Abstract
Introduction
Low BMD and fracture may be complications of type 1 diabetes. We sought to determine the roles of bone turnover and glycemic control in the etiology of low BMD.
Methods
Premenopausal women from the Wisconsin Diabetes Registry Study and matched controls were compared (n=75 pairs). Heel and forearm BMD were measured, and hip and spine BMD were measured in a subset. Markers of bone formation (osteocalcin) and resorption (NTx), and glycemic control (HbA1c) were determined.
Results
Age ranged from 18–50 years with a mean of 28, and 97% were Non-Hispanic white. Among women with diabetes, mean disease duration was 16 years and current HbA1c was 8%. Compared to controls, women with diabetes had a high prevalence of previous fracture (37% vs. 24%) and low BMD for age (heel or forearm: 49% vs. 31%), low heel and forearm BMD, and low osteocalcin levels. Levels of NTx were similar, suggesting uncoupled turnover favoring resorption. Poor glycemic control was associated with low BMD at all bone sites except the spine, and with low osteocalcin and NTx levels.
Conclusions
Optimal glycemic control may prevent low BMD and altered bone turnover in type 1 diabetes, and decrease fracture risk.
Keywords: bone mineral density; bone turnover; diabetes mellitus, type 1; glycemic control; premenopausal
Low BMD may be a complication of type 1 diabetes [1], and previous research points to higher risk of fracture associated with the disease [2]. With the incidence of type 1 diabetes rising worldwide [3], and improvements in life expectancy due to better disease management [4], type 1 diabetes could be an important contributor to the morbidities and costs associated with fracture. However, major questions remain unanswered which have been echoed in recent reviews of the literature [5–7]. For example, it is unclear what etiologic mechanisms, such as alterations in bone turnover and the effects of long-term glycemic control, mediate the association between type 1 diabetes and low BMD. Gaps in our understanding of clinical implications also remain, such as the effect of type 1 diabetes on BMD independent of changes in lifestyle, body composition, and health status associated with diabetes, and the actual prevalence of low BMD with type 1 diabetes [5].
Previous studies evaluating bone turnover by diabetes status have produced conflicting results [8–10], and studies on current glycemic control have not consistently detected an association between hemoglobin A1c (HbA1c) and BMD [11–13]. However, prior studies have used small clinic samples and very limited adjustment for confounders. One sufficiently powered study used a community-based sample of premenopausal women 35–55 years old and found similar levels of bone turnover by diabetes status, and no association between current HbA1c and BMD. However, this study did not adjust for such factors as age, weight, or medication use [14]. Results of research evaluating the effect of long-term glycemic control (disease durations ranging from one to 12 years within the sample) have been equivocal [15;16]. Much of the prior research on absolute BMD levels in those with type 1 has had similar methodological limitations (clinic samples, insufficient power, and limited adjustment for confounders) [8–10]. The few large studies based on representative samples with control groups found low BMD in individuals with type 1 diabetes compared to controls [14;17]. However, these studies did not collect data to control for important potential confounders such as dietary intake, comorbid conditions, reproductive history, or genetics. Therefore, questions on the pathophysiology and clinical relevance of bone fragility in those with type 1 diabetes remain.
The present study conducted in young women with type 1 diabetes was designed to address the above gaps in knowledge by using a sample homogenous with regard to gender, race, menopausal status, and disease duration, and is the first to use a matched control group with measurement of several possible confounders. It is also the first study to determine the effect of 10 years of glycemic control on BMD and bone turnover for an entire sample by utilizing data available since diagnosis. We hypothesized that type 1 diabetes has an independent clinically significant, negative association with BMD and bone turnover, and that poor glycemic control is related to low BMD and altered bone turnover. Such information may have implications for patient education and screening recommendations, and could aid future interventions to prevent bone loss and ultimately reduce fracture risk in those affected by type 1 diabetes.
Material and Methods
Population & Sample
The Wisconsin Diabetes Registry Study (WDRS) is a population-based cohort of incident cases with type 1 diabetes. Methods of ascertainment and recruitment have been published previously [18]. Briefly, new cases with type 1 diabetes diagnosed between May 1987 and April 1992, ≤30 years of age, and living in 28 contiguous counties in Wisconsin were identified and enrolled (n=597). Based on criteria in use during the time period WDRS participants were enrolled [19], diabetes was defined by the presence of polyuria and polydipsia with initiation of exogenous insulin use. Seven participants who were able to go off insulin after enrollment were dropped from the Registry.
Of the 597 participants originally enrolled in WDRS, 288 were women. Of these, 191 met our preliminary enrollment criteria in 2005 (continued participation in WDRS, 18–50 years of age, and living in Wisconsin). Thirteen women could not be contacted. Of those contacted, 17 women were ineligible due to menopause, or pregnancy or breastfeeding within the previous three months. Through the end of recruitment in December 2005, 89 eligible women with diabetes participated. The women were asked to identify a matched control (in priority order) as a full or half sister, female cousin, niece/aunt, or female friend without diabetes of the same race/ethnicity and within five years of age, up to 10 years if needed. Consequently, the pairs were also matched for many unmeasured life style factors. Seventy-five of the 89 eligible women with diabetes that participated had a matched control and form the sample for this analysis, providing 75 matched pairs.
The 75 women with diabetes in the current study were very similar to women within the same age range (18–50 years old in 2005) initially enrolled in the WDRS (n=266). There were no differences in baseline characteristics including age at diagnosis, mother’s level of education, number of insulin injections and blood glucose checks per day, and mean HbA1c during the first year of diabetes. However, the sample for the current study had a smaller percentage of minorities (3% vs. 8%) and slightly higher occupation-based socioeconomic status. Study approval was obtained from the Institutional Review Board at the University of Wisconsin-Madison and participants provided written informed consent at enrollment and at all subsequent visits.
Data Collection & Variables
Lifetime History of Fracture
Participants were asked via questionnaire about the occurrence of any previous “fracture or broken bone that had been confirmed by a physician or X-ray.” Fractures of the skull, face, metacarpals, metatarsals, fingers and toes were not considered [20].
