Abstract
Duplication of human chromosome 22q11.2 is associated with elevated rates of mental retardation, autism and many other behavioral phenotypes. However, because duplications cover 1.5–6 Mb, the precise manner in which segments of 22q11.2 causally affect behavior is not known in humans. We have now determined the developmental impact of over-expression of an ∼190 kb segment of human 22q11.2, which includes the genes TXNRD2, COMT and ARVCF, on behaviors in bacterial artificial chromosome (BAC) transgenic (TG) mice. BAC TG mice and wild-type (WT) mice were tested for their cognitive capacities, affect- and stress-related behaviors and motor activity at 1 and 2 months of age. An enzymatic assay determined the impact of BAC over-expression on the activity level of COMT. BAC TG mice approached a rewarded goal faster (i.e. incentive learning), but were impaired in delayed rewarded alternation during development. In contrast, BAC TG and WT mice were indistinguishable in rewarded alternation without delays, spontaneous alternation, prepulse inhibition, social interaction, anxiety-, stress- and fear-related behaviors and motor activity. Compared with WT mice, BAC TG mice had an ∼2-fold higher level of COMT activity in the prefrontal cortex, striatum and hippocampus. These data suggest that over-expression of this 22q11.2 segment enhances incentive learning and impairs the prolonged maintenance of working memory, but has no apparent effect on working memory per se, affect- and stress-related behaviors or motor capacity. High copy numbers of this 22q11.2 segment might contribute to a highly selective set of phenotypes in learning and cognition during development.
INTRODUCTION
The human genome contains many types of variation, ranging from single-nucleotide polymorphisms (SNPs) in individual genes to duplication or deletion of full chromosomes. A surprisingly large number of intermediate structural variations in the human genome, including kilo- to mega-base copy number variations (CNVs), also exist and are associated with autism spectrum disorders and schizophrenia (1–5). However, the precise manner in which these CNVs cause or remain silent in phenotypic variation is poorly understood.
Human chromosome 22q11.2 is considered a hotspot of CNVs (6). Children and adolescents with 22q11.2 duplications consistently exhibit cognitive and intellectual impairments during development, and they are often diagnosed with mental retardation and autism (7–20). Moreover, CNV screenings in individuals with autism (2,3,21–23) and mental retardation (24,25) have identified duplications in 22q11.2. Unlike human association studies of SNPs, the association between 22q11.2 duplications and developmental cognitive impairments is remarkably consistent and replicable (18,20). Published studies, excluding meeting abstracts, collectively show that 97% of individuals with 22q11.2 duplications exhibit cognitive deficits (18). However, because the diagnosis of mental retardation and autism include variations in diverse cognitive and intellectual capacities, the precise nature of cognitive impairments caused by 22q11.2 duplication remains unclear.
Duplications of 22q11.2 encompass 1.5 Mb or larger regions, making it impossible to determine whether segments or single genes are responsible for phenotypes in humans. Although association studies with SNPs have suggested that single-gene alleles in 22q11.2 might contribute to behavioral phenotypes, conflicting evidence has been reported. COMT is one of the most extensively studied 22q11.2 genes related to cognitive function. Many studies have implicated a high-activity allele of COMT in cognitive defects in humans (26). Moreover, haplotype analyses have indicated that a set of SNPs of a 22q11.2 region that includes thioredoxin reductase 2 (TXNRD2), catechol-O-methyltransferase (COMT) and armadillo repeat gene deletes in velocardiofacial syndrome (ARVCF) are over-transmitted in individuals with schizophrenia, a disorder characterized by specific cognitive impairments (27,28). However, other reports have failed to confirm these associations (29–32).
To determine the impact of a CNV of a specific 22q11.2 region on precise behavioral phenotypes, we tested mice that over-express an ∼190 kb human chromosomal segment, including TXNRD2, COMT and ARVCF, in a battery of assays that evaluate cognitive functions, affect- and stress-related behaviors and motor function. Because individuals with 22q11.2 duplications begin to exhibit many behavioral phenotypes early during development, our behavioral analysis primarily focused on the developmental period from 1 to 2 months. When no phenotype was seen during this time, we then tested the mice at 5 months of age. These ages correspond to developmental milestones in mice. Although strain differences exist, mice exhibit signs of sexual maturation at 1 month of age, but the development of many biological processes continues until they reach mature adulthood at 3 months. The period from 3 to 6 months of age is considered mature adulthood for C57BL/6J mice (33,34). Our data show that when this chromosomal segment is over-expressed, phenotypes related to a specific type of learning and memory are observed during development.
RESULTS
Mice were tested in a battery of tasks that are designed to evaluate cognition, learning, memory, affect- and stress-related behaviors and motor activity, as described earlier (35). Cognition, learning and memory were evaluated using rewarded approach, rewarded alternation, spontaneous alternation and prepulse inhibition (PPI). The affect-related traits were examined using social interaction, anxiety-related behaviors in the elevated plus maze and in an inescapable open field, a fear response in an acoustic startle task, and an affect- and stress-related behavior in a tail suspension task. Motor function was examined using horizontal spontaneous locomotor activity in an inescapable open field.
Rewarded approach
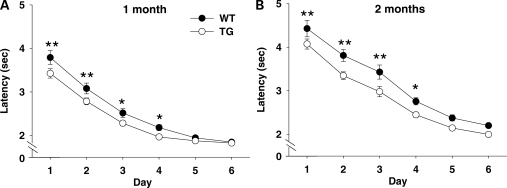
Mice gradually learned to reach a rewarded arm faster on an L-maze over time (Day, F5,3280 = 421.52, P < 0.01) and performed faster at the age of 1 month than at 2 months (Age, F1,656 = 89.14, P < 0.01) (Fig. 1). At both 1 and 2 months of age, bacterial artificial chromosome (BAC) transgenic (TG) mice exhibited a shorter latency to reach the goal than wild-type (WT) mice on early learning days than later learning days (Genotype, F1,656 = 21.99, P < 0.01; Genotype × Day, F5,3280 = 2.37, P < 0.05; Genotype × Age, F1,656 = 1.42, NS; Genotype × Age × Day, F5,3280 = 0.31, NS). Newman–Keuls post-hoc comparisons showed that BAC TG mice reached the goal faster on the first 4 days compared with WT mice.
Figure 1.
Rewarded approach at 1 month (A) and 2 months (B) of age. Time (means of square root values ± SEM) spent to reach a rewarded goal in the L-maze is shown. Because the homogeneity of variance was violated (Hartley's Fmax = 3469.68, P < 0.01), data were transformed and are presented as square root values. Asterisks indicate statistically significant differences from WT mice at 5% (*) and 1% (**) levels, as determined by Newman–Keuls comparisons. One month group: WT, n = 30; TG, n = 30. Two month group: WT, n = 24; TG, n = 26.
Rewarded alternation
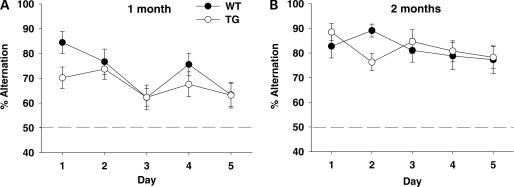
Given a choice between two arms following a visit to one of them during a preceding run, mice tend to visit a previously unvisited arm (i.e. rewarded alternation). Mice achieved better rewarded alternation at the age of 2 months than at 1 month (Age, F1,54 = 21.98, P < 0.01) and performed with higher percentages of alternation on earlier rather than later days (Day, F4,216 = 4.05, P < 0.01) (Fig. 2). WT and BAC TG mice performed rewarded alternation equally well at both 1 and 2 months of age (Genotype, F1,54 = 1.06, NS; Genotype × Age, F1,54 = 0.97, NS; Genotype × Age × Day, F4,216 = 1.53, NS).
