Abstract
Prosaposin is both a precursor of sphingolipid activator proteins and a secreted neurotrophic and myelinotrophic factor. Because peripheral nerve regeneration is impaired in diabetes mellitus, we measured prosaposin protein levels from control and streptozotocin-diabetic rats by collecting endoneurial fluid secreted into a bridging tube connecting the ends of transected sciatic nerve. Prosaposin protein levels were significantly reduced in endoneurial fluid from diabetic rats and increased in the proximal nerve stump compared to controls. To investigate whether a prosaposin-derived peptide could improve nerve regeneration, rats were treated with prosaptide TX14(A) following sciatic nerve crush. In control rats, TX14(A) was without effect in the uninjured nerve but shortened toe spread recovery time after nerve crush. In diabetic rats, efficacy of prosaptide TX14(A) was confirmed by correction of thermal hypoalgesia, formalin-evoked hyperalgesia and conduction slowing in the uninjured nerve. The peptide also prevented diabetes-induced abnormalities in nerve regeneration distance and mean axonal diameter of regenerated axons, whereas delayed recovery of toe spread was not improved. Muscle denervation atrophy was attenuated by TX14(A) in both control and diabetic rats. These results suggest that reduced prosaposin secretion after nerve injury may contribute to impaired regeneration rates in diabetic rats and that prosaptide TX14(A) can improve aspects of nerve regeneration.
Keywords: Diabetes, Nerve regeneration, Prosaposin, Prosaptide TX14(A)
INTRODUCTION
Peripheral nerve regeneration after physical injury is orchestrated by the sequential expression and release of a series of neurotrophic factors and cytokines from Schwann cells, invading macrophages and other cells adjacent to the injury site (1). Axonal survival, sprouting and maturation are regulated by members of the neurotrophin, GDNF and neuropoietic cytokine families, while assorted receptors on Schwann cells and other cellular components of the nerve trunk facilitate remyelination of the new axon and re-establishment of the regulated endoneurial microenvironment. Together, these systems allow peripheral nerve to reinnervate its original targets and restore function, although the recovery is usually imperfect with features such as internodal distance, axonal caliber and therefore conduction velocity being sub-optimal compared to the pre-injury condition.
It has been argued that the distal symmetrical polyneuropathy associated with diabetes mellitus represents an imbalance of neuronal survival and maintenance that is driven by increased distal degeneration and an impaired capacity to initiate and maintain the regenerative responses to injury. The epidermal nerves of diabetic patients exhibit a reduced regenerative capacity after physical trauma (2), while many studies in diabetic rodents have shown that recovery from nerve crush injury is delayed, slowed or morphologically disrupted (3). Interventions that can afford neuroprotection and stimulate regeneration have the potential to both shift the balance away from progressive degeneration and also enhance recovery of axons that are damaged but not yet dead.
Prosaposin is the precursor of saposin proteins involved in lysosomal sphingolipid degradation and is also found as a holoprotein in body fluids such as cerebrospinal fluid and milk. Prosaposin is secreted into the endoneurial fluid that accumulates distal to a peripheral nerve transection (4) and induces neuritogenesis in vitro (5), implicating it as a potential factor involved in nerve regeneration. Prosaptide TX14(A), a modified 14-mer peptide fragment of prosaposin that lacks saposin activity but retains neurotrophic properties, also induces neuritogenesis (6, 7). Prosaptide TX14(A) exhibits a number of neuroprotective properties in animal models of diabetic neuropathy, including prevention of conduction slowing and axonal atrophy in the peripheral nerve of diabetic rats (8, 9). Given the neuroprotective properties of prosaptide TX14(A) in diabetic rats and the potential role of prosaposin in peripheral nerve regeneration, we investigated whether the impaired nerve regeneration of diabetic rats is associated with an impediment of prosaposin secretion at the injury site and whether prosaptide TX14(A) has effects on indices of nerve regeneration in normal and diabetic rats.
MATERIALS AND METHODS
Animals
All experiments were performed on adult (starting weight 220–240 g) female Sprague-Dawley rats (Harlan Industries, San Diego, CA) using procedures approved by the local IACUC. Rats were allowed free access to food and water and were maintained on paper bedding with a 12-hour light-dark cycle.
Rats were made diabetic by the injection of streptozotocin (55 mg/kg i.p. in saline) following an overnight fast. Hyperglycemia was confirmed 4 days later by measuring glucose levels in a drop of tail vein blood using a strip-operated reflectance meter and at the end of the study by spectrophotometric assay (glucose assay kit, Sigma, St. Louis, MO) and only rats with a blood sugar of 15 mmol/l or higher were accepted as being diabetic.
Prosaposin Secretion
After 12 weeks of diabetes, rats were anesthetized using a mixture of sodium pentobarbital (12.5 mg/kg) and diazepam (1.25 mg/ml) in physiological saline (2 ml/kg i.p.), the hindquarters shaved and the surgery area prepared with betadine. Both sciatic nerves were exposed by a lateral incision in the thigh and the overlying muscles separated. Nerves were mobilized from the sciatic notch to the tibial-peroneal bifurcation and a 1-cm segment at the mid-thigh level resected and stored at −70° C. The proximal and distal stumps were sutured into the open-ends of a 14-mm-long silicon tube with 7-0 silk suture passing through the epineurium, so that the resulting gap between the severed stumps was 10-mm long. One or 9 days after nerve transection, animals were re-anesthetized, both sciatic nerves removed and both tubular fluid and 1 cm of proximal stump from both tubes of each animal collected. Nerve samples were homogenized into 200 μl of ice-cold homogenization buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.5% Triton X, 1 mM EDTA, 4 μg/ml protease inhibitor cocktail) and centrifuged at 14,000 g for 30 minutes at 4°C. After determination of sample protein concentration (BCA protein assay, Pierce, Rockford, IL), 4 μg of total extracted protein or 1.5 μl of tubular fluid samples diluted 30 times in PBS were subjected to SDS-PAGE using 12% Bis-tris gel (Invitrogen Life Technologies, Carlsbad, CA). Proteins were electrotransferred onto a nitrocellulose membrane (Hydron-C extra, Amersham Life Science, Arlington Heights, IL) and then incubated in 3% BSA/PBS-0.05% Tween 20 (BSA/PBST). Membranes were immunostained with an affinity-purified anti-saposin C antibody (1:10,000, GenWay Biotech Inc., San Diego, CA) or β-actin (1:2,000, Sigma). Blots were washed three times for ten minutes with PBST followed by a secondary anti-chicken antibody (1:20,000, Sigma) or anti-mouse antibody (1:10,000, Santa Cruz Biotechnology Inc., Santa Cruz, CA). After 3 further washes in PBST, immunodetection was performed using enhanced Chemiluminescence (ECL kit, Amersham). For repeated analysis of membranes, previously bound antibodies were removed using stripping buffer (0.2 M glycine pH 2.5, 0.05% Tween 20) for 45 minutes at 60°C. After immunostaining, the amount of prosaposin was determined densitometrically using Quantity One software. Sciatic nerve samples were normalized by calculating the ratio of the intensity of the band corresponding to prosaposin to the intensity of the band corresponding to actin. Prosaposin protein in endoneurial fluid was normalized to fluid volume.
