Abstract
Biological roles of ERK and MEK in signal transduction have been controversial. The aim of the current study was to determine the role of ERK1/2 in signaling through the ERK-MAPK cascade by using RNAi methodology. Transient transfection of erk1 or erk2 siRNA decreased the respective protein level to 3 - 8% in human lung fibroblasts. Interestingly, individual ERK isoform silencing resulted in a 2-fold reciprocal increase in phosphorylation of the alternate ERK isoform, with no change in respective total protein expression. Moreover, MEK was hyperphosphorylated as a result of combined ERK1 and ERK2 silencing, but was unaffected in individual ERK1 or ERK2 silenced cells. This hyperactivation of MEK was not due to activation of Raf family members, but rather was associated with PP2A downregulation. These data highlight the existence of a feedback loop in normal cells whereby ERK silencing is associated with decreased PP2A activity and consequent MEK activation.
Keywords: MAPK pathway, reciprocal ERK activation, MEK hyperactivation, protein phosphatases
Introduction
The ERK-MAPK pathway regulates numerous cellular processes in normal cells as well as in tumor cells, but its main function lies in the control of cell survival and cell proliferation [1,2]. The ERK-MAPK signaling cascade is organized hierarchically into a three-tiered module composed of MAPKKK(Raf), MAPKK(MEK1/2) and MAPK(ERK1/2). Although it is widely accepted that ERK1 and ERK2 are 84% identical at the protein level and ubiquitously expressed, several studies have highlighted either opposite or similar roles of ERK isoforms in cell proliferation [3-5]. Moreover, in disagreement with the current perception, a very recent study reported a previously uncharacterized role of MEK1 in downregulating MEK2-dependent ERK signaling, emphasizing that the role of MEK1 and MEK2 in growth factor-induced ERK phosphorylation may not be interchangeable [6]. Therefore, there is a clear need for a better understanding of signal transduction through the ERK-MAPK cascade.
Recent studies have addressed the specific role of ERK1, ERK2, and MEK1 in cell proliferation/growth by using RNAi methodology [3,7]. While silencing of ERK and MEK total protein was successful in vitro, abrogation of their active/phosphorylated forms was not achieved through either siRNA or shRNA transfection. For example, silencing ERK2 using siRNAs was accompanied by an increase in ERK1 phosphorylation in ERK1-deficient rat hepatocytes [7]. Similarly, a significant increase of ERK2 phosphorylation was noted in ERK1-silenced hepatocytes [7].
Activation and deactivation of the Raf/MEK/ERK signal transduction cascade is tightly regulated by kinases as well as phosphatases. Various phosphatases such as MKPs and/or PPs reverse phosphorylation on all components of the Raf/MEK/ERK cascade. PP2A is one of the major serine/threonine phosphatases involved in regulation of activation and deactivation of this cascade at multiple steps, as ascertained from in vitro studies, while its regulatory effects on MAPK signaling are cell type- or species-specific [8-11]. Although PP2A can inactivate MEK in vitro, there is no direct evidence to support that PP2A is a physiological MEK inactivator [12]. In keeping with earlier studies, here we report the presence of a reciprocal activation of ERK after individual ERK1 or ERK2 silencing. Importantly, we have identified a feedback activation of MEK as a result of combined ERK1 and ERK2 silencing. Furthermore, this increase of MEK phosphorylation/activity was independent of Raf family member activation. Finally, results of the present studies highlight the existence of a feedback loop in normal human lung fibroblasts whereby ERK silencing is associated with a decrease in PP2A activity.
Materials and Methods
Cell culture and chemical inhibitors
Human lung fibroblasts (HLFs) were grown as previously described [13]. Okadaic acid (OA), tautomycetin (TM), and protein phosphatase inhibitor 2 (a PP1 inhibitor) were obtained from EMD Chemicals and U0126 was from BioMol.
Transfection
HLFs were transfected with 0.5 - 1.0 nmole of the respective siRNA for a luciferase control and SMARTpool for Erk1 (NM_002746) and Erk2 (NM_138957, Dharmacon) as described previously [14].
