Abstract
Tick feeding modulates host immune responses. Tick-induced skewing of host CD4+ T cells towards a Th2 cytokine profile facilitates transmission of tick-borne pathogens that would otherwise be neutralized by Th1 cytokines. Tick-derived factors that drive this Th2 response have not previously been characterized. In the current study, we examined an I. scapularis cDNA library prepared at 18-24 hours of feeding and identified and expressed a tick gene with homology to Loxosceles spider venom proteins with sphingomyelinase activity. This I. scapularis sphingomyelinase-like (IsSMase) protein is a Mg+2-dependent, neutral (pH 7.4) form of sphingomyelinase. Significantly, in an in vivo TCR transgenic adoptive transfer assay IsSMase programmed host CD4+ T cells to express the hallmark Th2 effector cytokine IL-4. IsSMase appears to directly program host CD4 T cell IL-4 expression (as opposed to its metabolic by-products) because induced IL-4 expression was not altered when enzymatic activity was neutralized. TCR transgenic CD4 T cell proliferation (CFSE-dilution) was also significantly increased by IsSMase. Furthermore, a Th2 response is superimposed onto a virally-primed Th1 response by IsSMase. Thus, IsSMase is the first identified tick molecule capable of programming host CD4+ T cells to express IL-4.
Keywords: Ixodes scapularis, BALB/c, CD4, immune modulation
INTRODUCTION
Ticks transmit a variety of disease-causing infectious agents of medical and veterinary importance (1). Established, resurging and emerging tick-borne diseases of human health importance include spotted fevers, ehrlichioses, Lyme borreliosis, babesiosis, tick-borne encephalidides and hemorrhagic fevers (2, 3). Additionally, tick toxicoses and tick-transmitted theileriosis, babesiosis, cowdriosis and anaplasmosis are major constraints on livestock production (1).
Ticks feed on the blood of mammals over the course of several days, and have therefore evolved complex strategies to facilitate blood feeding. Tick saliva contains numerous pharmacologically active molecules that modulate host haemostasis, wound healing, pain and itch responses and inflammation (4, 5, 6, 7, 8). These tick-induced changes in host defenses that facilitate extended blood feeding also enhance pathogen transmission (4, 9). The salivary gland transcriptome of the tick Ixodes scapularis contains over 800 gene clusters of putatively secreted proteins, and many genes within a given cluster display high levels of polymorphism and are differentially expressed during the course of blood feeding (7).
The ability of ticks to modulate host immune responses in particular is thought to be central in facilitating pathogen transmission. In vitro studies have shown that Ixodes scapularis salivary gland extract contains an IL-2 binding protein (10) as well as the protein Salp15 that inhibits T cell proliferation (11, 12) and dendritic cell production of IL-6, IL-12p70 and TNF (13). In vitro studies have also shown that tick saliva can polarize CD4+ T cells towards a Th2 cytokine profile marked by IL-4 expression (4, 14, 15, 16, 17). It is likely that this Th2 response and concomitant suppression of Th1 responsiveness (marked by IFN-γ expression) facilitates the transmission of pathogens that would otherwise be neutralized by Th1 cytokines.
We recently developed an in vivo TCR transgenic adoptive transfer system to study the influence of I. scapularis feeding on host CD4+ T cell responses (18). Although this model system is normally strongly Th1 biased (19, 20), I. scapularis infestation or salivary gland extract (SGE) induced host CD4 T cells to express IL-4 (18). This tick-induced host CD4 T cell IL-4 expression is likely relevant to pathogen transmission because repeated infestation resulted in partially reduced IL-4 expression (18), and repetitive tick infestation is correlated with a reduced ability to modulate host immunity and to transmit pathogens (21). Furthermore, I. scapularis nymphs infected with Borrelia burgdorferi also display a partially reduced ability program host CD4 T cells to express IL-4 (18), illustrating that ticks and their transmitted pathogens drive antagonistic host immune responses.
Identification and characterization of the factor(s) present in tick saliva that drive host CD4 T cell IL-4 expression is of critical importance as this could inform strategies to prevent pathogen transmission. In fact, although factors that drive Th1 responsiveness have been extensively characterized, very few molecules have been identified that can drive Th2 responses (22, 23, 24). In the current study we examined an I. scapularis salivary gland cDNA library prepared at 18-24 hour of feeding and characterized a clone with homology to the dermonecrotic protein LiD1 found in the venom gland of the spider Loxosceles intermedia (25). LiD1 belongs to a family of venom proteins with sphingomyelinase (SMase) activity (26). The I. scapularis SMase-like clone was selected for study because of its expression early in blood feeding and due to the fact that membrane lipid rafts are specialized membrane microdomains containing sphingomyelin that are involved in T cell receptor signalling upon antigen engagement (27). Lipid rafts differ for Th1 and Th2 CD4 T cells, making T cell membrane lipids a target for modulation of T cell responses (28). Importantly, this I. scapularis SMase-like protein (IsSMase) can program host CD4 T cell IL-4 expression independently of its enzymatic activity.