BMD
Bilateral heel (calcaneus) and forearm (distal radius/ulna) BMD were measured in all participants by DXA using the Peripheral Instantaneous X-ray Imager (PIXI, GE Lunar, Madison, WI). The portable PIXI was selected for this study to make it possible to use the same apparatus to collect BMD data on women located throughout the state of Wisconsin. Previous research has demonstrated that heel BMD is a valid measure of bone health as it is predictive of future fracture [21] and identifies increased fracture risk almost as well as hip BMD [22]. Means of bilateral heel and forearm measurements were analyzed. To validate peripheral measurements, hip and spine BMD were measured in a convenience subgroup of women (n=19 pairs) by DXA using a Prodigy densitometer (GE Healthcare Lunar, Madison, WI) at the central study location. Lumbar spine BMD was determined using the antero-posterior projections and calculated as the mean of L1–L4 vertebrae. The proximal femur of the left hip was measured and BMD of the total hip and femoral neck are presented. All measurements were conducted by a single technologist. Coefficients of variation (CVs) of repeated measurements with repositioning were ≤3% at all sites.
Low BMD for Age
The classification of low BMD for age at each bone site was based on Z-scores (number of SDs from the mean value for the PIXI and Prodigy’s sex, race, and age-matched reference group) [23]. The current research study defined low BMD for age as a Z-score ≤−1.0 to capture changes in BMD of borderline risk not yet in the range for clinical diagnosis. Heel/forearm and hip low BMD for age were defined using the minimum Z-score of the right and left sites, and total hip and femoral neck, respectively.
Bone Turnover Markers
Bone turnover was assessed by measuring serum levels of osteocalcin and NTx as indicators of bone formation and resorption, respectively. Blood was collected during days 20–24 of the menstrual cycle (luteal phase), without regard to fasting, or time of day or year, and the serum was immediately separated onsite and frozen in a portable −80°C freezer. Osteocalcin was analyzed by Fairview Diagnostic Labs (Minneapolis, MN) using a chemiluminescent immunoassay. NTx was measured by Dynacare Labs (Milwaukee, WI) with an ELISA. The intra- and inter-assay CVs were ≤9% for osteocalcin, and ≤14% for NTx.
The Uncoupling Index (UI) was computed for the women with type 1 diabetes to assess the balance of formation and resorption during bone turnover. The method was originally suggested and validated by Eastell and colleagues and has been shown to be associated with bone outcomes such as osteoporosis in diverse samples [24]. Each osteocalcin and NTx level for the women with diabetes was first expressed as a Z-score (number of SDs from the mean of the 75 matched controls). The UI was then calculated as the osteocalcin Z-score minus NTx Z-score. A positive UI reflects bone turnover favoring formation; a negative UI reflects turnover favoring resorption. Controls therefore have a mean UI of zero and 50% have a negative UI.
Glycemic Control
Whole blood samples were analyzed for HbA1c, a measure of glucose control during the previous six to eight weeks, within seven days of collection by Fairview Diagnostic Labs (Minneapolis, MN). Automated high performance liquid chromatography was used following the Diabetes Control and Complications Trial (DCCT) reference method [25]. The nondiabetic range for this assay was 4.3–6.0%. The intra-assay coefficient of variation from blind split-samples was 0.6%. The inter-assay coefficient of variation reported by the lab was 2.5–3.3%.
Data on long-term glycemic control for the women with diabetes, as measured by total glycosylated hemoglobin (GHb) levels, came from previous follow-up of the WDRS. Total GHb is a measure of the total amount of hemoglobin in the blood that is glycosylated versus HbA1c which is a measure of a specific subtype of hemoglobin in the blood that is glycosylated. Total GHb and HbA1c are therefore highly correlated (r=0.98, unpublished data from WDRS, January 2002). However, the association is not linear. Based on a laboratory study of split samples by the WDRS, the following formula was developed: HbA1c = 0.786 + 0.797(GHb) − 0.006(GHb2).
During each previous WDRS follow-up exam, venipuncture was performed for determination of total GHb. Participants were also asked to submit a blood specimen from each routine visit to their clinic/physician, or every four months if no visit was scheduled. Prestamped mailing kits containing 5-ml EDTA-treated vacutainers were provided. Whole blood samples were analyzed for total GHb level within seven days of collection by Isolab GlycAffin microcolumn affinity chromatography (Isolab, Akron, OH) [26]. Internal standards from nondiabetic children and young adults had a mean (SD) total GHb level of 5.5 (0.8)%. Intra-assay variation was 1.1% for case samples and 0.9% for internal standards. The longitudinal measure of glycemic control for the women with diabetes was computed by taking the average of all total GHb determinations across the 10 years prior to the current study on bone health. The number of total GHb measurements ranged from one to 18, with a mean of 7.8.
Adjustment Variables
Height and weight were measured using a stadiometer height rod and physician beam scale (Healthometer, Bridgeview, IL), respectively. BMI was calculated as weight in kilograms divided by height in meters squared. Waist and hip circumference were measured twice and averaged to calculate the waist-to-hip ratio. Other potential confounders collected in detail by questionnaire included demographics, health behaviors (e.g. smoking history, daily alcohol and caffeine use, and weight change within the last year), reproductive health (e.g. age at menarche and average length of the menstrual cycle), and family and personal medical history (e.g. conditions and medications known to affect bone such as thyroid and celiac disease, family history of osteoporosis, and corticosteroid and hormonal contraceptive use). Dietary intake (e.g. calcium and vitamin D) was determined via a brief food frequency questionnaire [27]. Physical activity was measured using the Five-City Project physical activity assessment and quantified as daily total energy expenditure [28]. Because of bone marker variability, precision adjustment variables for bone marker levels included the time intervals since rising and since last food intake, timing in the menstrual cycle, and calendar month for the blood draw [29–31]. Long-term 10-year mean total GHb was adjusted for the number of blood samples used to calculate the mean to capture the varying precision of the estimates and possible health consciousness.
