Abstract
Cannabidiol (CBD) is a cannabinoid compound derived from Cannabis Sativa that does not possess high affinity for either the CB1 or CB2 cannabinoid receptors. Similar to other cannabinoids, we demonstrated previously that CBD suppressed interleukin-2 (IL-2) production from phorbol ester plus calcium ionophore (PMA/Io)-activated murine splenocytes. Thus, the focus of the present studies was to further characterize the effect of CBD on immune function. CBD also suppressed IL-2 and interferon-γ (IFN-γ) mRNA expression, proliferation, and cell surface expression of the IL-2 receptor alpha chain, CD25. While all of these observations support the fact that CBD suppresses T cell function, we now demonstrate that CBD suppressed IL-2 and IFN-γ production in purified splenic T cells. CBD also suppressed activator protein-1 (AP-1) and nuclear factor of activated T cells (NFAT) transcriptional activity, which are critical regulators of IL-2 and IFN-γ. Furthermore, CBD suppressed the T cell-dependent anti-sheep red blood cell immunoglobulin M antibody forming cell (anti-sRBC IgM AFC) response. Finally, using splenocytes derived from CB1-/-/CB2-/- mice, it was determined that suppression of IL-2 and IFN-γ and suppression of the in vitro anti-sRBC IgM AFC response occurred independently of both CB1 and CB2. However, the magnitude of the immune response to sRBC was significantly depressed in CB1-/-/CB2-/- mice. Taken together, these data suggest that CBD suppresses T cell function and that CB1 and/or CB2 play a critical role in the magnitude of the in vitro anti-sRBC IgM AFC response.
1. Introduction
Cannabinoids are a group of structurally-related compounds derived from the Cannabis Sativa plant, which is commonly known as marijuana. The primary psychoactive congener in marijuana is tetrahydrocannabinol (THC) [1]. Although THC is currently approved for medical use as Marinol®, there exists an ongoing debate in the United States as to whether smoking crude marijuana could be a medical necessity. This debate has sparked interest in determining the physiological properties of some of the other plant-derived cannabinoid compounds. One such compound is cannabidiol (CBD), which is one of the most abundant cannabinoids in the plant.
CBD possesses low affinity for both CB1 and CB2 cannabinoid receptors and therefore, does not produce the “high” associated with marijuana use [2, 3]. Despite this, CBD does exhibit immunosuppressive properties. In particular, CBD decreased IL-8 and the chemokines MIP-1α and MIP-1β from a human B cell line [4]. CBD has also been shown to suppress collagen-induced arthritis [5], and carrageenan-induced inflammation [6]. Importantly, CBD has been efficacious in combination with THC in treating neuropathic pain in multiple sclerosis, an autoimmune disease [7, 8].
Despite these reports that CBD possesses immunosuppressive actions, its effects on T lymphocytes have not been fully characterized. With our previous demonstration that CBD was one of the more potent plant-derived cannabinoids in suppressing IL-2 from PMA/Io-stimulated splenocytes [9], the focus of the present studies was to further investigate the effects of CBD on T lymphocyte function. The immunological endpoints include the determination of the effect of CBD on cytokine production (IL-2 and IFN-γ) from splenocytes activated through the T cell receptor, T and B cell proliferation, AFC responses, and direct effects on purified splenic T cells. As many reports in the literature suggest the involvement of a yet unidentified putative third cannabinoid receptor [10, 11], cannabinoid actions via other receptors [12-14], and that some effects of CBD can be reversed by the CB1 and CB2 receptor antagonists [15], we utilized splenocytes derived from CB1-/-/CB2-/- mice to address the role of CB1 and CB2 in the effects of CBD in T lymphocytes. Our results suggest that CBD suppresses T cell function via a mechanism that involves AP-1 and NFAT, and we have also discovered a putative critical role for CB1 and/or CB2 in the magnitude of the in vitro anti-sRBC IgM AFC response.
2. Materials and methods
2.1 Reagents
CBD and THC were provided by the National Institute on Drug Abuse (Bethesda, MD). All other reagents were obtained from Sigma (St. Louis, MO) unless otherwise noted.
2.2 Animals
Pathogen-free female B6C3F1 or C57BL/6 mice, 6 weeks of age, were purchased from Charles River Breeding Laboratories (Portage, MI). On arrival, mice were randomized, transferred to plastic cages containing sawdust bedding (5 animals/cage), and quarantined for 1 week. CB1-/-/CB2-/- mice were kindly provided by Dr. Andreas Zimmer (University of Bonn) and were bred at Michigan State University. Mice were given food (Purina Certified Laboratory Chow) and water ad libitum and were not used for experimentation until their body weight was 17-20 g. Animal holding rooms were kept at 21-24°C and 40-60% relative humidity with a 12-hr light/dark cycle. All procedures involving mice were performed in accordance with guidelines set forth by the Institutional Animal Care and Use Committee at Michigan State University.
2.3 Preparation of lymphocyte cultures
Mice were sacrificed and spleens were aseptically removed. Single cell suspensions were prepared and cells were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, 5 × 10-5 M 2-mercaptoethanol, and 2-10% bovine calf serum (BCS; Hyclone, Logan, UT). For immunofluorescence analysis, erythrocytes were lysed with ACK solution (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA). Jurkat cells (clone E6-1, ATCC, Manassas, VA) were maintained in RPMI 1640 medium supplemented with 2-10% BCS, 100 units/ml penicillin, 100 μg/ml streptomycin, 1× solutions of non-essential amino acids and sodium pyruvate (Invitrogen, Carlsbad, CA).
2.4 ELISA
Splenocytes (8 × 105 cells) were treated with CBD (0.1-20 μM) for 30 min at 37°C, followed by cellular activation for 24 hr in complete medium containing 2% BCS in 48-well culture plates at 0.8 ml/well. Cells were activated with either 40 nM/0.5 μM PMA/Io or 100 ng immobilized anti-CD3 plus 1 μg/ml soluble anti-CD28 (BD Biosciences, San Jose, CA). Alternatively, Jurkat cells (5 × 104 cells) were treated with CBD (0.1-10 μM) for 30 min at 37°C, followed by cellular activation for 24 hr in complete medium containing 2% BCS in 48-well culture plates at 0.25 ml/well. Jurkat cells were activated with 40 nM/0.5 μM PMA/Io. Cells were harvested and supernatants were collected and assayed for human IL-2, or murine IL-2 or IFN-γ production by ELISA. Recombinant purified human IL-2 or mouse IL-2 or IFN-γ (BD Biosciences, San Jose, CA) served as standards from which the amount of cytokine in the samples could be determined. Capture antibodies were purified anti-human IL-2 or anti-mouse IL-2 or IFN-γ and detection antibodies were biotinylated anti-human IL-2 or anti-mouse IL-2 or IFN-γ (BD Biosciences, San Jose, CA). Color development was performed using streptavidin peroxidase followed by tetramethylbenzidine (Fluka/Sigma, St. Louis, MO). Reactions were stopped with 6N H2SO4, after which samples were read at 450 nm.
2.5 Real time polymerase chain reaction (PCR)
Splenocytes (5 × 106 cells) were treated with CBD (0.5-10 μM) for 30 min at 37°C, followed by cellular activation for 6 hr in complete medium containing 2% BCS in 6-well culture plates at 5 ml/well. Cells were activated with 40 nM/0.5 μM PMA/Io. Cells were harvested and placed in TRI Reagent (Sigma, St. Louis, MO). Following phase separation with bromochlorophenol, RNA was precipitated from the aqueous phase with isopropanol. The remainder of the extraction, purification and DNase treatment was done using the Promega SV Total RNA Isolation System (Promega, Madison, WI). Total RNA was reversed transcribed using random primers with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). cDNA was amplified with Taqman primers and probe sets purchased from Applied Biosystems and analyzed using a 7900 HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA).