Nerve Regeneration
Two methods were used to assess nerve regeneration after injury, a direct measure of neuronal regeneration distance and an indirect measure by monitoring recovery of limb function. We have previously used both techniques to demonstrate that diabetes impedes nerve regeneration and limb functional recovery (10). Briefly, both sciatic nerves were exposed under anesthesia, as described above, and 1 sciatic nerve crushed for 5 seconds at the level of the obdurator tendon using watchmaker’s forceps, with the crush site marked by an epineurial suture. The contralateral nerve was mobilized, but not crushed, and served as a sham-operated control. After nerve crush, the skin incision was closed and the animal was allowed to recover. Nerve regeneration was allowed to proceed for 3, 7 or 32 days. Animals assigned to the 3- or 7-day regeneration time points were anesthetized and the crushed sciatic nerve re-exposed. Nerve regeneration distance was measured using a stimulating electrode (10V, 0.05 ms width 0.5Hz pulses from a Grass stimulator) initially placed to touch the nerve 30 mm distal to the crush site and then advanced in 0.5-mm increments towards the crush site until a reflex response was recorded in the musculature of the back. The distance from the stimulation point to the crush site was measured with calipers. Regeneration was measured in the animals assigned to the 32-day time point using recovery of toe spread in the injured limb. Both hind paws were coated with betadine and the animal encouraged to walk across paper with subsequent measurement of the distance between prints of the first and fifth toe of the injured and uninjured paw. Three prints of each paw were measured at each time point and the median toe spread calculated. Toe spread analysis was performed both before, and on a regular basis after, nerve injury and change in toe spread calculated at each time point relative to pre-injury values for each paw.
Other Assays
At the conclusion of the study, rats were anesthetized with halothane and nerve conduction velocity measured in both the sham-operated and injured sciatic nerves using stimulating electrodes (5 to 10 V, 0.05 ms pulse width) placed at the sciatic notch and Achilles tendon, and recording electrodes placed in the ipsilateral interosseus muscles. Nerve temperature was maintained at 37°C using a heating lamp. Resulting M and H waves were recorded on a digital storage oscilloscope and conduction velocity calculated as the distance between the stimulation sites divided by the time difference between the M or H wave peaks obtained from each stimulation site. Measurements were made in triplicate for each nerve and the median used to represent motor and sensory conduction velocity (MNCV and SNCV) for that nerve.
Paw withdrawal from noxious heat and flinching responses to injection of 0.5% formalin were measured as described in detail elsewhere (11) in the sham-operated limb 24 to 48 hours after the last treatment with prosaptide TX14(A) or vehicle. Briefly, rats were acclimated to an observation chamber on the surface of a modified Hargreaves apparatus (UARD, San Diego, CA). The time to withdraw the hind paw after initiating heating of the local surface below the paw was measured 4 times at least 5 minutes apart and the median of readings 2 to 4 calculated as the response time. We attempted measurements on the hind paw ipsilateral to the crush injury but all response times were above the upper cut off time used to prevent tissue damage (20 seconds); therefore, no data are presented. At least 4 hours after measuring responses to thermal stimulation, rats were acclimated to an observation chamber and 50 μl of 0.5% formalin was injected into the dorsum of the hind paw of the sham-operated limb. Formalin-evoked flinches were counted in 1-minute blocks every 5 minutes for up to 60 minutes, and the sum of flinches during minutes 0 to 5 (phase 1), 6 to 20 (Q phase) and 21 to 60 (phase 2) calculated.
At time of death, blood was collected for subsequent assay of plasma glucose content by spectrophotometric assay. A portion of the sciatic nerve 1 cm distal to the crush site was removed from the rats used for toe spread analysis (4 weeks untreated diabetes then 4 weeks of treatment with prosaptide TX14(A) or vehicle), fixed in 2.5% glutaraldehyde, post-fixed in osmium tetroxide and processed for light microscopy to allow computer-assisted morphometric analysis of myelinated fiber axonal diameter, exactly as previously described (9). The extensor digitorum longus (EDL) muscle was removed from both hind limbs, lightly dried on blotting paper and weighed.
Treatment
Groups of control and diabetic rats were treated with prosaptide TX14(A), a 14-mer peptide variant of the N terminal region of saposin C (TXLIDNNATEEILY; where X = D-alanine; Myelos Neurosciences, San Diego, CA). The peptide was dissolved in phosphate buffered saline and delivered on a thrice-weekly schedule (1 mg/kg i.p. on Monday, Wednesday and Friday) that began immediately following nerve crush injury. Separate groups of control and diabetic rats received an equivalent daily injection of vehicle alone.
Statistical Analysis
All measurements were made on coded animals to preclude observer bias. Data are presented as group mean ± SEM. Between-group comparisons were made either by unpaired t test or by 1-way ANOVA with post-hoc analysis using the Student Newman Keuls test to compare all groups or Dunnett’s test to selectively identify groups that were significantly different from the vehicle-treated control or the vehicle-treated diabetic group.