Statistical Analysis
Analyses were performed using SAS, version 8.0 (SAS Institute, Cary, NC). Statistical tests were considered significant at p<0.05. Differences in descriptive characteristics by diabetes status were tested by the paired t-test or McNemar’s test. The mean osteocalcin and NTx Z-score and UI for the women with diabetes were compared to zero by a one-group t-test. The proportion of women with diabetes with a negative UI was compared to 50% by a test of two proportions. Pearson correlations were computed to assess the consistency of BMD measurements, explore associations of BMD with key variables, and determine the association between current HbA1c and 10-year mean total GHb. Asymptotic or exact (hip and spine with sample size <30) 95% CIs were determined for the frequency of low BMD for age.
To assess associations of diabetes status with fracture and low BMD for age, conditional logistic regression models with maximum likelihood or exact (hip and spine) estimates were used. Linear regression models of differences were fitted to examine BMD and bone turnover markers by diabetes status. For example, the difference in BMD between matched women was regressed on the differences in adjustment variables. All adjusted models for low BMD for age and BMD included age and BMI. The potential confounding of all other variables measured was then assessed. Additional variables were included in the final adjusted models if the coefficient quantifying the association of interest changed by more than 10% in either direction, otherwise they were removed. However, models were not adjusted if adding covariates would have resulted in <10 events (e.g. cases of low BMD for age) or participants per variable [32] Sensitivity analyses limited the models of differences in BMD and bone turnover markers by diabetes status to the: 1) 44 pairs of sisters, 2) 19 pairs with central BMD, and 3) 38 pairs >=25 years old; results did not change and are presented using the entire sample.
To determine if differences in bone markers between groups could account for some of the variation in BMD by diabetes status, the differences between matched individuals in osteocalcin and NTx were added individually to each final BMD model. Linear regression models of group differences were also fitted to determine the effect of osteocalcin and NTx on BMD. To examine whether associations between bone turnover and BMD differed by diabetes status, two-way interactions between osteocalcin/NTx and diabetes status were modeled by entering the osteocalcin/NTx level of the woman with diabetes from each matched pair. The associations of BMD and bone turnover markers with UI, HbA1c and 10-year mean total GHb in the women with diabetes were analyzed using ordinary linear regression with adjustment for confounders as described above.
Results
For the 75 matched pairs (Table 1), mean age was 28 years and 97% were Non-Hispanic white. Of the controls, 71% were relatives and 29% were friends. The only significant differences between groups were that women with diabetes consumed less alcohol and drank more caffeine, and that a larger proportion had used thyroid replacement medication; height did not differ. Among women with diabetes, mean disease duration was 16 years, current HbA1c was 8.1%, and all reported exclusive use of insulin for their diabetes treatment. All the control women fell within the normal range for glycemic control (HbA1c ≤6%). None of the women in the study self-reported celiac disease.
Table 1.
Characteristics by diabetes status for 75 matched pairs of premenopausal women
| Characteristic | Type 1 Diabetes | No Diabetes |
|---|---|---|
| npairs=75 | ||
| Demographic | ||
| Age (years) | 27.7 (6.8) | 28.3 (8.2) |
| Range (years) | 18.1 – 45.4 | 18.0 – 50.7 |
| Non-Hispanic white (%) | 97.3 | 97.3 |
| Control relationship (%): | ||
| Full-sister | 58.7 | |
| First-cousin/niece | 12.0 | |
| Friend | 29.3 | |
| Anthropomorphic | ||
| Height (cm) | 165.2 (6.6) | 165.9 (7.1) |
| Weight (kg) | 76.7 (18.3) | 75.2 (19.7) |
| BMI (kg/m2) | 28.0 (5.8) | 27.2 (6.2) |
| Waist-to-hip ratio | 0.78 (0.05) | 0.77 (0.05) |
| Health behaviors/diet | ||
| Current smoker (%) | 20.0 | 28.0 |
| Current drinker (%) | 81.3 | 88.0 |
| Calcium (mg/day) | 1130 (749) | 966 (662) |
| Vitamin D (IU/day) | 279 (289) | 258 (216) |
| Alcohol (g/day) | 5.3 (7.2)* | 9.3 (15.3) |
| Caffeine (mg/day) | 156 (147)* | 98 (100) |
| Reproductive history | ||
| Current hormone use (%): | ||
| Estrogen + progesterone | 37.3 | 38.7 |
| Progesterone only | 9.3 | 1.3 |
| Average menstrual cycle length >30 days a (%) | 17.9 | 14.3 |
| Amenorrhea for ≥3 months a (%) | 7.1 | 7.1 |
| Ever pregnant (%) | 26.7 | 34.7 |
| Number of pregnancies b | 2.4 (1.1) | 2.9 (1.7) |
| Medical history | ||
| Ever had hypothyroidism (%) | 18.7* | 6.7 |
| Family history of osteoporosis (%) | 8.1 | 2.7 |
| Family history of fracture (%) | 68.9 | 71.6 |
| Diabetes | ||
| Current HbA1c (%) | 8.1 (1.7)* | 5.0 (0.3) |
| Range (%) | 5.6 –14.6 | 4.4 – 5.8 |
| 10-year mean total GHb (%) | 10.7 (2.0) | |
| Range (%) | 7.2 – 16.0 | |
| Age at diagnosis (years) | 11.9 (6.7) | |
| Range (years) | 1.1 – 29.5 | |
| Duration (years) | 16.1 (1.5) | |
| Range (years) | 13.2 – 18.4 | |
| Insulin administration (%): | ||
| <4 injections | 24.0 | |
| ≥4 injections | 30.7 | |
| Insulin pump | 45.3 | |
| Fracture | ||
| Ever fractured (%) | 37.3* | 24.0 |
| Ever fractured ≥18 years old (%) | 13.3 | 9.3 |
Data are means (SDs) and percentages
p<0.05
Matched pairs where neither woman was taking hormones, npairs =28
Matched pairs where both women had ever been pregnant, npairs =12
Women with type 1 diabetes had a higher frequency of lifetime fracture than did controls (37 vs. 24%, OR=2.3 [95% CI: 1.0–5.2]; Table 1). Significantly more women with diabetes had low BMD for age compared to matched controls peripherally in the heel or forearm (49 vs. 31%, OR=3.0; Table 2). Similarly, more women with diabetes had low BMD for age centrally in the hip or spine (32 vs. 11%, OR=5.0), but this difference was not statistically significant. After adjustment for age and BMI, the odds of low BMD for age at peripheral sites remained almost three times greater in women with diabetes.
