1016/S0278-5846(03)00020-4. [DOI] [PubMed] [Google Scholar]
- 18.Zhang B, Dong Y, Zhang G, et al. The inhalation anesthetic desflurane induces caspase activation and increases amyloid-beta protein levels under hypoxic conditions [published online ahead of print March 6, 2008] J Biol Chem. 2008;283(18):11866–11875. doi: 10.1074/jbc.M800199200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie Z, Dong Y, Maeda U, et al. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27(6):1247–1254. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohnen N, Warner MA, Kokmen E, Kurland LT. Early and midlife exposure to anesthesia and age of onset of Alzheimer's disease. Int J Neurosci. 1994;77(34):181–185. doi: 10.3109/00207459408986029. [DOI] [PubMed] [Google Scholar]
- 21.Muravchick S, Smith DS. Parkinsonian symptoms during emergence from general anesthesia. Anesthesiology. 1995;82(1):305–307. doi: 10.1097/00000542-199501000-00039. [DOI] [PubMed] [Google Scholar]
- 22.Lee TA, Wolozin B, Weiss KB, Bednar MM. Assessment of the emergence of Alzheimer's disease following coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty. J Alzheimers Dis. 2005;7(4):319–324. doi: 10.3233/jad-2005-7408. [DOI] [PubMed] [Google Scholar]
- 23.van Groen T, Puurunen K, Maki HM, Sivenius J, Jolkkonen J. Transformation of diffuse beta-amyloid precursor protein and beta-amyloid deposits to plaques in the thalamus after transient occlusion of the middle cerebral artery in rats. Stroke. 2005;36(7):1551–1556. doi: 10.1161/01.STR.0000169933.88903.cf. [DOI] [PubMed] [Google Scholar]
- 24.Xie Z, Dong Y, Maeda U, et al. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104(5):988–994. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Xie Z, Romano DM, Tanzi RE. Effects of RNAi-mediated silencing of PEN-2, APH-1a, and nicastrin on wild-type vs FAD mutant forms of presenilin 1. J Mol Neurosci. 2005;25(1):67–77. doi: 10.1385/JMN:25:1:067. [DOI] [PubMed] [Google Scholar]
- 26.Xie Z, Romano DM, Tanzi RE. RNA interference-mediated silencing of X11alpha and X11beta attenuates amyloid beta-protein levels via differential effects on beta-amyloid precursor protein processing. J Biol Chem. 2005;280(15):15413–15421. doi: 10.1074/jbc.M414353200. [DOI] [PubMed] [Google Scholar]
- 27.Matsuoka H, Kurosawa S, Horinouchi T, Kato M, Hashimoto Y. Inhalation anesthetics induce apoptosis in normal peripheral lymphocytes in vitro. Anesthesiology. 2001;95(6):1467–1472. doi: 10.1097/00000542-200112000-00028. [DOI] [PubMed] [Google Scholar]
- 28.Roesslein M, Frick M, Auwaerter V, et al. Sevoflurane-mediated activation of p38-mitogen-activated stresskinase is independent of apoptosis in Jurkat T-cells. Anesth Analg. 2008;106(4):1150–1160. doi: 10.1213/ane.0b013e3181683d37. [DOI] [PubMed] [Google Scholar]
- 29.Kvolik S, Glavas-Obrovac L, Bares V, Karner I. Effects of inhalation anesthetics halothane, sevoflurane, and isoflurane on human cell lines. Life Sci. 2005;77(19):2369–2383. doi: 10.1016/j.lfs.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 30.Wong CH, Liu TZ, Chye SM, et al. Sevoflurane-induced oxidative stress and cellular injury in human peripheral polymorphonuclear neutrophils. Food Chem Toxicol. 2006;44(8):1399–1407. doi: 10.1016/j.fct.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Loop T, Dovi-Akue D, Frick M, et al. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology. 2005;102(6):1147–1157. doi: 10.1097/00000542-200506000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Loop T, Scheiermann P, Doviakue D, et al. Sevoflurane inhibits phorbol-myristate-acetate-induced activator protein-1 activation in human T lymphocytes in vitro: potential role of the p38-stress kinase pathway. Anesthesiology. 2004;101(3):710–721. doi: 10.1097/00000542-200409000-00020. [DOI] [PubMed] [Google Scholar]
- 33.Xie Z, Dong Y, Maeda U, et al. Isoflurane-induced apoptosis: a potential pathogenic link between delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006;61(12):1300–1306. doi: 10.1093/gerona/61.12.1300. [DOI] [PubMed] [Google Scholar]
- 34.Thornberry NA. Caspases: key mediators of apoptosis. Chem Biol. 1998;5(5):R97–R103. doi: 10.1016/s1074-5521(98)90615-9. [DOI] [PubMed] [Google Scholar]
- 35.McLaughlin B, Hartnett KA, Erhardt JA, et al. Caspase 3 activation is essential for neuroprotection in preconditioning. Proc Natl Acad Sci U S A. 2003;100(2):715–720. doi: 10.1073/pnas.0232966100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campen MJ, Shimoda LA, O'Donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in C57BL/6J mice. J Appl Physiol. 2005;99(5):2028–2035. doi: 10.1152/japplphysiol.00411.2005. [DOI] [PubMed] [Google Scholar]