MATERIALS AND METHODS
Mice and adoptive transfer
6.5 TCR transgenic mice that express a TCR transgenic TCR specific for an I-Ed - restricted epitope deriving from hemagglutinin (HA) of influenza virus PR8 (110SFERFEIFPKE120) (29) were maintained on a BALB/c (H-2d) Thy1.1 background. Lymph node preparations from 6.5 TCR transgenic mice were depleted of CD8+ cells using magnetic beads, and the naive Thy1.1+ HA-specific TCR transgenic CD4 T cells were labelled with CFSE prior to adoptive transfer in Thy1.2+ BALB/c recipients (purchased from the Jackson Laboratory) that had been exposed to various forms of HA antigen as well as ticks or tick products, and subsequent functional analyses were performed as previously described (18, 19, 20). In short, adoptive transfer recipients were treated intradermally with either a bolus of soluble HA peptide (200 ug) or 105 PFU of a recombinant vaccinia virus expressing HA (viral-HA) at the site of tick feeding or injection of tick product. Four days post-transfer, adoptively transferred TCR transgenic CD4 T cells (identified as CD4+Thy1.1+) were recovered from the draining brachial and axillary lymph nodes, and the ability of the divided (CFSE-diluted) TCR transgenic CD4 T cells to express intracellular cytokines was measured by FACS following in vitro restimulation with 100 μg/ml HA peptide plus 5 μg/ml Brefeldin A (Sigma-Aldrich). All quantitative data are expressed as the mean ± SEM, and differences in cytokine expression levels between experimental groups were analyzed using an unpaired two-tailed Students t-test.
The University of Connecticut Health Center Institutional Animal Care and Use Committee approved all protocols used in this study.
Ticks and tick infestation
A pathogen-free I scapularis colony was maintained as previously described (30). In short, all life cycle stages are kept in sterile glass vials with mesh tops in desiccators over a saturated solution of potassium sulfate to maintain 97% relative humidity at 22°C with a 14 hour light: 10 hour dark photoperiod. Adult ticks blood feed on New Zealand white rabbits and nymphs and larvae are fed on mice.
Tick exposed mice were infested with pathogen-free I. scapularis nymphs confined within a capsule consisting of top half of a 1.5 ml microcentrifuge tube (Fisher Scientific) secured to clipped fur on the shoulder region with a 4:1 mixture (w/w) of calophonium (rosin, Sigma-Aldrich) and beeswax. Engorged nymphs were removed from the capsule as they detached from the mouse.
Salivary gland extract (SGE)
Adult I. scapularis fed for 4 days on white New Zealand rabbits (30), at which time females were removed for collection of salivary glands. Prior to dissection, ticks were washed with sterile distilled water and then surface sterilized with 70% ethanol. Salivary glands were removed and placed together into a minimal volume of 0.15 M Dulbecco’s phosphate buffered saline, pH 7.2 (1x PBS, Gibco) held on ice. Pooled salivary glands were sonicated at 55 kHz for 1 minute while held in an ice water bath followed by centrifugation at 14,000 g for 20 minutes at 4°C. Supernatant was collected as salivary gland extract (SGE) and protein concentration determined by bicinchoninic acid assay (31). SGE was divided into 50 μl aliquots and stored at -30°C. All SGE samples used in this study were frozen and thawed once.
Cloning and expression
A cDNA from the salivary glands of I. scapularis (7) encoding a putative sphingomyelinase-like (IsSMase) protein (NCBI accession Q202J4) was amplified by PCR using the forward primer 5′-GGGGGACAACTCTCTTGGGTTTTT-3′ and the reverse primer 5′-TATGTAGAAGGGGCTCAAAGGATT-3′. Amplified sequence was cloned into the insect cell expression vector pIB/V5-His-TOPO (Invitrogen), which contains a blasticidin resistance gene, a 14-amino acid epitope (V5) and a polyhistidine (6xHis) sequence at the C-terminus to facilitate purification. Integrity of the resulting construct was assessed by sequencing in both directions.
Endotoxin-free recombinant plasmids were purified using the EndoFree Plasmid Maxi Kit (Qiagen) and transfected into High 5, Trichopulsia ni, cells (Invitrogen) according to the manufacturer’s protocol. Stably transfected cells were grown in a 2 L Wave Bioreactor (GE Healthcare) at 27°C and maintained in the presence of blasticidin at a final concentration of 10 μg/ml. Cultures reaching densities 5-8 × 106 cells/ml were processed by centrifugation at 6,000xg to remove cells and particulate matter. Cell culture supernatants were buffer-exchanged with 1x PBS and concentrated at least 10X with a Vivaflow 200 tangential flow ultrafiltration system (Vivascience) fitted with low protein-binding regenerated cellulose membranes (10 kDa MWCO). Concentrates were loaded onto a Ni+2-nitriloacetic acid (NiNTA, Qiagen) column that was pre-equilibrated and washed with 50 mM NaH2PO4, 500 mM NaCl, 10-15 mM Imidazole, pH 8.0, and the His-tagged proteins were eluted with 50 mM NaH2PO4, 500 mM NaCl, 250 mM Imidazole, pH 8.0. Eluted fractions were extensively dialyzed against PBS and concentrated, using Ultrafree-4 concentrators (10 kDa MWCO, Amicon). Protein was quantified by the bicinchoninic acid (BCA) assay (31). Purity was assessed by discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (32) and gels were stained with Coomassie blue.
Sphingomyelinase activity assay
Functional activity of recombinant IsSMase in neutral (pH 7.4) or acidic (pH 5.0) conditions was determined by monitoring the rate of sphingomyelin hydrolysis at 37°C, using Amplex Red Sphingomyelinase Assay Kit (Invitrogen). Briefly, recombinant IsSMase (12.5 μg/ml) or controls were mixed in triplicate with 10 mM Amplex Red reagent (Invitrogen), 2 U/ml horseradish peroxidase, 0.2U/ml choline oxidase, 8 U/ml alkaline phosphatase, 0.5 mM sphingomyelin, and reaction buffer (100 mM Tris-HCl, pH 7.4) containing 10 mM MgCl2, 10 mM MnCl2, or 10 mM ZnCl2 in a final volume of 300 ul/well in 96-well microtiter plates. For each test, reaction buffer containing no enzyme was used as a negative control while sphingomyelinase from Staphylococcus aureus (0.02 U/ml, where one unit is defined as the amount of sphingomyelinase that will hydrolyse 1 μmole of TNPAL-sphingomyelin per minute at pH 7.4 at 37°C) was a positive control. Sphingomyelinase induced conversion of the Amplex Red reagent to red-fluorescent resorufin was detected using excitation and emission wavelengths of 530 nm and 590 nm, respectively. Fluorescence was recorded at 10 min intervals over a period of 3 h to follow the kinetics of the reaction. For each point, background fluorescence was corrected by subtracting the negative control values. For testing under acidic conditions, 100 mM Tris-HCL buffer was substituted for 50 mM sodium acetate, pH 5.0, and enzyme and substrate were mixed and incubated for 1 h before addition of Amplex Red reagent. The effects of using different metal ions was determined under similar conditions by substituting Mg+2 with Mn+2 or Zn+2 in the reaction buffer. In both instances, end-point measurements were taken after an additional 2 hr incubation period. To neutralize IsSMase enzymatic activity the protein was subjected to two freeze (-80°C) and thaw cycles.
