Abstract
More than 80% of the world’s HIV-infected adults live in sub-Saharan Africa, where heterosexual transmission is the predominant mode of spread. The virologic and immunologic correlates of female-to-male (FTM) and male-to-female (MTF) transmission are not well understood. A total of 1022 heterosexual couples with discordant HIV-1 serology results (one partner HIV infected, the other HIV uninfected) were enrolled in a prospective study in Lusaka, Zambia and monitored at 3-month intervals. A nested case-control design was used to compare 109 transmitters and 208 nontransmitting controls with respect to plasma HIV-1 RNA (viral load, VL), virus isolation, and CD4+ cell levels. Median plasma VL was significantly higher in transmitters than nontransmitters (123,507 vs. 51,310 copies/ml, p < 0.001). In stratified multivariate Cox regression analyses, the risk ratio (RR) for FTM transmission was 7.6 (95% CI: 2.3, 25.5) for VL ≥ 100,000 copies/ ml and 4.1 (95% CI: 1.2, 14.1) for VL between 10,000 and 100,000 copies/ml compared with the reference group of <10,000 copies/ml. Corresponding RRs for MTF transmission were 2.1 and 1.2, respectively, with 95% CI both bounding 1. Only 3 of 41 (7%) female transmitters had VL < 10,000 copies/ml compared with 32 of 93 (34%) of female nontransmitters (p < 0.001). The transmission rate within couples was 7.7/100 person-years and did not differ from FTM (61/862 person-years) and MTF (81/978 person-years) transmission. We conclude that the association between increasing plasma viral load was strong for female to male transmission, but was only weakly predictive of male to female transmission in Zambian heterosexual couples. FTM and MTF transmission rates were similar. These data suggest gender-specific differences in the biology of heterosexual transmission.
INTRODUCTION
IN 1999, an estimated 5.8 million people acquired human immunodeficiency virus (HIV) infections globally. Since the start of the epidemic, an estimated 80% of HIV-1-infected adults and 90% of infected children have come from Africa.1 Male homosexuals and injection drug users remain the largest risk groups in industrialized countries. Heterosexual transmission is the most rapidly growing risk exposure category in the United States and remains the predominant mode of HIV-1 spread in Africa.2 Subtype B is the most common HIV-1 subtype in industrialized countries and most virologic investigations have characterized this subtype. However, more than one-third of the world’s infections are estimated to occur with HIV-1 subtype C and its prevalence is increasing.3
Plasma HIV-1 RNA has been a consistent predictor of “contagion” for percutaneous4,5 and perinatal transmission.6-10 This relationship holds true for perinatal transmission in non-B subtype areas.11 Within the sexual transmission category, anal, oral, and vaginal intercourse bring different levels of risk.12 An anatomical difference is that semen is retained in the posterior vagina in much greater volume than vaginal fluid is retained postcoitus on the penis. Furthermore, since plasma HIV-1 RNA levels have been reported to be higher in HIV-1-infected men than in HIV-1-infected women13,14 and the diversity of transmitted viral variants may be greater in women than men,15 the correlates of female-to-male (FTM) and male-to-female (MTF) transmission may differ for penile-vaginal intercourse. Thus, incidence and predictors of transmission differ by route (percutaneous, sexual, perinatal) and may vary by gender15 or possibly HIV-1subtype.16
Until recently, studies of the relationship of plasma HIV-1 RNA levels to heterosexual transmission have focused on hemophiliac couples17 in whom the index case has mitigating conditions not found in other heterosexuals. Studies of non-hemophiliac couples have found higher plasma RNA levels,18 more frequent virus isolations,19 and lower CD4+ cell counts in transmitting compared with nontransmitting men.20 Conclusions regarding the independent contribution of plasma HIV-1 RNA to heterosexual transmission have been limited by small numbers, cross-sectional study designs, and in particular lack of female-to-male transmission pairs. Moreover, the significance of plasma viremia must include examination of other putative risk factors for transmission such as sexually transmitted diseases (STDs), frequency of sex, possibility of acquisition from a person outside the dyadic relationship, and lack of circumcision in men.21 A study of discordant couples in Uganda found increasing viral load to be predictive of HIV-1 transmission in an area with HIV-1 clades A and D.22 Epidemiologic linkage between the Ugandan couples was assumed but not confirmed. Significant differences in the relationship of viral load and MTF compared with FTM transmission were not found.22
This study presents data from a prospective cohort study of heterosexual couples from Lusaka, Zambia, where more than 90% of infections are with HIV-1 subtype C. Using a nested case-control design, men and women who transmitted HIV to their partners (“transmitters”) were compared with control “nontransmitters.” Phylogenetic analyses were used to confirm true transmission pairs, and the independent associations of plasma HIV RNA levels, HIV isolation rates, and calculated CD4+ cell levels with MTF and FTM transmission are described.
MATERIALS AND METHODS
Prospective study participants
Between February 1994 and November 2000, 1022 cohabiting couples with one HIV-1-infected and one HIV-1-uninfected partner (“discordant couples”) were enrolled in a prospective study through a voluntary HIV counseling and testing (VCT) center at the Zambia-UAB HIV Research Project in Lusaka, Zambia. Recruitment, counseling, and testing procedures have been detailed elsewhere.23,24 Briefly, the VCT service was promoted by community outreach workers, who delivered invitations door to door. Interested couples gathered at a central location near their residence each morning, and were provided with transportation to the center. A group session with video and discussion was followed by couples pretest counseling. Lunch was provided while two rapid HIV tests and syphilis serologies were performed. Joint posttest counseling and free syphilis treatment followed in the afternoon. Individuals wishing to be tested alone, that is, without involvement of their sexual partners, were referred to one of three other free VCT centers in Lusaka.
Of the 1022 discordant couples enrolled and monitored for 2 to 67 months (median, 15 months; mean, 22 months), 535 had HIV-infected men and 487 included HIV-1-infected women. Eligibility criteria included: (1) discordant HIV-1 serostatus (one partner HIV-1 antibody positive, the other antibody negative), (2) cohabiting in Lusaka for ≥6 months, and (3) women aged ≤48 years and men aged ≤65 years. The rationale for the age restriction was to include persons who were most sexually active.