Table 2.
Frequencies and ORs for low BMD for age (Z-score ≤ −1.0) for 75 matched pairs of premenopausal women with and without type 1 diabetes
| Low BMD for Age at Bone Sites |
Type 1 Diabetes (%) |
No Diabetes (%) |
OR |
|
|---|---|---|---|---|
| Unadjusted | Age & BMI Adjusted | |||
| npairs=75 | ||||
| Heel | 24 (14,34) | 7 (1, 12) | 4.3 (1.4, 12.6)* | 4.2 (1.1, 16.9)* |
| Forearm | 48 (37, 59) | 28 (18, 38) | 3.5 (1.4, 8.7)* | 3.1 (1.2, 8.1)* |
| Peripheral | 49 (38, 61) | 31 (20, 41) | 3.0 (1.3, 7.1)* | 2.7 (1.1, 6.8)* |
| npairs=19 | ||||
| Hip | 21 (6, 46) | 0 (0, 18) | ||
| Spine | 21 (6, 46) | 11 (1, 33) | 3.0 (0.2, 157.5) | |
| Central | 32 (13, 57) | 11 (1, 33) | 5.0 (0.6, 236.5) | |
Data are frequencies and ORs (95% CIs)
p<0.05
Hip and spine BMD significantly correlated with heel (r=0.64–0.70) and forearm BMD (r=0.47–0.55), irrespective of diabetes status. Age was not associated with BMD at any site except the spine (r=0.33). BMI was significantly associated with BMD at all sites for the control group (r=0.42–0.51). For the diabetes group, BMI was significantly related to heel and forearm BMD (r=0.42–0.47), but not hip or spine BMD (r=0.09–0.22).
Women with type 1 diabetes had significantly lower BMD levels at the heel, forearm, and femoral neck than did their matched controls (Table 3). With adjustment, BMD remained significantly lower in the heel and forearm; BMD levels were consistently, but not significantly, lower than among controls in the total hip, femoral neck, and spine. Adjustment for any differences between matched pairs in height, use of thyroid medication and progesterone-only contraceptives, and a family history of osteoporosis did not change the results and was therefore not applied to the final models. Bone areas of each site measured did not differ by diabetes status (data not shown).
Table 3.
Differences in BMD and bone turnover markers, and the potential mediating effects of bone turnover, for 75 matched pairs of premenopausal women with and without type 1 diabetes
| Type 1 Diabetes |
No Diabetes | Difference |
||
|---|---|---|---|---|
| Unadjusted | Adjusted a | |||
| BMD (g/cm2) at Bone Sites | ||||
| npairs=75 | ||||
| Heel | 0.495 (0.091) | 0.534 (0.094) | −0.039 (0.013)* | −0.053 (0.013)* |
| Forearm | 0.440 (0.048) | 0.465 (0.052) | −0.025 (0.007)* | −0.035 (0.007)* |
| npairs=19 | ||||
| Total Hip | 1.021 (0.130) | 1.083 (0.119) | −0.062 (0.037) | −0.057 (0.041) |
| Femoral Neck | 1.027 (0.146) | 1.094 (0.118) | 0.067 (0.033)* | −0.062 (0.038) |
| Spine | 1.210 (0.146) | 1.263 (0.155) | −0.053 (0.040) | −0.050 (0.043) |
| Bone Turnover Markers | ||||
| npairs=75 | ||||
| Osteocalcin (nmol/L) | 0.62 (0.29) | 0.79 (0.31) | −0.17 (0.05)* | −0.26 (0.05)* |
| NTx (nmol BCE/L) | 11.3 (4.6) | 11.6 (4.5) | −0.3 (0.7) | −0.4 (0.7) |
Data are means (SDs) and regression coefficients (SEs) quantifying differences by diabetes status (type 1 diabetes – no diabetes)
BCE, bone collagen equivalent
p<0.05
- BMD: age, BMI, weight (heel/forearm), alcohol and caffeine consumption (heel/forearm)
- Osteocalcin: alcohol consumption, time since rising, month of year
- NTx: BMI, weight change in last year, alcohol consumption, time since rising
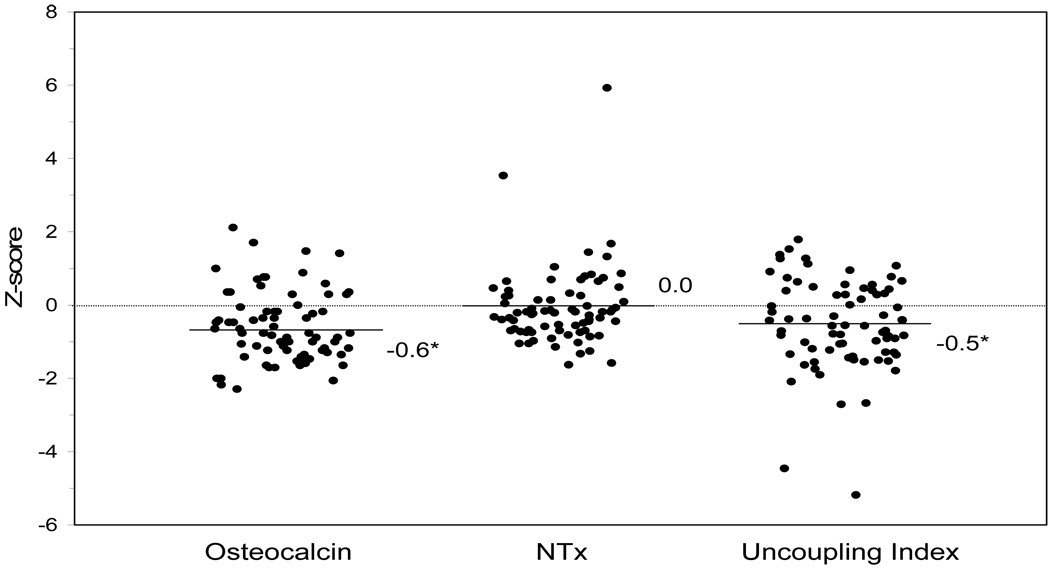
Bone formation, as measured by osteocalcin, was significantly lower in women with type 1 diabetes than in controls, both before and after adjustment (Table 3, Figure 1). However, bone resorption, as measured by NTx, was similar in the two groups. The mean UI for the premenopausal women with diabetes (Figure 1) was negative (−0.5) and significantly deviated from zero, indicating uncoupled bone turnover favoring resorption. Overall, 65% of the women with type 1 diabetes had a negative UI, significantly higher than the control group (p=0.009).