RESULTS
Molecular cloning of a SMase-like protein from I. scapularis
A BLAST search analysis of a cDNA library from the salivary glands of partially fed (18-24 h) I. scapularis females (7) revealed the presence of a cDNA clone with 39% nucleotide and 59% amino acid homology to the dermonecrotic protein LiD1(Accession No. AAQ16123) found in the venom gland of the spider Loxosceles intermedia (25). LiD1 is a 31 kDa protein belonging to a group of dermonecrotic proteins that cause severe skin lesions and other systemic effects through intense sphingomyelinase (SMase) activity against cellular matrix (26).
The putative protein product of the I. scapularis gene was named IsSMase. Predicted translation of the full-length sequence of IsSMase revealed an open reading frame (ORF) of 365 amino acids. A predicted signal peptide cleavage site was identified by the SignalP 3.0 program (33) and shown to reside between amino acids 17 (C) and 18 (Q) of the translated ORF, resulting in a theoretical 39.7 kDa mature protein with an isoelectric point of 6.03. Comparison of the translated sequence of IsSMase against the Conserved Domain Database (CDD) revealed motifs conserved in the glycerophosphoryl diester phosphodiesterase super-family UgpQ (COG0584). Alignment of spider and tick SMases is shown in Fig. 1 with conserved motifs indicated.
Figure 1.
Alignment of I. scapularis SMase-like protein (Q202J4) and the sphingomyelinase proteins from Loxosceles spp. spiders. Searches against the conserved domain databases (CDD) revealed motifs conserved in the glycerophosphoryl diester phosphodiesterases family UgpQ (COG0584). An alignment of the tick protein and the reported Loxosceles sp. SMases shows the conserved motif (red square) and the catalytically important residues H34, H70, C76, and C80 (arrows). Loxosceles intermedia (AAQ16123), L. similis (Q56JA9), L. boneti (Q5YD77). The predicted leader peptide for IsSMase is underlined.
Primary sequence comparison suggested that residues involved in the catalysis mechanism by spider SMases may be conserved in the tick protein. Tick SMase could display an (α/β)8 barrel fold as described for Loxosceles SMase D (34) with the active site formed by His29 and His70 and the amino acid residues Glu40, Asp51, and Asp109 involved in metal ion coordination. In addition, the catalytic loop could be stabilized by a disulfide bridge formed between residues Cys69 and Cys75 (arrows) as described for the spider SMase D (Fig. 1).
Protein expression and purification
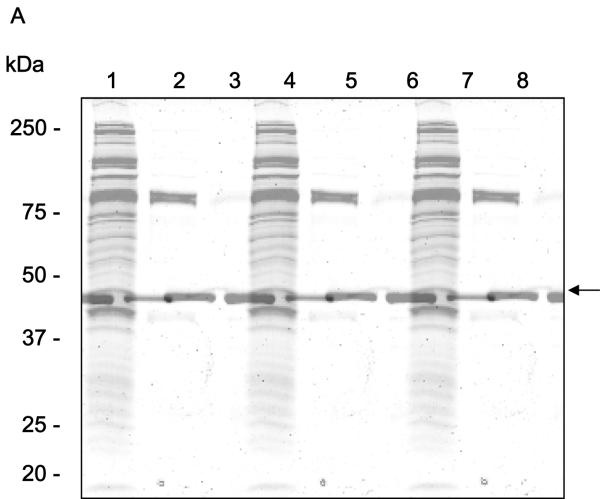
The final yield of recombinant IsSMase secreted by High 5 cells was in the range of 200-500 μg/L in harvested medium. SDS-PAGE analysis of recombinant IsSMase purified from insect cell culture supernatants (Fig. 2A) indicated an apparent size, including the 6x His and V5 tags, of ∼45 kDa that closely matched the size predicted from the cDNA sequence.
Figure 2.