All couples were monitored at 3-month intervals with repeat counseling as needed. At each visit the following was obtained: (1) documentation of sexual exposures (with and without a condom, with the study partner and/or with other partners), (2) interim medical history and physical examinations including screening for sexually transmitted diseases, and (3) repeat HIV serology for the seronegative partner. Seroconverters were invited for confirmatory blood testing and appropriately counseled.
All study procedures and consent forms were reviewed and approved by the University of Alabama at Birmingham Institutional Review Board, the University Teaching Hospital Research Ethics Committee in Lusaka, and the Office of Protection from Research Risks of the National Institutes of Health (Bethesda, MD). Participants signed written informed consent at the time of HIV testing and counseling, at the time of enrollment into the prospective studies, and again prior to sample procurement for the nested case-control studies. All consents were explained to both members of the couple together, and both partners signed each consent form. As per the procedure stated in the testing and counseling consent form, all participants were counseled as a couple concerning their test results, and were thus aware of their own and their partners’ HIV infection status.
Free condoms and free outpatient medical care were provided at the research clinic throughout the study. The study pharmacy included medications from the World Health Organization (WHO) essential drug list, in particular those listed in the National HIV/STD/TB/Leprosy Control Program. Medical staff were certified nurses and clinical officers (equivalent to physician’s assistants), as well as physicians from the University Teaching Hospital (UTH, Lusaka, Zambia). An insurance policy was also provided for hospitalization and specialty outpatient care at the UTH. Neither the UTH nor the study clinic pharmacy provided antiretroviral therapy.
Determination of epidemiological linkage
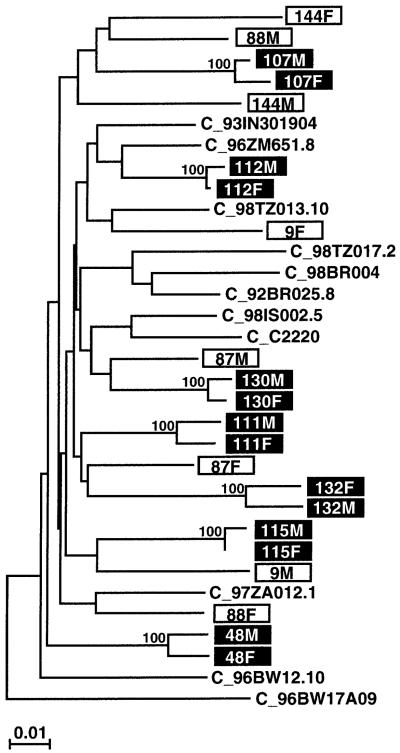
In 162 couples, an initially seronegative partner acquired HIV-1 during the prospective study. Epidemiological linkage was examined by sequence analysis of the infecting viral strains of both partners in 149 couples (the remaining 13 lacked adequate materials to perform the genetic analyses). Subgenomic gag, env, or long terminal repeat (LTR) fragments (ranging in size from 260 to 460 bp) were amplified from DNA extracted from uncultured patient peripheral blood mononuclear cells (PBMCs) or dried whole blood spots and sequenced directly without interim cloning. Viral sequences from each couple were then aligned and their percent nucleotide sequence differences calculated. These values were compared with the range and mean of sequence diversity determined for a set of well-characterized reference sequences (representing the same subtype) from the Los Alamos sequence database. Pairs with distances that fell below the distance range of the reference sequences were classified as likely linked; pairs with distances that fell within the distance range of the reference sequences were classified as likely unlinked. This preliminary classification was then confirmed by phylogenetic tree analysis. Using this combination of distance and phylogenetic approaches, we determined epidemiological linkage for 129 of 149 (87%) couples studied.25 Twenty couples were classified as unlinked (12 men and 8 women acquired HIV-1 from another source), and this was confirmed in each case by sequences analysis of a second genomic region. Figure 125a,b depicts a neighbor-joining tree constructed from partial HIV-1 gp41 sequences (389-bp consensus length) for a subset of putative transmission pairs. Of the 129 linked couples, 121 grouped within subtype C in the genomic regions analyzed, 3 within subtype A, 3 within subtype G, 1 within subtype D, and another within subtype J. All viruses from the 20 unlinked couples grouped within subtype C for both genomic regions analyzed.25
FIG. 1.
Epidemiological linkage analysis for a subset of putative HIV-1 transmission pairs. A phylogenetic tree was constructed from partial gp41 sequences (consensus length, 389 bp), using the neighbor-joining method25a and the Kimura two-parameter model.25b Horizontal branches are drawn to scale (the scale bar indicates 1% sequence divergence); vertical separation is for clarity only (the tree was rooted with A_U455). The bootstrap values at each node represent the percentage of 1000 bootstrap replicates that support the branching order (only values of 80% or higher are shown). Newly derived sequences from 22 different individuals (11 couples) are shown (boxed), along with 11 subtype C reference sequences (http://hiv-web.lanl.gov/HTML/alignments.html) from the Los Alamos sequence database (M and F indicate sequences derived from male and female partners, respectively). Viruses from seven couples (highlighted) are closely related to one other (0.5-2.7% nucleotide sequence diversity) and cluster together with significant bootstrap values (100%), thus indicating epidemiological linkage. By contrast, viruses from four other couples (boxed) do not cluster together and their range of within-couple diversity (5.9-10.1%) is comparable to that of the reference sequences (4.4-11.6%), thus making viral transmission between partners highly unlikely.
Sample collection
Of the 129 linked transmission pairs (cases), 109 (84%) had samples available for the nested case-control studies. A consecutive sample of 208 nontransmitting HIV-1+ partners (“controls”) were enrolled as they came in for their study visits. Plasma for quantitative HIV-1 RNA determination, whole blood lysate for CD4+ cell determination, and DNA for epidemiological linkage assessment were processed on site and stored at -70°C until batch shipment to the University of Alabama at Birmingham (UAB) on dry ice. Whole blood collected in acid-citrate-dextrose (ACD) tubes was flown from Lusaka, Zambia to Birmingham, Alabama for viral culture. The blood was processed and PBMCs were cultured in Birmingham between 36 and 40 hr after being drawn.