Immunoblotting
Immunoblotting was performed as previously described [15]. Antibodies used were as follows: ERK1/2, p-ERK1/2(thr202/tyr204), MEK1/2, p-MEK1/2(ser217/221), A-Raf, B-Raf, C-Raf, p-A-Raf(ser299), p-B-Raf(ser445), p-C-Raf(ser338) (Cell Signaling), p-p38(thr180/tyr182) and p-JNK(thr183/tyr185) (Santa Cruz) and α-tubulin (Sigma).
Immunoprecipitation (IP)
HLFs transfected with luciferase, erk1, erk2, or erk1+erk2 siRNA at 72hr post-transfection were washed twice in cold PBS and then lysed in RIPA buffer (Pierce). For anti-p-MEK immunoprecipitation, a Catch and Release Reverse IP System (Millipore) was utilized as previously reported [16].
PP2A activity assay
A nonradioactive assay system (Promega) was used for the detection of PP2A activity according to the manufacturer’s instructions. Cell extracts were isolated from HLFs at 72hr post-transfection with indicated siRNA and prepared as follows: cells were rinsed twice with ice-cold PBS and then lysed by RIPA buffer supplemented with protease inhibitors (Roche). After centrifugation, the resulting supernatant was passed through a Sephadex G-25 spin column (Promega) to remove free intracellular phosphate and then protein concentration for the phosphate-free cell extract was determined by BCA protein assay (Pierce). PP2A activity was determined at 30°C with 5 μg of the phosphate-free cell extract after a 30-min incubation in PP2A-specific reaction buffer. Where indicated, inhibitors of protein phosphatases (i.e., OA or PP1 inhibitor) were added 30-min prior to the initiation of assay reactions.
Statistics
GraphPad Prism 4 was used to perform statistical analysis among different experimental groups by either one way analysis of variance with a Tukey’s post-hoc test or a two tailed, unpaired t test.
Results and Discussion
Reciprocal ERK activation after ERK1 or ERK2 silencing
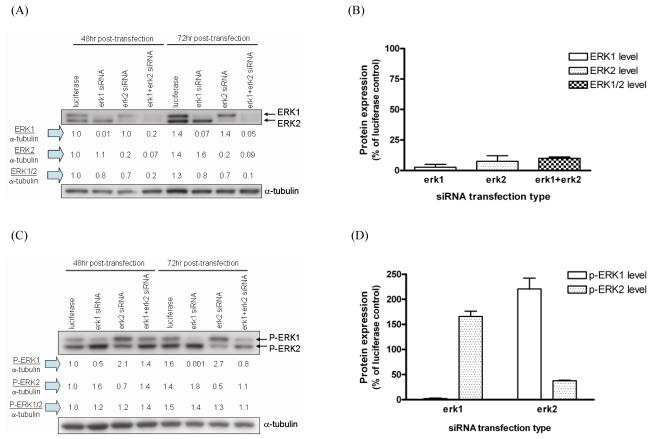
In order to better understand the role of the respective ERK isoform in signal transduction through the ERK-MAPK cascade, RNAi methodology was introduced to silence ERK1 and ERK2. Erk1 siRNA transfection decreased the expression level of ERK1 to 3% of that of control siRNA-transfected cells by 72hr post-transfection (Fig.1A-B). There was no change in expression of ERK2 under this condition suggesting the high specificity of erk1 siRNA for ERK1 knockdown. Similarly, an effective silencing of ERK2 by erk2 siRNA was achieved by 72hr post-transfection, with no compensatory effect on ERK1 expression. Co-transfection with erk1 and erk2 siRNA resulted in 80% and 90% knockdown of both ERK1 and ERK2 protein expression by 48 and 72hr post-transfection, respectively (Fig.1A-B). Transfection of luciferase siRNA had no effect on either p-ERK1/2 nor ERK1/2 protein expression (Suppl. Fig.1).
Fig. 1. Reciprocal ERK activation after ERK1 or ERK2 silencing.
