Abstract
L1-cell adhesion molecule (L1-CAM) belongs to a functionally conserved group of neural cell adhesion molecules that are implicated in many aspects of nervous system development. In many neuronal cells the adhesive function of L1-type CAMs induces cellular signaling processes that involves the activation of neuronal tyrosine protein kinases and among other functions regulates axonal growth and guidance. Mutations in the human L1-CAM gene are responsible for a complex neurodevelopmental condition, generally referred to as L1 syndrome. Several pathogenic L1-CAM mutations have been identified in humans that cause L1 syndrome in affected individuals without affecting the level of L1-CAM-mediated homophilic cell adhesion when tested in vitro. In this study, an analysis of two different pathogenic human L1-CAM molecules indicates that although both induce normal L1-CAM-mediated cell aggregation, they are defective in stimulating human epidermal growth factor receptor tyrosine kinase activity in vitro and are unable to rescue L1 loss-of-function conditions in a Drosophila transgenic model in vivo. These results indicate that the L1 syndrome-associated phenotype might involve the disruption of L1-CAM's functions at different levels. Either by reducing or abolishing L1-CAM protein expression, by interfering with L1-CAM's cell surface expression, by reducing L1-CAM's adhesive ability or by impeding further downstream adhesion-dependent signaling processes.
INTRODUCTION
L1-type neural cell adhesion molecules are widely expressed during metazoan nervous system development where they are involved in multiple cellular processes, ranging from neuronal differentiation and organization, neurite outgrowth and axonal pathfinding, to synapse formation and maintenance (1–3). Although non-vertebrate genomes encode only one L1-type protein, gene multiplication events generated four different L1-type genes in vertebrate species [referred to as L1-cell adhesion molecule (L1-CAM), close homologue of L1 (CHL1), Neurofascin and Ng-CAM related cell adhesion molecule (NrCAM)] (4). This may have facilitated a functional diversification of these paralogous L1-type proteins, including the addition of novel protein–protein interactions. However, several central L1 functions, such as the promotion of neurite outgrowth, axonal pathfinding and several cytoplasmic interactions are well conserved throughout the entire L1 gene family. One such conserved function is the adhesion-dependent activation of neuronal receptor tyrosine kinases (RTKs) receptors. On the basis of their results obtained from in vitro experiments Doherty, Walsh and coworkers postulated that in vertebrate neurons L1-CAM-mediated adhesion results in the activation of type I Fibroblast Growth Factor Receptor (FGFR) and ultimately in neurite outgrowth (5). Genetic results from the Drosophila system indicate that during pupal nervous system development the Drosophila L1-type protein Neuroglian (Nrg) mediates axonal growth and pathfinding of several sensory neurons through the activation of neuronal Epidermal Growth Factor (EGF) and FGFRs (6). Moreover, human L1-CAM rescues an RTK-mediated axonal growth and pathfinding phenotype in the developing Drosophila nervous system that is caused by neuroglian loss-of-function (LOF) conditions (7).
LOF conditions for L1-type genes in different species result in pleiotropic phenotypes, ranging from late embryonic lethality in Drosophila to mental retardation and neurological malformations in humans (8–10). Because of its genomic localization on the X chromosome in mice and humans, pathogenic mutations in the L1-CAM gene exhibit a typical X-linked inheritance in these species (11). As different mutations in the human L1-CAM gene exhibit a large phenotypic variance, they were originally reported under various designations, such as X-linked hydrocephalus, mental retardation, aphasia, shuffling gait, and adducted thumbs syndrome, X-linked agenesis of the corpus callosum and X-linked spastic paraplegia (12,13). These allelic disorders are now jointly referred to as L1 syndrome (1). Whereas all affected male individuals are mentally retarded, other neurological L1-associated phenotypes, such as hydrocephalus, agenesis of the corticospinal tract and the corpus callosum and clasp thumbs, exhibit variable penetrance and expressivity (14). The expression of these phenotypic traits not only depends on the type of molecular lesion and how it affects the expression and functionality of the L1-CAM protein, but likely appears also to be under considerable genetic modifier control. Well over 180 pathogenic mutations in the human L1-CAM gene have been analyzed at the DNA level. Many of these mutations cause major deletions or a premature termination of the L1-CAM protein. However, about one third of affected families have single missense mutations in the L1-CAM gene, which alters only one of the 1257 amino acid residues of the human neuronal L1-CAM protein. These pathogenic missense mutations are scattered over the entire length of the human L1-CAM protein implicating different L1-dependent functions in the pathophysiology of L1 syndrome. In general, carboxy-terminal mutations, which affect the cytoplasmic protein domain, exhibit a milder phenotype (15,16). Whereas many pathogenic L1-CAM mutations interfere with the protein's homo- or heterophilic adhesive function or result in defective protein trafficking, other L1-CAM missense mutations have been shown to mediate normal adhesion in various in vitro assay systems (14,17–21). These results indicate that functions other than homophilic adhesion might also contribute to the observed neurological defects in individuals with L1 syndrome.
Many molecular, as well as the developmental functions of human L1-CAM can be efficiently tested in Drosophila assay systems, e.g. wild-type human L1-CAM rescues the Drosophila L1-type protein nrg LOF phenotype in ocellar sensory neurons (7). Therefore, the fly can be used as a simple test system for probing the axonal growth and pathfinding function of L1-type proteins in vivo. In this study, we analyzed the functional capacity of two pathogenic human L1-CAM proteins (E309K alias H38 and Y1070C alias H1, Fig. 1), for which normal homophilic adhesion has previously been reported (17–19), to mediate downstream L1-CAM-dependent interactions and functions. The two amino acid residues that are affected by the H38 and the H1 mutation (E309 and Y1070) are both predicted to reside on the surface of their respective L1-CAM protein domain (22). Although these residues are not conserved in the Drosophila Neuroglian protein (T314 and E1072), both human mutations change the chemical nature of these amino acid residues considerably, either by introducing a positive charge (H38 E309K) or by introducing a protein surface exposed cysteine residue (H1 Y1070C). These changes may profoundly affect the tertiary structure of the L1-CAM protein or may only interfere with L1-CAM protein–protein interactions. On the basis of our previous observations that L1-CAM adhesion activates the epidermal growth factor receptor (EGFR) kinase (23) and that this interaction regulates axonal growth and pathfinding in the developing Drosophila nervous system in vivo (6,7), we investigated the functional capacity of these two mutant proteins to induce EGFR activity in a cell-based assay system and to rescue L1 LOF conditions in developing sensory neurons in vivo. We found that both pathogenic human L1-CAM proteins were fully competent to mediate homophilic cell adhesion in Drosophila cells, but exhibited a lower than wild-type ability to activate EGFR signaling and failed to rescue L1-deficient conditions in vivo. These results indicate that L1-CAM functionality during early nervous system development can be disrupted at different levels, firstly by deleting or truncating the L1-CAM protein, thereby creating protein null conditions; secondly by mutations that interfere with the proper cell surface expression of the L1-CAM protein; thirdly by mutations that diminish the adhesive properties of the protein; and finally by compromising molecular interactions that act downstream of L1-CAM-mediated adhesion, such as the activation of neuronal signaling processes.