2.6 Immunofluorescence analysis
Splenocytes (8 × 105 cells) were treated with CBD (0.2-20 μM) for 30 min at 37°C, followed by cellular activation with 40 nM/0.5 μM PMA/Io for 24 hr in complete medium containing 2% BCS in 48-well culture plates at 0.8 ml/well. Cells were harvested, stained with antibodies directed against CD3 or CD25 (CD3-FITC or CD25-PE; BD Biosciences, San Jose, CA), and analyzed using a FACSCalibur (BD Biosciences, San Jose, CA). Cells were gated based on forward and side scatter (FSC/SSC) and data were analyzed using CellQuest software (BD Biosciences, San Jose, CA).
2.7 Lymphoproliferation assays
Splenocytes (2 × 105 cells) were treated with CBD (0.2-20 μM) for 30 min at 37°C, followed by cellular activation in complete medium containing 2% (48 hr cultures) or 5% (72 hr cultures) BCS in 96-well culture plates at 0.2 ml/well. Cells were activated with either 40 nM/0.5 μM PMA/Io, 100 ng immobilized anti-CD3 plus 1 μg/ml soluble anti-CD28, or 10 μg/ml lipopolysaccharide (LPS). Splenocytes that were activated with LPS were cultured for 72 hr; splenocytes that were activated with PMA/Io or anti-CD3/CD28 were cultured for 48 hr. Cultures were pulsed with 1 μCi/well of [3H]-thymidine 18 hr prior to harvest, and the cells were harvested onto glass fiber filters using a PHD cell harvester (Cambridge Technology, Inc., Watertown, MA). Tritium incorporation was measured using a Packard Tri-Carb 2100TR Liquid Scintillation Analyzer (Packard Biosciences/Perkin-Elmer, Wellesley, MA).
2.8 Mixed lymphocyte response (MLR)
Splenocytes (1 × 105 cells) were treated with CBD (0.2-20 μM), followed by cellular activation with mitomycin C-treated non-self (DBA/2) splenocytes in complete medium containing 5% FBS in 96-well round bottom culture plates at 0.2 ml/well. The DBA/2 splenocytes were treated with 40 μg/ml mitomycin C for 60 min at 37°C, washed 4 times with RPMI and adjusted to the appropriate cell density such that the stimulator:responder ratio was 4:1 (4 × 105 mitomycin C-treated DBA/2 splenocytes: 1 ×105 B6C3F1 splenocytes). Cells were cultured for 96 hr. 18 hr prior to harvest, cultures were pulsed with 1 μCi/well of [3H]-thymidine and tritium incorporation was measured as described above.
2.9 Transient transfections
Jurkat cells (5 × 105 cells) were transfected using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) in complete medium containing 2% BCS. NFAT-luc, AP-1-luc and pTA-luc plasmids were purchased from Clontech (Mountain View, CA). Briefly, for every 5 × 105 cells to be transfected, pooled cells were incubated with 1.5 μg plasmid DNA and 3 μl Lipofectamine 2000 reagent, each delivered in 50 μl RPMI 1640. Plasmid DNA and Lipofectamine 2000 reagent were incubated in RPMI 1640 for 5 min, combined, and allowed to complex for 20 min prior to addition to cells. Transfected, pooled cells were then distributed to 48-well plates at 1 ml/well. Three hours post-transfection and plating, cells were treated with CBD (1-20 μM) for 30 min at 37°C, followed by cellular activation with 40 nM/0.5 μM PMA/Io for 24 hr. Supernatants were harvested and assessed for IL-2, and the cells were assessed for luciferase activity.
2.10 Luciferase assays
Luciferase activity was determined using the Luciferase Assay System and Reporter Lysis Buffer (RLB) from Promega (Madison, WI). Briefly, cells were washed once in PBS, then resuspended in 50 μl RLB per 5×105 cells. Cells were then frozen at -80°C for 10 min, thawed and directly transferred to opaque 96-well plates for luciferase activity determination. Luciferase substrate (100 μl) was added to each well using the Bio-Tek Synergy HT instrument with KC4 version 3.4 software (Winooski, VT). After a 2 sec delay, luciferase activity was detected over a 10 sec period and data are presented as relative light units (RLU) in counts per second (CPS). Protein determinations were performed using a Bicinchoninic Acid Assay (BCA; Sigma, St. Louis, MO).
2.11 T cell purifications
T cells were purified from whole splenocyte preparations using the Pan T Cell Isolation Kit according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). Briefly, spleens were collected and a single cell suspension was generated in MACS buffer (PBS, 0.5% BSA and 2 mM EDTA). Cells were then incubated with antibody cocktail and magnetic beads that allow for negative selection of T cells in the presence of a magnetic column. Purified T cells were subsequently used for ELISA analysis and proliferation. Purity of T cells was determined using immunofluorescence analysis with antibodies directed against CD3, and generally exceeded 98%.
2.12 In vitro antibody forming cell response (AFC)
Splenocytes (2.5-5 × 106 cells) were treated with CBD (1-20 μM), followed by cellular activation in complete medium containing 10% heat-inactivated BCS in 48-well culture plates at 0.5 ml/well. Cells were activated with either 6.5 × 106 sheep erythrocytes (sRBC; Colorado Serum, Denver, CO) or 100 μg/ml LPS. Splenocytes were cultured (sRBC, 5 days; LPS, 3 days) in a Bellco stainless steel tissue culture chamber pressurized to 5.5 psi with a blood-gas mixture containing 10% O2, 7% CO2, and 83% N2. The culture chamber was incubated at 37°C and rocked continuously for the duration of the culture period. Enumeration of the antibody forming cells was based on the Jerne plaque assay [16, 17]. Briefly, 50 or 100 μl aliquots of the cultured splenocytes were combined with 0.5% melted agar (Difco/BD, Franklin Lakes, NJ), guinea pig complement (Gibco/Invitrogen, Carlsbad, CA) and sheep erythrocytes, plated, covered with a 24 × 50 mm glass cover slip, and allowed to solidify. Plates were incubated for at least 3 hr at 37°C, after which AFCs were enumerated at 6.5× magnification using a Bellco plaque viewer (Bellco Glass Co., Vineland, NJ).
2.13 In vivo antibody forming cell response (AFC)
Mice (B6C3F1, 5 per treatment group) were administered CBD (25, 50 or 100 mg/kg) or THC (50 mg/kg) in corn oil by oral gavage for 3 days. On the second day, mice were sensitized with 5 × 108 sRBC per mouse by i.p. injection. Four days after sRBC sensitization, mice were sacrificed and total body, spleen, thymus and kidney weights were recorded. Single cell suspensions of splenocytes were then generated and used to determine the in vivo AFC response as described above.
2.14 Statistical analysis
The mean ± S.E. was determined for each treatment group. Differences between means were determined with a parametric analysis of variance. When significant differences were detected, treatment groups were compared to the appropriate control using Dunnett's two-tailed t test. Following a two-way analysis of variance, all groups were compared using Bonferroni's test. A two-tailed t test was used to determine statistical significance between stimulated groups in the anti-sRBC IgM AFC response between C57BL/6 and CB1-/-/CB2-/- mice. Statistical analyses were performed using GraphPad Prism version 4.0a for Macintosh OS X, GraphPad Software (San Diego, CA).