Erk1 erk2, and luciferase siRNA were transfected and protein lysates were isolated from transfected HLFs at 48 and 72hr post-transfection. Protein expression levels for (A,B) ERK1/2 and (C,D) p-ERK1/2 were measured by immunoblotting. One representative blot from four transfections is shown in (A) and (C) and numbers under the blot show individual protein expression normalized by α-tubulin. Densitometric analyses of ERK1, ERK2, ERK1/2, p-ERK1, or p-ERK2 proteins after indicated siRNA transfection are presented as bar plots in (B) and (D). Data are expressed as percent protein expression of luciferase control, and are the average ± SE from four experiments.
Notably, ERK1 silencing by erk1 siRNA transfection was accompanied by a consistent and significant increase in ERK2 phosphorylation at 48 and 72hr post-transfection (Fig.1C). This increase of p-ERK2 level after ERK1 silencing was 1.7-fold on average as compared to control transfection at 72hr post-transfection (Fig.1D). In a similar manner, ERK2 silencing by erk2 siRNA transfection was accompanied by an increase in ERK1 phosphorylation at 48 and 72hr post-transfection (Fig.1C). This increase in p-ERK1 after ERK2 silencing was 2.2-fold as compared to control transfection at 72hr post-transfection (Fig.1D). At 72hr post-transfection, p-ERK1 protein after erk1 siRNA transfection was completely abolished while approximately 65% of p-ERK2 protein after erk2 siRNA transfection was silenced (Fig.1D). Due to this apparent reciprocal ERK activation by silencing the other ERK isoform, there was 30% decrease in the level of combined p-ERK1 and p-ERK2 after combined ERK1/2 silencing at 72hr post-transfection (Fig.1C).
Similar findings on reciprocal ERK activation after either erk siRNA or shERK transfection were recently reported [3,7]. Consistent with our current data, chemical transfection with ERK1 or ERK2-specific siRNA was performed in primary hepatocytes and approximate 87% decrease of the expression level of each isoform was achieved by 72hr post-transfection. A significant increase of reciprocal ERK phosphorylation by individual ERK isoform silencing was noted [7]. Stable transfection with pSUPER-ERK1(sh-ERK1) or pSUPER-ERK2(sh-ERK2) with puromycin selection at 36hr post-transfection was carried out in NIH3T3 cells [3]. Again, consistent with our data, individual transfection with either sh-ERK1 or sh-ERK2 reduced the ERK1 or ERK2 level by about 92% in transfected cells. Silencing of ERK2 alone strongly reduced ERK2 phosphorylation while concomitantly increasing ERK1 phosphorylation compared to that in control cells. Furthermore, when both ERK1 and ERK2 protein expression were silenced simultaneously, the phosphorylation level of ERK1 and ERK2 was maintained, as also shown in the present study. Nevertheless, an increase in the p-ERK2 level after ERK1 silencing was not reported in this study [3]. No potential mechanism for reciprocal activation of ERK after ERK silencing was reported in the two studies mentioned above [3,7].
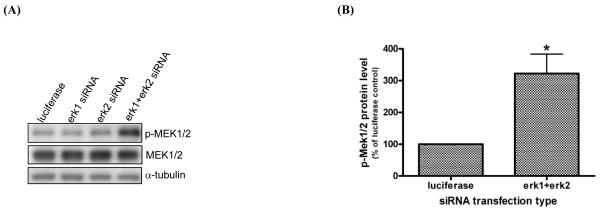
MEK1/2 is hyperactivated after combined ERK1 and ERK2 silencing
In order to explore the molecular mechanism(s) by which a reciprocal increase of p-ERK by alternate ERK isoform silencing sustains ERK phosphorylation after combined ERK1/2 silencing, we examined the direct upstream regulator of ERK phosphorylation, MEK, following individual or combined ERK silencing. MEK1/2 was hyperactivated, as evidenced by increased expression of p-MEK1/2(ser217/221), after erk1 and erk2 siRNA co-transfection (Fig.2A). However, there was no change in the p-MEK1/2 level after individual erk1 or erk2 siRNA transfection. Notably, MEK1/2 phosphorylation after combined ERK1/2 silencing was increased 3.2-fold compared to control cells (Fig.2B). Moreover, there was no change in the total MEK1/2 level under any transfection condition tested. These data suggest that the significant decrease in the combined level of ERK1 and ERK2 protein expression regulates a feedback signal to increase the MEK1/2 phosphorylation level, since the combined level of p-ERK1 and p-ERK2 is similar after both individual and combined silencing with erk1 and erk2 siRNA (Fig.1 A,C).