A total of 66 male and 43 female transmitters and 114 male and 94 female nontransmitters had plasma HIV-1 RNA (viral loads), viral culture data, and/or CD4+ cell level results (Table 1). Twenty transmitters were lost to follow-up prior to sample collection for the nested case-control studies. Unavailability of participants on the days scheduled for fresh blood draws resulted in some missing culture data. CD4+ cell levels were evaluated for a subset of 60 transmitters and 66 nontransmitters.
Table 1.
Number of Transmitters and Nontransmitters with Viral Load, Culture Results, and CD4+ Cell Levela
|
Transmitters |
Nontransmitters |
|||
|---|---|---|---|---|
| Men (n = 66) | Women (n = 43) | Men (n = 114) | Women (n = 94) | |
| Culture/CD4/viral load | 27 | 16 | 16 | 23 |
| Viral load/culture | 21 | 10 | 3 | 5 |
| Viral load/CD4 | 8 | 9 | 10 | 17 |
| Viral load only | 7 | 6 | 85 | 48 |
| Culture only | 3 | 2 | 0 | 1 |
| Total viral load | 63 | 41 | 114 | 93 |
| Total culture | 51 | 28 | 19 | 29 |
| Total CD4+ cell level | 35 | 25 | 26 | 40 |
Note: None of the subjects had only CD4+ cell level data.
Covariate measurement
Potential behavioral and clinical risk factors included age, genital ulcers, and genital inflammation reported or observed during routine physical examinations, reported number of unprotected sexual acts with partner in study in the last 3 months, and HIV disease stage. Measures of these covariates were taken from the time of transmission (in transmitters) or the time of sample collection (in nontransmitter controls).
The clinical status of HIV-1-infected participants was assessed and categorized according to the Kigali combined clinical and laboratory staging system developed by our group in Rwanda.26 This staging system uses sedimentation rate and hematocrit (rather than CD4+ cell counts, which are rarely available in resource-poor settings) combined with modified clinical criteria that reflect the common manifestations of HIV disease in Africa. Using this combined clinical and laboratory staging system, 3-year mortality among Rwandan women in stages I-II was <10%, compared with 29% in stage III and 62% in stage IV.26
Laboratory tests
HIV-1 infection was confirmed by testing positive on each of two rapid serological tests: the Dipstick HIV-1/HIV-2 antibody screening assay and the Capillus latex aggregation confirmatory test. We have previously published a comparison of this algorithm with a standard 2 ELISA algorithm.23 Quantitative plasma HIV-1 RNA levels were assessed with the Roche Amplicor 1.0 assay following the manufacturer’s instructions in a laboratory certified by the Virology Quality Assurance Program of the AIDS Clinical Trials Group (ACTG). We and others have determined that these primers can reliably detect and quantitate HIV-1 subtype C.27,28 CD4+ cell determination was made with the CD4 TRAx® assay, an ELISA system that measures total CD4 protein in whole blood lysates. In this assay, total CD4 protein from the subject is compared with a standard curve and the number of CD4+ cell equivalents is determined. Correlation between this method and standard measurement of CD4+ T cells by flow cytometry range from 0.87 to 0.95.29,30 The ACTG consensus method was used to isolate HIV-1 from the PBMCs of subjects as described by Japour et al.31
HIV incidence rate calculations
All person-years of observation in the prospective study were used to calculate overall HIV-1 incidence rates in the cohort. Seroconversion and transmission rates were calculated in three ways: (1) including all 162 seroconversions, (2) including only 129 linked transmissions, and (3) including linked (n = 129) and presumed linked (n = 13) transmissions (combined, n = 142). The rationale for the latter method is that we would expect 87% (or 11 of 13 transmissions) with missing linkage data to be truly linked. The two potentially misclassified cases would represent 1% of the total 142 and would thus not unduly inflate transmission rate calculations. Separate calculations were performed for MTF and FTM transmission. Exact distribution methods were used to calculate 95% confidence intervals.
Analysis of virologic and immunologic correlates of transmission
Median log viral loads and CD4+ cell levels were compared between transmitters and nontransmitters, together and stratified by gender using the Mann-Whitney t test. The χ2 test was used to compare proportions between transmitters and nontransmitters, together and stratified by gender.
Multivariate Cox regression models including behavioral and clinical covariates were employed to assess the independent contribution of viral load, culture, and CD4+ cell level to transmission. Stratified Cox regression models were performed for MTF and FTM transmission with the indicator variables for high (≥100,000 copies/ml) and medium (≥10,000 to <100,000 copies/ml) viral load, using low viral load (<10,000 copies/ml) as the reference. Separate Cox regression models were conducted with viral load as a continuous variable. An overall Cox regression model with interaction terms for gender and the categorical levels of viral load was also performed. In each case, model building was performed while retaining viral load and eliminating the least significant covariates one at a time, on the basis of the Wald χ2 p value.
RESULTS
Demographic and behavioral risk factors for transmitters and nontransmitters
Men who transmitted HIV-1 to their partners were on average 3 years younger than nontransmitters (Table 2), while age differences in women were not significant. Men’s and women’s age at first intercourse, number of lifetime partners, duration of union, and number of children living at the home or number of biological children were not different in transmitters and nontransmitters (Table 2). A history of STD in the 5 years prior to enrollment and positive RPR were more common in women who transmitted as compared with nontransmitters (p < 0.05), while the prevalence of trichomoniasis vaginalis was not different. The prevalence of circumcision among men was low (8%) and not significantly different in transmitting (6%) and nontransmitting men (10%).
Table 2.