Figure 2.
Rewarded alternation without delays. The percentage of correct choices is shown as means ± SEM. (A) One-month-old mice: WT, n = 15; TG, n = 19. (B) Two-month-old mice: WT, n = 11; TG, n = 13. The dashed line indicates 50%, i.e. chance.
Delayed rewarded alternation
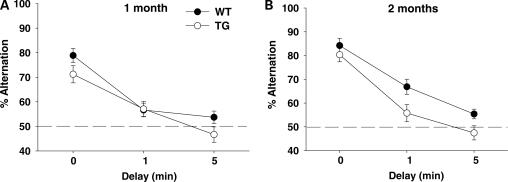
When delay intervals were imposed between the sampling run and the choice run in rewarded alternation, mice performed more poorly following longer delays (Delay, F2,304 = 87.87, P < 0.01) (Fig. 3). Two-month-old mice performed better than 1-month-old mice (Age, F1,152 = 6.55, P < 0.05). BAC TG mice performed more poorly than WT mice (Genotype, F1,152 = 13.69, P < 0.01).
Figure 3.
Rewarded alternation with delays. The percentage of correct choices is shown as means ± SEM. (A) One-month-old mice: WT, n = 15; TG, n = 11. (B) Two-month-old mice: WT, n = 13; TG, n = 13. The dashed line indicates 50%, i.e. chance.
Exploratory analyses of variance (ANOVAs) showed that BAC TG mice were impaired at 2 months (Genotype, F1,76 = 10.89, P < 0.01; Genotype × Delay, F2,152 = 0.69, NS), but not at 1 month of age (Genotype, F1,76 = 3.87, NS; Genotype × Delay, F2,152 = 1.12, NS). This impairment was due to a lack of developmental maturation of rewarded alternation; WT mice showed better performance at 2 months than at 1 month of age (Age, F1,82 = 6.39, P < 0.05), but such developmental maturation was absent in BAC TG mice (Age, F1,70 = 1.33, NS).
Spontaneous alternation
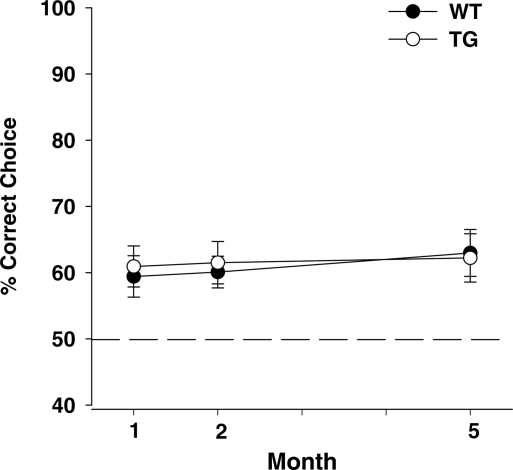
Spontaneous alternation is the innate tendency to visit a previously unvisited, rather than visited, arm, even without reward, in a T-maze. Genotype had no effect on choice accuracy (Genotype, F1,145 = 0.08, NS), and spontaneous alternation did not change during the developmental stages tested (Age, F2,145 = 0.29, NS) (Fig. 4).
Figure 4.
Spontaneous alternation. The percentage of correct choices (means ± SEM) is shown. One month: WT, n = 26; TG, n = 29. Two months: WT, n = 27; TG, n = 28. Five months: WT, n = 21; TG, n = 20. The dashed line indicates 50%, i.e. chance.
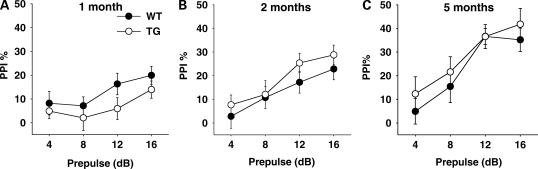
Prepulse inhibition
Mice showed higher levels of PPI at higher prepulse intensities (F3,474 = 46.40, P < 0.01), but genotype had no effect on PPI (F1,158 = 0.32, NS) (Fig. 5). Five-month-old mice exhibited considerably higher levels of PPI, compared with the other two age groups (Age, F2,158 = 7.39, P < 0.01; Age × Prepulse, F6,474 = 3.95, P < 0.01). No other effect was significant.
Figure 5.
Prepulse inhibition (PPI). The percentage of PPI (means ± SEM) is plotted against prepulse stimuli at 4, 8, 12 and 16 dB (69, 73, 77 and 81 dB presented with the 65 dB background noise. (A) One month: WT, n = 36; TG, n = 32. (B) Two months: WT, n = 27; TG, n = 28. (C) Five months: WT, n = 21, TG, n = 20.
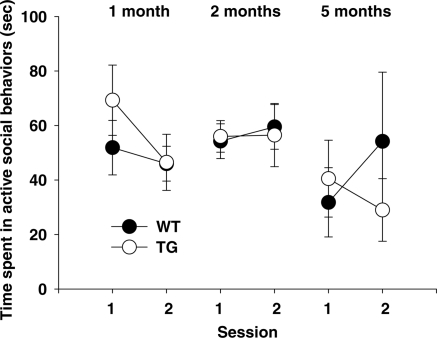
Social behavior
When a pair of non-littermate mice are placed into a home cage-like environment where neither is a resident, they engage in non-aggressive, affiliative social behaviors (35). Active affiliative social behaviors differed depending on age and session (Age × Session, F2,46 = 3.55, P < 0.01) (Fig. 6). Although the genotype alone did not affect active social interaction (F1,46 = 0.01, NS), it had a significant effect on this behavior together with the session (Genotype × Session, F1,46 = 6.64, P < 0.05). However, Newman–Keuls post hoc comparisons failed to detect a significant difference between WT and BAC TG mice at any session in any age group. No other single or interaction effect was significant.
Figure 6.
Active, affiliative social behaviors. Time (means ± SEM) spent in active social behavior is shown in two successive 5 min sessions. One month: WT, n = 12; TG, n = 12. Two months: WT, n = 8; TG, n = 8. Five months: WT, n = 6; TG, n = 6.
Mice exhibited very low levels of passive affiliative social interaction behaviors under our experimental conditions (data not shown). Neither genotype nor age had any effect on these behaviors (Genotype, F1,46 = 0.72, NS; Age, F2,46 = 0.50, NS). No other effect was significant.
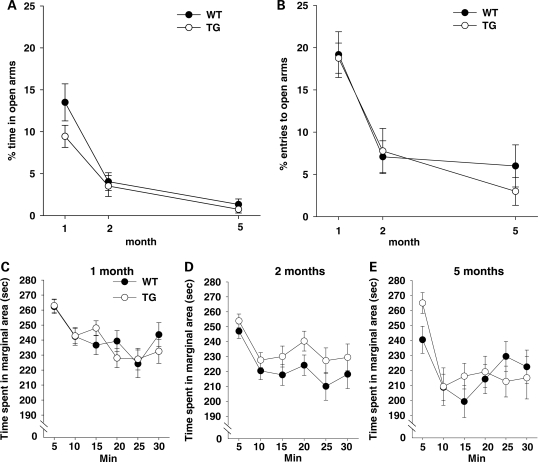
Elevated plus maze
Mice naturally avoid open arms in the elevated plus maze, which is thought to reflect anxiety, since it is attenuated by anxiolytic drugs (35). Mice avoided open arms in the elevated plus maze, as evidenced by <50% time spent in the open arms (Fig. 7A). Genotype had no effect on this anxiety-related behavior (Genotype, F1,113 = 2.17, NS), although this behavioral tendency was more pronounced at 2 and 5 months of age, compared with 1 month of age (Age, F2,113 = 29.62, P < 0.01).