Fig. 2. MEK1/2 is hyperactivated after combined ERK1 and ERK2 silencing.
Transfection was performed as described in Fig.1 and expression of p-MEK1/2(ser217/221) and MEK1/2 was examined in protein lysates isolated from HLFs transfected with the indicated siRNA at 72hr post-transfection. One representative blot from four transfections is shown in (A) and densitometric analysis of p-MEK1/2 protein expression after normalization by α-tubulin level is shown in (B). Data are expressed as percent protein expression of control, and are the average ± SE from four experiments. *: statistically significant from control at p<0.05.
Although a similar feedback increase in MEK phosphorylation has been previously reported, our current finding is unique since MEK hyperactivation was induced by an abrogation of ERK1/2 expression, and not by a downregulation of ERK1/2 phosphorylation as observed in other studies [17,18]. Endogenous and active MEK levels were found to be increased by pretreatment with the MEK inhibitors PD98059 or U0126 following 1 hr PMA (12-phorbol 13-myristate acetate) treatment in the presence of serum in NIH3T3 and WM35 cells [17]. However, in this latter report, no results were presented on the effect of the MEK inhibitor alone on p-MEK protein expression. Recently, a similar MEK hyperactivation was seen in DU145 cells after U0126 treatment [18]. Additionally, coexpression of MEK1 or MEK2 with MAP kinase phosphatase 1 (MKP-1) resulted in a 7-10-fold activation of MEK, which was attributed to a positive feedback regulation [19].
We next explored the potential crosstalk/feedback among the three MAPK pathways as a potential mechanism for the increase in MEK phosphorylation during ERK1/2 silencing. The activation status of the MAPKs, p38 and JNK was measured by probing for p-p38(thr180/tyr182) and p-JNK(thr183/tyr185), respectively. Neither individual ERK silencing nor simultaneous ERK1/ERK2 silencing had any effect on the activating phosphorylation levels of p38 and JNK (Suppl. Fig.2). This suggests that there is no crosstalk among the three MAPKs when ERK is silenced in HLFs.
MEK activation after ERK1/2 silencing is Raf-independent
In order to understand how MEK1/2 activity is increased in response to ERK1/2 silencing in HLFs, first we examined the activation state of the direct upstream MEK regulators, the Raf family members, under the condition of ERK1 and/or ERK2 silencing. It is known that p-serine 299, p-serine 445, and p-serine 338 play a pivotal role in A-Raf, B-Raf, and C-Raf activation, respectively. Thus, an indirect measure of Raf activity, i.e., the activating phosphorylation status of Raf, was examined by immunoblotting in ERK silenced cells with the phospho-specific and activation-specific antibodies, namely p-A-Raf(ser299), p-B-Raf(ser445), and p-C-Raf(ser338). In contrast to MEK1/2 activation, neither A-Raf, B-Raf nor C-Raf was significantly hyperphosphorylated in an activating manner by combined ERK1/2 silencing (Fig.3A-B). These data suggest that the phosphorylation/activation status of the Raf family was not associated with the p-MEK increase after combined ERK1/2 silencing.
Fig. 3. MEK activation after ERK1/2 silencing is independent of raf.
Transfection was performed as described in Fig.1 and expression of p-A-Raf(ser299), p-B-Raf(ser445), and p-C-Raf(ser338) was examined in protein lysates isolated from HLFs transfected with the indicated siRNA at 72hr post-transfection. One representative blot from three transfections with indicated siRNA is shown in (A) and densitometric analyses of protein expression of p-A-Raf(ser299), p-B-Raf(ser445), and p-C-Raf(ser338) after α-tubulin normalization are shown in (B). Data are expressed as percent protein expression of control, and are the average ± SE from three experiments. (C) Protein lysates from HLFs transfected with the indicated siRNA at 72hr post-transfection were immunoblotted for p-c-Raf(ser338) and p-MEK(217/221) from p-MEK immunoprecipitates. Non-specific rabbit IgG was used as the negative control. Left section of blot shows the expression of two proteins from lysates used as input.