Comparison of Demographic and Behavioral Risk Factors in Transmitters versus Nontransmitters Stratified by Gender
|
Women |
Men |
|||||
|---|---|---|---|---|---|---|
| Transmitters (n = 43) | Nontransmitters (n = 94) | p Value | Transmitters (n = 66) | Nontransmitters (n = 114) | p Value | |
| Age (years)a | 26 (18-42) | 27 (17-48) | 0.21 | 33 (24-54) | 36 (20-61) | 0.04b |
| Age (years) at first intercoursea | 17 (11-26) | 16 (11-32) | 0.91 | 18 (10-28) | 18 (10-51) | 0.6 |
| Lifetime partnersa | 4 (1-10) | 3 (1-10) | 0.57 | 11 (2-77) | 12 (1-97) | 0.81 |
| Duration of union (years)a | 4 (0-25) | 5 (0-34) | 0.36 | 7 (0-26) | 8 (0-31) | 0.13 |
| Number of children in the homea | 2 (0-10) | 3 (0-10) | 0.55 | 3 (0-12) | 4 (0-10) | 0.46 |
| Number of children togethera | 1 (0-9) | 1 (0-9) | 0.68 | 2 (0-8) | 2 (0-10) | 0.72 |
| Reported STD last 5 yearsc | 19/43 (44%) | 25/94 (27%) | 0.04b | 33/66 (50%) | 54/114 (47%) | 0.73 |
| RPR positive ≥1 : 2c | 15/43 (35%) | 17/93 (18%)d | 0.03b | 7/66 (11%) | 17/114 (15%) | 0.41 |
| Trichomoniasis vaginalisc | 3/37 (8%)e | 10/92 (11%)f | 0.76 | — | — | |
| Circumcisionc | 4/66 (6%) | 11/114 (10%) | 0.58 | |||
Mean (range).
Significance at ≤0.05.
n (%).
Rapid plasma reagin (RPR) data missing for one woman.
Trichomoniasis vaginalis data missing for six women at the time of sample collection because of pregnancy or menses.
Trichomoniasis vaginalis data missing for two women at the time of sample collection because of pregnancy or menses.
Fewer than 3% of couples used condoms prior to HIV testing and counseling (VCT), and this increased to more than 80% after VCT. Condom use remained consistently high through 24 months of follow-up, but most couples reported occasional unprotected sex (average about once per month).32
HIV-1 incidence rates
Seroconversion rates were 8.6/100 person-years, with no significant difference between FTM and MTF (Table 3).33 The seroconversion rate was highest between enrollment and the 3-month follow-up visit, reflecting the fact that some individuals were already infected prior to VCT but had not yet developed antibodies. When the first 3-month interval is eliminated from the analysis, 131 seroconversions occurred in 1629 person-years of follow-up, yielding a seroincidence of 8.0/100 PY (95% C.I.: 6.7, 9.5). Corresponding numbers for FTM were 7.3/100 person years (5.5, 9.3) and 8.l7/100 person years (6.8, 10.8) for MTF. If only the confirmed transmissions were included, the rate was 7.1/100 person-years, while the rate if presumed linked cases were included was 7.7/100 person-years. The latter estimate is likely to approximate most closely the true transmission rate between cohabiting partners, as it does not exclude those for whom we lacked confirmation of epidemiologic linkage.
TABLE 3.
Heterosexual Seroconversion and Within-Couple Transmission Rates
|
Female-to-male |
Male-to-female |
Overall |
||||
|---|---|---|---|---|---|---|
| n/PY | Rate/100 PY (95% CI) | n/PY | Rate/100 PY (95% CI) | n/PY | Rate/100 PY (95% CI) | |
| Seroconversion Rate | 73/888 | 8.2 (6.4, 10.2) | 89/996 | 8.9 (7.2, 10.9) | 162/1883 | 8.6 (7.3, 10.0) |
| Transmission Rate (linked only) | 53/854 | 6.2 (4.7, 8.0) | 0 76/975 | 7.8 (6.1, 9.6) | 129/1829 | 7.1 (5.9, 8.3) |
| Transmission Rate (including presumed linked) | 61/862 | 7.1 (5.4, 9.0) | 81/978 | 8.3 (6.6, 10.2) | 142/1840 | 7.7 (6.5, 9.0) |
Abbreviation: PY, Person-year.
Nested case-control analyses of plasma HIV-1 RNA levels, gender, and transmission
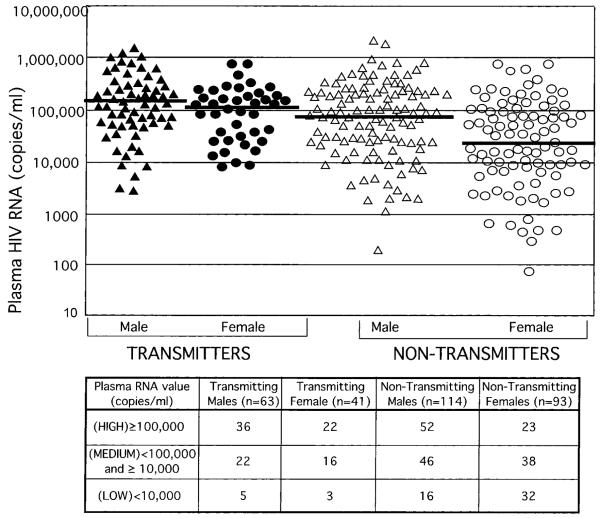
Transmitters had higher plasma RNA (viral loads) than nontransmitters (median of 123,507 vs. 51,310; p < 0.001). When stratified by gender, both transmitting men and women had significantly higher median viral loads (VLs) than nontransmitters (121,984 vs. 29,200 p < 0.002 for women and 132,278 vs. 85,015, p = 0.05 for men) (Fig. 2). Among women, the distribution in low medium, and high viral load categories differed markedly between transmitters and nontransmitters (Fig. 2, p = 0.001 for trend). In contrast, male transmitters and nontransmitters had comparatively similar distributions (p = 0.28). Only 7% of transmitting women compared with 34% of nontransmitters had VL <10,000 copies/ml. No transmitters had VL <1000 copies/ml; however, only eight nontransmitters (seven women and one man, <5% of the total) had VLs in this low range.
FIG. 2.
Plasma HIV RNA levels (copies/ml) of transmitters and nontransmitters stratified by gender: Male transmitters (triangles), female transmitters (circles), male nontransmitters (open triangles), female nontransmitters (open circles). Median plasma HIV RNA levels for each group are indicated by a solid line.
Multivariate Cox regression models
In the Cox regression model, both viral load ≥100,000 and viral load ≥10,000 to <100,000 were significantly associated with FTM transmission (risk ratios [RRs] of 7.6 and 4.1, respectively) compared with the referent group with <10,000 viral copies/ml (Table 4A). Corresponding RRs for men were 2.1 for high viral load and 1.2 for medium viral load (Table 4B), with confidence intervals bounding one in each case.