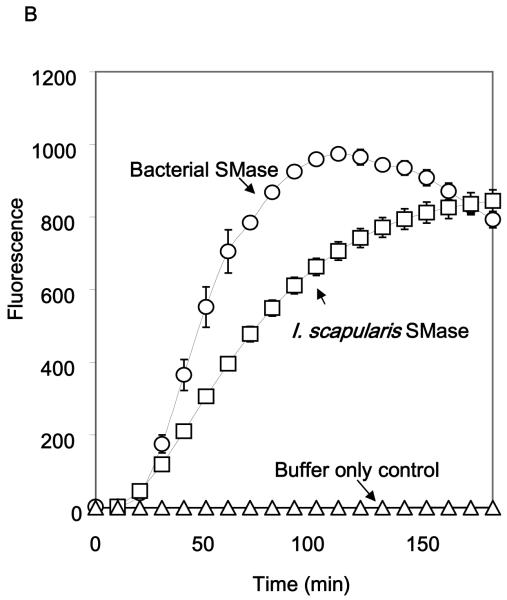
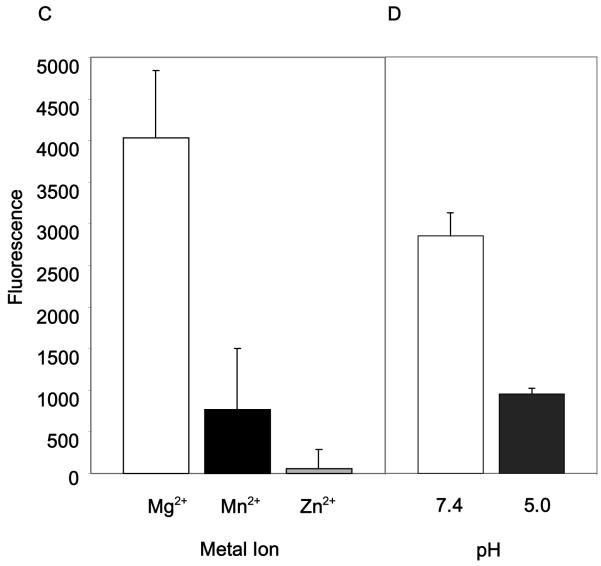
Expression and functional activity of IsSMase. A, Purified recombinant IsSMase was resolved by 10% discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie blue dye. Lane 1, molecular weight standard (kDa); Lane 2, flow-through; Lane 3, final wash; Lanes 4-8, eluates containing purified IsSMase (indicated by arrow). B, Functional activity of IsSMase was assessed by monitoring the rate of sphingomyelin hydrolysis at 37°C under standard conditions. C and D, effect of metal ions and pH on IsSMase activity. The sphingomyelinase activity of the IsSMase was recorded at multiple time points using excitation in the range of 530-560 nm and emission detection at 590 nm. Data plotted represent means ± SE (n=3). C, Activity of IsSMase at different pH.
Sphingomyelinase activity
In an in vitro sphingomyelinase assay, a concentration of 12.5 μg/ml of recombinant IsSMase resulted in a gradual increase in fluorescence intensity over the time interval assayed indicating hydrolysis of sphingomyelin which was similar to that observed when using 0.02 U/ml S. aureus sphingomyelinase (positive control) (Fig. 2B). Analysis of IsSMase in acidic conditions (50 mM sodium acetate, pH 5.0) resulted in significantly reduced fluorescence intensity (Fig. 2C). In addition, substitution of Mg+2 ions by Mn2+ resulted in 81.0 % decrease in sphingomyelinase activity while the use of Zn2+ ions almost completely abolished this activity (<1.0%) (Fig. 2D). These results suggest that IsSMase is likely a Mg+2-dependent, neutral (pH 7.4) form of sphingomyelinase.
IsSMase programs CD4 T cells to express IL-4
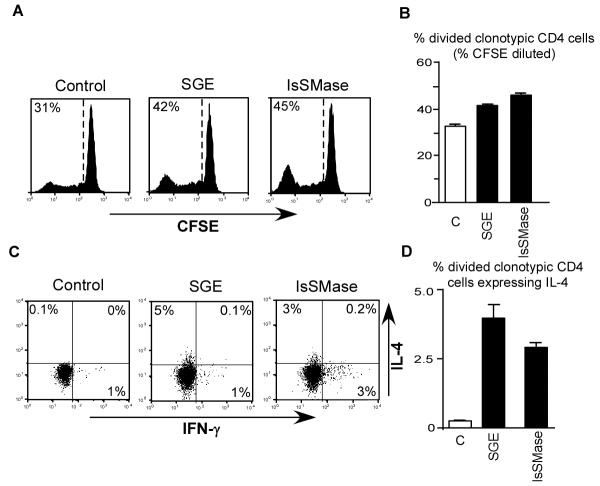
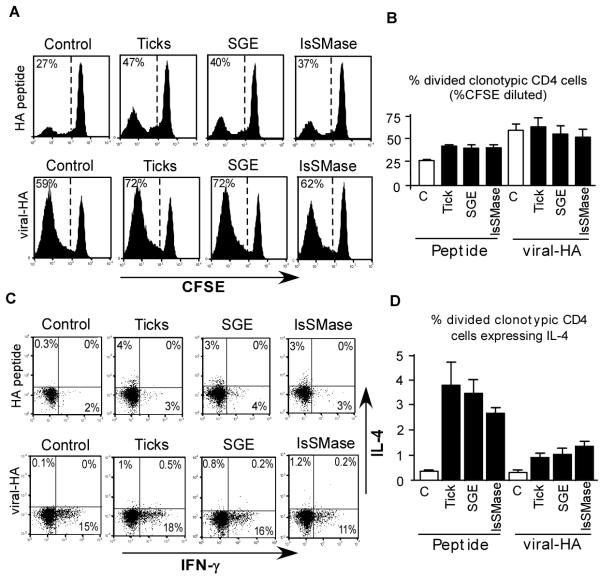
As previously observed in our TCR transgenic adoptive transfer model (18), intradermal injection of soluble HA peptide into control mice resulted in significant proliferation of TCR transgenic CD4 T cells as measured by CFSE dilution (Fig. 3, A and B). These divided TCR transgenic CD4 T cells, however, neither gained the ability to express the Th1 cytokine IFN-γ nor the Th2 cytokine IL-4 (Fig. 3C). This non-immunogenic response is consistent with the absence of pathogen-associated molecular patterns (PAMPs) or other adjuvant-like molecules (35). Intradermal injection of 5 μg SGE programmed a fraction of the divided TCR transgenic CD4 T cells to express IL-4 (Fig. 3 C and D). Although this fraction was relatively small (∼5%), this effect was highly reproducible (P<0.0001 compared to control) and similar in magnitude to our previous studies (18). A roughly similar level of IL-4 expression was induced by 5 μg recombinant IsSMase (P<0.0001 compared to control) (Fig 3 C and D). TCR transgenic CD4 T cell proliferation (CFSE-dilution) was also significantly increased by both SGE (P<0.004) and IsSMase (P<0.0001) over background (Fig. 3, A & B). Importantly, a recombinant Aedes aegypti salivary gland Kazal serine protease inhibitor protein expressed and purified in the same way as IsSMase lacked IL-4 polarizing activity (data not shown), indicating that IsSMase possesses specific Th2-skewing activity.