Research has shown that Ras-bound c-Raf is recruited to the plasma membrane for its proper activation [20]. Thus, we examined the possibility for differential enrichment of c-Raf or p-c-Raf(ser338) by immunoblotting of isolated membrane fractions from protein lysates after individual or combined ERK silencing. C-Raf protein was undetectable in a Triton-soluble compartment from ERK-silenced HLFs (data not shown). Furthermore, the formation of active c-Raf complexes with other proteins is critical to transduce its upstream signal to downstream effectors in the c-Raf/MEK/ERK cascade [20]. Therefore, we examined the binding of p-c-Raf(ser338) to p-MEK(ser217/221) at 72hr after individual or combined ERK silencing by co-immunoprecipitation. We postulated that if a physical interaction between these two phospho-proteins was critical to transduce an activation signal, then we should see increased p-c-Raf levels in p-MEK immunoprecipitates after combined ERK1/2 silencing. However, no correlative expression of p-c-Raf(ser338) from p-MEK immunoprecipitates was observed (Fig.3C). In contrast to our current findings, c-Raf was found to be activated after MEK inhibitor treatment or co-overexpression of MEK and MKP-1 [17,19]. Furthermore another recent study reported that B-Raf rather than C-Raf was found to play a critical role in the feedback regulation of MEK utilizing cancer cells with either wild-type B-Raf or B-RafV600E [18]. Our observation that MEK activation after combined ERK1/2 silencing is Raf-independent suggests a possibility for the presence of other MAPKKKs in HLFs. But, it is unlikely that Tpl2/Cot, another upstream regulator of MEK, increases MEK activity in ERK-silenced lung fibroblasts since this kinase is known to be expressed specifically in hematopoitic cells [21] and its mRNA was not expressed in HLFs, as found in our previous microarray analysis [13].
Role of phosphatases in MEK hyperphosphorylation
Signal transduction through the Raf/MEK/ERK cascade reflects a balance between the positive phosphorylation and the reverse dephosphorylation reactions. We hypothesized that dephosphorylation of p-MEK1/2 by protein phosphatases is either blocked or decreased in ERK1/2 silenced cells, thereby increasing the level of p-MEK1/2. Since MEK1/2 can be dephosphorylated by PP2A and PP1 [22,23] and p-MEK elevation by the MEK inhibitor U0126 treatment mimicked that of combined ERK1/2 silencing, we employed okadaic acid (OA, a PP2A inhibitor) and tautomycetin (TM, a PP1 inhibitor) to examine whether these PP inhibitors could alter the expression of p-MEK induced by U0126 in normal untransfected HLFs (Suppl. Fig.3). A single treatment of U0126 increased p-MEK1/2 expression by 4-fold at 1hr post-treatment (Suppl. Fig.3A). Co-treatment of U0126 and OA further enhanced p-MEK1/2 expression as early as 2hr post-treatment (Suppl. Fig.3A). Contrarily, a pre-treatment of TM decreased the expression level of p-MEK1/2 in U0126-treated cells (Suppl. Fig.3B) suggesting PP2A may play a role in MEK dephosphorylation in untransfected cells.
Next, the potential involvement of PP2A in MEK hyperactivation after combined ERK1/2 silencing was examined by direct measurement of its activity. The PP2A activity was measured in ERK1- or/and ERK2-silenced cells at 72hr post-transfection via a serine/threonine phosphatase assay system. The PP2A activity was decreased by approximately 50% when both ERK1 and ERK2 were silenced (Fig.4). However, the level of PP2A activity after individual ERK1 or ERK2 silencing was unchanged compared to that of the control. The magnitude of PP2A inhibition after combined ERK silencing was similar to that induced by 5μM OA in cell lysates from control siRNA-transfected cells. Moreover, pre-treatment of the control lysate with a PP1 inhibitor prior to the PP2A assay did not alter total measured PP2A activity confirming that this assay is specific for PP2A activity (Fig.4).