TABLE 4.
Multivariate Cox Regression Model for Female-to-Male and Male-to-Female Transmission
| Variable | Adjusted risk ratio | 95% CI | p Value |
|---|---|---|---|
| A. Female-to-Male Transmission | |||
| Viral load category (copies/ml)a | |||
| Low (<10,000) | 1.0 | ||
| Medium (≥10,000 to <100,000) | 4.1 | 1.2, 14.1 | 0.02 |
| High (≥100,000) | 7.6 | 2.3, 25.5 | 0.001 |
| Log viral load (per log10 increment)b | 2.5 | 1.5, 4.0 | 0.0002 |
| B. Male-to-Female Transmission | |||
| Viral load category (copies/ml)a,c | |||
| Low (<10,000) | 1.0 | ||
| Medium (≥10,000 to ,100,000) | 1.2 | 0.5, 3.4 | 0.67 |
| High (≥100,000) | 2.1 | 0.8, 5.6 | 0.12 |
| Log viral load (per log10 increment)b,d | 1.8 | 1.2, 2.8 | 0.004 |
Log viral loads were used in all models.
Separate Cox models were constructed, utilizing log viral load as a continuous variable.
Number of unprotected sexual acts in the last 3 months, genital inflammation, and age were also independently predictive of transmission.
Genital inflammation was independently predictive of transmission.
Table 4A and B also presents RRs for VL as a continuous variable. Similar to the model with categorical levels of viral load, the association of viral load with transmission was stronger in FTM transmission (RR, 2.5 per log increase in VL) than MTF transmission (RR, 1.8), although both were significant.
The significance of the differential effect of viral load on FTM versus MTF transmission was evaluated on the basis of an overall Cox regression model with interaction terms for gender and the categorical levels of viral load. Despite small numbers for testing interaction terms, there was an indication of interaction between high viral load and gender (p = 0.09).34 This finding was supported by the point estimate of the RR for high viral load in MTF transmission (2.1) not being within the 95% confidence interval (CI) for FTM transmission (2.3-25.5), and vice versa (7.6, not between 0.8 and 5.6) (Table 4A and B).
HIV-1 culture
HIV-1 cultures were performed in 127 subjects (79 transmitters, 48 nontransmitters). The isolation success rate was slightly higher in transmitters than nontransmitters (89 and 79%, p = 0.20). The difference in isolation rates between transmitting women and nontransmitting women was not substantial (93 and 86%, p = 0.67). Among men, the difference in isolation rates between transmitters and nontransmitters was greater, although it did not achieve statistical significance (86 and 68%, p = 0.16). Positive culture was not independently predictive of transmission in Cox regression analyses.
CD4+ cell levels
CD4+ cell levels were determined in 126 subjects (60 transmitters and 66 nontransmitters). More than 80% of our cohort had calculated CD4+ cell equivalents below 400 cells/mm3 and 16% had levels under 200 cells/mm3. Median CD4+ cell levels were similar in transmitters and nontransmitters (345 vs. 322 cells/mm3, p = 0.57). Analyses stratified by gender showed no significant difference between female transmitters and nontransmitters (333 vs. 336 cells/mm3, p = 0.24) and male transmitters and nontransmitters (347 vs. 271 cells/mm3, p = 0.54). Linear regression showed no significant correlation between CD4+ cell levels and log viral load in any analytic group, nor were CD4+ cell levels associated with positive viral cultures.
DISCUSSION
We studied the virologic and immunologic correlates of HIV-1 transmission in 1022 HIV-1-discordant cohabiting heterosexual couples from Zambia, who were monitored prospectively while being provided counseling, free condoms, and primary health care. In epidemiologically linked HIV-1 transmission pairs, 94% of whom were infected with clade C HIV-1,25 high viral load in the index partner was associated with seroconversion. The strength of association between increasing plasma viral load and transmission was much stronger for female-to-male (FTM) than for male-to-female (MTF) transmission. Neither HIV-1 isolation from PBMCs nor CD4+ cell levels was significantly predictive of transmission. The incidence of HIV-1 infection was 8.6/100 person-years, which is similar to that reported for other counseled discordant couples in Africa,35,36 but lower than the rate reported by Quinn et al.22 Eighty-seven percent of incident infections were acquired from the cohabiting partner.
Earlier studies of small numbers of subtype B transmission events suggested that plasma HIV-1 RNA levels were a key determinant of heterosexual transmission. This was confirmed in a discordant couples study from Uganda, where subtypes A and D of HIV predominate.22 Despite different study designs and HIV subtypes, the principal findings from the Zambia and Uganda studies are similar. In both studies, levels of plasma viremia were significantly higher among subjects whose partners seroconverted than among those whose partners did not seroconvert. Multivariate analyses in both studies found that there was a significant dose-response relation of increased transmission with increasing viral load. The Ugandan study found that each log increment in viral load was associated with a rate ratio of 2.45 for seroconversion in the uninfected partner when men and women were combined. Our study yielded similar risk ratios, but with differences between FTM (RR of 2.5) and MTF (RR of 1.8) transmission, not noted in Uganda. Gender differences were particularly striking at viral titers of 100,000 copies/ml or higher, with an RR of 7.6 for FTM transmission compared with only 2.1 for MTF transmission. One possible reason for the difference between the two studies is our confirmation of epidemiologic linkage in the Zambian transmission pairs. If some seroconvertors in the Uganda study acquired HIV from an outside partner, the resulting misclassification of their nontransmitter spouses into the transmitter group might have obscured some gender-related associations.
It has been suggested that there may be differences in transmissibility and pathogenicity of different clades of HIV-1, which might be due in part to differences in viral load. HIV-1 clade C-infected sex workers in Nairobi and Senegal showed higher viral loads37 and more advanced disease38 than those with subtype A or D infection. The viral loads in our study were also higher than those reported for Uganda, although we used an older version of the Roche Monitor HIV-1 PCR (1.0 vs. version 1.5 used in the Uganda study). Some studies have shown that the version 1.5 PCR assay yields slightly higher quantitative results for clade C and D samples (<0.5 log10) than version 1.0,27,39 while other studies show similar viral loads with the two assays.28 In contrast, the version 1.5 assay yields substantially higher viral loads with clade A samples.28,39 Had we used the version 1.5 assay that was used in Uganda, we might have obtained slightly higher viral loads, increasing the difference between the two cohorts even further.