Figure 3.
Recombinant IsSMase programs CD4 T cells responding to associated soluble antigen to develop the capacity to express IL-4. Adoptively transferred CFSE-labeled BALB/c HA-specific TCR transgenic CD4 T cells were recovered following adoptive transfer into BALB/c recipients that were simultaneously treated intradermally with soluble HA peptide and either SGE, IsSMase or PBS (Control or C). A, Representative CFSE-dilution histograms (left side, with the percentage of TCR transgenic CD4 T cells that have diluted CFSE-fluorescence shown) and B, quantitative data (mean ± SEM) corresponding to panel A. C, Representative IFN-γ vs IL-4 intracellular staining plots corresponding to the CFSE-divided TCR transgenic CD4 T cells in panel A. D, Quantitative analysis of intracellular IL-4 expression. N=4 to 6 for each group.
IsSMase superimposes a Th2 response onto a virally-primed Th1 response
As previously observed, TCR transgenic CD4 T cells primed by a recombinant vaccinia virus expressing HA (viral-HA) in the absence of tick infestation or intradermal injection of either IsSMase or SGE proliferated more vigorously (Fig. 4, A and B) and developed a greater capacity to express IFN-γ (Fig. 4C) compared to TCR transgenic CD4 T cells from soluble HA peptide administered mice. Similar to tick infestation and injection of SGE, injection of IsSMase did not alter either proliferation or IFN-γ in response to viral-HA but did increase IL-4 expression (P=0.004). The fraction of TCR transgenic CD4 T cells expressing IL-4 with IsSMase + viral-HA was somewhat lower than with peptide + IsSMase (Fig 4 C and D), suggesting a degree of antagonism in the activities of virus and IsSMase. Taken together, IsSMase is the first salivary gland molecule of any tick species to be demonstrated to cause Th2 polarization of host CD4+ T cells.
Figure 4.
Recombinant IsSMase superimposes IL-4 expression potential in CD4 T cells undergoing Th1 differentiation in response to viral immunization. Adoptively transferred CFSE-labeled BALB/c HA-specific TCR transgenic CD4 T cells were recovered 4 days following adoptive transfer into BALB/c recipients that were simultaneously injected intradermally with either soluble HA peptide or viral-HA at the site either infested with 10 nymphs on day -1 or intradermally injected with SGE, IsSMase or PBS (Control, C). A, Representative CFSE-dilution histograms and B, quantitative analysis. C, Representative IFN-γ vs IL-4 intracellular staining plots and D, quantitative analysis of IL-4 intracellular cytokine expression. N=4 for both groups.
IsSMase enzymatic activity is not required to program CD4 T cell IL-4 expression
To test whether SMase enzymatic activity is required to program host CD4 T cell IL-4 expression, we first tested bacterial SMase in an attempt to rule out sphingomyelinase activity as the sole reason for this effect (Fig. 2B). Intradermally administered 10 U bacterial SMase did not program TCR transgenic CD4 T cells to express IL-4 (Fig. 5A). Furthermore, IsSMase that was subjected to two freeze-thaw cycles and lost detectable enzymatic activity in the Amplex Red Sphingomyelinase Assay (data not shown) retained its capacity to drive CD4 T cells to express IL-4 (P=0.004) (Fig 5, A and B) whereas expression of IFN-γ was not significantly altered.
Figure 5.
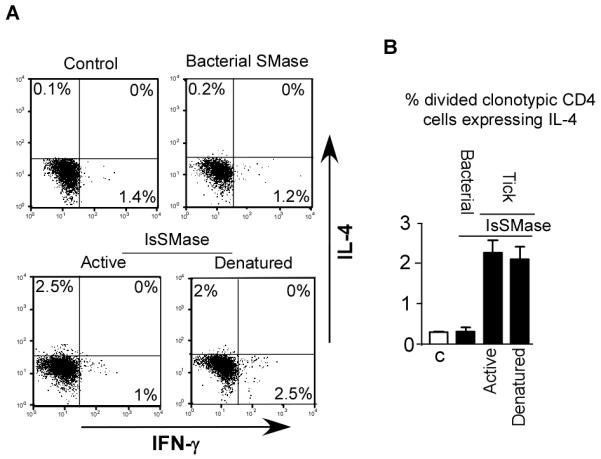
IsSMase enzymatic activity is not required to program CD4 T cell IL-4 expression. IL-4 expression potential in CD4 T cells exposed to bacterial SMase (10 U of Bacillus cereus SMase) and active vs denatured IsSMase. A, representative IFN-γ vs IL-4 intracellular staining plots and B, quantitative analysis. N=4 to 6 for each group.
DISCUSSION
Ticks remain attached, inject saliva and feed on host blood for 4 to 15 days with a typical adult female tick consuming approximately 4,000 mg of host blood (36). This intimate host contact resulted in evolution of differentially expressed salivary gland transcriptomes encoding hundreds of putatively secreted proteins that modulate or act as counter measures to multiple physiological components of host haemostasis, wound healing, and innate and specific acquired immune defences (4, 7, 8).