RESULTS
Nerve Prosaposin Content and Secretion After Injury
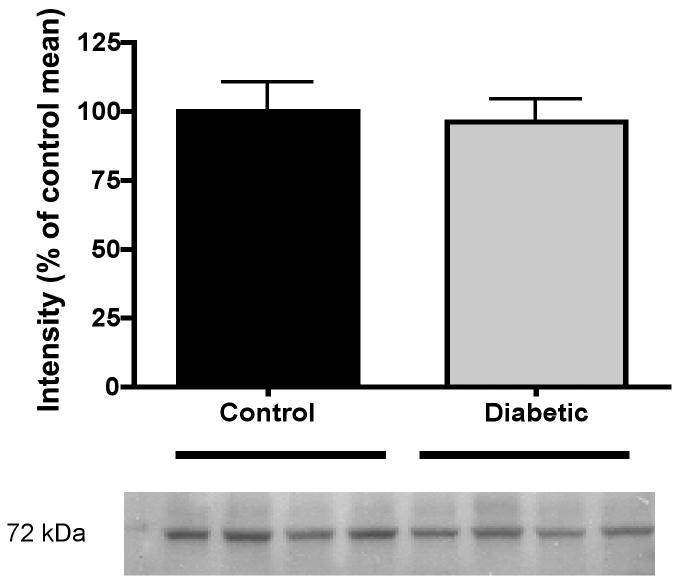
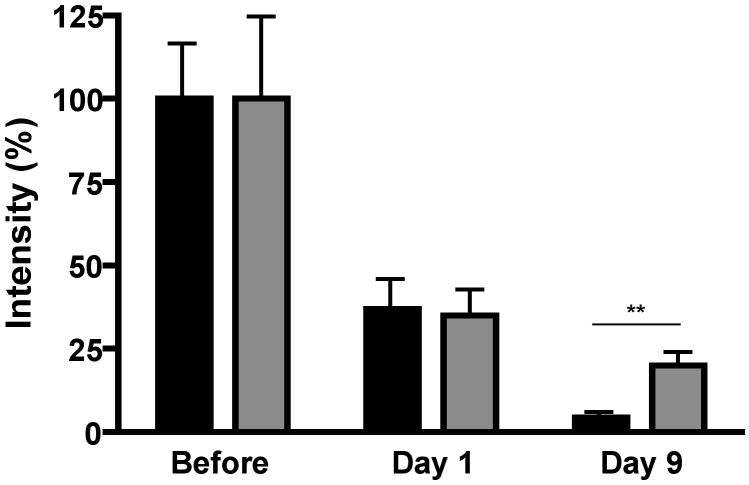
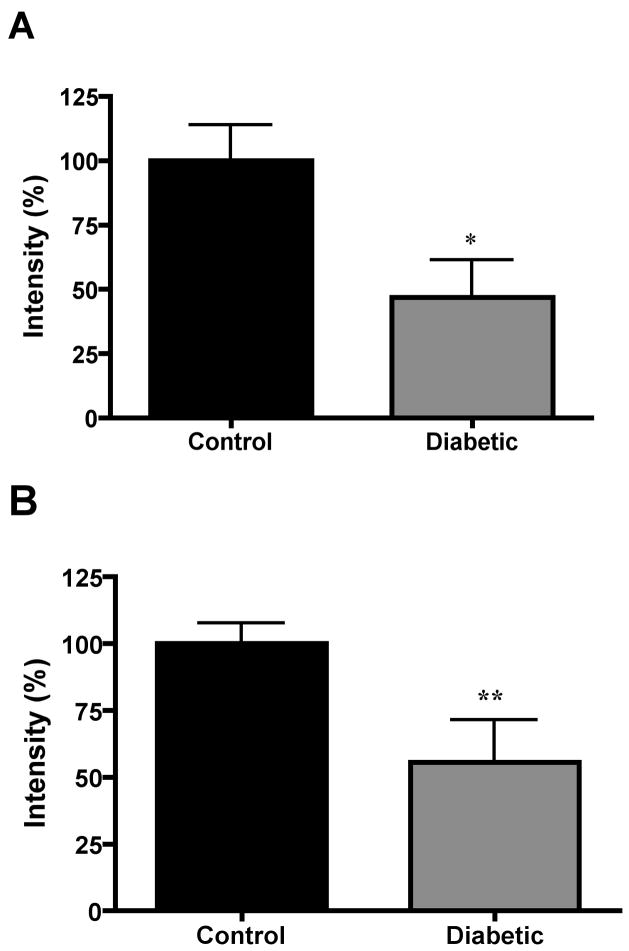
The prosaposin/saposin C antibody identified a single protein band at 72 kDa (Fig. 1), which is consistent with the molecular weight of whole prosaposin. No bands were detected at 12 to 16 kDa, the molecular weight of saposins (data not shown). Prosaposin content in the portion of sciatic nerve that was excised prior to implanting the connecting tube was similar in control and diabetic rats after 4 weeks of hyperglycemia (Fig. 1). The prosaposin content of the sciatic nerve immediately proximal to the bridging tube was markedly lower than that of the excised nerve (labeled ‘before’) on days 1 and 9 after transection in both control and diabetic rats (Fig. 2). Prosaposin protein content of this proximal nerve segment was similar between control and diabetic rats on day 1 after transection, but by day 9 this proximal nerve segment contained significantly (p < 0.01) less prosaposin protein in control rats than in diabetic rats (Fig. 2). In contrast, the prosaposin content of endoneurial fluid collected from the tube connecting the proximal and distal stumps was significantly (p < 0.05) greater in control rats compared to diabetic rats on both day 1 and day 9 (Fig. 3).
Figure 1.
Representative blot for prosaposin and densitometric quantification of prosaposin protein (72 kDa) in sciatic nerve of control and 12-week STZ-diabetic rats. Individual lanes were first normalized to their relative actin content and then values for each diabetic lane were normalized to the mean intensity of control lanes on the same blot to allow for inter-blot comparisons. Data are mean ± SEM (n = 10 to 14 per group).
Figure 2.
Prosaposin protein levels of the nerve proximal to the transection site in control (black) and diabetic (grey) rats before and 1 or 9 days after nerve transection. Each lane was initially normalized to sample actin levels and then to the mean intensity of control nerve segments collected during transection (labeled before) that were present on the same blot to allow for inter-blot comparisons. Data are mean ± SEM (n = 8 to 10 per group). **, p < 0.01 by unpaired t-test.
Figure 3.
Densitometric quantification of prosaposin protein level of endoneurial fluid from control and diabetic rats collected 1 (A) or 9 (B) days after transection. Data are mean ± SEM (n = 9 to 14 per group). *, p < 0.05, **, p < 0.01 vs control by unpaired t-test.