Figure 7.
The relative amounts (means ± SEM) of (A) time spent in and (B) frequency of visits to the open arms versus both the open and closed arms of the elevated plus maze are shown. One month: WT, n = 20; TG, n = 26. Two months: WT, n = 21; TG, n = 23. Five months: WT, n = 14; TG, n = 15. (C–E) Thigmotaxis. Time (means ± SEM) spent in the marginal area is shown over a period of 30 min. (C) One month: WT, n = 25; TG, n = 25. (D) Two months: WT, n = 27; TG, n = 29. (E) Five months: WT, n = 21; TG, n = 21.
Similarly, when analyzed for the frequency of visits to the open arms (Fig. 7B), genotype had no effect on this behavior (Genotype, F1,111 = 0.59, NS), but age was a significant factor (F2,111 = 23.90, P < 0.01).
Thigmotaxis
Thigmotaxis, a tendency to remain in the vicinity of a wall in an inescapable open field, is thought to reflect anxiety, stress and fear (35,36). Mice tended to stay in the vicinity of the wall more during the first 5 min than at later time points (Time interval, F5,705 = 22.66, P < 0.01) (Fig. 7C–E). Thigmotaxis was not affected by genotype (F1,141 = 1.20, NS) and was more sustained at 1 and 2 months of age, compared with 5 months of age (Age, F2,141 = 6.55, P < 0.01; Age × Time interval, F10,705 = 2.12, P < 0.05).
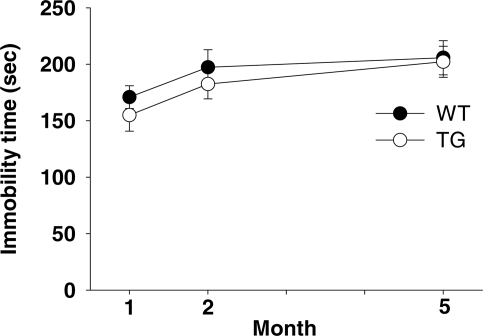
Tail suspension
Immobility during suspension by the tail is thought to reflect a basal affective state and a stress response (35,36). Genotype had no effect on immobility during this task (F1,83 = 0.85, NS), but mice tended to exhibit immobility longer at 2 and 5 months of age than at 1 month of age (F2,83 = 4.23, P < 0.05) (Fig. 8).
Figure 8.
Immobility during the tail suspension test. Time (means ± SEM) mice remained immobile is shown. One month: WT, n = 20; TG, n = 23. Two months: WT, n = 14; TG, n = 15. Five months: WT, n = 8; TG, n = 9.
Startle
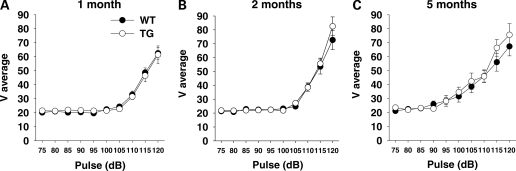
The sudden presentation of a loud acoustic stimulus causes a startle response. BAC TG and WT mice exhibited equally higher levels of startle in response to higher pulses (Genotype, F1,158 = 0.13, NS; Pulse, F9,1422 = 250.41, P < 0.01) (Fig. 9). One-month-old mice showed lower levels of startle at high levels of pulses than 2- and 5-month-old mice (Age, F2,158 = 4.72, P< 0.05; Age × Pulse, F18,1422 = 3.91, P < 0.01).
Figure 9.
Startle. (A–C) The magnitude of a startle response (V average, means ± SEM) is plotted against pulse stimuli (75–120 dB). (A) One month: WT, n = 31; TG, n = 30. (B) Two months: WT, n = 27; TG, n = 28. (C) Five months: WT, n = 21; TG, n = 20.
Spontaneous motor activity
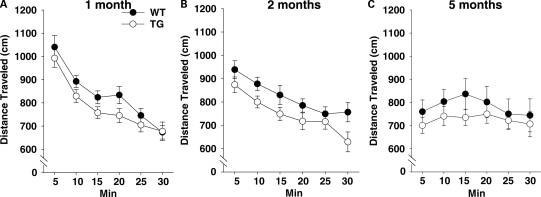
BAC TG mice exhibited lower levels of motor activity compared with WT mice (Genotype, F1,141 = 5.72, P < 0.05) (Fig. 10). Although 1- and 2-month-old mice gradually exhibited less locomotor activity within a session, such habituation was absent in 5-month-old mice, primarily due to lack of hyperactivity at the beginning of a session (Time interval, F5,705 = 35.30, P < 0.01; Age × Time interval, F10,705 = 9.61, P < 0.01). Despite the significant genotype effect, further exploratory ANOVAs failed to reveal a significant genotype effect at 1 month (Genotype, F1,48 = 2.65, NS), 2 months (Genotype, F1,54 = 3.68, NS) or 5 months of age (Genotype, F1,39 = 0.83, NS).
Figure 10.
Spontaneous locomotor activity in an inescapable open field. Horizontal distance traveled (means ± SEM) was measured for 30 min. Each tick on the x-axis represents a 5 min bin. (A) One month: WT, n = 25; TG, n = 25. (B) Two months: WT, n = 27; TG, n = 29. (C) Five months: WT, n = 21; TG, n = 21.
COMT activity
One or two copies of the BAC expressed in the brain were reflected in higher levels of COMT activity in various brain regions examined (Table 1). COMT activities were higher in BAC TG mice compared with WT mice (Genotype, F1,16 = 245.57, P < 0.01). Basal levels of COMT were higher in the prefrontal cortex compared with the striatum and hippocampus (Region, F2,32 = 187.54, P < 0.01). For each brain region examined, BAC TG mice exhibited ∼2-fold higher COMT activity than did WT mice.
Table 1.
COMT activity in various brain regions of WT and BAC TG mice
| Genotype | WT (n = 14) | TG (n = 4) |
|---|---|---|
| Prefrontal cortex | 39.32 (2.11) | 83.45 (1.45)* |
| Percentage | 100 | 212.2 |
| Striatum | 24.66 (0.95) | 49.26 (1.37)* |
| Percentage | 100 | 199.76 |
| Hippocampus | 20.26 (0.58) | 40.91 (1.08)* |
| Percentage | 100 | 201.92 |
Means (SEM) of COMT activity (activity/pmol/min/mg protein) in three brain regions. The number of age-matched mice used is indicated. WT, wild-type mice; TG, BAC transgenic mice.
*A statistically significant difference from WT mice, as determined by Newman–Keuls comparisons.
DISCUSSION
Our results demonstrate that over-expression of the ∼190 kb 22q11.2 human chromosomal region developmentally affects a select set of behaviors. BAC TG mice exhibited faster performance in rewarded approach, but were impaired in rewarded alternation with delay. These phenotypes are highly selective, since BAC TG mice did not exhibit any other phenotypes in rewarded alternation without delay, spontaneous alternation, PPI, social interaction, anxiety-, affect- or stress-related behaviors. Over-expression of the BAC resulted in a functional consequence in the brain, as evidenced by ∼2-fold higher levels of COMT enzymatic activity in the prefrontal cortex, striatum and hippocampus of BAC TG mice. We previously reported that over-expression of an adjacent ∼200 kb region of human chromosome 22q11.2, including Septin 5 (SEPT5), glycoprotein Ib (platelet), beta polypeptide (GP1BB), T-box 1 (TBX1) and guanine nucleotide-binding protein, beta polypeptide 1-like (GNB1L), caused antipsychotic-responsive behavioral sensitization in mice (37) and that Sept5 deficiency caused deficits in social interaction and rewarded approach in mice (35). Our data extend these findings by demonstrating that the ∼190 kb region with a different set of genes contributes to a unique set of behavioral phenotypes.