Figure 1.

Protein domain model of the L1-CAM protein indicating the positions of the H1 and H38 missense mutations.
RESULTS
The pathogenic human H1 and H38 L1-CAM proteins mediate normal homophilic adhesion in Drosophila Schneider 2 cells
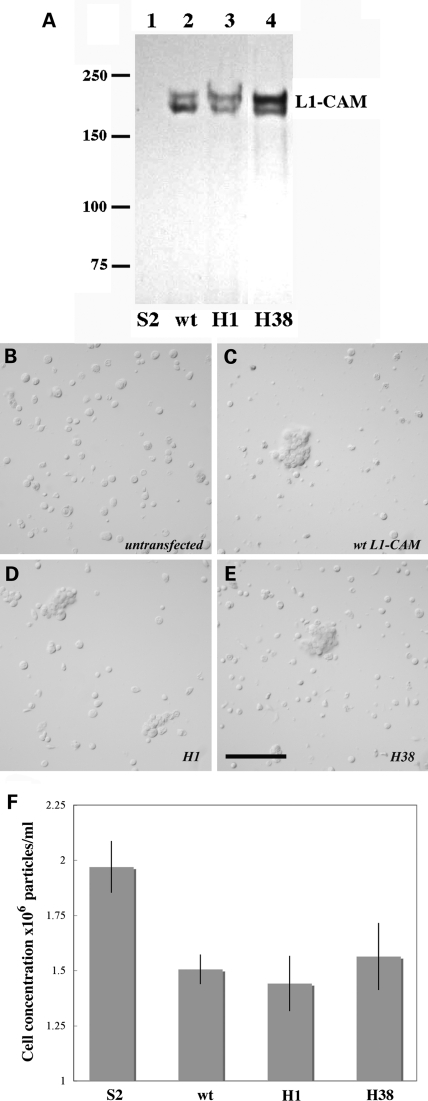
We first tested whether the pathogenic human L1-CAM proteins can be stably expressed by Drosophila Schneider 2 (S2) cells and whether these missense mutant L1-CAM proteins mediate wild-type levels of homophilic cell aggregation as had previously been reported in mammalian tissue culture cells (17,18). The H38 protein has a single lysine for glutamate change at position 309 in the third immunoglobulin domain of L1-CAM, whereas the H1 mutation results in a cysteine for tyrosine change at position 1070 in the fifth fibronectin type III domain. cDNAs encoding the neuronal isoform of wild-type or the two mutant forms of human L1-CAM were transfected into and expressed in Drosophila S2 cells. As shown in Figure 2A, following overnight induction of cDNA expression wild-type and mutant L1-CAM proteins were stably expressed in Drosophila S2 cells with an expected molecular weight of ∼200 kDa (24). Furthermore, unlike untransfected S2 cells that did not adhere to each other transfected S2 cells expressing wild-type of H1 or H38 mutant L1-CAM proteins over time formed robust cell aggregates (Fig. 2B–E). A quantitative analysis of S2 cell aggregation based on four independent experiments demonstrated that both H1 and H38 mutant L1-CAM protein mediate homophilic S2 cell aggregation to the same level as the wild-type protein (Fig. 2F). We also tested other pathogenic human L1-CAM proteins (H2, H12, H18, H46 and F5), which all exhibited a significantly lower adhesive ability when expressed in Drosophila S2 cells (not shown). This finding in Drosophila cells agrees with previous analyses using COS-7 and CHO cells, which also reported normal homophilic adhesion levels for the H1 and H38 pathogenic mutant L1-CAM proteins (17,18).
Figure 2.
Mutant H1 and H38 human L1-CAM protein both mediate wild-type level homophilic cell aggregation in Drosophila S2 cells. (A) Depicts a western blot of total S2 cell proteins that were isolated from the transfected S2 cell lines and subsequently tested for homophilic cell aggregation (B–F). (B–E) Show untransfected (B) or transfected S2 cells that express wild-type (B) or mutant (D—H1 and E—H38) human L1-CAM protein. The images show the presence of typical S2 cell aggregates for S2 cells expressing wild-type or mutant human L1-CAM. The size bar corresponds to 100 µm. (F) Depicts the quantification of homophilic S2 cell aggregation that is induced by the expression of the different human L1-CAM proteins. Shown are the results of five independent experiments. Compared with untransfected S2 cells P-values were calculated for wild-type L1-CAM transfected S2 cells as P = 1.3 × 10−4, for H1 transfected S2 cells as P = 0.5 × 10−4 and for H38 transfected S2 cells as P = 5.5 × 10−4.
Both H1 and H38 mutant L1-CAM proteins have a reduced ability to induce EGFR kinase activity
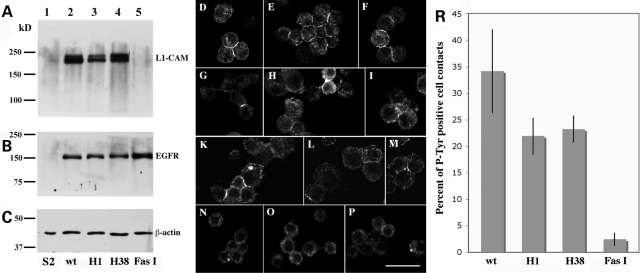
L1-type CAMs control neuronal development through their functional interactions with tyrosine kinase-regulated signaling pathways (1). We previously established and characterized an S2 cell assay to demonstrated that homophilic L1-CAM adhesion triggers EGFR tyrosine kinase activity (23) and genetic data are consistent with this interaction regulating axonal growth and guidance in Drosophila sensory neurons (6,7). To evaluate the ability of the H1 and H38 L1-CAM proteins to induce the adhesion-dependent activation of human EGFR, we generated double-transfected S2 cell lines, in which wild-type, H1 or H38 L1-CAM or Drosophila Fasciclin I was co-expressed with human EGFR. Drosophila Fasciclin I was used as a negative control as it has no reported functional interaction with the EGFR signaling pathway (23). The western blot analyses shown in Figure 3A and B demonstrate that human L1-CAM and EGFR proteins are stably expressed in induced S2 cells. For the analysis of EGFR tyrosine kinase activation, protein production was induced overnight and cells were briefly aggregated to generate small cell clusters, which were subsequently processed for immunocytochemistry with anti-phosphotyrosine antibodies. As previously reported (23), fluorescence staining indicating protein tyrosine phosphorylation of EGFR molecules was mainly restricted to cell contact areas. Individual cell–cell contact sites were carefully analyzed and scored by confocal microscopy. A quantitative analysis of four independent experiments using two independent transfected cell lines for each vector combination demonstrated a statistically significant reduction of EGFR activation for the H1 and H38 L1-CAM proteins (22 and 23%, respectively) when compared with wild-type L1-CAM protein (34%) (Fig. 3R). The Drosophila Fasciclin I induced a positive phosphotyrosine signal at only 2% of all cell–cell contacts. These results indicate that the ability of the H1 and H38 proteins to activate EGFR kinase signaling is not abolished, but significantly reduced, which may have physiological consequences under in vivo conditions.