Given the high viral loads, one might have predicted that transmission rates would be higher in Zambia compared with Uganda. However, unadjusted seroconversion rates in our Zambian discordant couples were lower than those reported in their Ugandan counterparts. In each case, the point estimate in one study was not in the 95% confidence intervals for the corresponding point estimate in the other study (personal communication, M. Wawer). This suggests that the lower seroconversion rates in Zambia are not likely to be due to chance. Although clade differences may play a role in heterosexual transmission, it is more likely that the differences in seroconversion rates between the Ugandan and Zambian discordant couple cohorts are due to differences in study design and resulting behavioral factors. The Uganda study was a nested cohort study in which couples were identified retrospectively, and although all participants had access to their HIV test results, the proportion that sought their own HIV infection status, and/or that of their partners, was less than 100% (M. Wawer, personal communication). Only 10-20% of participants in that study reported condom use at any time in the year prior to seroconversion.22 In our prospective study, all discordant couples were counseled about their HIV-1 test results together, and more than 80% of reported sexual contacts after enrollment included condom use.32
In this study, FTM and MTF transmission rates were similar. Two early studies reported that infected men were more likely to transmit HIV to their partners than infected women.40,41 These results fit “common sense” notions that women were more susceptible since a large inoculum of infected seminal fluid was deposited on a large susceptible surface area that may be abraded during sexual activity. In contrast, men were exposed to a relatively smaller volume of infected vaginal fluids on a less friable epithelial surface, for relatively shorter periods of time. However, these original European and North American studies included small numbers of transmission events and few HIV-1-infected women. Our study prospectively monitored a large cohort of discordant couples with substantial numbers of MTF and FTM transmission events. Moreover, our data on the equivalence of FTM and MTF transmission rates are in agreement with other large studies in Africa,22,35,36 Haiti,42 and even in Europe.43 Thus, new paradigms of the dynamics of heterosexual transmission are needed to explain the equivalent transmission rates noted here.
Most studies examining the relationship between plasma and female genital tract viral RNA or DNA levels have found moderate correlations.44-46 There is less consensus on the association between plasma viremia and male genital tract HIV-1 levels, where modest or no correlations have been reported.47-50 Our data indicate that there are gender differences in the relationship between plasma viral load and transmission. This suggests that the ratio of blood-to-genital compartment viral load may differ in men and women, and highlights the need for further research in this area.
As in other studies, we did not find CD4+ cell levels to be associated with MTF and FTM transmission.19,51 Studies of parenteral,4 perinatal,6,52 and heterosexual transmission19,51 have found viral load measurements to be more predictive of transmission than CD4+ cell counts. Limitations in our study included the limited dynamic range of the CD4 TRAx® ELISA compared with the standard flow cytometric assay, the inability of the TRAx® assay to distinguish between values equivalent to a cell count under 200/μl, and the low variability in CD4+ cell count in our largely healthy ambulatory population, almost two-thirds of whom were in the 200 to 400 CD4+ cell/μl range.
This study of heterosexual transmission of HIV-1 in Africa highlights the importance of plasma RNA levels and suggests that lowering plasma RNA could significantly reduce transmission risk, particularly for FTM transmission. One previous study showed no female-to-male transmission among women treated with zidovudine,53 although studies of the effects of antiretroviral therapy on HIV-1 levels in the genital tract have yielded inconsistent findings.50,54-56 Maintenance antiretroviral therapy is unlikely to be feasible for most resource-limited developing countries, where per capita annual health expenditures may be less than $30. Short-course antiretroviral therapy in acutely infected individuals has prompted reductions in set-point viral loads.57 However, the majority of the world’s HIV-1 infections are in the poorest countries, where early diagnosis and short-course antiviral therapy are likely to remain out of reach. The development of a vaccine, even a partially effective one that might lower the viral set point, is urgently needed to stem the HIV-1 pandemic, especially in resource-limited regions.58
ACKNOWLEDGMENTS
We thank the study participants, staff, interns, and Project Management Group members of the Zambia-UAB HIV Research Project in Lusaka, Zambia; technicians and students at the virology lab at UTH; the Aldrovandi laboratory at UAB; and the data analysis group in the Cancer Center at UAB.
Studies performed by the authors and reported have been funded in whole or in part with federal funds from the National Institutes of Health, under grants ROIAI40951, AI41530, NOIAI85338, NOIAI41530, and P30 AI27767.