- 37.Zhang G, Dong Y, Zhang B, et al. Isoflurane-induced caspase-3 activation is dependent on cytosolic calcium and can be attenuated by memantine. J Neurosci. 2008;28(17):4551–4560. doi: 10.1523/JNEUROSCI.5694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei H, Kang B, Wei W, et al. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res. 2005;1037(12):139–147. doi: 10.1016/j.brainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Zhang SD, Zhai J, Zhang H, Wan H, Li DZ. Protective effect of isoflurane and sevoflurane on ischemic neurons and expression of Bcl-2 and ICE genes in rat brain. Biomed Environ Sci. 2006;19(2):143–146. [PubMed] [Google Scholar]
- 40.Bouwman RA, Salic K, Padding FG, et al. Cardioprotection via activation of protein kinase C-delta depends on modulation of the reverse mode of the Na+/Ca2+ exchanger. Circulation. 2006;114(1suppl):I226–I232. doi: 10.1161/CIRCULATIONAHA.105.000570. [DOI] [PubMed] [Google Scholar]
- 41.Canas PT, Velly LJ, Labrande CN, et al. Sevoflurane protects rat mixed cerebro-cortical neuronal-glial cell cultures against transient oxygen-glucose deprivation: involvement of glutamate uptake and reactive oxygen species. Anesthesiology. 2006;105(5):990–998. doi: 10.1097/00000542-200611000-00021. [DOI] [PubMed] [Google Scholar]
- 42.Xia Z, Luo T. Sevoflurane or desflurane anesthesia plus postoperative propofol sedation attenuates myocardial injury after coronary surgery in elderly high-risk patients. Anesthesiology. 2004;100(4):1038–1039. doi: 10.1097/00000542-200404000-00050. [DOI] [PubMed] [Google Scholar]
- 43.Engelhard K, Werner C, Eberspacher E, et al. Sevoflurane and propofol influence the expression of apoptosis-regulating proteins after cerebral ischaemia and reper-fusion in rats. Eur J Anaesthesiol. 2004;21(7):530–537. doi: 10.1017/s0265021504007057. [DOI] [PubMed] [Google Scholar]
- 44.Lee HT, Kim M, Kim J, Kim N, Emala CW. TGF-beta1 release by volatile anesthetics mediates protection against renal proximal tubule cell necrosis. Am J Nephrol. 2007;27(4):416–424. doi: 10.1159/000105124. [DOI] [PubMed] [Google Scholar]
- 45.Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology. 2004;101(6):1313–1324. doi: 10.1097/00000542-200412000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Payne RS, Akca O, Roewer N, Schurr A, Kehl F. Sevoflurane-induced preconditioning protects against cerebral ischemic neuronal damage in rats. Brain Res. 2005;1034(12):147–152. doi: 10.1016/j.brainres.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Pape M, Engelhard K, Eberspacher E, et al. The long-term effect of sevoflurane on neuronal cell damage and expression of apoptotic factors after cerebral ischemia and reperfusion in rats. Anesth Analg. 2006;103(1):173–179. doi: 10.1213/01.ane.0000222634.51192.a4. [DOI] [PubMed] [Google Scholar]
- 48.Wei H, Liang G, Yang H. Isoflurane preconditioning inhibited isoflurane-induced neurotoxicity. Neurosci Lett. 2007;425(1):59–62. doi: 10.1016/j.neulet.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Velliquette RA, O'Connor T, Vassar R. Energy inhibition elevates beta-secretase levels and activity and is potentially amyloidogenic in APP transgenic mice: possible early events in Alzheimer's disease pathogenesis. J Neurosci. 2005;25(47):10874–10883. doi: 10.1523/JNEUROSCI.2350-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alkire MT, Gruver R, Miller J, McReynolds JR, Hahn EL, Cahill L. Neuroimaging analysis of an anesthetic gas that blocks human emotional memory. Proc Natl Acad Sci U S A. 2008;105(5):1722–1727. doi: 10.1073/pnas.0711651105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujie W, Kirino T, Tomukai N, Iwasawa T, Tamura A. Progressive shrinkage of the thalamus following middle cerebral artery occlusion in rats. Stroke. 1990;21(10):1485–1488. doi: 10.1161/01.str.21.10.1485. [DOI] [PubMed] [Google Scholar]
- 52.Nordborg C, Johansson BB. Secondary thalamic lesions after ligation of the middle cerebral artery: an ultrastructural study. Acta Neuropathol. 1996;91(1):61–66. doi: 10.1007/s004010050392. [DOI] [PubMed] [Google Scholar]
- 53.Dijkhuizen RM, Knollema S, van der Worp HB, et al. Dynamics of cerebral tissue injury and perfusion after temporary hypoxia-ischemia in the rat: evidence for region-specific sensitivity and delayed damage. Stroke. 1998;29(3):695–704. doi: 10.1161/01.str.29.3.695. [DOI] [PubMed] [Google Scholar]
- 54.Carmichael ST, Tatsukawa K, Katsman D, Tsuyuguchi N, Kornblum HI. Evolution of diaschisis in a focal stroke model. Stroke. 2004;35(3):758–763. doi: 10.1161/01.STR.0000117235.11156.55. [DOI] [PubMed] [Google Scholar]