Figure 3.
Quantitative evaluation of the ability of wild-type and mutant human L1-CAM proteins to induce human EGFR tyrosine kinase activity at S2 cell–cell contact sites. (A–C) Represent western blots of total protein extracts (50 µg per lane) from induced untransfected (lanes 1) or transfected (lanes 2–5) S2 cells. Blot A was incubated with anti-L1-CAM, blot B with anti-EGFR and blot C with anti-β-actin antibodies, respectively, and developed with enhanced chemiluminescence (ECL) reagent after an incubation with HRP-labeled secondary antibodies. (D–P) Show confocal microscopy images of small S2 cell aggregates that were incubated with an anti-phosphotyrosine antibody probe. S2 cells in (D–F) co-express wild-type human L1-CAM and EGFR, (G–I) mutant H1 L1-CAM and wild-type human EGFR, (K–M) mutant H38 L1-CAM and wild-type EGFR and (N–P) Drosophila Fasciclin I protein with wild-type human EGFR. The scale bar represents 20 µm. (R) depicts a quantitative analysis of cell contacts staining for phosphotyrosine for the four different cell lines depicted in the previous panels. These results were obtained from four separate experiments using two independently transfected S2 cell lines for each construct. At least 100 cell–cell contacts were evaluated per experiment for each transfected cell line. In comparison to wild-type L1-CAM transfected cells P= values were calculated for H1 transfected cells as P = 2.8 × 10−2, for H38 transfected cells as P = 3.6 × 10−2 and for Fasciclin I transfected cells as P = 0.3 × 10−2.
Expression of human L1-CAM proteins in Drosophila tissues
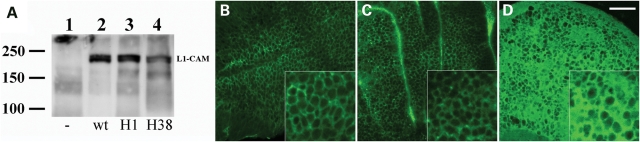
In order to investigate the functional ability of mutant human H1 and H38 L1-CAM proteins under in vivo conditions, we generated several independent Drosophila upstream activator sequence (UAS)-GAL4-inducible transgenic lines (25) for both human mutant L1-CAM proteins. Drosophila transgenic lines with a functional UAS-wt human L1-CAM insert had previously been generated and characterized (7). These lines were used to ascertain the expression and cell surface expression of human L1-CAM proteins in Drosophila tissues. Using western blot analysis, we found that wild-type, as well as mutant L1-CAM proteins were expressed in Drosophila tissues with the expected molecular weight of 200 kDa (Fig. 4A). The in vivo analysis of the cell surface expression of human L1-CAM proteins in imaginal disk epithelia in vivo, was analyzed by immunocytochemistry using anti-L1-CAM antibodies and revealed that wild-type and H1 proteins are expressed on the cell surface and at cell–cell contact regions in wing disks (Fig. 4B and C). In contrast, L1-CAM immunostaining for the H38 protein showed an accumulation of mutant H38 protein inside imaginal disk epithelial cells. This is not a surprising finding as H38 and other mutant L1-CAM proteins have previously been associated with intracellular trafficking defects and reduced cell surface expression (17–19,21).
Figure 4.
Expression of wild-type and mutant human L1-CAM in Drosophila imaginal disks. (A) Shows the result of a western blot Drosophila total protein (50 µg total protein per lane) that was incubated with anti-L1-CAM antibodies. (B–D) Show that expression of human L1-CAM proteins in Drosophila wing imaginal disks. (B) Depicts the expression from a wild-type human L1-CAM gene, whereas (C) and (D) show expression of the H1 and H38 mutant human L1-CAM proteins in wing imaginal disks, respectively. The inserts show sections of imaginal disk epithelia at a higher magnification. The scale bar represents 20 µm for the main images and 8 µm for the inserts.
Pathogenic mutant L1-CAM proteins fail to rescue nrg LOF conditions in Drosophila sensory neurons
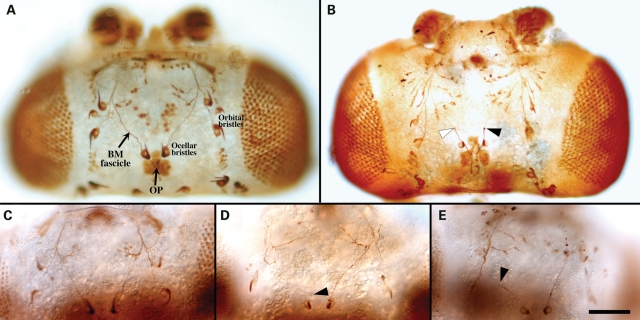
Many lines of evidence suggest that L1-type CAMs regulate axonal growth and pathfinding through the activation of neuronal tyrosine kinase signaling, including FGFR, EGFR and some non-RTKs (1). As several traits of L1 syndrome, e.g. agenesis of the corticospinal tract and the corpus callosum, might be caused by a dysfunction of this developmental L1-CAM function, we tested the ability of H1 and H38 mutant L1-CAM proteins to sustain axonal growth and guidance in vivo. We previously reported that ectopic expression of wild-type human L1-CAM rescues the nrg LOF phenotype in bristle mechanosensory (BM) and ocellar pioneer (OP) neurons, which constitute the ocellar sensory system (OSS) of the fly (7). This biological function involves the L1-dependent activation of neuronal RTK activity (6). As the L1- and the NCAM-type CAMs Neuroglian and Fasciclin II are co-expressed in OP neurons and exhibit an RTK-dependent functional redundancy during axonal growth and pathfinding (7), we focused our analysis on the functional capacity of H1 and H38 mutant proteins to rescue nrg LOF conditions in BM neurons, where Fasciclin II is not expressed. In wild-type animals, the ocellar BM fascicle contains converging axons from the single ocellar bristle and the ocellar microchaetes. Further anterior the BM fascicle converges with the orbital bristle nerve (Fig. 5A). A loss of the endogenous L1-type Nrg protein during OSS development often results in a stalling phenotype and less frequently in misguidance defects of ocellar and orbital BM axons (Fig. 5B). Sometimes only one or a subset of axons seems to stall within a nerve, although other axons extend normally (see the white arrowhead in Fig. 5B). As this situation can not always be distinguished from an abnormal thickening of axons, which is sometimes observed, only phenotypes in which all axons stopped growing were counted (see black arrowheads in Fig. 5B, D and E). In a few cases (<20% of phenotypic heads), BM axons separated from the epidermis without connecting with the orbital nerve or projected backwards or to an ectopic place. These cases were included in the quantitative evaluation that is presented in Table 1. The ectopic expression of wild-type and mutant human L1-CAM protein in a wild-type background results in a low, but not significant level (4–11%) of BM axonal defects. When the endogenous Drosophila L1-type CAM, Nrg, was removed using the temperature-sensitive nrg3 allele, BM axon defects were observed in 34% of all heads. Consistent with our previous results (7), the ectopic expression of wild-type human L1-CAM in these nrg LOF animals resulted in a statistically highly significant reduction of the BM phenotype (highlighted in bold in Table 1). In contrast to wild-type L1-CAM, the expression of H1 or H38 mutant L1-CAM protein did not rescue the nrg3 phenotype and the penetrance level of BM axon defects appeared to be additive from the nrg3 LOF and the human L1-CAM misexpression phenotype.