Figure 1.
Individual levels of bone turnover markers for 75 premenopausal women with type 1 diabetes (points jittered horizontally). Each level is expressed as a Z-score (number of SDs from the mean of 75 matched controls). The Uncoupling Index was calculated as the osteocalcin Z-score minus NTx Z-score. Lines with respective numbers are mean Z-scores or the UncouplingIndex. *p<0.05 statistical significance of the mean Z-score or Uncoupling Index compared to zero as assessed by one-group t-test.
When the difference in osteocalcin or NTx levels between matched pairs was added to the final models for BMD, the difference in BMD level by diabetes status did not change at any site. BMD at any bone site was also not associated with osteocalcin or NTx in either group, or the UI in the women with diabetes (data not shown).
Current HbA1c and 10-year mean total GHb in those with diabetes were strongly, but not perfectly, correlated (r=0.75). In the women with type 1 diabetes (Table 4), current and 10-year mean glycemic control had a strong, negative association with BMD at each bone site, except the spine, in both unadjusted and adjusted models. Osteocalcin and NTx levels also had a significant negative association with current HbA1c that became apparent with adjustment for relevant confounders. However, neither bone turnover marker was associated with 10-year mean total GHb.
Table 4.
The association of BMD and bone turnover markers with current and long-term glycemic control in 75 premenopausal women with type 1 diabetes
| Current HbA1c (%) |
10-year mean Total GHb (%) |
|||
|---|---|---|---|---|
| BMD (g/cm2) at Bone Sitesa | ||||
| n=75 | ||||
| Heel | ||||
| Unadjusted | −0.016 (0.006)* | −0.015 (0.005)* | ||
| Adjusted | −0.014 (0.005)* | −0.013 (0.005)* | ||
| Forearm | ||||
| Unadjusted | −0.012 (0.003)* | −0.009 (0.003)* | ||
| Adjusted | −0.010 (0.003)* | −0.006 (0.002)* | ||
| n=19 | ||||
| Total Hip | −0.037 (0.015)* | −0.043 (0.016)* | ||
| Femoral Neck | −0.040 (0.017)* | −0.053 (0.017)* | ||
| Spine | −0.022 (0.019) | −0.021 (0.021) | ||
| Bone Turnover Markersa | ||||
| n=75 | ||||
| Osteocalcin (nmol/L) | ||||
| Unadjusted | −0.03 (0.02) | −0.01 (0.02) | ||
| Adjusted | −0.04 (0.02)* | −0.01 (0.02) | ||
| NTx (nmol BCE/L) | ||||
| Unadjusted | −0.1 (0.2) | −0.1 (0.2) | ||
| Adjusted | −0.5 (0.2)* | −0.3 (0.2) | ||
| Uncoupling Index (1 Z-score) | ||||
| Unadjusted | −0.1 (0.1) | −0.1 (0.1) | ||
| Adjusted | 0.1 (0.1) | 0.0 (0.1) | ||
Data are regression coefficients (SEs) for associations with glycemic control HbA1c, hemoglobin A1c; GHb, glycosylated hemoglobin; BCE, bone collagen equivalent
p<0.05
- Heel/forearm BMD: age, BMI, energy expenditure (HbA1c), history of smoking (GHb)
- Osteocalcin: age (HbA1c), waist-to-hip ratio, weight change in last year (GHb), history of smoking, menstrual cycle length, years since menarche
- NTx: weight change in last year (HbA1c), history of smoking, years since menarche, medical conditions associated with BMD, time in menstrual cycle (HbA1c)
- Index: age, waist-to-hip ratio, weight change in last year (HbA1c), alcohol consumption (GHb), history of hormone use (HbA1c), menstrual cycle length (HbA1c), month of year (HbA1c)
- GHb: number of blood samples used to calculate mean GHb
Discussion
In response to several unanswered questions surrounding an association between type 1 diabetes and bone fragility, this study begins to systematically explore the potential mechanisms involved, and attempts to address remaining concerns over the effect of type 1 diabetes on bone health independent of confounding. The results are consistent with the study’s a priori hypotheses that type 1 diabetes has an independent negative association with BMD and bone turnover, and that among individuals with type 1 diabetes, poor glycemic control is related to low BMD and low bone turnover.
Specifically, bone turnover is negatively affected, with low bone formation and uncoupled bone turnover favoring resorption, in women with type 1 diabetes compared to well-matched controls. Two-thirds (65%) of the women with type 1 diabetes demonstrated uncoupled bone turnover favoring resorption. This may result in a condition where the quantity of bone formed does not balance the quantity of bone removed, thus reducing BMD. However, the process likely occurs over an extended period of time as there was no association between bone turnover markers and BMD in this group of young women. This is consistent with prior research on premenopausal women without diabetes [33]. Importantly, other research has demonstrated that uncoupled bone turnover favoring resorption leads to an increased risk of fracture in postmenopausal women that is independent of BMD [34]. Therefore, uncoupled bone turnover may play an etiologic role in increased susceptibility to fracture in those with type 1 diabetes [2].