Effects of Prosaptide TX14(A) on Rate of Nerve Regeneration
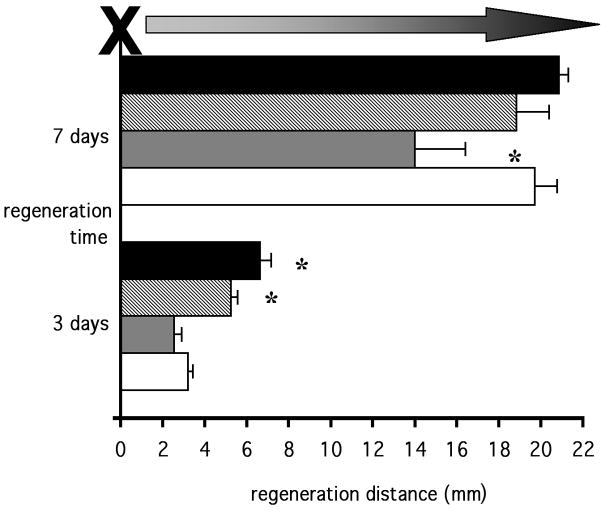
Six animals per group were studied on day 3 and day 7 after crush injury. Vehicle-treated diabetic rats exhibited weight loss (209 ± 5 g, mean ± SEM) and hyperglycemia (30.0 ± 2.0 mmol/l) compared to vehicle-treated controls (242 ± 2 g and 7.1 ± 0.4 mmol/l). Prosaptide TX14(A) did not affect these parameters in either control (224 ± 3 g and 7.0 ± 0.6 mmol/l) or diabetic (195 ± 4 g and 34.5 ± 1.2 mmol/l) rats. Three days after crush injury, vehicle-treated diabetic rats showed a significantly (p < 0.05) reduced distance of regeneration compared to vehicle-treated controls and a similar deficit was observed in diabetic rats treated with prosaptide TX14(A) (p < 0.05: Fig. 4). Nerve regeneration distance continued to be significantly (p < 0.05) reduced on day 7 after crush injury in vehicle-treated diabetic rats, but by this time the diabetic rats treated with TX14(A) showed a regeneration distance that was significantly (p < 0.05) higher than the vehicle-treated diabetic rats and not significantly different from control rats (Fig. 4). Prosaptide TX14(A) significantly (p < 0.05) slowed nerve regeneration distance in control rats on day 3, but not day 7 after injury (Fig. 4).
Figure 4.
Nerve regeneration distance at 3 or 7 days after crush injury in vehicle-treated control rats (black bars), prosaptide TX14(A)-treated control rats (hatched bars), vehicle-treated diabetic rats (grey bars) and prosaptide TX14(A)-treated diabetic rats (white bars). Data are mean ± SEM. Statistical analysis of all groups at each time by one-way ANOVA with the Student Newman Keuls post-hoc test, *, p < 0.05 vs all other groups.
Effects of Prosaptide TX14(A) on Nerve and Limb Function in Diabetes and After Crush Injury
Eight animals per group were followed for 4 weeks of diabetes and then 4 more weeks after sciatic nerve crush injury. After a total of 8 weeks of diabetes, rats showed hyperglycemia and reduced body weight compared to vehicle-treated controls, and treatment with prosaptide TX14(A) for the last 4 weeks did not alter these parameters in either control or diabetic rats (Table 1). Measurements made in the uninjured limb 48 hours after the last treatment with vehicle or prosaptide TX14(A) demonstrated that diabetes induced significant MNCV and SNCV slowing, thermal hypoalgesia and hyperalgesic behavior during phase 2 of the response to 0.5% paw formalin injection. Treatment with TX14(A) for the last 4 weeks of the study did not significantly alter any of these parameters in control rats. The uninjured limb of diabetic rats treated with prosaptide TX14(A) showed significant alleviation of MNCV and SNCV slowing, thermal hypoalgesia and formalin-evoked hyperalgesia compared to vehicle-treated diabetic rats, although the latter remained significantly different from control values (Table 1).
Table 1.
Effect of prosaptide TX14(A) on General Physiology and Uninjured Nerve Function After Treatment*
| Plasma glucose (mmol/l) | Body weight (g) | Sciatic MNCV (m/s) | Sciatic SNCV (m/s) | Thermal latency (s) | 0.5% formalin phase |
|||
|---|---|---|---|---|---|---|---|---|
| 1 | Q | 2 | ||||||
| (sum flinches) | ||||||||
| Control | 7.1±0.3a | 242±2a | 60.1±1.8a | 60.0±1.6a | 9.6±1.0a | 9±1 | 2±1a | 20±9a |
| Control + TX14(A) | 7.0±0.2a | 245±3a | 62.3±1.4a | 57.0±1.5a | 11.2±0.9a | 11±2 | 2±1a | 5±1a |
| Diabetic | 34.7±2.9b | 209±5b | 50.3±0.9b | 45.5±1.6b | 15.9±1.9b | 12±2 | 26±6b | 82±15b |
| Diabetic + TX14(A) | 33.5±2.2b | 195±9b | 57.9±1.3a | 53.6±3.0a | 8.2±0.6a | 7±2 | 24±6b | 46±3c |
| a vsb | a vsb | a vsb | a vsb | a vsb | a vsb | a,c vsb | ||
| p<0.001 | p<0.001 | p<0.001 | p<0.01 | p<0.01 | p<0.01 | p<0.01 | ||
| a vsc | ||||||||
| p<0.05 | ||||||||
, Treatment was for the last 4 weeks of an 8-week period of diabetes. Data are mean ± SEM. Statistical analysis by one-way ANOVA followed by the Student Newman Keuls post-hoc test.
The EDL muscle weight in the uninjured limb of control rats was significantly (p < 0.001) higher than that of diabetic rats, reflecting diabetes-induced muscle wasting (Table 2). Prosaptide TX14(A) treatment did not influence EDL muscle weight in either control or diabetic rats. The weight of the EDL muscle in the nerve-injured limb was markedly lower than that of the contralateral uninjured limb in all groups, suggesting denervation atrophy. The decline in muscle weight calculated as the EDL muscle weight as a percentage of the contralateral EDL muscle, however, was significantly attenuated by treatment with prosaptide TX14(A) in both the control and diabetic groups compared to their respective vehicle-treated groups.
Table 2.
Effects of Diabetes and Prosaptide TX14(A) on Responses of Muscle and Nerve to Nerve Crush Injury*
| EDL Muscle |
Regenerated Nerve | ||||
|---|---|---|---|---|---|
| uninjured (g) | injured (g) | atrophy (%) | MNCV (m/s) | MAD (μm) | |
| Control | 134.5±6.8a | 101.2±4.9a | 75.3±1.6a | 37.2±4.7 | 1.43±0.09a |
| Control + TX14(A) | 129.7±3.4a | 106.1±2.5a | 81.7±1.7b | 36.1±5.0 | 1.53±0.17a |
| Diabetic | 64.0±3.5b | 42.8±2.3b | 66.4±1.9c | 25.9±3.0 | 2.30±0.20b |
| Diabetic + TX14(A) | 65.2±4.7b | 47.7±4.4b | 72.6±2.3d | 24.4±3.3 | 1.75±0.13a |
| statistical significance | a vs.b | a vs.b | c vs.a.b.d | a vs.b | |
| p<0.001 | p<0.001 | &a.dvs. | p<0.05 | ||
| p<0.05 | |||||
Data are mean ± SEM. Statistical analyses by one-way ANOVA followed by the Student Newman Keuls post-hoc test.