Figure 5.
Neuroglian LOF phenotype in the adult Drosophila ocellar system and rescue by transgenic wild-type human L1-CAM. (A) Depicts the ocellar system of a wild-type fly. Neuronal cell bodies and axons were visualized by immunostaining with 22C10 antibody. (B) Shows the head of an nrg3 mutant fly that was incubated at non-permissive temperature during pupal development. The black arrowheads indicate a complete stalling phenotype of a BM axonal pathway, whereas the white arrowhead marks a partial stalling phenotype (not considered for data quantification). (C) Shows an example of the nrg LOF phenotype that was rescued by human L1-CAM expression, whereas (D) and (E) show a lack of rescue when H1 and H38 mutant human L1-CAM were expressed. The scale bar represents 100 µm.
Table 1.
Quantitative evaluation of the BM axonal stalling phenotype in wild-type or nrg LOF Drosophila heads that express wild-type or mutant human L1-CAM, respectively
| BM alterations All MS1075-Gal4/+ | No transgene | Human L1-CAM (wt) | Human L1-CAM (H1) | Human L1-CAM (H38) |
|---|---|---|---|---|
| Wild-type | 0/9 = 0% | 1/25 = 4% (P = 1.0000) | 2/18 = 11% (P = 0.5385) | 1/15 = 7% (P = 1.0000) |
| nrg3 | 25/74 = 34% | 5/51 = 10% (P = 0.0025) | 43/106 = 41% (P = 0.4349) | 25/54 = 46% (P = 0.1992) |
DISCUSSION
The variety of L1-CAM-associated developmental functions and the multitude of L1-interacting ligands correspond with the complexity of the L1 LOF phenotype in humans and in various model systems. Due to a strong genetic modifier effects on the penetrance and the expressivity, L1-associated phenotypes are highly variable. Therefore, it is difficult to equate specific molecular properties of L1-type CAMs or the lack thereof with experimental results obtained from in vitro test systems or with specific mutation-induced dysfunctions in vivo. As a consequence, it has remained unclear, why certain missense mutations in the human L1-CAM protein exhibit normal adhesive properties in vitro, but induce neurological abnormalities that overlap with those caused by protein null conditions. In this publication, we demonstrate that two human pathogenic L1-CAM proteins maintain their homophilic adhesiveness, but are deficient in vitro, as well as in vivo in a further downstream function, the adhesion-dependent induction of RTK tyrosine kinase activity.
As extra- and intracellular domains of L1-type proteins associate with a wide range of different protein ligands, heterophilic interactions might influence the expression of the L1 syndrome phenotype. De Angelis et al. analyzed the ability of H1 and H38 mutant L1-CAM protein to bind to two GPI-anchored, immunoglobulin-domain CAMs, Contactin/F11/F3 and TAG-1/Axonin-1. Both these Ig-domain CAMs are widely expressed in the developing nervous system where they mediate homophilic cell adhesion and also interact with L1-type proteins (26). Using a cell-free microsphere bead assay, De Angelis et al. (17,18) reported that the H38 L1-CAM protein has a reduced ability to interact with Contactin/F11 and TAG-1/Axonin-1. In contrast, they found that H1 L1-CAM protein exhibited an increased binding affinity for F11 and Axonin-1 isolated from chicken brain (17), but interacted at wild-type levels with human TAG-1 (18). However, these results do not reveal how L1-CAM's interactions with GPI-anchored CAMs might be compromised by the H1 and the H38 mutation and whether these interactions contribute to the phenotypic manifestations of L1 syndrome. The recent analyses of Contactin and TAG-1 knockout mice show no phenotypic overlap with L1-CAM mutations and therefore argue against a significant involvement of these heterophilic L1 ligands in the generation of the L1-CAM LOF phenotype. In TAG-1-deficient mice the gross anatomy of the nervous system is normal, but these mice exhibit a greater sensitivity to convulsant stimuli (27). In addition, TAG-1 is important for the organization of juxtaparanodal regions at nodes of Ranvier (28,29). The lack of this TAG-1 function is likely responsible for the impairment of cognitive functions and the reduction in motor activity that is observed in TAG-1 knockout mice (30). Mice lacking a functional contactin gene develop cerebellar defects, which result in a severe ataxia and death by postnatal Day 18 (31). Similar to TAG-1, Contactin is also involved in establishing functional paranodal junctions and is specifically important for the localization of Kv1.1 and 1.2 potassium channels at the nodes of Ranvier (32). The interaction between Contactin- and L1-type proteins at septate-type junctions is evolutionary well conserved (33) and has also been described in Drosophila epithelia and at neuron-glia cell contacts (34). However, as L1-CAM is not expressed at nodes of Ranvier in humans, this L1 function is not carried out by L1-CAM in mammalian species, but rather by its paralogs NrCAM and Neurofascin (35). The lack of phenotypic overlap between L1-CAM, Contactin and TAG-1 and the limited co-expression suggests a limited, if any contribution of these heterophilic L1-CAM ligands to the L1-CAM LOF phenotype.
Originally LOF conditions for L1-CAM in humans were described as four distinct disorders, which had only a limited phenotypic overlap (13). The expression of different phenotypic traits in humans with L1-CAM mutations is rather complex. Even within families that shares a common L1-CAM mutation, the expressivity of various phenotypic traits differ considerably between affected individuals (14,36). A strong influence of the genetic background on phenotype expression has also been confirmed in L1-CAM knockout mouse strains (37,38). Overall the L1-CAM knockout phenotype in mice resembles that of humans with L1-CAM mutations. It includes corticospinal defects, enlarged ventricles causing hydrocephalus, impaired locomotive functions, agenesis of the corpus callosum, hippocampal defects, a reduction in Schwann cell–axon interactions, a misorganization of dopaminergic neurons in the mouse mesencephalon and abnormal retinocollicular projections (10,37,39–45). Several of these traits, like the hypoplasticity of the corticospinal tract and malformations of ventricular structures resulting in hydrocephaly, are highly dependent on the genetic background (37,38,46). Interestingly, the importance of the genetic background for the penetrance and expressivity of the L1-CAM knockout phenotype is reminiscent of the situation described for knockout conditions of the mouse EGFR gene (47).