3. Results
3.1 CBD suppressed cytokine production in PMA/Io-stimulated splenocytes in a CB1 and CB2 receptor-independent manner
Previous work from our laboratory demonstrated that CBD was one of the most potent cannabinoids for suppression of PMA/Io-induced IL-2 production in splenocytes [9]. As seen in Figure 1B, CBD also suppressed PMA/Io-induced IFN-γ production, although the potency with which CBD suppressed IFN-γ was not as marked as for IL-2 (shown in Figure 1A as a comparative control). The CBD-induced suppression of both cytokines occurred at the level of mRNA (Figure 1C and D). With the demonstration that IL-2 is a sensitive target of suppression by CBD, we next determined the effect of CBD on expression of the IL-2 receptor α chain (CD25). CBD suppressed cell surface expression of CD25 in a concentration-dependent manner in PMA/Io-stimulated splenocytes (Figure 2). Interestingly, there was not a large population of CD25+ cells in the absence of stimulation, suggesting the primary effect of CBD on CD25 occurs during T cell activation as opposed to an effect on the T regulatory cell population. Finally, there was no difference in the ability of CBD to suppress PMA/Io-stimulated IL-2 and IFN-γ from splenocytes derived from either C57BL/6 wild type or CB1-/-/CB2-/- mice (Figure 3).
Figure 1.
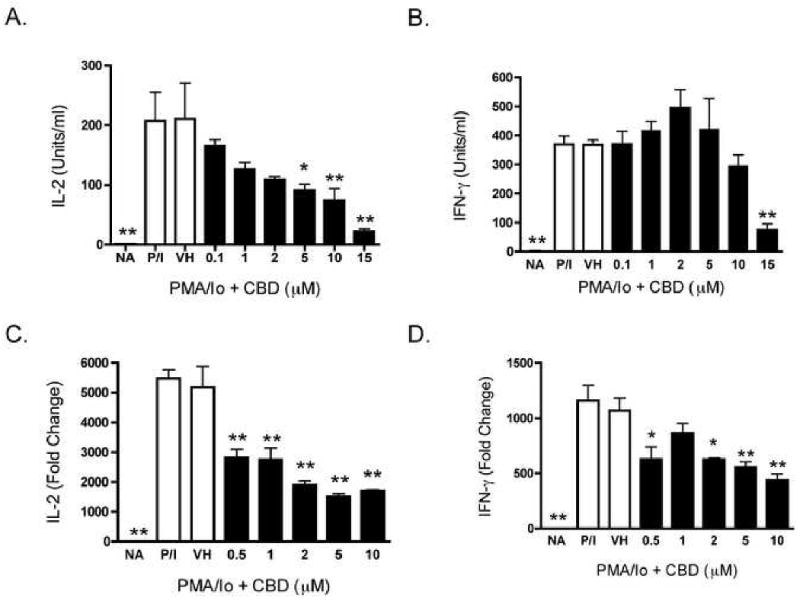
CBD suppressed cytokine production in PMA/Io-stimulated B6C3F1 splenocytes. A-B.) Splenocytes (8 × 105 cells) were treated with CBD (0.1-15 μM) for 30 min, followed by cellular activation with PMA/Io for 24 hr. Supernatants were harvested and the amount of IL-2 (A.) or IFN-γ (B.) was determined by ELISA. The data are expressed as the mean Units/ml ± SE of triplicate cultures. C-D.) Splenocytes (5 × 106 cells) were treated with CBD (0.5-10 μM) for 30 min, followed by cellular activation with PMA/Io for 6 hr. Real time PCR was performed for IL-2 and IFN-γ. Fold change was calculated as compared to NA. * or ** denotes values that are significantly different from the vehicle control at p < 0.05 or 0.01. Results are representative of at least two separate experiments. NA, naïve (untreated); VH, vehicle (0.1% ethanol).
Figure 2.
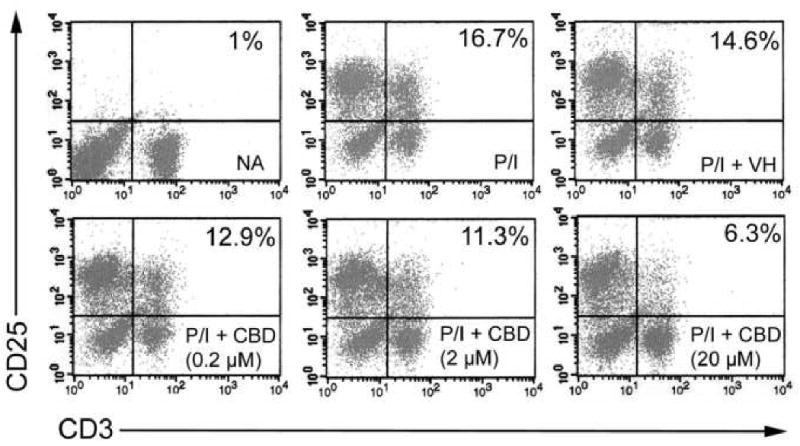
CBD suppressed CD25 cell surface expression in PMA/Io-stimulated B6C3F1 splenocytes. Splenocytes (8 × 105 cells) were treated with CBD (0.2-20 μM) for 30 min, followed by cellular activation with PMA/Io for 24 hr. Cells were harvested and stained with fluorescent antibodies directed against CD3 (FITC) or CD25 (PE). Cells were gated on FSC/SSC. Numbers denote percent gated events. Results are representative of three separate experiments. NA, naïve (untreated); VH, vehicle (0.1% ethanol).
Figure 3.
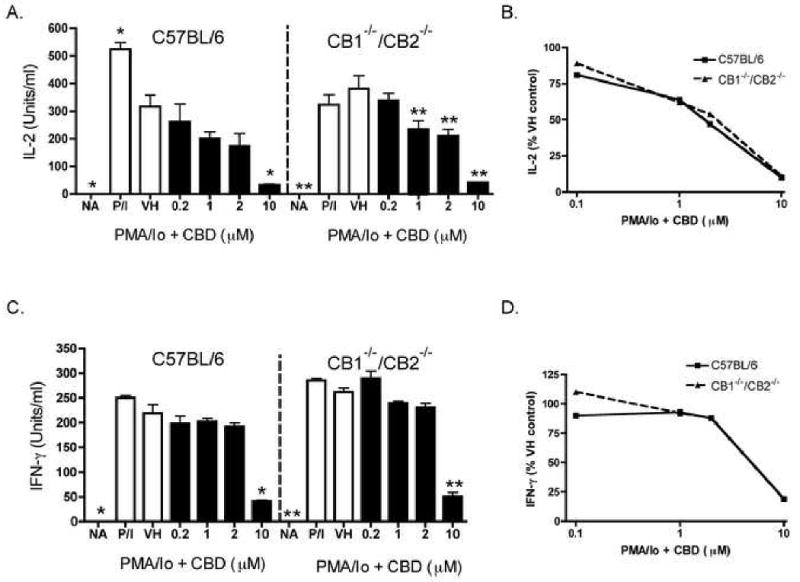
CBD suppressed cytokine production in wild type C57BL/6 and CB1-/-/CB2-/- splenocytes. Splenocytes (8 × 105 cells) were treated with CBD (0.2-10 μM) for 30 min, followed by cellular activation with PMA/Io for 24 hr. Supernatants were harvested and the amount of IL-2 (A-B.) or IFN-γ (C-D.) in wild type C57BL/6 and CB1-/-CB2-/- was determined by ELISA. The data are expressed as the mean Units/ml ± SE of triplicate cultures. * or ** denotes values that are significantly different from the respective vehicle control at p < 0.05 or 0.01. Data are presented as % VH control in B and D. Results are representative of two separate experiments. NA, naïve (untreated); VH, vehicle (0.1% ethanol).