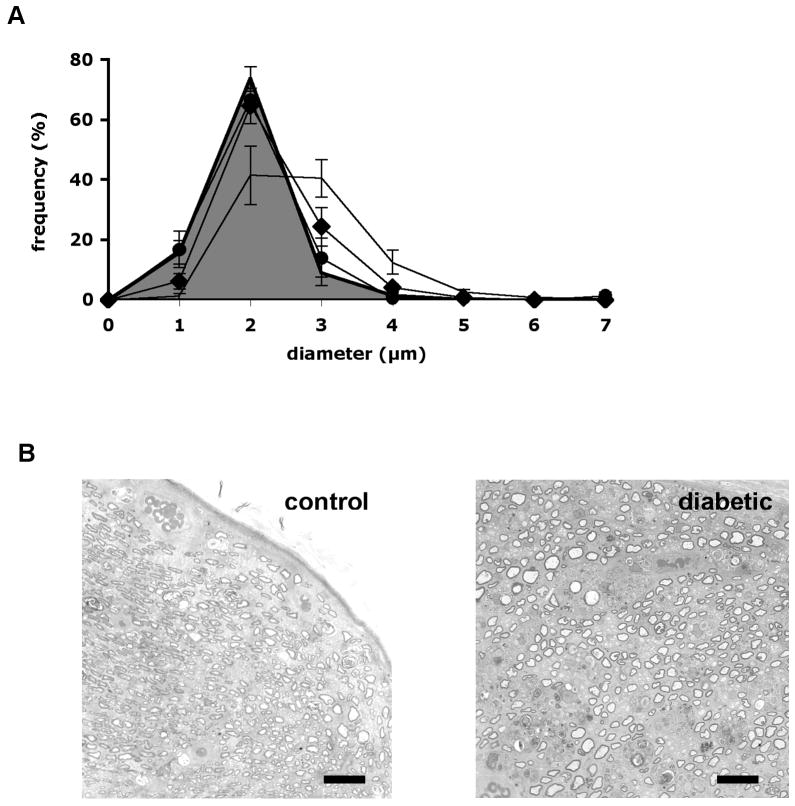
Motor nerve conduction velocity in the regenerated nerve of control rats measured one month after crush injury was markedly slower (Table 2) than in the uninjured contralateral nerve (Table 1) for all groups. The MNCV of diabetic rats tended (p > 0.05) to be lower than that of control rats and there was no obvious effect of prosaptide TX14(A) treatment on MNCV in the regenerated nerve of either control or diabetic rats (Table 2). H waves were not identified clearly; therefore, SNCV could not be calculated. The mean axonal diameter (MAD) of axons distal to the crush injury was significantly greater in the vehicle-treated diabetic rats compared to vehicle-treated control rats (p < 0.05: Table 2). The increased MAD of diabetic rats was prevented by prosaptide TX14(A) (p < 0.05 vs vehicle-treated diabetic rats), whereas prosaptide TX14(A) did not affect MAD of control rats (Table 2; Fig. 5).
Figure 5.
(A) Axonal size: frequency distribution in the sciatic nerve distal to the crush site measured 1 month after the injury in control rats (grey area under curve), control rats treated with prosaptide TX14(A) following crush injury (filled circles), diabetic rats (line without markers) and diabetic rats treated with prosaptide TX14(A) following crush injury (filled diamonds). Data are group mean ± SEM for each size bin. (B) Light microscopic images illustrating nerve distal to the crush site from control and diabetic rats used for morphometric analysis. Bar = 60 μm.
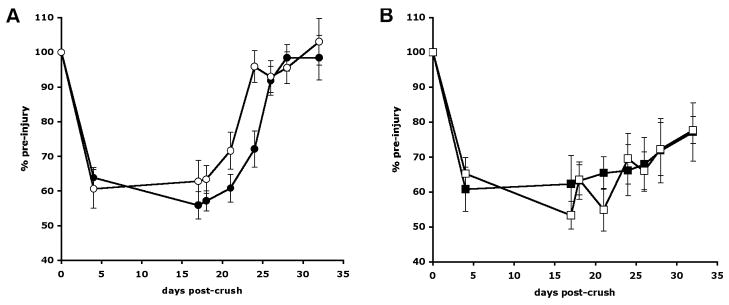
Before nerve crush or the onset of treatment, the toe-spread distance of control rats (21.9 ± 0.4 mm: cohort mean ± SEM) was significantly higher than that of diabetic rats (18.1 ± 0.5 mm) (p < 0.01 by unpaired t-test). Following crush injury, the toe-spread distance in the injured limb declined relative to pre-injury values in all groups (Fig. 6). In vehicle-treated control rats, recovery of toe spread occurred between days 26 and 28, whereas in control rats treated with prosaptide TX14(A) recovery was already clear by day 24 (p < 0.05 vs all other groups by 1-way ANOVA followed by Student Newman Keuls post-hoc test). Vehicle-treated diabetic rats did not show complete recovery of toe spread during the 32 days of the study, and a similar pattern was seen in diabetic rats treated with prosaptide TX14(A) such that on day 32 both diabetic groups were significantly different from both control groups (p < 0.05 by 1-way ANOVA followed by Student-Newman-Keuls post-hoc test).
Figure 6.
Toe spread in the nerve-injured limb of (A) vehicle-treated control rats (black circles) and prosaptide TX14(A)-treated control rats (white circles) and (B) vehicle-treated diabetic rats (black squares) and TX14(A)-treated diabetic rats (white squares). Data are group mean ± SEM (n = 8 per group). Statistical analysis was performed at days 24 and 32 and incorporated all 4 groups (see text for details).
Effects of Prosaptide TX14(A) on Nerve Prosaposin Expression
We measured nerve prosaposin content in sciatic nerve samples taken from control rats, rats after 8 weeks of untreated STZ-diabetes and rats treated with 1 mg/kg prosaptide TX14(A) thrice weekly for 8 weeks of STZ-induced diabetes to determine whether the effects of prosaptide TX14(A) on nerve regeneration were mediated by induction of nerve prosaposin expression. There was no significant difference in nerve prosaposin content between either of the two diabetic groups and the control group (untreated diabetic = 105 ± 9; treated diabetic = 103 ± 7: prosaposin per lane calculated relative to actin loading and group data calculated as percentage ± SEM of mean control values present on the same gel: n = 7/group). The impact of diabetes and efficacy of the prosaptide TX14(A) treatment regime on the biochemistry, function and structure of these nerves has been published elsewhere (8).