The above results are supported by basic science research showing that an uncoupling of bone turnover favoring resorption may be associated with type 1 diabetes. Animal and in vitro models of type 1 diabetes have consistently found fewer and more dysfunctional osteoblasts [35]. In contrast, osteoclast characteristics are often unchanged in animals with type 1 diabetes [35;36]. Bone formation may therefore be more impaired by type 1 diabetes than bone resorption leading to uncoupled bone turnover [37].
The study results also support the pathophysiologic role of hyperglycemia in bone fragility in those with type 1 diabetes. Women with type 1 diabetes and poor current glycemic control had low BMD and low bone turnover, including formation and resorption. This study is also the first to determine the effect of long-term glycemic control on bone health and found that poor control during the previous 10 years is also strongly associated with low BMD. Therefore, glycemic control appears to be associated with both the dynamic global process of bone remodeling on bone surfaces, and the more static quantity of bone mineral in regional sites throughout the body.
The adjusted coefficients quantifying a statistically significant decrease in BMD per one-percent increase in glycemic control range from 15–30% of the SD in BMD. For example, a one percent increase in current HbA1c is associated with a decrease equal to 15% of the SD in heel BMD. This may be clinically relevant as a one SD lower BMD is associated with a relative risk of fracture ranging from 1.4–2.6 in postmenopausal women [21]. An elevated HbA1c level may therefore have a detrimental effect on BMD and future fracture risk in addition to micro and macrovascular complications [25;38]. Again, the results are supported by basic science research which indicates that the low bone formation and deficient osteoblasts in type 1 diabetes described above, and consequent low BMD, may be a result of hyperglycemia [39]. For example, high levels of advanced glycated end-products of collagen have been found in animals with diabetes and are thought to directly inhibit osteoblast function by changing the bone cell-matrix interactions [39]. Lower collagen strength and bone brittleness may also result from glycosylation of collagen [37].
High blood glucose levels therefore appear to have a detrimental effect on bone health. However, recent experimental research demonstrating that osteocalcin can increase insulin sensitivity and pancreatic β-cell function [40] must also be considered. The low osteocalcin levels found in those with type 1 diabetes in the current study may therefore increase insulin resistance and subsequent blood glucose levels [40] in addition to hyperglycemia lowering bone formation. This recently discovered and potentially bidirectional association between glycemia and bone turnover may explain why only current and not long-term glycemic control was associated with bone turnover, particularly osteocalcin levels, in the current study. Clearly this area needs further longitudinal research.
Our study also found that heel and forearm BMD are low in premenopausal women with type 1 diabetes compared to controls after matching on unmeasured lifestyle factors and controlling for the confounding effects of body composition and health behaviors by statistical adjustment. The adjusted difference in heel and forearm BMD by diabetes status ranged from 57–70% of a SD in BMD. Again, this may be clinically relevant as a one SD lower BMD is associated with significant risk of fracture for postmenopausal women [21]. Our results are consistent with two well-designed previous studies which found low hip BMD in individuals with type 1 diabetes compared to controls, though they lacked data on the full spectrum of potential confounders [14;17]. These and previous findings therefore demonstrate that type 1 diabetes has an independent negative association with BMD in bone sites throughout the body with differing compositions of cortical and trabecular bone in premenopausal women, and of a magnitude that may increase fracture risk.
Further, we found that a higher proportion of women with type 1 diabetes had low BMD for age than did well-matched controls at the heel (24 vs. 7%) and forearm (48 vs. 28%). The largest prior study to estimate the frequency of low BMD associated with type 1 diabetes, whichused a clinic sample including premenopausal women and the World Health Organization criterion for osteopenia [41], found that 38% of individuals with diabetes had low BMD in either the hip or spine. Our estimate of 1/4 to 1/2 of premenopausal women with type 1 diabetes having peripheral BMD levels one SD or more below the mean BMD for their age is consistent with this finding.
The present study has several strengths. The sample of women with diabetes was homogenous with respect to gender, race, menopausal status, and disease duration. A concurrent control group without diabetes was utilized instead of relying on comparisons to the densitometer’s reference population which may systematically differ from the population of interest. To our knowledge, it is also the first study to use a matched control group consisting of relatives and friends without diabetes. The matching controlled for age, race/ethnicity, and many unmeasured confounders. The study also systematically measured and analyzed several potential confounders, and adjusted for those that were found to be most relevant. These strengths likely minimized differences in disease characteristics between women with diabetes, and in genetics [42] and lifestyle factors [43] between paired women with and without diabetes, to better estimate the true effect of the presence of type 1 diabetes on bone turnover and BMD. This study therefore has strong internal validity. The study was also sufficiently powered with its 75 well-matched pairs to detect clinically relevant differences in peripheral BMD.
There were however limitations to our research. The current study sample included primarily non-Hispanic white women. We do not know if the results also apply to men and persons of other ethnic groups with type 1 diabetes. If differences exist, prevention and treatment efforts for low BMD may be differentially targeted. The study included younger women who may not have reached peak bone mass. However, results did not change when the younger women were excluded. The study sample also included women who had and had not reached peak bone mass by diagnosis. Type 1 diabetes has been previously shown in well-designed studies to be associated with low BMD in individuals diagnosed in childhood [13] as well as after age 30 [17] when peak bone mass has been reached [44], indicating that the association occurs across the spectrum of age at onset. The effect of diabetes on BMD with childhood-onset may be function of both limited accrual of peak bone mass and accelerated loss after the peak is obtained, whereas the effect with adult-onset may be limited to the latter. A wide spectrum of age at diagnosis is needed within the same study to determine whether lower BMD is associated with a younger at onset; future analyses are planned for this sample to explore this question. The current study limited measurements of hip and spine BMD to a subgroup of participants as part of the validation of peripheral BMD. A systematic study of differences in hip and spine BMD by diabetes status, with thorough consideration of confounding and genetics, is needed. Information on medical conditions that affect bone and have a higher incidence in type 1 diabetes, such as thyroid and celiac disease, were limited to self-report. However, with the high use of health care by individuals with type 1 diabetes, such conditions may be more likely to be diagnosed and correctly reported. Finally, although the women who participated had similar baseline characteristics to the women originally enrolled in the WDRS, it is possible that diabetes care and disease progression may differ between the women who did and did not participate in the current study.