Compared with WT mice, BAC TG mice showed a shorter latency to reach the rewarded goal during an early training phase in rewarded approach. In this task, mice need to reach the baited goal arm in an L-maze. One critical element of this task is that the goal arm is pseudorandomly determined by the experimenter for each trial. Because the spatial memory for a previously visited arm provides no information as to which arm is baited next, and since the same arm is not baited more than twice in a row, this task minimizes spatial working and reference memory and maximally evaluates an approach behavior guided by local cues, represented by the only open arm regardless of its spatial location (i.e. incentive learning) (38). The faster approach in BAC TG mice does not seem to be due to improved motor capacity, because BAC TG mice and WT mice were indistinguishable in motor activity in an open field at any age tested (Fig. 10).
Because enhanced incentive learning was limited to an early training phase, some processes unique to the early phase of this learning might have been enhanced by over-expression of this 22q11.2 segment. Since COMT, a catalytic enzyme of dopamine, is encoded in the ∼190 kb BAC, increased COMT activity may have altered the early phase of dopamine transmission during rewarded behavior. In fact, how dopamine is released in the rodent brain depends on the amount of training. In rodents, when the delivery of reward is not fully expected, dopamine release occurs. When training progresses and the reward is predictable, such a response subsides (39,40). One potential explanation for the enhanced incentive learning is the postulated differential role of COMT on tonic and phasic dopamine release. It has been speculated that tonic and phasic dopamine release are terminated by COMT and the dopamine transporter, respectively, in the nucleus accumbens. According to this hypothesis, increased COMT activity is likely to amplify signaling initiated by reward-triggered phasic dopamine by removing the inhibitory action of tonically released dopamine on dopaminergic neurons (41).
Our findings provide insight into how the over-expressed chromosomal segment contributes to memory and cognitive functions. The absence of developmental improvement in delayed rewarded alternation is in contrast to normal rewarded alternation without delay and normal spontaneous alternation in BAC TG mice. Rewarded alternation and spontaneous alternation in the T-maze require working memory in which the animal temporarily retains information about a previously visited arm and subsequently chooses to visit the arm that does not match this arm. Our data suggest that BAC TG mice were selectively impaired in their cognitive capacity to retain working memory during delay.
The execution of rewarded alternation depends on multiple brain structures, including the prefrontal cortex, hippocampus and diencephalon, but the precise way each of the different structures contributes to elements of this task may not be identical (42). Over-expression of this 22q11.2 segment in the hippocampal system could influence the phenotype in delayed rewarded alternation. Damage to the hippocampal formation selectively impairs the delayed component of rewarded alternation and other working memory tasks in rodents, which is not seen with damage to the prefrontal cortex (43–48). Consistent with studies reporting that COMT mRNA is abundantly expressed in the human and rodent hippocampus (49–51), our data showed that COMT enzymatic activity also was present in the hippocampus. Although an ample body of evidence supports the role of COMT in the prefrontal cortex in cognition (26), our findings suggest the need for more studies to explore a possible link between COMT levels and hippocampal functions in prolonged retention of working memory.
There was no phenotype in short-term retention of working memory (Fig. 2), spontaneous alternation (Fig. 4), PPI (Fig. 5), social interaction (Fig. 6), anxiety-related behaviors in the elevated plus maze or thigmotaxis (Fig. 7), immobility in tail suspension (Fig. 8), startle (Fig. 9) or motor activity in an open field (Fig. 10) at any age tested. The absence of phenotypes in these tasks is unlikely to reflect a ceiling effect or a floor effect; performance showed further changes over days (Fig. 2), sessions (Figs 6, 7C and D and 10), age (Figs 7A and B and 8) and a range of prepulse (Fig. 5) and pulse (Fig. 9) intensities. Moreover, mice generally are capable of exhibiting much higher or lower levels of performance in all of these tasks (35). Although humans with 22q11.2 duplications variably exhibit affect-related phenotypes and delays in motor development (12,13,18), the chromosomal region we studied may not be responsible for these phenotypes. Alternatively, these phenotypes seen in humans with 22q11.2 duplication may not be well represented by the rodent tasks used in this study. It should be noted, however, that although not statistically significant at any age tested, there was an overall trend of lower motor activity in BAC TG mice. The three genes encoded in the BAC and other 22q11.2 genes may additively contribute to delays in motor development.
Our BAC TG mice over-express a human chromosomal segment that includes TXNRD2, COMT and ARVCF. Because these three genes are endogenously expressed in the developing mouse brain (51,52), behavioral phenotypes could reflect the impact of over-expression of any single human gene, more than one of the three human genes or an epistatic action among some or all of the three genes. Thus, our BAC TG mice cannot be directly compared with mice that over-express COMT alone (53). Moreover, our BAC TG mice exhibited an ∼2-fold increase in COMT activity in the brain, whereas COMT TG mice in the study by Papaleo et al. (53) showed an ∼16% increase in the levels of enzymatic activity in the frontal cortex, compared with WT mice. Duplication cases are expected to be associated with much higher levels of COMT activity compared with the ∼38% increase in enzymatic activity associated with a COMT Val allele versus Met allele in the human brain (54). In addition, adult COMT TG mice were tested at 3–7 months of age in the Papaleo et al. study (53), whereas our BAC TG mice were tested during development at 1 and 2 months of age, as well as during adulthood at 5 months of age.
Nevertheless, the differences and similarities between the phenotypes exhibited by our BAC TG mice and the COMT TG mice are interesting. Our BAC TG mice and the COMT TG mice were similarly impaired in the percentage of correct choices in discrete-trial rewarded alternation with delay. Moreover, COMT TG mice were impaired in only one version of an attentional shift task where the task difficulty is highest (53). High activity levels of COMT may exert a detrimental effect on the cognitive capacity when demand is high. More studies are needed to test whether COMT is involved in such a difficulty-specific deficit in many cognitive tasks.
COMT TG mice were reported to be impaired in rewarded alternation without delay (53), but our BAC TG mice were normal in this version of rewarded alternation. However, in the study of Papaleo et al. (53), data were shown as the number of days needed to reach a correct choice 80% of the time, and COMT TG mice needed more days to reach this criterion than did WT mice. Our data are consistent with their observation in that WT mice reached this criterion, but BAC TG mice never did at 1 month of age (Fig. 2A). COMT levels may affect the amount of training needed to reach a certain criterion more than the choice accuracy of working memory during development.
The apparent absence of PPI deficits in BAC TG mice is consistent with mouse studies reporting that the COMT TG mice, as well as Comt KO mice, were normal in PPI at 3–7 months (53,55). Our findings extend these studies by demonstrating that elevated levels of the three 22q11.2 genes, including COMT, are not associated with any PPI phenotype during development, as well as in adulthood. No study to date has reported analysis of PPI in humans with 22q11.2 duplication. If humans with 22q11.2 duplications exhibited a PPI phenotype, then over-expression of other 22q11.2 genes is likely to contribute to such a phenotype.