DISCUSSION
Prosaposin is the precursor for lysosomal saposins, but within the nervous system the holoprotein is the dominant form (12). Whole prosaposin is localized to neuronal cell bodies of the CNS and secreted into cerebrospinal fluid (12, 13). Prosaposin protein is also found in sensory cell bodies of the PNS and prosaposin mRNA is detected in non-neuronal cells within peripheral nerve trunks (8). An association with nerve repair processes is suggested by reports that prosaposin mRNA increases in the distal stump after nerve transection (14), while prosaposin protein is detected in the endoneurial fluid that bridges the transected nerve (4). Cell culture studies demonstrate that exogenous prosaposin induces neuritogenesis (5, 7) and that it promotes both the myelinating phenotype of Schwann cells (4, 15) and maturation of muscle (16). Addition of prosaposin into the region between proximal and distal stumps of a transected nerve dose-dependently increased the number of regenerating axonal sprouts (17). These data suggest that secreted whole prosaposin may have a role in the recovery of peripheral nerve and muscle after injury.
We measured nerve prosaposin protein levels using an anti-saposin C antibody. The 72 kDa molecular weight of the sole protein band detected with this antibody is consistent with that of whole prosaposin rather than saposin C (12 to 16 kDa), confirming a recent report that unprocessed prosaposin is the dominant form in the PNS (12). The relative decline of prosaposin protein in the proximal stump after transection compared to levels in uninjured nerve is consistent with decreased prosaposin protein in neuronal cell bodies of the facial nerve on days 7 and 14 post-transection (13), although the axonal transport dynamics of prosaposin in the PNS are not yet known. In contrast, prosaposin mRNA increases in the distal stump following nerve injury, implying injury-induced upregulation of subsequent prosaposin synthesis by non-neuronal cells (14). It will be important to determine whether there is a parallel upregulation of prosaposin mRNA and protein expression in the proximal stump and also the relative contributions of neuronal and non-neuronal prosaposin in the proximal stump to clarify the sources of prosaposin in the endoneurial fluid.
We also tested the hypothesis that the impaired nerve regeneration of diabetic rats (3, 13) is associated with reduced prosaposin availability. Neither 8 nor 12 weeks of diabetes altered the amount of prosaposin protein in the uninjured sciatic nerve. Prosaposin mRNA is increased in the sciatic nerve of rats after 8 weeks of diabetes (8), indicating that the 2-fold increase of mRNA does not result in a parallel elevation of protein. This apparent dichotomy could reflect diabetes-induced inefficiency in translation since amino acid uptake into nerve from diabetic rats is reduced (18) or alternatively that cells containing prosaposin mRNA in peripheral nerve (8) contribute only a small fraction of the measured prosaposin protein. An increase in the rate of prosaposin cleavage into lysosomal saposins is a less likely explanation, as we did not detect saposin C in the nerve or endoneurial fluid.
Prosaposin protein in the proximal stump of diabetic rats initially declined in parallel with the protein in control rats but this decline did not progress, so that there was significantly more prosaposin protein in the proximal stump of the sciatic nerve from diabetic rats compared to controls. Conversely, prosaposin protein levels in the endoneurial fluid collected beyond the point of transection were lower in diabetic rats compared to controls. One interpretation of these findings is that diabetes impedes secretion of neuronal prosaposin from the proximal stump so that it accumulates in the axon terminals and does not appear in the endoneurial fluid. The description of swollen and dystrophic regenerating axons projecting into the bridging tube of diabetic rats (19) may support this speculation. The impact of diabetes on prosaposin synthesis, anterograde axonal transport and turnaround at terminals by diabetic neurons requires further evaluation, however, before the attenuated decline of prosaposin in the proximal stump can be fully addressed. The contribution of prosaposin secreted from non-neuronal sources in both proximal and distal stumps must also be measured to clarify the cause of the diabetes-induced diminution of prosaposin in endoneurial fluid. Nevertheless, since prosaposin promotes neuritogenesis and neurite extension in cultured neurons and peripheral nerve (5, 17), the reduction of prosaposin in the endoneurial fluid through which regeneration takes place is consistent with the impaired rates and quality of regeneration seen in diabetic rats after nerve transection and re-growth through a similar bridging tube (19–21).
Prosaptide TX14(A) is a 14-mer peptide analog of the neurotrophic region of the saposin C domain of prosaposin (5). Like whole prosaposin, prosaptide TX14(A) induces neurite growth in transformed and adult primary sensory neurons that model neuronal regeneration after injury (6). Therefore, we investigated whether systemic prosaptide TX14(A) enhanced nerve regeneration and limb recovery after nerve crush injury. In control rats, the peptide was without effect on nerve function in the uninjured limb or indices of axonal growth and maturation in the injured nerve. It did, however, accelerate the time to recovery of limb function as measured by toe spread after nerve crush injury. This measurement likely represents a combination of nerve regeneration rate, which was unaffected by prosaposin up to 7 days after injury and by subsequent formation of functional neuromuscular synapses. The efficacy of prosaptide TX14(A) may therefore derive from enhanced motor end plate sprouting, as occurs after local injection of the peptide to muscle (6). The accelerated recovery of toe spread in control rats was accompanied by mitigation of EDL muscle atrophy in the ipsilateral limb with the latter potentially being a consequence of the former. Alternately, as prosaptide TX14(A) also attenuates atrophy of the EDL muscle after sciatic nerve ligation, a paradigm that does not allow re-innervation (16), the peptide may have a direct effect on the response of muscle to nerve injury that is independent of nerve regenerative capacity.
Systemic delivery of prosaptide TX14(A) protects peripheral nerve from diabetes-induced disorders of nerve structure and function (8, 9, 22, 23). Similar effects were seen in the present study, confirming the efficacy of the treatment regime without any reduction in the severity of diabetes. The peptide also prevented both the slowed nerve regeneration at 7 days post-crush and the increased MAD of regenerating axons measured one month after crush injury seen in diabetic rats. An increase in larger diameter axons has previously been reported in regenerating diabetic nerve after transection and was attributed to dystrophic accumulations (19) or a decrease in the relative proportion of smaller axons (20). Qualitative electron microscopic evaluation did not show any consistent occurrence of dystrophic accumulations of either organelles or cytoskeletal elements in the regenerating axons of diabetic rats (A.P. Mizisin, data not shown). Moreover, despite maintaining normal rates of regeneration over the first 7 days after injury in diabetic rats, it is notable that prosaptide TX14(A) did not prevent the ultimate failure of diabetic rats to recover toe spread distance. This may be because the efficacy of prosaptide TX14(A) in maintaining nerve regeneration rates in diabetic rats diminishes after day 7 or reflect the contribution of disorders other than nerve regeneration rate to recovery of toe spread that are not protected by the peptide. The capacity of prosaptide TX14(A) to partially protect EDL muscle weight of diabetic rats after crush injury, despite imperfect functional recovery of the limb, further supports the suggestion that prosaptide TX14(A) has direct protective effects on denervated muscle (16).