3.2 CBD suppressed cytokine production in T cells
In order to determine whether splenic T lymphocytes are direct targets of inhibition by CBD, splenocytes were activated with anti-CD3/anti-CD28, which exclusively stimulates T lymphocytes via the T cell receptor. Although not as marked as the inhibition of cytokines produced in response to PMA/Io, CBD also suppressed IL-2 and IFN-γ produced in response to anti-CD3/anti-CD28 from splenic T lymphocytes (Figures 4A and B). Furthermore, PMA/Io-induced IL-2 and IFN-γ production from purified splenic T cells (i.e., >95% purity) was also suppressed by CBD (Figures 4C and D). It is noteworthy that purified T cells were particularly sensitive to CBD in the presence of PMA/Io and therefore, lower concentrations of CBD were used for these studies.
Figure 4.
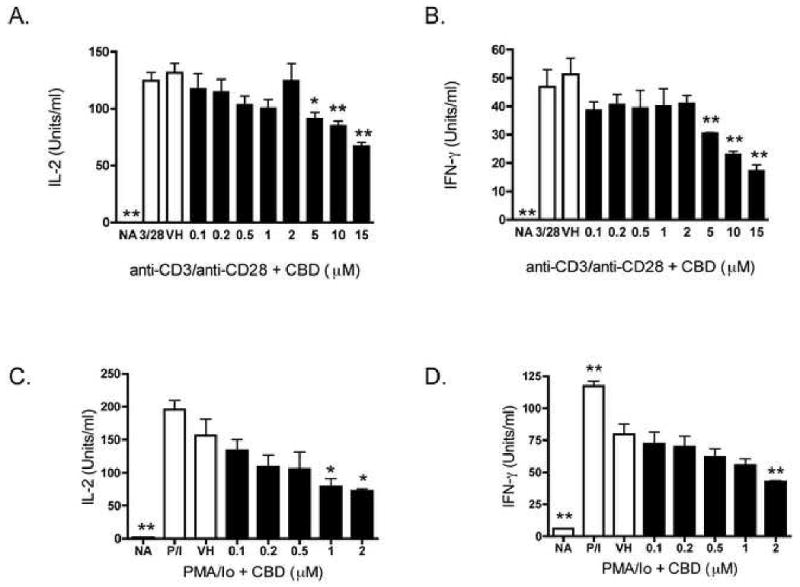
CBD suppressed cytokine production in B6C3F1 splenic T cells. A-B.) Splenocytes (8 × 105 cells) were treated with CBD (0.1-15 μM) for 30 min, followed by cellular activation with immobilized anti-CD3 plus soluble anti-CD28 for 24 hr. Supernatants were harvested and the amount of IL-2 (A.) or IFN-γ (B.) was determined by ELISA. C-D.) T cells purified from splenocytes (8 ×105 cells) were treated with CBD (0.1-2 μM) for 30 min, followed by cellular activation with PMA/Io for 24 hr. Supernatants were harvested and the amount of IL-2 (C.) or IFN-γ (D.) was determined by ELISA. The data are expressed as the mean Units/ml ± SE of triplicate cultures. * or ** denotes values that are significantly different from the vehicle control at p < 0.05 or 0.01. Results are representative of at least two separate experiments. NA, naïve (untreated); VH, vehicle (0.1% ethanol).
3.3. CBD suppressed cellular proliferation in several cell types
IL-2, which acts via the IL-2 receptor, is a critical cytokine for T cell proliferation; therefore we determined the effect of CBD on cellular proliferation. CBD suppressed PMA/Io-stimulated proliferation in a concentration-dependent manner (Figure 5A). However, as PMA/Io likely induces proliferation in most splenic cell types, various stimuli were utilized to target specific cell populations. Cellular proliferation in response to LPS, which predominantly activates B cells, was also suppressed in a concentration-dependent manner by CBD (Figure 5B). In order to address the effect of CBD on T cell proliferation, two different stimuli were used: anti-CD3/anti-CD28, or mitomycin C-treated allogeneic lymphocytes (MLR). In response to either anti-CD3/anti-CD28 or mitomycin C-treated allogeneic lymphocytes, both of which activate T cells via the T cell receptor, CBD suppressed T cell proliferation (Figures 5C and D).
Figure 5.
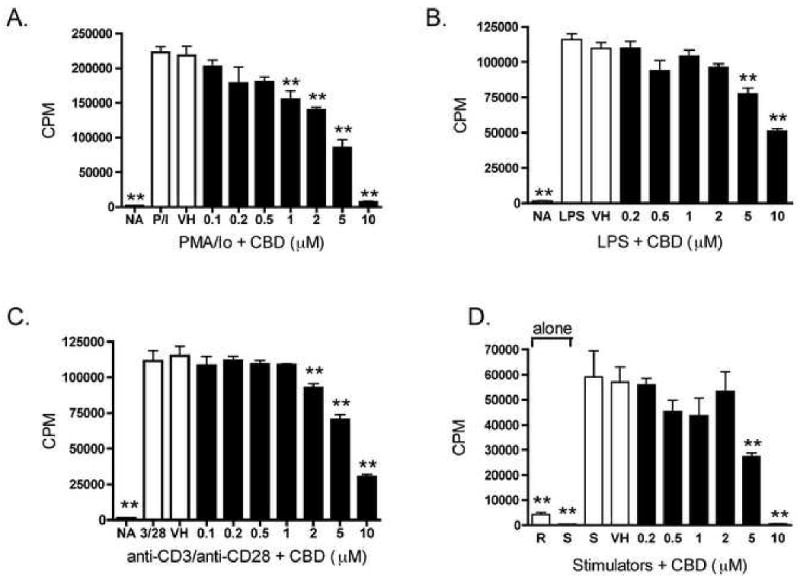
CBD suppressed cellular proliferation in B6C3F1 splenocytes in response to various stimuli. A-D.) Splenocytes (2 × 105 cells) were treated with CBD (0.2-10 μM) for 30 min, followed by cellular activation. 18 hours prior to harvest, cells were pulsed with 1 μCi 3H-thymidine. Cells were harvested onto glass fiber filters and tritium incorporation was measured with a liquid scintillation counter. Splenocytes were activated with A.) PMA/Io for 48 hr; B.) LPS for 72 hr; C.) immobilized anti-CD3 plus soluble anti-CD28 for 48 hr; D.) mitomycin C-treated allogeneic lymphocytes for 96 hr. The data are expressed as the mean CPM ± SE of quadruplicate cultures. * or ** denotes values that are significantly different from the vehicle control at p < 0.05 or 0.01. Results are representative of at least three separate experiments. R, responders; S, stimulators; NA, naïve (untreated); VH, vehicle (0.1% ethanol).
3.4 CBD suppressed the T cell-dependent AFC response
One functional immune endpoint that is sensitive to suppression by other plant-derived and synthetic cannabinoids is the T cell-dependent AFC response [18, 19]. As seen in Figure 6A, and consistent with THC, CBD suppressed the in vitro T cell-dependent anti-sRBC IgM AFC response, but did not affect the in vitro IgM AFC response to the polyclonal B cell activator, lipopolysaccharide (LPS; Figure 6B).
Figure 6.