Fig. 4. Role of protein phosphatases in MEK hyperactivation.
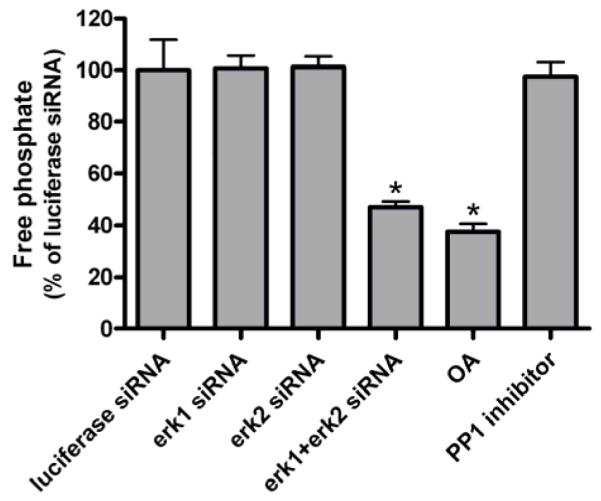
PP2A activity was measured for HLFs transfected with indicated siRNA by a Serine/Threonine Phosphatase Assay System. A standard curve for free phosphate was prepared for each experiment from Phosphate Standard and released phosphates in samples linearly correspond to absorbance readings with a 650nm filter. The amount of free phosphate released by PP2A from HLFs transfected with luciferase, erk1, erk2, or erk1+erk2 siRNA is shown as percent of control siRNA and is the average ± SE from three experiments. *: statistically significant from control siRNA at p<0.05 by two-tailed t-test. The amount of free phosphate released from control siRNA-transfected cells after pre-treatment of 5μM OA or 50ng/μL PP1 inhibitor is also shown.
PP2A is known to regulate c-Raf-MEK-ERK signaling through both positive and negative mechanisms via a feedback loop [22,24]. Ras-GTP induces kinase suppressor of Ras (KSR) dephosphorylation of ser 392 by generating active PP2A, resulting in translocation of KSR to the cell membrane. KSR in turn facilitates the phosphorylation of MEK by Raf [20]. Therefore, PP2A positively regulates the c-Raf/MEK/ERK cascade in this context. In contrast, critical phosphoserine residues on c-Raf, which are dephosphorylated by PP2A, reduce c-Raf catalytic activity and lower the ability of c-Raf to associate with Ras, which keeps signaltransduction through MEK-ERK low. ERK1/2 phosphorylates these same serine residues through a negative feedback mechanism [25]. Thus, the overall effect of PP2A on the status of c-Raf activity should not change in response to ERK silencing. In contrast, results of the current study suggest that PP2A plays a role in increased MEK phosphorylation in ERK1/2 silenced cells. The molecular mechanism by which PP2A regulates MEK phosphorylation is in need of further study.
The level of p-ERK1/2 was not completely diminished after combined ERK1/2 silencing, which may explain why a negative feedback from ERK to c-Raf was not found in the current studies. Other studies have reported that MEK hyperphosphorylation via Raf after MEK inhibitor treatment was consistently accompanied by a complete removal of p-ERK1/2 and no change in total ERK level [17,18]. We propose that when both ERK1 and ERK2 are abrogated after combined ERK1/2 silencing, cells need to quickly replenish the ERK protein pool, thus this short feedback loop of MEK-ERK, reported in the current study, becomes critical. However, the longer feedback loop of Raf-MEK-ERK may play a major role when ERK is dephosphorylated, but total protein is present.