A broadly recurrent theme among ixodid tick biology is that host T cell responses are redirected towards a Th2 profile marked by IL-4 expression, which often is accompanied with suppression of Th1 cytokines (4, 14, 15, 16, 17, 18). Furthermore, this Th2 response appears to facilitate pathogen transmission (37 38, 39, 40), while repeated infestations reduces the extent of both the degree of tick-induced host Th2 response and pathogen transmission (18, 21). Here, we report the cloning, expression and characterization of the first tick salivary gland molecule responsible for programming IL-4 expression in host CD4+ T cells.
Discovery of a cDNA clone with amino acid homology to L. intermedia dermonecrotic protein (25) was of interest due its presence in a salivary gland cDNA library prepared early in tick attachment and blood feeding. We hypothesized that the product of this gene contributed to formation of the hemorrhagic pool from which the tick feeds. IsSMase shares an identical glycerphosphoryl diester phosphodiesterase domain catalytic site with venom SMases of L. intermedia (26). This relationship is not surprising since spiders and ticks are both members of the class Arachnida. Tick recombinant IsSMase shows homology to a spider dermonecrotic protein; however, intradermal injection of 5 μg recombinant IsSMase did not induce any visually detectable cutaneous changes (data not shown) suggesting little if any dermonecrotic activity. Intense dermonecrosis at the tick attachment site would not be desirable due to potential disruption of tick feeding and creation of a site favorable for secondary bacterial and fungal infections. However, we cannot rule out the possibility that IsSMase enzymatic activity contributes to feeding lesion formation in combination with the many other enzymes in I. scapularis saliva (7).
SMase biological properties are relevant to tick feeding and pathogen transmission. SMases catalyze hydrolysis of sphingomyelin into phosphorylcholine and ceramide (41). The latter is then catabolized to sphingosine by ceramidase, and sphingosine kinase converts sphingosine to sphingosine-1-phosphate (42). Neutrophils are a significant component of the histopathological response to tick feeding (43, 44); however, ceramide suppresses neutrophil spreading and respiratory burst in the presence of TNF (45) which is known to be induced by I. scapularis feeding (15). Additionally, I. scapularis saliva reduces neutrophils, β2-integrin expression, and uptake and killing of B. burgdorferi spirochetes (46). Finally, sphingosine-1-phosphate acts on T cell differentiation during activation to decrease Th1 and enhance Th2 responses by reducing dendritic cell TNF and IL-12, which act to initiate Th1 differentiation (47).
The current study is the first to identify a tick molecule responsible for the programming of an IL-4 response by host CD4+ T cells. IsSMase enzymatic activity is not required to program IL-4 expression, therefore it is less likely that ceramide and sphingosine-1-phosphate are responsible for the observed IL-4 programming. We speculate that a freeze-thaw stable structure within IsSMase might bind a TLR or other receptor on APC or innate immune cells that in turn facilitate Th2 differentiation. It cannot be ruled out; however, that IsSMase enzymatic activity might contribute to modulation of certain facets of host immune responses such as lipid rearrangements involved in T cell receptor signalling (27). IsSMase may not be the only factor contained within SGE that can program host CD4 T cells to express IL-4, and it might be possible that multiple factors synergize to mediate this activity. An additional point to consider is that ticks obtain a blood meal over a period of approximately five days for a nymph and up to 14 days for an adult. The amount of saliva introduced varies during the course of feeding as does the amount of blood consumed (36). We also know that differential expression of salivary gland genes occurs during feeding (7), which could change the amount of a saliva protein introduced.
Development of a Th1 or Th2 CD4+ T cell response is primarily driven by pathogen-associated molecular patterns (PAMPs) from microbes and metazoan parasites that interact with specific TLRs on dendritic cells (23, 48). PAMP-TLR combinations that drive Th1 responses are well characterized, however, knowledge of combinations that elicit Th2 responses is extremely limited (49). The synthetic lipopeptide Pam3cys can drive Th2 responses via TLR2 (48), and other molecules that can elicit host IL-4 expression include: Schistosoma mansoni soluble egg antigen, the filarial nematode phosphorylcholine-containing glycoprotein ES-62, Toxoplasma gondii stimulated lipoxins, certain forms of Candida albicans, Porphyromonas gingivalis LPS and Nippostrongylus brasiliensis chitin (24, 48, 49, 50, 51). To this limited group of factors, we add I. scapularis sphingomyelinase (IsSMase).
ACKNOWLEDGEMENTS
We thank Mr. Keith Bouchard for tick colony maintenance and Dr. Saravanan Thangamani for the Aedes aegypti recombinant Kazal protein. This was supported by United States Army Medical Research and Materiel Command Award 0310075 (S.K.W.) and National Institutes of Health Grants AI062735 (S.K.W. and A.J.A.) and AI057441 (A.J.A.).