Although COMT TG mice exhibit less acoustic startle than WT controls (53), our BAC TG mice were normal in this fear-related behavior. Likewise, COMT TG mice exhibit lower levels of anxiety-like behavior compared with WT mice, whereas our BAC TG mice were normal in this measure. Because individual genes encoded in 22q11.2 may produce conflicting phenotypic elements, and the final phenotype is likely determined by complex interactions among them in large deletion/duplication cases (35), such hidden phenotypic elements could account for the apparent inconsistency in phenotypes associated with large chromosomal deletions/duplications and the functional variation of individual genes. Another possibility is that the genetic background influenced phenotypic expression. Our BAC TG mice were a congenic line with the genetic background of C57BL/6J mice. The COMT TG and WT mice were on a mixed genetic background of C57BL/6J and CD-1 (53). The flanking loci of the COMT transgene are probably not balanced between COMT TG and WT mice: more C57BL/6J than CD-1 alleles are likely present in COMT TG mice, and the opposite is likely to be true in WT controls. We recently reported that the phenotypic expression of mice deficient for Sept5, another 22q11.2 gene, depends on the genetic background (35). The phenotypic differences seen in the COMT TG mice and WT mice also might reflect this factor; the COMT TG mice may have shown a lower level of anxiety-related behavior than did WT mice (53) because C57BL/6 mice exhibit a lower level of anxiety compared with CD-1 mice (56). Consistent with this possibility, congenic Comt KO, HT and WT mice are indistinguishable in the percentage of time spent on open arms of the elevated plus maze (53).
Individuals with 22q11.2 duplications exhibit developmental cognitive impairments, including mental retardation, learning disabilities and autism (7–20). Moreover, when individuals with autism or mental retardation are screened for CNVs, 22q11.2 duplications encompassing the ∼190 kb region are found (2,21,23). Although there is no rodent model to globally recapitulate these neuropsychiatric disorders, our findings provide a translational element of cognitive functions that can be objectively tested in individuals with 22q11.2 duplication. Humans with 22q11.2 duplication would be expected to exhibit heightened incentive learning but impairments in working memory with delays.
There are a number of chromosomal abnormalities that are associated with a set of symptomatic elements of mental retardation and autism spectrum disorders, but each chromosomal abnormality also causes a separate set of specific symptomatic elements (57). When viewed from the impact of genes on specific cognitive impairments, the boundary between DSM-defined neuropsychiatric disorders blurs. Our data show that over-expression of the ∼190 kb segment of 22q11.2 affects prolonged retention of working memory, but working memory is non-specifically affected in many clinically defined neuropsychiatric disorders, including mental retardation, autism and schizophrenia. A major future challenge will be to critically evaluate how diverse symptomatic elements constitute, or even causally relate to, the clinically classified disease entities.
MATERIALS AND METHODS
Mice
The circular BAC DNA (BAC467.8), containing TXNRD2, COMT and ARVCF, was injected into the pronucleus of fertilized FVB zygotes. The TG mice expressed one to two copies of the BAC. The FVB inbred mice are homozygous for Pde6brd1, a recessive gene for retinal degeneration (58) that affects vision-guided behavior, learning and memory (59–61). Moreover, FVB mice are impaired in motor performance and learning (62). To avoid these non-specific effects of the genetic background, BAC TG mice were backcrossed to C57BL/6J mice for 10 generations. The genotypes of the mice were determined using tail tissues at the age of 7–10 days. We used two sets of PCR primers for genotyping: (i) AGG AAG ACC ATG TCC AGT GTG and TGT ATG GGT GTG TAA AGA TGG C and (ii) AAT GCT CAT CCG GAG TTC C and ACT GGT GAA ACT CAC CCA GG.
Animal handling and use followed a protocol approved by the Animal Care and Use Committee of Albert Einstein College of Medicine, in accordance with NIH guidelines. Mice were maintained under a 14 h light:10 h dark cycle and were tested during the light phase. Mice were maintained in a group of up to five in a cage and were given access to food and water ad libitum, unless otherwise specified. Male congenic BAC TG mice and their WT littermates were tested at the ages of 1 and 2 months, unless otherwise specified. If no phenotype was seen at these two age points, an additional group of 5-month-old mice was tested.
Behavioral analysis
Rewarded approach
Male BAC TG and WT littermates were tested at the ages of 1 or 2 months. Food deprivation started 2 days prior to testing. Food deprivation equally reduced body weights to 88–93% of baseline in WT and BAC TG mice during the rewarded approach and subsequent rewarded alternation (Day, F12,648 = 55.20, P < 0.01; Genotype, F1,54 = 0.25, NS; Age, F1,54 = 0.11, NS; Genotype × Day, F12,648 = 0.41, NS; Genotype × Age × Day, F12,648 = 1.62, NS).
The apparatus and procedure have been previously reported (35). Briefly, following habituation to the apparatus, individual mice were allowed to run from the start compartment of the T-maze to one goal arm while the other goal arm was blocked by a door (i.e. L-maze). The experimenter pseudorandomly designated one of the two arms as the goal, 50% of the cases for each day; the same arm was not chosen as the goal more than twice in a row. Because the previously visited arm provides no information, this procedure does not require spatial working or reference memory. Because the open goal arm served as a local cue that signaled the availability of reward, this task tests the animal's ability to reach the cued goal (i.e. incentive learning) (38). Six trials of up to 10 min were given per day for 6 days, with the inter-trial interval set within 10 min. The reward was sweetened, condensed milk (diluted 50/50 with water) placed in the food well at the end of the arm. Immediately after consuming the reward, mice were picked up from the goal compartment. Latency to the first lick of milk was recorded and used for analysis.
Rewarded alternation
After completion of the 6 day training in the rewarded approach, mice were trained for rewarded alternation on six trials per day for 5 days. We used the apparatus and the procedure described previously (35). Each discrete trial included a sampling run and a choice run, and six trials were given per day. The inter-trial interval was set within 10 min. During the sampling run for each mouse, the experimenter baited both goal arms, but pseudorandomly chose one arm as the only open arm and blocked the other arm with a closed door. The selection of the open arm differed for each mouse and day. Care was taken so that equal numbers of left and right goals were chosen as the goal for each day. Upon entering the goal arm, the mouse was left there for ∼11 s to consume the milk; it was then brought back to the start compartment. Both goal arms were opened 5 s later, and the choice run started. The previously unvisited arm was baited, and the previously visited arm was not. If a mouse did not enter the goal during the sampling or choice run within 2 min, the mouse was returned to the home cage and such trials were not used for analysis. The number of total arm visits and correct and incorrect visits were recorded and analyzed.
Delayed rewarded alternation
Mice were tested in this task with no delay, a 1 min delay and a 5 min delay imposed between the sampling run and the choice run. For each delay, mice were given six trials per day over a period of 3 days with the inter-trial interval set within 10 min. After completing testing with no delay, they were given testing with a 1 min delay and then a 5 min delay. Mice were returned to their home cages during delays. The delay was timed from the time the animal entered the goal arm, and the door was lowered to the time when animals were picked up from the home cages and placed in the start compartment. Otherwise, the procedure was identical to that of the rewarded alternation without delay, as described earlier.
Throughout delayed rewarded alternation, food deprivation significantly and equally reduced and maintained body weights of WT and BAC TG mice at 85–94% of their free feeding body weights at the ages of 1 and 2 months (Day, F16,768 = 54.49, P < 0.01; Genotype, F1,48 = 0.02, NS; Age, F1,48 = 0.04, NS; Genotype × Day, F16,768 = 0.85, NS; Genotype × Age × Day, F16,768 = 0.37, NS).
Spontaneous alternation
The apparatus used for rewarded alternation was used. Mice were placed in the start compartment facing away from the goal arms and confined there for 5 s before the door was opened. Both goal arms were open. Once mice chose and entered one of the goal arms, a door was gently lowered to confine mice in that arm, and 30 s later, they were removed from the arm. The mice were brought back to the start compartment, and the door was opened 5 s later for the next trial. They were allowed to choose either the goal arm just visited or the opposite goal arm. A total of 10 trials were given. If a mouse did not enter the goal during the first trial within 2 min, the mouse was returned to the start compartment and left there for 30 s before resuming a new trial. If a mouse did not enter the goal arms within 2 min during the subsequent trials, it was placed in the correctly alternated arm and confined there for 30 s. Such trials were not used for analysis. The percentage of alternated choices was used for analysis.