Different functional aspects of L1 gene family biology may contribute to the complexity of the observed L1 LOF phenotype. In vertebrate species, this complexity is increased by the expression of four L1-type genes (4). These paralogous L1-type genes and their protein products exhibit a significant overlap in their patterns of expression and their biological functions (4). As demonstrated by Sakurai et al. (48), these overlaps result in a genetic redundancy between different L1-type genes, which is only uncovered in multiple gene knockout animals. Other L1 mutant phenotypes appear to be more paralog-specific and are therefore additive in double mutant mice (49). Furthermore, even in species with only a single L1-type gene, such as Drosophila, a functional redundancy between Neuroglian and the NCAM-type Fasciclin II protein has been reported (7). These functional and molecular redundancies make it very difficult to pinpoint specific molecular functions such as L1-CAM's homophilic adhesion, as being responsible or dispensable for specific phenotypic traits.
The finding that certain human pathogenic L1-CAM mutations maintain their full homophilic binding ability can be interpreted in two ways. First, that L1-CAM-mediated homophilic adhesion has no or only a limited importance for L1-CAM functions. In support of this argument, Itoh et al. (38) reported that some phenotypic traits in an L1-CAM knockout mouse were ‘rescued’ by a homophilic adhesion-dead form of L1-CAM. Alternatively, L1-dependent processes that are downstream of its adhesive function may be specifically affected in these L1-CAM mutations. As sensory axon growth and guidance in the Drosophila ocellar system is regulated by the L1-mediated activation of neuronal RTKs (6,7), we tested this possibility by analyzing the ability of two mutant human L1-CAM proteins, H1 and H38, to activate EGFR signaling in vitro and to rescue the L1 LOF phenotype in vivo (Table 2). Both proteins can be stably expressed in Drosophila tissues and mediate a wild-type level of homophilic cell adhesion in S2 cells. However, the H38 protein also exhibits an abnormal intracellular location in Drosophila epithelial cells, indicating that its transport to the cell surface is impeded. Nevertheless, sufficient H38 protein reaches the cell surface to mediate a wild-type level of cell adhesion in S2 cells. Using an S2 cells EGFR activation assay, we found that both mutant L1-CAM proteins display a reduced ability to activate EGFR tyrosine kinase activity when compared with their wild-type counterpart. This in vitro result was confirmed by the inability of both mutant proteins to rescue LOF conditions for the Drosophila L1-type Neuroglian protein in vivo. Axonal growth and guidance of ocellar BM neurons depend on the L1-mediated activation of RTKs (6,7). Similar to the situation in the Drosophila OSS, axonal growth and misguidance defects have been reported as the cellular consequences for some of the phenotypic traits in L1-CAM knockout mice, specifically the agenesis of the corpus callosum and the corticospinal tract (39,42). Doherty and Walsh (5) demonstrated previously that the L1-CAM-mediated activation of neuronal type 1 FGFRs is an important mechanism to induce neurite outgrowth in vertebrate neurons. This L1 function appears to be well-conserved throughout evolution and governs some aspects of Drosophila nervous system development. We have shown that human L1-CAM activates Drosophila FGFR and EGFR activity to regulate axonal growth and guidance in Drosophila sensory neurons (7). Moreover, human L1-CAM specifically activates human EGFR in Drosophila cells (23). This indicates that the functional interaction between L1-type CAMs and neuronal RTKs constitutes an important functional L1 attribute in vivo. However, how much this L1 function and specifically the activation of EGFR contributes to the L1-CAM LOF phenotype in mice and humans is currently unknown and other signaling processes that are downstream of L1-CAM's hetero- and homophilic interactions may also exert an important influence on phenotypic expressivity. One such heterophilic interaction is the more recently described interaction between L1-CAM and the Semaphorin3A co-receptor Neuropilin-1 (50) and the reported dependency of Semaphorin3A signaling on L1-CAM expression (51). However, similar to TAG-1 and Contactin, Semaphorin3 knock out mice exhibit little phenotypic overlap in comparison with L1-CAM knock out mouse lines (52,53).
Table 2.
Summary of experimental results
| Human L1-CAM mutation | Protein domain affected | Amino acid change | Homophilic S2 cell aggregation | Adhesion-depend, EGFR activation in S2 cells | Protein expression in vivo | Cell surface expression in vivo | Genetic rescue of BM axonal pathfinding defect |
|---|---|---|---|---|---|---|---|
| Wild-type | wt | wt | wt | wt | Rescue | ||
| H1 | Fn 5 | Y1070C | wt | Reduced | wt | wt | No rescue |
| H38 | Ig3 | E309K | wt | Reduced | <wt | Partially intracellular | No rescue |
Accumulating evidence from several laboratories indicates that mutations in the L1-CAM gene/protein interfere with L1-CAM function at different levels. Many frame shift, nonsense and splice mutations create a non-function L1-CAM protein fragment and are therefore the equivalent of a protein null mutation. A second level of interfering with L1-CAM function is the aberrant cellular localization of L1-CAM protein, which has been reported for several L1-CAM point mutations (20,21). As reported here, this appears to be at least in part the case for the human H38 protein. Other mutant proteins are correctly transported to the neuronal cell surface, but are unable to fully engage in homo- and/or heterophilic interactions (17,18). In this report, we present evidence that L1-CAM functions downstream of its adhesive interactions might also be disabled in pathogenic mutant L1-CAM proteins and result in a specific phenotype. We previously demonstrated that homophilic L1-CAM interactions induce EGFR tyrosine kinase activity (23) and that this L1 function is well conserved between Drosophila Neuroglian and human L1-CAM (7). However, we cannot exclude that under in vivo conditions, other heterophilic L1 interactions also contribute to RTK activation and that pathogenic L1-CAM mutations also affect other, RTK-independent downstream interactions to cause some of the L1-associated phenotypic traits.
MATERIAL AND METHODS
Generation of plasmid vectors encoding mutant human L1-CAM proteins
cDNA clones encoding the entire open reading frame of the neuronal isoform of mutant human L1-CAM proteins were provided by Drs S. Kenwrick, T. Brümmendorf and F. Rathjen (17,18). The cDNA inserts were partially sequenced to verify the presence of the H1 and the H38 mutation, respectively. Subsequently, they were subcloned into the pRmHa-3 vector for the S2 cell transfection experiments and into the pUAST vector for the generation of transgenic Drosophila lines.
S2 cell transfection, protein expression and adhesion assays
S2 cells were kept in SFX HyQ Insect medium (Fisher Scientific, Pittsburgh, PA, USA) without fetal calf serum, but with penicillin/streptomycin (Life Technologies Corp., Carlsbad, CA, USA). Cell transfection using Lipofectin® (Life Technologies Corp.) and selection of transfected cell line using hygromycin resistance were preformed as previously described (23). For the quantitative aggregation assay, transfected S2 cells were induced overnight with 0.7 mm CuSO4, mechanically dissociated and incubated in a 50 ml test tube on a shaking platform at 200 rpm. Using a hemocytometer, cell aggregation was quantified by determining the single cell concentration after 8 h of incubation. Data points in Figure 2F represent the average of four independent experiments. Cell aggregation and subsequent immunostaining with the phosphotyrosine-specific mAb PY20 was performed as outlined by Islam et al. (23).