Footnotes
Disclosures
None
REFERENCES
- 1.Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129:S3–S14. doi: 10.1017/s0031182004005967. [DOI] [PubMed] [Google Scholar]
- 2.Walker DH. Tick-transmitted infectious diseases in the United States. Ann. Rev Public Health. 1998;19:237–269. doi: 10.1146/annurev.publhealth.19.1.237. [DOI] [PubMed] [Google Scholar]
- 3.Dennis DT, Piesman JF. Overview of tick-borne infections of humans. In: Goodman JL, Dennis DT, Sonenshine DE, editors. Tick-borne Diseases of Humans. American Society for Microbiology; Washington, DC: 2005. pp. 3–11. [Google Scholar]
- 4.Brossard M, Wikel SK. Tick immunobiology. Parasitology. 2004;129:S161–S176. doi: 10.1017/s0031182004004834. [DOI] [PubMed] [Google Scholar]
- 5.Valenzuela JG. Exploring tick saliva: from biochemistry to ‘sialomes’ and functional genomics. Parasitology. 2004;129:S83–S94. doi: 10.1017/s0031182004005189. [DOI] [PubMed] [Google Scholar]
- 6.Steen NA, Barker SC, Alewood PF. Proteins in the saliva of the Ixodidae (ticks): pharmacological features and biological significance. Toxicon. 2006;47:1–20. doi: 10.1016/j.toxicon.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro J, Alarcon-Chaidez F, Francischetti IMB, et al. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem Mol Biol. 2006;36:111–129. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Alarcon-Chaidez FJ, Sun J, Wikel SK. Construction and characterization of a cDNA library from the salivary glands of Dermacentor andersoni Stiles (Acari: Ixodidae) Insect Biochem Mol Biol. 2007;37:48–71. doi: 10.1016/j.ibmb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Wikel SK. Tick modulation of host immunity: An important factor in pathogen transmission. Int. J. Parasitol. 1999;29:851–859. doi: 10.1016/s0020-7519(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 10.Gillespie RD, Dolan MC, Piesman J, Titus RG. Identification of an IL-2 binding protein in the saliva of the Lyme disease vector tick, Ixodes scapularis. J Immunol. 2001;166:4319–4327. doi: 10.4049/jimmunol.166.7.4319. [DOI] [PubMed] [Google Scholar]
- 11.Urioste S, Hall LR, Telford SR, III, Titus RG. Saliva of the Lyme disease vector, Ixodes dammini, blocks cell activation by a nonprostaglandin E2-dependent mechanism. J Exp Med. 1994;180:1077–1085. doi: 10.1084/jem.180.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg R, Juncadella IJ, Ramamoorthi N, et al. Cutting edge: CD4 is the receptor for the tick saliva immunosuppressor, Salp15. J Immunol. 2006;177:6579–6583. doi: 10.4049/jimmunol.177.10.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hovius JWR, de Jong MAWP, den Dunnen J, et al. Salp 15 binding to DC-SIGN inhibits cytokine expression by impairing both nucleosome remodeling and RNA stabilization. PLoS Pathogens. 2008;4:e31. doi: 10.1371/journal.ppat.0040031. doi:10.1371/journal.ppat.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramachandra RN, Wikel SK. Modulation of host-immune responses by ticks (Acari: Ixodidae): effect of salivary gland extracts on host macrophages and lymphocyte cytokine production. J Med Entomol. 1992;29:818–826. doi: 10.1093/jmedent/29.5.818. [DOI] [PubMed] [Google Scholar]
- 15.Schoeler GB, Wikel SK. Modulation of host immunity by haematophagous arthropods. Ann Trop Med Parasitol. 2001;95:755–771. doi: 10.1080/0003498012011118. [DOI] [PubMed] [Google Scholar]
- 16.Kovar L, Kopecky J, Rihova B. Salivary gland extract from Ixodes ricinus tick polarizes the cytokine profile towards Th2 and suppresses proliferation of T lymphocytes in human PBMC culture. J Parasitol. 2001;87:1342–1348. doi: 10.1645/0022-3395(2001)087[1342:SGEFIR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Mejri N, Rutti B, Brossard M. Immunosuppressive effects of Ixodes ricinus tick saliva or salivary gland extracts on innate and acquired immune response of BALB/c mice. Parasitol. Res. 2002;88:192–197. doi: 10.1007/s00436-001-0515-1. [DOI] [PubMed] [Google Scholar]
- 18.Müller-Doblies UU, Maxwell SS, Boppana VD, et al. Feeding by the tick, Ixodes scapularis, causes CD4+ T cells responding to cognate antigen to develop the capacity to express IL-4. Parasite Immunol. 2007;29:485–499. doi: 10.1111/j.1365-3024.2007.00966.x. [DOI] [PubMed] [Google Scholar]
- 19.Higgins AD, Mihalyo MA, McGary PW, Adler AJ. CD4 T cell priming and tolerization are differentially programmed by APCs upon initial engagement. J Immunol. 2002;168:5573–5581. doi: 10.4049/jimmunol.168.11.5573. [DOI] [PubMed] [Google Scholar]
- 20.Doody ADH, Kovalchin JT, Mihalyo MA, Hagymasi AT, Drake CG, Adler AJ. Glycoprotein 96 can chaperone both MHC class I- and class II-restricted epitopes for in vivo presentation, but selectively primes CD8+ T cell effector function. J Immunol. 2004;172:6087–6092. doi: 10.4049/jimmunol.172.10.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wikel SK, Ramachandra RN, Bergman DK, Burkot TR, Piesman J. Infestation with pathogen-free nymphs of the tick Ixodes scapularis induces host resistance to transmission of Borrelia burgdorferi by ticks. Infect Immun. 1997;65:335–338. doi: 10.1128/iai.65.1.335-338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Pulendran B, Tang H, Denning TL. Division of labor, plasticity, and crosstalk between dendritic cell subsets. Curr Opin Immunol. 2008;20:61–67. doi: 10.1016/j.coi.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reese TA, Liang H-E, Tager AM, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–97. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalapothakis E, Araujo SC, de Castro CS, et al. Molecular cloning, expression and immunological properties of LiD1, a protein from the dermonecrotic family of Loxosceles intermedia spider venom. Toxicon. 2002;40:1691–1699. doi: 10.1016/s0041-0101(02)00201-5. [DOI] [PubMed] [Google Scholar]