The pathogenesis of impaired nerve regeneration in diabetes is initiated by increased glucose metabolism by aldose reductase (24–26) and includes muting of the initial response to traumatic injury and the subsequent switch to the regenerative phenotype (27–29). The response to nerve injury is orchestrated by a diverse array of trophic factors (1) and many aspects of these support mechanisms are reduced in the neuraxis of diabetic animals (30). The efficacy of prosaptide TX14(A) in restoring axonal regeneration rates may reflect replacement of the diminished support resulting from the reduced amounts of endogenous prosaposin present in endoneurial fluid after injury, with the peptide either acting at the regenerating sprouts, or at the cell body, as is seen in vitro (6). The initial site of action of systemically-delivered prosaptide TX14(A) is not known, however, and indirect effects of the peptide that lead to enhanced nerve regeneration cannot be discounted. Prosaptide TX14(A) did not induce prosaposin protein in nerve of diabetic rats, but other agents with neurotrophic properties have been reported to improve indices of nerve regeneration after injury; these include CNTF (10), insulin (31), C peptide (32), IGF (33), NGF (21) and gangliosides (34, 35). It remains plausible that prosaptide TX14(A) induces one or more of these factors in nerve or other tissues.
In summary, our studies show that impaired nerve regeneration in diabetic rats is accompanied by the reduced appearance of prosaposin in the environment through which regenerating axons grow. A peptide analog of prosaposin that shares neuritogenic properties with the holoprotein enhanced recovery of limb function in control rats and prevented indices of degenerative neuropathy and slowed regeneration in diabetic rats. Since diabetic neuropathy is characterized by both distal degeneration and an impaired regenerative capacity, prosaptide TX14(A) may both protect nerve from damage during diabetes and promote the recovery of damaged nerves.
Acknowledgments
Supported by University of California BioSTAR S96-06 and NIH grants NS38855 and DK057629 (NAC).
References
- 1.Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- 2.Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: Studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127:1606–15. doi: 10.1093/brain/awh175. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy JM, Zochodne DW. Impaired peripheral nerve regeneration in diabetes mellitus. J Peripher Nerv Syst. 2005;10:144–57. doi: 10.1111/j.1085-9489.2005.0010205.x. [DOI] [PubMed] [Google Scholar]
- 4.Hiraiwa M, Campana WM, Mizisin AP, Mohiuddin L, O’Brien JS. Prosaposin: A myelinotrophic protein that promotes expression of myelin constituents and is secreted after nerve injury. Glia. 1999;26:353–60. [PubMed] [Google Scholar]
- 5.O’Brien JS, Carson GS, Seo HC, Hiraiwa M, Kishimoto Y. Identification of prosaposin as a neurotrophic factor. Proc Natl Acad Sci U S A. 1994;91:9593–96. doi: 10.1073/pnas.91.20.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campana WM, Mohiuddin L, Misasi R, O’Brien JS, Calcutt NA. Prosaposin-derived peptides enhanced sprouting of sensory neurons in vitro and induced sprouting at motor endplates in vivo. J Peripher Nerv Syst. 2000;5:126–30. doi: 10.1046/j.1529-8027.2000.00019.x. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien JS, Carson GS, Seo HC, Hiraiwa M, Weiler S, Tomich JM, et al. Identification of the neurotrophic factor sequence of prosaposin. FASEB J. 1995;9:681–85. doi: 10.1096/fasebj.9.8.7768361. [DOI] [PubMed] [Google Scholar]
- 8.Calcutt NA, Campana WM, Eskeland NL, Mohiuddin L, Dines KC, Mizisin AP, et al. Prosaposin gene expression and the efficacy of a prosaposin-derived peptide in preventing structural and functional disorders of peripheral nerve in diabetic rats. J Neuropathol Exp Neurol. 1999;58:628–36. doi: 10.1097/00005072-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Mizisin AP, Steinhardt RC, O’Brien JS, Calcutt NA. TX14(A), a prosaposin-derived peptide, reverses established nerve disorders in streptozotocin-diabetic rats and prevents them in galactose-fed rats. J Neuropathol Exp Neurol. 2001;60:953–60. doi: 10.1093/jnen/60.10.953. [DOI] [PubMed] [Google Scholar]
- 10.Mizisin AP, Vu Y, Shuff M, Calcutt NA. Ciliary neurotrophic factor improves nerve conduction and ameliorates regeneration deficits in diabetic rats. Diabetes. 2004;53:1807–12. doi: 10.2337/diabetes.53.7.1807. [DOI] [PubMed] [Google Scholar]
- 11.Calcutt NA. Modeling diabetic sensory neuropathy in rats. Methods Mol Med. 2004;99:55–65. doi: 10.1385/1-59259-770-x:225. [DOI] [PubMed] [Google Scholar]
- 12.Hosoda Y, Miyawaki K, Saito S, Chen J, Bing X, Terashita T, et al. Distribution of prosaposin in the rat nervous system. Cell Tissue Res. 2007;330:197–207. doi: 10.1007/s00441-007-0464-9. [DOI] [PubMed] [Google Scholar]
- 13.Unuma K, Chen J, Saito S, Kobayashi N, Sato K, Saito K, et al. Changes in expression of prosaposin in the rat facial nerve nucleus after facial nerve transection. Neurosci Res. 2005;52:220–27. doi: 10.1016/j.neures.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Gillen C, Gleichmann M, Spreyer P, Muller HW. Differentially expressed genes after peripheral nerve injury. J Neurosci Res. 1995;42:159–71. doi: 10.1002/jnr.490420203. [DOI] [PubMed] [Google Scholar]