PPI and startle
Mice were tested for startle response and PPI in a startle chamber (S-R LAB, San Diego Instruments, San Diego, CA, USA). We used the procedure described previously (35). The amount of PPI was calculated as a percentage score for a prepulse trial: PPI% = 100 − [[(startle response for Prepulse + Pulse)/(startle response for Pulse-alone)] × 100]. Startle was elicited by pulses ranging from 75 to 120 dB, and startle magnitude was calculated as the average response to all of the Pulse-alone trials, excluding the first and last blocks of five Pulse-alone trials. PPI% and startle magnitude were analyzed.
Social interaction
Our experimental protocol was designed to optimally elicit affiliative social interaction and minimize aggressive behaviors (35). Non-littermate male WT and BAC TG pairs were tested. Each mouse was individually placed in a new home cage in the experimental room for 30 min before a pair was placed in a new, third home cage in two 5 min sessions, with a 30 min interval. Social interaction included active forms (i.e. tail rattle, bites, kicks, sideway offense, boxing, wrestling, mounting, pursuit, olfactory investigation and allogroom) and passive forms (i.e. escape, leap, side-by-side position and submissive posture). Time spent in active and passive social behaviors was rated by observers blinded to genotype (inter-rater correlation coefficient = 0.89, P < 0.0001) and was analyzed separately.
Elevated plus maze
The apparatus and procedure were identical to those described in our previous studies (35,36). Mice were brought into a room adjacent to the testing room at least 1 h before testing began. Individual mice were placed on the center platform facing one of the open arms and tested for 5 min. Behavior was videotaped from an adjacent room through an observation window. An observer blinded to the genotype of the mice rated the behavior. The percentage of (i) time spent in and (ii) entries into the open arms with respect to the total time spent in and the number of entries into the open and closed arms, respectively, was analyzed.
Tail suspension
The apparatus and the procedure were described previously (35,36). Briefly, mice were suspended for 6 min by the tail from a metal bar 30 cm above the platform. Behaviors were recorded by a video camera, and a rater blinded to genotype recorded the length of time each mouse remained immobile.
Locomotor activity
Spontaneous locomotor activity was tested in four sets of open-field apparatus (Truscan, Coulbourn Instruments, PA, USA) (35,36). Horizontal activity was recorded for 30 min. Distance traveled (cm) was used as an index of horizontal locomotor activity. Time (s) spent in the marginal area along the walls was used as an index of thigmotaxis.
COMT activity assay
Two- to 3-month-old mice were used for this analysis. COMT activity was determined by high-performance liquid chromatography with electrochemical detection using our standard method with a slight modification (63,64). The enzyme preparation was incubated at 37°C in 100 mm phosphate buffer (pH 7.4) containing 5 mm MgCl2, 200 mm S-adenosyl-l-methionine and 500 mm 3,4-dihydroxy benzoic acid (Sigma, St Louis, MO, USA). The reaction products, vanillic and isovanillic acid, were analyzed with high-performance liquid chromatography with electrochemical detection. The system consisted of a sample autoinjector (Jasco AS-2057, Tokyo, Japan), a pump (Merck Hitachi LaChrom L-7100, Tokyo, Japan) an RP-18 column (3 mm, 4.6 × 150 mm2; Waters Spherisorb, Milford, MA, USA), a coulometric detector (ESA Coulochem model 5100A detector and a model 5014B cell; ESA Inc., Chelmsford, MA, USA; detector potential −0.30 mV) and an integrator (Shimadzu C-R5A; Shimadzu Corporation, Kyoto, Japan). The mobile phase was 0.1 m Na2HPO4 (pH 3.3), 0.15 mm EDTA and 25% methanol with a flow rate of 0.8 ml/min. The protein concentration of the samples was determined with the Pierce protein assay kit, which is based on the bicinchoninic acid method (Pierce, Rockford, IL, USA). Specific activity of COMT is expressed as picomoles vanillic and isovanillic acid formed in 1 min per microgram of protein in the sample.
Statistical analysis
All data are presented as the mean ± SEM. Statistical significance was determined by ANOVA followed by Newman–Keuls post hoc comparisons. The minimum level of significance was set at 5%.
FUNDING
This work was supported by a Maltz NARSAD Independent Investigator Award and the National Institutes of Health grant (HD05311) to N.H.; the State of New York grant to S.A. and the Helsinki University Research funds, the Academy of Finland (210758) and Sigrid Juselius Foundation to P.T.M.
ACKNOWLEDGEMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the NIH. We thank Dr Daniel Weinberger of the NIMH for his insightful comments on an early draft of this paper.
Conflict of Interest statement. N.H. served as a consultant for IntraCellular Therapies, Inc. in 2008. M.A.G. holds an equity interest in San Diego Instruments, Inc.
REFERENCES
- 1.Sebat J., Lakshmi B., Malhotra D., Troge J., Lese-Martin C., Walsh T., Yamrom B., Yoon S., Krasnitz A., Kendall J., et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall C.R., Noor A., Vincent J.B., Lionel A.C., Feuk L., Skaug J., Shago M., Moessner R., Pinto D., Ren Y., et al. Structural variation of chromosomes in autism spectrum disorder. Am. J. Hum. Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christian S.L., Brune C.W., Sudi J., Kumar R.A., Liu S., Karamohamed S., Badner J.A., Matsui S., Conroy J., McQuaid D., et al. Novel submicroscopic chromosomal abnormalities detected in autism spectrum disorder. Biol. Psychiatry. 2008;63:1111–1117. doi: 10.1016/j.biopsych.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stefansson H., Rujescu D., Cichon S., Pietilainen O.P., Ingason A., Steinberg S., Fossdal R., Sigurdsson E., Sigmundsson T., Buizer-Voskamp J.E., et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–236. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagnamenta A.T., Wing K., Akha E.S., Knight S.J., Bolte S., Schmotzer G., Duketis E., Poustka F., Klauck S.M., Poustka A., et al. A 15q13.3 microdeletion segregating with autism. Eur. J. Hum. Genet. 2008 doi: 10.1038/ejhg.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korbel J.O., Urban A.E., Affourtit J.P., Godwin B., Grubert F., Simons J.F., Kim P.M., Palejev D., Carriero N.J., Du L., et al. Paired-end mapping reveals extensive structural variation in the human genome. Science. 2007;318:420–426. doi: 10.1126/science.1149504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelmann L., Pandita R.K., Spiteri E., Funke B., Goldberg R., Palanisamy N., Chaganti R.S., Magenis E., Shprintzen R.J., Morrow B.E. A common molecular basis for rearrangement disorders on chromosome 22q11. Hum. Mol. Genet. 1999;8:1157–1167. doi: 10.1093/hmg/8.7.1157. [DOI] [PubMed] [Google Scholar]
- 8.Ensenauer R.E., Adeyinka A., Flynn H.C., Michels V.V., Lindor N.M., Dawson D.B., Thorland E.C., Lorentz C.P., Goldstein J.L., McDonald M.T., et al. Microduplication 22q11.2, an emerging syndrome: clinical, cytogenetic, and molecular analysis of thirteen patients. Am. J. Hum. Genet. 2003;73:1027–1040. doi: 10.1086/378818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassed S.J., Hopcus-Niccum D., Zhang L., Li S., Mulvihill J.J. A new genomic duplication syndrome complementary to the velocardiofacial (22q11 deletion) syndrome. Clin. Genet. 2004;65:400–404. doi: 10.1111/j.0009-9163.2004.0212.x. [DOI] [PubMed] [Google Scholar]