Given the silent nature of bone loss and that fracture risk increases with age, there are several implications of our results. First, there is a need to educate health professionals and individuals living with type 1 diabetes regarding the risk posed to bone health, even at a young age. Only 10% of the women with diabetes in our study had been informed by a health care provider that diabetes may affect their bones. Second, it may be important to include type 1 diabetes as a risk factor when considering the need for BMD screening in women [45]. Finally, education and screening will not be effective without the means to prevent and treat low BMD in individuals with type 1 diabetes. Based on our results, intensive glycemic control may have a potential role in the treatment of weak bones and prevention of fracture. However, as good glycemic control is often difficult to achieve, pharmaceutical trials to improve BMD in women with type 1 diabetes are also needed. Antiresorptive medications (e.g. bisphosphonates) may not be useful in these women because of the normal levels of bone resorption demonstrated by the present study. Medications under development that increase bone formation may be more relevant. It will also be important to determine whether diabetes complications such as nephropathy mediate the effect of, or act in addition to, poor glycemic control on bone, in order to fully understand the mechanisms of bone fragility in type 1 diabetes and in turn develop effective interventions. These analyses are currently being conducted using data from previous follow-up of the WDRS.
In summary, BMD and bone turnover were negatively altered in premenopausal women with type 1 diabetes, despite better treatment and glycemic control compared to older studies. Poor glycemic control was associated with low BMD and altered bone turnover and these factors may contribute to the increased risk of fracture seen in this group [2]. Given the increasing incidence of type 1 diabetes [3], and that people may live longer with the disease [4], more individuals with type 1 diabetes may live long enough to be at risk for fracture. We anticipate that further research on skeletal risk in those with type 1 diabetes, will lead to improved bone health and decrease the costs of fracture. Importantly, efforts to prevent and treat bone fragility will need to be broadly targeted in women with type 1 diabetes as they age.
Acknowledgements
Funding: American Diabetes Association grant 1-05-CR-35, National Institutes of Health grant DK036904. We gratefully acknowledge the study participants and staff of the Wisconsin Women & Diabetes Study, Wisconsin Diabetes Registry Study, and Osteoporosis Clinic Research Program.
Footnotes
Disclosure statement: The authors have nothing to disclose.
The original publication is available at http://www.springerlink.com/openurl.asp?genre=article&id=doi:10.1007/s00198-008-0763-3.
References
- 1.Brown SA, Sharpless JL. Osteoporosis: an under-appreciated complication of diabetes. Clinical Diabetes. 2004;22:10–20. [Google Scholar]
- 2.Janghorbani M, Van Dam R, Willetd W, et al. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Amer J Epidemiol. 2007;166:495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 3.DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23:857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 4.Matsushima M, LaPorte RE, Maruyama M, et al. Geographic variation in mortality among individuals with youth-onset diabetes mellitus across the world. DERI Mortality Study Group. Diabetes Epidemiology Research International. Diabetologia. 1997;40:212–216. doi: 10.1007/s001250050665. [DOI] [PubMed] [Google Scholar]
- 5.Carnevale V, Romagnoli E, D'Erasmo E. Skeletal involvement in patients with diabetes mellitus. Diabetes Metab Res Rev. 2004;20:196–204. doi: 10.1002/dmrr.449. [DOI] [PubMed] [Google Scholar]
- 6.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 7.Hofbauer LC, Brueck CC, Singh SK, et al. Osteoporosis in patients with diabetes mellitus. J Bone Miner Res. 2007;22:1317–1328. doi: 10.1359/jbmr.070510. [DOI] [PubMed] [Google Scholar]
- 8.Gallacher SJ, Fenner JA, Fisher BM, et al. An evaluation of bone density and turnover in premenopausal women with type 1 diabetes mellitus. Diabet Med. 1993;10:129–133. doi: 10.1111/j.1464-5491.1993.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 9.Gunczler P, Lanes R, Paz-Martinez V, et al. Decreased lumbar spine bone mass and low bone turnover in children and adolescents with insulin dependent diabetes mellitus followed longitudinally. J Pediatr Endocrinol. 1998;11:413–419. doi: 10.1515/jpem.1998.11.3.413. [DOI] [PubMed] [Google Scholar]
- 10.Liu EY, Wactawski-Wende J, Donahue RP, et al. Does low bone mineral density start in post-teenage years in women with type 1 diabetes? Diabetes Care. 2003;26:2365–2369. doi: 10.2337/diacare.26.8.2365. [DOI] [PubMed] [Google Scholar]
- 11.Ponder SW, McCormick DP, Fawcett HD, et al. Bone mineral density of the lumbar vertebrae in children and adolescents with insulin-dependent diabetes mellitus. J Pediatr. 1992;120:541–545. doi: 10.1016/s0022-3476(05)82479-5. [DOI] [PubMed] [Google Scholar]
- 12.Miazgowski T, Czekalski S. A 2-year follow-up study on bone mineral density and markers of bone turnover in patients with long-standing insulin-dependent diabetes mellitus. Osteoporos Int. 1998;8:399–403. doi: 10.1007/s001980050082. [DOI] [PubMed] [Google Scholar]
- 13.Moyer-Mileur LJ, Dixon SB, Quick JL, et al. Bone mineral acquisition in adolescents with type 1 diabetes. J Pediatr. 2004;145:662–669. doi: 10.1016/j.jpeds.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 14.Strotmeyer ES, Cauley JA, Orchard TJ, et al. Middle-aged premenopausal women with type 1 diabetes have lower bone mineral density and calcaneal quantitative ultrasound than nondiabetic women. Diabetes Care. 2006;29:306–311. doi: 10.2337/diacare.29.02.06.dc05-1353. [DOI] [PubMed] [Google Scholar]