- 15.Hiraiwa M, Campana WM, Martin BM, O’Brien JS. Prosaposin receptor: Evidence for a G-protein-associated receptor. Biochem Biophys Res Commun. 1997;240:415–18. doi: 10.1006/bbrc.1997.7673. [DOI] [PubMed] [Google Scholar]
- 16.Rende M, Brizi E, Donato R, Provenzano C, Bruno R, Mizisin AP, et al. Prosaposin is immunolocalized to muscle and prosaptides promote myoblast fusion and attenuate loss of muscle mass after nerve injury. Muscle Nerve. 2001;24:799–808. doi: 10.1002/mus.1072. [DOI] [PubMed] [Google Scholar]
- 17.Kotani Y, Matsuda S, Sakanaka M, Kondoh K, Ueno S, Sano A. Prosaposin facilitates sciatic nerve regeneration in vivo. J Neurochem. 1996;66:2019–25. doi: 10.1046/j.1471-4159.1996.66052019.x. [DOI] [PubMed] [Google Scholar]
- 18.Thomas PK, Wright DW, Tzebelikos E. Amino acid uptake by dorsal root ganglia from streptozotocin-diabetic rats. J Neurol Neurosurg Psychiatry. 1984;47:912–16. doi: 10.1136/jnnp.47.9.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longo FM, Powell HC, Lebeau J, Gerrero MR, Heckman H, Myers RR. Delayed nerve regeneration in streptozotocin diabetic rats. Muscle Nerve. 1986;9:385–93. doi: 10.1002/mus.880090502. [DOI] [PubMed] [Google Scholar]
- 20.Tantuwaya VS, Bailey SB, Schmidt RE, Villadiego A, Tong JX, Rich KM. Peripheral nerve regeneration through silicone chambers in streptozocin-induced diabetic rats. Brain Res. 1997;759:58–66. doi: 10.1016/s0006-8993(97)00247-3. [DOI] [PubMed] [Google Scholar]
- 21.Whitworth IH, Terenghi G, Green CJ, Brown RA, Stevens E, Tomlinson DR. Targeted delivery of nerve growth factor via fibronectin conduits assists nerve regeneration in control and diabetic rats. Eur J Neurosci. 1995;7:2220–25. doi: 10.1111/j.1460-9568.1995.tb00643.x. [DOI] [PubMed] [Google Scholar]
- 22.Calcutt NA, Freshwater JD, O’Brien JS. Protection of sensory function and antihyperalgesic properties of a prosaposin-derived peptide in diabetic rats. Anesthesiology. 2000;93:1271–78. doi: 10.1097/00000542-200011000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Jolivalt CG, Ramos KM, Herbetsson K, Esch FS, Calcutt NA. Therapeutic efficacy of prosaposin-derived peptide on different models of allodynia. Pain. 2006;121:14–21. doi: 10.1016/j.pain.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Kamijo M, Merry AC, Akdas G, Cherian PV, Sima AA. Nerve fiber regeneration following axotomy in the diabetic biobreeding Worcester rat: The effect of ARI treatment. J Diabetes Complications. 1996;10:183–91. doi: 10.1016/1056-8727(95)00008-9. [DOI] [PubMed] [Google Scholar]
- 25.Love A, Cotter MA, Cameron NE. Impaired myelinated fiber regeneration following freeze-injury in rats with streptozotocin-induced diabetes: Involvement of the polyol pathway. Brain Res. 1995;703:105–10. doi: 10.1016/0006-8993(95)01070-x. [DOI] [PubMed] [Google Scholar]
- 26.Terada M, Yasuda H, Kikkawa R, Shigeta Y. Tolrestat improves nerve regeneration after crush injury in streptozocin-induced diabetic rats. Metabolism. 1996;45:1189–95. doi: 10.1016/s0026-0495(96)90234-6. [DOI] [PubMed] [Google Scholar]
- 27.Maeda K, Fernyhough P, Tomlinson DR. Regenerating sensory neurones of diabetic rats express reduced levels of mRNA for GAP-43, gamma-preprotachykinin and the nerve growth factor receptors, trkA and p75NGFR. Brain Res Mol Brain Res. 1996;37:166–74. doi: 10.1016/0169-328x(95)00303-a. [DOI] [PubMed] [Google Scholar]
- 28.Mohiuddin L, Tomlinson DR. Impaired molecular regenerative responses in sensory neurones of diabetic rats: gene expression changes in dorsal root ganglia after sciatic nerve crush. Diabetes. 1997;46:2057–62. doi: 10.2337/diab.46.12.2057. [DOI] [PubMed] [Google Scholar]
- 29.Xu G, Pierson CR, Murakawa Y, Sima AA. Altered tubulin and neurofilament expression and impaired axonal growth in diabetic nerve regeneration. J Neuropathol Exp Neurol. 2002;61:164–75. doi: 10.1093/jnen/61.2.164. [DOI] [PubMed] [Google Scholar]
- 30.Calcutt NA, Jolivalt CG, Fermyhough PF. Growth Factors as therapeutics for diabetic neuropathy. Curr Drug targets. 2008;9:47–59. doi: 10.2174/138945008783431727. [DOI] [PubMed] [Google Scholar]
- 31.Pierson CR, Zhang W, Murakawa Y, Sima AA. Insulin deficiency rather than hyperglycemia accounts for impaired neurotrophic responses and nerve fiber regeneration in type 1 diabetic neuropathy. J Neuropathol Exp Neurol. 2003;62:260–71. doi: 10.1093/jnen/62.3.260. [DOI] [PubMed] [Google Scholar]
- 32.Pierson CR, Zhang W, Sima AA. Proinsulin C-peptide replacement in type 1 diabetic BB/Wor-rats prevents deficits in nerve fiber regeneration. J Neuropathol Exp Neurol. 2003;62:765–79. doi: 10.1093/jnen/62.7.765. [DOI] [PubMed] [Google Scholar]
- 33.Ishii DN, Lupien SB. Insulin-like growth factors protect against diabetic neuropathy: Effects on sensory nerve regeneration in rats. J Neurosci Res. 1995;40:138–44. doi: 10.1002/jnr.490400116. [DOI] [PubMed] [Google Scholar]
- 34.Ekstrom PA, Tomlinson DR. Impaired nerve regeneration in streptozotocin-diabetic rats is improved by treatment with gangliosides. Exp Neurol. 1990;109:200–3. doi: 10.1016/0014-4886(90)90074-3. [DOI] [PubMed] [Google Scholar]
- 35.Triban C, Guidolin D, Fabris M, Marini P, Schiavinato A, Dona M, et al. Ganglioside treatment and improved axonal regeneration capacity in experimental diabetic neuropathy. Diabetes. 1989;38:1012–22. doi: 10.2337/diab.38.8.1012. [DOI] [PubMed] [Google Scholar]