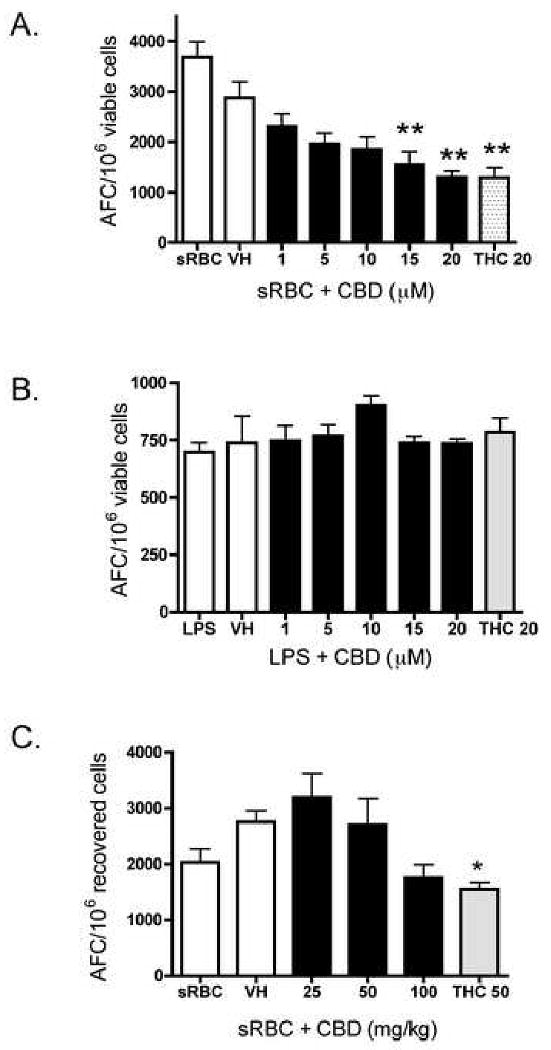
Effect of CBD on the IgM AFC response in B6C3F1 splenocytes. A-B.) Splenocytes (5 × 106 cells for sRBC; 2.5 × 106 cells for LPS) were treated with CBD (1-20 μM) or THC (20 μM) for 30 min, followed by stimulation with A.) sRBC for 5 days or B.) LPS for 72 hr. Cells were then incubated in a Bellco stainless steel tissue culture chamber pressurized to 5.5 psi with a gas mixture consisting of 10% O2, 7% CO2 and 83% N2. The culture chamber was placed at 37°C with constant rocking for the duration of the culture period. Enumeration of the AFC response was performed as described in Materials and Methods. The data are expressed as the mean AFC/106 viable cells ± SE of quadruplicate cultures. C.) B6C3F1 mice received CBD (25-100 mg/kg) or THC (50 mg/kg) by oral gavage for 3 days. On day 2, in addition to drug, mice received a single i.p. injection of sRBC (5 × 108 cells/mouse). Mice were sacrificed on day 6, after which the AFC response was enumerated as described in Materials and Methods. The data are expressed as the mean AFC/106 viable or recovered cells ± SE. Results are pooled from two separate experiments. * or ** denotes values that are significantly different from the vehicle control at p < 0.05 or 0.01.
As CBD suppressed the in vitro anti-sRBC IgM AFC response, the effect of CBD in vivo was determined. Oral administration of CBD produced a modest modulation of the IgM AFC response to sRBC (Figure 6C). Although none of the CBD treatments produced statistically significant modulation (at p < 0.05), the trend for CBD-induced suppression of the in vivo AFC response to sRBC at 100 mg/kg was consistent in two separate replicates of the experiment. The magnitude of suppression by 100 mg/kg CBD was similar to that produced by 50 mg/kg THC. There was no significant change in total body weight, spleen or thymus weights, or in the weight of the kidneys, presumably non-targets of immunosuppression by cannabinoids (data not shown).
Although CBD has been reported to possess low affinity for both CB1 and CB2 cannabinoid receptors [2, 3], we next attempted to discern and/or confirm the lack of a role for CB1 and CB2 in CBD-induced suppression of the anti-sRBC IgM AFC response by using splenocytes derived from CB1-/-/CB2-/- mice. As seen in Figure 7, CBD robustly suppressed the anti-sRBC IgM AFC response in C57BL/6 mice. There was a significant decrease (p < 0.001) in the overall magnitude of AFC induced by sRBC in the CB1-/-/CB2-/- mice versus C57BL/6 mice (379.7 ± 65.7 versus 3829 ± 443, respectively, over 4 separate experiments; one representative graph depicted in Figure 7A). Since there was such a large discrepancy in the magnitude of the immune response to sRBC in the CB1-/-/CB2-/- mice, the data are also expressed as percent of vehicle control for four separate experiments (Figure 7B). There was no difference in CBD-induced suppression of the anti-sRBC IgM response in either genotype with the exception of the 5 μM concentration, which was highly variable in the CB1-/-CB2-/- mice. It is likely that CB1 and/or CB2 are not involved in CBD-induced suppression of the in vitro anti-sRBC IgM AFC response. On the other hand, it is evident that either CB1 or CB2, or both, contribute to the overall magnitude of the in vitro anti-sRBC IgM AFC response.
Figure 7.
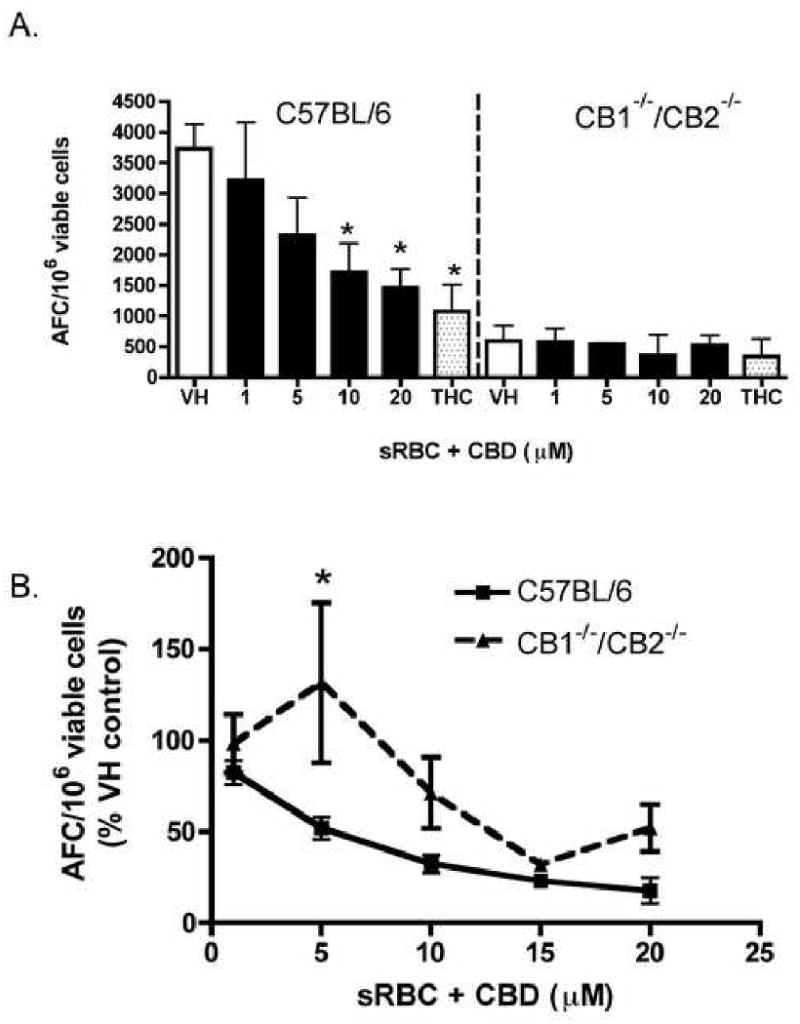
Effect of CBD on the in vitro AFC response in wild type C57BL/6 and CB1-/-/CB2-/- splenocytes. Splenocytes (5 × 106 cells) from C57BL/6 or CB1-/-/CB2-/- were treated with CBD (1-20 μM) or THC (20 μM) for 30 min, followed by stimulation with sRBC for 5 days. Cells were then cultured and the AFC were enumerated as stated in Figure 1. The data are expressed as the mean AFC/106 viable cells ± SE of quadruplicate cultures for one representative experiment (A.) or % VH results for CBD from four experiments are pooled, with the exception of the 15 μM group, which represents a single experiment (B.). * or ** denotes values that are significantly different from the respective vehicle control at p < 0.05 or 0.01.