- 10.Yobb T.M., Somerville M.J., Willatt L., Firth H.V., Harrison K., MacKenzie J., Gallo N., Morrow B.E., Shaffer L.G., Babcock M., et al. Microduplication and triplication of 22q11.2: a highly variable syndrome. Am. J. Hum. Genet. 2005;76:865–876. doi: 10.1086/429841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portnoi M.F., Lebas F., Gruchy N., Ardalan A., Biran-Mucignat V., Malan V., Finkel L., Roger G., Ducrocq S., Gold F., et al. 22q11.2 duplication syndrome: two new familial cases with some overlapping features with DiGeorge/velocardiofacial syndromes. Am. J. Med. Genet. A. 2005;137:47–51. doi: 10.1002/ajmg.a.30847. [DOI] [PubMed] [Google Scholar]
- 12.Brunet A., Gabau E., Perich R.M., Valdesoiro L., Brun C., Caballin M.R., Guitart M. Microdeletion and microduplication 22q11.2 screening in 295 patients with clinical features of DiGeorge/velocardiofacial syndrome. Am. J. Med. Genet. A. 2006;140:2426–2432. doi: 10.1002/ajmg.a.31499. [DOI] [PubMed] [Google Scholar]
- 13.Alberti A., Romano C., Falco M., Cali F., Schinocca P., Galesi O., Spalletta A., Di B.D., Fichera M. 1.5 Mb de novo 22q11.21 microduplication in a patient with cognitive deficits and dysmorphic facial features. Clin. Genet. 2007;71:177–182. doi: 10.1111/j.1399-0004.2007.00750.x. [DOI] [PubMed] [Google Scholar]
- 14.Mukaddes N.M., Herguner S. Autistic disorder and 22q11.2 duplication. World J. Biol. Psychiatry. 2007;8:127–130. doi: 10.1080/15622970601026701. [DOI] [PubMed] [Google Scholar]
- 15.Descartes M., Franklin J., de Stahl T.D., Piotrowski A., Bruder C.E., Dumanski J.P., Carroll A.J., Mikhail F.M. Distal 22q11.2 microduplication encompassing the BCR gene. Am. J. Med. Genet. A. 2008;146A:3075–3081. doi: 10.1002/ajmg.a.32572. [DOI] [PubMed] [Google Scholar]
- 16.Ou Z., Berg J.S., Yonath H., Enciso V.B., Miller D.T., Picker J., Lenzi T., Keegan C.E., Sutton V.R., Belmont J., et al. Microduplications of 22q11.2 are frequently inherited and are associated with variable phenotypes. Genet. Med. 2008;10:267–277. doi: 10.1097/GIM.0b013e31816b64c2. [DOI] [PubMed] [Google Scholar]
- 17.Ramelli G.P., Silacci C., Ferrarini A., Cattaneo C., Visconti P., Pescia G. Microduplication 22q11.2 in a child with autism spectrum disorder: clinical and genetic study. Dev. Med. Child Neurol. 2008;50:953–955. doi: 10.1111/j.1469-8749.2008.03048.x. [DOI] [PubMed] [Google Scholar]
- 18.Wentzel C., Fernstrom M., Ohrner Y., Anneren G., Thuresson A.C. Clinical variability of the 22q11.2 duplication syndrome. Eur. J. Med. Genet. 2008;51:501–510. doi: 10.1016/j.ejmg.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Yu S., Cox K., Friend K., Smith S., Buchheim R., Bain S., Liebelt J., Thompson E., Bratkovic D. Familial 22q11.2 duplication: a three-generation family with a 3-Mb duplication and a familial 1.5-Mb duplication. Clin. Genet. 2008;73:160–164. doi: 10.1111/j.1399-0004.2007.00938.x. [DOI] [PubMed] [Google Scholar]
- 20.Courtens W., Schramme I., Laridon A. Microduplication 22q11.2: a benign polymorphism or a syndrome with a very large clinical variability and reduced penetrance? Report of two families. Am. J. Med. Genet. A. 2008;146A:758–763. doi: 10.1002/ajmg.a.31910. [DOI] [PubMed] [Google Scholar]
- 21.The Autism Genome Project Consortium. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat. Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang K., Chen Z., Tadesse M.G., Glessner J., Grant S.F., Hakonarson H., Bucan M., Li M. Modeling genetic inheritance of copy number variations. Nucleic Acids Res. 2008;36:e138. doi: 10.1093/nar/gkn641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai G., Edelmann L., Goldsmith J.E., Cohen N., Nakamine A., Reichert J.G., Hoffman E.J., Zurawiecki D.M., Silverman J.M., Hollander E., et al. Multiplex ligation-dependent probe amplification for genetic screening in autism spectrum disorders: efficient identification of known microduplications and identification of a novel microduplication in ASMT. BMC Med. Genomics. 2008;1:50. doi: 10.1186/1755-8794-1-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menten B., Maas N., Thienpont B., Buysse K., Vandesompele J., Melotte C., de Ravel T., Van V.S., Balikova I., Backx L., et al. Emerging patterns of cryptic chromosomal imbalance in patients with idiopathic mental retardation and multiple congenital anomalies: a new series of 140 patients and review of published reports. J. Med. Genet. 2006;43:625–633. doi: 10.1136/jmg.2005.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engels H., Brockschmidt A., Hoischen A., Landwehr C., Bosse K., Walldorf C., Toedt G., Radlwimmer B., Propping P., Lichter P., Weber R.G. DNA microarray analysis identifies candidate regions and genes in unexplained mental retardation. Neurology. 2007;68:743–750. doi: 10.1212/01.wnl.0000256367.70365.e0. [DOI] [PubMed] [Google Scholar]
- 26.Tunbridge E.M., Harrison P.J., Weinberger D.R. Catechol-O-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol. Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Sanders A.R., Rusu I., Duan J., Vander Molen J.E., Hou C., Schwab S.G., Wildenauer D.B., Martinez M., Gejman P.V. Haplotypic association spanning the 22q11.21 genes COMT and ARVCF with schizophrenia. Mol. Psychiatry. 2005;10:353–365. doi: 10.1038/sj.mp.4001586. [DOI] [PubMed] [Google Scholar]
- 28.Mas S., Bernardo M., Parellada E., Garcia-Rizo C., Gasso P., Alvarez S., Lafuente A. ARVCF single marker and haplotypic association with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:1064–1069. doi: 10.1016/j.pnpbp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Ho B.C., Wassink T.H., O'Leary D.S., Sheffield V.C., Andreasen N.C. Catechol-O-methyl transferase Val158Met gene polymorphism in schizophrenia: working memory, frontal lobe MRI morphology and frontal cerebral blood flow. Mol. Psychiatry. 2005;10:229. doi: 10.1038/sj.mp.4001616. 287–298. [DOI] [PubMed] [Google Scholar]
- 30.Stefanis N.C., Van O.J., Avramopoulos D., Smyrnis N., Evdokimidis I., Stefanis C.N. Effect of COMT Val158Met polymorphism on the Continuous Performance Test, Identical Pairs Version: tuning rather than improving performance. Am. J. Psychiatry. 2005;162:1752–1754. doi: 10.1176/appi.ajp.162.9.1752. [DOI] [PubMed] [Google Scholar]
- 31.Tsai S.J., Yu Y.W., Chen T.J., Chen J.Y., Liou Y.J., Chen M.C., Hong C.J. Association study of a functional catechol-O-methyltransferase-gene polymorphism and cognitive function in healthy females. Neurosci. Lett. 2003;338:123–126. doi: 10.1016/s0304-3940(02)01396-4. [DOI] [PubMed] [Google Scholar]
- 32.Sanders A.R., Duan J., Levinson D.F., Shi J., He D., Hou C., Burrell G.J., Rice J.P., Nertney D.A., Olincy A., et al. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. Am. J. Psychiatry. 2008;165:497–506. doi: 10.1176/appi.ajp.2007.07101573. [DOI] [PubMed] [Google Scholar]
- 33.Bronson F.H., Dagg C.P., Snell G.D. In: Biology of the Laboratory Mouse. Green E.L., editor. New York: Dover Publications, Inc.; 2007. online publication. [Google Scholar]