- 15.Pascual J, Argente J, Lopez MB, et al. Bone mineral density in children and adolescents with diabetes mellitus type 1 of recent onset. Calcif Tissue Int. 1998;62:31–35. doi: 10.1007/s002239900390. [DOI] [PubMed] [Google Scholar]
- 16.Valerio G, del Puente A, Esposito-Del Puente A, et al. The lumbar bone mineral density is affected by long-term poor metabolic control in adolescents with type 1 diabetes mellitus. Horm Res. 2002;58:266–272. doi: 10.1159/000066441. [DOI] [PubMed] [Google Scholar]
- 17.Tuominen JT, Impivaara O, Puukka P, et al. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care. 1999;22:1196–1200. doi: 10.2337/diacare.22.7.1196. [DOI] [PubMed] [Google Scholar]
- 18.Palta M, LeCaire T, Daniels K, et al. Risk factors for hospitalization in a cohort with type 1 diabetes. Wisconsin Diabetes Registry. Amer J Epidemiol. 1997;146:627–636. doi: 10.1093/oxfordjournals.aje.a009328. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Diabetes Mellitus: Report of a WHO Study Group. Geneva: WHO; Technical Report Series 727. 1985 (ed) [PubMed]
- 20.Prince R, Sipos A, Hossain A, et al. Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res. 2005;20:1507–1513. doi: 10.1359/JBMR.050501. [DOI] [PubMed] [Google Scholar]
- 21.Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: scientific review. JAMA. 2002;288:1889–1897. doi: 10.1001/jama.288.15.1889. [DOI] [PubMed] [Google Scholar]
- 22.Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures: the Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 23.Binkley N, Bilezikian JP, Kendler DL, et al. Summary of the international society for clinical densitometry 2005 position development conference. J Bone Miner Res. 2007;22:643–645. doi: 10.1359/jbmr.070204. [DOI] [PubMed] [Google Scholar]
- 24.Eastell R, Robins SP, Colwell T, et al. Evaluation of bone turnover in type I osteoporosis using biochemical markers specific for both bone formation and bone resorption. Osteoporos Int. 1993;3:255–260. doi: 10.1007/BF01623829. [DOI] [PubMed] [Google Scholar]
- 25.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 26.Duck SC, Lee M, D'Alessio D. 24–42 month stability of internal blood standards for glycated hemoglobin analysis. Diabetes Res Clin Pract. 1990;9:195–199. doi: 10.1016/0168-8227(90)90112-7. [DOI] [PubMed] [Google Scholar]
- 27.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Amer J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 28.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Amer J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 29.Gorai I, Chaki O, Nakayama M, et al. Urinary biochemical markers for bone resorption during the menstrual cycle. Calcif Tissue Int. 1995;57:100–104. doi: 10.1007/BF00298428. [DOI] [PubMed] [Google Scholar]
- 30.Pasco JA, Henry MJ, Kotowicz MA, et al. Seasonal periodicity of serum vitamin D and parathyroid hormone, bone resorption, and fractures: the Geelong Osteoporosis Study. J Bone Miner Res. 2004;19:752–758. doi: 10.1359/JBMR.040125. [DOI] [PubMed] [Google Scholar]
- 31.Clowes JA, Khosla S, Eastell R. Potential role of pancreatic and enteric hormones in regulating bone turnover. J Bone Miner Res. 2005;20:1497–1506. doi: 10.1359/JBMR.050524. [DOI] [PubMed] [Google Scholar]
- 32.Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 33.Looker AC, Bauer DC, Chesnut CH, III, et al. Clinical use of biochemical markers of bone remodeling: current status and future directions. Osteoporos Int. 2000;11:467–480. doi: 10.1007/s001980070088. [DOI] [PubMed] [Google Scholar]
- 34.Garnero P, Hausherr E, Chapuy M-C, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS prospective study. J Bone Miner Res. 1996;11:1531–1538. doi: 10.1002/jbmr.5650111021. [DOI] [PubMed] [Google Scholar]
- 35.Verhaeghe J, van Herck E, Visser WJ, et al. Bone and mineral metabolism in BB rats with long-term diabetes. Decreased bone turnover and osteoporosis. Diabetes. 1990;39:477–482. doi: 10.2337/diab.39.4.477. [DOI] [PubMed] [Google Scholar]
- 36.Ward DT, Yau SK, Mee AP, et al. Functional, molecular, and biochemical characterization of streptozotocin-induced diabetes. J Am Soc Nephrol. 2001;12:779–790. doi: 10.1681/ASN.V124779. [DOI] [PubMed] [Google Scholar]
- 37.Bouillon R. Diabetic bone disease. Calcif Tissue Int. 1991;49:155–160. doi: 10.1007/BF02556109. [DOI] [PubMed] [Google Scholar]
- 38.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group (2005) Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katayama Y, Akatsu T, Kado TS, et al. Glycated bone collagen in diabetic rats and its effects on osteoblast functions (abstract 535) Bone. 1995;16:219S. [Google Scholar]
- 40.Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rozadilla A, Nolla JM, Montana E, et al. Bone mineral density in patients with type 1 diabetes mellitus. Joint Bone Spine. 2000;67:215–218. [PubMed] [Google Scholar]
- 42.Pocock NA, Eisman JA, Hopper JL, et al. Genetic determinants of bone mass in adults: a twin study. J Clin Invest. 1987;80:706–710. doi: 10.1172/JCI113125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sowers MR, Galuska DA. Epidemiology of bone mass in premenopausal women. Epidemiol Rev. 1993;15:374–398. doi: 10.1093/oxfordjournals.epirev.a036126. [DOI] [PubMed] [Google Scholar]
- 44.Henry YM, Fatayerji D, Eastell R. Attainment of peak bone mass at the lumbar spine, femoral neck and radius in men and women: relative contributions of bone size and volumetric bone mineral density. Osteoporos Int. 2004;15:263–273. doi: 10.1007/s00198-003-1542-9. [DOI] [PubMed] [Google Scholar]
- 45.U.S. Preventive Services Task Force. Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med. 2002;137:526–528. doi: 10.7326/0003-4819-137-6-200209170-00014. [DOI] [PubMed] [Google Scholar]