3.5 CBD suppressed NFAT and AP-1 reporter gene activity in PMA/Io-stimulated Jurkat cells
CBD suppressed IL-2 and IFN-γ cytokine production in various T cell preparations, both of which are regulated by several transcription factors, including AP-1 and NFAT [20-23]. Interestingly, both AP-1 and NFAT have been shown to be sensitive targets of inhibition by many cannabinoids [24-26]. Thus, we determined whether NFAT and AP-1 were also targeted by CBD using AP-1- and NFAT-driven luciferase reporter genes in human Jurkat T cells. As shown in Figure 8A, CBD did suppress human IL-2 production from PMA/Io-stimulated Jurkat T cells. Furthermore, the mechanism involves suppression of transcription as CBD suppressed PMA/Io-induced AP-1- and NFAT-luciferase expression in a concentration dependent manner (Figure 8B and 8C, respectively). There was no effect on the overall protein content (data not shown) in the various treatment groups, indicating, in fact, that the suppression of luciferase activity was due to CBD. Consistent with other cannabinoids [26], suppression of NFAT-luciferase was more robust than AP-1-luciferase.
Figure 8.
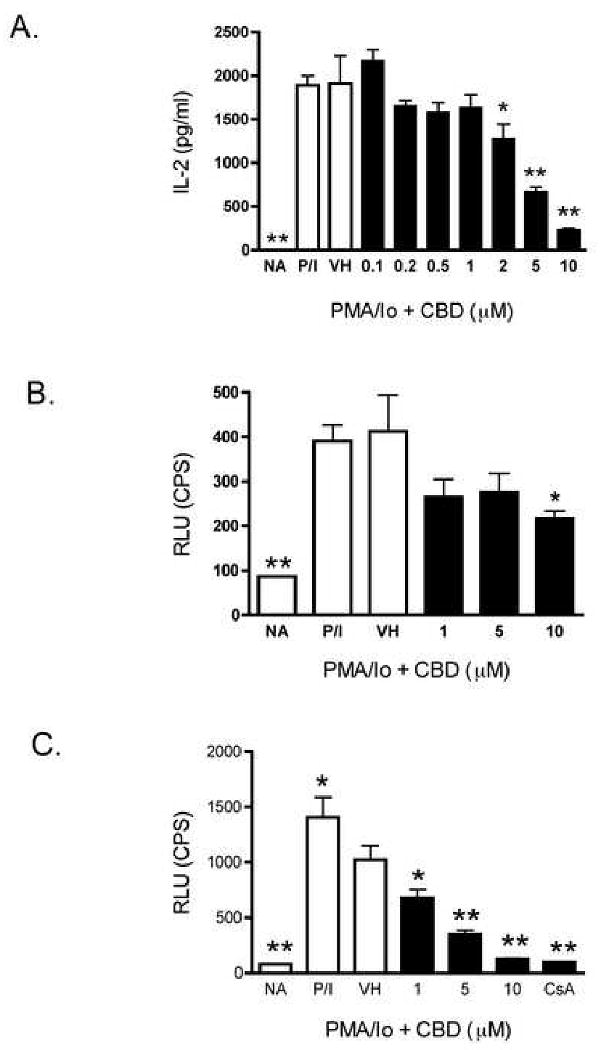
CBD suppressed IL-2 production and AP-1 and NFAT activity in PMA/Io-stimulated human Jurkat T cells. A.) Jurkat cells (5 × 104 cells) were treated with CBD (0.1-10 μM) for 30 min, followed by cellular activation with PMA/Io for 24 hr. Supernatants were harvested and the amount of IL-2 was determined by ELISA. The data are expressed as the mean Units/ml ± SE of triplicate cultures. B-C.) Jurkat cells (5 × 105 cells) were transiently transfected with either AP-1-luciferase (B.) or NFAT-luciferase (C.). Three hours later, cells were treated with CBD (1-10 μM) for 30 min, followed by cellular activation with PMA/Io for 21 hr. Luciferase activity was determined as described in Materials and Methods. The data are expressed as the mean CPS of triplicate cultures. * or ** denotes values that are significantly different from the vehicle control at p < 0.05 or 0.01. Results are representative of at least two separate experiments. NA, naïve (untreated); VH, vehicle (0.1% ethanol).
4. Discussion
CBD suppressed several immunological endpoints, with a profile of activity similar to other plant-derived, synthetic and endogenous cannabinoids [9, 18, 27-29]. Specifically, CBD suppressed cytokine production from activated primary mouse splenocytes in a concentration-dependent manner. Of note was the observation that this cytokine suppression occurred independently of either CB1 or CB2 as demonstrated in splenocytes derived from CB1-/-/CB2-/- mice. The major advantages of evaluating immunological endpoints using CB1-/-/CB2-/- mice, rather than the currently available CB1 and CB2 antagonists, are the absence of potential inverse agonism and/or direct effects of the antagonists, as has been reported [30, 31]. Despite potential activity with antagonists under certain conditions, the antagonists have been used to suggest that CBD might exert some of its effects via CB1 and/or CB2 [15]. There is also recent evidence that CBD might exert its effects via a yet unidentified cannabinoid receptor or the newly identified putative cannabinoid receptor, GPR55 [32]. Furthermore, there are reports that CBD binds the vanilloid receptor, VR1 [33], is an agonist at the serotonergic 5HT-1a receptor [34, 35], and is an agonist at A2a receptors in microglial cells [36]. In addition, Drysdale, et. al. demonstrated that the CBD-induced elevation in intracellular calcium in hippocampal cells was exacerbated in the presence of either a CB1 receptor antagonist or a VR1 receptor antagonist, suggesting signaling interactions between CBD and these receptors through an unknown mechanism [30]. Although we demonstrate that CBD-induced suppression of cytokine production from PMA/Io-stimulated splenocytes was independent of CB1 and CB2, we cannot exclude the possibility that CBD exerts its effects through one or more of the other aforementioned receptors at this time.
In addition to suppression of PMA/Io-stimulated IL-2 production, CBD suppressed IFN-γ production. The effect of CBD was more robust on IL-2 than IFN-γ from PMA/Io-stimulated splenocytes. However, in splenocytes that were stimulated through the T cell receptor using anti-CD3/anti-CD28, CBD exhibited similar potency on suppression of IL-2 and IFN-γ. The difference in sensitivity of CBD-induced suppression of IL-2 might be associated with the rapid elevation of intracellular calcium seen with PMA/Io, with PMA/Io-stimulated IL-2 being more sensitive to a rapid calcium rise than anti-CD3/anti-CD28-stimulated IL-2. This is supported by our previous observation that pretreatment of splenocytes with agents that elevate intracellular calcium resulted in suppression of PMA/Io-stimulated IL-2 [9], whereas the endogenous cannabinoid 2-arachidonoyl-glycerol (2-AG)-induced suppression of PMA/Io-stimulated IFN-γ could be partially reversed by increasing intracellular calcium [29]. The sensitivity of both IL-2 and IFN-γ, however, does suggest that there exists a common target of inhibition by CBD. One likely common target might be NFAT since NFAT is critical for both IL-2 and IFN-γ [37], and because NFAT DNA binding activity is markedly decreased in activated T cells when cultured in the presence of two other cannabinoids, CBN or 2-AG [24-26]. Indeed, CBD suppressed AP-1- and NFAT-luciferase gene expression. Although the CBD-induced suppression of NFAT-luciferase gene expression was more robust, it is notable that AP-1 proteins bind cooperatively with NFAT at many NFAT responsive elements in both IL-2 and IFN-γ [21, 23].