REFERENCES
- 1.UNAIDS-WHO Joint United National Programme on HIV/AIDS . AIDS Epidemic Update. World Health Organization; Geneva, Switzerland: 1999. [Google Scholar]
- 2.Allen S, et al. Confidential HIV testing and condom promotion in Africa. Impact on HIV and gonorrhea rates. JAMA. 1992;268:3338–3343. [PubMed] [Google Scholar]
- 3.Leitner T. Genetic subtypes of HIV-1. In: Myers G, Foley B, Mellors JW, Jeang KT, Wain-Hobson S, editors. Human Retroviruses and AIDS. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; Los Alamos, New Mexico: 1996. pp. III28–III40. [Google Scholar]
- 4.Busch MP, et al. Transfusion Safety Study Group Factors influencing human immunodeficiency virus type 1 transmission by blood transfusion. J Infect Dis. 1996;174:26–33. doi: 10.1093/infdis/174.1.26. [DOI] [PubMed] [Google Scholar]
- 5.Cardo DM, et al. Centers for Disease Control and Prevention Needlestick Surveillance Group A case-control study of HIV seroconversion in health care workers after percutaneous exposure. N Engl J Med. 1997;337:1485–1490. doi: 10.1056/NEJM199711203372101. see comments. [DOI] [PubMed] [Google Scholar]
- 6.Dickover RE, et al. Identification of levels of maternal HIV-1 RNA associated with risk of perinatal transmission. Effect of maternal zidovudine treatment on viral load. JAMA. 1996;275:599–605. [PubMed] [Google Scholar]
- 7.Coll O, et al. Vertical HIV-1 transmission correlates with a high maternal viral load at delivery. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:26–30. doi: 10.1097/00042560-199701010-00005. [DOI] [PubMed] [Google Scholar]
- 8.Mofenson LM, et al. Pediatric AIDS Clinical Trials Group Study 185 Team Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. N Engl J Med. 1999;341:385–393. doi: 10.1056/NEJM199908053410601. [DOI] [PubMed] [Google Scholar]
- 9.European Collaborative Study Maternal viral load and vertical transmission of HIV-1: An important factor but not the only one. The European Collaborative Study. AIDS. 1999;13:377–1385. [PubMed] [Google Scholar]
- 10.Boyer PJ, et al. Factors predictive of maternal-fetal transmission of HIV-1. Preliminary analysis of zidovudine given during pregnancy and/or delivery. JAMA. 1994;271:1925–1930. see comments. [PubMed] [Google Scholar]
- 11.Shaffer N, et al. Bangkok Collaborative Perinatal HIV Transmission Study Group Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: A randomised controlled trial. Lancet. 1999;353:773–780. doi: 10.1016/s0140-6736(98)10411-7. [DOI] [PubMed] [Google Scholar]
- 12.Leynaert B, Downs AM, de Vincenzi I, European Study Group on Heterosexual Transmission of HIV Heterosexual transmission of human immunodeficiency virus: Variability of infectivity throughout the course of infection. Am J Epidemiol. 1998;148:88–96. doi: 10.1093/oxfordjournals.aje.a009564. [DOI] [PubMed] [Google Scholar]
- 13.Farzadegan H, et al. Sex differences in HIV-1 viral load and progression to AIDS. Lancet. 1998;352:1510–1514. doi: 10.1016/S0140-6736(98)02372-1. [DOI] [PubMed] [Google Scholar]
- 14.Sterling TR, et al. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis. 1999;180:666–672. doi: 10.1086/314967. [DOI] [PubMed] [Google Scholar]
- 15.Long EM, et al. Gender differences in HIV-1 diversity at time of infection. Nat Med. 2000;6:71–75. doi: 10.1038/71563. [DOI] [PubMed] [Google Scholar]
- 16.Kunanusont C, et al. HIV-1 subtypes and male-to-female transmission in Thailand. Lancet. 1995;345:1078–1083. doi: 10.1016/s0140-6736(95)90818-8. [DOI] [PubMed] [Google Scholar]
- 17.Goedert JJ, et al. Heterosexual transmission of human immunodeficiency virus: Association with severe depletion of T-helper lymphocytes in men with hemophilia. AIDS Res Hum Retroviruses. 1987;3:355–361. doi: 10.1089/aid.1987.3.355. [DOI] [PubMed] [Google Scholar]
- 18.Fiore JR, et al. Biological correlates of HIV-1 heterosexual transmission. AIDS. 1997;11:1089–1094. doi: 10.1097/00002030-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Pedraza MA, et al. Heterosexual transmission of HIV-1 is associated with high plasma viral load levels and a positive viral isolation in the infected partner. J Acquir Immune Defic Syndr. 1999;21:120–125. [PubMed] [Google Scholar]
- 20.Lazzarin A, et al. Italian Study Group on HIV Heterosexual Transmission Man-to-woman sexual transmission of the human immunodeficiency virus. Risk factors related to sexual behavior, man’s infectiousness, and woman’s susceptibility. Arch Intern Med. 1991;151:2411–2416. [PubMed] [Google Scholar]
- 21.Royce RA, et al. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 22.Quinn TC, et al. Rakai Project Study Group Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. see comments. [DOI] [PubMed] [Google Scholar]
- 23.McKenna SL, et al. Rapid HIV testing and counseling for voluntary testing centers in Africa. AIDS. 1997;11(Suppl 1):S103–S110. [PubMed] [Google Scholar]
- 24.Allen S, N’Gandu KE, Tichacek A. The evolution of voluntary testing and counseling as an HIV prevention strategy. In: Gibney L, DiClemente RJ, Vermund SH, editors. Preventing HIV in Developing Countries: Biomedical and Behavioral Approaches. Plenum Press; New York: 1998. [Google Scholar]
- 25.Trask S, et al. Epidemiological linkage analysis in a heterosexual transmission cohort in Zambia. submitted. [Google Scholar]
- 25a.Saitou N, Nei N. The neighbor-joining method: A new method for reconstructing pylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 25b.Kimura M. A simple method for estimating evolutionary rates of base substitutions through competitive studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 26.Lifson AR, et al. Classification of HIV infection and disease in women from Rwanda. Evaluation of the World Health Organization HIV staging system and recommended modifications. Ann Intern Med. 1995;122:262–270. doi: 10.7326/0003-4819-122-4-199502150-00004. [DOI] [PubMed] [Google Scholar]
- 27.Hoesley C, et al. Comparative analysis of commercial PCR assays for the quantitation of plasma, HIV-1 RNA in patients infected with HIV-1 subtype C; 36th Annual Meeting of Infectious Diseases Society of America; Denver, Colorado. 1998; [DOI] [PubMed] [Google Scholar]
- 28.Alaeus A, et al. Assay of plasma samples representing different HIV-1 genetic subtypes: An evaluation of new versions of the Amplicor HIV-1 Monitor assay. AIDS Res Hum Retroviruses. 1999;15:889–894. doi: 10.1089/088922299310593. [DOI] [PubMed] [Google Scholar]