SDS-PAGE, antibodies, western blot analysis and immunostaining procedures
Proteins were separated in standard 10% SDS-PAGE gels and blotted onto Immobilon-P PVDF (Millipore Corp., Billerica, MA, USA) or BioTrace™ nitrocellulose membranes (Pall Corp., East Hills, NY, USA). Protein concentrations were determined using the BCA Protein Assay kit from Pierce Biotechnology Inc. (Rockford, IL, USA). Goat anti-L1-CAM and anti-EGFR polyclonal antisera were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Anti-β-actin and anti-phosphotyrosine PY20 mAbs were from Sigma-Aldrich (St. Louis, MO, USA) and from Cell Signaling Technology (Danvers, MA, USA), respectively. The Drosophila Fasciclin I-specific 5H7 mAb has been described by Hortsch et al. (54). For western blot analyses mAb ascites fluid was used at an 1:500 dilution, commercial polyclonal antisera at 1:300) and anti-β-actin antibody at 1:10 000. HRP- and FITC-labeled secondary antibodies were from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA, USA) and were used at an 1:2000 dilution for western blot analysis and at 1:200 for immunocytochemistry. Bound primary antibodies on western blots were visualized using the enhanced chemiluminescence (ECL) western blot detection kit from Amersham Pharmacia Biotech (now part of GE Healthcare, Chalfont St. Giles, UK). 3,3′-diaminobenzidine-based immunostainings of pupal heads with the 22C10 mAb were carried out as previously described (6). The VectastainABC kit from Vector Laboratories Inc. (Burlingame, CA, USA) was used to enhance the signal. Images of processed S2 cells and Drosophila imaginal disks were captured with a Nikon Optiphot 2 microscope (Nikon Corp., Tokyo, Japan) that is equipped with Nomarski optics and a Nikon DXM1200 digital camera system or using an Olympus FV confocal scanning laser microscope (Center Valley, PA, USA), which is housed in the Microscope Imaging Laboratory at the University of Michigan. Images of stained Drosophila heads were captured using an Eclipse 80i Nikon microscope with Nomarski optics and a Nikon DS-5M-L1 digital camera system.
Drosophila lines and in vivo expression of transgenic human L1-CAMs
Multiple transgenic lines were produced by microinjection of plasmid DNA into w1118 Drosophila embryos and the subsequent selection of w+ positive animals for each pUAST-mutant L1-CAM construct. Transgenic lines encoding wild-type human L1-CAM under UAS control have been described and were used in earlier publications (7,23). The MS1075 GAL4 driver line was previously characterized by Garcia-Alonso et al. (6) and used for nrg LOF rescue experiments by Kristiansen et al. (7). All fly lines except the nrg3 line were maintained and all experiments were performed at 25°C. The temperature-sensitive nrg3 allele was maintained at 18°C and shifted to 29°C as the non-permissive temperature (55). Details about the temperature shift experiments and the immunocytochemical staining procedure using the 22C10 mAb for the visualization of axonal pathway formation in the ocellar system have been published previously (6,7). The statistical analysis of the S2 cell experiments was performed using a Welch's t-test comparison for two independent samples and the two-tailed Fisher exact test was used for the analysis of the genetic experiments. The analysis in Table 1 represents the pooled results from two independent transgenic lines for each of the different constructs.
FUNDING
This work supported in part by grants from the National Institutes of Health (R01 NS32130 to M.H.); the National Science Foundation (IBN 0132819 to M.H.); and a GLOBAL Reach Research, Education and Collaboration in Health International faculty seed grant from the University of Michigan (M.H.). Grants from MCYT (SAF2001-1628, SAF2004-06593 to L.G.-A.); and Generalitat Valenciana (PROMETEO-2008-134 to L.G.-A.) supported the work in Spain.
ACKNOWLEDGEMENTS
We would like to acknowledge the advice and support of Drs Elisabeth Bock and Vladimir Berezin (University of Copenhagen) during the early phases of this project. We are also grateful for the help and technical support provided by Sigrid Baars (Universidad Miguel Hernandez) and Nicole Crisp (University of Michigan) and would like to thank Ruthi Hortsch (University of Michigan) for help with the statistical analysis and Drs Sue Kenwrick (University of Cambridge, Department of Medical Genetics, Addenbrooke's Hospital, Cambridge, England), Thomas Brümmendorf and Fritz Rathjen (Max-Delbrück-Centrum für Molekulare Medizin, Berlin, Germany) for providing to us the full size cDNA clones encoding the H1 and H38 mutant L1-CAM proteins and oligonucleotides for the resequencing of both mutations.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Panicker A.K., Buhusi M., Thelen K., Maness P.F. Cellular signalling mechanisms of neural cell adhesion molecules. Front. Biosci. 2003;8:D900–D911. doi: 10.2741/1014. [DOI] [PubMed] [Google Scholar]
- 2.Maness P.F., Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat. Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- 3.Hortsch M., Nagaraj K., Godenschwege T.A. The interaction between L1-type proteins and ankyrins—a master switch for L1-type CAM function. Cell. Mol. Biol. Lett. 2009;14:57–69. doi: 10.2478/s11658-008-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hortsch M. Structural and functional evolution of the L1-family: Are four adhesion molecules better than one? Mol. Cell. Neurosci. 2000;15:1–10. doi: 10.1006/mcne.1999.0809. [DOI] [PubMed] [Google Scholar]
- 5.Doherty P., Walsh F.S. CAM-FGF receptor interactions: a model for axonal growth. Mol. Cell. Neurosci. 1996;8:99–111. doi: 10.1006/mcne.1996.0049. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Alonso L., Romani S., Jimenez F. The EGF and FGF receptors mediate neuroglian function to control growth cone decisions during sensory axon guidance in Drosophila. Neuron. 2000;28:741–752. doi: 10.1016/s0896-6273(00)00150-1. [DOI] [PubMed] [Google Scholar]