The demonstration that CBD suppresses NFAT activity is further supported by our observation that CBD suppressed cell surface expression of CD25, which is also regulated by NFAT [38]. Interestingly, this effect appeared to be T cell-specific. Although CBD suppressed cell surface expression of CD25 on the CD3+ population, there was no effect on the CD3- (i.e., non-T cell, and presumably, mainly B cell) population. These results were also consistent with the observation that CBD suppressed the IgM AFC response to the T cell-dependent antigen, sRBC, but had no effect on the IgM AFC response to the polyclonal B cell activator, LPS. This is in contrast to our observation that CBD also suppressed LPS-induced proliferation, which has been classically identified as a B cell response. However, there is much evidence to suggest that T cells will proliferate in response to LPS and that T cells express the appropriate toll-like receptors important for this response [39, 40]. Alternatively, these results suggest that the mechanism of immunosuppression by CBD, and likely other cannabinoids for which this dichotomy has been observed [18], might involve a generalized suppression of cellular proliferation to certain stimuli. Overall, the ability of CBD to suppress PMA/Io-, LPS-, anti-CD3/anti-CD28-induced proliferation, in combination with suppression of the MLR, provides more evidence that CBD targets T cells.
The profile with which CBD targeted T cell cytokine production and proliferation was very similar to that previously reported for two other plant-derived cannabinoid compounds, THC and CBN [18, 19]. These results suggest that the mechanisms by which these three plant-derived cannabinoid compounds suppress cytokine production, at least in vitro, is similar and does not require CB1 or CB2. However, CBD only modestly suppressed the in vivo anti-sRBC IgM AFC response. These data support previous studies conducted in male CD-1 mice in which CBD (≤ 25 mg/kg) did not suppress the in vivo anti-sRBC IgM AFC response [41]. With the demonstration that CBD is efficacious in vivo in a variety of model systems [6, 7, 15, 42], these results suggest that the AFC response in vivo is rather refractory to inhibition by CBD. One explanation for the absence of suppression of the in vivo AFC response is that CBD might be rapidly metabolized in vivo to a non-functional metabolite, particularly because CBD was administered orally. The discrepancy between CBD and THC in vivo versus in vitro does implicate the involvement of one or both cannabinoid receptors in the anti-sRBC IgM AFC response. Further evidence of the requirement for CB1 and/or CB2 in the in vivo anti-sRBC IgM AFC is provided by our observations that the THC-induced suppression of this response was abolished in CB1-/-/CB2-/- mice (Springs, et. al., submitted for publication), suggesting that cannabinoids must possess affinity for either CB1 or CB2 to suppress the AFC response in vivo.
We also attempted to discern the role of CB1 and CB2 in CBD-induced suppression of the in vitro anti-sRBC IgM AFC response using splenocytes derived from CB1-/-/CB2-/- mice. Interestingly, the control anti-sRBC IgM AFC response by splenocytes isolated from CB1-/-/CB2-/- mice was remarkably low as compared to that observed with splenocytes isolated from wild type C57BL/6 mice, which limited our ability to absolutely conclude whether CB1 and/or CB2 played a critical role in CBD-induced suppression of the in vitro anti-sRBC IgM AFC response. However, upon examination of the data as percent of vehicle control, it appears unlikely that CB1 and/or CB2 are involved. Although there was a difference between genotypes at lower concentrations of CBD, the response was highly variable, which is likely related to the degree to which the CB1-/-/CB2-/- cells were stimulated in vitro with sRBC.
The above results also strongly suggest that CB1 and/or CB2 play a critical role in the in vitro anti-sRBC IgM AFC response. Currently, the reasons underlying the marked difference in the magnitude of the immune response to sRBC between splenocytes derived from CB1-/-/CB2-/- and wild type C57BL/6 mice remain to be fully elucidated. Part of the mechanism might involve compromised accessory cell function (eg. macrophages) in CB1-/-/CB2-/- mice since there was no difference in the magnitude of the in vitro IgM AFC response by purified B cells activated with CD40 ligand-expressing L cells, a stimulation which does not require T cells or macrophages (Springs, et. al., submitted for publication). Notably, no difference in the magnitude of the in vivo anti-sRBC IgM AFC response between CB1-/-/CB2-/- and wild type C57BL/6 mice was observed (Springs, et. al., submitted for publication), suggesting the existence of additional compensatory mechanisms in vivo.
Overall, CBD did not significantly alter the anti-sRBC IgM AFC response in vivo, but CBD did suppress a number of immune responses in vitro, specifically involving T cells as primary effectors. Using splenocytes derived from CB1-/-/CB2-/- mice, it was determined that CBD-induced suppression of cytokine production and suppression of the in vitro anti-sRBC IgM AFC response was CB1 and CB2 receptor-independent. More importantly, the observation of the significant difference in the control anti-sRBC IgM AFC response in CB1-/-/CB2-/- mice as compared to wild type C57BL/6 mice suggests that CB1 and/or CB2 play a critical role in the magnitude of the response to sRBC in vitro. These data provide a detailed characterization of CBD's effects on various immunological endpoints and provide further evidence that the mechanisms by which cannabinoids modulate immune function is both cannabinoid receptor-dependent and – independent. Finally, these data demonstrate that transcription factors in the IL-2 promoter are sensitive targets of inhibition by CBD.
Acknowledgments
We would like to thank Dr. Andreas Zimmer (University of Bonn) for kindly providing the CB1-/-/CB2-/- knockout mice. We would also like to thank Mr. Robert Crawford and Ms. Amber Crawford for excellent technical assistance and Mrs. Kimberly Hambleton for assistance in the preparation of the manuscript.
This work supported by NIH grant DA07908
Abbreviations
- AFC
antibody-forming cell
- CBD
cannabidiol
- THC
delta-9-tetrahydrocannabinol
- IL-2
interleukin-2
- IFN-γ
interferon-gamma
Footnotes
Classification: Inflammation and Immunopharmacology
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86(8):1646–1647. [Google Scholar]
- 2.Matsuda LA, Lolait SJ. Brownstein MJ, Young AC and Bonner TI, Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature (London) 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 3.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature (London) 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava MD, Srivastava BI, Brouhard B. Delta9 tetrahydrocannabinol and cannabidiol alter cytokine production by human immune cells. Immunopharmacology. 1998;40(3):179–85. doi: 10.1016/s0162-3109(98)00041-1. [DOI] [PubMed] [Google Scholar]
- 5.Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, Feldmann M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 2000;97(17):9561–6. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa B, Colleoni M, Conti S, Parolaro D, Franke C, Trovato AE, Giagnoni G. Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn Schmiedebergs Arch Pharmacol. 2004;369(3):294–9. doi: 10.1007/s00210-004-0871-3. [DOI] [PubMed] [Google Scholar]
- 7.Vaney C, Heinzel-Gutenbrunner M, Jobin P, Tschopp F, Gattlen B, Hagen U, Schnelle M, Reif M. Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study. Mult Scler. 2004;10(4):417–24. doi: 10.1191/1352458504ms1048oa. [DOI] [PubMed] [Google Scholar]