In conclusion, our current studies highlight the reciprocal ERK activation after ERK1 or ERK2 downregulation and MEK activation in response to both ERK1 and ERK2 downregulation. This MEK activation was Raf-independent and its activity was associated with a corresponding decrease in PP2A activity. The presence of a positive feedback regulatory mechanism involving MEK hyperactivation in response to ERK downregulation has potential clinical significance, since targeted cancer therapeutics have been found to show feedback regulation and drug resistance. Therefore, pharmaceuticals which target protein kinases whose activation is regulated by phosphorylation and dephosphorylation need to be thoroughly examined with respect to the activation status of target as well as upstream and downstream effectors due to the potential presence of multiple feedback regulatory mechanisms.
Supplementary Material
Acknowledgments
The authors acknowledge the support and encouragement of Dr. Steven Patierno. We thank Dr. Travis O’Brien, Dr. Gina Chun, Madhu Lal, Kristen Wright, and Laura Savery for helpful suggestions. This work was supported by NIH Grant CA 107972 to SC.
Abbreviations
- MEK
mitogen extracellular kinase
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen-activated protein kinase
- MAPKK
MAPK kinase
- MAPKKK
MAPK kinase kinase
- MKP
MAP kinase phosphatase
- PP
protein phosphatase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Pages G, Lenormand P, L’Allemain G, Chambard JC, Meloche S, Pouyssegur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc.Natl.Acad.Sci.U.S.A. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lavoie JN, L’Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J.Biol.Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- [3].Lefloch R, Pouyssegur J, Lenormand P. Single and combined silencing of ERK1 and ERK2 reveals their positive contribution to growth signaling depending on their expression levels. Mol.Cell Biol. 2008;28:511–527. doi: 10.1128/MCB.00800-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sanjo H, Hikida M, Aiba Y, Mori Y, Hatano N, Ogata M, Kurosaki T. Extracellular signal-regulated protein kinase 2 is required for efficient generation of B cells bearing antigen-specific immunoglobulin G. Mol.Cell Biol. 2007;27:1236–1246. doi: 10.1128/MCB.01530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vantaggiato C, Formentini I, Bondanza A, Bonini C, Naldini L, Brambilla R. ERK1 and ERK2 mitogen-activated protein kinases affect Ras-dependent cell signaling differentially. J.Biol. 2006;5:14.1–14.15. doi: 10.1186/jbiol38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Catalanotti F, Reyes G, Jesenberger V, Galabova-Kovacs G, de Matos SR, Carugo O, Baccarini M. A Mek1-Mek2 heterodimer determines the strength and duration of the Erk signal. Nat.Struct.Mol.Biol. 2009;16:294–303. doi: 10.1038/nsmb.1564. [DOI] [PubMed] [Google Scholar]
- [7].Fremin C, Ezan F, Boisselier P, Bessard A, Pages G, Pouyssegur J, Baffet G. ERK2 but not ERK1 plays a key role in hepatocyte replication: an RNAi-mediated ERK2 knockdown approach in wild-type and ERK1 null hepatocytes. Hepatology. 2007;45:1035–1045. doi: 10.1002/hep.21551. [DOI] [PubMed] [Google Scholar]
- [8].Adams DG, Coffee RL, Jr., Zhang H, Pelech S, Strack S, Wadzinski BE. Positive regulation of Raf1-MEK1/2-ERK1/2 signaling by protein serine/threonine phosphatase 2A holoenzymes. J.Biol.Chem. 2005;280:42644–42654. doi: 10.1074/jbc.M502464200. [DOI] [PubMed] [Google Scholar]
- [9].Kao G, Tuck S, Baillie D, Sundaram MV. C. elegans SUR-6/PR55 cooperates with LET-92/protein phosphatase 2A and promotes Raf activity independently of inhibitory Akt phosphorylation sites. Development. 2004;131:755–765. doi: 10.1242/dev.00987. [DOI] [PubMed] [Google Scholar]