- 26.Kalapothakis E, Chatzaki M, Goncalves-Dornelas H, et al. The Loxtox protein family in Loxosceles intermedia (Mello-Leitao) venom. Toxicon. 2007;50:938–946. doi: 10.1016/j.toxicon.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Kabouridis PS. Lipid rafts in T cell receptor signaling (review) Mol Membr Biol. 2006;23:49–57. doi: 10.1080/09687860500453673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Laethem F, Leo O. Membrane lipid rafts: new targets for immunoregulation. Curr Mol Med. 2002;2:557–570. doi: 10.2174/1566524023362122. [DOI] [PubMed] [Google Scholar]
- 29.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouchard KR, Wikel SK. Care, maintenance, and experimental infestation of ticks in the laboratory setting. In: Marquardt WC, Black WC IV, Freier JE, Hagedorn HH, Hemingway J, Higgs S, James AA, Kondratieff B, Moore CG, editors. Biology of Disease Vectors. 2nd edn. Elsevier Academic Press; San Diego, CA: 2005. pp. 705–711. [Google Scholar]
- 31.Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 34.Murakami MT, Fernandes-Pedrosa MF, Tambourgi DV, Arni RK. Structural basis for metal ion coordination and the catalytic mechanism of sphingomyelinases D. J Biol Chem. 2005;280:13658–13664. doi: 10.1074/jbc.M412437200. [DOI] [PubMed] [Google Scholar]
- 35.Adler AJ, Huang C-T, Yochum GS, Marsh DW, Pardoll DM. In vivo CD4+ T cell tolerance induction versus priming is independent of the rate and number of cell divisions. J Immunol. 2000;164:649–655. doi: 10.4049/jimmunol.164.2.649. [DOI] [PubMed] [Google Scholar]
- 36.Kaufman WR. Tick-host interaction: a synthesis of current concepts. Parasitol Today. 1989;5:47–56. doi: 10.1016/0169-4758(89)90191-9. [DOI] [PubMed] [Google Scholar]
- 37.Zeidner N, Dreitz M, Belasso D, Fish D. Suppression of acute Ixodes scapularis-induced Borrelia burgdorferi infection using tumor necrosis factor α, interleukin-2, and interferon-γ; J Infect Dis. 1996;173:187–195. doi: 10.1093/infdis/173.1.187. [DOI] [PubMed] [Google Scholar]
- 38.Zeidner N, Dolan M, Massung R, Piesman J, Fish D. Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis suppresses IL-2 and IFN-gamma production and promotes an IL-4 response in C3H/HeJ mice. Parasite Immunol. 2000;22:581–588. doi: 10.1046/j.1365-3024.2000.00339.x. [DOI] [PubMed] [Google Scholar]
- 39.Zeidner NS, Schneider BS, Nuncio MS, Gern L, Piesman J. Coinoculation of Borrelia spp. with tick salivary gland lysate enhances spirochete load in mice and is tick species-specific. J Parasitol. 2002;88:1276–1278. doi: 10.1645/0022-3395(2002)088[1276:COBSWT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 40.Zeidner NS, Schneider BS, Rutherford JS, Dolan MC. Suppression of Th2 cytokines reduces tick-transmitted Borrelia burgdorferi load in mice. J Parasitol. 2008;94:767–769. doi: 10.1645/GE-1416.1. [DOI] [PubMed] [Google Scholar]
- 41.Levade T, Jaffrezou JP. Signaling sphingomyelinases: which, where, how and why? Biochim Biophys Acta. 1999;1438:1–17. doi: 10.1016/s1388-1981(99)00038-4. [DOI] [PubMed] [Google Scholar]
- 42.Pandy S, Murphy RF, Agrawal DK. Recent advances in the immunobiology of ceramide. Exp Mol Pathol. 2007;82:298–309. doi: 10.1016/j.yexmp.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theis JH, Budwiser PD. Rhipicephalus sanguineus: sequential histopathology at the host-arthropod interface. Exp Parasitol. 1974;36:77–105. doi: 10.1016/0014-4894(74)90115-5. [DOI] [PubMed] [Google Scholar]
- 44.Gill HS, Walker AR. Differential cellular responses at Hyalomma anatolicum anatolicum feeding sites on susceptible and tick-resistant rabbits. Parasitology. 1985;91:591–607. doi: 10.1017/s0031182000062831. [DOI] [PubMed] [Google Scholar]
- 45.Fuortes M, Jin W, Nathan C. Ceramide selectively inhibits early events in the response of human neutrophils to tumor necrosis factor. J Leukoc Biol. 1996;59:451–460. doi: 10.1002/jlb.59.3.451. [DOI] [PubMed] [Google Scholar]
- 46.Montgomery RR, Lusitani D, de B, Chevance A, Malawista SE. Tick saliva reduces adherence and area of human neutrophils. Infect Immun. 2004;72:2989–2994. doi: 10.1128/IAI.72.5.2989-2994.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Von Wenckstern H, Zimmermann K, Kleuser B. The role of the lysopholipid sphingosine 1-phosphate in immune cell biology. Arch Immunol. Ther Exp. 2006;54:239–251. doi: 10.1007/s00005-006-0028-9. [DOI] [PubMed] [Google Scholar]
- 48.Agrawal S, Agrawal A, Doughty B, et al. Different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinases and c-Fos. J Immunol. 2003;171:4984–4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 49.Dillon S, Agrawal A, Van Dyke T, et al. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J Immunol. 2004;172:4733–4743. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 50.MacDonald AS, Straw AD, Bauman B, Pearce EJ. CD8- dendritic cell activation status plays an integral role in influencing Th2 response development. J Immunol. 2001;167:1982–1988. doi: 10.4049/jimmunol.167.4.1982. [DOI] [PubMed] [Google Scholar]
- 51.Whelan M, Harnett MM, Houston KM, Patel V, Harnett W, Rigley KP. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J Immunol. 2000;164:6453–6460. doi: 10.4049/jimmunol.164.12.6453. [DOI] [PubMed] [Google Scholar]