- 7.Kristiansen L.V., Velasquez E., Romani S., Baars S., Berezin V., Bock E., Hortsch M., Garcia-Alonso L. Genetic analysis of an overlapping functional requirement for L1- and NCAM-type proteins during sensory axon guidance in Drosophila. Mol. Cell. Neurosci. 2005;28:141–152. doi: 10.1016/j.mcn.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Bieber A.J., Snow P.M., Hortsch M., Patel N.H., Jacobs J.R., Traquina Z.R., Schilling J., Goodman C.S. Drosophila neuroglian: a member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell. 1989;59:447–460. doi: 10.1016/0092-8674(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 9.Kenwrick S., Watkins A., Angelis E.D. Neural cell recognition molecule L1: relating biological complexity to human disease mutations. Hum. Mol. Genet. 2000;9:879–886. doi: 10.1093/hmg/9.6.879. [DOI] [PubMed] [Google Scholar]
- 10.Fransen E., D'Hooge R., Van Camp G., Verhoye M., Sijbers J., Reyniers E., Soriano P., Kamiguchi H., Willemsen R., Koekkoek S.K., et al. L1 knockout mice show dilated ventricles, vermis hypoplasia and impaired exploration patterns. Hum. Mol. Genet. 1998;7:999–1009. doi: 10.1093/hmg/7.6.999. [DOI] [PubMed] [Google Scholar]
- 11.Djabali M., Mattei M.-G., Nguyen C., Roux D., Demengeot J., Denizot F., Moos M., Schachner M., Goridis C., Jordan B.R. The gene encoding L1, a neural adhesion molecule of the immunoglobulin family, is located on the X chromosome in mouse and man. Genomics. 1990;7:587–593. doi: 10.1016/0888-7543(90)90203-7. [DOI] [PubMed] [Google Scholar]
- 12.Jouet M., Rosenthal A., Armstrong G., MacFarlane J., Stevenson R., Paterson J., Metzenberg A., Ionasescu V., Temple K., Kenwrick S. X-linked spastic paraplegia (SPG1), MASA syndrome and X-linked hydrocephalus result from mutations in the L1 gene. Nat. Genet. 1994;7:402–407. doi: 10.1038/ng0794-402. [DOI] [PubMed] [Google Scholar]
- 13.Fransen E., Van Camp G., Vits L., Willems P.J. L1-associated diseases: clinical geneticists divide, molecular geneticists unite. Hum. Mol. Genet. 1997;6:1625–1632. doi: 10.1093/hmg/6.10.1625. [DOI] [PubMed] [Google Scholar]
- 14.Jouet M., Moncla A., Paterson J., McKeown C., Fryer A., Carpenter N., Holmberg E., Wadelius C., Kenwrick S. New domains of neural cell-adhesion molecule L1 implicated in X-linked hydrocephalus and MASA syndrome. Am. J. Hum. Genet. 1995;56:1304–1314. [PMC free article] [PubMed] [Google Scholar]
- 15.Fransen E., Van Camp G., D'Hooge R., Vits L., Willems P.J. Genotype-phenotype correlation in L1 associated diseases. J. Med. Genet. 1998;35:399–404. doi: 10.1136/jmg.35.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamasaki M., Thompson P., Lemmon V. CRASH syndrome: mutations in L1CAM correlate with severity of the disease. Neuropediatrics. 1997;28:175–178. doi: 10.1055/s-2007-973696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Angelis E., MacFarlane J., Du J.S., Yeo G., Hicks R., Rathjen F.G., Kenwrick S., Brümmendorf T. Pathological missense mutations of neural cell adhesion molecule L1 affect homophilic and heterophilic binding activities. EMBO J. 1999;18:4744–4753. doi: 10.1093/emboj/18.17.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Angelis E., Watkins A., Schäfer M., Brümmendorf T., Kenwrick S. Disease-associated mutations in L1 CAM interfere with ligand interactions and cell-surface expression. Hum. Mol. Genet. 2002;11:1–12. doi: 10.1093/hmg/11.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Cheng L., Lemmon V. Pathological missense mutations of neural cell adhesion molecule L1 affect neurite outgrowth and branching on an L1 substrate. Mol. Cell. Neurosci. 2004;27:522–530. doi: 10.1016/j.mcn.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Moulding H.D., Martuza R.L., Rabkin S.D. Clinical mutations in the L1 neural cell adhesion molecule affect cell-surface expression. J. Neurosci. 2000;20:5696–5702. doi: 10.1523/JNEUROSCI.20-15-05696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rünker A.E., Bartsch U., Nave K.A., Schachner M. The C264Y missense mutation in the extracellular domain of L1 impairs protein trafficking in vitro and in vivo. J. Neurosci. 2003;23:277–286. doi: 10.1523/JNEUROSCI.23-01-00277.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bateman A., Jouet M., MacFarlane J., Du J.S., Kenwrick S., Chothia C. Outline structure of the human L1 cell adhesion molecule and the sites where mutations cause neurological disorders. EMBO J. 1996;15:6050–6059. [PMC free article] [PubMed] [Google Scholar]
- 23.Islam R., Kristansen L.V., Romani S., Garcia-Alonso L., Hortsch M. Activation of EGF receptor kinase by L1-mediated homophilic cell interactions. Mol. Biol. Cell. 2004;15:2003–2012. doi: 10.1091/mbc.E03-05-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malhotra J.D., Tsiotra P., Karagogeos D., Hortsch M. Cis-activation of L1-mediated ankyrin recruitment by TAG-1 homophilic cell adhesion. J. Biol. Chem. 1998;273:33354–33359. doi: 10.1074/jbc.273.50.33354. [DOI] [PubMed] [Google Scholar]
- 25.Brand A.H., Dormand E.L. The GAL4 system as a tool for unravelling the mysteries of the Drosophila nervous system. Curr. Opin. Neurobiol. 1995;5:572–578. doi: 10.1016/0959-4388(95)80061-1. [DOI] [PubMed] [Google Scholar]
- 26.Karagogeos D. Neural GPI-anchored cell adhesion molecules. Front. Biosci. 2003;8:S1304–S1320. doi: 10.2741/1214. [DOI] [PubMed] [Google Scholar]
- 27.Fukamauchi F., Aihara O., Wang Y.J., Akasaka K., Takeda Y., Horie M., Kawano H., Sudo K., Asano M., Watanabe K., et al. TAG-1-deficient mice have marked elevation of adenosine A1 receptors in the hippocampus. Biochem. Biophys. Res. Commun. 2001;281:220–226. doi: 10.1006/bbrc.2001.4334. [DOI] [PubMed] [Google Scholar]
- 28.Poliak S., Salomon D., Elhanany H., Sabanay H., Kiernan B., Pevny L., Stewart C.L., Xu X., Chiu S.Y., Shrager P., et al. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J. Cell. Biol. 2003;162:1149–1160. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Traka M., Goutebroze L., Denisenko N., Bessa M., Nifli A., Havaki S., Iwakura Y., Fukamauchi F., Watanabe K., Soliven B., et al. Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J. Cell. Biol. 2003;162:1161–1172. doi: 10.1083/jcb.200305078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savvaki M., Panagiotaropoulos T., Stamatakis A., Sargiannidou I., Karatzioula P., Watanabe K., Stylianopoulou F., Karagogeos D., Kleopa K.A. Impairment of learning and memory in TAG-1 deficient mice associated with shorter CNS internodes and disrupted juxtaparanodes. Mol. Cell. Neurosci. 2008;39:478–490. doi: 10.1016/j.mcn.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 31.Berglund E.O., Murai K.K., Fredette B., Sekerkova G., Marturano B., Weber L., Mugnaini E., Ranscht B. Ataxia and abnormal cerebellar microorganization in mice with ablated contactin gene expression. Neuron. 1999;24:739–750. doi: 10.1016/s0896-6273(00)81126-5. [DOI] [PubMed] [Google Scholar]