- [10].Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem.Sci. 1999;24:186–191. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- [11].Strack S. Overexpression of the protein phosphatase 2A regulatory subunit Bgamma promotes neuronal differentiation by activating the MAP kinase (MAPK) cascade. J.Biol.Chem. 2002;277:41525–41532. doi: 10.1074/jbc.M203767200. [DOI] [PubMed] [Google Scholar]
- [12].Zheng CF, Guan KL. Cytoplasmic localization of the mitogen-activated protein kinase activator MEK. J.Biol.Chem. 1994;269:19947–19952. [PubMed] [Google Scholar]
- [13].Bae D, Camilli TC, Chun G, Lal M, Wright K, O’Brien TJ, Patierno SR, Ceryak S. Bypass of hexavalent chromium-induced growth arrest by a protein tyrosine phosphatase inhibitor: enhanced survival and mutagenesis. Mutat.Res. 2009;660:40–46. doi: 10.1016/j.mrfmmm.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bae D, Camilli TC, Ha NT, Ceryak S. Enhanced clonogenic survival induced by protein tyrosine phosphatase (PTP) inhibition after Cr(VI) exposure is mediated by c-Raf and Ras activity. Cell Signal. 2009;21:727–736. doi: 10.1016/j.cellsig.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ceryak S, Zingariello C, O’Brien T, Patierno SR. Induction of pro-apoptotic and cell cycle-inhibiting genes in chromium (VI)-treated human lung fibroblasts: lack of effect of ERK. Mol.Cell Biochem. 2004;255:139–149. doi: 10.1023/b:mcbi.0000007270.82431.3e. [DOI] [PubMed] [Google Scholar]
- [16].Lal MA, Bae D, Camilli TC, Patierno SR, Ceryak S. AKT1 mediates bypass of the G(1)/S checkpoint after genotoxic stress in normal human cells. Cell Cycle. 2009;8:1589–1602. doi: 10.4161/cc.8.10.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ahn NG, Nahreini TS, Tolwinski NS, Resing KA. Pharmacologic inhibitors of MKK1 and MKK2. Methods Enzymol. 2001;332:417–431. doi: 10.1016/s0076-6879(01)32219-x. [DOI] [PubMed] [Google Scholar]
- [18].Daouti S, Wang H, Li WH, Higgins B, Kolinsky K, Packman K, Specian A, Jr., Kong N, Huby N, Wen Y, Xiang Q, Podlaski FJ, He Y, Fotouhi N, Heimbrook D, Niu H. Characterization of a novel mitogen-activated protein kinase kinase 1/2 inhibitor with a unique mechanism of action for cancer therapy. Cancer Res. 2009;69:1924–1932. doi: 10.1158/0008-5472.CAN-08-2627. [DOI] [PubMed] [Google Scholar]
- [19].Shapiro PS, Ahn NG. Feedback regulation of Raf-1 and mitogen-activated protein kinase (MAP) kinase kinases 1 and 2 by MAP kinase phosphatase-1 (MKP-1) J.Biol.Chem. 1998;273:1788–1793. doi: 10.1074/jbc.273.3.1788. [DOI] [PubMed] [Google Scholar]
- [20].Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat.Rev.Mol.Cell Biol. 2005;6:827–837. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- [21].Waterfield MR, Zhang M, Norman LP, Sun SC. NF-kappaB1/p105 regulates lipopolysaccharide-stimulated MAP kinase signaling by governing the stability and function of the Tpl2 kinase. Mol.Cell. 2003;11:685–694. doi: 10.1016/s1097-2765(03)00070-4. [DOI] [PubMed] [Google Scholar]
- [22].Dent P, Jelinek T, Morrison DK, Weber MJ, Sturgill TW. Reversal of Raf-1 activation by purified and membrane-associated protein phosphatases. Science. 1995;268:1902–1906. doi: 10.1126/science.7604263. [DOI] [PubMed] [Google Scholar]
- [23].Gomez N, Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991;353:170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- [24].Robbins DJ, Zhen E, Owaki H, Vanderbilt CA, Ebert D, Geppert TD, Cobb MH. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J.Biol.Chem. 1993;268:5097–5106. [PubMed] [Google Scholar]
- [25].Dougherty MK, Muller J, Ritt DA, Zhou M, Zhou XZ, Copeland TD, Conrads TP, Veenstra TD, Lu KP, Morrison DK. Regulation of Raf-1 by direct feedback phosphorylation. Mol.Cell. 2005;17:215–224. doi: 10.1016/j.molcel.2004.11.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.