- 8.Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004;10(4):434–41. doi: 10.1191/1352458504ms1082oa. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan BL, Rockwell CE, Kaminski NE. Evidence for cannabinoid receptor-dependent and -independent mechanisms of action in leukocytes. J Pharmacol Exp Ther. 2003;306(3):1077–85. doi: 10.1124/jpet.103.051961. [DOI] [PubMed] [Google Scholar]
- 10.Begg M, Pacher P, Batkai S, Osei-Hyiaman D, Offertaler L, Mo FM, Liu J, Kunos G. Evidence for novel cannabinoid receptors. Pharmacol Ther. 2005;106(2):133–45. doi: 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Baker D, Pryce G, Davies WL, Hiley CR. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol Sci. 2005 doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Rockwell CE, Kaminski NE. A Cyclooxygenase Metabolite of Anandamide Causes Inhibition of Interleukin-2 Secretion in Murine Splenocytes. J Pharmacol Exp Ther. 2004;311(2):683–690. doi: 10.1124/jpet.104.065524. [DOI] [PubMed] [Google Scholar]
- 13.Rockwell CE, Snider NT, Thompson J, Vanden Heuvel J, Kaminski NE. Activation of PPAR-gamma by 2-arachidonylglycerol and 2-arachidonlyglcerol ether causes an inhibition of interleukin-2 in activated T cells. Submitted for publication. 2005 [Google Scholar]
- 14.Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci U S A. 1999;96(24):14136–41. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacerdote P, Martucci C, Vaccani A, Bariselli F, Panerai AE, Colombo A, Parolaro D, Massi P. The nonpsychoactive component of marijuana cannabidiol modulates chemotaxis and IL-10 and IL-12 production of murine macrophages both in vivo and in vitro. J Neuroimmunol. 2005;159(12):97–105. doi: 10.1016/j.jneuroim.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Kaminski NE, Holsapple MP. Inhibition of macrophage accessory cell function in casein-treated B6C3F1 mice. J Immunol. 1987;139(6):1804–10. [PubMed] [Google Scholar]
- 17.Jerne NK, Nordin AA. Plaque formation in agar by single antibody-producing cells. Science. 1963;140:405. [PubMed] [Google Scholar]
- 18.Herring AC, Koh WS, Kaminski NE. Inhibition of the cyclic AMP signaling cascade and nuclear factor binding to CRE and kappaB elements by cannabinol, a minimally CNS-active cannabinoid. Biochem Pharmacol. 1998;55(7):1013–23. doi: 10.1016/s0006-2952(97)00630-8. [DOI] [PubMed] [Google Scholar]
- 19.Schatz AR, Koh WS, Kaminski NE. Δ9-tetrahydrocanabinol selectively inhibits T-cell dependent humoral immune responses through direct inhibition of accessory T-cell function. Immunopharmacol. 1993;26:129–137. doi: 10.1016/0162-3109(93)90005-b. [DOI] [PubMed] [Google Scholar]
- 20.Sica A, Dorman L, Viggiano V, Cippitelli M, Ghosh P, Rice N, Young HA. Interaction of NF-kappaB and NFAT with the interferon-gamma promoter. J Biol Chem. 1997;272(48):30412–20. doi: 10.1074/jbc.272.48.30412. [DOI] [PubMed] [Google Scholar]
- 21.Sweetser MT, Hoey T, Sun YL, Weaver WM, Price GA, Wilson CB. The roles of nuclear factor of activated T cells and ying-yang 1 in activation-induced expression of the interferon-gamma promoter in T cells. J Biol Chem. 1998;273(52):34775–83. doi: 10.1074/jbc.273.52.34775. [DOI] [PubMed] [Google Scholar]
- 22.Shaw JP, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR. Identification of a putative regulator of early T cell activation genes. Science. 1988;241(4862):202–5. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- 23.Jain J, McCaffrey PG, Miner Z, Kerppola TK, Lambert JN, Verdine GL, Curran T, Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993;365(6444):352–5. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- 24.Faubert BL, Kaminski NE. AP-1 activity is negatively regulated by cannabinol through inhibition of its protein components, c-fos and c-jun. J Leukoc Biol. 2000;67(2):259–66. doi: 10.1002/jlb.67.2.259. [DOI] [PubMed] [Google Scholar]
- 25.Ouyang Y, Hwang SG, Han SH, Kaminski NE. Suppression of interleukin-2 by the putative endogenous cannabinoid 2-arachidonyl-glycerol is mediated through down-regulation of the nuclear factor of activated T cells. Mol Pharmacol. 1998;53(4):676–83. doi: 10.1124/mol.53.4.676. [DOI] [PubMed] [Google Scholar]
- 26.Yea SS, Yang KH, Kaminski NE. Role of nuclear factor of activated T-cells and activator protein-1 in the inhibition of interleukin-2 gene transcription by cannabinol in EL4 T-cells. J Pharmacol Exp Ther. 2000;292(2):597–605. [PubMed] [Google Scholar]
- 27.Condie R, Herring A, Koh WS, Lee M, Kaminski NE. Cannabinoid inhibition of adenylate cyclase-mediated signal transduction and IL-2 expression in the murine T-cell line, EL4.IL-2. J Biol Chem. 1996;271:13175–13183. doi: 10.1074/jbc.271.22.13175. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan BL, Ouyang Y, Herring A, Yea SS, Razdan R, Kaminski NE. Inhibition of leukocyte function and interleukin-2 gene expression by 2-methylarachidonyl-(2′-fluoroethyl)amide, a stable congener of the endogenous cannabinoid receptor ligand anandamide. Toxicol Appl Pharmacol. 2005;205(2):107–15. doi: 10.1016/j.taap.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan BL, Ouyang Y, Rockwell CE, Rao GK, Kaminski NE. 2-Arachidonoyl-glycerol suppresses interferon-gamma production in phorbol ester/ionomycin-activated mouse splenocytes independent of CB1 or CB2. J Leukoc Biol. 2005;77(6):966–74. doi: 10.1189/jlb.1104652. [DOI] [PubMed] [Google Scholar]
- 30.Drysdale AJ, Ryan D, Pertwee RG, Platt B. Cannabidiol-induced intracellular Ca(2+) elevations in hippocampal cells. Neuropharmacology. 2006;50(5):621–31. doi: 10.1016/j.neuropharm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Rao GK, Kaminski NE. Cannabinoid-Mediated Elevation of Intracellular Calcium: A Structure-Activity Relationship. J Pharmacol Exp Ther. 2006 doi: 10.1124/jpet.105.100503. [DOI] [PubMed] [Google Scholar]
- 32.Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007 doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134(4):845–52. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mishima K, Hayakawa K, Abe K, Ikeda T, Egashira N, Iwasaki K, Fujiwara M. Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism. Stroke. 2005;36(5):1077–82. doi: 10.1161/01.STR.0000163083.59201.34. [DOI] [PubMed] [Google Scholar]
- 35.Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30(8):1037–43. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- 36.Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci U S A. 2006;103(20):7895–900. doi: 10.1073/pnas.0511232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5(6):472–84. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 38.Schuh K, Twardzik T, Kneitz B, Heyer J, Schimpl A, Serfling E. The interleukin 2 receptor alpha chain/CD25 promoter is a target for nuclear factor of activated T cells. J Exp Med. 1998;188(7):1369–73. doi: 10.1084/jem.188.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuguchi T, Takagi K, Musikacharoen T, Yoshikai Y. Gene expressions of lipopolysaccharide receptors, toll-like receptors 2 and 4, are differently regulated in mouse T lymphocytes. Blood. 2000;95(4):1378–85. [PubMed] [Google Scholar]
- 40.Ulmer AJ, Flad H, Rietschel T, Mattern T. Induction of proliferation and cytokine production in human T lymphocytes by lipopolysaccharide (LPS) Toxicology. 2000;152(13):37–45. doi: 10.1016/s0300-483x(00)00290-0. [DOI] [PubMed] [Google Scholar]
- 41.Zimmerman S, Zimmerman AM, Cameron IL, Laurence HL. delta1-tetrahydrocannabinol, cannabidiol and cannabinol effects on the immune response of mice. Pharmacology. 1977;15(1):10–23. doi: 10.1159/000136658. [DOI] [PubMed] [Google Scholar]
- 42.Jan TR, Su ST, Wu HY, Liao MH. Suppressive effects of cannabidiol on antigen-specific antibody production and functional activity of splenocytes in ovalbumin-sensitized BALB/c mice. Int Immunopharmacol. 2007;7(6):773–80. doi: 10.1016/j.intimp.2007.01.015. [DOI] [PubMed] [Google Scholar]