- 34.Flurkey K., Currrer J.M., Harrison D.E. In: The Mouse in Biomedical Research. Fox J.G., editor. Vol. 3. Burlington, MA: Elsevier; 2007. pp. 637–672. [Google Scholar]
- 35.Suzuki G., Harper K.M., Hiramoto T., Sawamura T., Lee M., Kang G., Tanigaki K., Buell M., Geyer M.A., Trimble W.S., et al. Sept5 deficiency exerts pleiotropic influence on affective behaviors and cognitive functions in mice. Hum. Mol. Genet. 2009;18:1652–1660. doi: 10.1093/hmg/ddp086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu H., Lee M., Agatsuma S., Hiroi N. Pleiotropic impact of constitutive fosB inactivation on nicotine-induced behavioral alterations and stress-related traits in mice. Hum. Mol. Genet. 2007;16:820–836. doi: 10.1093/hmg/ddm027. [DOI] [PubMed] [Google Scholar]
- 37.Hiroi N., Zhu H., Lee M., Funke B., Arai M., Itokawa M., Kucherlapati R., Morrow B., Sawamura T., Agatsuma S. A 200-kb region of human chromosome 22q11.2 confers antipsychotic-responsive behavioral abnormalities in mice. Proc. Natl Acad. Sci. USA. 2005;102:19132–19137. doi: 10.1073/pnas.0509635102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crespi L.P. Quantitative variation of incentive and performance in the white rat. Am. J. Psychol. 1942;55:467–517. [Google Scholar]
- 39.Richardson N.R., Gratton A. Behavior-relevant changes in nucleus accumbens dopamine transmission elicited by food reinforcement: an electrochemical study in rat. J. Neurosci. 1996;16:8160–8169. doi: 10.1523/JNEUROSCI.16-24-08160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson N.R., Gratton A. Changes in medial prefrontal cortical dopamine levels associated with response-contingent food reward: an electrochemical study in rat. J. Neurosci. 1998;18:9130–9138. doi: 10.1523/JNEUROSCI.18-21-09130.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bilder R.M., Volavka J., Lachman H.M., Grace A.A. The catechol-O-methyltransferase polymorphism: relations to the tonic–phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- 42.Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci. Biobehav. Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- 43.Aggleton J.P., Neave N., Nagle S., Sahgal A. A comparison of the effects of medial prefrontal, cingulate cortex, and cingulum bundle lesions on tests of spatial memory: evidence of a double dissociation between frontal and cingulum bundle contributions. J. Neurosci. 1995;15:7270–7281. doi: 10.1523/JNEUROSCI.15-11-07270.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chudasama Y., Muir J.L. A behavioural analysis of the delayed non-matching to position task: the effects of scopolamine, lesions of the fornix and of the prelimbic region on mediating behaviours by rats. Psychopharmacology (Berl) 1997;134:73–82. doi: 10.1007/s002130050427. [DOI] [PubMed] [Google Scholar]
- 45.Mair R.G., Burk J.A., Porter M.C. Lesions of the frontal cortex, hippocampus, and intralaminar thalamic nuclei have distinct effects on remembering in rats. Behav. Neurosci. 1998;112:772–792. doi: 10.1037//0735-7044.112.4.772. [DOI] [PubMed] [Google Scholar]
- 46.Mogensen J., Hjortkjaer J., Ibervang K.L., Stedal K., Mala H. Prefrontal cortex and hippocampus in posttraumatic functional recovery: spatial delayed alternation by rats subjected to transection of the fimbria-fornix and/or ablation of the prefrontal cortex. Brain Res. Bull. 2007;73:86–95. doi: 10.1016/j.brainresbull.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Lee I., Kesner R.P. Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. J. Neurosci. 2003;23:1517–1523. doi: 10.1523/JNEUROSCI.23-04-01517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat. Rev. Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 49.Hong J., Shu-Leong H., Tao X., Lap-Ping Y. Distribution of catechol-O-methyltransferase expression in human central nervous system. Neuroreport. 1998;9:2861–2864. doi: 10.1097/00001756-199808240-00033. [DOI] [PubMed] [Google Scholar]
- 50.Matsumoto M., Weickert C.S., Akil M., Lipska B.K., Hyde T.M., Herman M.M., Kleinman J.E., Weinberger D.R. Catechol-O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience. 2003;116:127–137. doi: 10.1016/s0306-4522(02)00556-0. [DOI] [PubMed] [Google Scholar]
- 51.Maynard T.M., Haskell G.T., Peters A.Z., Sikich L., Lieberman J.A., LaMantia A.S. A comprehensive analysis of 22q11 gene expression in the developing and adult brain. Proc. Natl Acad. Sci. USA. 2003;100:14433–14438. doi: 10.1073/pnas.2235651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allen Brain Atlas. 2009 http://www.brain-map.org . [Google Scholar]
- 53.Papaleo F., Crawley J.N., Song J., Lipska B.K., Pickel J., Weinberger D.R., Chen J. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J. Neurosci. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J., Lipska B.K., Halim N., Ma Q.D., Matsumoto M., Melhem S., Kolachana B.S., Hyde T.M., Herman M.M., Apud J., et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am. J. Hum. Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gogos J.A., Morgan M., Luine V., Santha M., Ogawa S., Pfaff D., Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc. Natl Acad. Sci. USA. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kinsey S.G., Bailey M.T., Sheridan J.F., Padgett D.A., Avitsur R. Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Brain Behav. Immun. 2007;21:458–466. doi: 10.1016/j.bbi.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramocki M.B., Zoghbi H.Y. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature. 2008;455:912–918. doi: 10.1038/nature07457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang B., Hawes N.L., Hurd R.E., Davisson M.T., Nusinowitz S., Heckenlively J.R. Retinal degeneration mutants in the mouse. Vision Res. 2002;42:517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- 59.Wong A.A., Brown R.E. Visual detection, pattern discrimination and visual acuity in 14 strains of mice. Genes Brain Behav. 2006;5:389–403. doi: 10.1111/j.1601-183X.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 60.Brown R.E., Wong A.A. The influence of visual ability on learning and memory performance in 13 strains of mice. Learn. Mem. 2007;14:134–144. doi: 10.1101/lm.473907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wahlsten D., Cooper S.F., Crabbe J.C. Different rankings of inbred mouse strains on the Morris maze and a refined 4-arm water escape task. Behav. Brain Res. 2005;165:36–51. doi: 10.1016/j.bbr.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 62.McFadyen M.P., Kusek G., Bolivar V.J., Flaherty L. Differences among eight inbred strains of mice in motor ability and motor learning on a rotorod. Genes Brain Behav. 2003;2:214–219. doi: 10.1034/j.1601-183x.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 63.Reenila I., Tuomainen P., Männistö P.T. Improved assay of reaction products to quantitate catechol-O-methyltransferase activity by high-performance liquid chromatography with electrochemical detection. J. Chromatogr. B Biomed. Appl. 1995;663:137–142. doi: 10.1016/0378-4347(94)00433-6. [DOI] [PubMed] [Google Scholar]
- 64.Nissinen E., Männistö P. Determination of catechol-O-methyltransferase activity by high-performance liquid chromatography with electrochemical detection. Anal. Biochem. 1984;137:69–73. doi: 10.1016/0003-2697(84)90348-8. [DOI] [PubMed] [Google Scholar]