- 29.Paxton H, et al. Comparison of CD4 cell count by a simple enzyme-linked immunosorbent assay using the TRAx CD4 test kit and by flow cytometry and hematology. Clin Diagn Lab Immunol. 1995;2:104–114. doi: 10.1128/cdli.2.1.104-114.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saah AJ, et al. Helper T-lymphocyte count. TRAx CD4 test kit versus conventional flow cytometry. Arch Pathol Lab Med. 1997;121:960–962. [PubMed] [Google Scholar]
- 31.Japour AJ, et al. The RV-43 Study Group. the AIDS Clinical Trials Group Virology Committee Resistance Working Group Standardized peripheral blood mononuclear cell culture assay for determination of drug susceptibilities of clinical human immunodeficiency virus type 1 isolates. Antimicrob Agents Chemother. 1993;37:1095–1101. doi: 10.1128/aac.37.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meinzen-Derr J, et al. Self-report of condom use among cohabitating heterosexual discordant couples in Lusaka. Validation with biological markers; Zambia: submitted. [Google Scholar]
- 33.Brill I, et al. A SAS program for the computation of seroconversion rates in a prospective study of HIV discordant couples in Lusaka. Zambia: 2000. submitted. [Google Scholar]
- 34.Peterson B, George SL. Sample size requirements and length of study for testing interaction in a 2 × k factorial design when time-to-failure is the outcome. Control Clin Trials. 1993;14:511–522. doi: 10.1016/0197-2456(93)90031-8. [DOI] [PubMed] [Google Scholar]
- 35.Serwadda D, et al. The social dynamics of HIV transmission as reflected through discordant couples in rural Uganda. AIDS. 1995;9:745–750. doi: 10.1097/00002030-199507000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Allen S, et al. Effect of serotesting with counselling on condom use and seroconversion among HIV discordant couples in Africa. Br Med J. 1992;304:1605–1609. doi: 10.1136/bmj.304.6842.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neilson JR, et al. Subtypes of human immunodeficiency virus type 1 and disease stage among women in Nairobi, Kenya. J Virol. 1999;73:4393–4403. doi: 10.1128/jvi.73.5.4393-4403.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanki PJ, et al. Human immunodeficiency virus type 1 subtypes differ in disease progression. J Infect Dis. 1999;179:68–73. doi: 10.1086/314557. [DOI] [PubMed] [Google Scholar]
- 39.Triques K, Coste J, Perret JL, Segarra C, Mpoudi E, Reynes J, Delaporte E, Butcher A, Dreyer K, Herman S, Spadoro J, Peeters M. Efficiencies of four versions of the AMPLICOR HIV-1 MONITOR test for the quantification of different subtypes of human immunodeficiency virus. J Clin Microbiol. 1999;37:110–116. doi: 10.1128/jcm.37.1.110-116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Padian NS, et al. Heterosexual transmission of human immunodeficiency virus (HIV) in northern California: Results from a tenyear study. Am J Epidemiol. 1997;146:350–357. doi: 10.1093/oxfordjournals.aje.a009276. [DOI] [PubMed] [Google Scholar]
- 41.Saracco A, et al. Man-to-woman sexual transmission of HIV: Longitudinal study of 343 steady partners of infected men. J Acquir Immune Defic Syndr. 1993;6:497–502. [PubMed] [Google Scholar]
- 42.Deschamps MM, et al. Heterosexual transmission of HIV in Haiti. Ann Intern Med. 1996;125:324–330. doi: 10.7326/0003-4819-125-4-199608150-00011. [DOI] [PubMed] [Google Scholar]
- 43.de Vincenzi I, European Study Group on Heterosexual Transmission of HIV A longitudinal study of human immunodeficiency virus transmission by heterosexual partners. N Engl J Med. 1994;331:341–346. doi: 10.1056/NEJM199408113310601. [DOI] [PubMed] [Google Scholar]
- 44.Goulston C, McFarland W, Katzenstein D. Human immunodeficiency virus type 1 RNA shedding in the female genital tract. J Infect Dis. 1998;177:1100–1103. doi: 10.1086/517404. [DOI] [PubMed] [Google Scholar]
- 45.Iversen AK, et al. Distinct determinants of human immunodeficiency virus type 1 RNA and DNA loads in vaginal and cervical secretions. J Infect Dis. 1998;177:1214–1220. doi: 10.1086/515266. [DOI] [PubMed] [Google Scholar]
- 46.Hart CE, et al. Correlation of human immunodeficiency virus type 1 RNA levels in blood and the female genital tract. J Infect Dis. 1999;179:871–882. doi: 10.1086/314656. [DOI] [PubMed] [Google Scholar]
- 47.Coombs RW, et al. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: Evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis. 1998;177:320–330. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- 48.Vernazza PL, et al. Effect of antiviral treatment on the shedding of HIV-1 in semen. AIDS. 1997;11:1249–1254. doi: 10.1097/00002030-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Dyer JR, et al. Quantitation of human immunodeficiency virus type 1 RNA in cell free seminal plasma: Comparison of NASBA with Amplicor reverse transcription-PCR amplification and correlation with quantitative culture. J Virol Methods. 1996;60:161–170. doi: 10.1016/0166-0934(96)02063-0. [DOI] [PubMed] [Google Scholar]
- 50.Liuzzi G, et al. Analysis of HIV-1 load in blood, semen and saliva: Evidence for different viral compartments in a cross-sectional and longitudinal study. AIDS. 1996;10:F51–F56. doi: 10.1097/00002030-199612000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Ragni MV, Faruki H, Kingsley LA. Heterosexual HIV-1 transmission and viral load in hemophilic patients. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:42–54. doi: 10.1097/00042560-199801010-00006. [DOI] [PubMed] [Google Scholar]
- 52.Sperling RS, et al. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. N Engl J Med. 1996;335:1621–1629. doi: 10.1056/NEJM199611283352201. [DOI] [PubMed] [Google Scholar]
- 53.Nicolosi A, et al. Italian Study Group on HIV Heterosexual Transmission Risk factors for woman-to-man sexual transmission of the human immunodeficiency virus. J Acquir Immune Defic Syndr. 1994;7:296–300. [PubMed] [Google Scholar]
- 54.Gupta P, et al. High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J Virol. 1997;71:6271–6275. doi: 10.1128/jvi.71.8.6271-6275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayer KH, et al. Persistence of human immunodeficiency virus in semen after adding indinavir to combination antiretroviral therapy. Clin Infect Dis. 1999;28:1252–1259. doi: 10.1086/514775. [DOI] [PubMed] [Google Scholar]
- 56.Zhang H, et al. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998;339:1803–1809. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]
- 57.Rosenberg ES, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 58.Vermund SH. Rationale for the testing and use of a partially effective HIV vaccine. AIDS Res Hum Retroviruses. 1998;14(Suppl 3):S321–S323. [PubMed] [Google Scholar]