- 32.Boyle M.E., Berglund E.O., Murai K.K., Weber L., Peles E., Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:385–397. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 33.Hortsch M., Margolis B. Septate and paranodal junctions: Kissing cousins. Trends Cell. Biol. 2003;13:557–561. doi: 10.1016/j.tcb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Faivre-Sarrailh C., Banerjee S., Li J., Hortsch M., Laval M., Bhat M.A. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development. 2004;131:4931–4942. doi: 10.1242/dev.01372. [DOI] [PubMed] [Google Scholar]
- 35.Davis J.Q., Lambert S., Bennett V. Molecular composition of the node of Ranvier: Identification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain-) and NrCAM at nodal axon segments. J. Cell. Biol. 1996;135:1355–1367. doi: 10.1083/jcb.135.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fryns J.P., Spaepen A., Cassiman J.J., van den Berghe H. X linked complicated spastic paraplegia, MASA syndrome, and X linked hydrocephalus owing to congenital stenosis of the aqueduct of Sylvius: variable expression of the same mutation at Xq28. J. Med. Genet. 1991;28:429–431. doi: 10.1136/jmg.28.6.429-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dahme M., Bartsch U., Martini R., Anliker B., Schachner M., Mantei N. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat. Genet. 1997;17:346–349. doi: 10.1038/ng1197-346. [DOI] [PubMed] [Google Scholar]
- 38.Itoh K., Cheng L., Kamei Y., Fushiki S., Kamiguchi H., Gutwein P., Stoeck A., Arnold B., Altevogt P., Lemmon V. Brain development in mice lacking L1-L1 homophilic adhesion. J. Cell. Biol. 2004;165:145–154. doi: 10.1083/jcb.200312107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen N.R., Taylor J.S.H., Scott L.B., Guillery R.W., Soriano P., Furley A.J.W. Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr. Biol. 1997;8:26–33. doi: 10.1016/s0960-9822(98)70017-x. [DOI] [PubMed] [Google Scholar]
- 40.Rolf B., Kutsche M., Bartsch U. Severe hydrocephalus in L1-deficient mice. Brain Res. 2001;891:247–252. doi: 10.1016/s0006-8993(00)03219-4. [DOI] [PubMed] [Google Scholar]
- 41.Castellani V., Chedotal A., Schachner M., Faivre-Sarrailh C., Rougon G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron. 2000;27:237–249. doi: 10.1016/s0896-6273(00)00033-7. [DOI] [PubMed] [Google Scholar]
- 42.Jakeman L.B., Chen Y., Lucin K.M., McTigue D.M. Mice lacking L1 cell adhesion molecule have deficits in locomotion and exhibit enhanced corticospinal tract sprouting following mild contusion injury to the spinal cord. Eur. J. Neurosci. 2006;23:1997–2011. doi: 10.1111/j.1460-9568.2006.04721.x. [DOI] [PubMed] [Google Scholar]
- 43.Demyanenko G.P., Shibata Y., Maness P.F. Altered distribution of dopaminergic neurons in the brain of L1 null mice. Brain Res. Dev. Brain Res. 2001;126:21–30. doi: 10.1016/s0165-3806(00)00129-2. [DOI] [PubMed] [Google Scholar]
- 44.Demyanenko G.P., Tsai A.Y., Maness P.F. Abnormalities in neuronal process extension, hippocampal development, and the ventricular system of L1 knockout mice. J. Neurosci. 1999;19:4907–4920. doi: 10.1523/JNEUROSCI.19-12-04907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demyanenko G.P., Maness P.F. The L1 cell adhesion molecule is essential for topographic mapping of retinal axons. J. Neurosci. 2003;23:530–538. doi: 10.1523/JNEUROSCI.23-02-00530.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tapanes-Castillo A., Weaver E.J., Smith R.P., Kamei Y., Caspary T., Hamilton-Nelson K.L., Slifer S.H., Martin E.R., Bixby J.L., Lemmon V.P. A modifier locus on chromosome 5 contributes to L1 cell adhesion molecule X-linked hydrocephalus in mice. Neurogenetics. 2009;10 doi: 10.1007/s10048-009-0203-3. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Threadgill D.W., Dlugosz A.A., Hansen L.A., Tennenbaum T., Lichti U., Yee D., LaMantia C., Mourton T., Herrup K., Harris R.C., et al. Targeted disruption of mouse EGF receptor: Effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 48.Sakurai T., Lustig M., Babiarz J., Furley A.J., Tait S., Brophy P.J., Brown S.A., Brown L.Y., Mason C.A., Grumet M. Overlapping functions of the cell adhesion molecules Nr-CAM and L1 in cerebellar granule cell development. J. Cell. Biol. 2001;154:1259–1273. doi: 10.1083/jcb.200104122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heyden A., Angenstein F., Sallaz M., Seidenbecher C., Montag D. Abnormal axonal guidance and brain anatomy in mouse mutants for the cell recognition molecules close homolog of L1 and NgCAM-related cell adhesion molecule. Neuroscience. 2008;155:221–233. doi: 10.1016/j.neuroscience.2008.04.080. [DOI] [PubMed] [Google Scholar]
- 50.Castellani V., De Angelis E., Kenwrick S., Rougon G. Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. EMBO J. 2002;21:6348–6357. doi: 10.1093/emboj/cdf645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bechara A., Nawabi H., Moret F., Yaron A., Weaver E., Bozon M., Abouzid K., Guan J.L., Tessier-Lavigne M., Lemmon V., et al. FAK-MAPK-dependent adhesion disassembly downstream of L1 contributes to semaphorin3A-induced collapse. EMBO J. 2008;27:1549–1562. doi: 10.1038/emboj.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taniguchi M., Yuasa S., Fujisawa H., Naruse I., Saga S., Mishina M., Yagi T. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron. 1997;19:519–530. doi: 10.1016/s0896-6273(00)80368-2. [DOI] [PubMed] [Google Scholar]
- 53.Behar O., Golden J.A., Mashimo H., Schoen F.J., Fishman M.C. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- 54.Hortsch M., Goodman C.S. Drosophila Fasciclin I, a neural cell adhesion molecule, has a phosphatidylinositol lipid membrane anchor that is developmentally regulated. J. Biol. Chem. 1990;265:15104–15109. [PubMed] [Google Scholar]
- 55.Hall S.G., Bieber A.J. Mutations in the Drosophila neuroglian cell adhesion molecule affect motor neuron pathfinding and peripheral nervous system patterning. J. Neurobiol. 1997;32:325–340. [PubMed] [Google Scholar]